Abstract
Rationale
There is no consensus on criteria to include in an asthma remission definition in real life. Factors associated with achieving remission after biologic initiation remain poorly understood.
Objectives
To quantify the proportion of adults with severe asthma achieving multidomain-defined remission after biologic initiation and identify prebiologic characteristics associated with achieving remission that may be used to predict it.
Methods
This was a longitudinal cohort study using data from 23 countries from the International Severe Asthma Registry. Four asthma outcome domains were assessed in the 1 year before and after biologic initiation. A priori–defined remission cutoffs were: 0 exacerbations/yr, no long-term oral corticosteroid (LTOCS), partly/well-controlled asthma, and percent predicted FEV1 ⩾ 80%. Remission was defined using two (exacerbations + LTOCS), three (+control or +lung function), and four of these domains. The association between prebiologic characteristics and postbiologic remission was assessed by multivariable analysis.
Measurements and Main Results
A total of 50.2%, 33.5%, 25.8%, and 20.3% of patients met criteria for two-, three- (+control), three- (+lung function), and four-domain remission, respectively. The odds of achieving four-domain remission decreased by 15% for every additional 10 years of asthma duration (odds ratio, 0.85; 95% confidence interval, 0.73–1.00). The odds of remission increased in those with fewer exacerbations per year, lower LTOCS daily dose, better control, and better lung function before biologic initiation.
Conclusions
One in five patients achieved four-domain remission within 1 year of biologic initiation. Patients with less severe impairment and shorter asthma duration at initiation had a greater chance of achieving remission after biologic treatment, indicating that biologic treatment should not be delayed if remission is the goal.
Keywords: anti-IgE, anti-IL5/5R, anti-IL4Rα, exacerbation, lung function
At a Glance Commentary
Scientific Knowledge on the Subject
Asthma remission has been defined in many ways. Previous studies to identify predictors of remission have predominantly been retrospective or post hoc analyses from randomized controlled trials, limited to a single jurisdiction, have included relatively small numbers of patients, and/or investigated remission achievable with a single biologic.
What This Study Adds to the Field
In this longitudinal cohort real-life study including data from 23 countries, 20.3–50.2% of patients with severe asthma met criteria for clinical remission within 1 year of biologic treatment depending on domains included in the remission definition. Patients with less severe disease and shorter duration of asthma before biologic initiation had a better chance of achieving remission after biologic treatment. Our results suggest the need to consider earlier intervention with biologics for patients with severe asthma before significant and irreversible lung function impairment (partly as a consequence of repeated exacerbations) and before initiation of long-term oral corticosteroid treatment. Recognition that remission is more likely to occur if targeted earlier in the asthma life cycle may influence biologic prescription criteria and herald a paradigm shift away from targeting response in those with more severe asthma, toward the promotion of remission in those with less severe disease but at risk of developing severe asthma, but this will need to be confirmed.
Clinical studies and asthma treatment goals for adults with severe asthma have focused on biologic effectiveness and disease control, respectively, rather than remission as a therapeutic target (1). The existence of spontaneous remission in the population of adults with asthma (2–5), coupled with the chronic inflammatory nature of asthma and a similar treatment development trajectory as other chronic inflammatory conditions in which remission on treatment is well defined (6–8), led to the hope that the asthma management paradigm could undergo a similar shift from asthma control to asthma remission (9). Indeed, recently, there has been a shift in asthma management, with the concept of remission included in four national guidelines (10). To date, remission is not included as a therapeutic target by the Global Initiative for Asthma (GINA), although good control of symptoms, normal activity levels, and minimization of exacerbations, persistent airflow limitation, and side effects are listed as long-term goals (1).
Remission has been defined as “clinical,” “functional,” “immunological,” and “deep” (all criteria) remission (11). Expert consensus also defined clinical remission as the absence of asthma symptoms, optimization/stabilization of lung function, patient/provider agreement regarding disease remission, and no systemic oral corticosteroid (OCS; minimum duration of 12 mo). Objective resolution of asthma-related inflammation and, if appropriate, negative bronchial hyperresponsiveness was additionally required for complete remission (6). Recently updated national asthma guidelines from Germany, Spain, and Italy all agree on no exacerbations, no systemic corticosteroids, good asthma control, or no asthma-related symptoms and stable lung function as remission criteria (10). In Italy, OCS use was considered the central tenant of “partial” and “complete” clinical remission; the latter required the complete absence of asthma symptoms and exacerbations and stable lung function for ⩾12 months, and the former required any two of these criteria over the same time frame (12). These definitions will be part of the 2023 GINA Italy update (10).
There is, however, some variability in remission domains and cutoffs recommended by these guidelines. For example, a lung function criterion was not incorporated into the 2023 update of the Japanese Practical Guidelines for Asthma Management (10). Moreover, good asthma control definitions ranged from “no asthma-related symptoms” in the German and Spanish guidelines, to an Asthma Control Test (ACT) score of ⩾23 or ⩾20 in the Japanese and Italian guidelines, respectively (10). Like our study, others have used an Asthma Control Questionnaire (ACQ)-5 cutoff of less than 1.5 as corresponding to GINA partly or well-controlled (13). Most recently, a U.S. expert consensus panel increased the rigor of current definitions to also include no missed work and limited inhaled corticosteroid (ICS) dose (low to medium) and short-acting β2-agonist use (⩽1/mo) (14).
The achievement of clinical remission after biologic treatment has varied widely, ranging from 12–43% (11, 13, 15–22), most likely due to the wide range of criteria used to define it, but also due to differences in study methodology and heterogeneity among study populations. Identified predictors of remission have included younger age, shorter duration of asthma, less comorbidity, preserved lung function at biologic initiation, and no (or low-dose) maintenance OCS. Patients with elevated blood eosinophil counts (BECs) and FeNO concentrations have also reached remission more frequently (11, 13, 16, 17, 20). However, these studies have used retrospective or post hoc analyses and/or have included relatively small numbers of patients.
Further research is needed to explore and test consensus-derived remission definitions, to align on criteria to include in a global definition, to ascertain the impact of each domain included, and to identify factors that predict severe asthma remission after biologic treatment in real life. The International Severe Asthma Registry (ISAR) offers a unique opportunity to do that (23–26). Our study aimed to quantify the proportion of adult patients with severe asthma achieving multidomain-defined remission when treated with biologic therapy in real life (overall and by biologic class) and to identify prebiologic characteristics associated with remission in these patients. Some of the results of this study have been previously reported in the form of abstracts (27, 28).
Methods
Study Design and Data Source
This was a longitudinal, before-to-after biologic initiation cohort study including data from 23 countries that shared data with ISAR (see Table E1 in the online supplement) (23, 25, 29) from May 1, 2017 to January 25, 2023. Biologic class categorization was based on first biologic used during the study period, regardless of subsequent changes (stop or switch) during follow-up (intention-to-treat approach). Pre– and post–biologic initiation outcomes were described across four domains in the 1 year before biologic initiation and as close as possible to 1 year after biologic initiation (Figure E1 and Table 1).
Table 1.
Asthma Outcome Domain Definitions and Timing of Pre- and Postbiologic Assessment
| Outcome | Definition | Prebiologic | Postbiologic |
|---|---|---|---|
| Annualized exacerbation rate |
|
1 yr before biologic initiation (or 48 wk minimum) | Annualized after biologic initiation (number of events assessed for a minimum of 48 wk and a maximum of 80 wk after biologic initiation) |
| Asthma control* | At biologic initiation (or assessment closest to biologic initiation up to a maximum of 1 yr before biologic initiation) | Closest to 1 yr after biologic initiation (24 wk minimum and 80 wk maximum) | |
| Daily LTOCS dose† |
|
At biologic initiation | Closest to 1 yr after biologic initiation (24 wk minimum and 80 wk maximum) |
| Lung function‡ |
|
At biologic initiation (or assessment closest to biologic initiation up to a maximum of 1 yr before biologic initiation) | Closest to 1 yr after biologic initiation (24 wk minimum and 80 wk maximum) |
Definition of abbreviations: ACQ = Asthma Control Questionnaire; ACT = Asthma Control Test; ER = emergency room; GINA = Global Initiative for Asthma; LTOCS = long-term oral corticosteroid; OCS = oral corticosteroid; ppFEV1 = percent predicted FEV1.
Some countries use ACQ and/or ACT to assess control. In these instances, ACQ and/or ACT control categories were fitted to GINA 2020 control categories as follows: Mean ACQ: well controlled (⩽0.75), partly controlled (>0.75 to <1.5), uncontrolled (⩾1.5). Total ACT: well controlled (>19), partly controlled (>15 to ⩽19), uncontrolled (⩽15). A summary of control test used by each country is provided in the online supplement (Table E2).
In cases when there were different periods with different doses before biologic initiation, the most recent dose (i.e., closest to biologic initiation) was used. For postbiologic dose and if changed from prebiologic dose, the new dose closest to 1 year after biologic initiation (minimum 24 wk, maximum 80 wk) was used and the date of change used to calculate the follow-up time.
Postbronchodilator used if available and prebronchodilator used otherwise, while ensuring that pre- and postbiologic measures were both either pre- or post-bronchodilator. Post-bronchodilator measurements were used for 61.6% of patients with available prebiologic ppFEV1 (n = 2,705). The remaining 38.4% of patients were all treated with inhaled corticosteroid/long-acting β2-agonist (i.e., bronchodilator not specifically withheld).
Patients
Patients were required to be ⩾18 years old at biologic initiation and have severe asthma (i.e., receiving treatment at GINA 2018 step 5 or with uncontrolled asthma at GINA step 4) (30). Uncontrolled asthma for registry inclusion was defined as having severe asthma symptoms or frequent exacerbations (two or more per year) requiring OCS. Patients were also required to be treated with anti-IgE, anti-IL5/5R, or anti-IL4Rα; have available registry data before, or on, biologic initiation date for one or more study domain; and have follow-up data (as close to 1 year as possible). The presence of significant disease impairment at baseline was not required. Those with a history of bronchial thermoplasty were excluded.
Variables
Key patient demographic (e.g., age, sex, body mass index [BMI], smoking history) and prebiologic asthma clinical characteristics (e.g., asthma onset and duration, biomarker levels, treatment, and comorbidity history) were collected (Tables 2 and 3).
Table 2.
Patient Demographic and Clinical Characteristics before Biologic Initiation Overall and by Biologic Class
| Total (N = 3,717) | Anti-IgE (n = 1,390) | Anti-IL5/5R (n = 2,021) | Anti-IL4Rα (n = 306) | |
|---|---|---|---|---|
| Age at biologic initiation, yr, median (Q1, Q3) | 54 (43, 63) | 50 (40, 59) | 56 (46, 65) | 52 (41, 62) |
| Sex, N | 3,715 | 1,389 | 2,020 | 306 |
| Female | 2,305 (62.0) | 902 (64.9) | 1,214 (60.1) | 189 (61.8) |
| Ethnicity, N | 3,717 | 1,390 | 2,021 | 306 |
| White | 2,616 (70.4) | 982 (70.6) | 1,438 (71.2) | 196 (64.1) |
| Southeast Asian | 118 (3.2) | 59 (4.2) | 52 (2.6) | 7 (2.3) |
| Northeast Asian | 108 (2.9) | 25 (1.8) | 70 (3.5) | 13 (4.2) |
| African | 95 (2.6) | 36 (2.6) | 49 (2.4) | 10 (3.3) |
| Mixed | 68 (1.8) | 55 (4.0) | 7 (0.3) | 6 (2.0) |
| Other | 241 (6.4) | 89 (6.4) | 130 (6.4) | 22 (7.2) |
| Unknown/missing | 471 (12.7) | 144 (10.4) | 275 (13.6) | 52 (17.0) |
| BMI, kg/m2, N | 3,467 | 1,270 | 1,895 | 302 |
| Median (Q1, Q3) | 28.1 (24.4, 32.9) | 28.8 (25.1, 33.7) | 27.5 (24.0, 32.0) | 28.9 (24.8, 33.8) |
| Smoking status at Bx initiation, N | 2,692 | 978 | 1,479 | 235 |
| Current smoker | 74 (2.7) | 38 (3.9) | 29 (2.0) | 7 (3.0) |
| Ex-smoker | 791 (29.4) | 232 (23.7) | 479 (32.4) | 80 (34.0) |
| Never-smoker | 1,827 (67.9) | 708 (72.4) | 971 (65.7) | 148 (63.0) |
| Age of asthma onset, yr, N | 2,289 | 823 | 1,366 | 100 |
| Median (Q1, Q3) | 30 (14, 44) | 24 (10, 39) | 33 (18, 47) | 26 (10, 43) |
| Asthma duration,* yr, N | 2,289 | 823 | 1,366 | 100 |
| Median (Q1, Q3) | 19 (9, 34) | 20 (11, 34) | 18 (9, 34) | 22 (7, 34) |
| FEV1/FVC < 0.7, N | 2,646 | 1,390 | 1,433 | 238 |
| Yes | 1,398 (52.8) | 479 (49.1) | 811 (56.6) | 108 (45.4) |
| Pre-Bx highest BEC, 109 cells/L, N | 2,420 | 843 | 1,388 | 189 |
| Median (Q1, Q3) | 455 (230, 790) | 300 (200, 600) | 550 (300, 900) | 400 (200, 600) |
| Pre-Bx latest FeNO, ppb, N | 1,603 | 441 | 1,017 | 145 |
| Median (Q1, Q3) | 34 (18, 66) | 26 (14, 51) | 39 (21, 73) | 28 (16, 57) |
| Pre-Bx latest blood IgE count, IU/ml, N | 2,294 | 927 | 1,203 | 164 |
| Median (Q1, Q3) | 188 (75, 489) | 253 (114, 576) | 145 (53, 385) | 134 (33, 500) |
| Positive test to any allergen†, N | 1,730 | 739 | 892 | 99 |
| Yes | 1,378 (79.7) | 701 (94.9) | 609 (68.3) | 68 (68.7) |
| Medication use in the year preceding Bx initiation, N | 3,121 | 1,223 | 1599 | 299 |
| LAMA | 104 (3.3) | 46 (3.8) | 50 (3.1) | 8 (2.7) |
| Theophylline | 274 (8.8) | 114 (9.3) | 154 (9.6) | 6 (2.0) |
| LTRA | 1,378 (44.2) | 566 (46.3) | 659 (41.2) | 153 (51.2) |
| Macrolide | 368 (11.8) | 145 (11.9) | 170 (10.6) | 53 (17.7) |
| History of AR, N | 2430 | 987 | 1,186 | 257 |
| Yes | 1,274 (52.4%) | 600 (60.8%) | 570 (48.1%) | 104 (40.5%) |
| History of CRS, N | 2,860 | 1,063 | 1,543 | 254 |
| Yes | 1,471 (51.4) | 458 (43.1) | 880 (57.0) | 133 (52.4) |
| History of NP, N | 2,997 | 1,100 | 1,639 | 258 |
| Yes | 842 (28.1) | 196 (17.8) | 566 (34.5) | 80 (31.0) |
| History of osteoporosis, N | 3,154 | 1,259 | 1,604 | 291 |
| Yes | 485 (15.4) | 195 (15.5) | 258 (16.1) | 32 (11.0) |
| History of anxiety/depression, N | 3,172 | 1,226 | 1,669 | 277 |
| Yes | 481 (15.2) | 182 (14.8) | 245 (14.7) | 54 (19.5) |
| Eosinophilic gradient‡ (50), N | 2,901 | 714 | 2,021 | 166 |
| Grade 0 | 5 (0.2) | 5 (0.7) | 0 (0.0) | 0 (0.0) |
| Grade 1 | 62 (2.1) | 53 (7.4) | 0 (0.0) | 9 (5.4) |
| Grade 2 | 125 (4.3) | 109 (15.3) | 0 (0.0) | 16 (9.6) |
| Grade 3 | 2,709 (84.9) | 547 (76.6) | 2,021 (100.0) | 141 (84.9) |
Definition of abbreviations: AR = allergic rhinitis; BEC = blood eosinophil concentration; Bx = biologic; CRS = chronic rhinosinusitis; ISAR = International Severe Asthma Registry; LAMA = long-acting muscarinic antagonist; LTRA = leukotriene receptor antagonist; NP = nasal polyps; Q = quartile.
Data are presented as n (%) unless otherwise noted.
Age at biologic initiation minus reported age at asthma onset.
Except for the U.K. patients for whom no detail is available to ISAR (n = 471; 64.8% with a positive allergy test), ISAR collects data on test results for allergens in 11 categories: dust mite, grass mix, cat hair, mold mix, dog hair, Aspergillus, weed mix, trees, food mix, animal mix, and others. Patients with a reported positive test in at least one category were reported as positive; patients with at least one negative record and no positive records were reported as negative. A total of 1,230 patients had data available for at least two categories, of whom 256 (20.8%) were negative on all recorded tests, 250 (20.3%) were positive for one category only, and 724 (58.9%) were positive for at least two categories.
Note that patients receiving anti-IL5/5R were all categorized as “most likely” by the algorithm. Grade 0 (unlikely/noneosinophilic); Grade 1 (least likely); Grade 2 (likely); Grade 3 (most likely).
Table 3.
Prebiologic Asthma-related Outcomes Used in Remission Definitions
| Total (N = 3,717) | Anti-IgE (n = 1,390) | Anti-IL5/5R (n = 2,021) | Anti-IL4Rα (n = 306) | |
|---|---|---|---|---|
| Pre-Bx exacerbations,* N | 2,351 | 777 | 1,382 | 192 |
| 0 | 610 (25.9) | 221 (28.4) | 286 (20.7) | 103 (53.6) |
| 1 (not hospitalized) | 364 (15.5) | 126 (16.2) | 191 (13.8) | 47 (24.5) |
| 2 (not hospitalized) | 307 (13.1) | 100 (12.9) | 186 (13.5) | 21 (10.9) |
| ⩾1 (hospitalized) or ⩾3 in total | 1,070 (45.5) | 330 (42.5) | 719 (52.0) | 21 (10.9) |
| Pre-Bx LTOCS* dose, N | 3,094 | 1,076 | 1,824 | 194 |
| 0 mg/d (nonuser) | 1,852 (59.9) | 729 (67.8) | 974 (53.4) | 149 (76.8) |
| ⩽5 mg/d | 332 (10.7) | 98 (9.1) | 218 (12.0) | 16 (8.2) |
| >5 to 10 mg/d | 365 (11.8) | 100 (9.3) | 252 (13.8) | 13 (6.7) |
| >10 mg/d | 362 (11.7) | 105 (9.8) | 242 (13.3) | 15 (7.7) |
| User but missing dose | 183 (5.9) | 44 (4.1) | 138 (7.6) | 1 (0.5) |
| Pre-Bx asthma control,†‡ N | 1,808 | 637 | 1,095 | 76 |
| Well controlled | 189 (10.5) | 73 (11.5) | 104 (9.5) | 12 (15.8) |
| Partly controlled | 309 (17.1) | 88 (13.8) | 202 (18.4) | 19 (25.0) |
| Uncontrolled | 1,310 (72.5) | 476 (74.7) | 789 (72.1) | 45 (59.2) |
| Pre-Bx ppFEV1,†§ N | 2,705 | 995 | 1,472 | 238 |
| ⩾80% | 1,126 (41.6) | 412 (41.4) | 599 (40.7) | 115 (48.3) |
| <80% | 1,579 (58.4) | 583 (58.6) | 873 (59.3) | 123 (51.7) |
Definition of abbreviations: ACQ = Asthma Control Questionnaire; ACT = Asthma Control Test; Bx = biologic; GINA = Global Initiative for Asthma; LTOCS = long-term oral corticosteroid; ppFEV1 = percent predicted FEV1.
Data are presented as n (%) unless otherwise noted.
In the year preceding biologic initiation.
In the year preceding and closest to biologic initiation.
Assessed using either GINA control criteria (30), ACT (48), or ACQ (49). ACQ and/or ACT control categories were fitted to GINA 2020 control categories as follows: mean ACQ: well controlled (⩽0.75), partly controlled (>0.75 to <1.5), uncontrolled (⩾1.5). Total ACT: well controlled (>19), partly controlled (>15 to ⩽19), uncontrolled (⩽15).
Post-bronchodilator used if available, and prebronchodilator used otherwise, while ensuring that pre- and postbiologic measures were both either pre- or post-bronchodilator. Post-bronchodilator measurements were used for 61.6% of patients with available prebiologic ppFEV1 (n = 2,705). The remaining 38.4% of patients were all treated with inhaled corticosteroid/long-acting β2-agonist (i.e., bronchodilator not specifically withheld).
Asthma Outcome Domains, Timing of Assessments, and Remission Definitions
Definitions and timing of pre- and postbiologic outcomes are provided in Table 1. The asthma outcome domains used to define remission included exacerbation rate, long-term OCS (LTOCS) daily dose, asthma control (assessed using either GINA control criteria, ACT, or ACQ; Table E2), and percent predicted FEV1 (ppFEV1). ACQ and/or ACT control categories were fitted to GINA 2020 control categories as follows: mean ACQ: well controlled (⩽0.75), partly controlled (>0.75 to <1.5), uncontrolled (⩾1.5); total ACT: well controlled (>19), partly controlled (>15 to ⩽19), uncontrolled (⩽15). Similar cutoffs and correlations (31, 32) have been described and used by others (12, 13, 22). For FEV1, we used post-bronchodilator measures if available and prebronchodilator measures otherwise, while ensuring that pre- and postbiologic measures were both either before or after bronchodilator. Post-bronchodilator measurements were used for 61.6% of patients with available prebiologic ppFEV1 (n = 2,705). The remaining 38.4% of patients were all treated with ICS/long-acting β2-agonist (i.e., bronchodilator not specifically withheld).
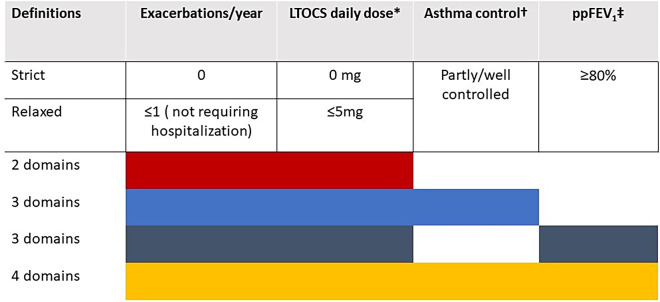
Domain choice was informed a priori by a previous ISAR study that examined pre- to postbiologic change in exacerbation rate, LTOCS use, asthma control, and lung function in patients categorized according to degree of prebiologic impairment and assessed the magnitude of improvement according to starting point and outcome assessed (33). Our domain choice and remission cutoffs were also informed by expert consensus (52 experts from 25 countries) (33) and aligned with findings of the expert consensus framework for asthma remission of Menzies-Gow and colleagues (i.e., 0 exacerbations, no LTOCS use, absence of significant symptoms, and optimized lung function) (6). Remission was characterized using two domains (i.e., exacerbation rate and LTOCS), three domains (i.e., exacerbation rate + LTOCS + asthma control OR exacerbation rate + LTOCS + ppFEV1), or all four asthma outcomes (Table 1). Remission cutoffs for each of these domains were also defined a priori and categorized as strict or relaxed (Figure 1). In this article, remission refers to strict remission in those who initiated biologics.
Figure 1.
Definitions of remission after biologic therapy using strict and relaxed domain cutoffs. *Prednisolone equivalent; †Control was assessed by Global Initiative for Asthma control criteria, Asthma Control Questionnaire, or Asthma Control Test; ‡Post-bronchodilator used if available, and prebronchodilator used otherwise, while ensuring that pre- and postbiologic measures were both either pre- or post-bronchodilator. Post-bronchodilator measurements were used for 61.6% of patients with available prebiologic percent predicted FEV1 (ppFEV1) (n = 2,705). The remaining 38.4% of patients were all treated with inhaled corticosteroid/long-acting β2-agonist (i.e., bronchodilator not specifically withheld). LTOCS = long-term oral corticosteroid.
Statistical Analyses
The statistical analysis plan was predefined. R version 4.1.0 (R Foundation for Statistical Computing) was used (34). The observed proportions of patients who met the criteria for each remission definition were described overall and by biologic class. A post hoc analysis was conducted to assess the proportion of patients meeting remission criteria in those with FEV1/FVC < 0.7 and FEV1/FVC ⩾ 0.7 No formal comparison between biologic classes was intended for these descriptive analyses. The associations between prebiologic characteristics and remission were analyzed using multivariable logistic regressions with remission (yes/no) as the outcome variable, using all proposed remission definitions. Patients with missing data for all asthma-related outcomes were excluded from the study, as well as patients with missing age and/or sex. However, patients with missing data for some but not all asthma-related outcomes were included in the analysis for the relevant outcomes. We did not conduct imputation of missing values. Significance was tested through log-likelihood ratios. Variables assessed for association with remission in the multivariable analyses included prebiologic characteristics that were statistically significant (P < 0.05) in a univariate analysis for any domain assessed (data not shown) or those informed by literature review and expert consensus. Analyses were adjusted for prebiologic asthma-related outcome included in the considered remission definition, age, and sex. Prebiologic asthma-related outcomes, biomarkers, asthma duration, and BMI were analyzed as continuous variables. The models were fitted overall and for each biologic class (not anti-IL4Rα because of small sample size). To test for difference between patients receiving anti-IgE and those receiving anti-IL5/5R, a single model was fitted in these patients, adding biologic class as an interaction term with the variables of interest.
Results
Patients
As of January 25, 2023, 14,284 patients were enrolled in ISAR. Of these, 6,816 initiated biologics, and 3,717 met all inclusion criteria and were included in one or more analyses (Figure E2). Most exclusions occurred because of lack of pre- (n = 715; 10.5%), or postbiologic data (n = 1,956; 28.7%) (Table E3). A total of 1,390, 2,021, and 306 patients received anti-IgE, anti-IL5/R, and anti-IL4Rα, respectively. The median duration of treatment was 1 year. Biologic interruption or switching was reported in 6.6% and 3.2% of patients, respectively (Table E4). The United States (n = 1,131; 30.4%), United Kingdom (n = 487; 13.1%), and Italy (n = 438; 11.8%) contributed the most patients (Table E1). The number of patients included in each analysis varied according to data availability for multiple domains (Figure E2).
Patient Demographic and Clinical Characteristics before Biologics
Patients were predominantly White (80.6%; n = 2,616/3,246), with a tendency for more females (62.0%; n = 2,305/3,715) and never-smokers (67.9%; n = 1,827/2,692), with a median age of 30 (quartile 1 [Q1], Q3: 14, 44) years at asthma onset and an asthma duration of 19 (Q1, Q3: 9, 34) years (Table 2). Median age and BMI at study entry were 54 (Q1, Q3: 43, 63) years and 28.1 (Q1, Q3: 24.4, 32.9) kg/m2, respectively. Biomarkers indicative of T2-high disease were all elevated, and 84.9% (n = 2,709/2,901) had an eosinophilic phenotype. Most patients (79.7%; n = 1,378/1,730) had a positive allergy test (i.e., to dust mite, grass mix, cat hair, mold mix, dog hair, Aspergillus, weed mix, trees, food mix, animal mix, and/or others), with 96.9% of patients (n = 1,040/1,073) with available data for at least one category (excluding the United Kingdom, which does not provide type of allergen data to ISAR) testing positive to an aeroallergen. The prevalence of T2-related comorbidities was 52.4% (n = 1,274/2,430), 51.4% (n = 1,471/2,860), and 28.1% (n = 842/2,997) for allergic rhinitis, chronic rhinosinusitis (CRS), and nasal polyposis, respectively (Table 2). In 2,278 patients with information on both allergic rhinitis and CRS, 700 (30.7%) reported both comorbidities. The prevalence of other comorbidities is provided in Table E1. Before biologic treatment, 45.5% of patients (n = 1,070/2,351) experienced one or more exacerbations requiring hospitalization or three or more exacerbations in total, 40.1% (n = 1,242/3,094) were treated with LTOCS, 72.5% (n = 1,310/1,808) had uncontrolled asthma, and 58.4% (n = 1,579/2,705) had a ppFEV1 < 80% (Table 3). Patients who subsequently initiated anti-IL5/5R tended to have more severe disease in terms of greater exacerbation burden and LTOCS use, and those who subsequently initiated anti-IL4Rα had less severe disease for all considered domains (Table 3). Those who subsequently achieved remission (any definition) after biologic initiation also had less severe disease at baseline than those who did not subsequently meet remission criteria, and they also tended to have a lower BMI, be older at asthma onset, have shorter disease duration, and have a higher BEC, a positive allergen test, and CRS before biologic treatment (Table E5).
Proportion of Patients in Remission
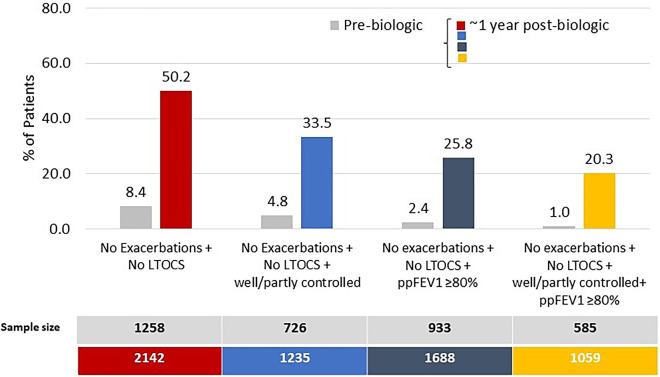
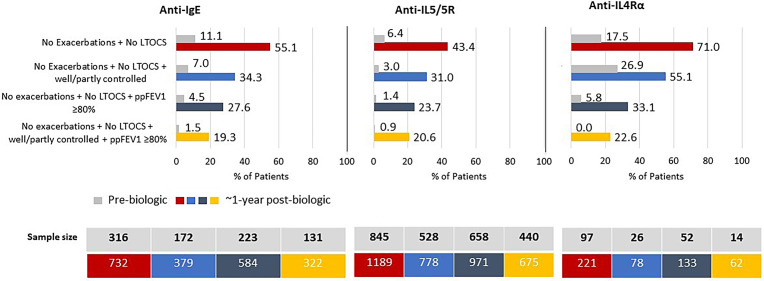
The percentage of patients in remission depended on the number of asthma outcome domains included in the definition, highest (50.2%; n = 1,076/2,142) for two-domain remission and lowest (20.3%; n = 215/1,059) for four-domain remission (Figure 2 and Table E6). The addition of lung function to the two-domain remission definition decreased the remission rate (25.8%; n = 435/1,688) to a greater degree than the addition of control status (33.5%; n = 414/1,235) (Figure 2 and Table E6). Remission was also achievable in those with evidence of irreversible airflow limitation, albeit less likely; 11.3% (n = 50/444) of those with prebiologic FEV1/FVC < 0.7 achieved four-domain remission, as did 25.4% (n = 88/347) of those with FEV1/FVC ⩾ 0.7 (Table E7). A small proportion of patients met remission criteria before biologic initiation, highest for two-domain remission (8.4%; n = 106/1,258) and lowest for four-domain remission (1.0%; n = 6/585) (Figure 2 and Table E8). Remission prevalence for patients treated with anti-IgE, anti-IL5/5R, and anti-IL4Rα ranged from 19.3–55.1%, 20.6–43.4%, and 22.6–71.0%, respectively (Figure 3).
Figure 2.
Percentage of patients in remission (strict criteria) before and after biologic treatment. ppFEV1 = percent predicted FEV1; LTOCS = long-term oral corticosteroid.
Figure 3.
Percentage of patients in remission (strict criteria) before and after treatment with anti-IgE, anti-IL5/5R, or anti-IL4Rα. LTOCS = long-term oral corticosteroid; ppFEV1 = percent predicted FEV1.
The prevalence of post–biologic initiation remission defined using the relaxed cutoffs was higher, ranging from 29.1% to 75.2% (Figure E3). Biologic class remission rates, using relaxed cutoffs, ranged from 25.7–78.0% for anti-IgE, 30.8–70.6% for anti-IL5/5R, and 29.0–90.0% for anti-IL4Rα (Figure E4). Tables E6 and E8 show a detailed breakdown of remission prevalence before and after biologic therapy.
Association between Prebiologic Characteristics and Remission (Multivariable Analyses)
Disease severity
In general, the odds of remission were increased in those with less severe disease evidenced by fewer exacerbations per year, lower LTOCS daily dose, better asthma control, and better lung function in the 1-year pre–biologic initiation period (Figures 4A and 4B and Table E9). For four-domain remission, the odds of remission decreased by 12% (95% confidence interval [CI], 0.80–0.97) for each additional exacerbation per year experienced before biologic initiation, and by 41% (95% CI, 0.45–0.77) for each additional 5-mg/d increment of LTOCS received before biologic initiation. The odds of achieving four-domain remission increased by 1.34 (95% CI, 0.91–1.97) and 1.29 (95% CI, 1.20–1.38) for each GINA control category improvement and each 5% ppFEV1 increment improvement before biologic initiation, respectively (Figure 4B). A similar association pattern was noted for two-domain (Figure E5A) and three-domain (+lung function) remission (Figure E5B) and for both anti-IgE and anti-IL5/5R, but generally with greater odds of remission for the latter (Figures E6–E9). Similar findings were also noted when results were adjusted by country, although the exacerbation odds ratio (OR) was attenuated (Table E10).
Figure 4.
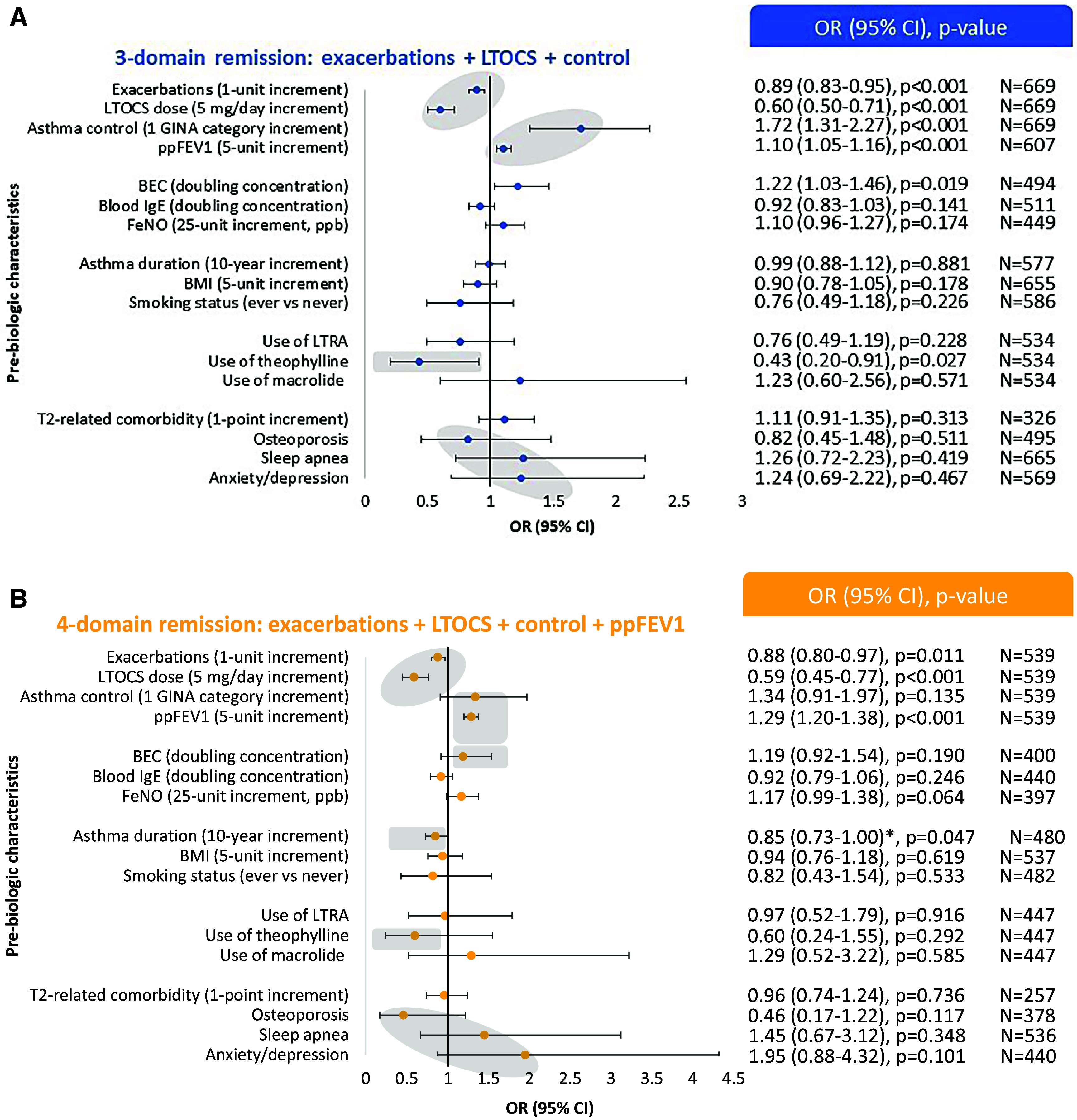
Association between selected prebiologic characteristics and (A) three-domain and (B) four-domain asthma remission in patients with severe asthma. Three-domain remission: 0 exacerbations/yr + no long-term oral corticosteroids (LTOCS) + well- or partly controlled asthma. Four-domain remission: 0 exacerbations/yr + no LTOCS + well- or partly controlled asthma + percent predicted FEV1 (ppFEV1) ⩾ 80%. Gray zones highlight association patterns. *Prebiologic lung function adjustment removed. Asthma duration: age at biologic initiation minus reported age at asthma onset. All odds ratios (ORs) were adjusted for prebiologic asthma-related outcome, including in the considered remission definition, as well as for age and sex. BEC = blood eosinophil count; BMI = body mass index; CI = confidence interval; GINA = Global Initiative for Asthma; LTRA = leukotriene receptor antagonist.
Biomarkers
Higher BEC (but not blood IgE or FeNO) concentration were associated with greater odds of remission (Figures 4, E5A, and E5B), particularly noted for anti-IL5/5R (Figures E6–E9), and slightly attenuated when adjusted by country, although the trend remained (Table E10).
Asthma duration
Shorter asthma duration was also associated with greater odds of remission (all definitions except three-domain remission (+control); Figures 4 and E5). Patients had 15% lower odds of achieving four-domain remission (OR, 0.85; 95% CI, 0.73–1.00) (Figure 4B). The same estimate was achieved when adjusted by country (Table E10). Similar findings were observed when restricting the study population to patients aged ⩾20 years at asthma onset (OR, 0.87; 95% CI, 0.67–1.14) and was not solely driven by lung function, being still apparent (although attenuated) when adjusted for pre–biologic initiation ppFEV1 (0.94; 95% CI, 0.79–1.13) (Table E9).
Other prebiologic variables
Neither BMI nor smoking status was associated with remission (any definition). Prescription for theophylline (but not leukotriene receptor antagonist or macrolide) was negatively associated with the odds of remission, with similar findings noted on country adjustment (Table E10). Although T2-related comorbidity score was not associated with remission (with or without country adjustment), those without a history of osteoporosis and with a history of sleep apnea or anxiety/depression tended to have a greater odds of achieving remission, although the confidence intervals were wide (Figures 4 and E5).
Discussion
To our knowledge, this is the largest study reporting prevalence of remission before and after biologic initiation and correlates of remission after biologic treatment for patients with severe asthma in real life. Multiple-domain severe asthma remission was achievable in real life, along a gradation according to number and type of domains included in its definition, in a broad, heterogeneous severe asthma population, many of whom would be excluded from randomized controlled trials. One in five patients with severe asthma met the criteria for clinical remission in all four domains within 1 year of biologic initiation, increasing to one in two patients when remission included exacerbation plus LTOCS outcome domains only (indicative of bronchial inflammation and most effectively targeted by biologic therapy). These findings lend further weight to GINA recommendations to avoid LTOCS, if possible, in severe asthma (i.e., because of potential for adverse events, many of which do not reverse upon discontinuation, plus now with a negative association with remission). Importantly, patients with less severe disease and shorter duration of asthma before biologic initiation had a better chance of achieving remission after biologic treatment.
To date, several studies have assessed the remission of severe asthma after biologic therapy (11, 13, 15–18, 21, 22). Three-domain biologic-associated remission rates (excluding lung function) were remarkably similar across studies: 37.6% using data from the German Asthma Net severe asthma cohort (17), 37.0% in a post hoc analysis using data from the REDES (Real-World Effectiveness and Safety of Mepolizumab) study (16), and 33.5% in the current study. Although remission definitions used in these studies frequently included the same domains, domain-specific criteria differed among them, making cross-comparisons difficult (10, 13–16). The prevalence of four-domain biologic-associated remission (including lung function) ranged from 14.5% to 43.0%, (20.3% in the current study) (13, 15–18, 20–22), varying according to lung function criterion applied, patient cohort, and biologic. Examples of previously used lung function remission criteria include ppFEV1 > 80 (as in the present study) (22), an objective assessment of normal lung function (2), and an FEV1 above the lower limit of normal or no more than 100 ml less than baseline (13). We consider inclusion of a high lung function hurdle an important component of clinical remission, as it is representative of lung function optimization (6) and may encourage earlier intervention with targeted treatment before irreversible lung damage. We also acknowledge the difficulty in achieving it in patients who frequently exhibit limited reversibility (35–37), the lack of consensus in defining lung function optimization/stabilization (38), and the ongoing debate on whether a lung function domain, used in sentinel remission papers (39) and national guidelines (10), should be included as a remission criterion. Of note, a reduced FEV1 can be due to other nonasthma factors and, therefore, be unrelated to the presence of remission.
Although severe asthma remission is achievable in some patients when treated with a biologic in real life, other patients receiving the same treatment failed to achieve it (13, 22). This is likely due to a complex interplay of factors, including the heterogeneity of asthma itself, the timing of biologic intervention and assessment of remission, the presence of nonreversible airflow obstruction, and the negative impact of comorbidities on asthma control (40). Understanding why certain patients with severe asthma treated with biologics fail to achieve remission is arguably just as important as predicting those who do achieve it. This represents an important unmet need, which requires consideration of the pathway to remission and national variability in biologic access (26), but may also warrant the adoption of an alternative concept of remission (e.g., personalized remission) and/or a different approach to achieve it (e.g., more effective or alternative interventions).
Some important points emerged when remission rate was assessed by biologic class. First, remission was noted for all classes assessed. Second, the addition of the lung function domain (to exacerbations plus LTOCS) had a consistently greater negative impact on remission rate than the addition of asthma control. And third, although the two- and three-domain remission (plus control or plus lung function) rates appeared higher for IL4Rα, caution in interpretation should be used because of small patient numbers, less severe impairment before biologic initiation, and the greater prevalence of patients in remission before treatment in this group. Notably, when the more stringent four-domain remission definition was applied, remission rates were similar across all biologic classes (approximately 20%), irrespective of inherent intergroup differences. We also noted a small proportion of patients in remission before biologic initiation (up to 1.5% for four-domain remission), which may be indicative of differences in biologics we use worldwide (26); an artifact of underreporting during the coronavirus disease (COVID-19) pandemic; better management in severe asthma centers, including optimization of inhaled treatments and comorbidity management; and improved adherence before biologic treatment (20). Also, it is possible that some patients were incorrectly categorized as being in remission before biologic initiation.
Prebiologic correlates of remission were consistent across remission definitions. However, in contrast to what has been formerly observed with biologic response, where greater response is associated with greater pre–biologic initiation disease severity (41–43), for remission, those with less impairment before biologic treatment had greater odds of achieving remission. Patients had 29% increased odds of achieving four-domain remission for every 5% greater ppFEV1 and were 41% less likely, respectively, to achieve remission for every additional 5 mg/d of LTOCS prescribed before biologic initiation. Others reported similar findings, but these studies have been small by comparison, national in scope, have investigated remission achievable with a single biologic, and/or assessed remission predictors by univariate analysis (11, 13, 16). A post hoc analysis of the REDES study, for example, found that compared with those who did not achieve clinical remission, those who achieved four-domain remission were more likely to have better prebiologic asthma control (ACT score: 15.9 vs. 13.7), lower median OCS dose (10.0 vs. 6.3 mg/d), and better lung function (ppFEV1: 71.2% vs. 86.9%) (16). Similarly, a study in Japanese patients with severe asthma found that those with a ppFEV1 ⩾ 75% were 3.38 times more likely to achieve three-domain clinical remission (11). A United Kingdom study found that the odds of remission were 7.44-fold higher in patients with high T2 biomarkers and lower for those who were female, obese, or had poorly controlled severe asthma before biologic initiation (13).
The shorter duration of asthma as a remission predictor in the current study is particularly relevant and could indicate that the path to remission should start as early as possible. Our finding has been corroborated by data from both the United Kingdom and Denmark, the former showing that the likelihood of remission reduced by 14% for every 10-year increase in disease duration (13, 22). Others reported that patients with an asthma diagnosis made after the age of 12 years were 1.9 times more likely to achieve three-domain clinical remission (17) and that greater improvements in lung function, when treated with tezepelumab compared with placebo, were observed in patients with a disease duration <20 years (44). This phenomenon is likely a consequence of accelerated lung function decline in those patients who frequently exacerbate (most marked in those <40 yr of age) (45) or due to limited efficacy of ICSs in preventing long-term lung function decline in some patients (or due to poor adherence or underprescription). Indeed, the ORs for asthma duration were attenuated when adjusted for prebiologic ppFEV1. In contrast to response, elevated FeNO concentrations were not consistently associated with increased odds of remission in our study, possibly as this biomarker may be better at predicting those who do badly without treatment rather than in predicting those who will do better while treated, or due to the fact that anti-IL4Rα is underrepresented in our study. An association with persistently high FeNO concentrations may have been observed but requires further study. The finding of a positive association of elevated BEC and higher odds of remission (particularly for anti-IL5/5R) is notable and an important treatable trait, although a selection bias for those with elevated BEC in the anti-IL5/5R group cannot be discounted.
Limitations of the current study include missing data, the relatively small number of anti-IL4Rα–treated patients, lack of patient matching between biologic classes, and the risk of multiplicity. Assessing generalizability is difficult, so although our study included a large cohort of patients with severe asthma from 23 countries, caution should be used when extrapolating results to the wider asthma population. Use of three tools to assess asthma control (i.e., GINA, ACT, and ACQ) could be considered a limitation. However, these are all validated with good intertest correlation (31, 32) and reflect intercountry variability in how asthma control is assessed in real life, including variability in control tools required for biologic eligibility and reimbursement, although this has been mitigated to some extent by adjusting for country. In addition, although remission can also be defined as a prolonged period with low to no disease activity, this goes beyond the scope of our study, which assessed disease activity at ∼1 year after biologic initiation. Inclusion of a patient-reported outcome measure in a remission definition may also strengthen our concept of what remission means to patients.
Strengths included the use of routinely collected clinical and functional domains to define remission, facilitating replication and validation globally. We included a large, real-life, and heterogeneous population with severe asthma treated with biologic therapy, with sufficient data to categorize remission using multiple domain definitions and using both strict and relaxed cutoffs for biologics overall and by class. The very low prevalence of remission before biologic initiation, coupled with the observed negative association of prebiologic impairment with odds of remission, indicates that the results were unlikely affected by inclusion of patients already in remission at baseline. Our study also investigated the likelihood of achieving remission using a large number of prebiologic variables used in routine management and included many patients not eligible for inclusion in randomized controlled trials. New directions and opportunities for future research include the assessment of remission duration (on treatment), because the occurrence of temporary remission cannot be discounted (46, 47). Remission prevalence at later time points and according to the American Thoracic Society definition (14), the persistence of remission upon treatment discontinuation, and the impact of earlier biologic initiation on disease trajectory should also be investigated. Future studies could also investigate the concepts of complete and long-term remission, including objective resolution of asthma-related inflammation and lung function stabilization (rather than optimization) as remission criteria, in line with the remission consensus framework (6) and recent national asthma management guidelines (10).
Our findings have tested the sensitivity of asthma remission definitions in the largest severe asthma cohort in the world, shown how the proportion of patients categorized as in remission is affected by some domains more than others, and, by identifying a wide range of prebiologic factors associated with remission, brought us one step closer to accurate remission prediction in real life. Although remission is the ultimate goal of asthma management, it occurs in a relatively small proportion of patients treated with current biologics. This may suggest the need to consider switching biologic therapies if remission is not achieved, use of biologic combinations, and use of biologics earlier to give patients the best chance of achieving remission, but further research is needed. If remission is the target, guidelines should reflect that, and treatment approaches/strategies in selected patients most likely to achieve it may be recommended (pending confirmation).
Supplemental Materials
Acknowledgments
Acknowledgment
The authors thank Drs. David Jackson and Diahn-Warng Perng for their contribution to study design and result interpretation as part of the Full BEAM (Biologic treatment rEsponders in severe AsthMa patients) working group; Drs. Kenneth Chapman, Susanne Hansen, Konstantinos Kostikas, and Jorge Maspero as members of the FULL BEAM working group and for review of early drafts of this article; Drs. Arnaud Bourdin, Deirdre Long, Nicolas Roche, Carlos Andres Celis Preciado, and Ivan Solarte Rodrigues for review of the article outline; Aaron Beastall for data interpretation and analyses; and Prof. William Henley for statistical advice. They also thank Dr. Ruth B. Murray (Medscript NZ Ltd) for assistance with drafting and editing this manuscript. The authors also thank the International Severe Asthma Registry collaborators (see online supplement).
Footnotes
Supported by Optimum Patient Care Global and AstraZeneca Ltd. This study was conducted by the Observational and Pragmatic Research Institute Pte Ltd. No funding was received by the Observational and Pragmatic Research Institute Pte Ltd for its contribution.
Author Contributions: Conceptualization and design: all authors. Formal analysis: G.S., L.B, and D.B.P. Data interpretation: all authors. Supervision: V.A.C. and D.B.P. Manuscript preparation and review: all authors.
A data supplement for this article is available via the Supplements tab at the top of the online article.
Originally Published in Press as DOI: 10.1164/rccm.202311-2192OC on May 3, 2024
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Global Initiative for Asthma. https://ginasthma.org/wp-content/uploads/2023/05/GINA-2023-Full-Report-2023-WMS.pdf
- 2. Tuomisto LE, Ilmarinen P, Niemelä O, Haanpää J, Kankaanranta T, Kankaanranta H. A 12-year prognosis of adult-onset asthma: Seinäjoki Adult Asthma Study. Respir Med . 2016;117:223–229. doi: 10.1016/j.rmed.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 3. Almqvist L, Rönmark E, Stridsman C, Backman H, Lindberg A, Lundbäck B, et al. Remission of adult-onset asthma is rare: a 15-year follow-up study. ERJ Open Res . 2020;6:00620–02020. doi: 10.1183/23120541.00620-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Westerhof GA, Coumou H, de Nijs SB, Weersink EJ, Bel EH. Clinical predictors of remission and persistence of adult-onset asthma. J Allergy Clin Immunol . 2018;141:104–109.e3. doi: 10.1016/j.jaci.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 5. Rönmark E, Lindberg A, Watson L, Lundbäck B. Outcome and severity of adult onset asthma: report from the obstructive lung disease in northern Sweden studies (OLIN) Respir Med . 2007;101:2370–2377. doi: 10.1016/j.rmed.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 6. Menzies-Gow A, Bafadhel M, Busse WW, Casale TB, Kocks JWH, Pavord ID, et al. An expert consensus framework for asthma remission as a treatment goal. J Allergy Clin Immunol . 2020;145:757–765. doi: 10.1016/j.jaci.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 7. Schlager L, Loiskandl M, Aletaha D, Radner H. Predictors of successful discontinuation of biologic and targeted synthetic DMARDs in patients with rheumatoid arthritis in remission or low disease activity: a systematic literature review. Rheumatology (Oxford) . 2020;59:324–334. doi: 10.1093/rheumatology/kez278. [DOI] [PubMed] [Google Scholar]
- 8. Smolen JS, Breedveld FC, Burmester GR, Bykerk V, Dougados M, Emery P, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis . 2016;75:3–15. doi: 10.1136/annrheumdis-2015-207524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nannini LJ. Treat to target approach for asthma. J Asthma . 2020;57:687–690. doi: 10.1080/02770903.2019.1591443. [DOI] [PubMed] [Google Scholar]
- 10. Lommatzsch M, Buhl R, Canonica GW, Ribas CD, Nagase H, Brusselle GG, et al. Pioneering a paradigm shift in asthma management: remission as a treatment goal. Lancet Respir Med . 2024;12:96–99. doi: 10.1016/S2213-2600(23)00415-0. [DOI] [PubMed] [Google Scholar]
- 11. Oishi K, Hamada K, Murata Y, Matsuda K, Ohata S, Yamaji Y, et al. A real-world study of achievement rate and predictive factors of clinical and deep remission to biologics in patients with severe asthma. J Clin Med . 2023;12:2900. doi: 10.3390/jcm12082900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Canonica GW, Blasi F, Carpagnano GE, Guida G, Heffler E, Paggiaro P, et al. Severe Asthma Network Italy definition of clinical remission in severe asthma: a Delphi consensus. J Allergy Clin Immunol Pract . 2023;11:3629–3637. doi: 10.1016/j.jaip.2023.07.041. [DOI] [PubMed] [Google Scholar]
- 13. McDowell PJ, McDowell R, Busby J, Eastwood MC, Patel PH, Jackson DJ, et al. UK Severe Asthma Registry Clinical remission in severe asthma with biologic therapy: an analysis from the UK Severe Asthma Registry. Eur Respir J . 2023;62:2300819. doi: 10.1183/13993003.00819-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blaiss M, Oppenheimer J, Corbett M, Bacharier L, Bernstein J, Carr T, et al. Consensus of an American College of Allergy, Asthma, and Immunology, American Academy of Allergy, Asthma, and Immunology, and American Thoracic Society workgroup on definition of clinical remission in asthma on treatment. Ann Allergy Asthma Immunol . 2023;131:782–785. doi: 10.1016/j.anai.2023.08.609. [DOI] [PubMed] [Google Scholar]
- 15. Menzies-Gow A, Hoyte FL, Price DB, Cohen D, Barker P, Kreindler J, et al. Clinical remission in severe asthma: a pooled post hoc analysis of the patient journey with benralizumab. Adv Ther . 2022;39:2065–2084. doi: 10.1007/s12325-022-02098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pavord I, Gardiner F, Heaney LG, Domingo C, Price RG, Pullan A, et al. Remission outcomes in severe eosinophilic asthma with mepolizumab therapy: analysis of the REDES study. Front Immunol . 2023;14:1150162. doi: 10.3389/fimmu.2023.1150162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Milger K, Suhling H, Skowasch D, Holtdirk A, Kneidinger N, Behr J, et al. Response to biologics and clinical remission in the adult GAN severe asthma registry cohort. J Allergy Clin Immunol Pract . 2023;11:2701–2712.e2. doi: 10.1016/j.jaip.2023.05.047. [DOI] [PubMed] [Google Scholar]
- 18. Portacci A, Iorillo I, Quaranta VN, Maselli L, Lulaj E, Buonamico E, et al. Severe asthma clinical remission after biologic treatment with anti-IL4/IL13: a real life experience. Respir Med . 2023;217:107348. doi: 10.1016/j.rmed.2023.107348. [DOI] [PubMed] [Google Scholar]
- 19. Numata T, Araya J, Okuda K, Miyagawa H, Minagawa S, Ishikawa T, et al. Long-term efficacy and clinical remission after benralizumab treatment in patients with severe eosinophilic asthma: a retrospective study. J Asthma Allergy . 2022;15:1731–1741. doi: 10.2147/JAA.S391807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lugogo NL, Mohan A, Akuthota P, Couillard S, Rhoads S, Wechsler ME. Are we ready for asthma remission as a clinical outcome? Chest . 2023;164:831–834. doi: 10.1016/j.chest.2023.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thomas D, McDonald VM, Stevens S, Harvey ES, Baraket M, Bardin P, et al. Biologics (mepolizumab and omalizumab) induced remission in severe asthma patients. Allergy . 2024;79:384–392. doi: 10.1111/all.15867. [DOI] [PubMed] [Google Scholar]
- 22. Hansen S, Søndergaard M, von Bülow A, Bjerrum A-S, Schmid J, Rasmussen LM, et al. Clinical response and remission in severe asthma patients treated with biologic therapies. Chest . 2024;165:253–266. doi: 10.1016/j.chest.2023.10.046. [DOI] [PubMed] [Google Scholar]
- 23. ISAR Study Group. International Severe Asthma Registry: mission statement. Chest . 2020;157:805–814. doi: 10.1016/j.chest.2019.10.051. [DOI] [PubMed] [Google Scholar]
- 24. Cushen B, Koh MS, Tran TN, Martin N, Murray R, Uthaman T, et al. ISAR Inventory Study Group Adult severe asthma registries: a global and growing inventory. Pragmat Obs Res . 2023;14:127–147. doi: 10.2147/POR.S399879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. FitzGerald JM, Tran TN, Alacqua M, Altraja A, Backer V, Bjermer L, et al. International severe asthma registry (ISAR): protocol for a global registry. BMC Med Res Methodol . 2020;20:212. doi: 10.1186/s12874-020-01065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Porsbjerg CM, Menzies-Gow AN, Tran TN, Murray RB, Unni B, Audrey Ang SL, et al. Global variability in administrative approval prescription criteria for biologic therapy in severe asthma. J Allergy Clin Immunol Pract . 2022;10:1202–1216.e23. doi: 10.1016/j.jaip.2021.12.027. [DOI] [PubMed] [Google Scholar]
- 27. Scelo G, Tran TN, Faregas M, Martin N, Menzies-Gow A, Wang E, et al. Clinical remission following biologic initiation in severe asthma: results of the International Severe Asthma Registry (ISAR) [abstract] Eur Respir J . 2023;62:PA1891. [Google Scholar]
- 28. Perez de Llano L, Scelo G, Tran TN, Le TT, Martin N, Fageras M, et al. Characteristics associated with clinical remission in patients with severe asthma who initiate biologics [abstract] Eur Respir J . 2023;62:PA1892. [Google Scholar]
- 29. Bulathsinhala L, Eleangovan N, Heaney LG, Menzies-Gow A, Gibson PG, Peters M, et al. Development of the International Severe Asthma Registry (ISAR): a modified Delphi study. J Allergy Clin Immunol Pract . 2019;7:578–588.e2. doi: 10.1016/j.jaip.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 30.Global Initiative for Asthma. 2020. https://ginasthma.org/wp-content/uploads/2020/04/GINA-2020-full-report_-final-_wms.pdf
- 31. Korn S, Both J, Jung M, Hübner M, Taube C, Buhl R. Prospective evaluation of current asthma control using ACQ and ACT compared with GINA criteria. Ann Allergy Asthma Immunol . 2011;107:474–479. doi: 10.1016/j.anai.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 32. Koolen BB, Pijnenburg MWH, Brackel HJL, Landstra AM, van den Berg NJ, Merkus PJFM, et al. Comparing Global Initiative for Asthma (GINA) criteria with the Childhood Asthma Control Test (C-ACT) and Asthma Control Test (ACT) Eur Respir J . 2011;38:561–566. doi: 10.1183/09031936.00173710. [DOI] [PubMed] [Google Scholar]
- 33. Perez-de-Llano L, Scelo G, Canonica GW, Chen W, Henley W, Larenas-Linnemann D, et al. Impact of pre-biologic impairment on meeting domain-specific biologic responder definitions in patients with severe asthma. Ann Allergy Asthma Immunol . doi: 10.1016/j.anai.2023.12.023. [DOI] [PubMed] [Google Scholar]
- 34.R Core Team. 2021. https://www.R-project.org/
- 35. Wang E, Wechsler ME, Tran TN, Heaney LG, Jones RC, Menzies-Gow AN, et al. Characterization of severe asthma worldwide: data from the International Severe Asthma Registry (ISAR) Chest . 2020;157:790–804. doi: 10.1016/j.chest.2019.10.053. [DOI] [PubMed] [Google Scholar]
- 36. McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, et al. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med . 2016;374:1842–1852. doi: 10.1056/NEJMoa1513737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hough KP, Curtiss ML, Blain TJ, Liu R-M, Trevor J, Deshane JS, et al. Airway remodeling in asthma. Front Med (Lausanne) . 2020;7:191. doi: 10.3389/fmed.2020.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Menzies-Gow AN, Price DB. Clinical remission in severe asthma: how to move from theory to practice. Chest . 2023;164:296–298. doi: 10.1016/j.chest.2023.03.001. [DOI] [PubMed] [Google Scholar]
- 39. Lommatzsch M, Brusselle GG, Canonica GW, Jackson DJ, Nair P, Buhl R, et al. Disease-modifying anti-asthmatic drugs. Lancet . 2022;399:1664–1668. doi: 10.1016/S0140-6736(22)00331-2. [DOI] [PubMed] [Google Scholar]
- 40. Pérez de Llano L, Marina Malanda N, Urrutia I, Martínez-Moragón E, Gullón-Blanco JA, Díaz-Campos R, et al. Factors associated with suboptimal response to monoclonal antibodies in severe asthma. Allergy . 2023;78:2305–2310. doi: 10.1111/all.15693. [DOI] [PubMed] [Google Scholar]
- 41. Casale TB, Chipps BE, Rosén K, Trzaskoma B, Haselkorn T, Omachi TA, et al. Response to omalizumab using patient enrichment criteria from trials of novel biologics in asthma. Allergy . 2018;73:490–497. doi: 10.1111/all.13302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Castro M, Zangrilli J, Wechsler ME, Bateman ED, Brusselle GG, Bardin P, et al. Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med . 2015;3:355–366. doi: 10.1016/S2213-2600(15)00042-9. [DOI] [PubMed] [Google Scholar]
- 43. Bleecker ER, Wechsler ME, FitzGerald JM, Menzies-Gow A, Wu Y, Hirsch I, et al. Baseline patient factors impact on the clinical efficacy of benralizumab for severe asthma. Eur Respir J . 2018;52:1800936. doi: 10.1183/13993003.00936-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Menzies-Gow A, Ambrose CS, Colice G, Hunter G, Cook B, Molfino NA, et al. Effect of tezepelumab on lung function in patients with severe, uncontrolled asthma in the phase 3 NAVIGATOR study. Adv Ther . 2023;40:4957–4971. doi: 10.1007/s12325-023-02659-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Soremekun S, Heaney LG, Skinner D, Bulathsinhala L, Carter V, Chaudhry I, et al. Asthma exacerbations are associated with a decline in lung function: a longitudinal population-based study. Thorax . 2023;78:643–652. doi: 10.1136/thorax-2021-217032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bahmer T. Daily, once-weekly, or no asthma controller therapy at all: the annoying issue with disease remission in clinical asthma trials. Am J Respir Crit Care Med . 2021;203:273–275. doi: 10.1164/rccm.202008-3161ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Psallidas I, Backer V, Kuna P, Palmér R, Necander S, Aurell M, et al. A phase 2a, double-blind, placebo-controlled randomized trial of inhaled TLR9 agonist AZD1419 in asthma. Am J Respir Crit Care Med . 2021;203:296–306. doi: 10.1164/rccm.202001-0133OC. [DOI] [PubMed] [Google Scholar]
- 48. Nathan RA, Sorkness CA, Kosinski M, Schatz M, Li JT, Marcus P, et al. Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol . 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 49. Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J . 1999;14:902–907. doi: 10.1034/j.1399-3003.1999.14d29.x. [DOI] [PubMed] [Google Scholar]
- 50. Heaney LG, Perez de Llano L, Al-Ahmad M, Backer V, Busby J, Canonica GW, et al. Eosinophilic and non-eosinophilic asthma: an expert consensus framework to characterize phenotypes in a global real life severe asthma cohort. Chest . 2021;160:814–830. doi: 10.1016/j.chest.2021.04.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.