Simple Summary
Cancer is a global health problem caused by uncontrolled cell growth and changes in how cells get and use energy. This review compares cancer’s hidden metabolic changes to dark matter and dark energy in the universe, which are mysterious and often ignored. It looks at how cancer cells alter their energy use, such as through the Warburg effect and changes in fat and protein production, driven by genetic mutations. These changes help cancer grow and survive. The review suggests that targeting these metabolic pathways could be a new way to treat cancer. It calls for more research to better understand these changes and develop new therapies by focusing on the "dark energy" that fuels cancer cells.
Keywords: cancer, metabolic reprogramming, Warburg effect
Abstract
Background: Cancer remains a global health challenge, characterized not just by uncontrolled cell proliferation but also by the complex metabolic reprogramming that underlies its development and progression. Objectives: This review delves into the intricate relationship between cancer and its metabolic alterations, drawing an innovative comparison with the cosmological concepts of dark matter and dark energy to highlight the pivotal yet often overlooked role of metabolic reprogramming in tumor evolution. Methods: It scrutinizes the Warburg effect and other metabolic adaptations, such as shifts in lipid synthesis, amino acid turnover, and mitochondrial function, driven by mutations in key regulatory genes. Results: This review emphasizes the significance of targeting these metabolic pathways for therapeutic intervention, outlining the potential to disrupt cancer’s energy supply and signaling mechanisms. It calls for an interdisciplinary research approach to fully understand and exploit the intricacies of cancer metabolism, pointing toward metabolic reprogramming as a promising frontier for developing more effective cancer treatments. Conclusion: By equating cancer’s metabolic complexity with the enigmatic nature of dark matter and energy, this review underscores the critical need for innovative strategies in oncology, highlighting the importance of unveiling and targeting the “dark energy” within cancer cells to revolutionize future therapy and research.
1. Introduction
Cancer represents a challenge on a global scale, exerting profound morbidity and mortality rates that strain healthcare financing and strategic planning worldwide [1]. The burden of cancer disproportionately impacts individuals from lower socioeconomic backgrounds and those experiencing adverse social determinants, underscoring the disease’s intricate interplay with broader societal inequalities [2,3,4]. The variability in cancer prognosis is attributed to the inherent complexity of the disease, which is further complicated by limited access to treatment and a lack of comprehensive research aimed at understanding its progression.
Cancer’s complexity is rooted in its nature as a multifaceted disease characterized by uncontrolled cell division and growth. This process is driven by a myriad of cellular and molecular mechanisms designed to evade the body’s homeostatic controls and exploit this aberrant behavior for the survival of cancer cells. Such complexity is further amplified by the disease’s heterogeneity, encompassing a vast array of types and subtypes, each defined by unique characteristics and behaviors. These often arise from the combination of genetic susceptibility and environmental exposures that cause mutations in key regulatory genes [5,6]. These alterations disrupt the balance between cell proliferation and apoptosis, enabling cancer cells to evade normal regulatory checks. Consequently, cancer cells acquire capabilities for sustained proliferative signaling, apoptosis resistance, and angiogenesis induction, which are critical for tumor progression.
Employing a metaphorical lens, the progression of cancer can be likened to the elusive concepts of “dark matter” or “dark energy” in cosmology. This analogy serves to illuminate the critical yet often obscured role of metabolic reprogramming in the survival and proliferation of cancer cells, akin to the foundational yet mysterious forces that govern the universe’s structure and expansion. The metabolic pathways of cancer, shrouded within complex biochemical networks, pose significant challenges for research, necessitating a strategic approach that transcends direct cellular targeting to include a nuanced understanding and manipulation of these metabolic processes.
Through an interdisciplinary lens that merges insights from biology, chemistry, physics, and computational sciences, we aim to highlight the intricate metabolic networks governing cancer cells. By drawing parallels with the study of dark matter and energy, we underscore the pivotal role of metabolic understanding in oncology, framing it as a frontier ripe with challenges yet abundant with potential for groundbreaking therapeutic advancements.
2. Metabolic Reprogramming in Cancer Cells
A hallmark feature of cancer is its metabolic reprogramming, which diverges significantly from the metabolic pathways observed in normal cells [7]. This reprogramming, exemplified by the Warburg effect, facilitates a preference for glycolysis over oxidative phosphorylation for adenosine triphosphate (ATP) production, despite the inefficiency of this process [8]. This metabolic shift is crucial for supporting the rapid energy and biomass production necessary for cancerous growth. Furthermore, cancer cells exhibit modifications in several other metabolic pathways, including oxidative phosphorylation, generation of reactive oxygen species, de novo lipid synthesis, fatty acid β-oxidation, glutaminolysis, and mitochondrial metabolism [9,10,11], highlighting the extensive metabolic flexibility that cancer cells employ to thrive.
Metabolism constitutes a balanced interplay between the assimilation and breakdown of molecular components orchestrated within the cytoplasm, mitochondria, and endoplasmic reticulum—key sites for a myriad of metabolic pathways. This equilibrium is essential for cell proliferation, necessitating upregulated metabolic activity to support the synthesis of proteins, nucleic acids, and lipids driven by growth factors [12]. A pivotal aspect of this regulatory mechanism is the enhanced uptake and utilization of glucose, culminating in glycolysis (Figure 1A) [13]. Under aerobic conditions, cells typically direct glucose-derived pyruvate through oxidative phosphorylation in the mitochondria for the efficient production of energy [14]. In contrast, under anaerobic conditions, pyruvate is used for lactate production [15].
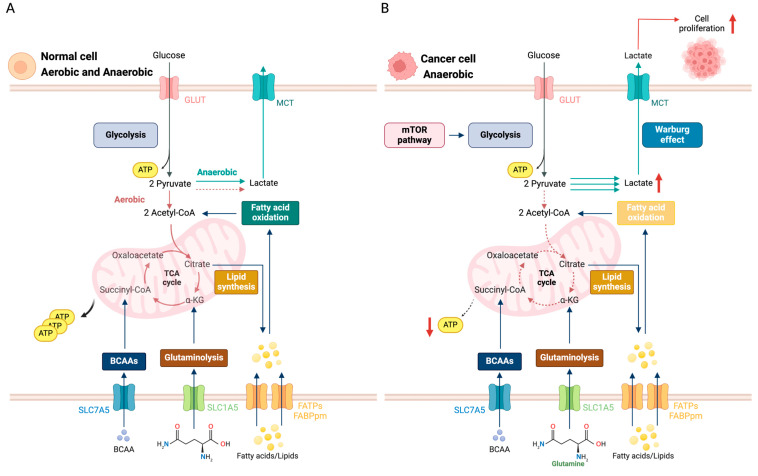
Figure 1.
A comparison of the major metabolic pathways in (A) normal and (B) cancer cells. In normal cells (A), glucose enters via GLUT transporters, fueling glycolysis and predominantly generating ATP through oxidative phosphorylation (OXPHOS) in the mitochondria. Fatty acid oxidation and glutaminolysis also contribute to ATP production and lipid synthesis. Pathways with relatively low activity, such as lactate production, are indicated by dashed lines. In contrast, cancer cells (B) demonstrate increased glucose uptake via upregulated GLUT transporters, resulting in enhanced glycolysis and the Warburg effect, where pyruvate is converted to lactate even in the presence of oxygen. This metabolic reprogramming supports rapid ATP production and proliferation. Despite the dominance of the Warburg effect, minimal TCA cycle activity and OXPHOS are retained, as indicated by the dashed lines. Abbreviations: glucose transporter type 1 (GLUT1); Monocarboxylate Transporter (MCT); adenosine triphosphate (ATP); mammalian target of rapamycin (mTOR); α-Ketoglutarate (α-KG); branched-chain amino acids (BCAAs); Solute Carrier Family 7 Member 5 (SLC7A5); Solute Carrier Family 1 Member 5 (SLC1A5); Fatty Acid Transport Protein (FATP); Plasma Membrane Fatty Acid-Binding Protein (FABPpm).
Cancer cells, however, exhibit a marked deviation from this metabolic blueprint, favoring glycolysis even under aerobic conditions—a phenomenon less efficient in ATP production known as the Warburg effect (Figure 1B) [16]. This metabolic idiosyncrasy is not merely a peculiarity but a strategic adaptation, enabling rapid energy production and the synthesis of biomolecules critical for unrestrained growth. The molecular underpinnings of the Warburg effect reveal an overexpression of glucose transporters facilitated by glucose transporter type 1 (GLUT1) and glucose transporter type 3 (GLUT3), enabling glucose internalization, alongside upregulations in key glycolytic enzymes, indicative of systemic reprogramming toward aerobic glycolysis, as observed in hepatocellular carcinoma [17,18].
The Warburg effect is a hallmark of cancer metabolism, characterized by the conversion of glucose to lactate, albeit in the presence of oxygen-rich environment/conditions and functional mitochondria [19]. In the original work of Otto Warburg in 1924, he observed that the tumor tissue has decreased cellular respiration and produces a high amount of lactate [20]. Eventually, in 1956, Warburg posited that defects in the mitochondria were responsible for cancer and neoplasia [21]. Scientists have investigated the Warburg effect for many years as an effect of this debate. However, this idea has been disregarded in recent studies.
Over the years, different ideas have been published that add to the facets of the Warburg effect as a cancer metabolic phenomenon. A paper published by Luengo et al. [22] explained a different take of what was truly happening during the event. They argued that aerobic glycolysis happens when there is more demand for NAD+ compared to ATP, and cells shift toward fermentation instead of oxidative phosphorylation [22]. Another viewpoint in this debate posits that the citric acid cycle, rather than glycolysis, is the primary driver of cancer cell proliferation [23]. Additionally, some hypotheses focus on the role of the immune system in cancer development. Tsai and colleagues proposed that T cell-mediated immunosurveillance increases glucose uptake to produce lactate while suppressing oxidative phosphorylation in tumor cells [24]. Meanwhile, Otto Warburg’s assertion that mitochondrial dysfunction plays a critical role in cancer has been supported by findings, but is more than a simple adaptation [25]. Mutations in Krebs cycle enzymes, such as succinate dehydrogenase (SDH), fumarate hydratase (FH), and isocitrate dehydrogenase (IDH), contribute to cancer progression [26]. Despite a century of research, the molecular mechanisms underlying the Warburg effect remain an enigma.
3. Metabolic Pathways and Genetic Dysfunctions in Cancer
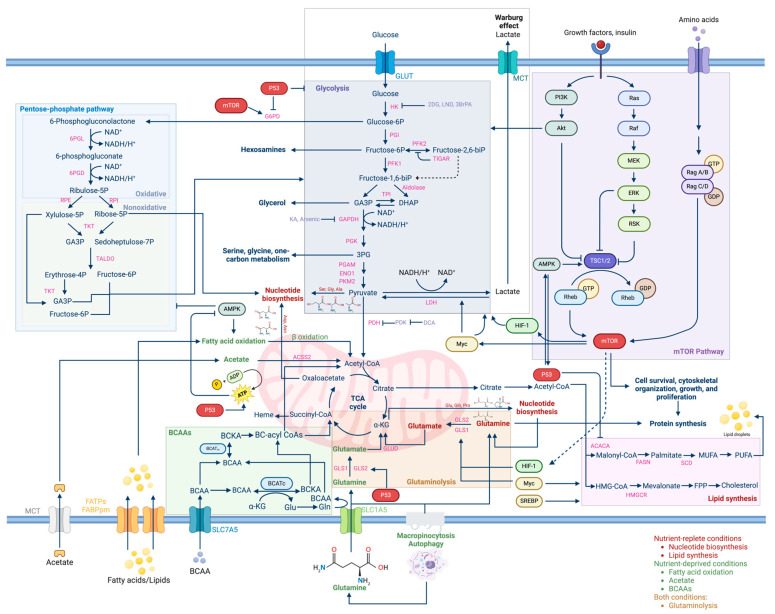
Beyond glycolysis, cancer metabolism encompasses a broad spectrum of altered pathways (Figure 2). The tricarboxylic acid cycle (TCA), while still operational for oxidative phosphorylation, exhibits an increased demand for the intermediates essential for rapid biosynthesis and energy production, a demand often met through mutations in enzymes like SDH, FH, and IDH, leading to TCA dysregulation [27,28,29]. Glutaminolysis also emerges as a critical pathway, with cancer cells demonstrating a heightened reliance on glutamine, facilitated by transporters such as the Alanine, Serine, Cysteine transporter 2 (ASCT2) and catalyzed by the upregulation of glutaminases—a type of reprogramming observed in various cancers including prostate cancer [30,31,32].
Figure 2.
An overview of the major metabolic pathways at work within cancer cells. Cell survival, growth, and proliferation require glucose to generate ATP, lipids, and amino acids through glycolysis, alongside other downstream reactions and pathways, including the pentose phosphate pathway, glutaminolysis, lipid synthesis, and branched-chain amino acid (BCAA) metabolism. The Warburg effect, characterized by increased glucose uptake and lactate production despite adequate oxygen, highlights metabolic reprogramming, supporting rapid tumor growth and survival even under oxidative conditions. The mTOR signaling pathway regulates cell growth, proliferation, survival, and cytoskeletal organization in response to insulin, growth factors, and other metabolic and cellular cues. Additionally, p53 plays an important role in promoting ATP production, facilitating citric acid cycle (also referred to as the TCA cycle or Krebs cycle) and glutamate synthesis, while regulating glycolysis and lipid synthesis. Dysregulation of mTOR signaling and p53 has been implicated in numerous diseases, including cancer and metabolic disorders. Moreover, the metabolic processes of cancer cells operate in distinct ways depending on the availability of nutrients. In situations where nutrients are abundant (nutrient-replete conditions), there is a focus on nucleotide production, lipid generation, and the utilization of glutamine. Conversely, under nutrient-deprived conditions, cancer cells favor fatty acid oxidation, acetate breakdown, the utilization of BCAAs, and glutaminolysis related to macropinocytosis and autophagy. Understanding the metabolic adaptations of cancer cells to diverse nutrient environmental conditions is vital for developing targeted therapies to combat disease progression. Abbreviations: Pentose phosphate pathway. glucose-6-phosphate dehydrogenase (G6PD); Ribulose 5-phosphate (Ribulose-5P); Xylulose 5-phosphate (Xylulose-5P); Ribose 5-phosphate (Ribose-5P); Glyceraldehyde 3-phosphate (G3P); Sedoheptulose 7-phosphate (sedoheptulose-7P); Transaldolase (TALDO); Erythrose 4-phosphate (Erythrose-4P); Fructose 6-phosphate (Fructose 6-p). Glycolysis. glucose-6-phosphate dehydrogenase (G6PD); Fructose 6-phosphate (Fructose 6-p); Fructose 1,6-biphosphate (Fructose 1,6-biP); Fructose 2,6-biphosphate (Fructose 2,6-biP); Glyceraldehyde 3-phosphate (GA3P); Dihydroxyacetone phosphate (DHAP); Glyceraldehyde-3-phosphate dehydrogenase (GAPDH); Phosphoglycerate mutase (PGAM); Pyruvate kinase M2 (PKM2); Lactate dehydrogenase (LDH). mTOR pathway. Phosphatidylinositol-3 kinase (PI3K); Protein kinase B (AKT); Rat sarcoma (Ras); Rapidly Accelerated Fibrosarcoma (Raf); Mitogen-Activated Protein Kinase (MEK); Extracellular Signal-Regulated Kinase (ERK); p90 Ribosomal S6 Kinase (RSK); Tuberous Sclerosis Complex1/2 (TSC1/2); Ras Homolog Enriched in Brain (Rheb); Guanosine Triphosphate (GTP); Ras-related GTP binding A/B (Rag A/B); Ras-related GTP binding C/D (Rag C/D); Guanosine Diphosphate (GDP); mammalian target of rapamycin (mTOR); Hypoxia-Inducible Factor 1 (HIF-1). branched-chain amino acid (BCAA). α-ketoglutarate (α-KG); glutamine (Gln); glutamate (Glu); Branched-chain Aminotransferases (BCAT); 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA); α-ketoisocaproate (KIC); branched-chain amino acid Aminotransferase (BCAT). Glutaminolysis. glutaminase (GLS); Glutamate Dehydrogenase (GLUD); Sodium-Dependent Neutral Amino Acid Transporter (SLC1A5). Lipid Synthesis. 3-hydroxyl3-methyl-glutaryl-coenzyme A reductase (HMG-CoA); Acetyl-CoA Carboxylase (ACACA); fatty acid synthase (FASN); 3-hydroxy-3-methylglutaryl-CoA Reductase (HMGCR); Farnesyl Pyrophosphate (FPP); Stearoyl-Coa Desaturase (SCD); Monounsaturated Fatty Acid (MUFA); Polyunsaturated Fatty Acid (PUFA).
Conversely, fatty acid β-oxidation acts as a bulwark against apoptosis, with the overexpression of enzymes like long-chain-fatty-acid-CoA ligase 4 (ACSL4) and alpha-methylacyl-CoA racemase (AMACR) enhancing mitochondrial integrity and cellular survival [33,34,35]. These processes are controlled genetically, and some of the key genes are listed in Table 1.
Despite advancements in our understanding, the metabolic landscape of cancer remains profoundly elusive, mirroring the mysterious nature of dark matter and dark energy in the cosmos. This elusiveness stems from a constellation of challenges that beset researchers endeavoring to decipher the complex pathophysiology underpinning cancer metabolism. The intricate metabolic processes operating within tumors are often as concealed as the cosmological phenomena, with only indirect evidence—such as the presence or absence of specific metabolites and enzymes—hinting at the underlying dysfunctions.
Cancer’s inherent heterogeneity further complicates this landscape. Each tumor presents a unique metabolic profile, influenced by its genetic makeup, microenvironment, and the specific mutations it harbors. This diversity means that while general patterns of metabolic reprogramming can be identified, as explored in this review, the reality is a mosaic of variations. Certain cancer cells, for instance, may preferentially utilize glucose or glutamine, reflecting a deviation from typical metabolic pathways [36]. Others, including gliomas, lung cancers, and leukemias, retain a reliance on oxidative phosphorylation to fuel rapid proliferation [37,38]. The targeting of reactive oxygen species (ROS) in chemotherapy highlights the unpredictable nature of cancer metabolism, with treatments sometimes exacerbating malignancy due to the varied redox responses across different tumors [39].
Table 1.
Genetic control and dysfunctions in cancer metabolic reprogramming.
| Metabolism | Oncogenic Protein | Metabolic Enzyme Targets | Mechanisms and Phenotype |
|---|---|---|---|
| Glycolysis, glutaminolysis, and amino acid synthesis | Myc | GLUT, HK2, and PFK [40,41]; LDH and MCT1 [42,43]; SLC1A5 and SLC38A5 [44]; GLS [45]; GLUD and transaminase [46,47]; G6PD and TKT [48] |
Gain-of-function mutation enhances cell cycle progression and metabolism in cancer by upregulating the expression of glucose transporters and the majority of glycolytic enzymes, promoting glycolysis and glutaminolysis. |
| Glycolysis, tricarboxylic acid cycle, and fatty acid oxidation | p53 | GLUT1/4 [49]; TIGAR [50]; LDH and PDH [51]; CPT1 and LPIN1 [52,53] | A loss of p53 alters the metabolism in cancer cells by downregulating several enzymes and transporters inhibiting mitochondrial respiration, glycolysis, and apoptosis. |
| Fatty acid synthesis and glycolysis | PTEN | PI3K/AKT [8] | Mutations or a loss of PTEN result in negative regulation of the PI3K/AKT signaling pathway and in turn intracellular metabolic reprogramming, promoting the growth and proliferation of cancer cells. |
| Glycolysis, glutaminolysis, and amino acid synthesis | Ras | PI3K/AKT/mTOR [8]; RAF/MEK/ERK [8]; Myc [8]; |
Oncogenic mutations lead to the upregulation of enzymes, resulting in tumor metabolic reprogramming and promotion of cell proliferation and survival. |
| Glycolysis and fatty acid synthesis | PIK3CA | PI3K/AKT [8] | Mutations lead to the activation of the PI3K/AKT pathway and enhance intracellular signal transduction, which leads to subsequent metabolic reprogramming of cancer cells. |
| Glycolysis and glutaminolysis | EGFR | PI3K/AKT; RAF/MEK/ERK [54] |
EGFR signaling pathways activate lipogenesis through PI3K/AKT and MAPK pathways, leading to increased de novo lipid synthesis and alterations in lipid metabolism that support cancer cell growth and proliferation. |
| Glycolysis | PDK1 | PDHC [55] | Activation promotes a shift from oxidative phosphorylation to glycolysis by inhibiting the pyruvate dehydrogenase complex, thereby redirecting cellular metabolism to support tumorigenesis and metastasis. |
| Glycolysis, de novo lipogenesis, and protein synthesis | NF1 | Neurofibromin [56] | Loss-of-function mutations alter neurofibromin expression, increase RAS and PI3K/AKT pathway signaling, constraining oxidative ATP production, restrict energetic flexibility, and increase glutamine influx into TCA intermediates, expanding lipid pools (especially triglycerides) and altering the synergy between metabolic inhibitors and traditional targeted inhibitors. |
| Glycolysis, tricarboxylic acid cycle, and fatty acid synthesis | HIF-1α | HK2, PDK1, LDHA [57,58] | In response to hypoxia, HIF-1α upregulates the activation of genes involved in glycolysis and metabolism, cell proliferation, angiogenesis, invasion, and metastasis. |
| Glycolysis, protein synthesis, and lipid metabolism | TSC2 | Rheb [59] | Loss-of-function mutations lead to abnormal activation of the mTOR pathway through increased Rheb activity. This results in altered protein synthesis, lipid metabolism, and glucose metabolism. |
| Glycolysis and fatty acid oxidation | SIRT1 | β-catenin [60] | When upregulated in response to glucose deficiency and oxidative stress, SIRT1 deacetylates β-catenin, causing its translocation from the nucleus to the cytoplasm, attenuates glycolysis, and positively correlates with fatty acid oxidation. This promotes the shift in glycolipid metabolism, facilitating tumor development in colorectal carcinoma. |
| Glycolysis, amino acid metabolism, lipid metabolism, and bile acid metabolism | YAP/TAZ | GLUT3 [61]; HK2 [62]; PFKFB3 [63]; SLC1A5 and SLC7A5 [64,65]; GOT1 and PSAT1 [66,67] | Overactivation promotes glycolysis by increasing GLUT3, HK2, and PFKFB3 expression, enhancing glutamine metabolism by upregulating transporters and enzymes. It modulates lipid and bile acid accumulation, aiding cancer metastasis. |
| Glycolysis and fatty acid oxidation | LKB1 | AMPK 1/2, MARK 1/2/3/4, SIK 1/2/3, NUAK 1/2, and SNRK [68] | LKB1 deficiency leads to the dysregulation of cellular energy homeostasis and contributes to the metabolic reprogramming of cancer cells, which induces excess glycolysis, the primary energy supply for cancer cells, enhancing their cellular growth and proliferation. |
| Glycolysis and tricarboxylic acid cycle | FH | PDHA1 [69] | Mutations lead to metabolic reprogramming characterized by increased glycolytic flux, a shift to glutamine as the primary carbon source, the induction of pseudohypoxia, alterations in lipid biosynthesis, and enhanced arginine metabolism, collectively promoting a favorable environment for cancer progression. |
| Glycolysis | PGAM1 | Wnt/β-catenin [70]; BCL-2, BAX, and caspase-3 [71]; ACTA2 [72,73] |
Overexpression results in dysregulated glycolysis, leading to altered bioenergetics characterized by increased aerobic glycolysis (Warburg effect), thereby promoting cancer cell growth, proliferation, and invasion. |
| Tricarboxylic acid cycle | IDH1/2 | TET2 [74]; JMJD2A [75] | Mutations lead to altered enzyme function, promoting the production of 2-hydroxyglutarate (2HG) which inhibits enzymes that cause differentiation in hematopoietic cells and histone methylation. |
Abbreviations: Myelocytomatosis oncogene (Myc); glucose transporter type 2 (GLUT2); Hexokinase 2 (HK2); Phosphofructokinase (PFK); Lactate Dehydrogenase (LDH); Monocarboxylate Transporter 1 (MCT1); Solute Carrier Family 1 Member 5 (SLC1A5); Solute Carrier Family 7 Member 5 (SLC7A5); Solute Carrier Family 38 Member 5 (SLC38A5); glutaminase (GLS); Glutamate Dehydrogenase (GLUD); glucose-6-phosphate dehydrogenase (G6PD); Transketolase (TKT); TP53-Induced Glycolysis and Apoptosis Regulator (TIGAR); Pyruvate Dehydrogenase (PDH); Carnitine Palmitoyltransferase 1 (CPT1); Lipid Phosphatase and Proteins Phosphatase 1 (LPIN1); Phosphatase and Tensin Homolog (PTEN); Phosphoinositide 3-Kinase/Protein Kinase B (PI3K/AKT); Rat Sarcoma (Ras); mammalian target of rapamycin (mTOR); Rapidly Accelerated Fibrosarcoma/Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase (RAF/MEK/ERK); Phosphoinositide 3-Kinase Catalytic Subunit Alpha (PIK3CA); Epidermal Growth Factor Receptor (EGFR); Pyruvate Dehydrogenase Kinase 1 (PDK1); Pyruvate Dehydrogenase Complex (PDHC); Neurofibromin 1 (NF1); Hypoxia-Inducible Factor 1 Alpha (HIF-1α); Lactate Dehydrogenase A (LDHA); Tuberous Sclerosis Complex 2 (TSC2); Ras Homolog Enriched in Brain (Rheb); Sirtuin 1 (SIRT1); Yes-Associated Protein/Transcriptional Coactivator with PDZ-Motif (YAP/TAZ); Phosphofructokinase Fructose-Bisphosphatase 3 (PFKFB3); Glutamate Oxidotransferase 1 (GOT1); Phosphoserine Aminotransferase 1 (PSAT1); Liver Kinase B1 (LKB1); AMP-Activated Protein Kinase 1/2 (AMPK 1/2); Microtubule Affinity-Regulating Kinase 1/2/3/4 (MARK 1/2/3/4); Salt-Inducible Kinase 1/2/3 (SIK 1/2/3); NUAK Family Kinase 1/2 (NUAK 1/2); Serine/Threonine/NORE1-Related Kinase (SNRK); Fumarate Hydratase (FH); Pyruvate Dehydrogenase Alpha 1 (PDHA1); Phosphoglycerate Mutase 1 (PGAM1); B-cell lymphoma 2 (BCL-2); Bcl-2-Associated X Protein (BAX); Alpha-Smooth Muscle Actin (ACTA2); Isocitrate Dehydrogenase 1/2 (IDH1/2); Tet Methylcytosine Dioxygenase 2 (TET2); Jumonji Domain-Containing 2A (JMJD2A).
Addressing this metabolic enigma requires innovative technological approaches. At the forefront are genetic technologies and structural biology, which offer insights into the genetic aberrations driving metabolic dysregulation in cancer cells [76]. Metabolomics, particularly when coupled with mass spectrometry, has emerged as a pivotal tool in mapping the cancer metabolome, facilitating both targeted and global analyses of cellular metabolites [77,78]. These methodologies, alongside isotope tracing techniques, are instrumental in tracing metabolic pathways, shedding light on how specific disruptions contribute to cancer progression [79,80].
4. The Tumor Microenvironment
As noted previously, each tumor presents a unique metabolic profile shaped by its genetic makeup, microenvironment, and specific mutations. This diversity creates a mosaic of variations, despite the identification of general patterns of metabolic reprogramming. A critical factor influencing these metabolic adaptations is the tumor microenvironment (TME), particularly under conditions of nutrient deprivation.
Central to this adaptability are key metabolic pathways, including fatty acid β-oxidation. Cancer cells have been shown to exploit lipid metabolism, enhancing lipid oxidation to thrive in hypoxic and nutrient-scarce conditions. This metabolic shift not only supports energy production but also alters the tumor microenvironment, promoting immunosuppression and cancer progression [81,82]. Oncogenes and mutated enzymes further contribute to these adaptations, enabling cancer cells to modify their microenvironment for continued growth and survival [83].
The pentose phosphate pathway (PPP) and fatty acid β-oxidation further illustrate cancer cells’ metabolic versatility. The PPP, vital for the synthesis of ribonucleotides and NADPH, induces an upregulation of glucose-6-phosphate dehydrogenase (G6PD) in cancerous tissues, a response to the oxidative stress endemic in tumor microenvironments [33,34,35,36,37].
Additionally, studies show that cancer proliferation often arises from competition between tumor cells and T cells for glucose in glucose-deficient environments, leading to increased glucose consumption by both cell types [25]. This metabolic tug of war underscores the adaptability of cancer cells in response to limited resources within the tumor microenvironment.
The TME is a complex and dynamic ecosystem that significantly influences cancer progression and therapeutic response. Within this milieu, cancer cells exhibit remarkable metabolic adaptability, allowing them to thrive under conditions of hypoxia and nutrient scarcity. The competition for limited resources, particularly glucose, between tumor cells and immune cells further complicates the metabolic landscape of the TME. This intricate interplay not only facilitates cancer cell survival and proliferation but also contributes to immunosuppression, thereby fostering a microenvironment conducive to tumor growth and metastasis. However, these changes are only part of the broader metabolic landscape of cancer. Another critical component in this reprogramming is the role of mitochondria, which, beyond their classical functions, have emerged as key players in cancer metabolism and will be the focus of the following section.
5. Mitochondria
Mitochondria play a multifaceted role in cancer evolution, influencing energy metabolism. They are vital in cellular signaling, apoptosis, and ROS production. While cancer cells primarily rely on aerobic glycolysis to support rapid proliferation, mitochondria remain crucial for biosynthesis, NAD+ regeneration, and redox balance [84,85]. Alterations in mitochondrial function can promote oncogenesis by generating excess ROS, which can cause DNA damage, activate oncogenes, and enhance genomic instability. This interplay between mitochondrial dysfunction and ROS production highlights mitochondria as key players in tumorigenesis [86].
The regulation of apoptosis by mitochondria is central to cancer development and resistance to therapy. In normal cells, mitochondrial outer membrane permeabilization (POMP) triggers the release of cytochrome c, leading to apoptosis. However, in cancer cells, this pathway is often suppressed through mutations in mitochondrial proteins or the overexpression of anti-apoptotic factors like B-cell lymphoma 2 (BCL-2) [87,88]. Such alterations allow cancer cells to evade cell death, contributing to their survival and resistance to conventional therapies, including chemotherapy and radiation [89]. Targeting mitochondrial pathways to restore apoptotic signaling is an emerging therapeutic strategy in cancer treatment, with promising compounds under investigation such as histone deacetylase inhibitors [90] and antisense oligonucleotides [91,92]. Some are shown in Table 2.
Table 2.
Compounds or drugs targeting mitochondrial pathways to restore apoptosis.
| Compound or Drug | Mechanism | Target | Specific Cancer |
Findings | Clinical Pipeline | Reference |
|---|---|---|---|---|---|---|
| Entinostat | Inhibits the function of histone deacetylases leading to more relaxed chromatin and gene expression | HDAC1/3 | Osteosarcoma | Shown to upregulate the expression of Fas, leading to decreased pulmonary metastasis and improved outcomes | Preclinical | [93] |
| Venetoclax | Selective BCL-2 inhibitor that releases pro-apoptotic proteins | BCL-2 | Acute myeloid leukemia | Overall response rate of 67% when combined with hypomethylating agents in elderly AML patients | FDA-approved, Phase III | [94] |
| MCL-1- specific inhibitor AZD5991 | Binds directly to Mcl-1 and induces rapid apoptosis in cancer cells by activating the Bak-dependent mitochondrial apoptotic pathway | MCL-1 | Myeloma and acute myeloid leukemia | Induces apoptosis in >80% of multiple MCL-1-dependent myeloma cell lines in vitro | Preclinical | [95] |
| Lenvatinib | Induces immunogenic cell death and activates TLR 3/4 ligands, enhancing immune response | TLR 3/4 | Hepatocellular carcinoma | Triggers immunogenic cell death, enhancing anti-tumor immunity; increases T-cell activation and infiltration by 40% in HCC models | Preclinical | [96] |
| Obatoclax | Directly induces apoptosis through the activation of BAX/BAK following their release from the pro-survival BCL-2 members | Pan-BCL -2 family | Hematologic malignancies | Demonstrates tolerability and partial responses in patients with chronic lymphocytic leukemia and Hodkins lymphoma | Phase I clinical trial | [97] |
Abbreviations: histone deacetylase 1/3 (HDAK1/3); B-cell lymphoma 2 (BCL-2); Myeloid Cell Leukemia 1 (MCL-1); acute myeloid leukemia (AML); Toll-like Receptors 3/4 (TLR3/4); hepatocellular carcinoma (HCC); Bcl-2-associated X protein/Bcl-2 antagonist/killer (BAX/BAK).
Another rapidly advancing area of research is mitochondrial dynamics. The processes of fission and fusion of mitochondria are closely linked to cancer progression and metastasis [98,99]. Mitochondrial fission is often upregulated in cancer cells, facilitating cell division, migration, and invasion [100]. Dysregulated fission, mediated by proteins such as dynamin-related protein 1 (DRP1), has been shown to promote metastasis in various cancers [98,99]. Targeting mitochondrial dynamics, along with mitochondrial DNA (mtDNA) mutations, represents a novel approach, as recent studies have demonstrated that disrupting mitochondrial fission can inhibit cancer cell proliferation and metastasis [100]. These findings indicate that mitochondria are not only essential for cancer cell metabolism but also for their survival, making them promising targets for cancer therapies.
6. Impact of Metabolic Reprogramming on Cancer Progression
Metabolic reprogramming plays a significant role in cancer progression, fueling the rapid proliferation and growth characteristics of malignancies. This reprogramming facilitates an enhanced reliance on glycolysis, providing a quick source of energy essential for tumor development. Glycolysis has been implicated in early tumorigenesis, driving epigenetic modifications, inhibiting cellular senescence, and enhancing DNA damage repair mechanisms, thereby supporting the survival and proliferation of cancer cells [101]. RAC-alpha serine/threonine-protein kinase (AKT), for instance, promotes glycolysis while exerting anti-apoptotic effects, further contributing to cancer cell survival [102]. Additionally, glycolytic metabolites such as lactate and pyruvate mediate interactions between cancer cells and their microenvironment, ultimately promoting tumor growth and metastasis [103]. Lactate, in particular, has been shown to induce polarization of tumor-associated macrophages toward an M2 phenotype, which supports tumor metastasis [104].
Amino acid metabolism also contributes to tumor metastasis and drug resistance. Hyperactivation of amino acid pathways is vital for biosynthesis in metastatic processes, with glutamine, serine, glycine, and proline playing key roles in maintaining metabolic intermediates essential for cancer development [105,106,107]. Proline catabolism, in particular, generates ROS that promote angiogenesis, signaling cascades, and epithelial-to-mesenchymal transition (EMT), which are hallmarks of aggressive cancer behavior [108].
Furthermore, the availability of amino acids is intricately linked to cancer cells’ epigenetic status and their capacity to develop drug-resistant phenotypes. For example, the increased uptake of glutamine alongside glucose has been associated with oral cancers’ resistance to cisplatin, while the production of putrescine via ornithine decarboxylase (ODC) contributes to erlotinib resistance [109,110]. Resistance to epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors, often achieved through metabolic reprogramming of branched-chain amino acids, further illustrates the complex interplay between metabolism and drug resistance [111].
7. Interdisciplinary Approaches in Cancer Metabolism Research
The multifaceted nature of cancer demands an interdisciplinary approach, bridging biology, chemistry, physics, and computational sciences with the personal dimensions of the disease and its economic implications. Moreso, the economic impact and investments required to translate research to therapies are considerations worthy of discussion.
The variability in metabolic profiles across cancer types necessitates precision metabolomics, integrating metabolic data with transcriptomics, genomics, and proteomics to tailor treatments more effectively. This multi-omics strategy has shown the roles of specific proteins in cancers, such as chromobox protein homologs (CBX2 and CBX7) in breast cancer, and has facilitated the development of targeted therapies for conditions like clear cell renal carcinoma [112,113]. Furthermore, metabolomics has advanced our understanding of the metabolic distinctions between normal and cancerous tissues, aiding in the identification of novel biomarkers and therapeutic avenues [114,115,116].
Nutrigenomics and dietary interventions explore the interactions among nutrition, genetics, and metabolism, highlighting how diet can influence epigenetic modifications and affect disease outcomes. This area of research promises to enhance the precision of medical treatments through dietary adjustments [117,118,119]. Additionally, computational systems biology has played a crucial role in elucidating the dynamics of metabolic reprogramming, offering tools for simulation and analysis that deepen our understanding of cancer’s metabolic complexity [120,121].
8. Therapeutic Implications and Future Directions
Our expanding knowledge of cancer’s metabolic reprogramming has led to the identification of numerous potential therapeutic targets. Compounds designed to inhibit specific metabolic pathways, such as glutaminase inhibitors and mTOR inhibitors, are showing promise in clinical settings, addressing the metabolic vulnerabilities of cancer cells [122,123,124]. The use of rapamycin and its analogs in various cancers exemplifies the potential of targeting metabolic pathways to inhibit tumor growth. Additionally, metformin, traditionally used for diabetes management, is being explored for its anticancer properties, demonstrating the crossover potential of drugs across different therapeutic areas [125,126,127]. Some more therapeutic compounds at different stages of investigation are listed in Table 3.
Table 3.
Therapeutic compounds or drugs and targets within cancer metabolism.
| Metabolism | Compound or Drug | Target | Mechanism | Findings | Clinical Pipeline | Reference |
|---|---|---|---|---|---|---|
| Glycolysis | 3-Bromopyruvate | Hexokinase II | Irreversibly alkylates HK2, resulting in the disruption of glucose metabolism, leading to cancer cell death | 3-BP (20 mg/kg) reduced tumor size by 75–80% in animals and induced apoptosis and necrosis in drug-treated tumor tissues | Animal Studies | [128] |
| TCA cycle | 6-Methoxydihydroavicine (6-ME) | Oxaloacetic Acid Metabolism | Disrupts OAA metabolism, leading to ROS accumulation and resulting in disrupted mitochondrial homeostasis, ultimately driving apoptosis in ovarian cancer cells | 6-ME significantly reduced tumor growth in a nude mouse model of ovarian cancer without causing physiologically harmful effects on the animal | Animal Studies | [129] |
| Glycolysis | AZD3965 | MCT-1 | Inhibition of MCT1-mediated lactic acid efflux during T-cell lymphocyte proliferation | The drug showed rapid oral absorption, nearly complete bioavailability, nonlinear pharmacokinetics, and potential involvement in enterohepatic circulation (EHC), with evidence of target-mediated drug disposition (TMDD) | Phase I | [130] |
| Glycolysis | Benserazide (Benz) | Hexokinase II | Competitive and noncompetitive binding to selectively inhibit HK2 | In vivo, it suppresses tumor growth in mice without toxicity; when formulated as liposomal nanoparticles, Benz enhances tumor targeting and efficacy at lower doses | Animal Studies | [131] |
| Glutaminolysis | CB839 (Telaglenastat) | Glutamine oxidase | Block glutamine-to-glutamate conversion, reducing the number of immunosuppressive cells and reshaping the tumor microenvironment | The study established a recommended phase II dose (RP2D) for telaglenastat, demonstrating safety, strong GLS inhibition, and early anticancer activity, prompting further investigation | Phase I |
[132] |
| Glycolysis | Curcumin (Cur) + Thymoquinone (TQ) | Caspase-3 and PI3K/AKT | Induces apoptosis and cell cycle arrest, and decreases proliferation, colony formation, and migration of MCF7 and MDA-MB-231 cells | Cur and TQ significantly inhibited cancer cell growth and migration, increased apoptosis (73.96% for Cur, 75.76% for Cur + TQ), and reduced S-phase values compared to controls | In vitro | [133] |
| Glycolysis | Demethylzeylasteral (DML) | Lactate |
Dose-dependent decrease in intracellular lactate levels Regulation of histone acetylation via H3K9la and H3K56la modification sites |
DML treatment significantly inhibited tumor growth in vivo, as shown by slower tumor growth rates in treated groups compared to controls, and regulated Pan Kla expression, correlating with decreased cancer cell proliferation | In vitro | [134] |
| Glycolysis | Fenbendazole (FZ) |
Microtubules p53 Hexokinase II |
Disruption of microtubule dynamics Increases p53 translocation to mitochondria, which is suggested to induce cell death Inhibition of HK2 activity, leading to apoptosis |
FZ administration significantly reduced tumor size and weight in A549 xenografted nude mice | Animal studies | [135] |
| TCA cycle | Ivosidenib | Isocitrate dehydrogenase 1 | Inhibits IDH1 catalysis of the oncometabolite 2HG that disrupts epigenetic regulation, blocks cellular differentiation, and contributes to tumorigenesis | Ivosidenib effectively suppresses plasma 2-HG in IDH1-mutated cholangiocarcinoma and chondrosarcoma, supporting a dose of 500 mg QD for advanced solid tumors | Phase I | [136] |
| Amino acid synthesis | JPH203 (Nanvuranlat) | L-type Amino Acid Transporter 1 | LAT1 inhibition | The drug showed significant improvement in progression-free survival in patients with advanced, refractory biliary tract cancers compared to placebo, with a disease control rate of 25%; the treatment was found to be safe and well tolerated | Phase II | [137,138] |
| Glycolysis | Marinopyrrole derivative MP1 | Myc and mTOR signaling | Modulate global gene expression and inhibit Myc-associated transcriptional targets including translation/mTOR targets. Inhibit tumor growth and Myc expression | MP1 is an orally bioavailable compound with favorable pharmacokinetics and pharmacodynamics, crossing the blood–brain barrier and achieving concentrations above IC50 in tumors, including in the brain, with good tolerability and no significant toxicity | Animal studies | [139,140] |
| Oxidative phosphorylation | Metformin | NADH | Increased flux of glucose carbons via the pentose phosphate pathway, leading to the inhibition of complex I (NADH:ubiquinone oxidoreductase) | Proneural BTICs respond better to metformin, while mesenchymal BTICs are more glycolytic and less responsive; glycolysis targeting may be more effective for mesenchymal BTICs. | Phase II | [141] |
| Glycolysis | Dimethylaminomicheliolide (DMAMCL), a Micheliolide derivative | Pyruvate kinase | Covalent binding at residue cysteine424 to promote tetramer formation and selectively activate PKM2 | DMAMCL significantly suppresses tumor growth in vivo by activating PKM2, showing potential as a novel anticancer therapeutic drug, with optimal effects observed at 10 μg/mL | Discovery | [142] |
| Fatty acid synthesis | Omeprazole | Fatty acid synthase (FASN), which is a rate-limiting enzyme in synthesizing fatty acids | Proton pump inhibitors selectively inhibit FASN activity and induce apoptosis in Triple-Negative Breast Cancer (TNBC) cell lines via AKT and HIF-1 under hypoxic stress, allowing for adaptations in the tumor microenvironment | Omeprazole, when added to neoadjuvant AC-T, can safely inhibit FASN and shows a promising pCR rate, though further confirmation is needed | Phase II | [143] |
| Glycolysis | Oxamate | Lactate Dehydrogenase A | Induces inhibition of LDHA which suppress glucose uptake, lactate secretion, invasion, and proliferation in GH3 cells via the downregulation of GLUT1 and MMP2 expression and the inhibition of the Akt-GSK-3β-cyclinD1 pathway | Oxamate significantly inhibits the invasion and proliferation of primary pituitary PA cells derived from patients after transsphenoidal resection, confirming its potential as a therapeutic agent against human-invasive PA cells | Discovery | [144] |
| One-carbon metabolism | Methotrexate (MTX), Pemetrexed (PTX) | Serine hydroxymethyltransferases | Inhibit growth of cancer cells by cutting off the supply of 5,10-meTHF (utilized for nucleotide biosynthesis and hyperactivated in cancer) | PTX binds deeper in SHMTs than MTX due to its unique P-moiety structure, making it a more potent inhibitor; polyglutamylation significantly enhances the inhibitory activity of antifolates like PTX and MTX against SHMTs in vivo | Drug repurposing | [145] |
| Glycolysis | Shikonin | Pyruvate kinase | Decreases the PKM2-mediated aerobic glycolysis switch in tumor cells, thereby inhibiting tumor proliferation | Shikonin suppresses tumor growth in a dose-dependent manner in a mouse model of B16 melanoma at concentrations of 1 mg/kg and 10 mg/kg | Animal studies | [146] |
| Fatty acid synthesis | TVB 2640 (Denifanstat) + bevacizumab | Fatty acid synthase | Alternation of fatty acid synthase signaling which can drive phenotypic plasticity and cell fate decisions, mitochondrial regulation of cell death, immune escape, and organ-specific metastatic potential | TVB-2640 combined with bevacizumab significantly improved progression-free survival (PFS) in patients with recurrent high-grade astrocytoma compared to historical bevacizumab monotherapy, demonstrating a favorable safety profile and promising efficacy | Phase II | [147] |
| Amino acid synthesis | Venetoclax with azacitidine | BCL-2 | Inhibition of BCL-2 which leads to the suppression of oxidative phosphorylation | Venetoclax and azacitidine show high response rates in treatment-naive patients, but relapsed patients exhibit reduced sensitivity due to metabolic adaptations in leukemic stem cells, indicating potential for targeting fatty acid metabolism | Phase I | [148] |
Abbreviations: Hexokinase 2 (HK2); Oxaloacetic acid (OAA); reactive oxygen species (ROS); Monocarboxylate Transporter 1 (MCT1); Phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT); Michigan Cancer Foundation-7 (MCF-7); MD Anderson-Metastatic Breast-231 (MDA-MB-231); Histone H3 lysine 9 lactylation (H3K9la); Histone H3 at lysine 56 lactylation (H3K56la); tumor protein 53 (p53); Isocitrate dehydrogenase 1 (IDH1); 2-hydroxyglutarate (2HG); L-type Amino Acid Transporter 1 (LAT1); Myelocytomatosis oncogene (Myc); mechanistic target of rapamycin (mTOR); Nicotinamide Adenine Dinucleotide (NADH); Pyruvate kinase M2 (PKM2); fatty acid synthase (FASN); Lactate Dehydrogenase A (LDHA); Hypoxia-Inducible Factor 1 Subunit Alpha (HIF1); growth hormone (GH3); glucose transporter type 1 (GLUT1); 5;10-methylenetetrahydrofolate (5;10-meTHF); Matrix Metallopeptidase 2 (MMP2); B-cell lymphoma 2 BCL-2 (BCL-2); Anthracycline–Taxane-based Chemotherapy (AC-T); Pathologic Complete Response (pCR); Pituitary Adenoma (PA); Serine Hydroxymethyl Transferase (SHMT); progression-free survival (PFS).
9. Challenges and Limitations in Understanding and Targeting Cancer Metabolism
Recent insights into cancer biology have unveiled a complex and intricately organized structure governing tumor progression, challenging the traditional view of cancer as a disordered and chaotic entity. This organization suggests that similar to the cosmic influence of dark matter and dark energy, a hidden order underpins the metabolic reprogramming of cancer cells, offering novel perspectives for cancer medicine. Despite these advancements, the integration of cancer’s metabolic “dark energy” into clinical practice remains an elusive goal, reflecting the complexity of the disease and the limitations of current therapeutic approaches.
It is now evident that cancer’s metabolic network is not reliant on a singular pathway but involves complex interactions among multiple inter-related pathways [149,150]. This heterogeneity is compounded by genetic alterations in oncogenes and tumor suppressor genes, which drive the metabolic rewiring essential for tumorigenesis and malignant transformation [12,151]. Yet, the translation of this knowledge into effective therapies is hindered by the complex nature of cancer metabolism and the inherent limitations of existing treatment modalities.
Traditional cancer therapies, such as chemotherapy and radiotherapy, suffer from significant drawbacks, including systemic toxicity and a lack of selectivity, often damaging healthy tissues alongside cancer cells [152]. This issue is exacerbated by the cancer cells’ ability to activate self-renewal pathways and metabolic shifts that favor tumor growth, further complicating the development of targeted therapies. Although small-molecule inhibitors have shown promise, their clinical application is limited by issues such as nonspecific toxicity and poor solubility [153]. The redundancy and crosstalk among signaling pathways necessitate a multifaceted therapeutic approach, rather than targeting single pathways. For example, CD147 or the extracellular matrix metalloproteinase inducer (EMMPRIN) is one target candidate in hematological malignancies [154]. Single-cell metabolomics, focusing on metabolic rewiring at the cellular level, is also an approach worth researching, as this may have potential, as shown in hematologic malignancies [155].
A critical challenge in cancer treatment is tumor heterogeneity and the dynamic nature of cancer metabolism, which vary not only among patients but also within individual tumors. This metabolic flexibility allows cancer cells to adapt to environmental pressures and treatment interventions, making them moving targets for therapy. The variability in metabolic programming underscores the need for personalized treatment strategies that consider the unique metabolic landscape of each patient’s cancer.
To overcome these challenges, a comprehensive understanding of the molecular mechanisms underlying cancer’s resilience and adaptability is essential. As our knowledge of the cosmos expands, so too must our understanding of cancer’s complexity. Future therapeutic strategies must prioritize the development of more accurate models that reflect the tumor microenvironment’s intricacy and the pivotal role of metabolism in cancer progression. Embracing this complexity and adopting a more nuanced approach to cancer metabolism will be crucial for advancing the development of effective, clinically translatable cancer therapies.
10. Conclusions
By drawing parallels with cosmological concepts like dark matter and dark energy, this review presents the hidden dimensions of cancer cell metabolism and highlights the importance of understanding its complexities. Moving forward, interdisciplinary research efforts and innovative strategies are needed to fully exploit this frontier. Ultimately, unveiling and targeting the “dark energy” within cancer cells could revolutionize future therapy and research, offering hope for more effective and clinically sound treatments.
Acknowledgments
The authors wish to express their sincere gratitude for the support and contributions that made this study possible. Special thanks go to the National Science and Technology Council for their financial support through grants NSTC 112-2314-B-038-099-MY3 and MOST 111-2314-B-038-072, and the Higher Education Sprout Project by the Ministry of Education (MOE) of Taiwan awarded to Ching-Feng Chiu. These grants have been instrumental in facilitating the research presented in this manuscript.
Author Contributions
C.-F.C. and S.-Y.H. were responsible for study conception/design; C.-F.C., J.J.G.G., R.R.H.R. and C.S. wrote the manuscript; C.-F.C., J.J.G.G. and R.R.H.R. reviewed and analyzed the data reported in the literature; C.D.D.C., E.M.O., D.C.I.L., P.C.E., J.Z., Y.-W.L. and K.I.N. were responsible for the collection of research; C.D.D.C., E.M.O., D.C.I.L. and P.C.E. prepared the tables; J.J.G.G., J.Z. and K.I.N. curated the figures. P.C.E. made a thorough check of the manuscript. All authors read, reviewed, revised, and approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no potential conflicts of interest.
Funding Statement
This study was funded by the National Science and Technology Council (NSTC), Grant/Award Number: 112-2314-B-038-099-MY3, the Ministry of Science and Technology (MOST), Grant/Award Number: 111-2314-B-038-072.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Gaidai O., Yan P., Xing Y. Future world cancer death rate prediction. Sci. Rep. 2023;13:303. doi: 10.1038/s41598-023-27547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pineda E., Benavente R., Gimmen M.Y., DeVille N.V., Taparra K. Cancer Disparities among Pacific Islanders: A Review of Sociocultural Determinants of Health in the Micronesian Region. Cancers. 2023;15:1392. doi: 10.3390/cancers15051392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Syrnioti G., Eden C.M., Johnson J.A., Alston C., Syrnioti A., Newman L.A. Social Determinants of Cancer Disparities. Ann. Surg. Oncol. 2023;30:8094–8104. doi: 10.1245/s10434-023-14200-0. [DOI] [PubMed] [Google Scholar]
- 4.Aguadé-Gorgorió G., Costa J., Solé R. An oncospace for human cancers. BioEssays. 2023;45:2200215. doi: 10.1002/bies.202200215. [DOI] [PubMed] [Google Scholar]
- 5.Imodoye S.O., Adedokun K.A., Bello I.O. From complexity to clarity: Unravelling tumor heterogeneity through the lens of tumor microenvironment for innovative cancer therapy. Histochem. Cell Biol. 2024;161:299–323. doi: 10.1007/s00418-023-02258-6. [DOI] [PubMed] [Google Scholar]
- 6.Xu X., Peng Q., Jiang X., Tan S., Yang Y., Yang W., Han Y., Chen Y., Oyang L., Lin J., et al. Metabolic reprogramming and epigenetic modifications in cancer: From the impacts and mechanisms to the treatment potential. Exp. Mol. Med. 2023;55:1357–1370. doi: 10.1038/s12276-023-01020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nong S., Han X., Xiang Y., Qian Y., Wei Y., Zhang T., Tian K., Shen K., Yang J., Ma X. Metabolic reprogramming in cancer: Mechanisms and therapeutics. MedComm. 2023;4:e218. doi: 10.1002/mco2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lasorsa F., di Meo N.A., Rutigliano M., Ferro M., Terracciano D., Tataru O.S., Battaglia M., Ditonno P., Lacerelli G. Emerging hallmarks of metabolic reprogramming in prostate cancer. Int. J. Mol. Sci. 2023;24:910. doi: 10.3390/ijms24020910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee J., Shin D., Roh J.L. Lipid metabolism alterations and ferroptosis in cancer: Paving the way for solving cancer resistance. Eur. J. Pharmacol. 2023;941:175497. doi: 10.1016/j.ejphar.2023.175497. [DOI] [PubMed] [Google Scholar]
- 10.Li Y.J., Zhang C., Martincuks A., Herrmann A., Yu H. STAT proteins in cancer: Orchestration of metabolism. Nat. Rev. Cancer. 2023;23:115–134. doi: 10.1038/s41568-022-00537-3. [DOI] [PubMed] [Google Scholar]
- 11.DeBerardinis R.J., Lum J.J., Hatzivassiliou G., Thompson C.B. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 12.Mason E.F., Rathmell J.C. Cell metabolism: An essential link between cell growth and apoptosis. Biochim. Biophys. Acta. 2011;1813:645–654. doi: 10.1016/j.bbamcr.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cantor J.R., Sabatini D.M. Cancer cell metabolism: One hallmark, many faces. Cancer Discov. 2012;2:881–898. doi: 10.1158/2159-8290.CD-12-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neugent M.L., Goodwin J., Sankaranarayanan I., Yetkin C.E., Hsieh M.H., Kim J.W. A new perspective on the heterogeneity of cancer glycolysis. Biomol. Ther. 2018;26:10. doi: 10.4062/biomolther.2017.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abbaszadeh Z., Çeşmeli S., Avcı Ç.B. Crucial players in glycolysis: Cancer progress. Gene. 2020;726:144158. doi: 10.1016/j.gene.2019.144158. [DOI] [PubMed] [Google Scholar]
- 17.Feng J., Li J., Wu L., Yu Q., Ji J., Wu J., Guo C. Emerging roles and the regulation of aerobic glycolysis in hepatocellular carcinoma. J. Exp. Clin. Cancer Res. 2020;39:126. doi: 10.1186/s13046-020-01629-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Z., Li T., Sun L., Yuan Y., Zhu Y. Potential mechanisms of cancer-associated fibroblasts in therapeutic resistance. Biomed. Pharmacother. 2023;166:115425. doi: 10.1016/j.biopha.2023.115425. [DOI] [PubMed] [Google Scholar]
- 19.Warburg O. The metabolism of carcinoma cells. J. Cancer Res. 1925;9:148–163. doi: 10.1158/jcr.1925.148. [DOI] [Google Scholar]
- 20.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 21.Luengo A., Li Z., Gui D.Y., Sullivan L.B., Zagorulya M., Do B.T., Ferreira R., Naamati A., Ali A., Lewis C.a., et al. Increased demand for NAD+ relative to ATP drives aerobic glycolysis. Mol. Cell. 2021;81:691–707. doi: 10.1016/j.molcel.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martínez-Reyes I., Cardona L.R., Kong H., Vasan K., McElroy G.S., Werner M., Kihshen H., Reczek C.R., Weinberg S.E., Gao P., et al. Mitochondrial ubiquinol oxidation is necessary for tumour growth. Nature. 2020;585:288–292. doi: 10.1038/s41586-020-2475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsai C.H., Chuang Y.M., Li X., Yu Y.R., Tzeng S.F., Teoh S.T., Lindblad K.E., Matteo M.D., Cheng W.-C., Hsueh P.-C., et al. Immunoediting instructs tumor metabolic reprogramming to support immune evasion. Cell Metab. 2023;35:118–133. doi: 10.1016/j.cmet.2022.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang C.H., Qiu J., O’Sullivan D., Buck M.D., Noguchi T., Curtis J.D., Chen Q., Gindin M., Gubin M.M., ven der Windt G.J.W., et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–1241. doi: 10.1016/j.cell.2015.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vaupel P., Multhoff G. Revisiting the Warburg effect: Historical dogma versus current understanding. J. Physiol. 2021;599:1745–1757. doi: 10.1113/JP278810. [DOI] [PubMed] [Google Scholar]
- 26.Chen J.Q., Russo J. Dysregulation of glucose transport, glycolysis, TCA cycle and glutaminolysis by oncogenes and tumor suppressors in cancer cells. Biochim. Et Biophys. Acta (BBA)–Rev. Cancer. 2012;1826:370–384. doi: 10.1016/j.bbcan.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Eniafe J., Jiang S. The functional roles of TCA cycle metabolites in cancer. Oncogene. 2021;40:3351–3363. doi: 10.1038/s41388-020-01639-8. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt C., Sciacovelli M., Frezza C. Fumarate hydratase in cancer: A multifaceted tumour suppressor. Semin. Cell Dev. Biol. 2020;98:15–25. doi: 10.1016/j.semcdb.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Z., Liu F., Fan N., Zhou C., Li D., Macvicar T., Dong Q., Bruns C.J., Zhao Y. Targeting glutaminolysis: New perspectives to understand cancer development and novel strategies for potential target therapies. Front. Oncol. 2020;10:589508. doi: 10.3389/fonc.2020.589508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shah R., Chen S. Metabolic signaling cascades prompted by glutaminolysis in cancer. Cancers. 2020;12:2624. doi: 10.3390/cancers12092624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan T., Gao L., Wu G., Shen G., Xie S., Wen H., Yang J., Zhou Y., Tu Z., Qian W. Elevated expression of glutaminase confers glucose utilization via glutaminolysis in prostate cancer. Biochem. Biophys. Res. Commun. 2015;456:452–458. doi: 10.1016/j.bbrc.2014.11.105. [DOI] [PubMed] [Google Scholar]
- 32.Patra K.C., Hay N. The pentose phosphate pathway and cancer. Trends Biochem. Sci. 2014;39:347–354. doi: 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zha S., Ferdinandusse S., Hicks J.L., Denis S., Dunn T.A., Wanders R.J., Luo J., De Marzo A.M., Isaacs W.B. Peroxisomal branched chain fatty acid β–oxidation pathway is upregulated in prostate cancer. Prostate. 2005;63:316–323. doi: 10.1002/pros.20177. [DOI] [PubMed] [Google Scholar]
- 34.Padanad M.S., Konstantinidou G., Venkateswaran N., Melegari M., Rindhe S., Mitsche M., Yang C., Batten K., Huffman K.E., Liu J., et al. Fatty acid oxidation mediated by Acyl–CoA synthetase long chain 3 is required for mutant KRAS lung tumorigenesis. Cell Rep. 2016;16:1614–1628. doi: 10.1016/j.celrep.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osthus R.C., Shim H., Kim S., Li Q., Reddy R., Mukherjee M., Xu Y., Wonsey D., Lee L.A., Dang C.V. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J. Biol. Chem. 2000;275:21797–21800. doi: 10.1074/jbc.C000023200. [DOI] [PubMed] [Google Scholar]
- 36.Ye X.Q., Li Q., Wang G.H., Sun F.F., Huang G.J., Bian X.W., Yu S.C., Qian G.S. Mitochondrial and energy metabolism–related properties as novel indicators of lung cancer stem cells. Int. J. Cancer. 2011;129:820–831. doi: 10.1002/ijc.25944. [DOI] [PubMed] [Google Scholar]
- 37.Roy D., Sheng G.Y., Herve S., Carvalho E., Mahanty A., Yuan S., Sun L. Interplay between cancer cell cycle and metabolism: Challenges, targets and therapeutic opportunities. Biomed. Pharmacother. 2017;89:288–296. doi: 10.1016/j.biopha.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 38.Benfeitas R., Uhlen M., Nielsen J., Mardinoglu A. New challenges to study heterogeneity in cancer redox metabolism. Front. Cell Dev. Biol. 2017;5:65. doi: 10.3389/fcell.2017.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biswas D., Khan M.W. New techniques in understanding cancer biology and metabolism. Technol. Cancer Res. Treat. 2020;19:1533033820943248. doi: 10.1177/1533033820943248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim J.W., Zeller K.I., Wang Y., Jegga A.G., Aronow B.J., O’Donnell K.A., Dang C.V. Evaluation of myc E-box phylogenetic footprints in glycolytic genes by chromatin immunoprecipitation assays. Mol. Cell. Biol. 2004;24:5923–5936. doi: 10.1128/MCB.24.13.5923-5936.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis B.C., Prescott J.E., Campbell S.E., Shim H., Orlowski R.Z., Dang C.V. Tumor induction by the c-Myc target genes rcl and lactate dehydrogenase A. Cancer Res. 2000;60:6178–6183. [PubMed] [Google Scholar]
- 42.Doherty J.R., Yang C., Scott K.E., Cameron M.D., Fallahi M., Li W., Hall M.A., Amelio A.L., Mishra J.K., Li F. Blocking lactate export by inhibiting the Myc target MCT1 disables glycolysis and glutathione synthesis. Cancer Res. 2014;74:908–920. doi: 10.1158/0008-5472.CAN-13-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wise D.R., DeBerardinis R.J., Mancuso A., Sayed N., Zhang X.Y., Pfeiffer H.K., Nissin I., Daikhin E., Yudkoff M., McMahon S.B., et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gao P., Tchernyshyov I., Chang T.C., Lee Y.S., Kita K., Ochi T., Zeller K.I., De Marzo A.M., Van Eyk J.E., Mendell J.T., et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qing G., Li B., Vu A., Skuli N., Walton Z.E., Liu X., Mayes P.A., Wise D.R., Thompson C.B., Maris J.M., et al. ATF4 regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer Cell. 2012;22:631–644. doi: 10.1016/j.ccr.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korangath P., Teo W.W., Sadik H., Han L., Mori N., Huijts C.M., Wildes F., Bharti S., Zhang Z., Santa-Maria C.A., et al. Targeting glutamine metabolism in breast cancer with aminooxyacetate. Clin. Cancer Res. 2015;21:3263–3273. doi: 10.1158/1078-0432.CCR-14-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang R., Dillon C.P., Shi L.Z., Milasta S., Carter R., Finkelstein D., McCormick L.L., Fitzgerald P., Munger J., Green D.R. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity. 2011;35:871–882. doi: 10.1016/j.immuni.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang C., Liu J., Liang Y., Wu R., Zhao Y., Hong X., Lin M., Yu H., Liu L., Levine A.J., et al. Tumour-associated mutant p53 drives the Warburg effect. Nat. Commun. 2013;4:2935. doi: 10.1038/ncomms3935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bensaad K., Tsuruta A., Selak M.A., Vidal M.N.C., Nakano K., Bartrons R., Gottlieb E., Vousdan K.H. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 50.Contractor T., Harris C.R. p53 negatively regulates transcription of the pyruvate dehydrogenase kinase Pdk2. Cancer Res. 2012;72:560–567. doi: 10.1158/0008-5472.CAN-11-1215. [DOI] [PubMed] [Google Scholar]
- 51.Sanchez-Macedo N., Feng J., Faubert B., Chang N., Elia A., Rushing E.J., Tsuchihara K., Bungard D., Berger S.L., Jones R.G., et al. Depletion of the novel p53-target gene carnitine palmitoyltransferase 1C delays tumor growth in the neurofibromatosis type I tumor model. Cell Death Differ. 2013;20:659–668. doi: 10.1038/cdd.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Assaily W., Rubinger D.A., Wheaton K., Lin Y., Ma W., Xuan W., Brown-Endres L., Tsuchihara K., Mak T.W., Benchimol S., et al. ROS-mediated p53 induction of Lpin1 regulates fatty acid oxidation in response to nutritional stress. Mol. Cell. 2011;44:491–501. doi: 10.1016/j.molcel.2011.08.038. [DOI] [PubMed] [Google Scholar]
- 53.Eltayeb K., La Monica S., Tiseo M., Alfieri R., Fumarola C. Reprogramming of lipid metabolism in lung cancer: An overview with focus on EGFR–mutated non–small cell lung cancer. Cells. 2022;11:413. doi: 10.3390/cells11030413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Padder R.A., Bhat Z.I., Ahmad Z., Singh N., Husain M. DRP1 promotes BRAFV600E–driven tumor progression and metabolic reprogramming in colorectal cancer. Front. Oncol. 2021;10:592130. doi: 10.3389/fonc.2020.592130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Philpott C., Tovell H., Frayling I.M., Cooper D.N., Upadhyaya M. The NF1 somatic mutational landscape in sporadic human cancers. Hum. Genom. 2017;11:13. doi: 10.1186/s40246-017-0109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim J.W., Gao P., Liu Y.C., Semenza G.L., Dang C.V. Hypoxia-inducible factor 1 and dysregulated c-Myc cooperatively induce vascular endothelial growth factor and metabolic switches hexokinase 2 and pyruvate dehydrogenase kinase 1. Mol. Cell. Biol. 2007;27:7381–7393. doi: 10.1128/MCB.00440-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qing G., Skuli N., Mayes P.A., Pawel B., Martinez D., Maris J.M., Simon M.C. Combinatorial regulation of neuroblastoma tumor progression by N-Myc and hypoxia inducible factor HIF-1α. Cancer Res. 2010;70:10351–10361. doi: 10.1158/0008-5472.CAN-10-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cho J., Lee J., Kim J., Kim S.T., Lee S., Kim S.Y., Ha S.Y., Park C.-K., Lim H.Y. Loss of tuberous sclerosis complex 2 (TSC2) as a predictive biomarker of response to mTOR inhibitor treatment in patients with hepatocellular carcinoma. Transl. Oncol. 2016;9:466–471. doi: 10.1016/j.tranon.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei Z., Xia J., Li J., Cai J., Shan J., Zhang C., Zhang L., Wang T., Qian C., Liu L. SIRT1 promotes glucolipid metabolic conversion to facilitate tumor development in colorectal carcinoma. Int. J. Biol. Sci. 2023;19:1925–1940. doi: 10.7150/ijbs.76704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cosset E., Ilmjärv S., Dutoit V., Elliott K., von Schalscha T., Camargo M.F., Reiss A., Moroishi T., Seguin L., Gomez G., et al. Glut3 addiction is a druggable vulnerability for a molecularly defined subpopulation of glioblastoma. Cancer Cell. 2017;32:856–868. doi: 10.1016/j.ccell.2017.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Song L., Tang H., Liao W., Luo X., Li Y., Chen T., Zhang X. FOXC2 positively regulates YAP signaling and promotes the glycolysis of nasopharyngeal carcinoma. Exp. Cell Res. 2017;357:17–24. doi: 10.1016/j.yexcr.2017.04.019. [DOI] [PubMed] [Google Scholar]
- 62.Zheng X., Han H., Liu G.P., Ma Y.X., Pan R.L., Sang L.J., Li R.-H., Yang L.-J., Marks J.R., Wang W., et al. LncRNA wires up Hippo and Hedgehog signaling to reprogramme glucose metabolism. EMBO J. 2017;36:3325–3335. doi: 10.15252/embj.201797609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hansen C.G., Ng YL D., Lam WL M., Plouffe S.W., Guan K.L. The Hippo pathway effectors YAP and TAZ promote cell growth by modulating amino acid signaling to mTORC1. Cell Res. 2015;25:1299–1313. doi: 10.1038/cr.2015.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Edwards D.N., Ngwa V.M., Wang S., Shiuan E., Brantley-Sieders D.M., Kim L.C., Reynolds A.B., Chen J. The receptor tyrosine kinase EphA2 promotes glutamine metabolism in tumors by activating the transcriptional coactivators YAP and TAZ. Sci. Signal. 2017;10:eaan4667. doi: 10.1126/scisignal.aan4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang C.S., Stampouloglou E., Kingston N.M., Zhang L., Monti S., Varelas X. Glutamine-utilizing transaminases are a metabolic vulnerability of TAZ/YAP-activated cancer cells. EMBO Rep. 2018;19:e43577. doi: 10.15252/embr.201643577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bertero T., Oldham W.M., Cottrill K.A., Pisano S., Vanderpool R.R., Yu Q., Zhao J., Tai Y., Tang Y., Zhang Y.Y., et al. Vascular stiffness mechanoactivates YAP/TAZ-dependent glutaminolysis to drive pulmonary hypertension. J. Clin. Investig. 2016;126:3313–3335. doi: 10.1172/JCI86387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y., Meng Q., Sun Q., Xu Z., Zhou H., Wang Y. LKB1 deficiency–induced metabolic reprogramming in tumorigenesis and non–neoplastic diseases. Mol. Metab. 2021;44:101131. doi: 10.1016/j.molmet.2020.101131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gonçalves E., Sciacovelli M., Costa A.S., Tran M.G., Johnson T.I., Machado D., Frezza C., Saez-Rodriguez J. Post–translational regulation of metabolism in fumarate hydratase deficient cancer cells. Metab. Eng. 2018;45:149–157. doi: 10.1016/j.ymben.2017.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu X., Tan X., Liu P., Wu Y., Qian S., Zhang X. Phosphoglycerate mutase 1 (PGAM1) promotes pancreatic ductal adenocarcinoma (PDAC) metastasis by acting as a novel downstream target of the PI3K/Akt/mTOR pathway. Oncol. Res. 2018;26:1123. doi: 10.3727/096504018X15166223632406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wen Y.A., Zhou B.W., Lv D.J., Shu F.P., Song X.L., Huang B., Wang C., Zhao S.C. Phosphoglycerate mutase 1 knockdown inhibits prostate cancer cell growth, migration, and invasion. Asian J. Androl. 2018;20:178–183. doi: 10.4103/aja.aja_57_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang D., Jin N., Sun W., Li X., Liu B., Xie Z., Qu J., Xu J., Yang X., Su Y., et al. Phosphoglycerate mutase 1 promotes cancer cell migration independent of its metabolic activity. Oncogene. 2017;36:2900–2909. doi: 10.1038/onc.2016.446. [DOI] [PubMed] [Google Scholar]
- 72.Ishikawa M., Inoue T., Shirai T., Takamatsu K., Kunihiro S., Ishii H., Nishikata T. Simultaneous expression of cancer stem cell-like properties and cancer-associated fibroblast-like properties in a primary culture of breast cancer cells. Cancers. 2014;6:1570–1578. doi: 10.3390/cancers6031570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Figueroa M.E., Abdel-Wahab O., Lu C., Ward P.S., Patel J., Shih A., Li Y., Bhagwat N., Vasanthakumar A., Fernandez H.F., et al. Leukemic IDH1 and IDH2 mutations result in a hypermethylation phenotype, disrupt TET2 function, and impair hematopoietic differentiation. Cancer Cell. 2010;18:553–567. doi: 10.1016/j.ccr.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chowdhury R., Yeoh K.K., Tian Y.M., Hillringhaus L., Bagg E.A., Rose N.R., Leung I.K.H., Li X.S., Woon E.C.Y., Yang M., et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011;12:463–469. doi: 10.1038/embor.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alam M.M., Lal S., FitzGerald K.E., Zhang L. A holistic view of cancer bioenergetics: Mitochondrial function and respiration play fundamental roles in the development and progression of diverse tumors. Clin. Transl. Med. 2016;5:3. doi: 10.1186/s40169-016-0082-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xu J., Yu T., Zois C.E., Cheng J.X., Tang Y., Harris A.L., Huang W.E. Unveiling cancer metabolism through spontaneous and coherent Raman spectroscopy and stable isotope probing. Cancers. 2021;13:1718. doi: 10.3390/cancers13071718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Danzi F., Pacchiana R., Mafficini A., Scupoli M.T., Scarpa A., Donadelli M., Fiore A. To metabolomics and beyond: A technological portfolio to investigate cancer metabolism. Signal Transduct. Target. Ther. 2023;8:137. doi: 10.1038/s41392-023-01380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jones C.L., Inguva A., Jordan C.T. Targeting energy metabolism in cancer stem cells: Progress and challenges in leukemia and solid tumors. Cell Stem Cell. 2021;28:378–393. doi: 10.1016/j.stem.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Grima–Reyes M., Martinez–Turtos A., Abramovich I., Gottlieb E., Chiche J., Ricci J.E. Physiological impact of in vivo stable isotope tracing on cancer metabolism. Mol. Metab. 2021;53:101294. doi: 10.1016/j.molmet.2021.101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Sá Junior P.L., Câmara D.A.D., Porcacchia A.S., Fonseca P.M.M., Jorge S.D., Araldi R.P., Ferreira A.K. The roles of ROS in cancer heterogeneity and therapy. Oxidative Med. Cell. Longev. 2017;2017:2467940. doi: 10.1155/2017/2467940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bader J.E., Voss K., Rathmell J.C. Targeting metabolism to improve the tumor microenvironment for cancer immunotherapy. Mol. Cell. 2020;78:1019–1033. doi: 10.1016/j.molcel.2020.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ohshima K., Morii E. Metabolic reprogramming of cancer cells during tumor progression and metastasis. Metabolites. 2021;11:28. doi: 10.3390/metabo11010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang Z., Wu X., Chen H.N., Wang K. Amino acid metabolic reprogramming in tumor metastatic colonization. Front. Oncol. 2023;13:1123192. doi: 10.3389/fonc.2023.1123192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Martínez-Reyes I., Chandel N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020;11:102. doi: 10.1038/s41467-019-13668-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang C., Shao L., Pan C., Ye J., Ding Z., Wu J., Du Q., Ren Y., Zhu C. Elevated level of mitochondrial reactive oxygen species via fatty acid β-oxidation in cancer stem cells promotes cancer metastasis by inducing epithelial–mesenchymal transition. Stem Cell Res. Ther. 2019;10:175. doi: 10.1186/s13287-019-1265-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Indran I.R., Tufo G., Pervaiz S., Brenner C. Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2011;1807:735–745. doi: 10.1016/j.bbabio.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 87.Chong SJ F., Low IC C., Pervaiz S. Mitochondrial ROS and involvement of Bcl-2 as a mitochondrial ROS regulator. Mitochondrion. 2014;19:39–48. doi: 10.1016/j.mito.2014.06.002. [DOI] [PubMed] [Google Scholar]
- 88.Zong W.X., Rabinowitz J.D., White E. Mitochondria and cancer. Mol. Cell. 2016;61:667–676. doi: 10.1016/j.molcel.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ellis L., Bots M., Lindemann R.K., Bolden J.E., Newbold A., Cluse L.A., Scott C.L., Strasser A., Atadja P., Lowe S.W., et al. The histone deacetylase inhibitors LAQ824 and LBH589 do not require death receptor signaling or a functional apoptosome to mediate tumor cell death or therapeutic efficacy. Blood. 2009;114:380–393. doi: 10.1182/blood-2008-10-182758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rudin C.M., Salgia R., Wang X., Hodgson L.D., Masters G.A., Green M., Vokes E.E. Randomized phase II Study of carboplatin and etoposide with or without the bcl-2 antisense oligonucleotide oblimersen for extensive-stage small-cell lung cancer: CALGB 30103. J. Clin. Oncol. 2008;26:870–876. doi: 10.1200/JCO.2007.14.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yan C., Gu J., Zhang Y., Ma K., Lee R.J. Efficient delivery of the Bcl-2 antisense oligonucleotide G3139 via nucleus-targeted aCD33-NKSN nanoparticles. Int. J. Pharm. 2022;625:122074. doi: 10.1016/j.ijpharm.2022.122074. [DOI] [PubMed] [Google Scholar]
- 92.Ferreira-da-Silva A., Valacca C., Rios E., Pópulo H., Soares P., Sobrinho-Simoes M., Scorrano L., Maximo V., Campello S. Mitochondrial dynamics protein Drp1 is overexpressed in oncocytic thyroid tumors and regulates cancer cell migration. PLoS ONE. 2015;10:e0122308. doi: 10.1371/journal.pone.0122308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Schimmer A.D., O’Brien S., Kantarjian H., Brandwein J., Cheson B.D., Minden M.D., Kamel-Reid S., Berger M., Viallet J., Borthakur G., et al. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin. Cancer Res. 2008;14:8295–8301. doi: 10.1158/1078-0432.CCR-08-0999. [DOI] [PubMed] [Google Scholar]
- 94.Kiany S., Harrison D., Gordon N. The histone deacetylase inhibitor Entinostat/Syndax 275 in osteosarcoma. Curr. Adv. Osteosarcoma Clin. Perspect. Past Present Future. 2020;1257:75–83. doi: 10.1007/978-3-030-43032-0_7. [DOI] [PubMed] [Google Scholar]
- 95.DiNardo C.D., Pratz K., Pullarkat V., Jonas B.A., Arellano M., Becker P.S., Letai A., Chyla B., Potluri J., Pollyea D.A., et al. Venetoclax combined with decitabine or azacitidine in treatment-naive, elderly patients with acute myeloid leukemia. Blood J. Am. Soc. Hematol. 2019;133:7–17. doi: 10.1182/blood-2018-08-868752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tron A.E., Belmonte M.A., Adam A., Aquila B.M., Boise L.H., Chiarparin E., Secrist J.P., Clark E.A., Wilson D.M., Hird A.W., et al. Discovery of Mcl-1-specific inhibitor AZD5991 and preclinical activity in multiple myeloma and acute myeloid leukemia. Nat. Commun. 2018;9:5341. doi: 10.1038/s41467-018-07551-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhou C., Yang Z.F., Sun B.Y., Yi Y., Wang Z., Zhou J., Fan J., Gan W., Ren N., Qiu S.J. Lenvatinib induces immunogenic cell death and triggers toll-like receptor-3/4 ligands in hepatocellular carcinoma. J. Hepatocell. Carcinoma. 2023;10:697–712. doi: 10.2147/JHC.S401639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin X.H., Qiu B.Q., Ma M., Zhang R., Hsu S.J., Liu H.H., Chen J., Gao D.M., Cui J.F., Ren Z.G., et al. Suppressing DRP1-mediated mitochondrial fission and mitophagy increases mitochondrial apoptosis of hepatocellular carcinoma cells in the setting of hypoxia. Oncogenesis. 2020;9:67. doi: 10.1038/s41389-020-00251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhao J., Zhang J., Yu M., Xie Y., Huang Y., Wolff D.W., Abel P.W., Tu Y. Mitochondrial dynamics regulates migration and invasion of breast cancer cells. Oncogene. 2013;32:4814–4824. doi: 10.1038/onc.2012.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Inoue-Yamauchi A., Oda H. Depletion of mitochondrial fission factor DRP1 causes increased apoptosis in human colon cancer cells. Biochem. Biophys. Res. Commun. 2012;421:81–85. doi: 10.1016/j.bbrc.2012.03.118. [DOI] [PubMed] [Google Scholar]
- 101.Ediriweera M.K., Jayasena S. The role of reprogrammed glucose metabolism in cancer. Metabolites. 2023;13:345. doi: 10.3390/metabo13030345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lei P., Wang W., Sheldon M., Sun Y., Yao F., Ma L. Role of glucose metabolic reprogramming in breast cancer progression and drug resistance. Cancers. 2023;15:3390. doi: 10.3390/cancers15133390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xie S.Z., Pan J.J., Xu J.F., Zhu W.W., Qin L.X. The critical function of metabolic reprogramming in cancer metastasis. Aging Cancer. 2022;3:20–43. doi: 10.1002/aac2.12044. [DOI] [Google Scholar]
- 104.Jin H.R., Wang J., Wang Z.J., Xi M.J., Xia B.H., Deng K., Yang J.L. Lipid metabolic reprogramming in tumor microenvironment: From mechanisms to therapeutics. J. Hematol. Oncol. 2023;16:103. doi: 10.1186/s13045-023-01498-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Reina-Campos M., Diaz-Meco M.T., Moscat J. The complexity of the serine glycine one–carbon pathway in cancer. J. Cell Biol. 2019;219:e201907022. doi: 10.1083/jcb.201907022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hipólito A., Martins F., Mendes C., Lopes-Coelho F., Serpa J. Molecular and metabolic reprogramming: Pulling the strings toward tumor metastasis. Front. Oncol. 2021;11:656851. doi: 10.3389/fonc.2021.656851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aggarwal V., Tuli H.S., Varol A., Thakral F., Yerer M.B., Sak K., Varol M., Jain A., Khan M.A., Sethi G. Role of reactive oxygen species in cancer progression: Molecular mechanisms and recent advancements. Biomolecules. 2019;9:735. doi: 10.3390/biom9110735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chen X., Chen S., Yu D. Metabolic reprogramming of chemoresistant cancer cells and the potential significance of metabolic regulation in the reversal of cancer chemoresistance. Metabolites. 2020;10:289. doi: 10.3390/metabo10070289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jang W.J., Choi B., Song S.H., Lee N., Kim D.J., Lee S., Jeong C.H. Multi-omics analysis reveals that ornithine decarboxylase contributes to erlotinib resistance in pancreatic cancer cells. Oncotarget. 2017;8:92727. doi: 10.18632/oncotarget.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang Y., Zhang J., Ren S., Sun D., Huang H.Y., Wang H., Jin Y., Li F., Zheng C., Yang L., et al. Branched-chain amino acid metabolic reprogramming orchestrates drug resistance to EGFR tyrosine kinase inhibitors. Cell Rep. 2019;28:512–525. doi: 10.1016/j.celrep.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 111.Iqbal M.A., Siddiqui S., Ur Rehman A., Siddiqui F.A., Singh P., Kumar B., Saluja D. Multiomics integrative analysis reveals antagonistic roles of CBX2 and CBX7 in metabolic reprogramming of breast cancer. Mol. Oncol. 2021;15:1450–1465. doi: 10.1002/1878-0261.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Weiss R.H. Metabolomics and metabolic reprogramming in kidney cancer. Semin. Nephrol. 2018;38:175–182. doi: 10.1016/j.semnephrol.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Draguet A., Tagliatti V., Colet J.M. Targeting Metabolic Reprogramming to Improve Breast Cancer Treatment: An In Vitro Evaluation of Selected Metabolic Inhibitors Using a Metabolomic Approach. Metabolites. 2021;11:556. doi: 10.3390/metabo11080556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li B., Sui L. Metabolic reprogramming in cervical cancer and metabolomics perspectives. Nutr. Metab. 2021;18:93. doi: 10.1186/s12986-021-00615-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Soumoy L., Schepkens C., Krayem M., Najem A., Tagliatti V., Ghanem G.E., Saussez S., Colet J.M., Journe F. Metabolic reprogramming in metastatic melanoma with acquired resistance to targeted therapies: Integrative metabolomic and proteomic analysis. Cancers. 2020;12:1323. doi: 10.3390/cancers12051323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Klotz L.O., Carlberg C. Nutrigenomics and redox regulation: Concepts relating to the Special Issue on nutrigenomics. Redox Biol. 2023;68:102920. doi: 10.1016/j.redox.2023.102920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Malcomson F.C., Mathers J.C. Translation of nutrigenomic research for personalised and precision nutrition for cancer prevention and for cancer survivors. Redox Biol. 2023;62:102710. doi: 10.1016/j.redox.2023.102710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rahman A., Chakraborty S., Kabir Y. Harnessing personalized nutrigenomics for cancer prevention and treatment through diet-gene interaction. In: Kabir Y., editor. Functional Foods in Cancer Prevention and Therapy. Academic Press; New York, NY, USA: 2020. pp. 387–403. [Google Scholar]
- 119.Aghakhani S., Zerrouk N., Niarakis A. Metabolic reprogramming of fibroblasts as therapeutic target in rheumatoid arthritis and cancer: Deciphering key mechanisms using computational systems biology approaches. Cancers. 2020;13:35. doi: 10.3390/cancers13010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yizhak K., Le Dévédec S.E., Rogkoti V.M., Baenke F., De Boer V.C., Frezza C., Schulze A., van de Water B., Ruppin E. A computational study of the Warburg effect identifies metabolic targets inhibiting cancer migration. Mol. Syst. Biol. 2014;10:744. doi: 10.15252/msb.20145746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Meric–Bernstam F., Lee R.J., Carthon B.C., Iliopoulos O., Mier J.W., Patel M.R., Tannir N.M., Owonikoko T.K., Haas N.B., Voss M.H., et al. CB–839, a glutaminase inhibitor, in combination with cabozantinib in patients with clear cell and papillary metastatic renal cell cancer (mRCC): Results of a phase I study. J. Clin. Oncol. 2019;37((Suppl. S7)):549. doi: 10.1200/JCO.2019.37.7_suppl.549. [DOI] [Google Scholar]
- 122.Mueller C., Al–Batran S., Jaeger E., Schmidt B., Bausch M., Unger C., Sethuraman N. A phase IIa study of PEGylated glutaminase (PEG–PGA) plus 6-diazo-5-oxo-L-norleucine (DON) in patients with advanced refractory solid tumors. J. Clin. Oncol. 2008;26((Suppl. S15)):2533. doi: 10.1200/jco.2008.26.15_suppl.2533. [DOI] [Google Scholar]
- 123.Li J., Kim S.G., Blenis J. Rapamycin: One drug, many effects. Cell Metab. 2014;19:373–379. doi: 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Navarro C., Ortega A., Santeliz R., Garrido B., Chacín M., Galban N., Vera I., De Sanctis J.B., Bermúdez V. Metabolic reprogramming in cancer cells: Emerging molecular mechanisms and novel therapeutic approaches. Pharmaceutics. 2022;14:1303. doi: 10.3390/pharmaceutics14061303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Phan L.M., Yeung S.C.J., Lee M.H. Cancer metabolic reprogramming: Importance, main features, and potentials for precise targeted anti-cancer therapies. Cancer Biol. Med. 2014;11:1. doi: 10.7497/j.issn.2095-3941.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Cioce M., Pulito C., Strano S., Blandino G., Fazio V.M. Metformin: Metabolic rewiring faces tumor heterogeneity. Cells. 2020;9:2439. doi: 10.3390/cells9112439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Xiao Y., Yu T.J., Xu Y., Ding R., Wang Y.P., Jiang Y.Z., Shao Z.M. Emerging therapies in cancer metabolism. Cell Metab. 2023;35:1283–1303. doi: 10.1016/j.cmet.2023.07.006. [DOI] [PubMed] [Google Scholar]
- 128.Roy S., Dukic T., Bhandary B., Tu K.J., Molitoris J., Ko Y.H., Shukla H.D. 3-Bromopyruvate inhibits pancreatic tumor growth by stalling glycolysis, and dismantling mitochondria in a syngeneic mouse model. Am. J. Cancer Res. 2022;12:497. [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang H., Shangguan F., Zhang L., Ma N., Song S., Ma L., Liu C., Liu M., An J., Li H., et al. A novel mechanism of 6-methoxydihydroavicine in suppressing ovarian carcinoma by disrupting mitochondrial homeostasis and triggering ROS/MAPK mediated apoptosis. Front. Pharmacol. 2023;14:1093650. doi: 10.3389/fphar.2023.1093650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Guan X., Morris M.E. Pharmacokinetics of the monocarboxylate transporter 1 inhibitor AZD3965 in mice: Potential enterohepatic circulation and target-mediated disposition. Pharm. Res. 2020;37:5. doi: 10.1007/s11095-019-2735-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Li W., Zheng M., Wu S., Gao S., Yang M., Li Z., Min Q., Sun W., Chen L., Xiang G., et al. Benserazide, a dopadecarboxylase inhibitor, suppresses tumor growth by targeting hexokinase 2. J. Exp. Clin. Cancer Res. 2017;36:58. doi: 10.1186/s13046-017-0530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Harding J.J., Telli M., Munster P., Voss M.H., Infante J.R., DeMichele A., Dunphy M., Le M.H., Molineaux C., Orford K., et al. A phase I dose-escalation and expansion study of telaglenastat in patients with advanced or metastatic solid tumors. Clin. Cancer Res. 2021;27:4994–5003. doi: 10.1158/1078-0432.CCR-21-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Saddiq A.A., El-Far A.H., Mohamed S.A., Almaghrabi O.A., Mousa S.A. Curcumin and thymoquinone combination attenuates breast cancer cell lines’ progression. Integr. Cancer Ther. 2022;21:15347354221099537. doi: 10.1177/15347354221099537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Pan L., Feng F., Wu J., Fan S., Han J., Wang S., Yang L., Liu W., Wang C., Xu K. Demethylzeylasteral targets lactate by inhibiting histone lactylation to suppress the tumorigenicity of liver cancer stem cells. Pharmacol. Res. 2022;181:106270. doi: 10.1016/j.phrs.2022.106270. [DOI] [PubMed] [Google Scholar]
- 135.Dogra N., Kumar A., Mukhopadhyay T. Fenbendazole acts as a moderate microtubule destabilizing agent and causes cancer cell death by modulating multiple cellular pathways. Sci. Rep. 2018;8:1–5. doi: 10.1038/s41598-018-30158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fan B., Mellinghoff I.K., Wen P.Y., Lowery M.A., Goyal L., Tap W.D., Pandya S.S., Manyak E., Jiang L., Liu G., et al. Clinical pharmacokinetics and pharmacodynamics of ivosidenib, an oral, targeted inhibitor of mutant IDH1, in patients with advanced solid tumors. Investig. New Drugs. 2020;38:433–444. doi: 10.1007/s10637-019-00771-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Furuse J., Ikeda M., Ueno M., Furukawa M., Morizane C., Takehara T., Nishina T., Todaka A., Okano N., Hara K., et al. A Phase II Placebo-Controlled Study of the Effect and Safety of Nanvuranlat in Patients with Advanced Biliary Tract Cancers Previously Treated by Systemic Chemotherapy. Clin. Cancer Res. 2024;30:3990–3995. doi: 10.1158/1078-0432.CCR-24-0461. [DOI] [PubMed] [Google Scholar]
- 138.Furuse J., Ikeda M., Ueno M., Furukawa M., Morizane C., Takehara T., Nishina T., Todaka A., Okano N., Hara K., et al. Nanvuranlat, an L-type amino acid transporter (LAT1) inhibitor for patients with pretreated advanced refractory biliary tract cancer (BTC): Primary endpoint results of a randomized, double-blind, placebo-controlled phase 2 study. J. Clin. Oncol. 2023;41((Suppl. S4)):494. doi: 10.1200/JCO.2023.41.4_suppl.494. [DOI] [Google Scholar]
- 139.Coulter D.W., Chhonker Y.S., Kumar D., Kesherwani V., Aldhafiri W.N., McIntyre E.M., Alexander G., Ray S., Joshi S.S., Li R., et al. Marinopyrrole derivative MP1 as a novel anti–cancer agent in group 3 MYC–amplified Medulloblastoma. J. Exp. Clin. Cancer Res. 2024;43:18. doi: 10.1186/s13046-024-02944-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.McGuire T.R., Coulter D.W., Bai D., Sughroue J.A., Li J., Yang Z., Qiao Z., Liu Y., Murry D.J., Chhonker Y.S., et al. Effects of novel pyrrolomycin MP1 in MYCN amplified chemoresistant neuroblastoma cell lines alone and combined with temsirolimus. BMC Cancer. 2019;19:837. doi: 10.1186/s12885-019-6033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Seliger C., Meyer A.L., Leidgens V., Rauer L., Moeckel S., Jachnik B., Proske J., Dettmer K., Rothhammer-Hampl T., Kaulen L.D., et al. Metabolic heterogeneity of brain tumor cells of proneural and mesenchymal origin. Int. J. Mol. Sci. 2022;23:11629. doi: 10.3390/ijms231911629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li J., Li S., Guo J., Li Q., Long J., Ma C., Ding Y., Yan C., Li L., Wu Z., et al. Natural product Micheliolide (MCL) irreversibly activates pyruvate kinase M2 and suppresses leukemia. J. Med. Chem. 2018;61:4155–4164. doi: 10.1021/acs.jmedchem.8b00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Sardesai S.D., Thomas A., Gallagher C., Lynce F., Ottaviano Y.L., Ballinger T.J., Schneider B.P., Storniolo A.M., Bauchle A., Althouse S.K., et al. Inhibiting fatty acid synthase with omeprazole to improve efficacy of neoadjuvant chemotherapy in patients with operable TNBC. Clin. Cancer Res. 2021;27:5810–5817. doi: 10.1158/1078-0432.CCR-21-0493. [DOI] [PubMed] [Google Scholar]
- 144.An J., Zhang Y., He J., Zang Z., Zhou Z., Pei X., Zheng X., Zhang W., Yang H., Li S. Lactate dehydrogenase A promotes the invasion and proliferation of pituitary adenoma. Sci. Rep. 2017;7:4734. doi: 10.1038/s41598-017-04366-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ruszkowski M., Sekula B., Ruszkowska A., Contestabile R., Nogues I., Angelaccio S., Szczpaniak A., Dauter Z. Structural basis of methotrexate and pemetrexed action on serine hydroxymethyltransferases revealed using plant models. Sci. Rep. 2019;9:19614. doi: 10.1038/s41598-019-56043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhao X., Zhu Y., Hu J., Jiang L., Li L., Jia S., Zen K. Shikonin inhibits tumor growth in mice by suppressing pyruvate kinase M2–mediated aerobic glycolysis. Sci. Rep. 2018;8:14517. doi: 10.1038/s41598-018-31615-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kelly W., Diaz Duque A.E., Michalek J., Konkel B., Caflisch L., Chen Y., Pathuri S.C., Madhusudanannair-Kunnuparampil V., Floyd J., II, Brenner A. Phase II investigation of TVB-2640 (denifanstat) with bevacizumab in patients with first relapse high-grade astrocytoma. Clin. Cancer Res. 2023;29:2419–2425. doi: 10.1158/1078-0432.CCR-22-2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jones C.L., Stevens B.M., D’Alessandro A., Reisz J.A., Culp-Hill R., Nemkov T., Pei S., Khan N., Adane B., Ye H., et al. Inhibition of amino acid metabolism selectively targets human leukemia stem cells. Cancer Cell. 2018;34:724–740. doi: 10.1016/j.ccell.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Xiao Z., Dai Z., Locasale J.W. Metabolic landscape of the tumor microenvironment at single cell resolution. Nat. Commun. 2019;10:3763. doi: 10.1038/s41467-019-11738-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Stine Z.E., Schug Z.T., Salvino J.M., Dang C.V. Targeting cancer metabolism in the era of precision oncology. Nat. Rev. Drug Discov. 2022;21:141–162. doi: 10.1038/s41573-021-00339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vogelstein B., Kinzler K.W. Cancer genes and the pathways they control. Nat. Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 152.Dragu D.L., Necula L.G., Bleotu C., Diaconu C.C., Chivu-Economescu M. Therapies targeting cancer stem cells: Current trends and future challenges. World J. Stem Cells. 2015;7:1185. doi: 10.4252/wjsc.v7.i9.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Borah A., Raveendran S., Rochani A., Maekawa T., Kumar D.S. Targeting self-renewal pathways in cancer stem cells: Clinical implications for cancer therapy. Oncogenesis. 2015;4:e177. doi: 10.1038/oncsis.2015.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Spinello I., Labbaye C., Saulle E. Metabolic function and Therapeutic Potential of CD147 for Hematological Malignancies: An overview. Int. J. Mol. Sci. 2024;24:9178. doi: 10.3390/ijms25179178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zuo F., Yu J., He X. Single-cell metabolomics in hematopoiesis and hematological malignancies. Front. Oncol. 2022;12:931393. doi: 10.3389/fonc.2022.931393. [DOI] [PMC free article] [PubMed] [Google Scholar]