Abstract
Aim Poly (ADP-ribose) polymerase (PARP) is a nuclear repair enzyme whose role is widely depicted in various physiological and pathological processes. In the present study, we wanted to check the status of PARP and the role of various cell death proteases involved in apoptotic and non-apoptotic forms of cell death during transient focal cerebral ischemia in rat model. The activation of these proteases can result in the production of PARP fragments which can be treated as specific signature fragments to the particular protease involved in the pathology and hence the type of cell death. Results In the ischemic samples, we observed activation of calpain, cathepsin-b, caspase-3, and granzyme-b which were known to act on and cleave PARP to produce specific signature fragments by Western blot and immunohistochemical analysis. Cresyl violet staining showed the presence of apoptotic and necrotic cell deaths. Further we observed interaction of AIF and gra-b with PARP in double immunofluorescence and co-immunoprecipitation experiments. Conclusion Activation of calpains, cathepsin-b, caspase-3, and granzyme-b correlated with either apoptotic or necrotic cell deaths in cresyl violet staining. The appearance of PARP signature fragments gives a clear idea on the involvement of particular protease in the pathology. Appearance of signature fragments like 89- and 50-kDa indicates the involvement of apoptotic and necrotic cell death in the pathology. Further interaction of AIF and gra-b with PARP also indicates the involvement of non-apoptotic modes of cell death during the pathology of focal cerebral ischemia.
Keywords: Cerebral ischemia, PARP, Granzyme-b, AIF, Apoptosis, Necrosis
Introduction
Cell death during cerebral ischemia is heterogeneous and is complex due to the involvement of several apoptogenic proteins (Yamashima 2000; Chaitanya and Babu 2008). A clear demarcation of apoptosis or necrosis based on biochemical and morphological events has become increasingly difficult. The involvement of several proteases of different families harbored at different locations, shuffling to other places during pathological events (Hayashi and Abe 2004), their cross talks at different time intervals (Neumar et al. 2003), ability to execute different modes of cell death independent or dependent of each other (Cho and Toledo-Pereyra 2008; Van Wijk and Hageman 2005; Dawson and Dawson 2004), inadequate knowledge of suitable markers for prior identification of the perspective victim, renders pharmacological intervention inefficient for the etiology of cerebral ischemia. Despite having adequate data on the involvement of several proteases in the pathology of cerebral ischemia, practically there are no effective pharmacological agents available for the diseased. Moreover, some of the proteases, once classically thought to be the guardians or protectors of the cell, have gradually emerged to have deleterious consequences upon continuous or over activation and Poly (ADP-ribose) polymerase (PARP) is one among them (Van Wijk and Hageman 2005).
PARP-1, a 113-kDa protein, is an abundant nuclear protein that is present in all nucleated cells of multi-cellular eukaryote organisms. On average, approximately one molecule of this enzyme is present per 1000 base pairs of DNA (De Murcia and Menissier de Murcia 1994). PARP-1 is involved in a variety of physiological and pathological events such as DNA replication, DNA repair, gene expression, cellular differentiation, chromatin decondensation, malignant transformation, inflammation, developmental aspect and apoptosis (Smith 2001; Herzeg and Wang 2001; Ziegler and Oei 2001; Hassa and Hottiger 2002). Activation of PARP-1 was demonstrated as the earliest and the most sensitive response of a cell to DNA damage and it was recognized as “a molecular nick sensor” (De Murcia and Menissier de Murcia 1994). PARP-1 accounts for at least 85% of maximal cellular PARP activity (Virag and Szabo 2002). The remaining activity is due to other PARP species, the physiological functions of which are less well characterized. In the scenario of cell death, PARP is one of the favorite substrates for a large number of suicidal proteases like caspase, calpain, cathepsin, and granzyme-b. Further it has been recently shown that cell death mediated by AIF independent of caspase is modulated by PARP-1 (Yu et al. 2006). Moreover, the ability of PARP-1 to interact and modulate the transcription factor NF-kB makes it crucial in the decisions of life and death of a cell (Hassa and Hottiger 2002).
It is well known that caspases mediate apoptotic cell death and calpains and cathepsin mediate both apoptotic and necrotic cell death depending on the intensity of the insult (Artal-Sanz et al. 2006; Xiuli et al. 2004; Stefan Reid and Yigong 2004). Granzyme-b, which was thought to play a role in autophagy or apoptosis, has also been shown to participate in necrotic death (O’Connell and Stenson 2007; Young et al. 2007). PARP is one of the preferred substrates for these proteases and generates fragments of different molecular weights which are considered to be the signature fragments of these proteases. Hence, a careful observation of the fragments can lead to an idea of the protease involved and to identify the type of cell death in progress. Hence, in the present study, we investigated the status of PARP and its fragments, the status of suicidal proteases that mediate the generation of PARP signature fragments and the type of cell death that was under operation during transient focal cerebral ischemia in rat.
Materials and Methods
Antibodies
Calpain antibody used for immunohistochemical analysis was purchased from RDI (Research Diagnostics Inc.), Flanders, NJ, and the antibody used for Western blot analysis was a gift from Dr. Spencer, University of California, LA. Cathepsin-b antibody was purchased from Oncogene, Sandiego, CA. Cleaved caspase-3 antibody, which detects p-20 fragment, used for immunohistochemical analysis and caspase-3 antibody for Western blot analysis and PARP antibody was purchased from CST (Cell Signaling and Technology), Beverly, MA. Granzyme-b antibody was purchased from Calbiochem, Germany. AIF primary rabbit polyclonal antibody was purchased from Oncogene, Sandiego, CA.
Surgical Procedure
All experiments were performed according to the guidelines of Institutional Animal Ethical Committee (IAEC). Male Wistar rats, weighing 300–350 g, were used in the present study. Rats were randomly divided into 3 groups. 1, sham group; 2, 1-h reperfused; 3, 1-day reperfused groups which were 3-h middle cerebral artery (MCA) occluded. MCAo was achieved by the nylon suture method (Longa et al. 1989; Kawamura et al. 1991), in which the left MCA was occluded by inserting a nylon monofilament through the external carotid artery to occlude origin of MCA for 3 h. The animals were anesthetized using N2O-halothane mixture through facemask. Briefly, a midline incision was made on the neck, and bifurcation of the left common carotid artery, left external carotid artery, and left internal carotid artery was separated. The external carotid artery was ligated distally and a 3-0 monofilament suture (50 mm in length) was inserted through an arteriectomy of the external carotid artery. The nylon suture was gently advanced from the external carotid artery into the internal carotid artery. The path of the suture toward the base of the skull was visualized. Approximately 17.5–18 mm of suture was inserted past the common carotid artery bifurcation to block the origin of the left MCA. The occlusion of the MCA was felt. For the sham-operated rats, the carotid arteries were exposed; suture was inserted but not extended to occlude the MCA. The animal was allowed to recover from anesthesia. After the occlusion period, the animal was re-anaesthetized and the filament was removed from the artery.
Three rats from 1-h reperfusion time period were perfusion-fixed with saline followed by 4% paraformaldehyde and the brains were dissected out for immunohistochemical analysis. Four rats from each group were killed with an overdose of pentobarbital, brains were removed quickly, and the ipsilateral ischemic regions were processed for immunoblotting.
Behavioral Tests
After the recovery of the animal from anesthesia, an observer who was masked to the experimental conditions performed behavioral functional tests (Murakami et al. 1998). Neurological deficits were scored as follows: no neurological deficit normal (0); failure to extend the right forepaw fully (1); circling to the right (2); unable to walk spontaneously (3); and dead (4). Animals showing no deficit were not taken into the study.
Western Blots
After decapitation, rat brains (n = 4) were dissected immediately and ipsilateral hemispheres were separated, snap frozen in liquid nitrogen, and stored at −80°C until analysis. Tissues were homogenized in the radio immunoprecipitation assay buffer containing 50 mM Tris–HCl pH 8.0, 150 mM NaCl, 1 mM EDTA, 0.4% deoxy chlolate, 1% non-idet P-40 containing protease inhibitors including 1 mM PMSF and phosphatase inhibitors including 10 mM β-glycerophosphate, 10 mM NaF, 0.3 mM Na3Vo4. The lysates were sonicated for 2 min in the equal intervals of time period and then centrifuged at 14,000g for 15 min at 4°C. Supernatants were collected into pre-chilled eppendorphs and used as protein samples for further analysis. Equal amounts of protein were separated using SDS-PAGE and further transferred onto nitro-cellulose membrane. The transferred immunoblots were blocked in non-fat skimmed milk (5%) in tris buffer saline (TBS; 10 mM Tris pH 7.5, 150 mM NaCl) for 1 h. The membranes were incubated for 12–14 h in primary antibodies raised against calpain, cleaved caspase-3, cathepsin-b, PSD-95, and spectrin. Blots were re-incubated with secondary antibodies conjugated to alkaline peroxidase (ALP) (anti-rabbit and anti-mouse IgG conjugated to ALP obtained from Genei Pvt Ltd, Bangalore, India), for 1 h at room temperature. Before and after incubation of blots with secondary antibodies, blots were washed with TBS and TBST (TBS containing 0.1% Tween 20). Immunoreactivity was visualized by incubating the blots in BCIP/NBT substrate.
Cresyl Violet Staining
Formalin-fixed, paraffin-embedded ischemic rat brain sections were deparaffinized in xylene, rehydrated in alcohol series, and incubated in 0.1% Cresyl violet solution for 3–5 min. The sections were then rinsed in distilled water and differentiated in 95% alcohol, followed by dehydration in 100% alcohol. The sections were then cleared in xylene and mounted using DPX mounting medium.
Immunohistochemistry
For immunohistochemical analysis (n = 3), rats after MCA occlusion were perfusion-fixed with saline and then with 4% paraformaldehyde solution. Brains were removed and post-fixed in the same fixative for another 24 h. Then each tissue block was dehydrated, embedded in paraffin, and cut into 3–4-μm-thick coronal sections. Paraffin was removed from slides using xylene, followed by rehydration in an alcohol dilution series. Antigen retrieval was performed using a microwave method. Slides were incubated for 20 min after slow boiling for 10 min and rinsed in PBS. Slides were soaked in 0.1% Triton-X 100 in PBS for 5 min to increase permeability of fixed tissue, followed by rinsing in 1× PBS. Endogenous peroxidase was blocked by incubation for 45 min in methanol containing 1.5% hydrogen peroxide and blocked using 10% normal goat serum for 1 h. The sections were then stained with monoclonal antibodies raised against calpain, cleaved caspase-3 (p-20 fragment) and cathepsin-b (diluted 1:100). After they were washed, the sections were overlaid for 1 h with peroxidase goat anti-mouse and anti-rabbit antibodies followed by developing with DAB complex (DAKO-kit). All incubations were performed under humidified conditions, and slides were washed 4 times for 5 min each in PBS between steps. Contralateral hemisphere, omission of primary or secondary antibody served as controls.
Calpain and Caspase Activity Assays
Calpain Activity
Calpain activity was measured using azocasein (Sigma–Aldrich) as substrate (Takeuchi et al. 1992; Shukla et al. 2006) at a final concentration 0.6% in 1.0 ml of reaction mixture containing 0.02% β-mercaptoethanol, 100 mM Tris–acetate buffer (pH 7.5), 10 mM KCl, 5 mM CaCl2, and 50 μg of enzyme solution. The control tubes were treated the same way except CaCl2 was replaced by EGTA (5 mM). The reaction was incubated for 30 min at 37°C and was stopped by the addition of 0.4 ml of 20% trichloro acetic acid and left on ice for 30 min. The samples were centrifuged at 6000 rpm for 1 h and absorbance of supernatant was read at 366 nm using Shimadzu spectrophotometer.
Caspase Activity
Caspase activity of the cells undergoing apoptosis was determined using Ac-DEVD-AFC hydrolysis in the tissue lysates. In total, 50 μg of protein from ipsilateral and contralateral tissue lysates was incubated in caspase assay buffer consisting of 20 mM Tris–HCl buffer (pH 7.2) containing 1 mM Mg2+, 80 mM KCl, and 1 mM DTT) and 5 μg of Ac-DEVD-AFC and the volume was made up to 500 μl. The reaction mixture was incubated at 37°C for 60 min. Ac-DEVD-AFC hydrolysis was monitored by fluorescence emission of the released AFC (excitation, 400 nm; emission, 500 nm) using a Fluoromax-3, Jobin Yvon, Horiba spectrofluorometer as described earlier (Yadaiah et al. 2007; Bhuyan et al. 2001).
Results
Existence of Apoptotic and Necrotic Cell Deaths During Cerebral Ischemia
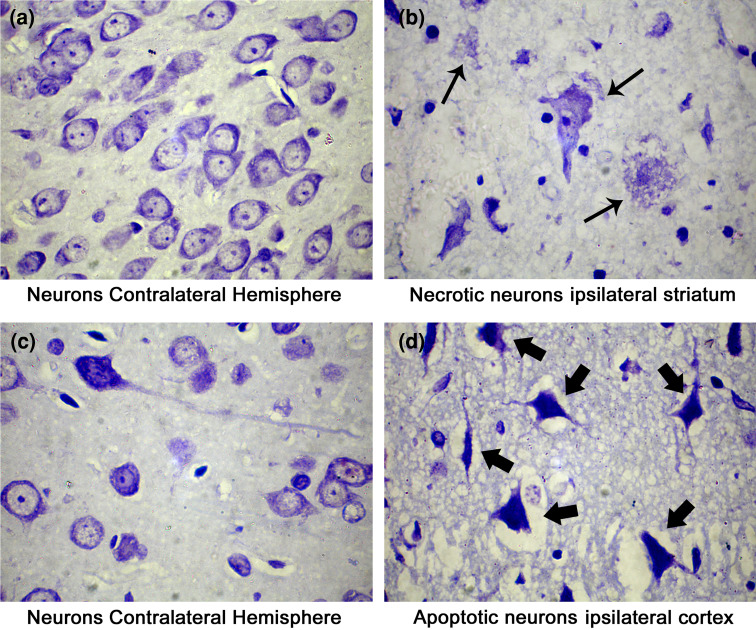
Cerebral ischemia results in an infarct comprising of necrotic core and apoptotic penumbra. Recent studies have pointed out that necrosis rather apoptosis might be a key player in the etiology of cerebral ischemia. We observed differential modes of cell death, i.e. apoptotic and necrotic cell deaths, during cerebral ischemia. Cresyl violet staining of the ischemic brain sections revealed necrotic cells identified by cellular oncosis, loss of membrane integrity, and burst of the cell in the striatum. Apoptotic cells identified with their shrunken shape, vacuolation, and dense staining were significantly high in the cortical region (Fig. 1). The presence of heterogeneous cell deaths in different regions of the ischemic brain points toward the involvement of different proteases in mediating different modes of cell death in different regions of the ischemic brain.
Fig. 1.
Cresyl violet stained rat brain sections. Cresyl violet stains the nissil granules of the neurons and renders purple color to neurons. Necrotizing neurons are indicated by the small arrows in the striatum of ipsilateral hemisphere, apoptotic neurons are indicated by large arrows in the cortex of the ipsilateral hemisphere, and normal neurons in the striatum and cortex of contralateral hemisphere can be observed. Bright field images were taken with Olympus UCTR30-2 fluorescent microscope
Elevation of Calpains, Cathepsin-b, Granzyme-b, and Active Caspase-3 Levels During Cerebral Ischemia
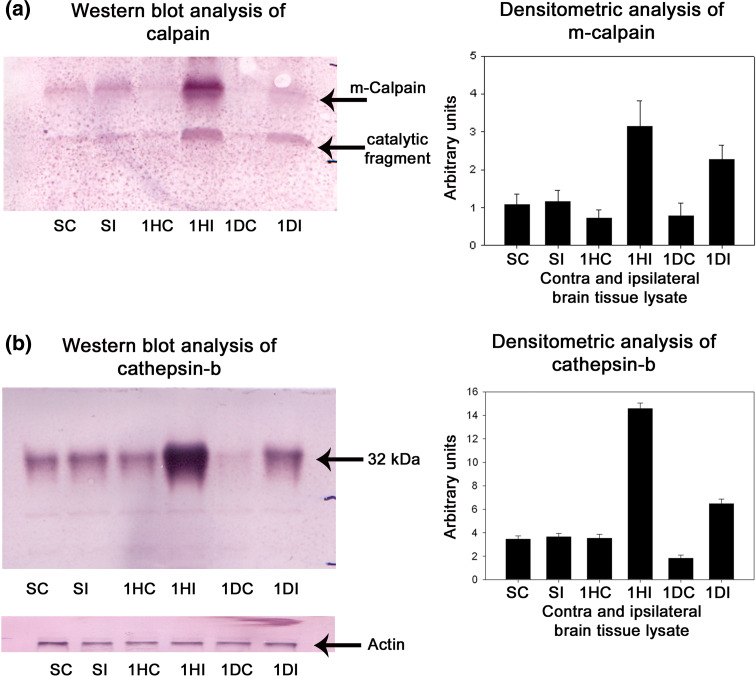
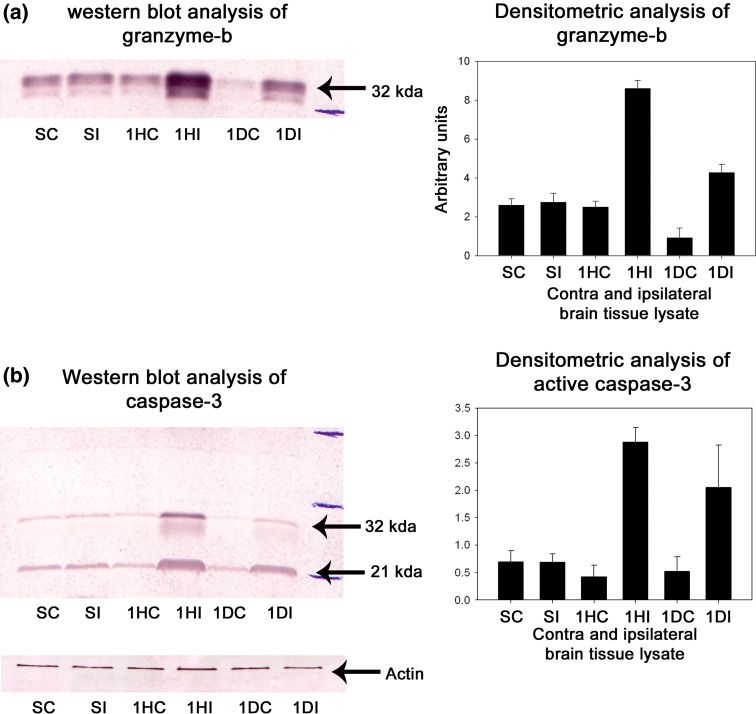
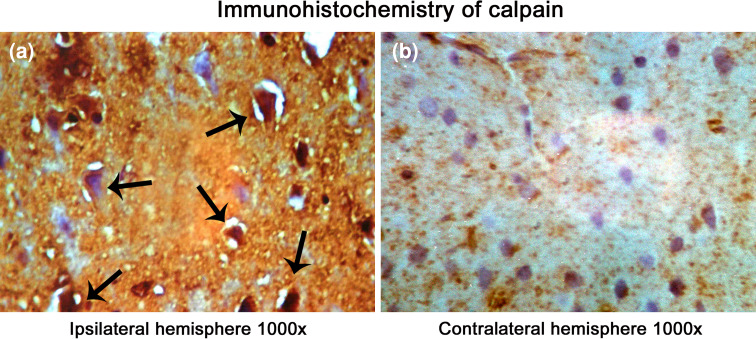
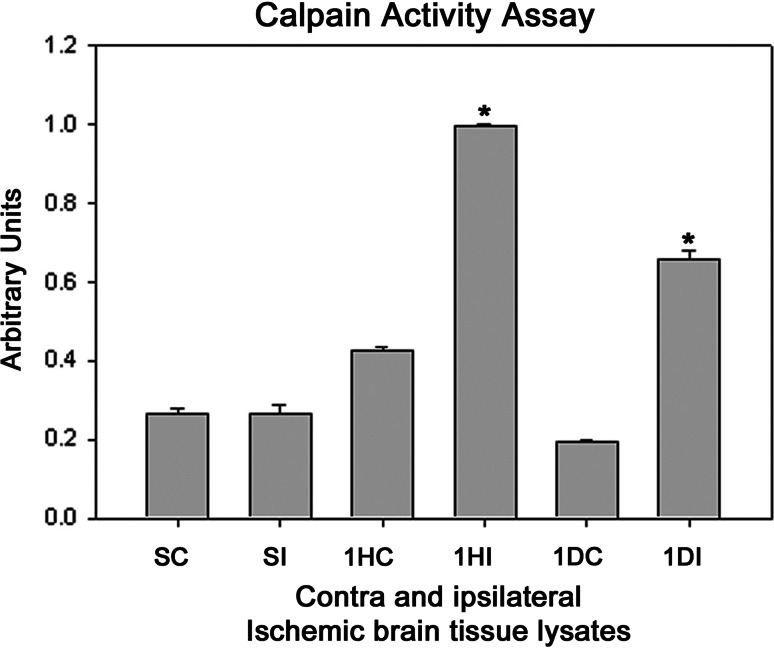
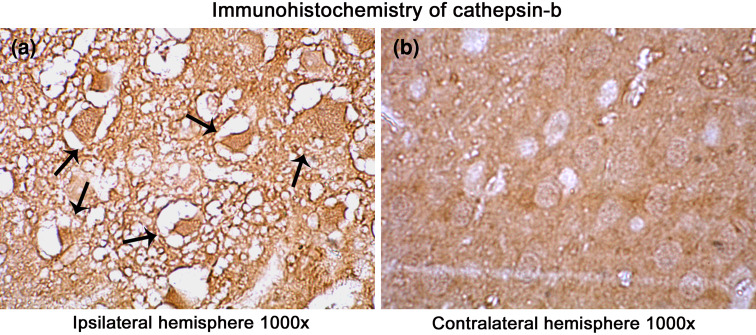
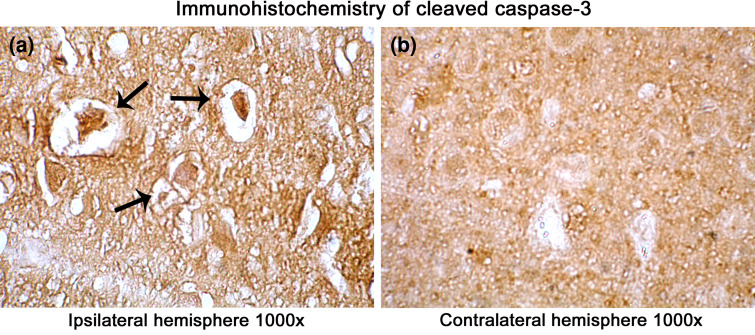
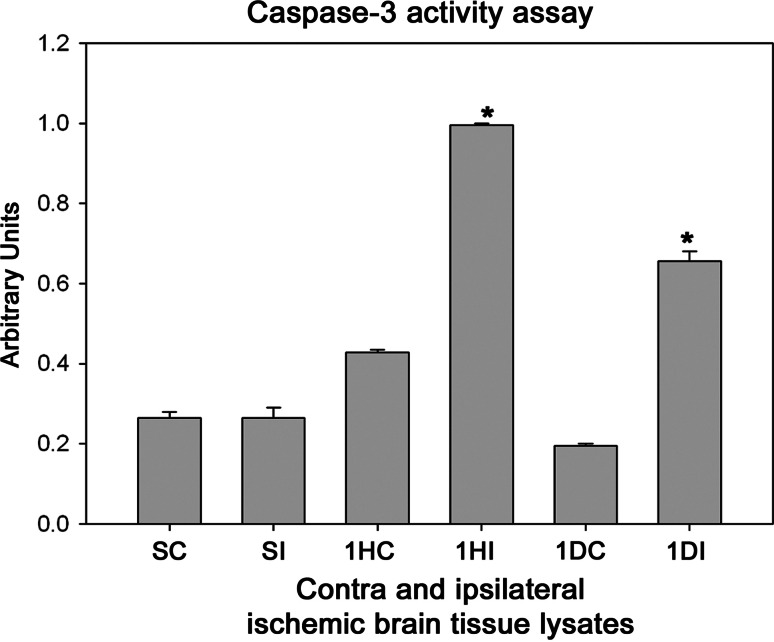
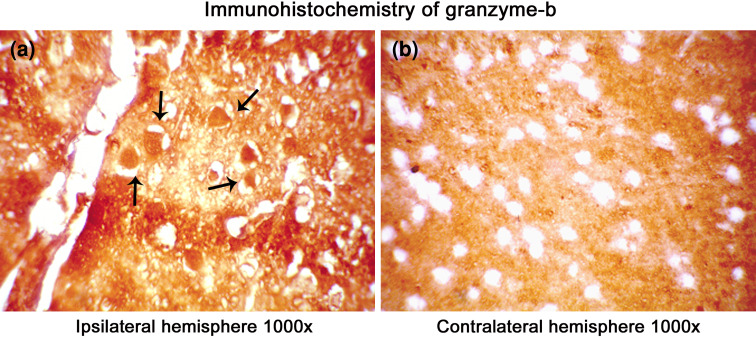
Several protease mediating apoptotic and necrotic cell deaths were found to be up-regulated during the ischemia/reperfusion in ischemic rat brain. Western blot analysis revealed the dramatic elevation of calpains, cathepsin-b, granzyme-b which can modulate both apoptotic and necrotic cell deaths by 1-h reperfusion time period, respectively (Figs. 2a, b and 3a). Active caspase-3, which is known to mediate apoptotic cell death, was also found to be elevated by 1-h reperfusion time period lasting till 1 day of reperfusion (Fig. 3b). The elevation of these proteases was robust at 1-h reperfusion time period. Immunohistochemical analysis with calpain, cathepsin-b, caspase-3, and granzyme-b revealed differential localization of these proteases. Calpain immunohistochemistry has shown the presence of this protease mostly in the striatum correlating with necrotic cell death identified by cresyl violet staining (Fig. 4). Further calpain activity was maximal at 1-h followed by 1-day reperfusion time periods in the ipsilateral hemispheres over the contralateral hemisphere correlating with their elevated levels in the Western blot analysis (Fig. 5). Cathepsin-b immunohistochemistry has shown the presence of this protease mostly in the striatum correlating with calpain immunohistochemistry and necrotic cell death identified by cresyl violet staining (Fig. 6). Active caspase-3 immunohistochemistry revealed the presence of this protease mostly in the cortical region correlating with apoptotic cell death (Fig. 7) and its activity was also maximal at 1-h and 1-day reperfusion time periods in the ipsilateral hemispheres over the contralateral hemisphere correlating with the elevated level cleaved caspase-3 levels in the Western blot analysis (Fig. 8). Immunohistochemistry with granzyme-b antibody has shown the presence of this protease both in cortical and striatal regions of the ischemic infarct (Fig. 9). The elevation of these suicidal proteases correlated with either apoptotic or necrotic cell deaths or both of them cell deaths that were observed with cresyl violet staining during cerebral ischemia.
Fig. 2.
a, b Western blot and densitometric analysis of calpain and cathepsin-b levels during cerebral ischemia in rat brain, respectively. Representative blot of three individual Western blots of calpain and cathepsin-b. A significant increase in the calpain and cathepsin-b levels was observed in ischemic ipsilateral samples over the contralateral samples starting from 1 h of reperfusion till 1-day reperfusion. Densitometric analysis for calpain was performed on full-length m-calpain. SC sham contralateral, SI sham ipsilateral, 1HC 1 h contralateral, 1HI 1 h ipsilateral, 1DC 1 day contralateral, 1DI 1 day ipsilateral samples, respectively, after 3 h occlusion. Densitometric analysis was performed using NIH image analysis software
Fig. 3.
a, b Western blot and densitometric analysis of gra-b and caspase-3 levels in ischemic rat brain. Representative blot of four individual Western blots of gra-b and caspase-3. A significant increase in the gra-b and caspase-3 levels was observed in ischemic ipsilateral samples over the corresponding contralateral samples starting from 1-h reperfusion till 1 day of reperfusion. SC sham contralateral, SI sham ipsilateral, 1HC 1 h contralateral, 1HI 1 h ipsilateral, 1DC 1 day contralateral, 1DI 1 day ipsilateral reperfused samples, respectively, after 3 h occlusion. Densitometric analysis was performed using NIH image analysis software
Fig. 4.
Immunohistochemical localization of calpain in the ischemic rat brain. A significant increase in the localization of calpain in the ipsilateral hemisphere than contralateral is visible. Images were taken under a magnification of 1000× to have a clear view of cellular localization of calpains in the infarct. Pyknotic cells and few shrunken cells resembling apoptotic cells stained positive for calpain in striatum and cortex (a) of ipsilateral hemisphere over contralateral hemisphere (b) was observed. Arrows point toward the degenerating cells ion the ipsilateral hemisphere. Photographs were taken with Nikon Alphaphot YS2 microscope
Fig. 5.
Calpain activity in the contralateral and ipsilateral ischemic rat brain tissue lysates. Activity was determined by spectrophotomery using azocasein as the substrate. The bars are normalized with reference to maximal absorbance of the released trichloroacetic acid-soluble peptides from azocasein after the addition of 50 μg of tissue lysate. SC sham contralateral, SI sham ipsilateral, 1HC 1 h contralateral, 1HI 1 h ipsilateral, 1DC 1 day contralateral, 1DI 1 day ipsilateral reperfused samples, respectively, after 3 h occlusion. P < 0.05 was considered significant
Fig. 6.
Immunohistochemical localization of cathepsin-b in the ischemic rat brain. A significant increase in the localization of cathepsin-b in the ipsilateral hemisphere than contralateral is visible. Images were taken under a magnification of 1000× to have a clear view of regional and cellular localization of cathepsin-b in the infarct. Arrows point toward the degenerating cells in the ipsilateral hemisphere (a) over the contralateral hemisphere (b). Bright field images were taken with Olympus UCTR30-2 fluorescent microscope
Fig. 7.
Immunohistochemical localization of cleaved caspase-3 in the ischemic rat brain. A significant increase in the localization of active caspase-3 in the ipsilateral hemisphere than contralateral is visible. However, increase in the immunoreactivity is mostly confined to apoptotic cells in the cortex than striatum. Images were taken under a magnification of 1000× to have a clear view of regional and cellular localization of active caspase-3 in the infarct. Arrows point toward the degenerating cells in the ipsilateral hemisphere (a) over the contralateral hemisphere (b). Bright field images were taken with Olympus UCTR30-2 fluorescent microscope
Fig. 8.
Caspase activity in the contralateral and ipsilateral hemispheres of the ischemic rat brain tissue lysates using Ac-DEVD-AFC hydrolysis. The bars are normalized with reference to the maximal hydrolysis (fluorescence) observed after addition of 50 μg of tissue lysate. SC sham contralateral, SI sham ipsilateral, 1HC 1 h contralateral, 1HI 1 h ipsilateral, 1DC 1 day contralateral, 1DI 1 day ipsilateral reperfused samples, respectively, after 3 h occlusion. P < 0.05 was considered significant
Fig. 9.
Immunohistochemical localization of gra-b in the ischemic rat brain. A significant increase in the localization of gra-b in the ipsilateral hemisphere compared to the contralateral hemisphere is visible. Immunoreactivity was not found in the cells of the contralateral hemisphere. Images were taken under a magnification of 1000× to give a clear view of regional and cellular localization of gra-b in the infarct. Arrows point toward the degenerating cells in the ipsilateral hemisphere (a) over the contralateral hemisphere (b). Bright field images were taken with an Olympus UCTR30-2 fluorescent microscope
Interaction of PARP with AIF
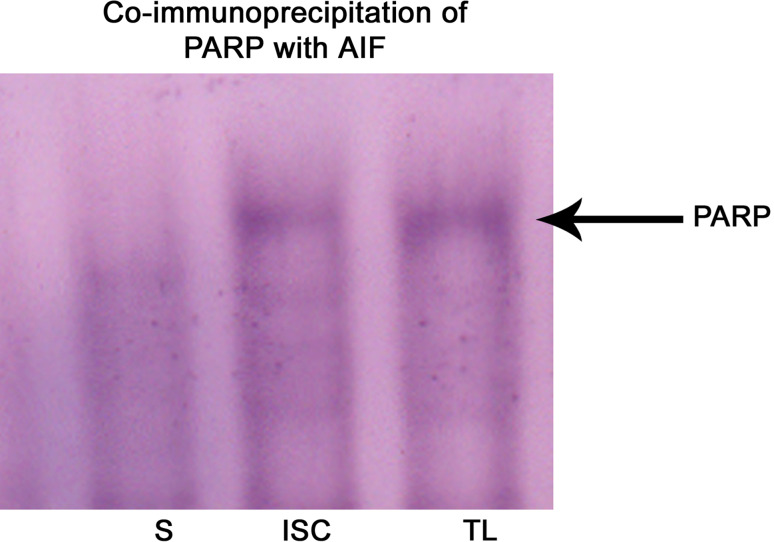
PARP, apart from its main function of repairing DNA, was known to mediate necrotic cell death as well as cell death mediated by AIF. Co-immunoprecipitation experiment with AIF revealed the interaction of PARP with AIF during cerebral ischemia (Fig. 10). PARP was known to interact with AIF and helps in the nuclear translocation of AIF, resulting in large-scale DNA fragmentation. Hence, this observation indicates that PARP acts as a double-edged sword depending on the intensity of cellular insults.
Fig. 10.
Co-immunoprecipitation of PARP-1 with AIF respectively. AIF antibody was used to isolate the antibody-antigen complex and Western blot analysis was performed to identify the interacting proteins. Western blot analysis showed the presence of PARP interacting with AIF in the ipsilateral hemisphere during cerebral ischemia. S sham-operated ipsilateral tissue lysate, I tissue lysate from 1-h reperfused ischemic sample, TL whole tissue lysate of ipsilateral hemisphere which was known to contain elevated levels of PARP. TL without immunoprecipitation was used as positive control
Differential Cleavage of PARP
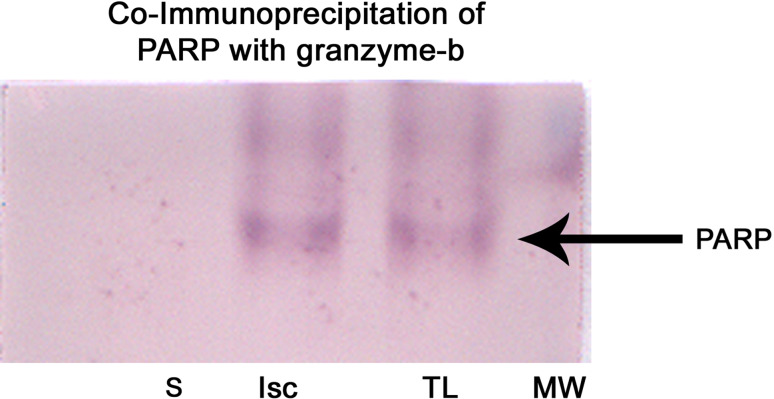
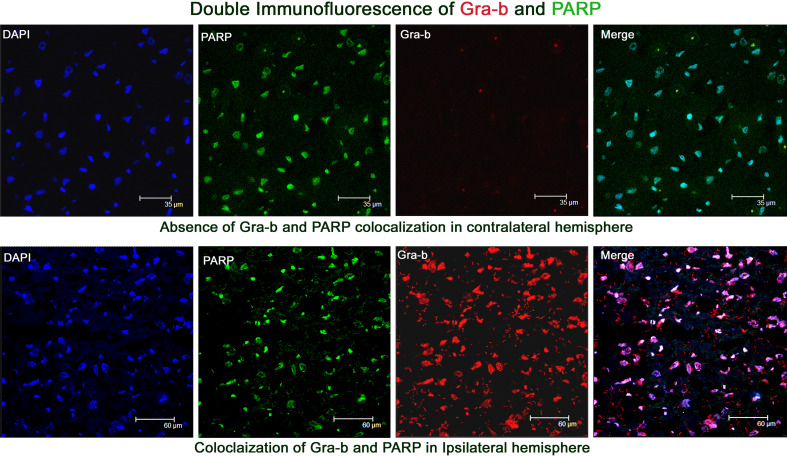
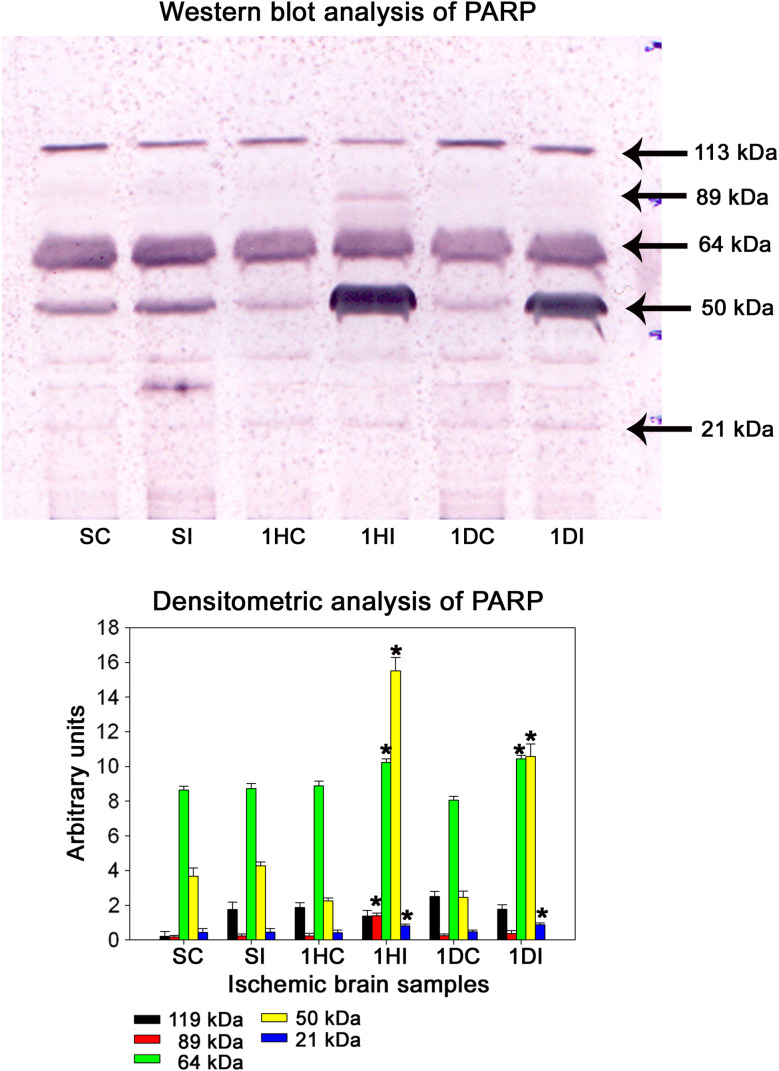
PARP is one of the substrates which stands in the front row for a variety of suicidal proteases. Co-immunoprecipitation (Fig. 11) and double immunofluorescence analysis revealed the interaction of granzyme-b with PARP during the pathology of cerebral ischemia (Fig. 12). Caspases, calpains, cathepsin, and granzymes were the most important proteases which were known to cleave PARP and liberate specific signature fragments. The presence of these signature fragments also gives an idea about the involvement of the suicidal protease particular to pathology. We observed PARP break-down signature fragments of molecular weights 89-, 64-, 50-, and 21-kDa (Fig. 13). The presence of 89- and 21-kDa signature fragments correlated with the activation of caspase-3 and the presence of apoptotic cell deaths and presence of 50-kDa fragment correlated with cathepsin-b and necrotic cell death and the presence of 64-kDa fragment correlated with the elevated levels of granzyme-b.
Fig. 11.
Co-immunoprecipitation of PARP with gra-b. Gra-b antibody was used to isolate the antibody-antigen complex and Western blot analysis was performed to identify the interacting proteins. Western blot analysis showed the presence of PARP as one of the proteins interacting with gra-b during cerebral ischemia. S sham-operated ipsilateral tissue lysate, I tissue lysate from 1-h reperfused ischemic sample, TL whole tissue lysate of ipsilateral hemisphere which was known to contain elevated levels of PARP. TL without immunoprecipitation was used as positive control
Fig. 12.
Double immunofluorescence analysis of PARP and granzyme-b. DAPI fluorescence was depicted in blue, PARP immunofluorescence was depicted in green, gra-b immunofluorescence was depicted in red and merged image showing the co-localization of gra-b with PARP. Co-localization of gra-b with PARP was observed in ipsilateral hemisphere over the contralateral hemisphere
Fig. 13.
Western blot analysis of PARP in ischemic rat brain. Representative blot of three individual Western blots of PARP. A significant increase in the 89, 64, 50, and 21 kDa fragments was observed in ischemic samples as compared to the sham-operated samples starting from the 1-h reperfusion till 1-day reperfusion in the 3-h MCA occluded samples. Densitometric analysis was performed on 89, 64, 50, and 21 kDa fragments using NIH image analysis software. P > 0.05
Discussion
Mechanical occlusion of any blood vessel carrying blood to the brain results in an infarct comprising of central necrotic core surrounded by transient region and apoptotic penumbra (Mehta et al. 2007; Graham and Chen 2001). Ischemic cell death is very complex due to the activation of several suicidal proteases, difference in activation time periods, complex cross talks between them activating one another and their ability to execute different modes of cell death independent or dependent of each other (Cho and Toledo-Pereyra 2008; Neumar et al. 2003). Calpains and cathepsins were known to mediate both apoptotic and necrotic cell deaths, whereas caspases were implicated only in apoptotic cell deaths (Artal-Sanz et al. 2006; Xiuli et al. 2004; Stefan Reid and Yigong 2004). Recently, the serine protease granzyme-b, which is known to be secreted by activated CTLs and NK cells, was shown to mediate non-apoptotic mode of cell death (necrotic) apart from classical apoptotic cell death which is dependent of caspase activation (Young et al. 2007).
Understanding molecular mechanisms during acute ischemic insult which results in heterogeneous cell death is very difficult. Recent data suggest that necrosis rather than apoptosis appears to be the crucial component of the damage to the nervous system during human ischemic injuries and neurodegenerative diseases (Haunstetter and Izumo 1998). Studies in primates indicate that damage to the lysosomal membrane is inflicted enzymatically by activated calpains. Calpains localize to lysosomal membranes after the onset of ischemic episodes with subsequent spillage of cathepsins into the cytoplasm (Syntichaki et al. 2005). This observation led to the formulation of the “calpain–cathepsin hypothesis,” whereby the calcium-mediated activation of calpains results in the rupture of lysosomes and leakage of killer cathepsins that eventually dismantle the cell (Xiuli et al. 2004; Artal-Sanz et al. 2006). Calpains, caspases, and granzymes cleave many common substrates including cytoskeletal and regulatory proteins (Yamashima et al. 2003). In addition, these protease systems appear to modulate each other via calpain-mediated cleavage and activation of caspases 3, 7, 8, 9 (Wang 2000) and caspase-3-mediated cleavage of calpastatin, which is endogenous inhibitor of calpain, thereby activating calpain (Kato et al. 2000) and granzyme-b-mediated activation of caspase-3 directly or via Bid. Cathepsin-b has also been shown to activate caspase either directly or indirectly. Taking together these data it becomes clearly understood that the ischemic cell death is modulated by a network of suicidal proteases, and inhibition of any single protease might not be sufficient to mitigate the ischemic injury.
Recent reports have shown that PARP is cleaved to 89- and 21-kDa signature fragments by caspases, 70- and 40-kDa signature fragment by calpains, 50-kDa necrotic fragment by cathepsin-b, and 64-kDa signature fragments by granzyme-b (Froelich et al. 1996; Gobeil et al. 2001; Ferrer and Planas 2003). The presence or elevation of these fragments gives a clear indication of the protease involved and indirectly the type of death involved. However, as some of the proteases mentioned earlier participate in caspase-independent apoptotic cell deaths and necrotic cell deaths, it becomes quite difficult to elucidate the type of cell death accurately. We observed significant increase in the levels of calpains, cathepsin-b, active caspase-3, and granzyme-b levels indicating involvement of multiple protease systems and the possibility of heterogeneous cell death. The presence of necrotic cells correlated with the activation of calpains and cathepsin-b, and the presence of apoptotic cells correlated with the activation of caspase-3. Further the appearance of 89- and 21-kDa apoptotic fragments and 50-kDa necrotic fragment indicates the existence of heterogeneous cell death in the infarct of focal cerebral ischemia. Moreover, calpains, cathepsin-b, and granzyme-b can also be involved in the apoptotic cell death. Further the elevation of granzyme-b levels, which is known to be secreted from CTLs and NK cells, gives an indication of the involvement of immune responses in the pathology of cerebral ischemia (Hurn et al. 2007; Yilmaz et al. 2006).
Multi-factorial functions have been attributed to PARP which include cell death as well as cell survival (Smith 2001). Recent experimental data suggest that post-ischemic activation of PARP-1 occurs practically in every cell type of the brain-affected region and significantly contributes to the extension of the final damage. Genetic suppression of either PARP-1 or PARP-2 or administration of appropriate doses of PARP inhibitors within a reasonable time drastically reduces the infarct volume of stroke in rodent models, thus suggesting that PARP inhibitors may reduce stroke-induced neurological damage (Ikeda et al. 2005; Koh et al. 2004). Further PARP has been implicated in inflammatory responses and as a modulator of AIF-mediated cell death (Hassa and Hottiger 2002; Yu et al. 2006). Hence, cleavage of PARP by caspases might be beneficial in reducing inflammatory responses and cell death mediated by PARP-1 (Nicotera et al. 1998; Ha and Snyder 1999). Though granzyme-b mediated 74-kDa fragment has been shown to have activity, its role contributing to cell death remains unknown (Froelich et al. 1996). Further it is known that calpains and cathepsin-b can activate caspase-3 either directly or indirectly by their cross talks (Yamashima et al. 2003; Lomgren et al. 2001), but why and how are these proteases set apart from activating caspase-3 during acute necrotic cell death needs to be investigated. Shedding more light into this aspect will help in further identifying putative molecules whose alteration can be of high use in the pathology of cerebral ischemia.
Focal cerebral ischemia results in cell death, which is considered to be chaotic. In the present study, the status of PARP during transient focal cerebral ischemia has been studied. The presence of specific PARP signature fragments generated by caspase, cathepsin-b, and granzyme-b correlates with the activation of these proteases and the type of cell death they are known to modulate. Hence, differential PARP cleavage during cerebral ischemia indicates the involvement of multiple protease systems and differential mode of cell death. Further its cleavage pattern can be used as one of the markers for the existence of specific proteases in the etiology and the type of cell death being executed.
Acknowledgments
GVC acknowledges financial help from UGC, New Delhi, India, in the form of SRF. Financial assistance from DBT, ICMR is gratefully acknowledged. Generous gift of Dr. Spencer for calpain antibodies is acknowledged. Mrs. M. Pushpanjali’s help from Prof. K. V. A. Ramaiah Laboratory, University of Hyderabad, India, in performing caspase activity assay is gratefully acknowledged.
References
- Artal-Sanz M et al (2006) Lysosomal biogenesis and function is critical for necrotic cell death in Caenorhabditis elegans. J Cell Biol 173:231–239. doi:10.1083/jcb.200511103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhuyan AK et al (2001) Resting membrane potential as a marker of apoptosis: studies on Xenopus oocytes microinjected with cytochrome c. Cell Death Differ 8:63–69. doi:10.1038/sj.cdd.4400773 [DOI] [PubMed] [Google Scholar]
- Chaitanya GV, Babu PP (2008) Activation of calpain, cathepsin-b and caspase-3 during transient focal cerebral ischemia in rat model. Neurochem Res 33:2178–2186 [DOI] [PubMed] [Google Scholar]
- Cho BB, Toledo-Pereyra LH (2008) Caspase-independent programmed cell death following ischemic stroke. J Invest Surg 21:141–147. doi:10.1080/08941930802029945 [DOI] [PubMed] [Google Scholar]
- Dawson VL, Dawson TM (2004) Deadly conversations: nuclear-mitochondrial cross-talk. J Bioenerg Biomembr 36:287–294. doi:10.1023/B:JOBB.0000041755.22613.8d [DOI] [PubMed] [Google Scholar]
- De Murcia G, Menissier de Murcia J (1994) Poly (ADP-ribose) polymerase: a molecular nick-sensor. Trends Biochem Sci 19:172–176. doi:10.1016/0968-0004(94)90280-1 [DOI] [PubMed] [Google Scholar]
- Ferrer I, Planas AM (2003) Signaling of cell death and cell survival following focal cerebral ischemia: life and death struggle in the penumbra. J Neuropathol Exp Neurol 62:329–339 [DOI] [PubMed] [Google Scholar]
- Froelich CJ et al (1996) Granzyme B/perforin-mediated apoptosis of Jurkat cells results in cleavage of poly (ADP-ribose) polymerase to the 89-kDa apoptotic fragment and less abundant 64-kDa fragment. Biochem Biophys Res Commun 23:658–665. doi:10.1006/bbrc.1996.1565 [DOI] [PubMed] [Google Scholar]
- Gobeil S et al (2001) Characterization of the necrotic cleavage of poly(ADP-ribose) polymerase (PARP-1): implication of lysosomal proteases. Cell Death Differ 8:588–594. doi:10.1038/sj.cdd.4400851 [DOI] [PubMed] [Google Scholar]
- Graham SH, Chen J (2001) Programmed cell death in cerebral ischemia. J Cereb Blood Flow Metab 21:99–109. doi:10.1097/00004647-200102000-00001 [DOI] [PubMed] [Google Scholar]
- Ha HC, Snyder SH (1999) Poly (ADP-ribose) polymerase is a mediator of necrotic cell death by ATP depletion. Proc Natl Acad Sci USA 96:13978–13982. doi:10.1073/pnas.96.24.13978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassa PO, Hottiger MO (2002) The functional role of poly (ADP-ribose) polymerase 1 as novel coactivator of NF-kappaB in inflammatory disorders. Cell Mol Life Sci 59:1534–1553. doi:10.1007/s00018-002-8527-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haunstetter A, Izumo S (1998) Apoptosis: basic mechanisms and implications for cardiovascular disease. Circ Res 82:1111–1129 [DOI] [PubMed] [Google Scholar]
- Hayashi T, Abe K (2004) Ischemic neuronal cell death and organellae damage. Neurol Res 26:827–834. doi:10.1179/016164104X3770 [DOI] [PubMed] [Google Scholar]
- Herzeg Z, Wang ZQ (2001) Functions of poly(ADP-ribose) polymerase (PARP) in DNA repair, genomic integrity and cell death. Mutat Res 477:97–110. doi:10.1016/S0027-5107(01)00111-7 [DOI] [PubMed] [Google Scholar]
- Hurn PD et al (2007) T and B cell deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab 27:1798–1805. doi:10.1038/sj.jcbfm.9600482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y et al (2005) Neuroprotective effects of KCL-440, a new poly(ADP-ribose) polymerase inhibitor, in the rat middle cerebral artery occlusion model. Brain Res 1060:73–80. doi:10.1016/j.brainres.2005.08.046 [DOI] [PubMed] [Google Scholar]
- Kato M et al (2000) Caspases cleaves the amino-terminal calpain inhibitory unit of calpastatin during apoptosis in human jurkat T cells. J Biochem 127:297–305 [DOI] [PubMed] [Google Scholar]
- Kawamura S et al (1991) Rat middle cerebral artery occlusion using an intraluminal thread technique. Acta Neurochir (Wien) 109:126–132. doi:10.1007/BF01403007 [DOI] [PubMed] [Google Scholar]
- Koh SH et al (2004) The effect of PARP inhibitor on ischaemic cell death, its related inflammation and survival signals. Eur J Neurosci 20:1461–1472. doi:10.1111/j.1460-9568.2004.03632.x [DOI] [PubMed] [Google Scholar]
- Lomgren K et al (2001) Synergistic activation of caspase-3 by m-calpain after neonatal hypoxia-ischemia: a mechanism of “pathologic apoptosis”. J Biol Chem 276:10191–10198. doi:10.1074/jbc.M007807200 [DOI] [PubMed] [Google Scholar]
- Longa EZ et al (1989) Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20:84–91 [DOI] [PubMed] [Google Scholar]
- Mehta SL et al (2007) Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Brain Res Rev 54(1):34–66. doi:10.1016/j.brainresrev.2006.11.003 [DOI] [PubMed] [Google Scholar]
- Murakami K et al (1998) Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci 18:205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumar RW et al (2003) Cross talk between Calpain and Caspase proteolytic systems during neuronal apoptosis. J Biol Chem 16:1462–1467 [DOI] [PubMed] [Google Scholar]
- Nicotera P et al (1998) Intracellular ATP, a switch in the decision between apoptosis and necrosis. Toxicol Lett 102–103:139–142. doi:10.1016/S0378-4274(98)00298-7 [DOI] [PubMed] [Google Scholar]
- O’Connell AR, Stenson CC (2007) A more serine way to die: defining the characteristics of serine protease-mediated cell death cascades. Biochim Biophys Acta 1773:1491–1499. doi:10.1016/j.bbamcr.2007.08.002 [DOI] [PubMed] [Google Scholar]
- Shukla M et al (2006) Activation of calpains, calpastatin and spectrin cleavage in the brain during the pathology of fatal murine cerebral malaria. Neurochem Int 48:108–113. doi:10.1016/j.neuint.2005.09.001 [DOI] [PubMed] [Google Scholar]
- Smith S (2001) The world according to PARP. Trends Biochem Sci 26:174–179. doi:10.1016/S0968-0004(00)01780-1 [DOI] [PubMed] [Google Scholar]
- Stefan Reid J, Yigong S (2004) Molecular mechanisms of Caspases during apoptosis. Nat Rev Mol Cell Biol 5:897–907. doi:10.1038/nrm1496 [DOI] [PubMed] [Google Scholar]
- Syntichaki P et al (2005) The vacuolar H+ ATPase mediates intracellular acidification required for neurodegeneration in C. elegans. Curr Biol 15:1249–1254. doi:10.1016/j.cub.2005.05.057 [DOI] [PubMed] [Google Scholar]
- Takeuchi KH et al (1992) Immunoassay and activity of calcium-activated neutral proteinase (mCANP): distribution in soluble and membrane-associated fractions in human and mouse brain. J Neurochem 58:1526–1532. doi:10.1111/j.1471-4159.1992.tb11374.x [DOI] [PubMed] [Google Scholar]
- Van Wijk SJ, Hageman GJ (2005) Poly(ADP-ribose) polymerase-1 mediated caspase-independent cell death after ischemia/reperfusion. Free Radic Biol Med 39:81–90 [DOI] [PubMed] [Google Scholar]
- Virag L, Szabo C (2002) The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev 54:375–429. doi:10.1124/pr.54.3.375 [DOI] [PubMed] [Google Scholar]
- Wang KK (2000) Calpain and caspase: can you tell the difference? Trends Neurosci 23:20–26. doi:10.1016/S0166-2236(99)01479-4 [DOI] [PubMed] [Google Scholar]
- Xiuli L et al (2004) The role of Calpain in oncotic cell death. Annu Rev Pharmacol Toxicol 44:349–370. doi:10.1146/annurev.pharmtox.44.101802.121804 [DOI] [PubMed] [Google Scholar]
- Yadaiah M et al (2007) High affinity binding of Bcl-xL to cytochrome c: possible relevance for interception of translocated cytochrome c in apoptosis. Biochim Biophys Acta 1774:1370–1379 [DOI] [PubMed] [Google Scholar]
- Yamashima T (2000) Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates. Prog Neurobiol 62:273–295. doi:10.1016/S0301-0082(00)00006-X [DOI] [PubMed] [Google Scholar]
- Yamashima T et al (2003) Sustained calpain activation is associated with lysosomal rupture executes necrosis of the post ischemic CA1 neurons in primates. Hippocampus 13:791–800. doi:10.1002/hipo.10127 [DOI] [PubMed] [Google Scholar]
- Yilmaz G et al (2006) Role of T lymphocytes and interferon-gamma in ischemic stroke. Circulation 113:2105–2112. doi:10.1161/CIRCULATIONAHA.105.593046 [DOI] [PubMed] [Google Scholar]
- Young HK et al (2007) Granzyme B leakage-induced apoptosis is a crucial mechanism of cell death in nasal-type NK/T-cell lymphoma. Lab Invest 87:241–250. doi:10.1038/labinvest.3700517 [DOI] [PubMed] [Google Scholar]
- Yu SW et al (2006) Apoptosis-inducing factor mediates poly(ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad Sci USA 103:18314–18319. doi:10.1073/pnas.0606528103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler M, Oei SL (2001) A cellular survival switch: poly(ADP-ribosyl)ation stimulates DNA repair and silences transcription. Bioessays 23:543–548. doi:10.1002/bies.1074 [DOI] [PubMed] [Google Scholar]