Abstract
The CEACAM1 glycoproteins (formerly called biliary glycoproteins; BGP, C-CAM, CD66a, or MHVR) are members of the carcinoembryonic antigen family of cell adhesion molecules. In the mouse, splice variants of CEACAM1 have either two or four immunoglobulin (Ig) domains linked through a transmembrane domain to either a short or a long cytoplasmic tail. CEACAM1 has cell adhesion activity and acts as a signaling molecule, and long-tail isoforms inhibit the growth of colon and prostate tumor cells in rodents. CEACAM1 isoforms serve as receptors for several viral and bacterial pathogens, including the murine coronavirus mouse hepatitis virus (MHV) and Haemophilus influenzae, Neisseria gonorrhoeae, and Neisseria meningitidis in humans. To elucidate the mechanisms responsible for the many biological activities of CEACAM1, we modified the expression of the mouse Ceacam1 gene in vivo. Manipulation of the Ceacam1 gene in mouse embryonic stem cells that contained the Ceacam1a allele yielded a partial knockout. We obtained one line of mice in which the insert in the Ceacam1a gene had sustained a recombination event. This resulted in the markedly reduced expression of the two CEACAM1a isoforms with four Ig domains, whereas the expression of the two isoforms with two Ig domains was doubled relative to that in wild-type BALB/c (+/+) mice. Homozygous (p/p) Ceacam1a-targeted mice (Ceacam1aΔ4D) had no gross tissue abnormalities and were viable and fertile; however, they were more resistant to MHV A59 infection and death than normal (+/+) mice. Following intranasal inoculation with MHV A59, p/p mice developed markedly fewer and smaller lesions in the liver than +/+ or heterozygous (+/p) mice. The titers of virus produced in the livers were 50- to 100-fold lower in p/p mice than in +/p or +/+ mice. p/p mice survived a dose 100-fold higher than the lethal dose of virus for +/+ mice. +/p mice were intermediate between +/+ and p/p mice in susceptibility to liver damage, virus growth in liver, and susceptibility to killing by MHV. Ceacam1a-targeted mice provide a new model to study the effects of modulation of receptor expression on susceptibility to MHV infection in vivo.
The CEACAM1 glycoproteins, formerly called biliary glycoproteins (BGP, Bgp, mmCGM1, C-CAM, CD66a, or MHVR), are members of the carcinoembryonic antigen (CEA) family in the immunoglobulin (Ig) superfamily (4, 22, 44, 51, 70). The nomenclature for the CEA gene family has recently been unified (9), and seven genes are now referred to as the CEACAM genes. CEACAM1, the most conserved gene of the CEA gene family, is found as a single copy on human chromosome 19q13.2 (30) and in the rat genome (24). However, mouse chromosome 7, in the 7A2-A3 region near the centromere, a region syntenic to the region encoding CEACAM1 on human chromosome 19q, contains two highly related Ceacam genes, called Ceacam1 and Ceacam2 (formerly called Bgp1 and Bgp2, respectively) (48, 49, 58). The human, rat, and mouse CEACAM1 genes have highly conserved structures (4, 24, 49), 66% identity between their respective promoters, similar but not identical patterns of mRNA splicing, and similar patterns of expression in different tissues (27, 32, 44, 49, 52, 53).
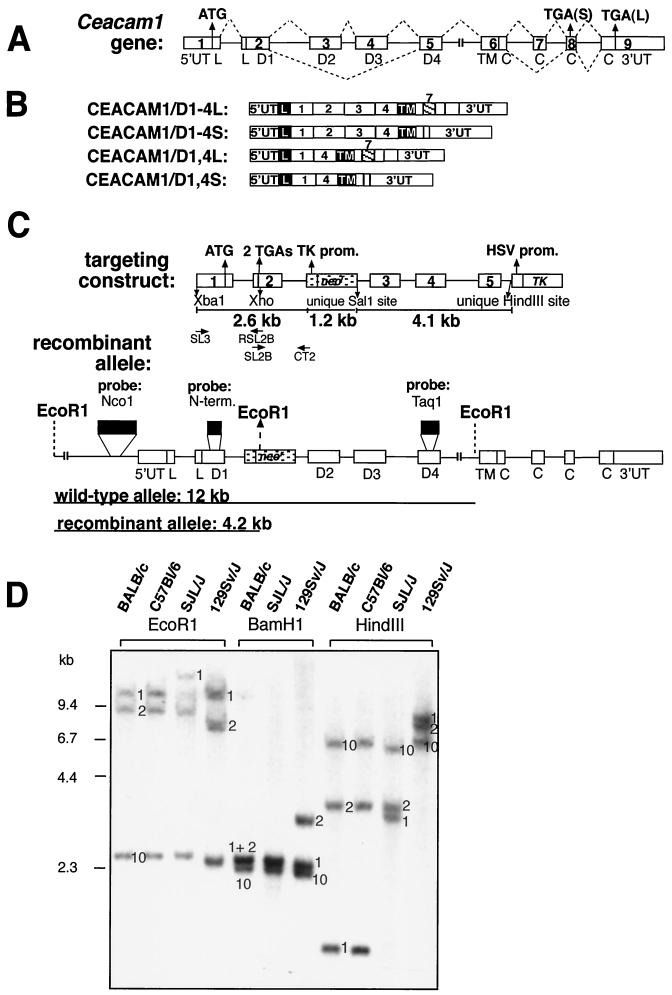
Mouse CEACAM1 isoforms are transmembrane glycoproteins that have either two or four Ig domains produced by alternative splicing of the primary transcript (Fig. 1A and B) (43, 44). CEACAM1 isoforms with four Ig domains, denoted CEACAM1/D1-4, include the N domain (D1) attached to three constant Ig domains (D2, D3, and D4). Splice isoforms with two Ig domains (CEACAM1/D1,4) link D1 to D4. The exodomains are linked via a transmembrane domain to either of two cytoplasmic tails. The short cytoplasmic tail (CEACAM1-S) contains 10 amino acids (aa) rich in Ser and Gly residues. The long cytoplasmic tail (CEACAM1-L) results from inclusion of 53-bp Ceacam1 exon 7, which shifts the open reading frame (ORF) for the tail at aa 453 to yield a 73-aa tail (Fig. 1A) (43, 49).
FIG. 1.
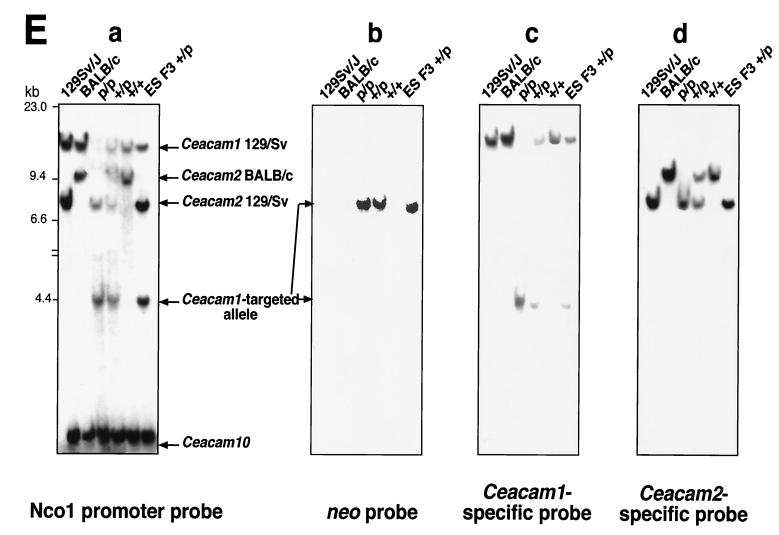
Targeting of the Ceacam1 gene. (A) The mouse Ceacam1 gene encodes nine exons. The ATG initiation codon is located in the first exon, whereas two stop codons can be alternatively used, one in exon 8 [TGA(S), used in the translation of isoforms encoding a short cytoplasmic domain], or another in exon 9 [TGA(L), used in the translation of isoforms encoding a long cytoplasmic domain]. The broken lines over and under the gene structure represent the alternative splicing events that produce CEACAM1 isoforms with either two or four Ig domains and either a short or a long cytoplasmic domain. (B) The Ceacam1 gene produces four major alternatively splice variants. The CEACAM1/D1-4 variants contain D1 to D4 Ig domains (identified in the boxes) that are linked through a transmembrane domain (TM) to either a short (S) or a long (L) tail. This alternative splicing event is due to the inclusion of 53-bp exon 7 (hatched box with exon number over the box), resulting in a shift of the ORF and the translation of a 73-aa tail. (C) Targeting construct. The targeting construct that led to a recombination event in ES cells had the TK-neor selection cassette (stippled) inserted into a unique SalI site in the second intron. The construct contained two engineered TGA stop codons at the beginning of exon 2 that were inserted by overlapping mutagenesis with the oligonucleotides indicated beneath the construct. These TGA codons were eliminated in the recombination event. A herpes simplex virus thymidine kinase-negative selection cassette was included in the unique HindIII site within intron 5. Three probes, identified by the black boxes, were used in Southern analyses. The recombinant allele producing a novel EcoRI-cleaved fragment of 4.2 kb, due to the insertion of TK-neor, was detected with the NcoI promoter probe. prom., promoter; N-term., N terminal. (D) Southern analyses of genomic DNAs from different inbred strains of mice. Mice express three Ceacam-related genes: Ceacam1 (1), Ceacam2 (2), and Ceacam10 (10). The numbers next to the various restriction fragments in the gels indicate the position of each gene in the particular digest of genomic DNA. Samples of 5 μg of genomic DNA extracted from each mouse strain were digested with either EcoRI, BamHI or HindIII restriction enzyme and run on 0.75% agarose gels. The DNA fragments were transferred to GeneScreen Plus and probed with a 32P-labeled 395-bp NcoI fragment located in the proximal promoter region. Note that the 129Sv/J Ceacam2-specific EcoRI restriction fragment is shorter than that of other mouse strains. The 1.7-kb Ceacam1-specific HindIII fragment present in BALB/c and C57BL/6 mice is not found in strain 129Sv/J. Instead, this fragment migrates as an 8.0-kb fragment due to the mutation of the HindIII site present in intron 1 of other inbred strains. (E) Genotyping of ES cells and mice. Recombination events in ES cells and genotypes of mice obtained from heterozygous matings were detected by cleaving genomic DNA with EcoRI. The samples were run on 0.75% agarose gels and hybridized with 32P-labeled probes described in Materials and Methods and shown in panel C. (Panel a) The NcoI probe is located in the upstream promoter region and hybridizes to all three mouse Ceacam-related genes (1, 2, and 10). The targeted allele in the F3 ES cells and mice is shortened from the original 12 kb to 4.2 kb. (Panel b) The neo probe detects one band of approximately 8.0 kb. Only one integration site was detected in the F3 ES cell line. The genomic DNA from +/+ mice did not hybridize with this probe, whereas the +/p and p/p mice were positive. (Panel c) The Ceacam1-specific probe, located in the N-terminal domain of the gene (shown in panel C), detected a 12-kb EcoRI fragment in genomic DNA prepared from all mice except p/p mice. In addition, this probe hybridized to the targeted 4.2-kb fragment in the F3 ES cell line and in +/p and p/p mice. (Panel d) The Ceacam2-specific probe, also from the N-terminal domain of its respective gene, hybridized to either a 9.6-kb fragment or a 7.7-kb fragment, depending on the contribution from the BALB/c or 129Sv/J mice, respectively.
The CEACAM1 glycoproteins are abundantly expressed on apical membranes of epithelial cells in the gastrointestinal and respiratory tracts, in bile canaliculi, and on the proximal tubules of the kidneys (44, 50, 71). They are also found on small vascular endothelial cells, in hemopoietic cells (B cells, neutrophils, macrophages, monocytes, platelets, and activated T cells), and in thymic stromal cells (17, 27, 46, 47). CEACAM1 isoforms are also expressed at the apical surfaces of epithelial cells in the reproductive tissues (uterus, ovary, breast, and prostate) (34, 68, 71) and at low levels on glial cells in the nervous system (27, 62). CEACAM1 is abundantly expressed in endodermal and mesenchymal derivatives during early mouse embryonic development (19).
CEACAM1 performs many important cellular functions. It is a cell adhesion molecule (44, 52, 59) and a signaling molecule (10, 31, 51) that regulates the growth of tumor cells (34, 40). Recently, CEACAM1 was shown to be a potent angiogenic factor (25). In addition, CEACAM1 is a receptor for bacterial and viral pathogens. The opa surface proteins of pathogenic strains of Neisseria gonorrhoeae and Neisseria meningitidis and membrane proteins of Haemophilus influenzae bind specifically to the human CEACAM1 protein and several other human CEA-related glycoproteins (29, 72–74). In this study, we explored the role in viral pathogenesis of murine CEACAM1a, a receptor for mouse hepatitis virus (MHV).
MHV strains are murine coronaviruses that cause respiratory and enteric infections, hepatitis, splenolysis, immune dysfunction, acute encephalitis, and chronic demyelinating disease of the brain and spinal cord (5, 16). MHV infection of mice varies from inapparent and self-limited infection to severe disease and death, depending on the age, immune status, strain, and route of inoculation of the mouse and on the dose and strain of the virus. In some mouse strains, inapparent MHV infection disrupts normal patterns of cytokine expression for 5 months after infection (18). Many, but not all, of the murine tissues that express CEACAM1 are natural targets for MHV infection.
Williams (76), Williams et al. (77), and Dveksler et al. (22) identified and cloned the cDNA encoding the CEACAM1/D1-4 MHV receptor. When this murine Ceacam1a cDNA was transfected into hamster cell lines, which are not susceptible to MHV, expression of the mouse CEACAM1a glycoprotein rendered the hamster cells susceptible to MHV (22). The sequence of the virus receptor was found to be identical to that of the biliary glycoprotein identified as a CEA-related cell adhesion glycoprotein (44). Adult SJL mice, which are highly resistant to MHV infection (7, 39, 67), are homozygous for CEACAM1b, an allele of CEACAM1a that differs by 27 of 108 aa in the N domain (D1) (21, 78). The spike (S) glycoprotein of MHV attaches to the N domain (D1) of CEACAM1a (23). Most inbred strains of mice (BALB/c, C57BL/6, C3H, 129Sv, and so forth) are susceptible to MHV infection and are homozygous for the CEACAM1a allele, whereas outbred CD1 mice express both CEACAM1a and CEACAM1b and are susceptible to infection.
All MHV strains tested to date utilize the murine CEACAM1a proteins as receptors (15, 21). Mutational analyses showed that the virus binds to the B-C-C′ region of domain 1 of the CEACAM1a protein (57, 75). A monoclonal antibody (CC1) directed against D1 of CEACAM1a blocks virus attachment and prevents infection in vitro and in vivo (23, 65). All four isoforms of murine CEACAM1a serve as receptors for MHV A59 when expressed at high levels in hamster cells (21). Interestingly, the expression of high levels of murine CEACAM1b in hamster cells also makes the cells susceptible to MHV-A59 infection (21). When expressed at high levels in BHK cells, murine CEACAM2 also serves as an MHV receptor, although it is a markedly less efficient MHV A59 receptor than CEACAM1a (48). Soluble CEACAM2 also has much less virus neutralization activity than soluble CEACAM1a (80).
The surface density of CEACAM1a/D1,4 with the short cytoplasmic tail affects the susceptibility of cells to infection with the MHV JHM strain. HeLa cells that expressed low levels of recombinant murine CEACAM1a/D1,4 were susceptible to infection with MHV JHM but did not exhibit cytopathic effects, such as cell fusion and death (55). In contrast, HeLa cells that expressed high levels of murine CEACAM1a/D1,4 with the short tail on the cell surface were killed within 14 h of infection with MHV JHM. Complexes formed between the CEACAM1a receptor protein and the viral S glycoprotein in the endoplasmic reticulum and Golgi apparatus (56). MHV infection or expression of the viral S glycoprotein on murine cells rapidly led to the selection of cells that expressed reduced levels of CEACAM1a (14, 55, 63). Persistent infection of murine cells in vitro with MHV A59 leads to the selection of cells resistant to wild-type virus and to the selection of small-plaque viral mutants that have mutations in the receptor-binding N-terminal domain of the viral S glycoprotein, and some of these small-plaque mutants have an extended host range (2, 3, 28, 63, 64).
The goal of this research was to derive Ceacam1 knockout mice for the study of CEACAM1 functions in normal and infected animals. We therefore disrupted the mouse Ceacam1a gene in 129Sv mouse embryonic stem (ES) cells in order to generate knockout animals and examined the genotypes and phenotypes of the resulting heterozygous and homozygous mice. Normally this technique completely abrogates or knocks out the expression of the targeted gene in homozygous mice. The only line of mice that resulted from this knockout strategy showed only partial, incomplete reduction in CEACAM1a expression; expressed markedly altered ratios of CEACAM1a isoforms in different murine tissues; but had normal phenotype, fertility, and life span. Expression of the CEACAM1a/D1-4 isoforms was reduced by 90 to 95% in these mice, whereas the CEACAM1a/D1,4 isoforms were expressed at levels higher than normal. Following intranasal inoculation with MHV A59, homozygous (p/p) Ceacam1a-targeted mice failed to develop clinical signs of viral infection, developed fewer and smaller lesions in the liver, and produced less virus in the liver than wild-type (+/+) mice. Thus, reducing the level of expression of CEACAM1a/D1-4 proteins and altering the ratios of two- and four-domain CEACAM1a isoforms in vivo resulted in mice with significantly decreased susceptibility to MHV infection. These results also suggest that the four-domain CEACAM1a isoforms are the principal MHV receptors in vivo.
MATERIALS AND METHODS
Generation of the targeting vector.
The mouse 129Sv Ceacam1a gene was isolated from a 129Sv genomic library graciously provided by Christian Benoît and Diane Mathis (Strasbourg, France). This genomic library was prepared from the D3 ES cell line; partial MboI restriction fragments of approximately 10 to 15 kb were inserted into a lambda GEM12 vector. A total of 5 × 105 PFU of this genomic library were screened with three distinct probes identified in Fig. 1C. A proximal promoter fragment (NcoI probe; 395-bp fragment found at nucleotides 975 to 1370 in the Ceacam1 promoter) (49) hybridized to the mouse Ceacam1a, Ceacam1b, Ceacam2, and Ceacam10 genes at high stringency (Fig. 1D). The Ceacam1a gene was specifically detected using the full-length Ceacam1a cDNA or a TaqI probe (nucleotides 1150 to 1295 in the Ceacam1a cDNA) when hybridization was done at high stringency (49). Two clones encompassing the Ceacam1a gene were obtained. The identity of the gene was confirmed by restriction digestion and DNA sequence analyses of relevant exons.
The scheme for producing the targeting vector illustrated in Fig. 1C was designed to insert the pTK-neor selection cassette (69) downstream of exon 2. Two major restriction fragments were used to prepare the targeting vector. The first was a 2.6-kb XbaI-SalI fragment encompassing the first two exons and the first intron of the mouse Ceacam1a gene. This fragment served as a template for PCRs to amplify the 5′ arm of the targeting vector, consisting of two shorter fragments, of 1.1 and 1.5 kb. Mutated nucleotides are indicated in bold in the following sequences. The mutated 1.1-kb fragment was produced by PCR amplifications with oligonucleotide SL3, located at the 5′ end of the 5′ untranslated region (sense: 5′CGGAGTATGTTCTAGAACACTG) and oligonucleotide RSL2B, located within exon 2 at the end of the DNA sequence that encodes the signal sequence (antisense: 5′GACTCGAGCAGTGGTGGCAGGTCATCAGGAG). The mutated 1.5-kb fragment was generated by PCR with oligonucleotide SL2B (sense: 5′CTCCTGATGACCTGCCACCACTGCTCGAGTC) and oligonucleotide CT2, located at a unique SalI site within intron 2 (antisense: 5′AGACACAATCTGTCGACTCCTC). The SL2B and RSL2B oligonucleotides introduced six nucleotides as substitutions that encode two TGA stop codons in the ORF (bold in the oligonucleotide sequence) and a unique XhoI site at the beginning of exon 2 which encodes the N-terminal domain, D1. The two fragments were joined together by overlapping PCRs using oligonucleotides SL3 and CT2. The 2.6-kb 5′ arm of the targeting vector was linked to the pTK-neor selection cassette, originating from the pMC1-neor-poly(A) vector (69). The resulting 3.8-kb fragment was cloned upstream of the remaining 4.1-kb Ceacam1a gene fragment that constituted the 3′ arm of the targeting vector. Finally, a herpes simplex virus thymidine kinase-negative selection cassette (69) was introduced in a unique HindIII site downstream of the targeting vector. To determine whether the Ceacam1a-targeted ES cell line DNA enclosed the added TGA stop codons, PCR amplifications were performed with ES cell genomic DNA. An EcoRI-tagged oligonucleotide in Ceacam1a intron 1, located upstream of the stop codons (CT3; sense: 5′GGAATTCCTAAGATTGATAGGTCTTTC), and an oligonucleotide positioned within the neor gene (RTKneo1; antisense: 5′GGAAACATTCCAGGCCT) were used in this procedure. The amplified 1.7-kb fragment was cloned and subjected to DNA sequence analyses.
DNA analyses.
Genomic DNA was prepared from ES cell clones grown in 24-well dishes using standard procedures (69). Genotyping was performed using <1 cm of tails clipped from 3-week-old pups; genomic DNA was prepared using a QIAamp DNA kit (Qiagen). Approximately 5 μg of genomic DNA was cleaved with restriction endonuclease EcoRI and separated on 0.75% agarose gels. The DNA was transferred to GeneScreen Plus membranes (NEN-Life Science Products, Boston, Mass.) and hybridized at 42°C for 18 h with 2 × 106 to 4 × 106 dpm of random-primed [α-32P]dATP-labeled restriction fragments (49). Membranes were washed at a final stringency of 65°C in a 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate (SDS) solution. The NcoI promoter fragment of 395 bp was used as a probe (49). An N-terminal domain fragment specific for the Ceacam1a gene (102 bp) or a Ceacam2-specific fragment (153 bp), amplified by PCR as previously described (48, 49), was used to distinguish the Ceacam1a and Ceacam2 genes. The 125-bp TaqI restriction fragment located within exon 5 is specific for the Ceacam1 gene and does not hybridize with the Ceacam2 gene at high stringency. A 700-bp HindIII-NaeI fragment prepared from the coding region of the neor gene was used to define the number of neo integration sites in the ES cell clones by Southern hybridization. A 360-bp cDNA fragment corresponding to exons that encode the long or short cytoplasmic tails was also used to validate the integrity of the Ceacam1 gene locus at its 3′ end.
ES cell culturing.
Twenty-five micrograms of the NotI-linearized targeting vector described above was electroporated into 1 × 107 129Sv R1 ES cells, graciously provided by Andras Nagy (Samuel Lunenfeld Research Institute, Toronto, Ontario, Canada). ES cells were maintained on mitomycin-inactivated G418-resistant mouse embryonic fibroblasts in leukemia inhibitory factor-containing Dulbecco modified Eagle medium (DMEM; Life Technologies, Grand Island, N.Y.) with 15% fetal bovine serum (FBS) (HyClone Laboratories, Logan, Utah) as previously described (69). G418 (300 μg/ml; active form) and ganciclovir (2 μM) selections were carried out 48 h posttransfection, and clones were isolated and amplified after 9 days in selective medium.
Generation and breeding of chimeric mice.
Chimeric mice were generated by microinjection of Ceacam1a-targeted ES cells (at passage 20) into BALB/c blastocysts as described previously (12). Chimeric males were crossed with either BALB/c or C57BL/6 females. Heterozygous mice were obtained and crossed to generate Ceacam1a homozygous mice. Owing to the partial knockout phenotype and to avoid confusion between animals that may be generated in the future with a complete Ceacam1a gene ablation, we use the terminology +/+ for wild-type mice, +/p for heterozygous mice, and p/p for homozygous Ceacam1aΔ4D mice described in this paper. Experiments were performed with +/p and/or p/p Ceacam1a-targeted mice in a BALB/c background and +/+ BALB/c mice in the same genetic background. All animals used in these experiments were between 6 and 12 weeks of age.
Sampling and preparation of tissues.
The mice were sacrificed by cervical dislocation, and the tissues were removed and washed in phosphate-buffered saline (PBS). The intestine was dissected, cleared of debris, and washed in PBS; it was then divided into sections of equal lengths corresponding to the duodenum, jejunum, and ileum. The colon was sampled distal to the cecum. All tissues were immediately snap-frozen on dry ice for subsequent DNA, RNA, or protein analyses or fixed in 4% paraformaldehyde–PBS and processed for histological analyses and immunohistochemical analyses.
Histological analyses.
Paraformaldehyde-fixed tissues were dehydrated in ethanol and paraffin embedded. Tissue sections of 6-μm thickness were stained with anti-CEACAM1 antibodies (19) and counterstained with hematoxylin according to standard histological procedures.
Antibodies, immunoblotting, and immunoprecipitations.
Fresh tissues were excised from 2- to 6-month-old Ceacam1aΔ4D +/+, +/p, or p/p mice, snap-frozen on dry ice, and made into powder using a mortar and pestle. The powder was resuspended in 500 to 1,000 μl of lysis buffer (35). Proteins were separated by SDS–8% polyacrylamide gel electrophoresis (PAGE) and transferred to Immobilon membranes (Millipore, Nepean, Ontario, Canada). Expression of the CEACAM1 isoforms was detected by immunoblotting 75 to 200 μg of total proteins with CEACAM1-specific rabbit polyclonal antibody 231 or 2456 (44). To detect CEACAM1 proteins expressing the long cytoplasmic domain, proteins were immunoprecipitated with 5 μg of rabbit polyclonal antibody 836 IgG fraction (60). Immune complexes were visualized using either 125I-labeled protein A or enhanced chemiluminescence detection (Amersham Pharmacia Biotech, Baie d'Urfé, Quebec, Canada). Controls for detection of the various splice isoforms were prepared from previously described NIH 3T3 cell clones that express Ceacam1a cDNAs encoding CEACAM1a/D1-4 isoforms with either the long or short cytoplasmic tail or constructs encoding CEACAM1a/D1,4 isoforms (19). Labeled proteins were quantified using a Fuji BioImager 2000 system; results are expressed as fold expression ± standard deviation.
Biochemical and hematological analyses.
Mice were anesthetized with a ketamine-xylazine-acepromazine mixture, and blood was collected from the jugular vein. Samples were processed for standard biochemical parameters at the Jewish General Hospital, Montreal, Quebec, Canada, with a Hitachi clinical biochemistry analyzer (model 917). For hematological studies, blood was collected in heparinized tubes. Blood cells were counted and analyzed on a Baker CBC model 9000 apparatus (Animal Resources Centre, McGill University).
MHV preparations and intranasal inoculation of mice.
The MHV A59 strain used in these experiments was propagated in the spontaneously transformed 17 Cl 1 line of BALB/c 3T3 cells as previously described (26). The supernatant medium was collected at 24 h after inoculation, centrifuged to remove cellular debris, divided into aliquots, quickly frozen, and stored at −80°C. Titers of infectious virus were determined by plaque assays with 17 Cl 1 cells (26). Ceacam1aΔ4D p/p, +/p, and +/+ mice 8 to 12 weeks old were inoculated intranasally with 15 μl of virus at 104, 106, or 108 PFU in DMEM with 10% FBS. Control, uninfected +/+, +/p, or p/p mice were sham inoculated intranasally with 15 μl of DMEM with 10% FBS. The mice were observed daily for clinical signs of illness, such as lethargy, ruffled fur, hunched posture, or paresis. A numerical scale of clinical symptoms was used to assess the degree of illness of the animals.
Analyses of tissues of inoculated mice.
At intervals of 3, 4, 5, 7, 9, and 31 days after inoculation, mice were sacrificed, serum samples were collected for evaluation of antiviral antibody, and livers were collected and processed to determine the yield of infectious virus and for histopathological studies. To quantitate infectious virus in the liver, portions of the liver removed at necropsy were rinsed in Dulbecco-Vogt PBS, weighed, homogenized in DMEM with 10% FBS, and rapidly frozen and thawed at 37°C three times. Cell debris was removed by centrifugation. The virus titer per gram of liver in the supernatant medium was determined by plaque assays with 17 Cl 1 cells as described previously (26). Tissues were fixed in neutral buffered formalin or 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin. Sections were examined by light microscopy, and the numbers and sizes of lesions in comparable areas of the liver sections were determined. Virus-infected cells in the liver were identified by immunostaining with a monoclonal antibody directed against viral nucleocapsid protein N, kindly provided by Julian Leibowitz (Texas A&M University, College Station), followed by peroxidase-labeled anti-mouse Ig. Controls, which showed no immunostaining, included the following: antibody treatment and peroxidase-conjugated anti-mouse IgG treatment of liver sections from sham-inoculated mice and incubation of liver sections from infected mice with a monoclonal antibody directed against an irrelevant antigen followed by peroxidase-conjugated anti-mouse IgG. Antiviral antibody titers in sera collected from sham-inoculated mice and +/p and p/p mice at 31 days after virus inoculation were determined by enzyme-linked immunoassays on plates coated with sucrose density gradient-purified MHV A59 virions.
Statistical analyses.
A multinomial logistic regression model was used to investigate the effect of genotype on the number of lesions observed. Three variables (inoculum, days postinoculation, and number of lesions observed) were considered relative to the genotype of the mice. The number of lesions was categorized as low (0 to 2 lesions inclusive), medium (3 to 10 lesions inclusive), and high (11 lesions or more), with the low category being defined as the comparison category (the model chi-square P value was <0.0001).
RESULTS
Identification of the 129Sv Ceacam1a gene and generation of Ceacam1a-targeted p/p mice.
We previously analyzed the BALB/c mouse genome for Ceacam-related genes and found three highly homologous genes located on mouse chromosome 7, identified as the Ceacam1, Ceacam2, and Ceacam10 genes (Fig. 1D) (38, 48, 49). The murine Ceacam1 and Ceacam2 genes are adjacent on the chromosome and share a high degree of homology in their exon-intron organization as well as in their nucleotide sequences (48, 49). The Ceacam10 gene is different in many of its features and positioned approximately 0.7 cM distal to the Ceacam1 and Ceacam2 genes (38, 81). As shown in Fig. 1D, the migration patterns of restriction fragments corresponding to the genes vary slightly in different inbred mouse strains.
For genetic manipulation experiments, we isolated the Ceacam1a gene of 129Sv mice (Fig. 1A). Four nucleotide differences in the 5′ untranslated region located in exon 1 and two point mutations in the 3′ untranslated region (exon 9) were noted relative to the published sequence of BALB/c Ceacam1a cDNA (GenBank accession number X15351). No mutations were found in the coding region of the gene. However, a HindIII polymorphism between the BALB/c and 129Sv/J strains (Fig. 1D) was demonstrated by sequencing intron 1. The HindIII site present in the first intron of the Ceacam1a gene from BALB/c mice was absent in 129Sv/J mice, as demonstrated by an approximately 8.0-kb HindIII fragment (Fig. 1D). The exon-intron distribution and the lengths of the introns were identical between the Ceacam1a genes of the two mouse strains.
All CEACAM1a splice isoforms (Fig. 1B) express the N-terminal domain, D1. Most of the functions for this protein, except inhibition of tumor growth and spread, are mediated by D1 (11, 23, 37, 42, 74). We therefore prepared three targeting vectors designed to eliminate exon 2 of Ceacam1a in order to completely abrogate the expression of Ceacam1a (43). In all of the targeting vectors, two TGA stop codons were inserted in exon 2, at nucleotide positions encoding the end of the signal sequence. In the first targeting construct that we tried, most of exon 2 was replaced with the 1.2-kb TK-neor selection cassette. The second targeting vector had the 1.2-kb TK-neor selection cassette inserted into the characteristic BamHI site present further downstream in exon 2. From the 3,000 ES clones generated with these two constructs, no Ceacam1a +/p ES cell lines was identified.
The third strategy relied on the insertion of the selection cassette into a unique SalI site further downstream in intron 2. As illustrated in Fig. 1C, the 5′ arm of the targeting vector consists of 2.6 kb of isogenic 129Sv DNA within the recombinant fragment, and the 3′ end matches 4.1 kb of the 129Sv Ceacam1a gene. From the 800 G418-resistant clones produced using the targeting construct described in Fig. 1C, one Ceacam1a +/p ES cell clone (F3) was identified. Analyses of EcoRI-cleaved genomic DNA of the F3 ES cell line with three probes revealed the characteristic Ceacam1a-targeted allele. These probes are an NcoI promoter-specific probe specific for all three Ceacam-related genes (Fig. 1E, panel a, fragment of 4.2 kb), a probe binding to the neor gene coding region (Fig. 1E, panel b, fragment of 8.0 kb), or a Ceacam1a-specific labeled restriction fragment (Fig. 1E, panel c, fragment of 4.2 kb). The targeting event was also confirmed with other characteristic restriction digests of the F3 genomic DNA (data not shown). The integrity of the mutant versus the normal Ceacam1a genomic locus was confirmed by hybridizing restriction fragments of ES cell genomic DNA with representative probes located 5′ or 3′ to the recombination site (data not shown). No modifications were found in the Ceacam2 or Ceacam10 genes in the ES cell line (Fig. 1E, panels a and d). As the position of the selection cassette was located far from the engineered TGA stop codons, we also used the PCR-based sequencing assay described in Materials and Methods to investigate whether the targeted Ceacam1a allele included the engineered TGA stop codons. We found that the recombination event had occurred between the stop codons and the selection cassette, thus eliminating the stop codons from the F3 ES cell Ceacam1a-targeted allele. Because the TGA stop codons were removed, there was a high probability that in homozygous mice, some CEACAM1a proteins might be expressed from this targeted gene.
Chimeric mice were generated by microinjection of the ES cell line into BALB/c mouse blastocysts. Six chimeric male mice were obtained, one of which transmitted the +/p Ceacam1a-targeted allele through the germ line. The heterozygous Ceacam1a +/p progeny mice were mated with each other to produce homozygous (p/p) mice. Mice were genotyped by Southern analyses with the NcoI promoter probe (Fig. 1E, panel a). The nontargeted Ceacam1a restriction fragment of 12 kb in the +/p mice appeared with half the hybridization intensity of that in the wild-type +/+ mice. This fragment was absent from genomic DNA of the p/p mice, with a concomitant increase in the 4.2-kb targeted restriction fragment (Fig. 1E, panels a, b, and c). Interestingly, in the Ceacam1a p/p mice, the BALB/c Ceacam2 alleles were replaced by the 129Sv Ceacam2 alleles (Fig. 1E, panel d). The frequency of germ line transmission was calculated to be 3.7% in the BALB/c background. Mating of heterozygous mice produced expected Mendelian ratios of Ceacam1a p/p offspring (1.0 +/+:1.6 +/p:1.0 p/p). CEACAM1 is expressed in the ovary and prostate; however, the progeny had approximately equal numbers of males and females (46.3% males, 53.7% females).
General health status of the offspring.
We have maintained for 18 months a sizeable colony (approximately 350 individuals) of targeted mice in both the BALB/c and the C57BL/6 backgrounds and have not noticed any reduction of fertility, bone or cartilage abnormalities, tumors, or abnormal behavior.
Expression of CEACAM1 glycoproteins in Ceacam1a-targeted mice.
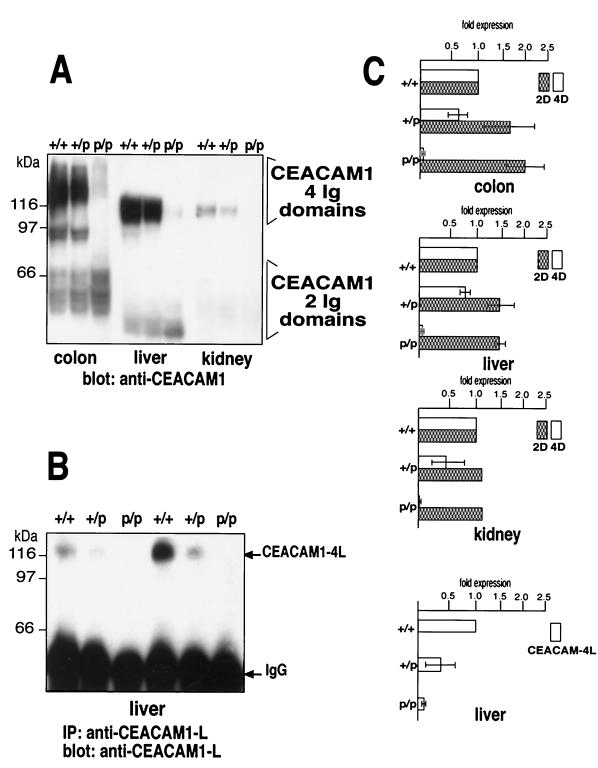
The CEACAM1 glycoprotein isoforms are heavily glycosylated proteins with up to 16 N-linked sugar chains attached to the extracytoplasmic Ig domains. We examined the expression of the CEACAM1a isoforms in mouse colon, liver, and kidney tissues by immunoblotting total proteins from several different mice with monoclonal and polyclonal anti-CEACAM1a antibodies (Fig. 2A). In these tissues, expression of the two CEACAM1a/D1-4 isoforms was decreased in the Ceacam1a p/p mice to 6% ± 2%, 3% ± 1%, and 2% ± 1% in colon, liver, and kidney tissues, respectively, relative to expression in the wild-type +/+ mice (Fig. 2A and C). The expression of the two CEACAM1a/D1,4 isoforms was increased in these tissues in p/p mice relative to +/+ mice. The colon showed a 2.0-fold higher ratio relative to the results for controls. Interestingly, because normal +/+ kidney tissue expressed a high (4D > 2D) ratio of CEACAM1a, the kidneys of p/p mice exhibited the most significant overall decrease in CEACAM1a expression.
FIG. 2.
Expression of CEACAM1 in various tissues. (A) Expression of CEACAM1 proteins in various mouse tissues. The colon, liver, and kidneys were excised from +/+, +/p, and p/p mice and snap-frozen. The frozen tissues were powdered and extracted with lysis buffer. Samples of 200 μg were analyzed by SDS–8% PAGE and transferred to Immobilon membranes. The CEACAM1 isoforms were detected by immunoblotting with rabbit anti-mouse 231 or 2456 polyclonal antibody and 125I-labeled protein A. (B) Expression of CEACAM1-L isoforms. Total lysate protein samples of 1 mg prepared from the livers of +/+, +/p, and p/p mice were immunoprecipitated with 5 μg of antibody 836 IgG, raised to CEACAM1-L. Immune complexes were collected and separated by SDS-PAGE, and the CEACAM1-L protein was detected with the same antibody. (C) Quantitation of CEACAM1 isoforms. The proteins on blots from several representative experiments were quantified using a Fuji BioImaging system, and the results were computed relative to the expression in +/+ mice. Error bars indicate standard deviations. D, domains.
The tumor suppressor and signaling functions of CEACAM1 rely on the expression of its long cytoplasmic domain, generated by alternative splicing of the 53-bp exon 7 included in the transcript (Fig. 1A and B) (10, 31, 34, 35, 40). Consequently, we verified the expression of CEACAM1a-L isoforms in Ceacam1a-deficient mice by immunoprecipitation with a long-tail-specific antibody (Fig. 2B). CEACAM1a-L expression was decreased in the p/p liver (Fig. 2B and C; 9% ± 4% wild-type expression) and colon (data not shown; 15% ± 4% wild-type expression). These results suggest that insertion of the neo selection cassette in intron 2 of the Ceacam1a gene modified the normal alternative splicing pattern, shifting it preferentially to the expression of Ceacam1a/D1,4 mRNAs (Fig. 1A and B). In addition, splicing of the cytoplasmic domain exons was not affected, as the ratio of CEACAM1a-L to CEACAM1a-S isoforms remained constant in the p/p and +/+ mice. These results also indicated that Ceacam1a-specific alternative splicing patterns differed slightly from tissue to tissue.
Histological data.
Paraffin-embedded tissue sections were stained for routine histologic analysis and immunostained with anti-CEACAM1 polyclonal antibody (231 or 2456) and monoclonal antibody (CC1). No histological differences were noted for the colon, liver, and kidneys of p/p mice and +/+ mice (Fig. 3). When immunostained with a polyclonal anti-CEACAM1a antibody, tissues from +/+ animals exhibited strong expression of the luminal membrane surface and crypt epithelium (Fig. 3a). Bile canaliculi of the liver and proximal tubules of the kidneys were also strongly positive for CEACAM1a in +/+ mice (Fig. 3c and e). In contrast, in p/p mice, colonic epithelial cells showed fainter staining of the crypts. The low signal corroborated the immunoblotting analyses and could be explained by the expression of the two-Ig-domain-containing isoforms (Fig. 3b). This finding was confirmed by antibody dilution experiments, which indicated that a 20-fold dilution of the anti-CEACAM1 antibody could not detect CEACAM1a proteins in the p/p mouse colon, whereas positive staining was still observed in the colon of +/+ mice incubated with the same antibody dilution. The bile canaliculi of the p/p mouse hepatocytes showed much weaker labeling than that seen in the +/+ mice (Fig. 3d). The collecting tubules in the kidneys of p/p mice were faintly or not labeled with anti-CEACAM1a antibody (Fig. 3f). Other tissues that normally express CEACAM1a (small intestine, endometrium, ovary, prostate, stomach, spleen, thymus, and lungs) also displayed weaker or no immunostaining in p/p mice. No histological abnormalities were observed in any of these tissues.
FIG. 3.
CEACAM1 immunostaining of tissue sections from wild-type and p/p mice. Tissues were immunostained with CEACAM1-specific polyclonal antibody 231 and lightly counterstained with hematoxylin. (a, c, and e) Sections from +/+ mice. (b, d, and f) Sections from p/p mice. (a and b) Colon. Cross-sections of colonic crypts in p/p mice (b) have weaker CEACAM1 immunostaining of the luminal membrane (arrowheads) than those in +/+ mice (a). (c and d) Liver. Hepatocyte bile canaliculi contacts (arrowheads) are CEACAM1 positive in +/+ mice (c), whereas they express CEACAM1 only weakly or not at all in p/p mice (d). (e and f) Kidneys. Collecting tubules of the kidneys were strongly positive for CEACAM1 in +/+ mice (arrowheads) (e), whereas those of p/p mice were faint and generally negative (f). Magnification, approximately ×37.
It has recently been reported that CEACAM1 plays a role in the down-regulation of the cytolytic functions of activated human intestinal intraepithelial lymphocytes (47). Sections of intestines from Ceacam1aΔD1-4 +/+, +/p, or p/p mice were carefully examined by a pathologist (S.J.) and immunologists (Vladimir Baranov and Sten Hammarstrom, Umeå, Sweden) in order to detect any possible intestinal structural alterations or differences in CEACAM1a expression. The degrees of leukocyte infiltration in the lamina propria of the small and large intestines were similar in p/p and +/+ mice. No morphological changes were noted for liver or kidney tissues excised from either younger (9 weeks to 3 months) or older (5 months to 1.5 years) p/p mice.
p/p mice had no obvious morphological differences from +/+ mice and were equally fertile. No increase in the incidence of tumors relative to the data for +/+ mice was observed as p/p animals aged. Taken together, the studies described above show that the phenotypes of Ceacam1aΔD4 p/p mice are similar to those of normal, +/+ mice.
Biochemical analyses.
CEACAM1a is expressed in many tissues important for biochemical homeostasis, such as liver, kidneys, and the gastrointestinal tract. Because a decrease in the expression of major CEACAM1a isoforms might cause a physiological imbalance, biochemical markers in serum samples taken from either fasting or nonfasting animals were analyzed. Urinalysis was also performed. No significant changes were noted for characteristic enzyme markers, electrolytes, or glucose, cholesterol, or protein levels.
Hematological analyses.
CEACAM1a is expressed in a number of blood cells (platelets, macrophages, granulocytes, B lymphocytes, and activated T lymphocytes) (17, 27, 46). Because elimination or reduction of major CEACAM1a isoforms might cause immunological deficiencies, mice were subjected to hematological analyses. Blood samples from 10 different +/+, +/p, and p/p siblings were tested for percentages of mature blood cells as well as total numbers of different blood cell types. Parameters were similar when siblings were compared.
Susceptibility to MHV infection.
Because murine CEACAM1a isoforms are receptors for MHV (21, 22, 79), we assessed the susceptibility of Ceacam1a-targeted mice to MHV infection. Three-month-old p/p, +/p, and +/+ mice were inoculated intranasally with 104, 106, or 108 PFU of the hepatotropic MHV A59 strain/mouse. The mice were observed daily for the development of clinical signs of illness, such as lethargy, ruffled fur, dehydration and paresis. They were sacrificed 3 to 31 days postinoculation or immediately if showing significant distress. The histopathologic characteristics of the livers, the results of immunolabeling with antiviral antibody, and the production of infectious virus from the livers of +/+, +/p, and p/p mice inoculated with MHV A59 were compared.
Signs of illness were readily apparent in normal, +/+ BALB/c mice within 2 days after intranasal inoculation with 106 PFU/mouse. The animals were hunched, shivering, and lethargic and had ruffled fur. In marked contrast, p/p mice inoculated with even 108 PFU/mouse did not show signs of illness at up to 7 days after virus inoculation. +/p mice showed signs of virus disease at about 5 days after inoculation with 106 PFU/mouse, but most +/p mice did not succumb to infection. The +/+ mice inoculated with 106 PFU/mouse either died or had to be euthanatized for ethical reasons by day 3 or 4 after virus inoculation. Serum harvested at 31 days postinoculation from +/p and p/p mice inoculated with MHV at 106 or 108 PFU/mouse showed high titers of antiviral antibody in an enzyme-linked immunoassay.
Lesions observed in the livers of these animals correlated with the severity of clinical signs (Fig. 4). Livers of +/+ mice 3 days after inoculation with 106 PFU/mouse had small patches of normal hepatocytes scattered among large confluent lesions. There was extensive coagulative necrosis, with large areas of fibrin deposits, mild infiltration of mononuclear inflammatory cells, and scattered apoptotic cells (Fig. 4A and B). Livers of p/p mice 3 days after inoculation with 106 PFU/mouse (Fig. 4F and G) were mostly normal, with scattered small focal lesions that consisted of small aggregates of inflammatory cells. Cells expressing viral antigens were found at the periphery of the lesions (data not shown). Few apoptotic cells were present, and no large areas of necrosis were observed. Livers of +/p mice at 3 days after inoculation (Fig. 4C and D) had lesions that were intermediate in severity between those seen in the +/+ and p/p mouse livers. Figure 4E shows that, at 5 days after inoculation with 106 PFU/mouse, liver lesions in +/p mice had increased in size and developed large areas of necrosis, while liver lesions in p/p mice remained small (Fig. 4H).
FIG. 4.
Representative liver lesions of MHV A59-infected mice at 3 days after inoculation. +/+, +/p, and p/p mice were inoculated intranasally with 106 PFU of hepatotropic MHV A59 and sacrificed at 3 or 5 days postinoculation (dpi). (A and B) Very large areas of necrosis in the livers of +/+ mice. (C and D) Intermediate-sized lesions in the livers of +/p mice. (F and G) Scattered small aggregates of inflammatory cells (arrows) with no areas of hepatocyte necrosis in p/p mice. (E and H) +/p mice (E) and p/p mice (H) were inoculated intranasally with 106 PFU of MHV A59 and sacrificed at 5 dpi. At 5 dpi, areas of hepatocyte necrosis in +/p mice (F) had increased in size relative to the lesions at day 3 (C), while the lesions in p/p livers remained very small. Magnifications: A, C, E, F, and H, ×91; B, D, and G, approximately ×164.
Data on the number of lesions per section of liver are summarized in Fig. 5. Three experiments, done with an inoculum of 106 PFU/mouse (grouped together in the middle panel of Fig. 5), showed that +/+ mice had more lesions per unit area at 3 days after inoculation, after which they died. The +/p mice survived and developed a larger number of lesions at day 5 after inoculation. Lesions were resolving or gone in +/p mice by day 7. In marked contrast, p/p mice had many fewer lesions than +/+ or +/p animals, most appearing by day 5 and being resolved by day 7. Interestingly, the number of lesions in livers of p/p mice at day 5 after inoculation did not increase even with a dose of 108 PFU/mouse (Fig. 5, bottom panel). When +/+ mice were inoculated with a low dose, 104 PFU/mouse (Fig. 5, top panel), they survived longer than +/+ mice given 106 PFU/mouse, a lethal dose. At 104 PFU/mouse and 5 days after inoculation, +/p mice had few signs of illness and markedly fewer lesions than +/p animals inoculated with 106 PFU/mouse.
FIG. 5.
Quantitation of lesions per section of liver from MHV A59-inoculated mice. +/+, +/p, and p/p mice were inoculated intranasally with 104, 106, or 108 PFU of virus per mouse, and livers were harvested at 3, 5, or 7 days postinoculation. Several experiments are illustrated. The number of lesions per section of mouse liver is shown on the y axis. Each bar indicates the number of lesions per liver section harvested from one mouse at the time indicated on the x axis. The following were not done: +/+ mice inoculated with 104 PFU at day 7 and 108 PFU; and +/p mice inoculated with 104 and 108 PFU at day 7. +/+ animals inoculated with 106 PFU died or were euthanatized by day 3 or 4, whereas +/p and p/p mice survived. p/p mice were highly resistant to disease, even at doses of 108 PFU/mouse. Plus signs over the bars indicate livers that had more than 50 lesions. Asterisks on the x axis indicate no liver lesions.
Statistical analyses with a multinomial logistic regression model were carried out to evaluate the number of lesions obtained relative to inoculum, days postinoculation, and genotype. The number of lesions was found to depend only on genotype, with the p/p group used as the comparison category (model chi-square P, <0.0001). The variables corresponding to inoculum and number of days were not found to significantly affect the number of lesions. The model suggests that the genotype +/+ (P = 0.014) and +/p (P = 0.008) mice significantly more often experience a high number of lesions (as defined in Materials and Methods) than do the genotype p/p mice.
The small size and low number of lesions observed in the livers of p/p mice at 5 days after inoculation with 106 PFU/mouse correlated with the relatively low yield of infectious virus. At 5 days after inoculation with 106 PFU/mouse, p/p mice produced, on average, 410 PFU of infectious particles per g of liver. The livers of +/p mice at 5 days after inoculation had much larger lesions than those of p/p mice (Fig. 4) and had virus yields ranging from 850 to 120,000 PFU/g, with an average of 22,100 PFU/g. +/+ mice inoculated with 106 PFU/mouse produced approximately 30,000 PFU/g of liver at day 3, and all animals were dead at day 5. Infectious virus was not recovered from the livers of any of the surviving experimental animals on day 7 after inoculation, even in animals given 108 PFU/mouse. Thus, altering the concentrations and isoforms of MHV receptors expressed at the surface of hepatocytes in vivo greatly reduced the susceptibility of the hepatocytes to infection, reduced the yield of infectious virus in the liver, and ultimately reduced the severity of disease.
DISCUSSION
Three targeting vectors were used to abrogate the expression of the Ceacam1a gene. These were based on the insertion of stop codons within exon 2 and replacement by the neor selection cassette of most or all of exon 2 (enclosing the D1 domain of the protein). It is unclear at present why this approach was problematic. One possibility is that the genomic DNA of the targeting construct was isolated from a library produced from D3 ES cells. However, three independent ES cell lines (J1, RW4, and R1) were transfected with these constructs, without success. As we have recently generated ES cells with a complete knockout of Ceacam1a expression using a different targeting construct that nonetheless was engineered with the same D3 genomic DNA (N. Beauchemin et al., unpublished observations), we postulate that higher-order genomic structures are likely to exist within intron 1 or exon 2 of the Ceacam1a gene and that they prevent efficient recombination within this region.
Extensive characterization of the phenotypes of the homozygous (p/p) and heterozygous (+/p) Ceacam1aΔ4D mice bred in the backgrounds of two different inbred mouse lines (BALB/c and C57BL/6) has so far failed to reveal any significant structural or physiological differences between these and normal, +/+ animals. Thus, although the total amounts of the two CEACAM1a/D1-4 isoforms are decreased by about 90% in p/p animals, the residual CEACAM1a/D1-4 proteins and/or the slightly increased levels of the two CEACAM1a/D1,4 isoforms are probably able to provide sufficient amounts of CEACAM1 proteins to accomplish the essential tasks of these glycoproteins during development.
Most experiments on the functions of CEACAM1 proteins have been done using heterologous cell lines transfected with cDNAs encoding only a single CEACAM1 isoform. Yet, because CEACAM1 isoforms are expressed on most adherent cell lines, it is likely that the transfected cells used to express a recombinant CEACAM1a glycoprotein simultaneously express homologous CEACAM glycoproteins encoded by their own genomes. Indeed, most mouse cell lines coexpress more than one isoform of CEACAM1a, and these may be found on the membranes as monomers, homodimers, or heterodimers (36). The sites on the cell membrane where the CEACAM1 isoforms are expressed differ considerably from one cell type to another and may vary with the physiological state of the cell (61). Most studies on functions of CEACAM1 proteins have focused either on the functions of the exodomains or the functions of the cytoplasmic tails. There is as yet little information about the relative levels of expression and the functions of each of the four isoforms in different murine cell types and tissues.
Studies on cultured cell lines have shown that the level of expression of a virus receptor can affect the susceptibility of the cells to virus infection. In vivo, several experiments of nature suggest that reduced levels of expression of a virus receptor or coreceptor can also reduce susceptibility to virus infection and disease. For example, globoside is a receptor for human parvovirus B19, and individuals who do not express this receptor are profoundly resistant to B19 infection (13). In addition, humans who are heterozygous for a mutant CCR-5 delta 32 allele that encodes a defective chemokine coreceptor for human immunodeficiency virus type 1 show increased resistance to virus infection and a decreased rate of disease progression (1). Experimentally, the biological significance of reduced levels of receptor expression as a determinant of virus susceptibility has been explored by using monoclonal antibodies to the receptor to block infection and also by constructing transgenic mice that express different levels of a human receptor for a specific virus and comparing the specific infectivities of the virus for the different transgenic lines (54). To our knowledge, this study is the first example of gene manipulation to explore the significance for virus susceptibility of reduced levels or altered ratios of splice variants of a virus receptor in the natural host of the virus.
Comparison of MHV A59 infection of the Ceacam1aΔ4D p/p, +/p, and +/+ mice shows that the reduced expression and/or altered ratio of splice isoforms in the apparently healthy and phenotypically normal p/p mice have rendered the animals highly resistant to even very high doses (108 PFU/mouse) of virulent, hepatotropic MHV A59 delivered by a natural (intranasal) route of inoculation. These p/p animals develop fewer lesions than +/p or +/+ animals, have much smaller lesions, with correspondingly less liver damage and lower titers of virus in the liver, and show little clinical evidence of virus infection. Perhaps the transmission of MHV infection from a p/p mouse with a brief, inapparent infection to another p/p mouse would also be reduced due to the low titers of virus produced in p/p mice.
The mechanism(s) by which this manipulation of the Ceacam1a gene makes the p/p mice resistant to MHV disease is not yet clear. Following intranasal inoculation with virus, the virus first replicates locally in epithelial cells at the site of inoculation and then spreads to other tissues along nerves and/or through the bloodstream either as free virions or in infected leukocytes (6, 41). To reach the liver, virus from the blood would likely infect Kupffer cells and/or endothelial cells and spread to hepatocytes, causing expanding focal lesions. The diameter of the lesions in p/p mice was markedly smaller than that in +/+ mice or +/p mice, and the lesions in the livers of p/p mice expanded very little in diameter from day 3 to day 5 postinoculation; however, liver lesions in +/p mice expanded markedly from day 3 to day 5 (Fig. 4). The lower level and/or altered ratio of isoforms of CEACAM1a in the p/p hepatocytes probably limit the spread of viral infection from hepatocyte to hepatocyte as well as the spread of virus to the liver. Another factor that may contribute to the larger liver lesions observed in +/+ and +/p mice is induction by MHV A59 of monocyte procoagulant activity, a prothrombinase encoded by the fgl2 gene (45). Similar very large necrotic lesions in the liver are seen in MHV strain 3-induced fulminant hepatitis, and the pathogenesis of these lesions has been shown to be due to the induction of fgl2 expression by MHV strain 3 infection (20). The expression of fgl2 leads to microthrombus formation and hypoxia in the liver, rapidly followed by extensive necrosis and death. Perhaps because the yield of MHV A59 in the livers of infected p/p mice is much lower than the yield of virus in +/+ and +/p mice, p/p mice may express less fgl2 than infected +/+ or +/p mice and consequently have smaller liver lesions.
To study the effects of altered CEACAM1a expression on the immune response to MHV infection, we plan to cross p/p mice with mouse strains that have known immunodeficiencies and then evaluate changes in the pathogenesis of MHV in the offspring. In addition, we plan to evaluate the effects of age of the mouse, strain of the virus, and route of inoculation upon the pathogenesis of hepatitis in +/+, +/p, and p/p mice.
As far as we know, p/p animals are normal except that they are highly resistant to MHV infection. Transgenic plants that are resistant to multiple plant viruses have been engineered based on transgenically expressed viral proteins and/or posttranscriptional gene silencing (8, 66). Genetically engineered, disease-resistant crops and animals can have substantial economic impact. MHV causes frequent epizootics in colonies of laboratory mice, and the virus can be persistently shed by immunosuppressed animals (16). Because inapparent MHV infection can alter the normal responses of mice to a variety of experimental procedures and cause death of some immunosuppressed animals, laboratory mouse colonies must invest in expensive surveillance programs to detect MHV infection. If an epizootic is detected, to eliminate the virus from infected colonies, breeding must cease, and the importation of new, susceptible animals into a colony is prohibited for months. Valuable, irreplaceable mouse strains infected with MHV must be rederived by cesarean delivery, and sometimes all mice in an infected colony must be euthanatized. Numerous strains of MHV that elicit strain-specific immune responses have been detected in mouse colonies, and animals are susceptible to repeated infections with different strains (16, 33). All MHV strains appear to utilize CEACAM1a isoforms as their principal receptors. Therefore, a strategy designed to reduce the availability of the receptor glycoproteins in inbred mice appears more likely to prevent epizootics of MHV than other approaches, such as immunization. This article demonstrates the feasibility of genetically engineering inbred strains of mice for increased resistance to MHV, without introducing detectable changes in the development, physiology, fecundity, or longevity of the inbred mice.
ACKNOWLEDGMENTS
Dianna M. Blau and Claire Turbide contributed equally to this work.
We are grateful to Bonnie Bullis for technical assistance, to Christian Benoît and Diane Mathis for providing the 129Sv genomic library, to Janet Stephens (University of Colorado Health Sciences Center) and Julian Leibowitz (Texas A & M University) for helpful discussions, and to Fabrice Rouah (Department of Mathematics, McGill University) for statistical analyses. We express our sincere appreciation to the following collaborators: Sten Hammarström and Vladimir Baranov (Umea, Sweden), the staff of the McGill Animal Care Facility (Randy Gullbrandsen, Maria Ribeiro, Hélène Ste-Croix, Lynn Matsumiya, and Richard Latt), and the staff of the University of Colorado Health Sciences Center Animal Facility.
This work was supported by the Canadian Institutes for Health Research (grant MOP42501 to N.B.) and the National Institutes of Health (grant AI25231 to K.V.H.). Dianna M. Blau was supported by a training grant from the National Institutes of Health (T32 AI07537), and Stéphanie Létourneau was supported by a training studentship from the Fonds de la Recherche en Santé du Québec. Michel Tremblay is a scientist supported by the Medical Research Council of Canada, and Nicole Beauchemin is a senior scientist at the Fonds de la Recherche en Santé du Québec.
REFERENCES
- 1.Balfe P, Churcher Y, Penny M, Easterbrook P J, Goodall R L, Galpin S, Gotch F, Daniels R S, McKeating J A. Association between a defective CCR-5 gene and progression to disease in HIV infection. AIDS Res Hum Retrovir. 1998;14:1229–1234. doi: 10.1089/aid.1998.14.1229. [DOI] [PubMed] [Google Scholar]
- 2.Baric R S, Sullivan E, Hensley L, Yount B, Chen W. Persistent infection promotes cross-species transmissibility of mouse hepatitis virus. J Virol. 1999;73:638–649. doi: 10.1128/jvi.73.1.638-649.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baric R S, Yount B, Hensley L, Peel S A, Chen W. Episodic evolution mediates interspecies transfer of a murine coronavirus. J Virol. 1997;71:1946–1955. doi: 10.1128/jvi.71.3.1946-1955.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnett T R, Kretschmer A, Austen D A, Goebel S J, Hart J T, Elting J J, Kamarck M E. Carcinoembryonic antigens: alternative splicing accounts for the multiple mRNAs that code for novel members of the carcinoembryonic antigen family. J Cell Biol. 1989;108:267–276. doi: 10.1083/jcb.108.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barthold S W. Mouse hepatitis virus biology and epizootiology. In: Bhatt P N, Jacoby R O, Morse III H C, New A E, editors. Viral and mycoplasmal infections of laboratory rodents. Effects on biomedical research. Orlando, Fla: Academic Press, Inc.; 1986. pp. 571–601. [Google Scholar]
- 6.Barthold S W. Olfactory neural pathway in mouse hepatitis virus nasoencephalitis. Acta Neuropathol (Berlin) 1988;76:502–506. doi: 10.1007/BF00686390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barthold S W, Smith A L. Response of genetically susceptible and resistant mice to intranasal inoculation with mouse hepatitis virus JHM. Virus Res. 1987;7:225–239. doi: 10.1016/0168-1702(87)90030-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baulcombe D. Viruses and gene silencing in plants. Arch Virol Suppl. 1999;15:189–201. doi: 10.1007/978-3-7091-6425-9_14. [DOI] [PubMed] [Google Scholar]
- 9.Beauchemin N, Chen T, Draber P, Dveksler G, Gold P, Gray-Owen S, Grunert F, Hammarström S, Holmes K V, Karlson A, Kuroki M, Lin S H, Lucka L, Najjar S M, Neumaier M, Öbrink B, Shively J E, Skubitz K M, Stanners C P, Thomas P, Thompson J A, Virji M, von Kleist S, Wagener C, Watt S, Zimmermann W. Redefined nomenclature for members of the carcinoembryonic antigen family. Exp Cell Res. 1999;252:243–249. doi: 10.1006/excr.1999.4610. [DOI] [PubMed] [Google Scholar]
- 10.Beauchemin N, Kunath T, Robitaille J, Chow B, Turbide C, Daniels E, Veillette A. Association of biliary glycoprotein with protein tyrosine phosphatase SHP-1 in malignant colon epithelial cells. Oncogene. 1997;14:783–790. doi: 10.1038/sj.onc.1200888. [DOI] [PubMed] [Google Scholar]
- 11.Beauchemin N, Lin S H. Role of C-CAM as a tumor suppressor. In: Stanners C P, editor. Cell adhesion and communication mediated by the CEA family. Amsterdam, The Netherlands: Harwood Academic Publishers; 1998. pp. 155–175. [Google Scholar]
- 12.Bradley A. Production and analysis of chimeric mice. In: Robertson E J, editor. Teratocarcinomas and embryonic stem cells: a practical approach. Oxford, England: IRL Press; 1987. p. 113-152. [Google Scholar]
- 13.Brown K E, Hibbs J R, Gallinella G, Anderson S M, Lehman E D, McCarthy P, Young N S. Resistance to parvovirus B19 infection due to lack of virus receptor (erythrocyte P antigen) N Engl J Med. 1994;330:1192–1196. doi: 10.1056/NEJM199404283301704. [DOI] [PubMed] [Google Scholar]
- 14.Chen W, Baric R S. Molecular anatomy of mouse hepatitis virus persistence: coevolution of increased host cell resistance and virus virulence. J Virol. 1996;70:3947–3960. doi: 10.1128/jvi.70.6.3947-3960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Compton S R. Enterotropic strains of mouse coronavirus differ in their use of murine carcinoembryonic antigen-related glycoprotein receptors. Virology. 1994;203:197–201. doi: 10.1006/viro.1994.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Compton S R, Barthold S W, Smith A L. The cellular and molecular pathogenesis of coronaviruses. Lab Anim Sci. 1993;43:15–28. . (Erratum, 43:203.) [PubMed] [Google Scholar]
- 17.Coutelier J P, Godfraind C, Dveksler G S, Wysocka M, Cardellichio C B, Noel H, Holmes K V. B lymphocyte and macrophage expression of carcinoembryonic antigen-related adhesion molecules that serve as receptors for murine coronavirus. Eur J Immunol. 1994;24:1383–1390. doi: 10.1002/eji.1830240622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cray C, Mateo M O, Altman N H. In vitro and long-term in vivo immune dysfunction after infection of BALB/c mice with mouse hepatitis virus strain A59. Lab Anim Sci. 1993;43:169–174. [PubMed] [Google Scholar]
- 19.Daniels E, Létourneau S, Turbide C, Kuprina N, Rudinskaya T, Yazova A C, Holmes K V, Dveksler G S, Beauchemin N. Biliary glycoprotein 1 expression during embryogenesis: correlation with events of epithelial differentiation, mesenchymal-epithelial interactions, absorption, and myogenesis. Dev Dyn. 1996;206:272–290. doi: 10.1002/(SICI)1097-0177(199607)206:3<272::AID-AJA5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 20.Ding J W, Ning Q, Liu M F, Lai A, Leibowitz J, Peltekian K M, Cole E H, Fung L S, Holloway C, Marsden P A, Yeger H, Phillips M J, Levy G A. Fulminant hepatic failure in murine hepatitis virus strain 3 infection: tissue-specific expression of a novel fgl2 prothrombinase. J Virol. 1997;71:9223–9230. doi: 10.1128/jvi.71.12.9223-9230.1997. . (Erratum, 72:3504, 1998.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dveksler G S, Dieffenbach C W, Cardellichio C B, McCuaig K, Pensiero M N, Jiang G S, Beauchemin N, Holmes K V. Several members of the mouse carcinoembryonic antigen-related glycoprotein family are functional receptors for the coronavirus mouse hepatitis virus-A59. J Virol. 1993;67:1–8. doi: 10.1128/jvi.67.1.1-8.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dveksler G S, Pensiero M N, Cardellichio C B, Williams R K, Jiang G S, Holmes K V, Dieffenbach C W. Cloning of the mouse hepatitis virus (MHV) receptor: expression in human and hamster cell lines confers susceptibility to MHV. J Virol. 1991;65:6881–6891. doi: 10.1128/jvi.65.12.6881-6891.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dveksler G S, Pensiero M N, Dieffenbach C W, Cardellichio C B, Basile A A, Elia P E, Holmes K V. Mouse hepatitis virus strain A59 and blocking antireceptor monoclonal antibody bind to the N-terminal domain of cellular receptor. Proc Natl Acad Sci USA. 1993;90:1716–1720. doi: 10.1073/pnas.90.5.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Edlund M, Gaardsvoll H, Bock E, Öbrink B. Different isoforms and stock-specific variants of the cell adhesion molecule C-CAM (cell-CAM 105) in rat liver. Eur J Biochem. 1993;213:1109–1116. doi: 10.1111/j.1432-1033.1993.tb17860.x. [DOI] [PubMed] [Google Scholar]
- 25.Ergün S, Kilik N, Ziegeler G, Hansen A, Nollau P, Gotze J, Wurmbach J H, Horst A, Weil J, Fernando M, Wagener C. CEA-related cell adhesion molecule 1: a potent angiogenic factor and a major effector of vascular endothelial growth factor. Mol Cell. 2000;5:311–320. doi: 10.1016/s1097-2765(00)80426-8. [DOI] [PubMed] [Google Scholar]
- 26.Frana M F, Behnke J N, Sturman L S, Holmes K V. Proteolytic cleavage of the E2 glycoprotein of murine coronavirus: host-dependent differences in proteolytic cleavage and cell fusion. J Virol. 1985;56:912–920. doi: 10.1128/jvi.56.3.912-920.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Godfraind C, Langreth S G, Cardellichio C B, Knobler R, Coutelier J P, Dubois-Dalcq M, Holmes K V. Tissue and cellular distribution of an adhesion molecule in the carcinoembryonic antigen family that serves as a receptor for mouse hepatitis virus. Lab Investig. 1995;73:615–627. [PubMed] [Google Scholar]
- 28.Gombold J L, Hingley S T, Weiss S R. Identification of peplomer cleavage site mutations arising during persistence of MHV-A59. Adv Exp Med Biol. 1993;342:157–163. doi: 10.1007/978-1-4615-2996-5_25. [DOI] [PubMed] [Google Scholar]
- 29.Gray-Owen S D, Dehio C, Haude A, Grunert F, Meyer T F. CD66 carcinoembryonic antigens mediate interactions between Opa-expressing Neisseria gonorrhoeae and human polymorphonuclear phagocytes. EMBO J. 1997;16:3435–3445. doi: 10.1093/emboj/16.12.3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hammarström S, Olsen A, Teglund S, Baranov V. The nature and expression of the human CEA family. In: Stanners C P, editor. Cell adhesion and communication mediated by the CEA family. Amsterdam, The Netherlands: Harwood Academic Publishers; 1998. pp. 1–30. [Google Scholar]
- 31.Hauck C R, Meyer T F, Lang F, Gulbins E. CD66-mediated phagocytosis of Opa52 Neisseria gonorrhoeae requires a Src-like tyrosine kinase- and Rac1-dependent signalling pathway. EMBO J. 1998;17:443–454. doi: 10.1093/emboj/17.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hauck W, Nédellec P, Turbide C, Stanners C P, Barnett T R, Beauchemin N. Transcriptional control of the human biliary glycoprotein gene, a CEA gene family member down-regulated in colorectal carcinomas. Eur J Biochem. 1994;223:529–541. doi: 10.1111/j.1432-1033.1994.tb19022.x. [DOI] [PubMed] [Google Scholar]
- 33.Homberger F R, Zhang L, Barthold S W. Prevalence of enterotropic and polytropic mouse hepatitis virus in enzootically infected mouse colonies. Lab Anim Sci. 1998;48:50–54. [PubMed] [Google Scholar]
- 34.Hsieh J T, Luo W, Song W, Wang Y, Kleinerman D I, Van N T, Lin S H. Tumor suppressive role of an androgen-regulated epithelial cell adhesion molecule (C-CAM) in prostate carcinoma cell revealed by sense and antisense approaches. Cancer Res. 1995;55:190–197. [PubMed] [Google Scholar]
- 35.Huber M, Izzi L, Grondin P, Houde C, Kunath T, Veillette A, Beauchemin N. The carboxyl-terminal region of biliary glycoprotein controls its tyrosine phosphorylation and association with protein-tyrosine phosphatases SHP-1 and SHP-2 in epithelial cells. J Biol Chem. 1999;274:335–344. doi: 10.1074/jbc.274.1.335. [DOI] [PubMed] [Google Scholar]
- 36.Hunter I, Sawa H, Edlund M, Öbrink B. Evidence for regulated dimerization of cell-cell adhesion molecule (C-CAM) in epithelial cells. Biochem J. 1996;320:847–853. doi: 10.1042/bj3200847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Izzi L, Turbide C, Houde C, Kunath T, Beauchemin N. cis-Determinants in the cytoplasmic domain of CEACAM1 responsible for its tumor inhibitory function. Oncogene. 1999;18:5563–5572. doi: 10.1038/sj.onc.1202935. [DOI] [PubMed] [Google Scholar]
- 38.Keck U, Nédellec P, Beauchemin N, Thompson J, Zimmermann W. The cea10 gene encodes a secreted member of the murine carcinoembryonic antigen family and is expressed in the placenta, gastrointestinal tract and bone marrow. Eur J Biochem. 1995;229:455–464. doi: 10.1111/j.1432-1033.1995.0455k.x. [DOI] [PubMed] [Google Scholar]
- 39.Knobler R L, Haspel M V, Oldstone M B. Mouse hepatitis virus type 4 (JHM strains) induced fatal central nervous system disease. I. genetic control and murine neuron as the susceptible site of disease. J Exp Med. 1981;153:832–843. doi: 10.1084/jem.153.4.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunath T, Ordoñez-Garcia C, Turbide C, Beauchemin N. Inhibition of colonic tumor cell growth by biliary glycoprotein. Oncogene. 1995;11:2375–2382. [PubMed] [Google Scholar]
- 41.Lavi E, Fishman P S, Highkin M K, Weiss S R. Limbic encephalitis after inhalation of a murine coronavirus. Lab Investig. 1988;58:31–36. [PubMed] [Google Scholar]
- 42.Luo W, Earley K, Tantingco V, Hixson D C, Liang T C, Lin S H. Association of an 80 kDa protein with C-CAM1 cytoplasmic domain correlates with C-CAM1-mediated growth inhibition. Oncogene. 1998;16:1141–1147. doi: 10.1038/sj.onc.1201619. [DOI] [PubMed] [Google Scholar]
- 43.McCuaig K, Rosenberg M, Nédellec P, Turbide C, Beauchemin N. Expression of the Bgp gene and characterization of mouse colon biliary glycoprotein isoforms. Gene. 1993;127:173–183. doi: 10.1016/0378-1119(93)90716-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McCuaig K, Turbide C, Beauchemin N. mmCGM1a: a mouse carcinoembryonic antigen gene family member, generated by alternative splicing, functions as an adhesion molecule. Cell Growth Differ. 1992;3:165–174. [PubMed] [Google Scholar]
- 45.McGilvray I D, Lu Z, Wei A C, Dackiw A P, Marshall J C, Kapus A, Levy G, Rotstein O D. Murine hepatitis virus strain 3 induces the macrophage prothrombinase fgl-2 through p38 mitogen-activated protein kinase activation. J Biol Chem. 1998;273:32222–32229. doi: 10.1074/jbc.273.48.32222. [DOI] [PubMed] [Google Scholar]
- 46.Möller M J, Kammerer R, Grunert F, von Kleist S. Biliary glycoprotein (BGP) expression on T cells and on a natural-killer-cell sub-population. Int J Cancer. 1996;65:740–745. doi: 10.1002/(SICI)1097-0215(19960315)65:6<740::AID-IJC5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 47.Morales V M, Christ A, Watt S M, Kim H S, Johnson K W, Utku N, Texieira A M, Mizoguchi A, Mizoguchi E, Russell G J, Russell S E, Bhan A K, Freeman G J, Blumberg R S. Regulation of human intestinal intraepithelial lymphocyte cytolytic function by biliary glycoprotein (CD66a) J Immunol. 1999;163:1363–1370. [PubMed] [Google Scholar]
- 48.Nédellec P, Dveksler G S, Daniels E, Turbide C, Chow B, Basile A A, Holmes K V, Beauchemin N. Bgp2, a new member of the carcinoembryonic antigen-related gene family, encodes an alternative receptor for mouse hepatitis viruses. J Virol. 1994;68:4525–4537. doi: 10.1128/jvi.68.7.4525-4537.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nédellec P, Turbide C, Beauchemin N. Characterization and transcriptional activity of the mouse biliary glycoprotein 1 gene, a carcinoembryonic antigen-related gene. Eur J Biochem. 1995;231:104–114. doi: 10.1111/j.1432-1033.1995.tb20676.x. [DOI] [PubMed] [Google Scholar]
- 50.Öbrink B. C-CAM (cell-CAM 105)–a member of the growing immunoglobulin superfamily of cell adhesion proteins. Bioessays. 1991;13:227–234. doi: 10.1002/bies.950130505. [DOI] [PubMed] [Google Scholar]
- 51.Öbrink B. CEA adhesion molecules: multifunctional proteins with signal-regulatory properties. Curr Opin Cell Biol. 1997;9:616–626. doi: 10.1016/S0955-0674(97)80114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ocklind C, Odin P, Öbrink B. Two different cell adhesion molecules—cell-CAM 105 and a calcium-dependent protein—occur on the surface of rat hepatocytes. Exp Cell Res. 1984;151:29–45. doi: 10.1016/0014-4827(84)90353-7. [DOI] [PubMed] [Google Scholar]
- 53.Prall F, Nollau P, Neumaier M, Haubeck H D, Drzeniek Z, Helmchen U, Loning T, Wagener C. CD66a (BGP), an adhesion molecule of the carcinoembryonic antigen family, is expressed in epithelium, endothelium, and myeloid cells in a wide range of normal human tissues. J Histochem Cytochem. 1996;44:35–41. doi: 10.1177/44.1.8543780. [DOI] [PubMed] [Google Scholar]
- 54.Racaniello V R, Ren R, Bouchard M. Poliovirus attenuation and pathogenesis in a transgenic mouse model for poliomyelitis. Dev Biol Stand. 1993;78:109–116. [PubMed] [Google Scholar]
- 55.Rao P V, Gallagher T M. Mouse hepatitis virus receptor levels influence virus-induced cytopathology. Adv Exp Med Biol. 1998;440:549–555. doi: 10.1007/978-1-4615-5331-1_71. [DOI] [PubMed] [Google Scholar]
- 56.Rao P V, Gallagher T M. Intracellular complexes of viral spike and cellular receptor accumulate during cytopathic murine coronavirus infections. J Virol. 1998;72:3278–3288. doi: 10.1128/jvi.72.4.3278-3288.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rao P V, Kumari S, Gallagher T M. Identification of a contiguous 6-residue determinant in the MHV receptor that controls the level of virion binding to cells. Virology. 1997;229:336–348. doi: 10.1006/viro.1997.8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rettenberger G, Zimmermann W, Klett C, Zechner U, Hameister H. Mapping of murine YACs containing the genes Cea2 and Cea4 after B1-PCR amplification and FISH-analysis. Chromosome Res. 1995;3:473–478. doi: 10.1007/BF00713961. [DOI] [PubMed] [Google Scholar]
- 59.Rojas M, deMarte D L, Screaton R A, Stanners C P. Radical differences in functions of closely related members of the human carcinoembryonic antigen gene family. Cell Growth Differ. 1996;7:655–662. [PubMed] [Google Scholar]
- 60.Rosenberg M, Nédellec P, Jothy S, Fleiszer D, Turbide C, Beauchemin N. The expression of mouse biliary glycoprotein, a carcinoembryonic antigen-related gene, is down-regulated in malignant mouse tissues. Cancer Res. 1993;53:4938–4945. [PubMed] [Google Scholar]
- 61.Sadekova S, Lamarche-Vane N, Li X, Beauchemin N. The CEACAM1-L glycoprotein associates with the actin cytoskeleton and localizes to cell-cell contact through activation of Rho-like GTPases. Mol Biol Cell. 2000;11:65–77. doi: 10.1091/mbc.11.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sawa H, Kamada K, Sato H, Sendo S, Kondo A, Saito I, Edlund M, Öbrink B. C-CAM expression in the developing rat central nervous system. Mol Brain Res. 1994;78:35–43. doi: 10.1016/0165-3806(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 63.Sawicki S G, Lu J H, Holmes K V. Persistent infection of cultured cells with mouse hepatitis virus (MHV) results from the epigenetic expression of the MHV receptor. J Virol. 1995;69:5535–5543. doi: 10.1128/jvi.69.9.5535-5543.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schickli J H, Zelus B D, Wentworth D E, Sawicki S G, Holmes K V. The murine coronavirus mouse hepatitis virus strain A59 from persistently infected murine cells exhibits an extended host range. J Virol. 1997;71:9499–9507. doi: 10.1128/jvi.71.12.9499-9507.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Smith A L, Cardellichio C B, Winograd D F, de Souza M S, Barthold S W, Holmes K V. Monoclonal antibody to the receptor for murine coronavirus MHV-A59 inhibits viral replication in vivo. J Infect Dis. 1991;163:879–882. doi: 10.1093/infdis/163.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Song E K, Koh H K, Kim J K, Lee S Y. Genetically engineered transgenic plants with the domain 1 sequence of tobacco mosaic virus 126 kDa protein gene are completely resistant to viral infection. Mol Cell. 1999;9:569–575. [PubMed] [Google Scholar]
- 67.Stohlman S A, Frelinger J A. Resistance to fatal central nervous system disease by mouse hepatitis virus strain JHM. I. Genetic analysis. Immunogenetics. 1978;6:277–281. [Google Scholar]
- 68.Svalander P C, Odin P, Nilsson B O, Öbrink B. Expression of cellCAM-105 in the apical surface of rat uterine epithelium is controlled by ovarian steroid hormones. J Reprod Fertil. 1990;88:213–221. doi: 10.1530/jrf.0.0880213. [DOI] [PubMed] [Google Scholar]
- 69.Thomas K R, Capecchi M R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- 70.Thompson J A, Grunert F, Zimmermann W. Carcinoembryonic antigen gene family: molecular biology and clinical perspectives. J Clin Lab Anal. 1991;5:344–366. doi: 10.1002/jcla.1860050510. [DOI] [PubMed] [Google Scholar]
- 71.Turbide C, Rojas M, Stanners C P, Beauchemin N. A mouse carcinoembryonic antigen gene family member is a calcium-dependent cell adhesion molecule. J Biol Chem. 1991;266:309–315. [PubMed] [Google Scholar]
- 72.Virji M, Evans D, Griffith J, Hill D, Serino L, Hadfield A, Watt S M. Carcinoembryonic antigens are targeted by diverse strains of typable and nontypable Haemophilus influenzae. Mol Microbiol. 2000;36:784–795. doi: 10.1046/j.1365-2958.2000.01885.x. [DOI] [PubMed] [Google Scholar]
- 73.Virji M, Makepeace K, Ferguson D J, Watt S M. Carcinoembryonic antigens (CD66) on epithelial cells and neutrophils are receptors for Opa proteins of pathogenic neisseriae. Mol Microbiol. 1996;22:941–950. doi: 10.1046/j.1365-2958.1996.01551.x. [DOI] [PubMed] [Google Scholar]
- 74.Virji M, Watt S M, Barker S, Makepeace K, Doyonnas R. The N-domain of the human CD66a adhesion molecule is a target for Opa proteins of Neisseria meningitidis and Neisseria gonorrhoeae. Mol Microbiol. 1996;22:929–939. doi: 10.1046/j.1365-2958.1996.01548.x. [DOI] [PubMed] [Google Scholar]
- 75.Wessner D R, Shick P C, Lu J H, Cardellichio C B, Gagneten S E, Beauchemin N, Holmes K V, Dveksler G S. Mutational analysis of the virus and monoclonal antibody binding sites in MHVR, the cellular receptor of the murine coronavirus mouse hepatitis virus strain A59. J Virol. 1998;72:1941–1948. doi: 10.1128/jvi.72.3.1941-1948.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Williams R K. Receptor for mouse hepatitis virus is a member of the carcinoembryonic antigen family of glycoproteins. Proc Natl Acad Sci USA. 1991;88:5533–5536. doi: 10.1073/pnas.88.13.5533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams R K, Jiang G S, Snyder S W, Frana M F, Holmes K V. Purification of the 110-kilodalton glycoprotein receptor for mouse hepatitis virus (MHV) A59 from mouse liver and identification of a nonfunctional, homologous protein in MHV-resistant SJL/J mice. J Virol. 1990;64:3817–3823. doi: 10.1128/jvi.64.8.3817-3823.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yokomori K, Lai M M. The receptor for mouse hepatitis virus in the resistant mouse strain SJL is functional: implications for the requirement of a second factor for viral infection. J Virol. 1992;66:6931–6938. doi: 10.1128/jvi.66.12.6931-6938.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yokomori K, Lai M M. Mouse hepatitis virus receptors: more than a single carcinoembryonic antigen. Arch Virol Suppl. 1994;9:461–471. doi: 10.1007/978-3-7091-9326-6_45. [DOI] [PubMed] [Google Scholar]
- 80.Zelus B D, Wessner D R, Williams R K, Pensiero M N, Phibbs F T, deSouza M, Dveksler G S, Holmes K V. Purified, soluble recombinant mouse hepatitis virus receptor, Bgp1(b), and Bgp2 murine coronavirus receptors differ in mouse hepatitis virus binding and neutralizing activities. J Virol. 1998;72:7237–7244. doi: 10.1128/jvi.72.9.7237-7244.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zimmermann W. The nature and expression of the rodent CEA families: evolutionary considerations. In: Stanners C P, editor. Cell adhesion and communication mediated by the CEA family. Amsterdam, The Netherlands: Harwood Academic Publishers; 1998. pp. 31–56. [Google Scholar]