Abstract
Artificial intelligence (AI) is increasingly becoming integral to medical practice, potentially enhancing outcomes in thoracic surgery. AI-driven models have shown significant accuracy in diagnosing non-small-cell lung cancer (NSCLC), predicting lymph node metastasis, and aiding in the efficient extraction of electronic medical record (EMR) data. Moreover, AI applications in robotic-assisted thoracic surgery (RATS) and perioperative management reveal the potential to improve surgical precision, patient safety, and overall care efficiency. Despite these advancements, challenges such as data privacy, biases, and ethical concerns remain. This manuscript explores AI applications, particularly machine learning (ML) and natural language processing (NLP), in thoracic surgery, emphasizing their role in diagnosis and perioperative management. It also provides a comprehensive overview of the current state, benefits, and limitations of AI in thoracic surgery, highlighting future directions in the field.
Keywords: thoracic surgery, AI, artificial intelligence, VATS, RATS, precision medicine, precision surgery
1. Introduction
Artificial intelligence is increasingly becoming an integral part of medical practice, improving patient and healthcare team outcomes, lowering costs, and influencing public health [1]. Surgery is predicted to be impacted by current advancements in AI; however, due to several drawbacks, it has not yet reached its full potential in the discipline. The amount of data generated today outpaces the human ability to handle it cognitively, and AI is poised to play a significant role in supporting the provision of tailored healthcare (Figure 1 and Figure 2). For instance, current advances in AI have demonstrated great levels of accuracy in imaging and signal detection tasks. AI technologies in thoracic surgery are proving to be valuable in precise diagnoses, improving treatment planning, and optimizing postoperative care mainly using machine learning (ML) and natural language processing (NLP) techniques [2]. NLP is revolutionizing how clinical information is processed and analyzed. Additionally, ML algorithms are increasingly being used for in-depth analysis of imaging data, aiding in the lung cancer diagnosis and classification [3]. These result in numerous advantages, including reduced medical errors and quicker, more efficient patient care [3,4]. Certain challenges, however, have been noted, some of which include limited generalizability, data privacy concerns, the need for well-annotated and homogenous datasets for training, potential biases, and ethical considerations [2]. This article explores the current state of AI in thoracic surgery, covering diagnosis, operative planning, and postoperative management, while highlighting AI’s potential advantages and limitations.
Figure 1.
Subcategories of artificial intelligence [5].
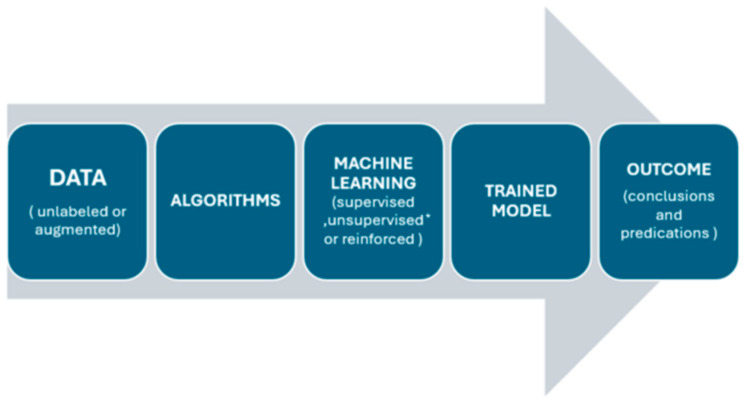
Figure 2.
Process and steps in artificial intelligence [5]. * Unsupervised methods are used for pattern recognition and clustering rather than generating predictive models.
2. Methodology
A comprehensive search was performed using the PubMed/MEDLINE, Cochrane, and Google Scholar databases. The articles were then screened for relevant studies by reviewing their abstracts with the following criteria: (1) topics: AI in healthcare and surgery, AI and respiratory medicine, AI and pathology, and AI and thoracic surgery; (2) published in English and in a peer-reviewed journal. Next, two authors (M.U.A. and J.A.K.) comprehensively reviewed the full manuscripts for inclusion. There was no year limit. The inclusion criteria prioritized but were not limited to clinical trials and meta-analyses on the role of artificial intelligence in thoracic surgery published from 2021 onwards. Articles of interest that had been cited by the articles identified in the initial search were also reviewed.
3. Discussion
3.1. Machine Learning and Natural Language Processing Applications in Thoracic Surgery
Thoracic surgery practice can be greatly aided by ML and NLP models, which aid in the diagnostic analysis of common and widespread diseases like non-small-cell lung cancer (NSCLC). About 84% of lung cancer cases are NSCLC, posing a great burden on healthcare, and is one of the greatest risks to human health due to its low 5-year relative survival rate of 25.0% [6]. The staging of NSCLC poses a great challenge to pulmonologists and thoracic surgeons because of the limitations of invasive modalities such as mediastinoscopy and ultrasound-guided transbronchial needle aspiration [7]. While such modalities have better diagnostic capabilities over non-invasive modalities such as CT and PET scans, they are not routinely used in screening or in clinical practice on patients with severe comorbidities [8]. Researchers have investigated employing statistical analyses or machine learning techniques to acquire nontrivial knowledge on full patient attributes and lymph node metastasis status in order to achieve exact staging [8,9,10,11,12,13,14,15]. The majority of clinical data, including tumor size, lymph node, tumor density, pleural indentation, and other information, are recorded in free-text format in electrical medical records (EMRs), which makes it difficult to manually extract data. Manual extraction takes a lot of time and is prone to human error. Thus, one major issue is how to efficiently extract these data to aid in later tasks like LNM prediction [16]. There has been an upsurge in the use of NLP models to extract this information automatically. Unfortunately, to date, this has not been widely adopted in thoracic surgery. In contrast, some medical fields have successfully implemented this technology. For instance, Chen et al. [17] computed the Cancer of the Liver Italian Program score by extracting data from various clinical notes, such as CT reports and operation notes. To determine the TNM and clinicopathological stage of colorectal cancer in Australian patients, Martinez et al. [18] took data from pathology records. To evaluate patients’ chances of survival, Yuan et al. [19] extracted several features from EMRs using NLP methods. Another recent study by Hu et al. [7] developed a lymph node metastasis prediction model for patients with NSCLC by integrating NLP and ML with EMR systems. They concluded that every machine learning model in their study outperformed the clinician’s assessment and the size requirements. Their experimental results also demonstrated that the NLP model can effectively extract information from CT reports, aiding in the lymph node metastasis prediction model’s development and updates, thereby facilitating its use in clinical settings.
Other areas where ML models have rapidly been adopted and show potential in thoracic surgery are diagnostic imaging and predictive analysis of surgical outcomes. These models will serve as a great addition for surgeons to foresee the surgical outcomes in patients undergoing high-risk surgeries or in patients with severe comorbidities. Kunze et al. [20] evaluated ML algorithms’ ability to predict clinically significant outcomes after orthopedic surgery and found that the currently available algorithms can easily discriminate the propensity to achieve clinically significant outcomes using the minimal clinically important difference with fair-to-good performance as evidenced by C-statistics ranging from 0.6 to 0.95 in most of the analyses. Another study by Stam et al. [21] found that AI algorithms can precisely predict surgical complications in major abdominal surgeries, provided they are thoroughly tested and validated and provided they are relying on a complete, balanced database. A 2023 study by Rana et al. [22] highlighted MRI and X-ray imaging as key imaging modalities for surgical disease detection using ML and DL techniques, with MATLAB and SVM as commonly used tools. Convolutional neural networks and random forest were found to outperform other algorithms, suggesting that the use of DL models with denoising approaches can improve accuracy. A common glossary of artificial intelligence is reported in Table 1.
Table 1.
| Algorithm | Models’ Creation from Mathematical Approaches to Data |
|---|---|
| Model | A mathematical function produced by an algorithm using a training set of data. |
| Artificial intelligence | A branch of computer science that focuses on teaching machines to perform tasks that traditionally require human intellect. |
| Machine Learning | A subfield of artificial intelligence where computers can learn from experience and recognize patterns in data without the need for explicit programming. |
| Deep learning | An area of machine learning that uses multilayer neural networks to examine data and create representations that resemble human thought processes. |
| Supervised learning | A subdivision of ML where the algorithm learns from labeled training data for the purpose of the classification or prediction of new data. |
| Unsupervised learning | A subset of machine learning where computer algorithms are trained on unlabeled training data to find patterns and draw conclusions. *Unsupervised methods are used for pattern recognition and clustering rather than generating predictive models. |
| Computer vision | An artificial intelligence field where computers to identify, understand, and react to visual input data. |
| Natural language processing | An AI field that enables computers to understand, interpret, and react to voice or text input. |
3.2. Advanced AI Applications in Thoracic Surgery
3.2.1. Identification of High-Risk Patients
Predictive analyses for the identification of high-risk patients are one of the most common applications of artificial intelligence. Several supervised and unsupervised ML models are utilized for the purpose of predicting binary events like readmission and mortality. A few examples of such incentives are the risk stratification index (RSI) [25], the American College of Surgeons National Surgical Quality Program (ACS NSQIP) [26], the Revised Cardiac Risk Index [27,28], and the Preoperative Score to Predict Postoperative Mortality [29]. An artificial neural network (ANN) method was created using genome sequencing data to guide safer and more successful warfarin dosing; in patients with international normalized ratios (INRs) > 3.5, the algorithm predicted the therapeutic dose with an accuracy of 83% [30]. For instance, an ANN-based model used to stratify postoperative bleeding risk in patients undergoing cardiac pulmonary bypass had a 92% accuracy rate [31]. To forecast the requirement for prolonged ventilation during coronary bypass grafting (AUC = 0.71–0.73), another ANN algorithm was created [32]. Patient safety could be significantly improved in these circumstances with early intervention. Other predictive models have deployed ML techniques for the purpose of estimating the postoperative discharge destination, down to the floor, within 24 h of surgery [33]. To increase these models’ predicted accuracy, full and real-time patient data are an absolute necessity.
3.2.2. Predicting and Detecting Complications
AI-powered sensors and continuous monitoring would play a massive role in aiding a thoracic surgeon in the early detection of complications. Thoracic surgery involves several postoperative pulmonary complications, such as respiratory function and infections that frequently develop after major surgery, adding to the mortality rates, prolonged hospital stays, and increased costs [34,35,36]. Moreover, early-stage treatment of lung disorders may decrease the probability of bad outcomes, further emphasizing the need for early detection. To minimize such complications and aid in early detection, AI can assist in the development of prediction algorithms and decision support systems, while novel sensors and ongoing monitoring can aid in the collection of substantial volumes of physiological or electronic health record data [37]. The most frequently utilized classical model in thoracic surgery is the Assess Respiratory Risk in Surgical Patients in Catalonia (ARISCAT) score. ARISCAT was developed by Canet et al. [38] to foresee PPC in surgical patients using logistic regression (LR), which is the only score scale that maintains discriminator power for external acknowledgment [39]. The ARISCAT score attained an AUROC of 0.80 in a prospective validation study with 5859 patients [39]. Furthermore, Bolourani et al. [40] created an ML model to predict respiratory failure following pulmonary lobectomy in 4062 patients. The AUROC was not given, even though the sensitivity and specificity were 83.3% and 94.5%, respectively. Chen et al.’s study [41] examined several machine learning methods, such as LR, SVM, RF, adaptive boosting, and GBM, to predict the risk of pneumonia in 786 patients following orthotopic liver transplantation. Their study identified 14 factors, including laboratory and clinical variables, that were linked to postoperative pneumonia. Furthermore, opioid-induced respiratory depression frequently occurs in the postoperative general care unit. Early diagnoses of such respiratory episodes are now possible due to new portable and wearable monitoring technologies [42,43,44]. Since these events occur way before true code-blue events, early identification and intervention may provide an opportunity to avert disastrous consequences [45]. Score scales such as Prediction of Opioid-Induced Respiratory Depression in Patients Monitored by Capnography are the first step in the prediction of the risk of respiratory depression, using multivariable regression modeling on continuous oximetry and capnography data, followed by the next step, pattern detection with DL techniques [46].
Timely and effective treatment can make the crucial difference between saving a life and losing it. Such crucial calls can sometimes be mishandled by the surgeon in charge, but AI-driven decision support systems may help prevent these errors. Even during routine clinical care, interventions based on high-quality evidence are routinely miscalculated and not provided. Such high-quality evidence and patient data can be combined by a decision support system to provide point-of-care recommendations. For instance, Joosten et al. [47] demonstrated a positive effect on neurocognitive recovery by precisely titrating liquids, analgesics, and anesthesia using three closed-loop devices. AI can assist in the development of professional societies’ guidelines and recommendations under a lack of high-quality evidence. It can also assist in the development of guideline-based decision support systems. Using decision assistance and image-based navigational tools, hospitals can address inefficiencies and clinical issues faced by physicians during surgery by implementing AI and predictive analytics in conventional ORs [37]. Certain AI systems can assist in anticipating complications, ensuring smoother surgeries and quicker recovery by assessing the probability of problems even before a patient is transferred into the operating room. AI is already proving to be an extremely important aid by improving the identification of target areas, such as through the OR "black box" platform, which records and analyzes surgical procedures to identify potential issues. For instance, one hospital discovered frequent OR door openings during surgery due to the suture cart being outside the room, leading to its relocation back inside [37]. Complex OR environments can benefit from digitalization and AI integration, as seen with the Triton system, which uses AI and infrared technology to analyze sponge photos and quantify blood loss [48].
3.2.3. Perioperative Management
Artificial intelligence can serve as a potent tool in perioperative thoracic surgery management by enhancing diagnostic processes, performing tumor staging, facilitating predictive analysis, enabling robotic-assisted surgeries, and reducing postoperative complications and mortality rates. Rapid Diagnostic Assessment and Tumor Staging can make the difference between survival and death during preoperative management. A recent study conducted by Ye et al. experimented with a deep learning radiomics model to predict high-risk pathologic pulmonary nodules in patients through CT semantics [49]. Their proposed combined model yielded area-under-the-receiver-operating-characteristic-curve (AUC) values of 0.872 and 0.814 in the training and external validation cohorts, respectively. Decision curve analysis (DCA) assures that the proposed models provide a net benefit in predicting high-risk pathological pulmonary nodules early on, which helps in providing rapid aid [49]. Similarly, such radiomic and clinical features can be combined to form comprehensive models to predict recurrence risk in NSCLC patients. A study extracted a total of 1562 radiomic features and retained 29 features after feature selection [50]. A COX multivariate regression model determined the N stage as the independent risk factor for postoperative recurrence. The AUC values of the radiomic–clinical comprehensive model were 0.972 and 0.937 in the training and test cohorts, respectively; these values far surpass those of the standalone clinical and radiomic models [50]. Another study conducted by Blüthgen et al. evaluated CT-derived radiomics for the thymic-epithelial-tumor stage and the presence of myasthenia gravis (MG) and concluded its usefulness as an imaging biomarker for thymic-epithelial-tumor histology and TNM staging [51]. Deep learning models’ efficiency in determining tumor stages was further cemented by two other studies conducted by Chen et al. [52,53], where both studies, respectively, dwelled on the use of CT image analytics through machine learning models for the identification of lung adenocarcinoma and high-risk tumor areas. The promising results from their computational study confirm that such a method provides a reliable basis for adenocarcinoma diagnoses supplementary to pathological examinations.
Intraoperative surgical management has also been flooded with artificial intelligence and deep learning model uses. Predictive analyses and robotic assistance throughout treatment have provided a massive upper hand to surgeons, allowing them to perform procedures with ease and smoothness. A deep learning prognostic model could assist in predicting the need for additional intraoperative placement of chest drainage after thoracoscopic lobectomy to prevent the risk of tension pneumothorax [54]. It was found that the incidence risk of tension pneumothorax was 4.53%, and the nomogram makes it possible to decide on the intraoperative installation of an additional pleural drainage tube and prevent complications associated with postoperative lung collapse. Prolonged air leakage is a serious and frequent complication post lung resection surgery. AI was used to develop a useful risk predictor model to help recognize patients who might benefit from additional preventive procedures, and the model was successfully able to confirm significant prognostic risk factors and protective factors for prolonged air leakage [55]. However, after internal validation, the C-statistic value was found to be 0.63, which is too low to generate a reliable score in clinical practice [55]. The use of AI in intraoperative planning and surgical management remains a field for further study and development.
Artificial intelligence (AI), machine learning (ML), and deep learning models are all equally transformative in postoperative surgical management in thoracic patients. Continuous monitoring of patients for predicting future potential complications is a major leverage point of AI for a thoracic surgeon. In a recent study conducted by Kadomatsu et al. [56], an elastic-net regularized generalized linear model that calculates a novel comorbidity risk score built especially for lung resection procedures was created. Data from 2018 and 2019 were used to validate this model, which examined 40 variables such as surgical methods, tumor-related characteristics, and patient status. The final 20-variable model performed noticeably better in predicting postoperative complications, with an AUC of 0.734, as opposed to 0.521 for the Charlson Comorbidity Index (CCI). Furthermore, AI models have been significantly developed for other critical care settings. For instance, a study by Frades et al. [57] aimed to create a mortality predictor for Intermediate Respiratory Care Unit (IRCU) patients using machine learning models. In the first year of implementation, the resulting model’s combination with multivariate logistic regression analysis considerably lowered failure rates by 50%, indicating its efficacy in mortality risk detection and real-time monitoring. As AI technologies continue to evolve, their integration into postoperative care protocols will likely become increasingly sophisticated, further enhancing recovery and long-term health outcomes for thoracic surgery patients.
3.2.4. Immunotherapy Guidance
AI has further emerged as a revolutionizing tool in immunotherapeutic guidance for thoracic surgery, particularly through model development for recognizing DNA methylation biomarkers, RNA sequencing, selecting effective drug targets, and gene classification. Deep learning AI models may be able to meet the unmet demand for non-invasive testing for the early diagnosis of lung malignancies by integrating imaging, clinical, and DNA methylation biomarkers to enhance the classification of pulmonary nodules [58]. In a prospective study conducted across 24 hospitals in China, He et al. [58] proposed a combined model, PulmoSeek Plus, which integrates clinical, imaging, and cell-free DNA methylation biomarkers to improve the classification of pulmonary nodules. The model achieved an AUC of 0.85 in the validation cohorts, outperforming the standalone CIBM and PulmoSeek models, and had a sensitivity of 0.98 for early-stage lung cancer and a DCA indicating a substantial reduction in unnecessary surgeries and delayed treatments. In addition, AI has highlighted the role of dendritic cells in lung adenocarcinoma. Zhang et al. [59] conducted a study on lung adenocarcinoma and numerous other cancer patients undergoing immunotherapy, identifying 83 DC marker genes; this led to the development of a seven-gene signature that predicts prognoses and treatment responses. It was found that patients with lower risk scores responded better to immunotherapy and targeted therapies, and Cathepsin H emerged as a new protective biomarker for lung adenocarcinoma [59]. Similarly, an eight-gene risk model was successfully developed to predict esophageal squamous cell carcinoma induced by the tricarboxylic acid (TCA) cycle [60]. The function assay suggested the CTTN gene’s role in cell proliferation and invasion through the EMT pathway and highlighted the importance of the TCA cycle’s importance in tumor immunity. The role of AI predictive and analytic models is further solidified by studies such as Li et al.’s [61], where cuproptosis was studied as a promising therapeutic modality for LUAD patients who had grown resistant to radiotherapy and chemotherapy. By identifying cuproptosis-related genes (CRGs) and constructing a three-layer ANN risk model, a robust prognostic value was demonstrated where low-risk group patients had immune "hot" tumors with anticancer activity and were more sensitive to immunotherapy, while high-risk group patients had immune "cold" tumors and were more sensitive to chemotherapy. Furthermore, another comprehensive study by Chen at al. [62] utilized machine learning techniques to develop an autophagy-related gene (ARG) classifier based on eight key ARGs. This classifier showed excellent diagnostic and prognostic performance (AUC > 0.85) across multiple datasets, outperforming traditional markers like procalcitonin and C-reactive protein. The model also correlated significantly with immune cell infiltration, immune pathways, and cytokine levels, effectively reflecting the immune microenvironment during sepsis [62]. Areas of AI applications in thoracic surgery are demonstrated in Table 2.
Table 2.
Areas of AI applications in thoracic surgery [63].
| Application Areas | Examples |
|---|---|
| Preoperative phase | Early detection of lung cancer; genetic review and decision-making in chemo- vs. immunotherapy |
| Diagnosis | Improving diagnostic accuracy and reducing false-positive rates in radiology, liquid biopsy, and histology |
| Operative phase | Enhancing surgical safety, accuracy, and decision-making in robotic-assisted surgery |
| Surgical skill assessment | |
| Surgical planning optimization | |
| Postoperative phase | Predicting complications and mortality risks post-surgery |
| Enhancing risk stratification | |
| Supporting clinical decision-making | |
| Education | Providing educational support and surgical training feedback |
| Management | Improving operating room scheduling and efficiency |
| Optimizing overall resource utilization | |
| Enhancing cost-effectiveness |
3.3. Limitations and Ethical Implications of Utilizing AI
While AI can have numerous advantages, as we highlighted, it also comes with its risks [64].
Regardless of the extent to which ML is employed, a robot is believed to be unable to achieve fully autonomous thoughts. It is assumed that robots consistently replicate human cognitive processes, albeit with greater speed and logical consistency. As a result, human intuition and experience remain crucial factors. Surgeons frequently rely on instinct, and AI will most likely not be able to replace a human approach, at least not fully yet [64]. Another major limiting factor for using AI in surgery is the financial burden of operating such advanced technology. Generalization and uniform standards of treatment will be impaired because not all regions can afford the technology. It is possible that an algorithm created in one institution will not apply to other universities directly. The model will be effectively tailored to reflect the clinical experience of that institution through the development of the algorithm. This presents advantages as well as disadvantages for prospective consumers [65]. The quantity and quality of the data that are provided determine how well AI responds. The degree to which AI algorithms may be applied to different subgroups depends heavily on a number of criteria, including outliers, missing data, and how representative the included populations are. In fact, it is critical to regularly update algorithms with fresh patient data so that they can adjust how decisions are made [66]. The types and quality of the data that are accessible to the algorithm restrict its outputs; for example, lung cancers that affect Caucasians in the EU do not share the same epidemiologic features as lung cancers that affect persons in Asia. Nonsmoking reasons account for a larger percentage of cancer cases in Asia, especially in women [67]. Therefore, there is a chance that selection bias will affect projections if specific populations or sexes are underrepresented.
Moreover, most of the uses of AI-driven technology in surgery are yet in their infancy stage. The autonomous execution of complicated surgical procedures is not the same as that of simple independent tasks. ML is not always accurate and may produce inaccurate results, and hence, it is a must to overview whatever decisions the AI software may suggest. IBM’s Watson for Oncology Cognitive Computing system, created in 2012, employs AI algorithms to provide therapy suggestions for a range of illnesses, including lung tumors. Oncologists at the Memorial Sloan Kettering Cancer Center (New York, NY, USA) trained the program to recognize important information related to a patient’s cancer, such as blood test results, pathology and imaging reports, and the existence of genetic mutations. The available treatment options broadly align with the standards of the National Comprehensive Cancer Network [66]. However, in 2018, IBM’s Watson faced criticism for making incorrect treatment recommendations in certain situations, potentially endangering the lives of patients [68]. It is strictly contraindicated for a patient with lung squamous cell carcinoma to use bevacizumab, as recommended by the system. In June 2019, the American Society for Clinical Oncology received an abstract about IBM’s Watson for Oncology Cognitive Computing system, which hinted at its potential to support multidisciplinary tumor boards’ decision-making [69]. Another example backing this argument is a study that attempted to use ML for early pneumonia diagnoses: two sets of chest radiographs—one with pneumonia and the other without—were given to the algorithms so they could learn to distinguish between the two. The mark on the radiograph used to designate the right and left sides, which turned out to differ across the two hospitals, was the greatest predictive feature of pneumonia, according to the algorithm, which immediately determined this [70]. Because of this, ML still requires supervision at this point in its development.
To fully achieve the potential of AI in thoracic surgery, some key ethical concerns must be tackled, including obtaining informed consent for data use, ensuring safety and transparency, addressing fairness and biases in algorithms, and safeguarding data privacy [71]. Overall, the legality of ML models also remains debated (Resolution of the European Parliament, 16 February 2017) [72]. The primary motive is to counteract the ethically challenging circumstances brought about by implementing AI in thoracic surgery [73]. Most legal discussions around artificial intelligence have focused on the issue of algorithmic transparency. The use of AI in high-risk scenarios emphasizes the need for transparent, fair, and responsible design, with essential components being clarity and accessibility [74]. It is common for information regarding the operation of algorithms to be purposefully difficult to access [75]. Machines that can learn new behavioral patterns and function according to lose norms are said to pose a danger to our ability to assign blame to their creators or operators. We might not have anyone to hold responsible for any harm caused if AI is used [74,75]. The scale of the threat is uncertain, and using machines will drastically restrict our capacity to assign responsibility and take charge of the decision-making process [76]. Modern computing approaches can obscure the reasoning behind the outputs of an AI system, making it difficult for non-technical clinical users to understand [77]. While AI systems like IBM’s Watson for Oncology are designed to support clinical decision-making by evaluating information and recommending patient care, the complexity of these systems can create challenges [74,75]. If widely adopted, AI systems could revolutionize clinical decision-making and reshape healthcare dynamics, making it crucial for clinicians to ensure the safe implementation of these technologies [78,79,80]. However, as this study tries to clarify, it is possible that AI will be able to get beyond these restrictions; at the moment, one can only hypothesize as to how AI will be able to get around some ethical and moral conundrums. A summary of the key topics discussed in the above paragraphs regarding the identification of high-risk patients, predicting and detecting complications, perioperative management, and immunotherapy guidance is highlighted in Table 3.
Table 3.
Summary of key topics related to AI applications in thoracic surgery.
| Section | No. of Studies | Main Outcomes | Advantages | Disadvantages |
|---|---|---|---|---|
| Identification of High-Risk Patients | 7 | Models accurately predict mortality, readmission, bleeding, and ventilation needs, e.g., 83% warfarin dose accuracy and 92% postoperative bleeding prediction. | Improved patient safety and outcomes through early intervention; high accuracy in stratifying risk. | Real-time data are required for higher prediction accuracy; generalization to all institutions is challenging due to the differences in the available data and resources. |
| Predicting and Detecting Complications | 12 | Successful use of ML for predicting respiratory failure, pneumonia, and opioid-induced depression; ARISCAT and OR "black box" systems showed utility. | Early identification of complications, real-time monitoring, and decision support; reduce surgical errors and improve patient outcomes. | Requires large datasets for validation; not always reliable when applied across diverse clinical settings. Computational costs may be prohibitive. |
| Perioperative Management | 6 | AI-assisted diagnosis (CT-based radiomics) improves tumor staging, and reduces complications (e.g., air leaks); DL models show AUC values of up to 0.97 for predicting recurrence and risk stratification. | Enhances diagnostic accuracy and intraoperative decisions; AI-assisted surgery improves precision and reduces complications. | Low external validation in some cases (AUC of 0.63 for some predictions); potential for low accuracy when generalizing models to different patient populations or regions. |
| Postoperative Management | 4 | Predictive models (e.g., elastic-net regularized models) improve the early detection of complications and reduce mortality; for example, 50% reduction in failure rates in IRCU patients. | Continuous monitoring and prediction of future complications; faster recovery and improved patient outcomes. | Data from select regions may not generalize well; updates to models are needed as well as new patient data to maintain accuracy. |
| Immunotherapy Guidance | 6 | Models predict biomarkers for immunotherapy (e.g., DC markers), classify pulmonary nodules, and enhance early cancer diagnosis, prognosis, and drug target identification. | Non-invasive testing; improved classification of cancer subtypes; personalized immunotherapy guidance with high predictive accuracy. | Limited validation across broader populations; potential issues with data privacy when using genetic markers and large-scale sequencing. |
4. Conclusion
Artificial intelligence (AI) has shown the potential to revolutionize thoracic surgery by enhancing diagnostic accuracy, optimizing treatment plans, and improving postoperative care. AI models, such as those predicting lymph node metastasis in non-small-cell lung cancer, have demonstrated comparable performance over traditional methods, aiding in precise staging and better clinical decision-making. Moreover, AI-driven robotic surgeries, perioperative intelligence, and predictive models for identifying high-risk patients and complications have paved the way for safer and more efficient surgical procedures.
Despite these advancements, however, the integration of AI in thoracic surgery faces some challenges, including the need for extensive datasets, problems of data privacy, potential biases in AI models, and ethical concerns. Addressing these limitations requires continuous refinement of AI models, adherence to ethical guidelines, and the establishment of transparent and accountable AI systems. Looking ahead, the future of AI in thoracic surgery holds promise; there is the potential for more sophisticated AI-driven systems, advanced perioperative management models, and decision support systems that can integrate seamlessly into the clinical environment. More research and external validation will be essential to harness AI’s full potential and ensure its effective application in thoracic surgery.
Author Contributions
Conceptualization: M.M., M.U.A. and J.A.K.; Methodology: M.U.A. and J.A.K.; Validation: M.M., A.Y. and W.S.; Formal Analysis: M.M.; Investigation: M.M. and A.Y.; Resources: M.U.A., J.A.K. and B.N.S.; Data Curation: M.M., M.U.A., J.A.K. and B.N.S.; Writing—Original Draft Preparation: M.U.A., J.A.K., B.N.S. and M.M.; Writing—Review and Editing: M.U.A., J.A.K., B.N.S., M.M., A.Y. and W.S.; Visualization: M.M., M.U.A. and J.A.K.; Supervision: M.M., A.Y. and W.S.; Project Administration: M.M. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Matheny M.E., Whicher D., Thadaney Israni S. Artificial Intelligence in Health Care: A Report from the National Academy of Medicine. JAMA. 2020;323:509–510. doi: 10.1001/jama.2019.21579. [DOI] [PubMed] [Google Scholar]
- 2.Abbaker N., Minervini F., Guttadauro A., Solli P., Cioffi U., Scarci M. The future of artificial intelligence in thoracic surgery for non-small cell lung cancer treatment a narrative review. Front. Oncol. 2024;14:1347464. doi: 10.3389/fonc.2024.1347464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Norwegian Centre for E-health Research Artificial Intelligence in Health Care—The Hope, the Hype, the Promise, the Peril. [(accessed on 8 June 2024)]. Available online: https://ehealthresearch.no/en/reports/other/artificial-intelligence-in-health-care-the-hope-the-hype-the-promise-the-peril.
- 4.Case N. How to Become a Centaur. [(accessed on 8 June 2024)];J. Des. Sci. 2018 Available online: https://jods.mitpress.mit.edu/pub/issue3-case/release/6. [Google Scholar]
- 5.Vaidya Y.P., Shumway S.J. Artificial intelligence: The future of cardiothoracic surgery. [(accessed on 10 June 2024)];J. Thorac. Cardiovasc. Surg. 2024 doi: 10.1016/j.jtcvs.2024.04.027. Available online: https://www.jtcvs.org/article/S0022-5223(24)00371-4/fulltext. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Facts & Figures 2021|American Cancer Society. [(accessed on 21 August 2024)]. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html.
- 7.JMIR Medical Informatics—Using Natural Language Processing and Machine Learning to Preoperatively Predict Lymph Node Metastasis for Non–Small Cell Lung Cancer with Electronic Medical Records: Development and Validation Study. [(accessed on 10 June 2024)]. Available online: https://medinform.jmir.org/2022/4/e35475#ref1. [DOI] [PMC free article] [PubMed]
- 8.Development of a Nomogram for Preoperative Prediction of Lymph Node Metastasis in Non-Small Cell Lung Cancer: A SEER-Based Study—Zhang—Journal of Thoracic Disease. [(accessed on 10 June 2024)]. Available online: https://jtd.amegroups.org/article/view/41481/html. [DOI] [PMC free article] [PubMed]
- 9.Lv X., Wu Z., Cao J., Hu Y., Liu K., Dai X., Yuan X., Wang Y., Zhao K., Lv W., et al. A nomogram for predicting the risk of lymph node metastasis in T1–2 non-small-cell lung cancer based on PET/CT and clinical characteristics. Transl. Lung Cancer Res. 2021;10:430–438. doi: 10.21037/tlcr-20-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Development and Validation of a Clinical Prediction Model for N2 Lymph Node Metastasis in Non-Small Cell Lung Cancer—The Annals of Thoracic Surgery. [(accessed on 10 June 2024)]. Available online: https://www.annalsthoracicsurgery.org/article/S0003-4975(13)01366-0/fulltext. [DOI] [PubMed]
- 11.Occult Mediastinal Lymph Node Metastasis in FDG-PET/CT Node-Negative Lung Adenocarcinoma Patients: Risk Factors and Histopathological Study—Miao—2019—Thoracic Cancer—Wiley Online Library. [(accessed on 10 June 2024)]. Available online: https://onlinelibrary.wiley.com/doi/10.1111/1759-7714.13093. [DOI] [PMC free article] [PubMed]
- 12.Prediction Model for Nodal Disease among Patients with Non-Small Cell Lung Cancer—Abstract—Europe PMC. [(accessed on 10 June 2024)]. Available online: https://europepmc.org/article/MED/30710518.
- 13.A Clinical Prediction Rule to Estimate the Probability of Mediastinal Metastasis in Patients with Non-small Cell Lung Cancer—Journal of Thoracic Oncology. [(accessed on 10 June 2024)]. Available online: https://www.jto.org/article/S1556-0864(15)31627-0/fulltext. [PubMed]
- 14.A Prediction Model for Pathologic N2 Disease in Lung Cancer Patients with a Negative Mediastinum by Positron Emission Tomography—Journal of Thoracic Oncology. [(accessed on 10 June 2024)]. Available online: https://www.jto.org/article/S1556-0864(15)33473-0/fulltext. [DOI] [PubMed]
- 15.Novel Approach for Predicting Occult Lymph Node Metastasis in Peripheral Clinical Stage I Lung Adenocarcinoma—Song—Journal of Thoracic Disease. [(accessed on 10 June 2024)]. Available online: https://jtd.amegroups.org/article/view/27808/20883. [DOI] [PMC free article] [PubMed]
- 16.Yim W.-W., Yetisgen M., Harris W.P., Kwan S.W. Natural Language Processing in Oncology: A Review. JAMA Oncol. 2016;2:797–804. doi: 10.1001/jamaoncol.2016.0213. [DOI] [PubMed] [Google Scholar]
- 17.Chen L., Song L., Shao Y., Li D., Ding K. Using natural language processing to extract clinically useful information from Chinese electronic medical records. Int. J. Med. Inf. 2019;124:6–12. doi: 10.1016/j.ijmedinf.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 18.Cross-Hospital Portability of Information Extraction of Cancer Staging Information—ScienceDirect. [(accessed on 11 June 2024)]. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0933365714000669?via%3Dihub. [DOI] [PubMed]
- 19.Performance of a Machine Learning Algorithm Using Electronic Health Record Data to Identify and Estimate Survival in a Longitudinal Cohort of Patients with Lung Cancer|Artificial Intelligence|JAMA Network Open|JAMA Network. [(accessed on 11 June 2024)]. Available online: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2781685. [DOI] [PMC free article] [PubMed]
- 20.Kunze K.N., Krivicich L.M., Clapp I.M., Bodendorfer B.M., Nwachukwu B.U., Chahla J., Nho S.J. Machine Learning Algorithms Predict Achievement of Clinically Significant Outcomes after Orthopaedic Surgery: A Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2022;38:2090–2105. doi: 10.1016/j.arthro.2021.12.030. [DOI] [PubMed] [Google Scholar]
- 21.Stam W.T., Goedknegt L.K., Ingwersen E.W., Schoonmade L.J., Bruns E.R.J., Daams F. The prediction of surgical complications using artificial intelligence in patients undergoing major abdominal surgery: A systematic review. Surgery. 2022;171:1014–1021. doi: 10.1016/j.surg.2021.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Rana M., Bhushan M. Machine learning and deep learning approach for medical image analysis: Diagnosis to detection. Multimed. Tools Appl. 2023;82:26731–26769. doi: 10.1007/s11042-022-14305-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salna M. The Promise of Artificial Intelligence in Cardiothoracic Surgery. J. Chest Surg. 2022;55:429–434. doi: 10.5090/jcs.22.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scoping Review of Artificial Intelligence Applications in Thoracic Surgery|European Journal of Cardio-Thoracic Surgery|Oxford Academic. [(accessed on 21 August 2024)]. Available online: https://academic.oup.com/ejcts/article/61/2/239/6380645?login=false.
- 25.Broadly Applicable Risk Stratification System for Predicting Duration of Hospitalization and Mortality|Anesthesiology|American Society of Anesthesiologists. [(accessed on 13 June 2024)]. Available online: https://pubs.asahq.org/anesthesiology/article/113/5/1026/10000/Broadly-Applicable-Risk-Stratification-System-for. [DOI] [PubMed]
- 26.Development and Evaluation of the Universal ACS NSQIP Surgical Risk Calculator: A Decision Aid and Informed Consent Tool for Patients and Surgeons—ScienceDirect. [(accessed on 13 June 2024)]. Available online: https://www.sciencedirect.com/science/article/abs/pii/S1072751513008946. [DOI] [PMC free article] [PubMed]
- 27.2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines|Journal of the American College of Cardiology. [(accessed on 13 June 2024)]. Available online: https://www.jacc.org/doi/abs/10.1016/j.jacc.2014.07.944. [DOI] [PubMed]
- 28.Fronczek J., Polok K., Devereaux P.J., Górka J., Archbold R.A., Biccard B., Duceppe E., Le Manach Y., Sessler D.I., Duchińska M., et al. External validation of the Revised Cardiac Risk Index and National Surgical Quality Improvement Program Myocardial Infarction and Cardiac Arrest calculator in noncardiac vascular surgery. Br. J. Anaesth. 2019;123:421–429. doi: 10.1016/j.bja.2019.05.029. [DOI] [PubMed] [Google Scholar]
- 29.Preoperative Score to Predict Postoperative Mortality (POSPOM)|Anesthesiology|American Society of Anesthesiologists. [(accessed on 13 June 2024)]. Available online: https://pubs.asahq.org/anesthesiology/article/124/3/570/14280/Preoperative-Score-to-Predict-Postoperative.
- 30.Pavani A., Naushad S.M., Kumar R.M., Srinath M., Malempati A.R., Kutala V.K. Artificial Neural Network-Based Pharmacogenomic Algorithm for Warfarin Dose Optimization. Pharmacogenomics. 2016;17:121–131. doi: 10.2217/pgs.15.161. [DOI] [PubMed] [Google Scholar]
- 31.Huang R.S.P., Nedelcu E., Bai Y., Wahed A., Klein K., Tint H., Gregoric I., Patel M., Kar B., Loyalka P., et al. Post-Operative Bleeding Risk Stratification in Cardiac Pulmonary Bypass Patients Using Artificial Neural Network. Ann. Clin. Lab. Sci. 2015;45:181–186. [PubMed] [Google Scholar]
- 32.Wise E.S., Stonko D.P., Glaser Z.A., Garcia K.L., Huang J.J., Kim J.S., Kallos J.A., Starnes J.R., Fleming J.W., Hocking K.M., et al. Prediction of Prolonged Ventilation after Coronary Artery Bypass Grafting: Data from an Artificial Neural Network. Heart Surg. Forum. 2017;20:E007–E014. doi: 10.1532/hsf.1566. [DOI] [PubMed] [Google Scholar]
- 33.Khanna A.K., Shaw A.D., Stapelfeldt W.H., Boero I.J., Chen Q., Stevens M., Gregory A., Smischney N.J. Postoperative Hypotension and Adverse Clinical Outcomes in Patients Without Intraoperative Hypotension, After Noncardiac Surgery. Anesth. Analg. 2021;132:1410. doi: 10.1213/ANE.0000000000005374. [DOI] [PubMed] [Google Scholar]
- 34.Opioid-Induced Respiratory Depression Increases Hospital Costs and Length of Stay in Patients Recovering on the General Care Floor|BMC Anesthesiology. [(accessed on 13 June 2024)]. Available online: https://link.springer.com/article/10.1186/s12871-021-01307-8. [DOI] [PMC free article] [PubMed]
- 35.Epidemiology, practice of ventilation and outcome for patients at increased risk of postoperative pulmonary complications. Eur. J. Anaesthesiol. 2017;34:492–507. doi: 10.1097/EJA.0000000000000646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Serpa Neto A., Hemmes S.N.T., Barbas C.S.V., Beiderlinden M., Fernandez-Bustamante A., Futier E., Hollmann M.W., Jaber S., Kozian A., Licker M., et al. Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: A systematic review and meta-analysis. Lancet Respir. Med. 2014;2:1007–1015. doi: 10.1016/S2213-2600(14)70228-0. [DOI] [PubMed] [Google Scholar]
- 37.Maheshwari K., Cywinski J.B., Papay F., Khanna A.K., Mathur P. Artificial Intelligence for Perioperative Medicine: Perioperative Intelligence. Anesth. Analg. 2023;136:637. doi: 10.1213/ANE.0000000000005952. [DOI] [PubMed] [Google Scholar]
- 38.Canet J., Gallart L., Gomar C., Paluzie G., Vallès J., Castillo J., Sabaté S., Mazo V., Briones Z., Sanchis J., et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113:1338–1350. doi: 10.1097/ALN.0b013e3181fc6e0a. [DOI] [PubMed] [Google Scholar]
- 39.Mazo V., Sabaté S., Canet J., Gallart L., de Abreu M.G., Belda J., Langeron O., Hoeft A., Pelosi P. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology. 2014;121:219–231. doi: 10.1097/ALN.0000000000000334. [DOI] [PubMed] [Google Scholar]
- 40.Bolourani S., Wang P., Patel V.M., Manetta F., Lee P.C. Predicting respiratory failure after pulmonary lobectomy using machine learning techniques. Surgery. 2020;168:743–752. doi: 10.1016/j.surg.2020.05.032. [DOI] [PubMed] [Google Scholar]
- 41.Chen C., Yang D., Gao S., Zhang Y., Chen L., Wang B., Mo Z., Yang Y., Hei Z., Zhou S. Development and performance assessment of novel machine learning models to predict pneumonia after liver transplantation. Respir. Res. 2021;22:94. doi: 10.1186/s12931-021-01690-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khanna A.K., Hoppe P., Saugel B. Automated continuous noninvasive ward monitoring: Future directions and challenges. Crit. Care. 2019;23:194. doi: 10.1186/s13054-019-2485-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khanna A.K., Ahuja S., Weller R.S., Harwood T.N. Postoperative ward monitoring—Why and what now? Best Pract. Res. Clin. Anaesthesiol. 2019;33:229–245. doi: 10.1016/j.bpa.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Saugel B., Hoppe P., Khanna A.K. Automated Continuous Noninvasive Ward Monitoring: Validation of Measurement Systems Is the Real Challenge. Anesthesiology. 2020;132:407–410. doi: 10.1097/ALN.0000000000003100. [DOI] [PubMed] [Google Scholar]
- 45.Lee L.A., Caplan R.A., Stephens L.S., Posner K.L., Terman G.W., Voepel-Lewis T., Domino K.B. Postoperative Opioid-induced Respiratory Depression: A Closed Claims Analysis. Anesthesiology. 2015;122:659–665. doi: 10.1097/ALN.0000000000000564. [DOI] [PubMed] [Google Scholar]
- 46.Khanna A.K., Bergese S.D., Jungquist C.R., Morimatsu H., Uezono S., Lee S., Ti L.K., Urman R.D., McIntyre R.J., Tornero C., et al. Prediction of Opioid-Induced Respiratory Depression on Inpatient Wards Using Continuous Capnography and Oximetry: An International Prospective, Observational Trial. Anesth. Analg. 2020;131:1012. doi: 10.1213/ANE.0000000000004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anesthetic Management Using Multiple Closed-loop Systems and Delayed Neurocognitive Recovery|Anesthesiology|American Society of Anesthesiologists. [(accessed on 13 June 2024)]. Available online: https://pubs.asahq.org/anesthesiology/article/132/2/253/108808/Anesthetic-Management-Using-Multiple-Closed-loop.
- 48.The Association between the Introduction of Quantitative Assessment of Postpartum Blood Loss and Institutional Changes in Clinical Practice: An Observational Study—International Journal of Obstetric Anesthesia. [(accessed on 13 June 2024)]. Available online: https://www.obstetanesthesia.com/article/S0959-289X(19)30070-6/abstract. [DOI] [PubMed]
- 49.Development and Validation of a Deep Learning Radiomics Model to Predict High-Risk Pathologic Pulmonary Nodules Using Preoperative Computed Tomography—Academic Radiology. [(accessed on 4 August 2024)]. Available online: https://www.academicradiology.org/article/S1076-6332(23)00461-0/abstract. [DOI] [PubMed]
- 50.Application of a Comprehensive Model Based on CT Radiomics and Clinical Features for Postoperative Recurrence Risk Prediction in Non-small Cell Lung Cancer—Academic Radiology. [(accessed on 4 August 2024)]. Available online: https://www.academicradiology.org/article/S1076-6332(23)00662-1/fulltext. [DOI] [PubMed]
- 51.Computed Tomography Radiomics for the Prediction of Thymic Epithelial Tumor Histology, TNM Stage and Myasthenia Gravis|PLoS ONE. [(accessed on 5 August 2024)]. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0261401. [DOI] [PMC free article] [PubMed]
- 52.Machine Vision-Assisted Identification of the Lung Adenocarcinoma Category and High-Risk Tumor Area Based on CT Images: Patterns. [(accessed on 5 August 2024)]. Available online: https://www.cell.com/patterns/fulltext/S2666-3899(22)00044-7?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS2666389922000447%3Fshowall%3Dtrue. [DOI] [PMC free article] [PubMed]
- 53.Chen L., Qi H., Lu D., Zhai J., Cai K., Wang L., Liang G., Zhang Z. A deep learning based CT image analytics protocol to identify lung adenocarcinoma category and high-risk tumor area. STAR Protoc. 2022;3:101485. doi: 10.1016/j.xpro.2022.101485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pikin O.V., Ryabov A.B., Alexandrov O.A., Larionov D.A., Martynov A.A., Toneev E.A. Predictive model for additional intraoperative placement of chest drainage after thoracoscopic lobectomy. Khirurgiia (Sofiia) 2023:14–25. doi: 10.17116/hirurgia202312114. [DOI] [PubMed] [Google Scholar]
- 55.Divisi D., Pipitone M., Perkmann R., Bertolaccini L., Curcio C., Baldinelli F., Crisci R., Zaraca F., Italian VATS group Prolonged air leak after video-assisted thoracic anatomical pulmonary resections: A clinical predicting model based on data from the Italian VATS group registry, a machine learning approach. J. Thorac. Dis. 2023;15:849–857. doi: 10.21037/jtd-21-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kadomatsu Y., Emoto R., Kubo Y., Nakanishi K., Ueno H., Kato T., Nakamura S., Mizuno T., Matsui S., Chen-Yoshikawa T.F. Development of a machine learning-based risk model for postoperative complications of lung cancer surgery. Surg. Today. 2024 doi: 10.1007/s00595-024-02878-y. [DOI] [PubMed] [Google Scholar]
- 57.Patient Management Assisted by a Neural Network Reduces Mortality in an Intermediate Care Unit. [(accessed on 5 August 2024)]. Available online: http://www.archbronconeumol.org/en-linkresolver-patient-management-assisted-by-neural-S0300289619305940. [DOI] [PubMed]
- 58.Accurate Classification of Pulmonary Nodules by a Combined Model of Clinical, Imaging, and Cell-Free DNA Methylation Biomarkers: A Model Development and External Validation Study—The Lancet Digital Health. [(accessed on 5 August 2024)]. Available online: https://www.thelancet.com/journals/landig/article/PIIS2589-7500(23)00125-5/fulltext. [DOI] [PubMed]
- 59.Zhang L., Guan M., Zhang X., Yu F., Lai F. Machine-Learning and combined analysis of single-cell and bulk-RNA sequencing identified a DC gene signature to predict prognosis and immunotherapy response for patients with lung adenocarcinoma. J. Cancer Res. Clin. Oncol. 2023;149:13553–13574. doi: 10.1007/s00432-023-05151-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liang Y., Tan B., Du M., Wang B., Gao Y., Wang M. A tricarboxylic acid cycle-based machine learning model to select effective drug targets for the treatment of esophageal squamous cell carcinoma. Front. Pharmacol. 2023;14:1195195. doi: 10.3389/fphar.2023.1195195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frontiers|Deep Learning Reveals Cuproptosis Features Assist in Predict Prognosis and Guide Immunotherapy in Lung Adenocarcinoma. [(accessed on 6 August 2024)]. Available online: https://www.frontiersin.org/journals/endocrinology/articles/10.3389/fendo.2022.970269/full. [DOI] [PMC free article] [PubMed]
- 62.Chen Z., Zeng L., Liu G., Ou Y., Lu C., Yang B., Zuo L. Construction of Autophagy-Related Gene Classifier for Early Diagnosis, Prognosis and Predicting Immune Microenvironment Features in Sepsis by Machine Learning Algorithms. J. Inflamm. Res. 2022;15:6165–6186. doi: 10.2147/JIR.S386714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bellini V., Valente M., Rio P.D., Bignami E. Artificial intelligence in thoracic surgery: A narrative review. J. Thorac. Dis. 2021;13:6963. doi: 10.21037/jtd-21-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gupta A., Singla T., Chennatt J.J., David L.E., Ahmed S.S., Rajput D. Artificial intelligence: A new tool in surgeon’s hand. J. Educ. Health Promot. 2022;11:93. doi: 10.4103/jehp.jehp_625_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Use of Artificial Intelligence for the Preoperative Diagnosis of Pulmonary Lesions—ScienceDirect. [(accessed on 1 August 2024)]. Available online: https://www.sciencedirect.com/science/article/abs/pii/0003497589905924.
- 66.Etienne H., Hamdi S., Roux M.L., Camuset J., Khalife-Hocquemiller T., Giol M., Debrosse D., Assouad J. Artificial intelligence in thoracic surgery: Past, present, perspective and limits. Eur. Respir. Rev. 2020;29:200010. doi: 10.1183/16000617.0010-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.The International Epidemiology of Lung Cancer: Geographical Distribution and Secular Trends—Journal of Thoracic Oncology. [(accessed on 1 August 2024)]. Available online: https://www.jto.org/article/S1556-0864(15)30445-7/fulltext. [DOI] [PubMed]
- 68.Ross C., Swetlitz I. IBM’s Watson supercomputer recommended ‘unsafe and incorrect’cancer treatments, internal documents show. Stat. 2018;25:1–10. [Google Scholar]
- 69.A Prospective Blinded Study of 1000 Cases Analyzing the Role of Artificial Intelligence: Watson for Oncology and Change in Decision Making of a Multidisciplinary Tumor Board (MDT) from a Tertiary Care Cancer Center.|Journal of Clinical Oncology. [(accessed on 1 August 2024)]. Available online: https://ascopubs.org/doi/10.1200/JCO.2019.37.15_suppl.6533.
- 70.Rajpurkar P., Irvin J., Zhu K., Yang B., Mehta H., Duan T., Ding D., Bagul A., Langlotz C., Shpanskaya K., et al. CheXNet: Radiologist-Level Pneumonia Detection on Chest X-Rays with Deep Learning. arXiv. 2017 doi: 10.48550/arXiv.1711.05225.1711.05225 [DOI] [Google Scholar]
- 71.Ethical and Legal Challenges of Artificial Intelligence-Driven Healthcare—ScienceDirect. [(accessed on 14 June 2024)]. Available online: https://www.sciencedirect.com/science/article/pii/B9780128184387000125?via%3Dihub.
- 72.Rodrigues R. Legal and human rights issues of AI: Gaps, challenges and vulnerabilities. J. Responsible Technol. 2020;4:100005. doi: 10.1016/j.jrt.2020.100005. [DOI] [Google Scholar]
- 73.The Ethics of AI in Health Care: A Mapping Review—ScienceDirect. [(accessed on 14 June 2024)]. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0277953620303919?via%3Dihub.
- 74.Naik N., Hameed B.M.Z., Shetty D.K., Swain D., Shah M., Paul R., Aggarwal K., Ibrahim S., Patil V., Smriti K., et al. Legal and Ethical Consideration in Artificial Intelligence in Healthcare: Who Takes Responsibility? Front. Surg. 2022;9:862322. doi: 10.3389/fsurg.2022.862322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Migliore M. VATS surgery for anatomical lung resection: A different approach for every surgeon. Video-Assist. Thorac. Surg. 2016;1:31. doi: 10.21037/vats.2016.10.01. [DOI] [Google Scholar]
- 76.Albrecht J.P. REPORT on the Proposal for a Regulation of the European Parliament and of the Council on the Protection of Individuals with Regard to the Processing of Personal Data and on the Free Movement of Such Data (General Data Protection Regulation)|A7-0402/2013|European Parliament. [(accessed on 14 June 2024)]. Available online: https://www.europarl.europa.eu/doceo/document/A-7-2013-0402_EN.html.
- 77.There Is No Techno-Responsibility Gap|Philosophy & Technology. [(accessed on 14 June 2024)]. Available online: https://link.springer.com/article/10.1007/s13347-020-00414-7.
- 78.Clinical AI: Opacity, Accountability, Responsibility and Liability|AI & SOCIETY. [(accessed on 14 June 2024)]. Available online: https://link.springer.com/article/10.1007/s00146-020-01019-6.
- 79.Migliore M. Video-assisted thoracic surgery techniques for lung cancer: Which is better? Future Oncol. 2016;12:1–4. doi: 10.2217/fon-2016-0465. [DOI] [PubMed] [Google Scholar]
- 80.Migliore M., Halezeroglu S., Mueller M.R. Making precision surgical strategies a reality: Are we ready for a paradigm shift in thoracic surgical oncology? Future Oncol. 2020;16:1–5. doi: 10.2217/fon-2020-0279. [DOI] [PubMed] [Google Scholar]