Abstract
Cells that lack p53 signaling frequently occur in ulcerative colitis (UC) and are considered early drivers in UC-associated colorectal cancer (CRC). Epithelial injury during colitis is associated with transient stem cell reprogramming from the adult, homeostatic to a “fetal-like” regenerative state. Here, we use murine and organoid-based models to study the role of Trp53 during epithelial reprogramming. We find that p53 signaling is silent and dispensable during homeostasis but strongly up-regulated in the epithelium upon DSS-induced colitis. While in WT cells this causes termination of the regenerative state, crypts that lack Trp53 remain locked in the highly proliferative, regenerative state long-term. The regenerative state in WT cells requires high Wnt signaling to maintain elevated levels of glycolysis. Instead, Trp53 deficiency enables Wnt-independent glycolysis due to overexpression of rate-limiting enzyme PKM2. Our study reveals the context-dependent relevance of p53 signaling specifically in the injury-induced regenerative state, explaining the high abundance of clones lacking p53 signaling in UC and UC-associated CRC.
Loss of p53 locks cells in the fetal-like regenerative state in the context of colitis.
INTRODUCTION
The colonic epithelium is organized into clonal crypts, which, under homeostatic conditions, show a distinct cellular organization with proliferative Lgr5+ stem cells in the crypt base giving rise to short-lived differentiated secretory and absorptive lineages (1). Mucosal injury causes transient reprogramming of the epithelium into a highly regenerative state (2, 3), characterized by an expansion of proliferative cells at the expense of differentiation—a state that resembles the fetal epithelium (4). Upon resolution of injury, the regenerative state is terminated, enabling the reacquisition of homeostasis. Inflammatory bowel diseases such as ulcerative colitis (UC) are characterized by inflammation and recurring disruption of the mucosal barrier. The epithelium of patients with UC shows chronic up-regulation of regenerative fetal-like gene signatures (4, 5), suggesting that it persists in the regenerative state long-term.
Reactivation of fetal-like programs is also a feature of cancer (6). Patients with UC have a higher risk of developing colorectal carcinoma (CRC) (7, 8). Previous insights into the mutational landscape of the epithelium of patients with UC have revealed a selection and expansion of clones that lack p53 activity due to sporadic mutations (9–12). Furthermore, loss or inactivation of p53 is frequently observed in UC-associated CRC (13). The mutational cascade of UC-associated CRC differs both in frequency and sequence from sporadic CRC: Mutations in p53 that go along with loss of p53 signaling (14, 15) are observed earlier and more frequently in UC-associated CRC (16–20). These loss-of-function mutations have been identified as likely drivers of carcinogenesis in patients with UC (21). However, it has so far not been investigated why selection and expansion of clones that lack p53 signaling occur specifically in the context of colitis and UC-associated CRC, and whether and how such clones affect epithelial function.
In the present study, we investigated how loss of p53 signaling affects the murine colonic epithelium in the context of colitis. We demonstrate that TRP53 is induced in the murine epithelium upon entry into the regenerative state after chemical colitis. By integrating comprehensive proteome and transcriptome analyses, we discover that epithelium lacking p53 fails to reestablish homeostatic crypt architecture and lineage diversity and instead remains locked in a fetal-like regenerative state. We dissect this finding mechanistically by using organoid cultures. Bioenergetic profiling reveals altered metabolic activity in Trp53 knockout (KO) cells that is characterized by high dependence on glycolysis with overexpression of the glycolytic rate-limiting enzyme pyruvate kinase M2 (PKM2). We show that the regenerative state can be blocked by inhibition of glycolysis, which specifically affects Trp53 KO cells locked in the regenerative state.
RESULTS
Trp53 signaling is activated after colitis-associated injury but is dispensable for colonic homeostasis
Mutation of Trp53 has previously been shown to change stem cell dynamics in dextran sodium sulfate (DSS) colitis but not in healthy tissue (22), hinting at a context-dependent role of Trp53 in the colonic epithelium. We therefore wanted to determine the activity of TRP53 in the colonic epithelium during homeostasis and regeneration. To this end, we first treated wild-type (WT) mice with 2% (w/v) DSS dissolved in drinking water for 5 days and euthanized them 5 to 7 days or 2 months later to capture early and late responses to DSS. Histology and immunofluorescence (IF) analyses confirmed that at early time points, the epithelium showed known features of the regenerative state such as a thickening of the epithelial layer, pseudostratified crypts (fig. S1A), a proliferative burst within the epithelium (fig. S1B), activation of regenerative yes-associated protein 1 (YAP) signaling throughout the crypt (fig. S1C), and loss of differentiated cells (fig. S1, D and E). After 2 months, the epithelium had fully regained its normal architecture and lineage compartmentalization and returned to the homeostatic state (fig. S1, A to E, bottom). These findings are in line with published data for DSS colitis (4), as confirmed by gene set enrichment analysis (GSEA) (fig. S1F). We also harnessed published expression data of epithelial cells from two different DSS time course datasets (23, 24) and a published dataset of stromal pseudobulk RNA expression (25) to investigate the signaling cues involved in regeneration. We found that Wnt/β-catenin signaling, including Wnt targets like Cd44 and Axin2, and the proliferation marker Ki-67 are up-regulated early after injury (fig. S1, G to I). In concordance with this epithelial response, entry into the regenerative state is linked to stromal up-regulation of the Wnt signaling enhancers Rspo3 and Rspo1 and down-regulation of the Wnt inhibitor Dkk3. We also analyzed mice with a KO of Rspo3 in myofibroblasts, the primary producers of this ligand in colon stroma. We treated mice with DSS and found that lack of Rspo3 prevents the epithelium from entering the regenerative state, as indicated by the absence of YAP up-regulation (fig. S1K). These data demonstrate the importance of Wnt/Rspo signaling after DSS-induced damage to allow for initiation of regeneration in colonic epithelium.
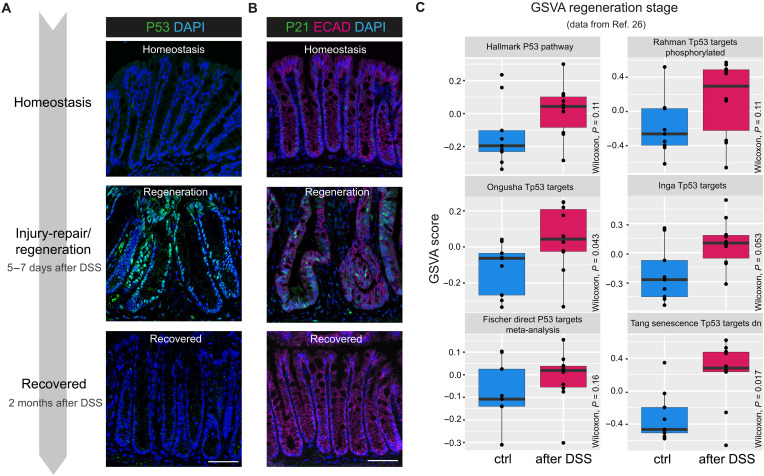
Next, we performed IF staining for p53 and its target p21 (CDKN1A) at time points representing homeostasis, regeneration (5 to 7 days after DSS), and recovery (2 months after DSS) to investigate the time course of p53 activation during and after colitis injury. While epithelial expression of p53 and p21 protein was almost completely absent during homeostasis, both were present in almost all nuclei of regenerative crypts (Fig. 1, A and B). By 2 months after the initial injury, expression had returned to baseline levels. We confirmed our findings on the transcriptome level by performing gene set variation analysis (GSVA) on a published dataset from a time series DSS experiment with similar conditions to our experimental setup (26), which revealed a substantial increase in p53 signaling during regeneration (5 days DSS plus 7 days recovery) across several gene sets (Fig. 1C). Thus, we conclude that p53 signaling is largely absent during homeostasis but becomes activated after colitis-associated injury.
Fig. 1. Trp53 signaling is activated after epithelial injury.
(A) Schematic for DSS-induced colitis and IF staining for P53 (green) and DAPI (blue). (B) IF staining for P21 (green), E-cadherin (ECAD; magenta), and 4′,6-diamidino-2-phenylindole (DAPI; blue). (C) Box and whisker plot for GSVA score for p53-related pathways (26) in control (blue) versus DSS-treated colon in regeneration phase (5 days DSS with 7 days of recovery) (magenta). Scale bars, 50 μm. n = 5 mice for homeostasis, n = 6 mice for regeneration, and n = 3 mice for 2-month recovery.
To confirm that p53 activity is dispensable for maintenance of homeostatic crypts, we analyzed the colonic epithelium of uninjured Trp53 KO mice. Histological and IF analysis failed to reveal obvious defects: Overall crypt architecture remained intact (fig. S2, A and F), and while there was a slight but not statistically significant increase in the proliferative compartment as revealed by KI-67 staining (fig. S2, B and G), other markers such as active YAP (aYAP) (regeneration), MUC2 (goblet cell lineage), and KRT20 (absorptive/colonocyte lineage) remained unaltered (fig. S2, C to E and I). The absence of Trp53 was confirmed by genotyping and IF (fig. S2J).
Loss of Trp53 prevents return to homeostasis after colitis-associated injury
As we observed up-regulation of TRP53 expression specifically in the epithelial lining during regeneration, we next wanted to understand its role in epithelial cells during injury repair. To this end, we generated a conditional knockout (cKO) model (Axin2 CreERT2 Trp53 flox/flox) in which Trp53 can be deleted specifically in epithelial stem/progenitor cells that give rise to all other epithelial lineages present in the colonic crypt. The KO was induced by injection of tamoxifen 7 days before DSS treatment for 5 days, followed by 2 months of recovery (Fig. 2A).
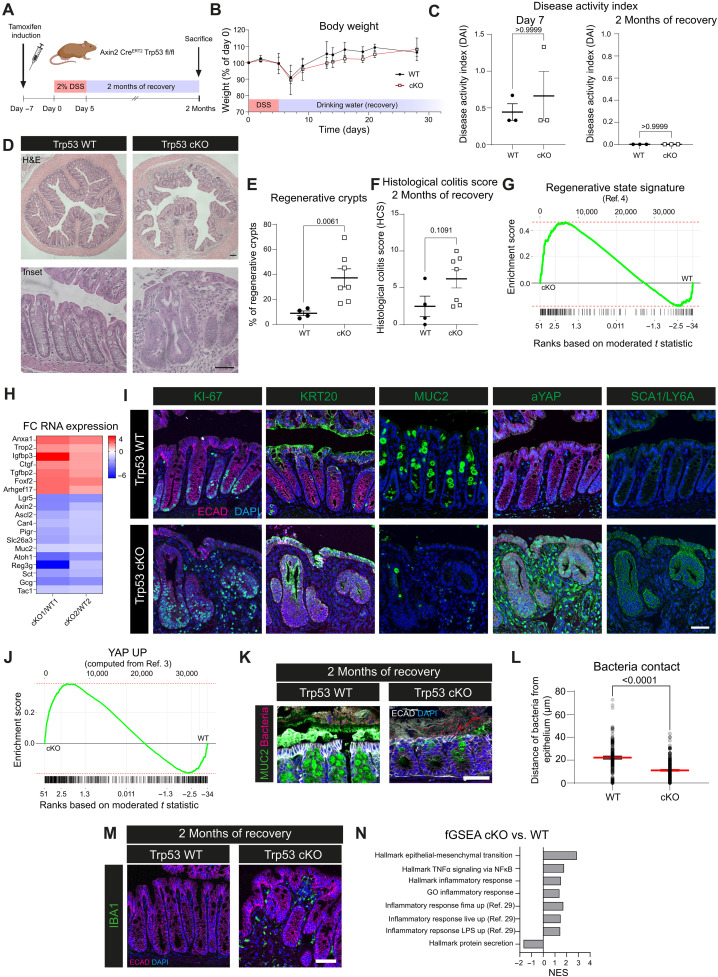
Fig. 2. Loss of Trp53 prevents return to tissue homeostasis after colitis-associated injury and locks the epithelium in a regenerative state.
(A) Schematic: DSS treatment and recovery. (B) Body weight curve. (C) Disease activity index (DAI) for day 7 and 2 months of recovery. (D) Hematoxylin and eosin (H&E) staining for WT and cKO with 2 months of recovery. (E) Quantification of regenerative crypts after 2 months of recovery. (F) Histological colitis score at 2 months recovery. (G) Enrichment plot for the regenerative signature (4) cKO versus WT [false discovery rate (FDR) = 0.015, P = 0.00038, enrichment score (ES) = 0.47, normalized enrichment score (NES) = 2]. (H) Fold change (FC) of RNA expression for regenerative and epithelial lineage markers in cKO versus WT. Results from one WT versus one cKO mouse each. (I) IF of WT and cKO for KI-67, KRT20, MUC2, aYAP, or SCA1/LY6a (green), E-cadherin (magenta), and DAPI (blue). (J) Enrichment plot for a YAP signature based on data from (3) for cKO versus WT (FDR = 0.015, P = 0.00036, ES = 0.38, NES = 1.8). (K) RNA-ISH and IF for bacteria (magenta), MUC2 (green), E-cadherin (white), and DAPI (blue) in WT and cKO mice with 2 months of recovery. (L) Quantification of (K). Median and SEM. (M) IF for IBA1 (green) in WT and cKO mice with 2 months of recovery (E-cadherin, magenta; DAPI, blue). (N) NES for GSEA of Molecular Signatures Database (MSigDB) gene sets of mice with 2 months of recovery. Scale bars, 100 μm [overview in (C)] and 50 μm (others). (B and C) n = 3 mice for WT and cKO. (E, F, and L) n = 2 mice for WT; n = 3 for cKO; data points represent quantification from different colon cross sections. n = 2 mice for WT; n = 3 for cKO for all stainings. n = 2 mice for RNA expression data. TNFα, tumor necrosis factor–α; NF-κB, nuclear factor κB; GO, Gene Ontology; LPS, lipopolysaccharide.
During the injury phase, Trp53 cKO mice lost weight to the same extent as WT mice but regained weight more slowly thereafter (Fig. 2B). Despite weight loss, both WT and cKO mice showed only mild clinical signs during injury, which were similar in both groups (Fig. 2C, left), with no disease activity evident after 2 months of recovery (Fig. 2C, right). To exclude escaper crypts emerging, we stained colon tissues from mice euthanized early after DSS and found that, while WT mice had high nuclear signal, p53 expression was entirely absent in cKO mice (fig. S3J).
We then analyzed the tissue of mice euthanized after 2 months of recovery and found that, while the WT epithelium was fully recovered, the colon of mice lacking epithelial Trp53 remained in a regenerative state even 2 months after DSS treatment, with large stretches of epithelium still thickened and pseudostratified (Fig. 2, D and E). When investigating tissue sections, we observed a trend toward a higher histological colitis score in Trp53 cKO mice (Fig. 2F). To explore this phenotype further, we performed a transcriptome analysis comparing bulk mRNA from WT and Trp53 cKO colon and found that Trp53 cKO mice showed increased expression of regenerative markers such as Anxa1, Igfbp3, and Ctgf, while markers of homeostatic epithelium were decreased, such as the homeostatic stem cell marker Lgr5 along with differentiation markers for colonocytes (e.g., Slc26a3), secretory cells (e.g., Atoh1), and enteroendocrine cells (e.g., Gcg). GSEA analysis revealed a significant enrichment for a previously defined signature of the regenerative state (4) in Trp53 cKO mice (Fig. 2, G and H). These findings were confirmed at the protein level using IF staining (Fig. 2I): At 2 months post-DSS, the epithelium of Trp53 cKO mice showed notable similarities to the regenerative epithelium of WT mice early after injury (compare to fig. S1, A to E). We observed increased numbers of proliferating cells labeled by KI-67, loss of the differentiation markers KRT20 and MUC2, and increased expression of the regenerative markers aYAP and Stem cells antigen-1 (SCA1) [Fig. 2I, representative cross sections in fig. S3A, and quantification in fig. S3 (B to D)]. We then combined two published signatures (3) into one gene signature for up-regulated YAP signaling (YAP UP), which showed an enrichment in the colon of Trp53 cKO mice compared to WT mice (Fig. 2J).
As Wnt/Rspo signaling is also up-regulated in the recovery phase (fig. S1G) (27) and crucial for activating regenerative YAP signaling (fig. S1K), we next aimed to decipher the Wnt landscape in the “locked regenerative” Trp53 cKO tissue. We performed in situ hybridization for Rspo3 and Axin2 to investigate the Wnt/Rspo signaling activity over time in Trp53 WT and cKO mice. As expected, Rspo3 was up-regulated strongly during injury repair after DSS both in WT and cKO but stayed slightly enriched in cKO mice after 2 months of recovery (fig. S3, E and F). The same was true for Axin2 expression, which remained elevated in cKO mice after 2 months of recovery (fig. S3, G and H). To eliminate the possibility that Wnt signaling was elevated due to a mutation in the Wnt/β-catenin pathway, we stained for β-catenin in tissue sections of Trp53 WT and cKO mice and used adenomatous polyposis coli (APC) KO assembloids (28) as a control. While assembloids generated from APC KO organoids showed nuclear accumulation of β-catenin, no such accumulation was visible in any of the tissue sections (fig. S3I). Together, these data suggest an elevation of regenerative signaling pathways, including Wnt/Rspo signaling in Trp53 cKO tissues.
As we observed ongoing regeneration in Trp53 cKO mice even at 2 months postinjury, we wondered whether this affected tissue functionality. We therefore used methacarn-fixed colonic specimens and coimaged the microbiota and epithelial mucus by using a combined approach of RNA in situ hybridization (RNA-ISH) and IF. While in WT mice a thick mucus barrier separated the microbiota from the epithelium, mucus was largely absent in Trp53 cKO mice, and bacteria were found in close contact with epithelial cells (Fig. 2, K and L). We next checked for immune infiltration and found IBA1, a marker for macrophages also known as AIF1 (29–32), expressed in the regenerative areas of Trp53 cKO but not WT mice (Fig. 2M). These findings were reflected in our transcriptome data, in which inflammatory pathways were enriched in Trp53 cKO versus WT mice, particularly pathways involved in macrophage responses (Fig. 2N) (33).
We therefore conclude that following colonic injury, mice lacking epithelial Trp53 fail to restore colonic homeostasis, show ongoing up-regulation of Wnt/Rspo signaling, and are locked in the regenerative state. This leads to impaired barrier integrity and fuels chronic inflammation.
Wnt supplementation induces the regenerative state and Trp53 activation in organoid cultures
Next, we wanted to further characterize the “locked” regenerative state found in epithelium lacking Trp53 and understand the underlying signaling events. For this, we chose the organoid model, as it faithfully recapitulates cell lineages present in the colonic crypt and allows for fine-tuning of cell states by manipulating growth factor supplementation. The standard medium composition for organoid cultures includes supraphysiological levels of Wnt-activating ligands [surrogate Wnt (sWnt) and CHIR99021 (Chir)], and, thus, organoid cultures per se can be considered to represent the regenerative state. We thus first adjusted our culture conditions to mimic homeostasis (absence or withdrawal of Wnt ligands) and regeneration (supplementation of Wnt ligands) as observed during DSS colitis in mice. We used organoids derived from the small intestine (SI), which can grow without artificial activation of Wnt signaling through medium supplementation (34), therefore resembling the homeostatic tissue with both stem cells and differentiated cells present. By adding sWnt to the culture, we observed a well-described phenotypic shift of crypt-like to spheroid growth (fig. S4A) alongside mRNA expression profiles that copy the phenotype observed in colon epithelium during the regenerative state: decreased expression of differentiation markers and up-regulation of regenerative markers (fig. S4B). In concordance with the increased expression of p53 and its target p21 observed in regenerative colon tissue (see Fig. 1, A and B), we also saw up-regulation of p53 target genes in regenerative SI organoids (fig. S4B, right).
We then repeated the experiment using colon organoids. As colon organoids cannot form in the absence of Wnt signaling (35), we allowed them to establish in Wnt-activating medium, before transferring them to medium with (+WM) or without Wnt ligands (−WM) for 2 days (Fig. 3A). Again, Wnt supplementation boosted transcription of regenerative markers while inhibiting expression of differentiation markers (Fig. 3B, left and second from left). Unlike in SI organoids (fig. S4B, second from the right), stem cell and Wnt signaling markers were increased in +WM medium (Fig. 3B, second from the right), which might reflect the higher dependence on external Wnt, since colon organoids lack Wnt ligand–producing Paneth cells (34). p53 signaling was also strongly increased in regeneration-inducing +WM medium (Fig. 3B, right), confirming that the regenerative state is associated with elevated p53 signaling also in colon organoids.
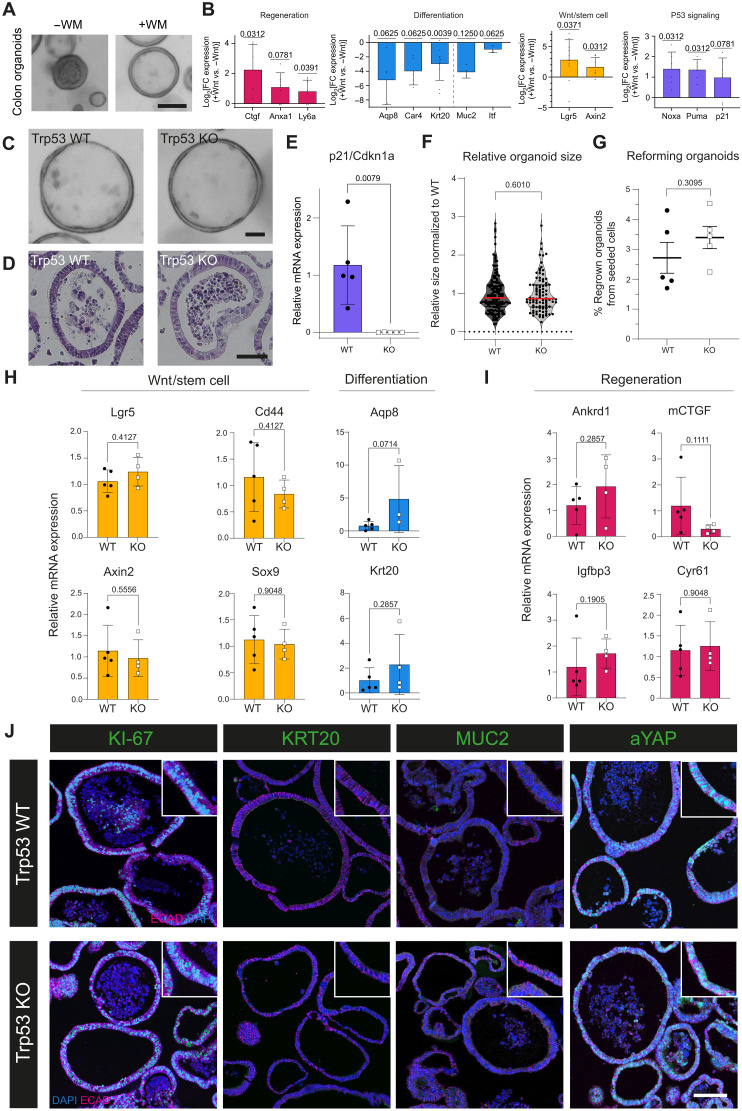
Fig. 3. Trp53 KO organoids do not differ from WT organoids in culture conditions mimicking regeneration.
(A) Brightfield (BF) images of colon organoids kept in medium without (−WM) or with (+WM) supplementation of sWnt after organoid formation. Scale bar, 100 μm. Representative images from n > 10. (B) LogFC of mRNA expression of regeneration, differentiation, and Wnt/stem cell markers and p53 signaling for +WM versus −WM organoids. n = 4 to 10 biological replicates. (C) BF images of Trp53 WT and KO organoids in +WM medium. Scale bar, 50 μm. Representative images from n > 10 biological replicates. (D) H&E images of Trp53 WT and KO organoids. Scale bar, 50 μm. Representative images from n = 3 biological replicates. (E) mRNA expression of p21 in WT and KO organoids in +WM medium. n = 5 biological replicates. (F) Quantification of organoid size in +WM condition normalized to WT. Median (red line) and quartiles (black lines). n = 4 biological replicates. (G) Percentage of organoids regrowing from seeded cells for WT and KO organoids in +WM condition. n = 5 biological replicates. (H) mRNA expression of Wnt signaling/stem cell markers and differentiation markers of Trp53 WT and KO organoids in +WM medium. n = 3 to 5 biological replicates. (I) mRNA expression of regeneration markers of Trp53 WT and KO organoids grown in +WM medium. n = 4 to 5 biological replicates. (J) IF images for WT and KO organoids in +WM condition for KI-67, KRT20, MUC2, and aYAP (all green) and counterstaining for E-cadherin (magenta) and DAPI (blue). Representative images from n = 3 biological replicates. Scale bar, 100 μm.
Loss of p53 does not alter colon organoid functionality or gene expression in the regenerative state
As TRP53 is activated in regeneration-inducing +WM cultures, we asked what effects its deletion would cause in colon organoids. For this, we used a tamoxifen-inducible epithelial KO model (VillinCreERT2 Trp53 flox/flox) and generated stable Trp53 KO lines, as verified by genotyping (fig. S4C) and evaluation of p21 (Cdkn1a) expression by quantitative polymerase chain reaction (qPCR; Fig. 3E). In the regeneration-inducing +WM medium condition, Trp53 KO and WT organoids did not differ in terms of morphology (Fig. 3, C and D), size (Fig. 3F), or organoid reforming capacity (Fig. 3G and fig. S4D). Also functionally, KO organoids did not outcompete WT organoids in a competition assay using coculture with fluorescent WT organoids (fig. S4, E and F). Examining both mRNA (via qPCR) and protein expression (via IF) of markers for stem cells/Wnt signaling, differentiation, and regeneration, we did not find any significant regulation due to Trp53 KO (Fig. 3, H to J). In summary, loss of Trp53 does not have an obvious effect on organoids grown in conditions that drive the regenerative state.
Trp53 KO organoids remain in the regenerative state after reinduction of homeostatic conditions
While Trp53 does not appear to be required for regeneration in organoids, our in vivo experiments had shown TRP53 up-regulation after injury in the regenerative epithelium, and its loss prevented a return to homeostasis. Therefore, we hypothesized that TRP53 may play a role in termination of the regenerative state. We tested this hypothesis in colonic Trp53 KO and WT organoids, by first inducing the regenerative state using external Wnt activation (+WM) and then reinducing homeostasis by withdrawing Wnt signals (−WM) (Fig. 4A, top). While WT organoids morphologically changed toward smaller, thick-walled organoids typical of homeostatic conditions, Trp53 KO organoids kept their spheroidal shape (Fig. 4A, bottom). qPCR for differentiation markers revealed that Trp53 KO organoids did not up-regulate Krt20 and Aqp8 expression to the same extent as WT organoids (Fig. 4B), and on the transcriptome level, they also showed a global negative enrichment for a published enterocyte gene set (Fig. 4C, upper left) (36). Despite removal of external Wnt factors, the transcriptome of Trp53 KO organoids showed enrichment for the intestinal stem cell signature (37) and for the regenerative colonic signature and for the colonic “fetal-like” state signature (Fig. 4C) (4).
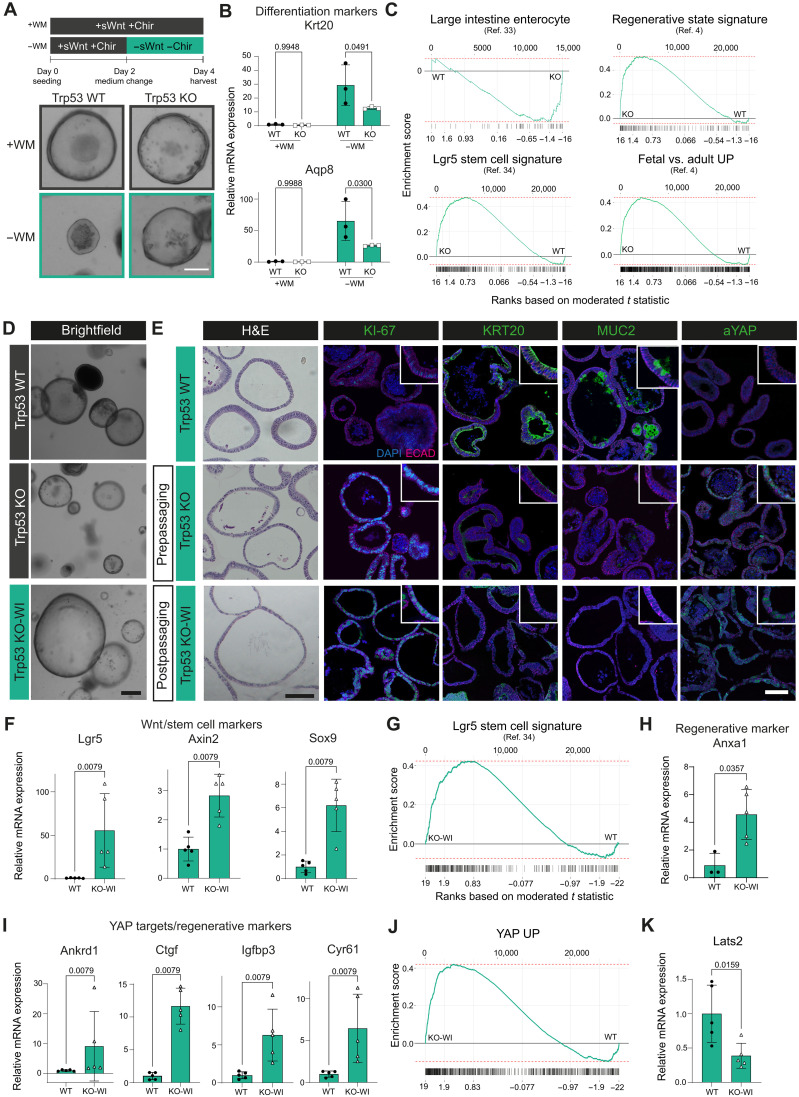
Fig. 4. Trp53 KO organoids remain in the regenerative state after reinduction of homeostatic conditions.
(A) Treatment schematic and BF images of Trp53 WT and KO organoids. Scale bar, 50 μm. n > 6 biological replicates. (B) mRNA expression of differentiation markers for WT and KO in +WM and −WM conditions, n = 3 biological replicates. (C) Enrichment plots for KO versus WT in −WM conditions of large intestine enterocytes (36), Lgr5 stem cell (37), regenerative state, and fetal-like signature (4) (enterocytes FDR = 0.031, P = 0.0017, ES = −0.49, NES = −1.9; Lgr5 stem cell FDR = 0.012, P = 0.00026, ES = 0.48, NES = 2; regenerative FDR = 0.012, P = 0.00028, ES = 0.51, NES = 2; fetal versus adult FDR = 0.012, P = 0.00022, ES = 0.42, NES = 2), n = 2 biological replicates per genotype. (D) BF images of WT, KO organoids (in −WM condition), and KO in −WM medium (KO-WI). Scale bar, 250 μm. n > 6 biological replicates. (E) H&E and IF images for WT, KO, and KO-WI in −WM condition. KI-67, KRT20, MUC2, aYAP (all green), E-cadherin (magenta), and DAPI (blue). Scale bars, 100 μm. n = 3 biological replicates. (F) mRNA expression of Wnt/stem cell markers for WT and KO-WI in −WM condition. n = 5 biological replicates. (G) Enrichment plots for KO-WI versus WT in −WM conditions for Lgr5 signature (FDR = 0.019, P = 0.00048, ES = 0.42, NES = 1.9), n = 2 biological replicates per genotype. (H and I) mRNA expression of regenerative markers in WT and KO-WI in −WM condition. n = 3 to 5 biological replicates. (J) Enrichment plots for KO-WI versus WT in −WM conditions for YAP UP signature (FDR = 0.019, P = 5 × 10−04, ES = 0.42, NES = 2), n = 2 biological replicates per genotype. (K) Lats2 mRNA expression in WT and KO-WI in −WM conditions. n = 5 biological replicates.
To test whether the up-regulation of regenerative pathways was of any functional relevance, we passaged Trp53 KO organoids maintained in medium without Wnt supplementation. Unexpectedly, Trp53 KO organoids were now able to continue growing in −WM medium and could be maintained in this medium long-term (Fig. 4D and fig. S5A). We termed these organoids growing without external Wnt activation Wnt-independent Trp53 KO (KO-WI) organoids. Trp53 KO-WI organoids were characterized by a larger size compared to WT or KO organoids (Fig. 4, D and E, and fig. S5B) and lack of polarization (Fig. 4E, first column). For all lineage markers investigated, Trp53 KO-WI organoids showed even higher expression than KO organoids compared to WT, both on mRNA and protein levels: massive up-regulation of proliferation (KI-67), aYAP and YAP target genes (e.g., Anxa1, Ankrd1, and Ctgf), as well as the Wnt signaling/stem cell markers Lgr5 and Axin2 (Fig. 4, E, F, H, and I). At the same time, differentiation markers (e.g., Krt20 and Muc2) were down-regulated (Fig. 4E and fig. S5, F, H, and I). Transcriptome analysis of Trp53 KO-WI, Trp53 KO, and WT organoids also revealed that on a global level, pathways enriched in Trp53 KO organoids were even more enriched in Trp53 KO-WI organoids: the Lgr5 stem cell signature, “YAP UP,” and fetal signature (Fig. 4, G and J, and fig. S5D). Simultaneously, differentiation gene sets for absorptive and secretory lineages were negatively enriched (fig. S5, G and J).
Next, we examined whether the loss of Trp53 directly acts on components of the Hippo/YAP pathway. We found reduced mRNA expression of the effector kinase Lats2 (Fig. 4K) but not its paralog Lats1 (fig. S5E), in KO-WI organoids, which could point to a specific mechanistic link of p53 signaling intercepting with YAP signaling during return to homeostasis.
To characterize Trp53 KO-WI organoids functionally, we repeated the competition experiment described above and cocultured KO-WI or WT organoids 1:1 with fluorescently labeled WT organoids in +WM and −WM conditions (fig. S5K). Trp53 KO-WI organoids outcompeted WT cells not only in the −WM condition but also in the +WM condition (fig. S5, L to N), likely reflecting the competitive advantage during injury and regeneration described by others (22). The number of fluorescent WT organoids growing in the +WM condition remained unchanged irrespective of whether they were cocultured with WT or KO-WI organoids, indicating that the growth advantage is intrinsic to regenerative KO-WI cells and not due to an inhibitory effect on WT bystander cells.
We then performed whole genome sequencing (WGS) in two clones of KO-WI organoids to ask whether they had acquired classic Wnt-activating mutations that could lead to the observed phenotype. However, no evidence of coding mutations in the various Wnt or YAP-related genes was detected (fig. S5O). We also performed Sanger sequencing for Exon 3 of β-catenin, in which mutations are known to occur frequently in models of colorectal cancer. Again, no mutations were detected in any of the five KO-WI replicates sequenced (file S1).
Overall, we conclude that the Trp53 KO epithelium fails to return to homeostasis following regeneration. Once induced, the Trp53 KO epithelium remains fixed in the regenerative state long-term even in the absence of the growth factors that initially drive entry into this state with Trp53 KO cells outcompeting WT cells.
Activation of Trp53 transiently suppresses Wnt signaling during regeneration
To understand the interaction of p53 and Wnt signaling observed in KO and KO-WI organoids, we applied the reverse approach and activated p53 signaling in SI and colon organoids by treatment with the mouse double minute 2 homolog (MDM2) inhibitor Nutlin-3a. In SI organoids, ongoing Wnt supplementation in +WM medium attenuated any effect of Nutlin, probably due to the high potency of the artificial Wnt ligand used. Thus, we changed the experimental setup as shown in fig. S6A: SI organoids were Wnt-boosted into a regenerative state for 3 days and subsequently changed to −WM medium to allow for wash out of Wnt signal. Six and 14 hours before harvesting, organoids were treated with 10 μm of Nutlin-3a or vehicle control [dimethyl sulfoxide (DMSO)]. As expected, we observed a robust increase of Trp53 signaling as revealed by elevated expression of the Trp53 target genes p21 and Puma (fig. S6B). Wnt target expression was reduced as early as 6 hours (fig. S6C), whereas an increase in differentiation marker expression only started from 14 hours onward (fig. S6D). Cultures of colon organoids were treated for 2 hours with 10 μm of Nutlin-3a or vehicle (fig. S6E). Consistent with the SI organoid data, we saw an increase in Trp53 target gene expression, down-regulation of Wnt signaling, and up-regulation of differentiation markers (fig. S6F). These data show that activation of p53 transiently suppresses Wnt signaling during both SI and colon regeneration, which is then followed by epithelial differentiation. Thus, our data indicate that activation of Trp53 during regeneration terminates the regenerative state by suppressing Wnt signaling and reinitiating differentiation. In this way, p53 could serve as a sensor to prevent excessive regeneration and enable the highly proliferative fetal-like epithelium to return to homeostasis with physiological crypt compartmentalization and lineage differentiation.
Locked regenerative Trp53 KO epithelium remains in a high glycolytic metabolic state
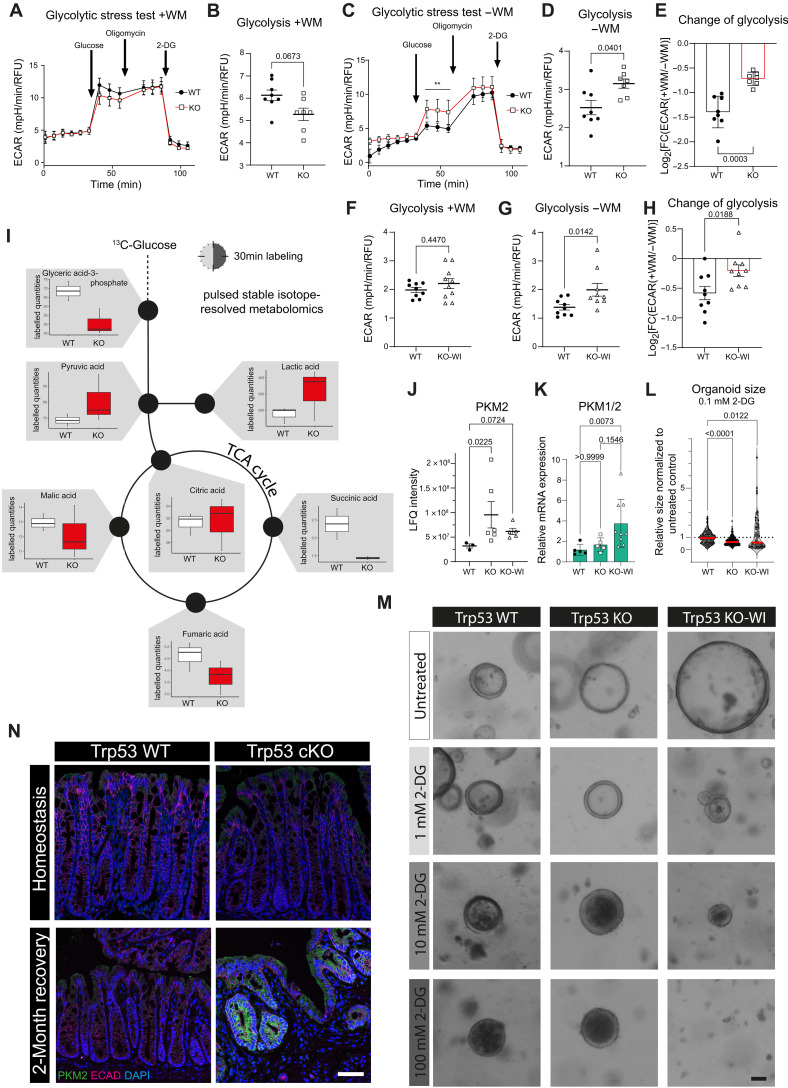
Medium color change indicated a more rapid acidification in Trp53 KO and KO-WI organoid cultures in the −WM medium experiments. We thus wondered whether their higher regenerative capacity could be explained by different metabolic regulation compared to WT organoids. Seahorse metabolic assay did not indicate significant differences in a glycolytic stress test assay for WT and Trp53 KO organoids grown in +WM medium enforcing the regenerative state, with KO organoids even trending toward lower glycolysis levels (Fig. 5, A and B). While overall levels of glycolysis decreased with application of −WM medium, Trp53 KO organoids maintained significantly higher glycolytic rates compared to WT organoids (Fig. 5, C and D), which becomes even more obvious when calculating the logarithmic fold change between the regenerative (+WM) and homeostasis-reinstated (−WM) conditions (Fig. 5E). Likewise, Trp53 KO-WI organoids maintained higher levels of glycolysis compared to WT organoids in the same conditions (Fig. 5, F to H).
Fig. 5. Locked regenerative Trp53 KO epithelium remains in a high glycolytic metabolic state.
(A) Extracellular acidification rate (ECAR) plot for Seahorse glycolytic stress test assay: WT and Trp53 KO organoids in +WM medium. (B) Rate for glycolysis in WT and KO from (A). (C) ECAR plot for WT and KO organoids in −WM medium. (D) Rate for glycolysis in WT and KO from (C). (E) LogFC for glycolysis in WT and KO in +WM versus −WM medium. (F and G) Rate for glycolysis in WT and Trp53 KO-WI organoids in +WM medium and in −WM medium. (H) LogFC for glycolysis in WT and KO-WI organoids in +WM versus −WM medium. (I) Labeled quantities plot for WT and KO organoids from pulsed stable isotope resolved metabolomics (pSIRM) (n = 3 technical replicates). (J) Protein expression of PKM2 in WT, KO, and KO-WI organoids (n = 3 biological replicates). (K) mRNA expression of PKM1/2 in WT, KO, and KO-WI organoids (n = 5 biological replicates, plotted are one to two experimental replicates per biological replicate). (L) Organoids’ size treated with 0.1 mM 2-deoxyglucose (2-DG) for 2 days. Red line: mean. n = 6 biological replicates for WT, n = 4 biological replicates for KO, n = 2 biological replicates for KO-WI. (M) BF images of WT, KO, and KO-WI organoids treated with different concentrations of 2-DG for 2 days (n = 2 to 6 biological replicates). Scale bar, 50 μm. (N) IF staining for PKM2 in homeostasis (n = 2 mice) or 2 months after DSS in WT and KO mice (WT, n = 2 mice; KO, n = 3 mice). (A, D, and E) Mean ± SEM; individual data points represent technical replicates of n = 3 biological replicates. (F, G, and H) Mean ± SEM; individual data points represent technical replicates of n = 5 (WT) or n = 4 (KO-WI) biological replicates. RFU, relative fluorescence units. TCA, tricarboxylic acid.
To explore this in more detail, we developed a protocol for pulsed stable isotope-resolved metabolomics (pSIRM) with organoids. We labeled WT and KO organoids for 30 min with 13C-glucose and analyzed the label incorporation of metabolites by gas chromatography–time-of-flight–mass spectrometry (GC-ToF-MS). pSIRM revealed higher labeled quantities of the glycolysis products pyruvate and lactate in KO compared to WT organoids (Fig. 5I). Together with the Seahorse assay data, the accumulation of these metabolites indicates an increased glycolytic flux in Trp53 KO organoids.
Next, we performed a proteome analysis of WT, Trp53 KO, and Trp53 KO-WI organoids to investigate the expression of glycolytic enzymes and found a rate-limiting enzyme, PKM2, to be up-regulated both in KO and in KO-WI compared to WT organoids (Fig. 5J). For KO-WI but not for KO organoids, this was also the case on the mRNA level (Fig. 5K). To investigate the functional relevance of elevated glycolysis, we applied 2-deoxyglucose (2-DG), a glucose analog that inhibits glycolysis, to WT, Trp53 KO, and Trp53 KO-WI organoids. We observed concentration-dependent inhibitory effects of 2-DG treatment in all three genotypes, but KO-WI organoids were particularly sensitive to the treatment (Fig. 5, L and M). We then went back to our in vivo samples and performed IF staining for PKM2 on homeostatic and 2-month postinjury samples of WT and Trp53 cKO mice. While PKM2 was uniformly but weakly expressed in homeostatic tissues, it was up-regulated in the locked regenerative epithelium of Trp53 cKO mice (Fig. 5N).
These data suggest that Trp53 KO epithelium, once it has entered the regenerative state, remains locked in this regenerative state also metabolically, characterized by enhanced glycolysis. By targeting the glycolytic pathway, growth inhibition of locked regenerative Trp53 KO-WI organoids can be achieved, suggesting that inhibiting glycolysis pharmacologically could be a means to target the regenerative state in patients with UC to prevent fixation or chronification.
In summary, our data provide insight into how p53 signaling deficiency could become selected in the context of colitis. Once it enters the regenerative state, p53 KO epithelium commits to a trajectory that is characterized by enhanced proliferation, loss of differentiation, lack of epithelial functionality, and increased glycolytic metabolism. Its inability to escape the regenerative state once it becomes independent of the Wnt signals that drive regeneration could be a hallmark for chronification of the disease and a carcinogenic trajectory. This could potentially be exploited for screening and therapy, for example, through inhibition of glycolysis.
DISCUSSION
In this study, we provide evidence for a critical role of p53 signaling in terminating regeneration after acute colitis. While p53 signaling is activated during regeneration, it is not required for homeostasis or induction of the regenerative state. Instead, it is required for terminating the regenerative state, and loss of Trp53 prevents the epithelium from returning to homeostasis even 2 months after the initial injury in a mouse model of colitis. Trp53 KO cells remain locked in a fetal-like regenerative state characterized by activation of YAP signaling and high glycolytic metabolism driven by elevated levels of the rate-limiting enzyme PKM2.
Loss of p53 is often observed in UC and UC-associated cancers (16–19, 38), and even gain-of-function mutations can result in a loss of physiological p53 signaling (14, 15, 39). Previous studies have revealed that loss of p53 contributes to UC-associated CRC (16, 40), but the mechanism for this context-dependent effect of p53 is not clear.
In this study, we reveal that p53 is dispensable in epithelial homeostasis but is the main driver triggering a switch back to the homeostatic state following regeneration. Both in vivo and in vitro, we observed up-regulation of p53 signaling by induction of the regenerative state that is driven by Wnt and down-regulation during homeostatic conditions. We have shown previously that a transient increase in Wnt/R-Spondin signaling is essential for injury repair upon DSS (27). The present results demonstrate that Wnt signaling not only induces the fetal-like regenerative state but simultaneously activates p53—a function that has not been previously recognized and that is crucial for reestablishing homeostasis.
In our study, loss of p53 resulted in massive enrichment of regenerative signaling pathways such as YAP. In vitro, we could show that once they enter the regenerative state, Trp53 KO organoids remain in this state with elevated stemness and regenerative capacity even when Wnt signals are depleted. In KO organoids growing Wnt independently (Trp53 KO-WI), gene signatures of the regenerative state were enriched even further. They also expressed lower levels of the effector kinase Lats2, which inhibits YAP through phosphorylation (41, 42), hinting at a mechanistic link between p53 and YAP signaling on the transcriptional level. LATS2 and p53 interact through a positive feedback loop (43, 44), suggesting that down-regulation of Lats2 via decreased p53 signaling could cause the observed sustained activation of YAP. In addition, it has been proposed that loss of p53 signaling can release the mutual exclusivity of Wnt and YAP signaling activation in cancers (45) that is presumed to exist in the healthy colon (4). We observed a simultaneous activation of Wnt/stem cell and YAP/regenerative markers in our locked regenerative Trp53 KO organoids.
Our data reveal that the regenerative state is associated with high levels of glycolytic metabolism, at least partly due to increased levels of PKM2, the final rate-limiting enzyme of glycolysis, which is typically expressed in fetal tissues and cancers (46–48). In the normal colonic epithelium, proliferative stem cells rely more on glycolysis than on oxidative phosphorylation to meet their energy requirements, and reversely, differentiated enterocytes are more dependent on oxidative phosphorylation than on glycolysis (49). In the SI, glycolysis was found to be crucial for homeostatic stem cell self-renewal (50), but so far, its role in the homeostatic versus regenerative colon has not been investigated in detail. Here, we show that colon cells gain increased glycolytic capacity when they enter the regenerative state, which is down-regulated again once they return to homeostasis. This likely enables cells to meet their increased energy needs during the proliferative burst after injury while later reestablishing a slower cell turnover during homeostasis by reducing glycolytic capacity again. Cells that lack Trp53, however, are fixed in the higher-energetic state and are thus not restrained in their proliferation after external cues limit regenerative signaling in WT cells. Both Wnt and YAP/TAZ signaling, enriched in Trp53 KO cells, can induce glycolysis (51–54). This highlights the importance of p53 for inducing the metabolic switch after regeneration, suppressing glycolysis and allowing metabolic reprogramming toward differentiated colonocytes.
Our data provide an explanation for how p53 mutant cells may outcompete WT cells specifically in the context of the injury-regeneration cycles associated with colitis. During ongoing regeneration in Trp53 cKO mice, we observed malfunction of the epithelial architecture and barrier. Functionally, crypt architecture was not restored, and the differentiated compartment was lacking, e.g., secretory goblet cells. Differentiated cell types contribute to homeostasis, and their loss is linked to tissue dysfunction (55, 56). Trp53-deficient mice showed reduced mucus production and a disrupted mucosal barrier. As UC is characterized by cycles of inflammation and regeneration, lack of goblet cells, and barrier disruption, loss of p53 may contribute to the manifestation and chronification of UC via positive selection of mutant clones, which lack the ability to differentiate. We found that regenerative Trp53 KO-WI organoids were able to outgrow WT cells in a competition assay in medium conditions mimicking regeneration. In addition, the observed ongoing inflammation and the fact that bacteria and their potentially genotoxic products (57) can come into contact with the epithelium after loss of p53 could also pave the way for accumulation of further mutations and a carcinogenic trajectory. This idea is supported by the high proportion of UC-associated tumors with loss-of-function or dominant-negative mutations of p53 combined with a loss of heterozygosity, which suggests negative selection against WT early during carcinogenesis (21, 22). It would be of great interest to monitor regeneration of Trp53 KO epithelium for longer periods of time, as previous studies in murine models of chronic colitis have reported enhanced development of colonic neoplasia and tumors in Trp53 KO mice (58, 59) but did not investigate epithelial reprogramming during regeneration. Another study found an increased tumor burden after deleting Trp53 in Lgr5-positive stem cells only if genotoxic damage and multiple cycles of DSS were combined but not with genotoxic stress alone (60), highlighting the context-dependent role of Trp53 during chronic inflammatory damage. Investigating the lasting changes after injury in Trp53 KO epithelium might help to understand chronification and the carcinogenic cascade in UC-associated CRC.
Collectively, our data demonstrate that while dispensable during epithelial homeostasis per se, p53 activation is critical for reestablishment of homeostasis after colitis-associated injury. If cells with loss of Trp53 enter the regenerative state, they are unable to exit this state again. Their higher proliferative potential then likely allows them to outcompete WT cells following injury, thereby becoming fixed in the mucosa. As affected epithelium fails to reinstate homeostasis but remains highly proliferative and shows long-term inflammation and immune-infiltration, loss of p53 signaling may contribute to progressive dysfunction, chronification of colitis, and ultimately to carcinogenesis. Our data provide a mechanistic explanation of why early loss of p53 signaling is frequently observed in UC-associated colorectal carcinogenesis.
MATERIALS AND METHODS
Study design
No statistical methods were used to predetermine sample size.
Mouse experiments
The institutional and legal authorities at Charité–Universitätsmedizin Berlin and Landesamt für Gesundheit und Soziales (LaGeSo) Berlin approved all procedures involving animals (licence nos. G0084_17 and T-CH0032-20). B6.129S2-Trp53tm1Tyj/J mice and age-matched WT controls were used to investigate the impact of global loss of Trp53 in homeostasis. C57BL/6 mice were used for investigation of Trp53 expression during and after colitis. Axin2CreERT2 mice (61) or VillinCreERT2 mice (62) were crossed with Trp53 flox/flox mice (63) to generate tamoxifen-inducible strains in which Trp53 could be knocked out in the colonic stem cell compartment or in epithelial cells, respectively. RSPO KO mice were generated by crossing Myh11CreERT2 mice (64) with Rspo3 flox/flox mice (65) in which induction with tamoxifen leads to depletion of Rspo3 in myofibroblasts. All animals were kept in a specific pathogen–free environment in individually ventilated cages and provided with drinking water and standard chow ad libitum. The mice were bred and maintained at the Charité animal care facility on a 12-hour light/12-hour dark cycle in a controlled temperature (22.5° ± 2.5°C) and humidity (50 ± 5%) environment. Males were used for all experiments; the presented conclusions thus might not be applicable for females. To induce KO, tamoxifen (Sigma-Aldrich, 4 mg/25 g body weight, dissolved in 200 μl of corn oil) was injected intraperitoneally into mice at the indicated time points. DSS [2% (w/v)] (MP Biomedicals; 36,000 to 50,000 molecular weight) was dissolved in drinking water and provided to mice for the indicated period (5 to 7 days). For recovery experiments, DSS was replaced by normal drinking water. The disease activity index was monitored as described in (66). At the end of the experiment, mice were euthanized, and the colon was isolated. Samples for IF, RNA-ISH, and RNA isolation were collected and processed as described below.
Tissue processing and hematoxylin and eosin staining
For formalin-fixed paraffin embedding (FFPE), the colon was dissected, removing feces and all visible fat tissue and blood vessels. Pieces were fixed in 2% paraformaldehyde (PFA; Merck) for 24 hours. The Charité Core Unit Immunopathology for Experimental Models performed paraffin embedding, sectioning, and hematoxylin and eosin (H&E) staining. Brightfield images were taken with a Zeiss Observer 7 microscope and analyzed with ZEN v3.3.89 (Zeiss).
Quantitative reverse transcription PCR
RNA was extracted from snap-frozen colon tissue or in-well lysed organoids using the NucleoSpin RNA isolation kit (Macherey & Nagel) including on-column deoxyribonuclease digestion. cDNA was generated using the iScript cDNA synthesis kit (Bio-Rad), and qPCR was performed with SYBR-green (Thermo Fisher Scientific) and the QuantStudio Real-Time PCR System (Thermo Fisher Scientific). The data were collected with QuantStudio Design and Analysis software v1.4.3. Fold change expression was determined following the delta Ct method and normalized to Gapdh, β-microglobulin, or Hprt1. Primer sequences are listed in table S1.
Immunofluorescence
FFPE sections were rehydrated and, following an antigen retrieval step with either citrate- or tris-EDTA buffer, blocked for 1 hour in blocking buffer [0.1% Tween 20 in phosphate-buffered saline (PBS) supplemented with 5% fetal bovine serum]. Subsequently, slides were incubated with primary antibodies at indicated concentrations (for antibody information, see table S2) overnight at 4°C in a humidified chamber, followed by three washes in Phosphate Buffered Saline with 0.1% Tween-20 (PBS-T) and incubation with secondary antibody at 1:300 (see table S3) for 2 to 3 hours at room temperature in a humidified chamber. Nuclei were stained with 1:1000 4′,6-diamidino-2-phenylindole (DAPI; Cell Signaling). Slides were washed again three times in PBS-T and subsequently mounted with ImmuMount (Epredia). Samples were imaged with a Leica SP8-Falcon or a Leica Stellaris8-Falcon-STED confocal microscope. Images were analyzed with the Leica LasX software v3.5.6.21594 and ImageJ/Fiji v2.1.0. As a staining control for p53, we used human colorectal cancer specimens, which were a gift of the Charité Universitätsmedizin pathology department.
RNA in situ hybridization
To visualize the microbiota, methacarn-fixed tissue was processed as previously described (67). The Charité Core Unit Immunopathology for Experimental Models performed paraffin embedding, sectioning, and H&E staining. Tissue sections were rehydrated, allowed to dry, and overlaid with EUB388-Cy3 probe (1 μg/μl; Biomers) in hybridization solution [20 mM tris-HCl (pH 7.4), 0.9 M NaCl, and 0.1% (w/v) SDS]. A cover slide was used to prevent drying, and slides were incubated at 50°C overnight in a humidified chamber. Slides were washed in PBS three times for 5 min and blocked for 30 min before primary antibody application and overnight incubation. After washing, secondary antibody staining and counterstaining with 1:1000 DAPI was performed for 2 to 3 hours. The slides were washed three times in PBS and mounted with ImmuMount. Imaging was conducted at an SP8-Falcon confocal microscope (Leica). For single-molecule RNA-ISH, the RNAscope Red Detection Kit was used following the manufacturer’s instructions (Advanced Cell Diagnostics) for Rspo3 (catalog no. 402011) and Axin2 (catalog no. 400331).
Image analysis
Images were analyzed with the software indicated in the description of staining procedures. Crypt lengths were measured manually for all visible crypts in available cross sections by using the measurement tool in ZEN software. Counting of positive cells was conducted with the counting tool of Fiji software. Samples were evaluated in a blinded manner. The histological colitis score was calculated according to a previously described method (68).
Genotyping
For genotyping, tail or ear punch biopsies of mice or organoids washed in PBS/0.1% bovine serum albumin (BSA) were lysed in proteinase-K containing lysis buffer and incubated at 56°C for several hours. Samples were diluted with water, and PCR was set up with the MyTaq HS DNA Polymerase Red Mix (Bioline). Products were evaluated on a 2% agarose gel using HDGreen plus DNA Stain (Intas Science Imaging), HyperLadder 100-bp ladder (Meridian), and a ChemiDoc MP imager (Bio-Rad). Genotyping primer sequences are listed in table S1.
Organoid cell culture
The mouse colon or SI (jejunum part) was dissected to remove fat tissue and blood vessels and opened longitudinally. For colon organoid preparation, the tissue was cut into 5-mm pieces and washed three times in cold PBS (Gibco), followed by incubation for 20 min in 10 mM EDTA (Invitrogen) in PBS supplemented with 0.5 mM dithiothreitol (DTT) (Sigma-Aldrich) at 37°C. Buffer was changed to cold PBS, and the tube was vigorously shaken for 2 min to isolate crypts. After two washes in PBS, the supernatant containing crypts was collected and centrifuged at 400g for 5 min at 4°C. The number of colonic crypts was counted, and 300 crypts per 10 μl Cultrex BME (Cultrex Reduced Growth Factor Basement Membrane Extract, Type R1, R&D Systems) were resuspended and seeded in droplets. Cultrex was polymerized at 37°C for 20 min, and then colon medium was added: Advanced Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Gibco) supplemented with 10 mM Hepes (Gibco), 2 mM GlutaMAX (Gibco), penicillin/streptomycin (100 U/ml; Gibco), 1.25 mM N-acetylcysteine (Sigma-Aldrich), 1× B-27 (Gibco), 1× N2 (Gibco), mouse epidermal growth factor (EGF) (50 ng/ml; Invitrogen), 0.5 μM A83-01 (transforming growth factor–β inhibitor, Sigma-Aldrich), 0.328 nM sWnt (U-Protein Express), 3 μM CHIR-99021 (Chir, Selleckchem), 25% R-spondin 1–conditioned medium, and mouse noggin (100 ng/ml; PeproTech) for regeneration-inducing +WM medium condition. For −WM medium, sWnt and Chir were not added. Y-27632 (10 μM; ROCK inhibitor, Hoelzel) was added after the initial seeding and after passaging until the first medium change. Organoids were incubated at 37°C, 5% CO2 in a humidified incubator. The medium was replaced every 2 to 3 days. Every 4 to 6 days, the organoids were passaged as single cells generated by a 7-min incubation in TryplE (Gibco) at 37°C, mixing with 0.1% BSA in PBS and shearing with a 100 Sterican 27G canula (Braun) and syringe (Braun). Six thousand single cells were seeded per 10 μl Cultrex. The organoids were cultured for at least one passage before conducting experiments.
For SI organoids, the surface of the opened intestine was gently streaked with a coverslip to get rid of villi. The intestine was then cut into 5-mm pieces and washed 10 times in PBS. Pieces were incubated for 5 min at room temperature in 10 mM EDTA/PBS followed by 30 min in 2 mM EDTA/PBS with 0.25 mM DTT (4°C with agitation). The supernatant was discarded, and tissue pieces were shaken rigorously for 2 min in 5 ml of Hanks’ balanced salt solution (HBSS; with Mg and Cl2, Gibco). The supernatant of four washing cycles was collected and added to 5 ml of HBSS (with Mg and Cl2). The suspension was filtered with a 70-μm filter (Greiner Bio-One). Fractions with visible crypts were pooled, centrifuged at 1000g for 4 min at 4°C, and resuspended in PBS, and crypts were counted. Two hundred crypts per 10 μl of Cultrex BME Type 2 (Cultrex Reduced Growth Factor Basement Membrane Extract, Type 2 PathClear, R&D Systems) were seeded. Cultrex was polymerized at 37°C for 20 min, and then SI organoid medium was added: Advanced DMEM/F12 (Gibco) supplemented with 10 mM Hepes (Gibco), 2 mM GlutaMAX (Gibco), penicillin/streptomycin (100 U/ml; Gibco), 1.25 mM N-acetylcysteine (Sigma-Aldrich), 1× B-27 (Gibco), 1× N2 (Gibco), mouse EGF (50 ng/ml; Invitrogen), 25% R-spondin 1–conditioned medium, and mouse noggin (100 ng/ml; PeproTech) for −Wnt standard medium. To induce regeneration, +Wnt medium containing 0.328 nM sWnt was added for indicated time spans. The organoids were incubated at 37°C, 5% CO2 in a humidified incubator. The medium was replaced every 2 to 3 days. Every 5 to 7 days, the organoids were passaged by disrupting with a 100 Sterican 27G canula (Braun) and splitting them at ratios of 1:2 to 1:4. The organoids were cultured for at least one passage before conducting experiments.
To generate Trp53 KO organoids, 800 nM 4-OH-tamoxifen (Sigma-Aldrich) was added to cultures from VillinCreERT2 x Trp53 flox/flox mice for 2 days before splitting. Trp53 KO organoids were selected by treating cultures with 10 mM Nutlin-3a (Merck) for several passages until WT organoids were depleted and purity of KO cultures was confirmed by genotyping. For p53 induction experiments, 10 mM Nutlin-3a was added to WT colon and SI organoid cultures for indicated periods with DMSO-treated cells as control. For glycolysis inhibition, 2-DG was dissolved to 1, 10, or 100 mM in medium and administered to organoids for 2 days. For competition experiments, organoids were seeded in a 1:1 mix with fluorescent organoids generated from Actin-dsRed mice provided by F. Heymann. Cultures were fixed in 4% PFA for 20 min, washed with PBS, permeabilized with 0.1% Triton X-100 in PBS for 3 min, and counterstained in-well with 1:1000 DAPI for 10 min. After two washes in PBS, organoids were imaged in-plate with a Zeiss Observer 7 microscope and quantified manually with ZEN v3.3.89 software (Zeiss). Brightfield images of cultures were taken with an EVOS M5000 microscope (Thermo Fisher Scientific), and organoid size was manually measured with ZEN software.
Assembloids
APC KO organoids were a gift from D. Stange and were cultured as described above but in the absence of sWnt and Chir supplementation. Assembloids were generated and processed as described previously (28) by coculturing APC KO organoids with stroma cells from C57BL/6 mice.
Organoid histology and staining
Organoids were harvested in organoid harvesting solution (Gibco) and incubated for 20 min on ice with two washing steps with 0.1% BSA/PBS. Organoids were transferred to 4% formaldehyde (Roth) in PBS and fixed for 1 hour at room temperature followed by washing in 0.1% BSA in PBS, embedding in 2% agarose, dehydration, and embedding in paraffin. Four-micrometer sections were deparaffinized and rehydrated, followed by antigen retrieval in citrate or tris buffer. Nonspecific antibody binding was blocked by incubation in blocking buffer for 1 hour, followed by overnight incubation with primary antibodies (see table S2). The next day, slides were washed and incubated with secondary antibodies (see table S3) for 2 to 3 hours at room temperature and counterstained with DAPI (1:1000). Slides were mounted with ImmuMount, and IF images were acquired with the confocal laser scanning microscope SP8-Falcon (Leica). Images were collected and analyzed with Leica LasX software, ZEN (Zeiss), and ImageJ/Fiji v2.1.0.
Microarray analysis
RNA from organoids or tissue samples was extracted with the Nucleospin RNA Kit (Machery Nagel). Quality control and quantification of total RNA were carried out using a 2100 bioanalyzer (Agilent Technologies) and a NanoDrop 1000 ultraviolet-visible spectrophotometer (Kisker). Microarray experiments were performed as independent dual-color dye-reversal color-swap hybridizations using two biological replicates each. Total RNA was amplified and labeled with a dual-color Quick-Amp labeling kit (Agilent Technologies). Briefly, mRNA was reverse-transcribed and amplified using an oligo-dT-T7 promoter primer and labeled with cyanine 3-cytidintriphosphate (CTP) or cyanine 5-CTP. After precipitation, purification, and quantification, 0.75 g of each labeled cRNA was fragmented and hybridized to whole-genome mouse GE v2 multipack microarrays (8x60k; Agilent Technologies, 074809) according to the supplier’s protocol (Agilent Technologies). Scanning of microarrays was performed at a resolution of 3 μm using a G2565CA high-resolution laser microarray scanner (Agilent Technologies). Microarray image data were processed with Image Analysis/Feature Extraction software G2567AA vA.11.5.1.1 (Agilent Technologies) using default settings and the GE2_1105_Oct12 extraction protocol. The extracted dual-color raw data .txt files were further analyzed using R and the associated BioConductor package limma or with the Rosetta Resolver software (Rosetta Inpharmatics LLC). Microarray data have been deposited in the Gene Expression Omnibus (GEO; www.ncbi.nlm.nih.gov/geo/) of the National Center for Biotechnology Information and can be assessed with the GEO accession number GSE254312.
To analyze the human gene sets, the DGE data were restricted to probe sets that have a homologous gene in mice and humans. For all probe sets, the one with the highest t score and rank in the resulting list was selected and subsequently used for GSEA analysis using R package Fast Gene Set Enrichment Analysis (fGSEA) (69). Expression data were analyzed as follows: For each of the selected comparisons, the replicates of the target condition were compared to the corresponding control using limma or Rosetta Resolver, producing differential expression statistics for all genes and comparisons. Analyses were performed as individual two-group unpaired comparisons. P values were adjusted for multiple testing by a global false discovery rate (FDR) according to the method described by Benjamini and Hochberg.
Results were plotted using Microsoft Excel, and the software R v4.2 (70) was used to create the heatmaps and gene set enrichment plots. For visualization, the R packages ggplot2 and pheatmap (71) were used.
Bioinformatic analysis of published datasets
Data from published studies of mouse acute colitis DSS models were retrieved from GSE214600 (26), GSE168053 (24), and E-MTAB-5249 (4). Differential expression was computed using R package limma (72), GSEA analysis was performed using R package fGSEA, and for GSVA, R package GSVA (73) was used. Single-cell RNA (scRNA) expression data from a mouse DSS model [GSE201723 (23)] was generated using CellRanger v6.01 from published raw data, and expression values were processed and analyzed using R package Seurat after sample integration with CCA.
Whole genome sequencing
Organoids were grown as described above and harvested in Organoid Harvesting Solution (Gibco). DNA was isolated using the Qubit dsDNA HS Assay Kit (Thermo Fisher Scientific) according to the manufacturer’s protocol with elution in elution buffer (EB) buffer (Qiagen). WGS was performed by the Genomics Platform at the Max Delbrück Center on a NovaSeq X Plus (Illumina) with a 10B FlowCell [paired-end (PE) 2 × 150bp]. For analysis of WGS data, reads were aligned with BWA-MEM to the GRCm38 reference genome. Variants were called with Mutect2 version 2.2 from Genome Analysis Toolkit (GATK), with WT as the “normal” and KO-WI as the “tumor.” Variants were filtered with FilterMutectCalls and annotated with RefSeq genes and mutation consequences using Annotate Variation (ANNOVAR).
Liquid chromatography–MS proteomics
Organoids were harvested and washed two times with PBS, and pellets were snap-frozen in liquid nitrogen. Samples were stored at −70°C until further processing. The pellets were resuspended in 150 μl of rea buffer [8 M urea and 100 mM tris-HCl (pH 8.2)]. Cells were lysed on a Bioruptor sonicator (Diagenode), using 5 cycles of sonication (45-s on, 15-s off). Protein concentration was determined by bicinchoninic acid colorimetric assay (Pierce), and a 50-μg aliquot of each protein sample was reduced with 10 mM DTT for 45 min at 30°C and alkylated with 100 mM iodoacetamide for 25 min at 25°C. Proteins were diluted with 5 volumes of 50 mM tris-HCl buffer and digested using Lys-C/trypsin mixture [Promega, 1:25 (w/w) overnight under gentle shaking at 37°C]. Digestion was stopped through acidification with 5 μl of trifluoroacetic acid (Merck). Fifteen micrograms of each resulting peptide mixture was then desalted on Stage Tip (74), and the eluates were dried and reconstituted to 15 μl in 0.5% acetic acid.
For all samples, 5 μl was injected on an liquid chromatography–tandem MS system (EASY-nLC 1200 coupled to a QExactive HF, Thermo Fisher Scientific), using a 130-min gradient ranging from 2 to 60% of solvent B (80% acetonitrile, 0.1% formic acid; solvent A = 0.1% formic acid in water). For the chromatographic separation, a 20-cm-long capillary (75-μm inner diameter) packed with 1.9-μm C18 beads (Reprosil-AQ, Dr. Maisch HPLC) was used. A nanospray tip on one end of the capillary was generated using a laser puller, allowing fritless packing (P-2000 Laser Based Micropipette Puller, Sutter Instruments). The nanospray source was operated with a spray voltage of 2.2 kV and an ion transfer tube temperature of 260°C. Data were acquired in data-dependent mode, with one survey MS scan in the Orbitrap mass analyzer [120,000 resolution at 200 mass/charge ratio (m/z)] followed by up to 10 MS/MS scans (30,000 resolution at 200 m/z) on the most intense ions. Normalized collision energy was set to 26. Once selected for fragmentation, ions were excluded from further selection for 30 s to increase new sequencing events.
Raw data were analyzed using the MaxQuant proteomics pipeline (v.1.6.17.0) and the built-in Andromeda search engine (75) with the UniProt Mouse database (downloaded on 19 April 2021 from uniport.org). Heavy or light carbamidomethylation of cysteines was chosen as labels, and oxidation of methionine and acetylation of N terminus were chosen as variable modifications. No fixed modification was used. The search engine peptide assignments were filtered at 1% FDR, and the feature match between runs was enabled. For protein quantification, label-free quantitation (LFQ) intensities calculated by MaxQuant were used (76). The minimum LFQ ratio count was set to 2, and an MS/MS spectrum was always required for LFQ comparison of the precursor ion intensities. Data quality was inspected using the in-house developed tool PTXQC (77).
Pulsed stable isotope-resolved metabolomics
Organoids were grown in −WM condition and labeled with U-13C glucose (Campro Scientific) for 30 min by adding respective amounts of labeled glucose to glucose-free medium (Bio-West). After labeling, organoids were harvested, washed two times with Hepes (pH 7.4) buffer, and snap-frozen in liquid nitrogen. Additional wells of organoids were treated in the same way and sheared into single cells to count the total live cell number after the labeling procedure to allow for normalizing metabolite content to cell number.
Samples were extracted using ice-cold methanol:chloroform:water mix (2:1:2, v/v/v; Sigma-Aldrich) with the internal standard cinnamic acid (2 μg/ml, Sigma-Aldrich). Samples were shaken for 60 min at 4°C and centrifuged for 10 min at 1400g and 4°C. Polar phases were collected and dried under vacuum. The extracts were then resuspended in 20% methanol, shaken for 30 min, centrifuged for 10 min at 1400g and 4°C, aliquoted in fresh vials, and dried under vacuum. Quantification standards, composed of 67 metabolites (produced in-house) in different dilution ranges, were extracted with a methanol:chloroform:water mix (5:2:1, v/v/v). Dried sample extracts, quantification, and identification standards (78) were derivatized as follows: A derivatization solution with 20 mg methoxamine (Sigma-Aldrich) per 1000 μl of pyridine (Sigma-Aldrich) was prepared and added to the samples, followed by a 60-min incubation at 30°C and 1400 rpm in a thermal shaker. Next, N-methyl-N-trimethylsilyltrifluoracetamid (VWR) and alkane standards (1:100, v/v; produced in-house) were added, followed by a 120-min incubation at 37°C and 1400 rpm in a thermal shaker. Samples were then centrifuged for 10 min at 14,000g and 21°C and transferred to glass vials for GC-MS.
Measurements were performed on a GC-ToF-MS (Pegasus BT from Leco, equipped with an Agilent 7890 GC and an Multi-Purpose Sampler (MPS) robotic from Gerstel). One microliter of derivatized samples or identification/quantification standards was injected in a temperature-controlled injector in a 1:5 split mode. The gas chromatographic separation was carried out on an Rxi-5 ms GC column (Restek) of 30-m length and 0.25-mm inner diameter, using the following temperature gradient: 68°C hold for 5 min, followed by a first temperature gradient with 5°C/min until 120°C, then a second temperature gradient of 7°C/min until 200°C, then a third gradient with 12°C/min until 320°C, and a final hold for 6 min at 320°C. Helium was used as the carrier gas with a constant flow rate of 1.20 ml/min. Electron ionization was used with an electron energy of 70 eV. The transfer line temperature was set to 250°C. Spectra were recorded at 20 spectra/s and within a mass range of 60 to 550 m/z. Raw data were processed with ChromaTOF from Leco (version 5.55.41_BT). Retention times were aligned using the alkane standards added during derivatization. Next, an in-house library was used for metabolite annotation. Further analysis was performed using the in-house developed R package MetaLabs from the Kempa Lab, allowing for absolute quantification of metabolites based on the quantification standards and the calculation of 13C label incorporation.
Bioenergetic profiling
Seahorse glycolysis stress test assays were performed for colon organoids, adapted from a recently described protocol for intestinal organoids (79). Briefly, organoids were seeded in the Seahorse assay plate as described above. Medium and inhibitor solutions were prepared and applied as in (79). As a Seahorse XFe96 (96 wells, Agilent Technologies) was used, and volumes were reduced accordingly. For normalization, the CyQUANT Cell Proliferation Assay (Thermo Fisher Scientific) and a SpectraMax i3x plate reader with the respective software SoftMaxPro v7.0 were used. Data analysis was conducted with the WAVE v2.6.3 (Agilent Technologies) software.
Statistics and reproducibility
Data are expressed as mean ± SD unless indicated otherwise. P < 0.05 was considered significant. Unpaired Mann-Whitney test was used to determine differences between two groups. Kruskal-Wallis test followed by respective post hoc analysis (Dunn’s test) was used for statistical comparison of more than two groups. Microscopic images were chosen from representative regions. GraphPad Prism 10 and R 4.2 were used for data visualization and statistical analysis.
Acknowledgments
We thank S. Müllerke, J. Wolff, Y. Giesecke, and P. Seidler for excellent technical support. We also thank J. Grobe for technical support with proteomic and metabolomic experiments and I. Wagner for technical support regarding microarray setup. We thank the Charité–Universitätsmedizin Berlin pathology department for providing human colorectal cancer specimens and D. Stange for providing APC KO organoids. We thank R. Zietlow for scientific editing of the manuscript.
Funding: This work was supported by Berlin School of Integrative Oncology (BSIO) grant for emerging projects 2022 (K.H.), German Research Foundation Si 1983 3/1 (M.Si.), German Research Foundation Si 1983 4/1 (M.Si.), European Research Council ERC-Starting Grant REVERT (M.Si.), German Federal Ministry of Education and Research PACE Therapy Consortium (M.Si.), Einstein Center EC3R grant (M.Si.), German Research Foundation 2546/7-1 (M.Sc. and R.E.F.), and German Federal Ministry of Education and Research MSTARS–Multimodal Clinical Mass Spectrometry to Target Treatment Resistance (S.K. and S.B.).
Author contributions: Conceptualization: K.H., M.Si., M.Sc., L.L., C.E., S.K.,H.-J.M., and M.L. Data curation: K.H., H.B., S.G., H.-J.M., G.M., and M.F. Formal analysis: K.H., H.B., M.Si., S.B., G.M., S.G., M.F., B.J.E.M., C.E., H.-J.M., and M.Sc. Funding acquisition: M.Si., K.H., and M.Sc. Investigation: K.H., C.H., A.W., M.L., A.-S.F., G.B., H.-J.M., and M.M. Methodology: K.H., C.H., H.-J.M., S.G., G.M., R.E.F., M.L., M.M., S.K., M.Sc., and M.Si. Project administration: M.Si., K.H., F.T., M.Sc., and A.D.S. Resources: M.Si., S.K., A.D.S., F.T., C.E., S.G., H.-J.M., A.-S.F., G.M., and M.Sc. Software: H.B., M.F., S.G., G.M., B.J.E.M., H.-J.M., and K.H. Supervision: M.Si., M.Sc., S.K., A.D.S., C.E., F.T., and H.-J.M. Validation: K.H., L.L., C.E., S.K., H.-J.M., M.M., M.Sc., and B.J.E.M. Visualization: K.H., H.B., S.G., B.J.E.M., C.E., H.-J.M., A.D.S., and M.Si. Writing—original draft: K.H., M.Si., H.-J.M., and M.L. Writing—review and editing: All authors.
Competing interests: The authors declare that they have no competing interests.
Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. All transcriptomic data generated in this study have been deposited in the National Center for Biotechnology Information Gene Expression Omnibus (GEO) under accession code GSE254312. Sequencing data have been deposited in the Sequence Read Archive with submission ID SUB14582177 and BioProject ID PRJNA1131196. Code has been deposited on Zenodo: Berger, H., Hartl, K., & Sigal, M. (2024). Scripts used for analysis of microarray, RNA-Seq, scRNA-Seq and WGS data in Hartl et al., “p53 terminates the regenerative fetal-like state after colitis-associated injury”. Zenodo. https://doi.org/10.5281/zenodo.13754713.
Supplementary Materials
The PDF file includes:
Figs. S1 to S6
Tables S1 to S3
Legend for data S1
Other Supplementary Material for this manuscript includes the following:
Data S1
REFERENCES AND NOTES
- 1.Barker N., van Es J. H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P. J., Clevers H., Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Ayyaz A., Kumar S., Sangiorgi B., Ghoshal B., Gosio J., Ouladan S., Fink M., Barutcu S., Trcka D., Shen J., Chan K., Wrana J. L., Gregorieff A., Single-cell transcriptomes of the regenerating intestine reveal a revival stem cell. Nature 569, 121–125 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Gregorieff A., Liu Y., Inanlou M. R., Khomchuk Y., Wrana J. L., Yap-dependent reprogramming of Lgr5+ stem cells drives intestinal regeneration and cancer. Nature 526, 715–718 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Yui S., Azzolin L., Maimets M., Pedersen M. T., Fordham R. P., Hansen S. L., Larsen H. L., Guiu J., Alves M. R. P., Rundsten C. F., Johansen J. V., Li Y., Madsen C. D., Nakamura T., Watanabe M., Nielsen O. H., Schweiger P. J., Piccolo S., Jensen K. B., YAP/TAZ-dependent reprogramming of Colonic Epithelium Links ECM Remodeling to Tissue Regeneration. Cell Stem Cell 22, 35–49.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarvestani S. K., Signs S. A., Lefebvre V., Mack S., Ni Y., Morton A., Chan E. R., Li X., Fox P., Ting A., Kalady M. F., Cruise M., Ashburn J., Stiene J., Lai W., Liska D., Xiang S., Huang E. H., Cancer-predicting transcriptomic and epigenetic signatures revealed for ulcerative colitis in patient-derived epithelial organoids. Oncotarget 9, 28717–28730 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma A., Blériot C., Currenti J., Ginhoux F., Oncofetal reprogramming in tumour development and progression. Nat. Rev. Cancer 22, 593–602 (2022). [DOI] [PubMed] [Google Scholar]
- 7.Lutgens M. W. M. D., Vleggaar F. P., Schipper M. E. I., Stokkers P. C. F., van. der Woude C. J., Hommes D. W., de Jong D. J., Dijkstra G., Bodegraven A. A., Oldenburg B., Samsom M., High frequency of early colorectal cancer in inflammatory bowel disease. Gut 57, 1246 (2008). [DOI] [PubMed] [Google Scholar]
- 8.Eaden J. A., Abrams K. R., Mayberry J. F., The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut 48, 526–535 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leedham S. J., Graham T. A., Oukrif D., McDonald S. A. C., Rodriguez–Justo M., Harrison R. F., Shepherd N. A., Novelli M. R., Jankowski J. A. Z., Wright N. A., Clonality, founder mutations, and field cancerization in human ulcerative colitis–associated neoplasia. Gastroenterology 136, 542–550.e6 (2009). [DOI] [PubMed] [Google Scholar]
- 10.Kakiuchi N., Yoshida K., Uchino M., Kihara T., Akaki K., Inoue Y., Kawada K., Nagayama S., Yokoyama A., Yamamoto S., Matsuura M., Horimatsu T., Hirano T., Goto N., Takeuchi Y., Ochi Y., Shiozawa Y., Kogure Y., Watatani Y., Fujii Y., Kim S. K., Kon A., Kataoka K., Yoshizato T., Nakagawa M. M., Yoda A., Nanya Y., Makishima H., Shiraishi Y., Chiba K., Tanaka H., Sanada M., Sugihara E., Sato T.-A., Maruyama T., Miyoshi H., Taketo M. M., Oishi J., Inagaki R., Ueda Y., Okamoto S., Okajima H., Sakai Y., Sakurai T., Haga H., Hirota S., Ikeuchi H., Nakase H., Marusawa H., Chiba T., Takeuchi O., Miyano S., Seno H., Ogawa S., Frequent mutations that converge on the NFKBIZ pathway in ulcerative colitis. Nature 577, 260–265 (2020). [DOI] [PubMed] [Google Scholar]
- 11.Hussain S. P., Amstad P., Raja K., Ambs S., Nagashima M., Bennett W. P., Shields P. G., Ham A.-J., Swenberg J. A., Marrogi A. J., Harris C. C., Increased p53 mutation load in noncancerous colon tissue from ulcerative colitis: A cancer-prone chronic inflammatory disease. Cancer Res. 60, 3333–3337 (2000). [PubMed] [Google Scholar]
- 12.Takaku H., Ajioka Y., Watanabe H., Hashidate H., Yamada S., Yokoyama J., Kazama S., Suda T., Hatakeyama K., Mutations of p53 in morphologically non-neoplastic mucosa of long-standing ulcerative colitis. Jpn. J. Cancer Res. 92, 119–126 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kern S. E., Redston M., Seymour A. B., Caldas C., Powell S. M., Kornacki S., Kinzler K. W., Molecular genetic profiles of colitis-associated neoplasms. Gastroenterology 107, 420–428 (1994). [DOI] [PubMed] [Google Scholar]
- 14.Rajamäki K., Taira A., Katainen R., Välimäki N., Kuosmanen A., Plaketti R. M., Seppälä T. T., Ahtiainen M., Wirta E. V., Vartiainen E., Sulo P., Ravantti J., Lehtipuro S., Granberg K. J., Nykter M., Tanskanen T., Ristimäki A., Koskensalo S., Renkonen-Sinisalo L., Lepistö A., Böhm J., Taipale J., Mecklin J. P., Aavikko M., Palin K., Aaltonen L. A., Genetic and epigenetic characteristics of inflammatory bowel disease-associated colorectal cancer. Gastroenterology 161, 592–607 (2021). [DOI] [PubMed] [Google Scholar]
- 15.Liebl M. C., Hofmann T. G., The role of p53 signaling in colorectal cancer. Cancer 13, 2125 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baker A.-M., Cross W., Curtius K., Al Bakir I., Choi C.-H. R., Davis H. L., Temko D., Biswas S., Martinez P., Williams M. J., Lindsay J. O., Feakins R., Vega R., Hayes S. J., Tomlinson I. P. M., McDonald S. A. C., Moorghen M., Silver A., East J. E., Wright N. A., Wang L. M., Rodriguez-Justo M., Jansen M., Hart A. L., Leedham S. J., Graham T. A., Evolutionary history of human colitis-associated colorectal cancer. Gut 68, 985–995 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin J., Harpaz N., Tong Y., Huang Y., Laurin J., Greenwald B. D., Hontanosas M., Newkirk C., Meltzer S. J., p53 Point mutations in dysplastic and cancerous ulcerative colitis lesions. Gastroenterology 104, 1633–1639 (1993). [DOI] [PubMed] [Google Scholar]
- 18.Galandiuk S., Rodriguez–Justo M., Jeffery R., Nicholson A. M., Cheng Y., Oukrif D., Elia G., Leedham S. J., McDonald S. A. C., Wright N. A., Graham T. A., Field cancerization in the intestinal epithelium of patients with Crohn’s ileocolitis. Gastroenterology 142, 855–864.e8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brentnall T. A., Crispin D. A., Rabinovitch P. S., Haggitt R. C., Rubin C. E., Stevens A. C., Burmer G. C., Mutations in the p53 gene: An early marker of neoplastic progression in ulcerative colitis. Gastroenterology 107, 369–378 (1994). [DOI] [PubMed] [Google Scholar]
- 20.Yoshida T., Mikami T., Mitomi H., Okayasu I., Diverse p53 alterations in ulcerative colitis-associated low-grade dysplasia: Full-length gene sequencing in microdissected single crypts. J. Pathol. 199, 166–175 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Petitjean A., Achatz M. I., Borresen-Dale A. L., Hainaut P., Olivier M., TP53 mutations in human cancers: Functional selection and impact on cancer prognosis and outcomes. Oncogene 26, 2157–2165 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Vermeulen L., Morrissey E., van der Heijden M., Nicholson A. M., Sottoriva A., Buczacki S., Kemp R., Tavaré S., Winton D. J., Defining stem cell dynamics in models of intestinal tumor initiation. Science 342, 995–998 (2013). [DOI] [PubMed] [Google Scholar]
- 23.Xie L., Fletcher R. B., Bhatia D., Shah D., Phipps J., Deshmukh S., Zhang H., Ye J., Lee S., Le L., Newman M., Chen H., Sura A., Gupta S., Sanman L. E., Yang F., Meng W., Baribault H., Vanhove G. F., Yeh W.-C., Li Y., Lu C., Robust colonic epithelial regeneration and amelioration of colitis via FZD-specific activation of Wnt signaling. Cell. Mol. Gastroenterol. Hepatol. 14, 435–464 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C. Y., Girish N., Gomez M. L., Dubé P. E., Washington M. K., Simons B. D., Polk D. B., Transitional anal cells mediate colonic re-epithelialization in colitis. Gastroenterology 162, 1975–1989 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinchen J., Chen H. H., Parikh K., Antanaviciute A., Jagielowicz M., Fawkner-Corbett D., Ashley N., Cubitt L., Mellado-Gomez E., Attar M., Sharma E., Wills Q., Bowden R., Richter F. C., Ahern D., Puri K. D., Henault J., Gervais F., Koohy H., Simmons A., Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell 175, 372–386.e17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peters L. A., Friedman J. R., Stojmirovic A., Hagen J., Houten S., Dodatko T., Amaro M. P., Restrepo P., Chai Z., Rodrigo Mora J., Raymond H. A., Curran M., Dobrin R., Das A., Xiong H., Schadt E. E., Argmann C., Losic B., A temporal classifier predicts histopathology state and parses acute-chronic phasing in inflammatory bowel disease patients. Commun. Biol. 6, 95 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harnack C., Berger H., Antanaviciute A., Vidal R., Sauer S., Simmons A., Meyer T. F., Sigal M., R-spondin 3 promotes stem cell recovery and epithelial regeneration in the colon. Nat. Commun. 10, 4368 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin M., Hartl K., Heuberger J., Beccaceci G., Berger H., Li H., Liu L., Müllerke S., Conrad T., Heymann F., Woehler A., Tacke F., Rajewsky N., Sigal M., Establishment of gastrointestinal assembloids to study the interplay between epithelial crypts and their mesenchymal niche. Nat. Commun. 14, 3025 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Imai Y., Ibata I., Ito D., Ohsawa K., Kohsaka S., A novel Geneiba1in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem. Biophys. Res. Commun. 224, 855–862 (1996). [DOI] [PubMed] [Google Scholar]
- 30.Mikkelsen H. B., Huizinga J. D., Larsen J. O., Kirkeby S., Ionized calcium-binding adaptor molecule 1 positive macrophages and HO-1 up-regulation in intestinal muscularis resident macrophages. Anat. Rec. 300, 1114–1122 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donovan K. M., Leidinger M. R., McQuillen L. P., Goeken J. A., Hogan C. M., Harwani S. C., Flaherty H. A., Meyerholz D. K., Allograft inflammatory factor 1 as an immunohistochemical marker for macrophages in multiple tissues and laboratory animal species. Comp. Med. 68, 341–348 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Utans U., Arceci R. J., Yamashita Y., Russell M. E., Cloning and characterization of allograft inflammatory factor-1: A novel macrophage factor identified in rat cardiac allografts with chronic rejection. J. Clin. Invest. 95, 2954–2962 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou Q., Amar S., Identification of signaling pathways in macrophage exposed to porphyromonas gingivalis or to Its purified cell wall components. J. Immunol. 179, 7777–7790 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Sato T., van Es J. H., Snippert H. J., Stange D. E., Vries R. G., van den Born M., Barker N., Shroyer N. F., van de Wetering M., Clevers H., Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature 469, 415–418 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukuda M., Mizutani T., Mochizuki W., Matsumoto T., Nozaki K., Sakamaki Y., Ichinose S., Okada Y., Tanaka T., Watanabe M., Nakamura T., Small intestinal stem cell identity is maintained with functional Paneth cells in heterotopically grafted epithelium onto the colon. Genes Dev. 28, 1752–1757 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gao S., Yan L., Wang R., Li J., Yong J., Zhou X., Wei Y., Wu X., Wang X., Fan X., Yan J., Zhi X., Gao Y., Guo H., Jin X., Wang W., Mao Y., Wang F., Wen L., Fu W., Ge H., Qiao J., Tang F., Tracing the temporal-spatial transcriptome landscapes of the human fetal digestive tract using single-cell RNA-sequencing. Nat. Cell Biol. 20, 721–734 (2018). [DOI] [PubMed] [Google Scholar]
- 37.Munoz J., Stange D. E., Schepers A. G., van de Wetering M., Koo B. K., Itzkovitz S., Volckmann R., Kung K. S., Koster J., Radulescu S., Myant K., Versteeg R., Sansom O. J., van Es J. H., Barker N., van Oudenaarden A., Mohammed S., Heck A. J., Clevers H., The Lgr5 intestinal stem cell signature: Robust expression of proposed quiescent ‘+4’ cell markers. EMBO J. 31, 3079–3091 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kobayashi K., Tomita H., Shimizu M., Tanaka T., Suzui N., Miyazaki T., Hara A., p53 expression as a diagnostic biomarker in ulcerative colitis-associated cancer. Int. J. Mol. Sci. 18, 1284 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brighenti E., Calabrese C., Liguori G., Giannone F. A., Trerè D., Montanaro L., Derenzini M., Interleukin 6 downregulates p53 expression and activity by stimulating ribosome biogenesis: A new pathway connecting inflammation to cancer. Oncogene 33, 4396–4406 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ullman T. A., Itzkowitz S. H., Intestinal inflammation and cancer. Gastroenterology 140, 1807–1816 (2011). [DOI] [PubMed] [Google Scholar]
- 41.Hao Y., Chun A., Cheung K., Rashidi B., Yang X., Tumor suppressor LATS1 is a negative regulator of oncogene YAP*. J. Biol. Chem. 283, 5496–5509 (2008). [DOI] [PubMed] [Google Scholar]
- 42.Guo C., Liang C., Yang J., Hu H., Fan B., Liu X., LATS2 inhibits cell proliferation and metastasis through the Hippo signaling pathway in glioma. Oncol. Rep. 41, 2753–2761 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aylon Y., Yabuta N., Besserglick H., Buganim Y., Rotter V., Nojima H., Oren M., Silencing of the Lats2 tumor suppressor overrides a p53-dependent oncogenic stress checkpoint and enables mutant H-Ras-driven cell transformation. Oncogene 28, 4469–4479 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aylon Y., Michael D., Shmueli A., Yabuta N., Nojima H., Oren M., A positive feedback loop between the p53 and Lats2 tumor suppressors prevents tetraploidization. Genes Dev. 20, 2687–2700 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee Y., Kim N. H., Cho E. S., Yang J. H., Cha Y. H., Kang H. E., Yun J. S., Cho S. B., Lee S.-H., Paclikova P., Radaszkiewicz T. W., Bryja V., Kang C. G., Yuk Y. S., Cha S. Y., Kim S.-Y., Kim H. S., Yook J. I., Dishevelled has a YAP nuclear export function in a tumor suppressor context-dependent manner. Nat. Commun. 9, 2301 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vander Heiden M. G., Locasale J. W., Swanson K. D., Sharfi H., Heffron G. J., Amador-Noguez D., Christofk H. R., Wagner G., Rabinowitz J. D., Asara J. M., Cantley L. C., Evidence for an alternative glycolytic pathway in rapidly proliferating cells. Science 329, 1492–1499 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christofk H. R., Vander Heiden M. G., Harris M. H., Ramanathan A., Gerszten R. E., Wei R., Fleming M. D., Schreiber S. L., Cantley L. C., The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452, 230–233 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Puzio-Kuter A. M., The role of p53 in metabolic regulation. Genes Cancer 2, 385–391 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaiko G. E., Ryu S. H., Koues O. I., Collins P. L., Solnica-Krezel L., Pearce E. J., Pearce E. L., Oltz E. M., Stappenbeck T. S., The colonic crypt protects stem cells from microbiota-derived metabolites. Cell 165, 1708–1720 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li C., Zhou Y., Wei R., Napier D. L., Sengoku T., Alstott M. C., Liu J., Wang C., Zaytseva Y. Y., Weiss H. L., Wang Q., Evers B. M., Glycolytic regulation of intestinal stem cell self-renewal and differentiation. Cell. Mol. Gastroenterol. Hepatol. 15, 931–947 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X., Zhao H., Li Y., Xia D., Yang L., Ma Y., Li H., The role of YAP/TAZ activity in cancer metabolic reprogramming. Mol. Cancer 17, 134 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang W., Xiao Z.-D., Li X., Aziz K. E., Gan B., Johnson R. L., Chen J., AMPK modulates Hippo pathway activity to regulate energy homeostasis. Nat. Cell Biol. 17, 490–499 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vallée A., Lecarpentier Y., Vallée J.-N., The key role of the WNT/β-catenin pathway in metabolic reprogramming in cancers under normoxic conditions. Cancers 13, 5557 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pate K. T., Stringari C., Sprowl-Tanio S., Wang K., TeSlaa T., Hoverter N. P., McQuade M. M., Garner C., Digman M. A., Teitell M. A., Edwards R. A., Gratton E., Waterman M. L., Wnt signaling directs a metabolic program of glycolysis and angiogenesis in colon cancer. EMBO J. 33, 1454–1473 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sigal M., Meyer T. F., Coevolution between the human microbiota and the epithelial immune system. Dig. Dis. 34, 190–193 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Iftekhar A., Sigal M., Defence and adaptation mechanisms of the intestinal epithelium upon infection. Int. J. Med. Microbiol. 311, 151486 (2021). [DOI] [PubMed] [Google Scholar]
- 57.Hartl K., Sigal M., Microbe-driven genotoxicity in gastrointestinal carcinogenesis. Int. J. Mol. Sci. 21, 7439 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fujii S., Fujimori T., Kawamata H., Takeda J., Kitajima K., Omotehara F., Kaihara T., Kusaka T., Ichikawa K., Ohkura Y., Ono Y., Imura J., Yamaoka S., Sakamoto C., Ueda Y., Chiba T., Development of colonic neoplasia in p53 deficient mice with experimental colitis induced by dextran sulphate sodium. Gut 53, 710–716 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang W.-C. L., Coudry R. A., Clapper M. L., Zhang X., Williams K.-L., Spittle C. S., Li T., Cooper H. S., Loss of p53 enhances the induction of colitis-associated neoplasia by dextran sulfate sodium. Carcinogenesis 28, 2375–2381 (2007). [DOI] [PubMed] [Google Scholar]
- 60.Davidson L. A., Callaway E. S., Kim E., Weeks B. R., Fan Y.-Y., Allred C. D., Chapkin R. S., Targeted deletion of p53 in Lgr5-expressing intestinal stem cells promotes colon tumorigenesis in a preclinical model of colitis-associated cancer. Cancer Res. 75, 5392–5397 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Amerongen R., Bowman A. N., Nusse R., Developmental stage and time dictate the fate of Wnt/β-catenin-responsive stem cells in the mammary gland. Cell Stem Cell 11, 387–400 (2012). [DOI] [PubMed] [Google Scholar]
- 62.El Marjou F., Janssen K.-P., Hung-Junn Chang B., Li M., Hindie V., Chan L., Louvard D., Chambon P., Metzger D., Robine S., Tissue-specific and inducible Cre-mediated recombination in the gut epithelium. Genesis 39, 186–193 (2004). [DOI] [PubMed] [Google Scholar]
- 63.Marino S., Vooijs M., van der Gulden H., Jonkers J., Berns A., Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 14, 994–1004 (2000). [PMC free article] [PubMed] [Google Scholar]
- 64.Herring B. P., Hoggatt A. M., Burlak C., Offermanns S., Previously differentiated medial vascular smooth muscle cells contribute to neointima formation following vascular injury. Vasc. Cell 6, 21 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neufeld S., Rosin J. M., Ambasta A., Hui K., Shaneman V., Crowder R., Vickerman L., Cobb J., A conditional allele of Rspo3 reveals redundant function of R-spondins during mouse limb development. Genesis 50, 741–749 (2012). [DOI] [PubMed] [Google Scholar]
- 66.Yang C., Merlin D., Unveiling colitis: A journey through the dextran sodium sulfate-induced model. Inflamm. Bowel Dis. 30, 844–853 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Earle K. A., Billings G., Sigal M., Lichtman J. S., Hansson G. C., Elias J. E., Amieva M. R., Huang K. C., Sonnenburg J. L., Quantitative imaging of gut microbiota spatial organization. Cell Host Microbe 18, 478–488 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nusrat A., Parkos C. A., Garcia-Hernandez V., Neumann P.-A., Koch S., Lyons R., Systematic scoring analysis for intestinal inflammation in a murine dextran sodium sulfate-induced colitis model. J. Vis. Exp. 14, 10.3791/62135 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.A. S. Alexey, An algorithm for fast preranked gene set enrichment analysis using cumulative statistic calculation. bioRxiv 060012 [Preprint] (2016). 10.1101/060012. [DOI]
- 70.R Core Team (R Foundation for Statistical Computing, 2022).
- 71.H. Wickham, ggplot2 - Elegant Graphics for Data Analysis (Springer-Verlag, 2016). [Google Scholar]
- 72.Ritchie M. E., Phipson B., Wu D., Hu Y., Law C. W., Shi W., Smyth G. K., limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 43, e47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hänzelmann S., Castelo R., Guinney J., GSVA: Gene set variation analysis for microarray and RNA-seq data. BMC Bioinformatics 14, 7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rappsilber J., Mann M., Ishihama Y., Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat. Protoc. 2, 1896–1906 (2007). [DOI] [PubMed] [Google Scholar]
- 75.Cox J., Neuhauser N., Michalski A., Scheltema R. A., Olsen J. V., Mann M., Andromeda: A peptide search engine integrated into the MaxQuant Environment. J. Proteome Res. 10, 1794–1805 (2011). [DOI] [PubMed] [Google Scholar]
- 76.Cox J., Hein M. Y., Luber C. A., Paron I., Nagaraj N., Mann M., Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ*. Mol. Cell. Proteomics 13, 2513–2526 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bielow C., Mastrobuoni G., Kempa S., Proteomics quality control: Quality control software for MaxQuant results. J. Proteome Res. 15, 777–787 (2016). [DOI] [PubMed] [Google Scholar]
- 78.Opialla T., Kempa S., Pietzke M., Towards a more reliable identification of isomeric metabolites using pattern guided retention validation. Metabolites 10, 457 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ludikhuize M. C., Meerlo M., Burgering B. M. T., Rodriguez Colman M. J., Protocol to profile the bioenergetics of organoids using Seahorse. STAR Protoc. 2, 100386 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figs. S1 to S6
Tables S1 to S3
Legend for data S1
Data S1