Abstract
Viral infection of the central nervous system (CNS) can result in perturbation of cell-to-cell communication involving the extracellular matrix (ECM). ECM integrity is maintained by a dynamic balance between the synthesis and proteolysis of its components, mainly as a result of the action of matrix metalloproteinases (MMPs) and the tissue inhibitors of metalloproteinases (TIMPs). An MMP/TIMP imbalance may be critical in triggering neurological disorders, in particular in virally induced neural disorders. In the present study, a mouse model of brain infection using a neurotropic strain of canine distemper virus (CDV) was used to study the effect of CNS infection on the MMP/TIMP balance and cytokine expression. CDV replicates almost exclusively in neurons and has a unique pattern of expression (cortex, hypothalamus, monoaminergic nuclei, hippocampus, and spinal cord). Here we show that although several mouse brain structures were infected, they exhibited a differential pattern in terms of MMP, TIMP, and cytokine expression, exemplified by (i) a large increase in pro-MMP9 levels, in particular in the hippocampus, which occurred mainly in neurons and was associated with in situ gelatinolytic activity, (ii) specific and significant upregulation of MT1-MMP mRNA expression in the cortex and hypothalamus, (iii) an MMP/TIMP imbalance, suggested by the upregulation of TIMP-1 mRNA in the cortex, hippocampus, and hypothalamus and of TIMP-3 mRNA in the cortex, and (iv) a concomitant region-specific large increase in expression of Th1-like cytokines, such as gamma interferon, tumor necrosis factor alpha, and interleukin 6 (IL-6), contrasting with weaker induction of Th2-like cytokines, such as IL-4 and IL-10. These data indicate that an MMP/TIMP imbalance in specific brain structures, which is tightly associated with a local inflammatory process as shown by the presence of immune infiltrating cells, differentially impairs CNS integrity and may contribute to the multiplicity of late neurological disorders observed in this viral mouse model.
Viral replication in the central nervous system (CNS) can result in transient or permanent impairment of cellular machinery and functions and neural cell death. These disorders can be induced directly by viral products or indirectly by virally induced molecules. Since cells receive signals from their microenvironment via the extracellular matrix (ECM), the ECM may constitute a molecular substrate for perturbation of cell-cell communication (40). ECM integrity is maintained by a dynamic balance between the synthesis and degradation of its components, which is mediated by matrix metalloproteinases (MMPs) and their endogenous inhibitors, the tissue inhibitors of metalloproteinases (TIMPs) (41, 43, 44). The MMPs constitute a family of Zn-proteinases classified according to substrate specificity, the main substrates being ECM components. The MMP/TIMP equilibrium may reflect the net proteolytic activity involved in numerous physiological processes (42, 61), with disruption of this balance resulting in serious diseases, such as arthritis, and tumor growth and metastasis.
An MMP/TIMP imbalance may play a critical role in neurological disorders, as suggested by the overexpression of certain MMPs in Alzheimer's disease (1), multiple sclerosis (MS) (14, 42), and amyotrophic lateral sclerosis (36). An MMP/TIMP imbalance may also be involved in the virally induced neural damage seen in patients suffering from viral meningitis or human immunodeficiency virus (HIV)-associated encephalitis (13, 34) and in patients suffering from tropical spastic paraparesis/human T-cell leukemia virus type 1 (HTLV-1)-associated myelopathy (TSP/HAM) (20, 23). Indeed, our previous work has shown that MMP-9 and TIMP-3 are preferentially upregulated in the cerebrospinal fluid (CSF) and parenchyma of TSP/HAM patients compared to that in asymptomatic virus carriers (35), these data being consistent with the upregulation of MMP-3, MMP-9, TIMP-1, and TIMP-3 expression seen in neural cells following contact with HTLV-1-infected T lymphocytes (21, 22).
To obtain a better understanding of the virus-neural cell relationship associated with CNS disorders, it is crucial to investigate in vivo the exact role of viral infection on MMP and TIMP expression in the CNS. To study the in vivo effects of CNS infection on the MMP/TIMP equilibrium, we have used a mouse model of brain infection employing canine distemper virus (CDV) (8), a potentially neurotropic morbillivirus related to the human measles virus (27, 56, 63). Replication and persistence of CDV in the CNS result in a biphasic disease (6). Mice surviving the acute encephalitis develop a late neurological disorder showing neuroendocrinological or motor impairment. During the acute phase of the disease, a unique pattern of CDV replication is seen which is restricted to a few structures in the CNS (7). Viral transcripts are almost exclusively localized in the cortex, hypothalamus, monoaminergic nuclei, hippocampus, most of the limbic system, and the spinal cord. At the cellular level, CDV transcripts are predominantly found in neurons and their processes (7). Proinflammatory cytokines are concomitantly detected in neurons in CDV-targeted brain areas (4), suggesting the importance of neural cell activation in the inflammatory state.
The goal of this work was to examine the expression of MMPs and TIMPs in different CNS structures of CDV-infected mice during the acute encephalitic phase. Since previous studies have indicated that the transcription of MMPs and TIMPs in neural cells is tightly regulated by cytokines (19, 25, 26), the expression of proinflammatory (interleukin 6 [IL-6], tumor necrosis factor alpha [TNF-α], and gamma interferon [IFN-γ]) and anti-inflammatory (IL-4 and IL-10) cytokines was studied. We show that the expression of MMP-2, MMP-9, membrane type 1-MMP (MT1-MMP), TIMP-1, and TIMP-3 was differentially upregulated in different cerebral regions. In addition, upregulation of MMPs and TIMPs occurred concomitantly with the expression of proinflammatory cytokines, supporting the idea of a functional link between these molecules and their involvement in the pathological process that occurs following viral infection.
MATERIALS AND METHODS
Experimental design. (i) Animals.
Four-week-old female outbred Swiss mice (Harlan-France, Gannat, France) were housed according to European Economic Community (86/609/EEC) and French (Decree 87-848) animal care regulations in a temperature-controlled room (22°C), with a fixed 12 h/12 h light/dark cycle. Animals were supplied with standard laboratory chow and water ad libitum. A total of 42 animals was used in this work.
(ii) Virus.
A neurotropic variant of CDV was obtained from the Onderstepoort vaccinal strain serially passaged in suckling mouse brain (neuroadapted CDV strain) (8). A neonatal mouse brain suspension containing 200 to 1,000 PFU of the neuroadapted CDV strain (12th-passage homogenate used as viral stock) was inoculated intracerebrally (10 to 20 μl) into the mice. Control (sham-inoculated) animals were inoculated with brain homogenates from noninfected neonatal mice.
Transcript and protein analyses were carried out on microdissected brain structures from infected and sham-inoculated mice sacrificed during the early stage of acute meningoencephalitis concomitant with active viral replication, i.e., on 7, 12, 14, 16, and 17 days postinoculation (dpi).
(iii) Brain tissue preparation.
Mice were deeply anesthetized by intraperitoneal injection of 6% pentobarbital (1 μl/g of body weight) and then perfused with ice-cold 0.1 M phosphate buffer (PB), pH 7.4, through the left ventricle. For protein or RNA extraction, the brains were rapidly removed and the frontal cortex, mesencephalon, hippocampus, hypothalamus, brain stem, and cerebellum were microdissected on ice, cut sagittally, snap-frozen in liquid nitrogen, and stored at −80°C until use. Dissections were always performed in the same order using the same landmarks to optimize reproducibility, and subsequent histological controls of the remaining tissue confirmed the accuracy of dissection.
For MMP, TIMP, and cytokine mRNA analyses, samples were obtained from three series of infection experiments (a total of 14 mice) at 14, 16, and 17 dpi by pooling microdissected brain structures from sham-inoculated and infected mice (2, 3, and 2 mice, respectively). For RNase protection assay (RPA) experiments, a further 2 mice from each group (sham-inoculated and infected mice) were used at 14 dpi. In addition, individual hypothalami from sham-inoculated (5) and infected (3) mice were used at 14 dpi for MT1-MMP, MMP-9, TIMP-1, and TIMP-3 reverse transcription (RT)-PCR analysis. For CD4 and CD8 mRNA analysis, a further 2 mice from each group (sham-inoculated and infected mice) were used at 7 and 14 dpi.
For simultaneous analyses of protein and transcript levels, we used two samples obtained by pooling samples from 3 sham-inoculated and 3 infected mice sacrificed at 16 dpi (see above). The brains were cooled to 4°C and cut sagitally into two hemispheres, one of which was used for protein analyses and the other for transcript experiments. Lysates of microdissected brain structures from infected (2) and sham-inoculated (2) mice at 7 and 14 dpi were also tested for gelatinase activity.
Semiquantitative assay of CDV NP, MMP, and TIMP mRNAs using RT-PCR.
RT-PCR was used for the semiquantitative assay of mRNAs coding for MMP-2, MMP-7, MMP-9, MT1-MMP, TIMP-1, TIMP-2, TIMP-3, proinflammatory cytokines (TNF-α, IL-6, and IFN-γ), and anti-inflammatory cytokines (Il-4 and Il-10) in the microdissected brain structures from both sets of mice. The mRNA levels were normalized to those for the housekeeping genes, cyclophilin (CyP) and G3PDH. In addition, nucleoprotein (NP)-CDV transcript levels and glial fibrillary acidic protein (GFAP) gene expression were used, respectively, as indicators of viral replication and astrocyte activation.
Total RNAs were extracted using RNA B (Bioprobe, Montreuil, France) according to the manufacturer's instructions. RNA integrity was checked using denaturing 1% agarose gel electrophoresis and ethidium bromide staining. The concentration and purity of the RNAs were estimated on a spectrophotometer (Beckman), using the optical density at 260 nm and the ratio of the optical densities at 260 and 280 nm (1.8 to 2.0), respectively.
RT was performed using 500 ng of total RNas from microdissected brain structures. Denatured RNAs (10 min at 70°C) were first stranded at 42°C for 90 min in a final volume of 20 μl containing 22 U of RNasin (Promega, Madison, Wis.), 10 mM dithiothreitol, a 0.5 mM concentration of each deoxynucleoside triphosphate (dATP, dTTP, dGTP, and dCTP), 5 ng of oligo(dT)12-18 primer (Pharmacia Biotech)/μl, RT buffer (final concentration of 50 mM Tris-HCl [pH 8.3], 75 mM KCl, 3 mM MgCl2), and 200 U of Moloney murine leukemia virus RT (Gibco BRL, Life Technologies). In the negative control, the RNA was omitted.
PCR was performed on a Biomed thermocycler or a Robocycler (to determine the optimal annealing temperature) (Stratagene) using 10 μl of a 1:10 dilution of the above cDNA samples obtained by oligo(dT) priming. In pilot experiments, the efficacy of each amplification stage was checked to ensure exponential amplification, and the number of cycles, MgCl2 concentration, and annealing temperature for each set of primers were optimized as described in Table 1. Primer pairs and the internal probe for each transcript were designed from GenBank sequences (Table 1), and their specificity was verified using FASTA 3 (Pearson and Lipman). These oligonucleotides were synthesized by Eurogentec (Seraing, Belgium). The PCR mixture (final volume, 50 μl) consisted of PCR buffer (final concentration of 20 mM Tris-HCl [pH 8.4], 50 mM KCl; Gibco BRL, Life Technologies), 1.5 to 3 mM MgCl2, a 0.2 mM concentration of each deoxynucleoside triphosphate, a 0.4 μM concentration of each specific 3′ and 5′ primer (Table 1), and 2 U of Taq DNA polymerase (Gibco BRL, Life Technologies). Samples were subjected to PCR (prior hot start to minimize mispriming) using the conditions of 22 to 35 cycles of 95°C for 45 s, 55 to 62°C for 45 s, and 72°C for 60 s, with a final elongation step of 72°C for 9 min. Lack of contamination was verified by omission of cDNA. To maximize the reliability of quantification of amplified products, all samples to be compared were processed simultaneously using the same master mix. A 10-μl sample of the amplified products was then analyzed on a 1.6% agarose gel. The amplicons were covalently bound to a 0.2-μm Hybond N+ nylon membrane (Pharmacia Biotech) by electrotransfer (15 V, 45 min) and were subjected to Southern blot hybridization as previously described (6). Briefly, membrane-bound DNA was denatured with 0.4 N NaOH for a few minutes, neutralized for 5 min at room temperature in 6× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), and prehybridized for 45 min at 42°C in 6× SSC containing 1× Denhardt, 25 mM phosphate buffer, 25 mM EDTA, 250 μg of salmon sperm DNA/ml, and 0.1% sodium dodecyl sulfate (SDS). The membrane-bound DNA was then hybridized for 30 min at 42°C in the same buffer containing 5′-labeled ([γ-32P]ATP) specific internal probes for the MMPs, TIMPs, and cytokines analyzed (Table 1). Membranes from the three sets of experiments were hybridized with the same probes, i.e., with the same specific radioactivities. Viral replication was estimated from the NP-CDV mRNA. All transcript levels were normalized with respect to mRNA coding for the housekeeping CyP and G3PDH genes (57). The hybridized membranes were then exposed for phosphorimage quantification (PhosporImager; Molecular Dynamics). The signals were expressed as arbitrary units, calculated as the ratio of the value for a given molecule to that for G3PDH. These normalized values were used to estimate region-specific modulation of target mRNAs in infected versus sham-inoculated mice and expressed as relative units.
TABLE 1.
Oligonucleotides used in RT-PCRa
| cDNA | Accession no. or reference | Sequence (5′-3′)
|
|
|---|---|---|---|
| Forward | Reverse | ||
| MMP-2 | M84324 | AAGATTGACGCTGTGTAGAGG | CACGACAGCATCCAGGTTATCAGG |
| MMP-9 | D12712 | TTCGACACTGACAAGTGGGG | TCACACGCCAGAAGAATTTGCC |
| MMP-7 | L36244 | CCAAACAGTCCAAAATGGCATTCC | TTCATGGGTGGCAGCAAACAGG |
| MT1-MMP | X83536 | CCACATTAAGGAGCTTGTCCGAGG | TGGCAGTAAAGCAGTCGCTTGG |
| TIMP-1 | X04684 | GCTAAAAGGATTCAAGGCTGTGGG | AAAGCTCTTTGCTGAGCAGGGC |
| TIMP-2 | X62622 | ATCAGAGCCAAAGCAGTGAGCG | GGTAATGTGCATCTTGCCATCTCC |
| TIMP-3 | L19622 | AAGCAGATGAAGATGTACCGAGGC | CCCAAAATTGGAGAGCATGTCG |
| TNF-α | M11731 | TCTCATCAGTTCTATGGCCC | GGGAGTAGACAAGGTACAAC |
| IL-6 | J03783 | GTTCTCTGGGAAATCGTGGA | TGTACTCCAGGTAGCTATGG |
| IL-4 | M13238 | TCGGCATTTTGAACGAGGTC | GAAAAGCCCGAAAGAGTCTC |
| IL-10 | M37897 | ATGCAGGACTTTAAGGGTTACTTG | TAGACACCTTGGTCTTGGAGCTTA |
| IFN-γ | M28621 | GCTCTGAGACAATGAACGCT | AAAGAGATAATCTGGCTCTGC |
| GAPDH | 57 | TGAAGGTCGGTGTGAACGGATTTGGC | CATGTAGGCATGAGGTCCACCAC |
| Cyp | M60456 | CTTCAGTGAGAGCAGAGATTACAGG | ATAATGGCACTGGCGGCAGG |
| NP-CDV | 56 | AGGCTGGTTAGAGAATAAGG | AATCCACCTTCTCATCTCCGAGTCG |
| Lyt-2 | M12825 | AGGATGCTCTTGGCTCTTCC | TCACAGGCGAAGTCCAATCC |
| L3T4 | M13816 | AGCAACTCTAAGGTCTCTAACC | AGAGTCAGAGTCAGGTTGCC |
| Sequence (5′–3′) of internal probe | Amplicon | Annealing temp (°C) | No. of cycles |
|---|---|---|---|
| TCCAGAGTGCTGGCAGAATAGACC | 300 | 60 | 31 |
| CCATACAGATACTGGATGCC | 967 | 60 | 35 |
| ATATCCGCAGTCCCCCCACTAACC | 351 | 56 | 32 |
| TCCAGAAGAGAGCTGTATCG | 522 | 60 | 30 |
| ATATGCCCACAAGTCCCAGAACCG | 213 | 58 | 25 |
| TCCTTCTTTCCTCCAACG | 243 | 58 | 26 |
| CACTCATTCTTGGAGGTCAC | 327 | 58 | 26 |
| TTCGAGTGACAAGCCTGTAG | 212 | 55 | 30 |
| CCAGTTTGGTAGCATCCATC | 208 | 55 | 30 |
| TGTGAGGACGTTTGGCACAT | 216 | 55 | 30 |
| ACAGGGGAGAAATCGATGAC | 254 | 55 | 30 |
| TTGCAGCTCTTCCTCATGG | 227 | 55 | 30 |
| GGCTAAGCAGTTGGTGGT | 979 | 62 | 22 |
| CAAGACTGAATGGCTGGATGGC | 395 | 55–62 | 22 |
| AGTATCAGGAGCAGTCACCG | 159 | 55 | 25 |
| CGAGTTGCTGATGACTGAGC | 398 | 62 | 35 |
| GCAGTTCCAGAAGTCGCTGT | 466 | 62 | 35 |
Expected size of each amplified product (amplicon) is given in nucleotides. Optimum annealing temperature and cycle numbers for each amplification are also given. Sequences were selected from DNA databanks and their specificity verified by comparison with other known rodent sequences and the corresponding oligonucleotides synthethized by Eurogentec.
Statistical analysis.
mRNA levels were compared in the sham-inoculated and infected groups using the nonparametric two-tailed Mann-Whitney U test, based on rank sums calculated for each variable (Statview 4.5 Software; Abacus Concepts). Normalized values were used in the analyses. To examine the relationship between two variables, each observation was matched by pairs (infected versus sham-inoculated mice) and all pairs were tested using Spearman's Rho correlation. P values of <0.05 were considered significant.
RPA.
The production of sets of RPA probes for the detection of MMP and TIMP gene expression has been described previously (48, 49). Briefly, the MMP probe set included probes for stromelysins 1, 2, and 3, matrilysin, metalloelastase, gelatinases A and B, collagenase 3, and MT1-MMP. The TIMP probe set contained probes for TIMP-1, TIMP-2, TIMP-3, and α2-macroglobulin. A fragment of the RPL32-4A gene (16) served as an internal loading control. RPAs (using 6 μg of total RNA) were performed as described previously (49).
Gelatinase purification and detection.
The presence of type IV collagenases/gelatinases in microdissected brain structures was assessed by gelatin zymography after protein extraction and enzyme enrichment.
All procedures were carried out at 4°C. Brain structures were homogenized in a Polytron in 1 ml of working buffer (50 mM Tris-HCl [pH 7.6], 150 mM NaCl, 5 mM CaCl2, 1% Triton X-100, 0.05% BRIJ-35; Sigma, St. Louis, Mo.) and centrifuged (12,000 rpm for 5 min; Sigma 2K15 centrifuge), and the supernatants were stored in aliquots at −80°C. The total protein concentration was measured using a modified Lowry assay (Bio-Rad kit) with bovine serum albumin solutions of known concentrations as standards. Because of the small amount of gelatinases in homogenates, the enzymes were enriched using the method described by Zhang and Gottschall (67). Briefly, 500 μl of supernatant containing 100 to 300 μg of total protein was incubated for 60 min with gentle shaking with 50 μl of gelatin-Sepharose 4B (Pharmacia Biotech) and then was centrifuged (12,000 rpm for 5 min; Sigma 2K15 centrifuge), and the supernatant was discarded. The gelatin-Sepharose beads were washed once with 500 μl of working buffer and then were incubated for 30 min with 150 μl of elution buffer (10% dimethyl sulfoxide in working buffer) and centrifuged (12,000 for 5 min; Sigma 2K15 centrifuge). The supernatants containing the gelatinases were stored in aliquots at −80°C until used for substrate gel electrophoresis.
SDS-polyacrylamide gel electrophoresis zymography under nonreducing conditions on a gelatin gel was used to detect the enzymatic activities of the type IV collagenases, MMP-2 (gelatinase A) and MMP-9 (gelatinase B), as described previously (21). Briefly, purified samples (18 μl) were mixed with 6 μl of loading buffer (0.125 mM Tris-HCl [pH 6.8], 4% sucrose, 10% SDS, 0.003% bromophenol blue) and electrophoresed (100 V for 2 h) on an SDS–9% (wt/vol) polyacrylamide gel containing 0.07% gelatin G2500 (Sigma). Following electrophoresis, the gels were washed twice for 20 min at room temperature with 2.5% Triton X-100 to remove SDS and to renature the enzymes. The catalytic sites were activated by incubating the gels for 20 h at 37°C in 100 mM Tris (pH 7.4)–15 mM CaCl2, and then the gels were stained for 15 min with 0.1% Coomassie blue R250 in 30% methanol–10% acetic acid and destained in 30% methanol–10% acetic acid until clear bands corresponding to areas of gelatin degradation were seen. Note that the zymography technique classically gives two bands for MMP-2 (proenzyme and enzyme forms; 72 and 65 kDa, respectively) and MMP-9 (proenzyme and enzyme forms; 92 and 85 kDa, respectively). As controls, active recombinant MMP-9 protein was electrophoresed in the same gel together with cell culture supernatant of the BHK21 cell line treated with phorbol myristate acetate (PMA), which contains MMP-2 active form, and of TNF-α-treated neural DEV cells (21), which show a gelatinolytic band corresponding to active MMP-9. Furthermore, treatment of the lysates with 1 μM p-aminophenylmercuric acetate (APMA; Sigma), which converts the proenzyme to the active form, allowed us to determine the form of purified gelatinases (proenzyme versus active forms). The gels were scanned, and the resulting images were subjected to densitometric analysis to estimate the relative protein content in brain structures from infected and sham-inoculated mice.
The gelatinolytic activity of MMP-9 (gelatinase B) was also measured using the Biotrak activity assay (RPN 2630; Pharmacia Biotech) according to the manufacturer's instructions.
In situ analysis. (i) IHC.
Immunohistochemistry (IHC) was performed for the detection of viral proteins (already described [6]), gelatinases MMP-2 and MMP-9, CD4- and CD8-specific (L3T4 and Lyt-2) T-cell antigens, and CD45 and CD11b, cell surface markers for immune cells.
To localize MMP protein expression, after perfusion with PB the mice were perfused with PB containing 1% paraformaldehyde (PFA). Following decapitation, the brains were rapidly removed, postfixed in 1% PFA (1 h, room temperature), immersed in 15% saccharose (at least overnight at 4°C), frozen in isopentane cooled to −60°C, and stored at −80°C until use. Coronal brain sections (14 to 20 μm thick) from mice sacrificed at 12 and 14 dpi (2 sham-inoculated and 2 infected mice for each time point) were prepared using a cryostat microtome (−16°C) and were collected on silane-coated glass slides. They were then treated with reagents eliminating any nonspecific labeling due to endogenous biotin and avidin (30 min at room temperature; avidin-biotin blocking kit; Vector) and incubated for 2 days at 4°C with rabbit polyclonal antibodies raised against MMP-2 and MMP-9 (1:2,000 in 0.1 M PB [pH 7.4], 0.3% Triton X-100, 0.01% Thimerosal [PB-T]; Chemicon, Temecula, Calif.). After three washes in PB-T, the samples were incubated sequentially with biotinylated polyclonal anti-rabbit immunoglobulin G (IgG) antibodies (1:5,000 dilution, 2 h at room temperature; Jackson ImmunoResearch Laboratories) and avidin-biotin peroxidase complex (ABC; Vectastain Elite kit SP 2001; 1 h at room temperature). Immunoreactivity was visualized by 3,3′-diaminobenzidine (DAB) staining (2 mg of DAB in 10 ml of 50 mM Tris [pH 7.6], 0.02% H2O2). Omission of the primary or secondary antibodies resulted in no signal.
To identify the cell type expressing MMP-9, double labeling was also performed on brain sections from sham-inoculated and infected mice (at 12 and 14 dpi) which were pretreated as described above and then incubated for 2 days at 4°C with rabbit polyclonal anti-MMP-9 antibodies (1:2,000; Chemicon) and mouse monoclonal anti-microtubule-associated protein 2 (MAP-2) antibody (a specific marker for the neuronal phenotype) (1:100; Sigma). After washes and incubation with biotinylated secondary antibodies against rabbit IgG (2 h at room temperature, 1:5,000 dilution), immune complexes were revealed using a streptavidin-Alexa Fluor 488 conjugate (for MMP-9 detection, 1:1,000 dilution; Molecular Probes) and Alexa Fluor 546-conjugated goat anti-mouse IgG (for MAP-2 detection, 1:1,000 dilution; Molecular Probes).
For the immunodetection of immune cell surface molecules, fresh unfixed brain sections from sham-inoculated and infected mice at 14 dpi were fixed in cold ethanol (95%, 10 min at room temperature) and then treated as described above using antibodies against L3T4 and Lyt-2 (1:100; BD PharMingen) and CD45R and CD11b (1:100; BD PharMingen and Cedarlane, respectively).
(ii) ISZ.
In situ zymography (ISZ) was performed on brain sections to detect proteolytic activity at the cellular level. Fresh, unfixed brain sections (20 μm) on aminoalkylsilane-treated slides were incubated in a dark moist chamber for 48 h at 37°C with reaction buffer (0.05 M Tris HCl, 0.15 M NaCl, 5 mM CaCl2, 0.2 mM sodium azide [pH 7.6]) containing fluorolabeled gelatin (100 μg/ml, EnzCheck gelatinase/collagenase assay; Molecular Probes) and 0.2% agarose. The specificity of the reaction was demonstrated using a wide-spectrum metalloproteinase inhibitor, phenanthroline monohydrate (100 to 500 μg/ml).
(iii) Histopathology.
To obtain further information on histological changes occurring in the mouse brain during the acute stage of infection corresponding to the active phase of viral replication, sections from infected and sham-inoculated mice at 12 and 14 dpi were stained with classical hematoxylin-eosin, methyl green, and cresyl violet stains, which visualize the cell soma, processes, or nuclei. Recruitment of infiltrating immune cells was also visualized using CD45R and CD11b immunodetection (as described above), which, together with CD4 and CD8 detection, allowed us to identify the phenotype of the cell.
RESULTS
Region-specific replication of CDV.
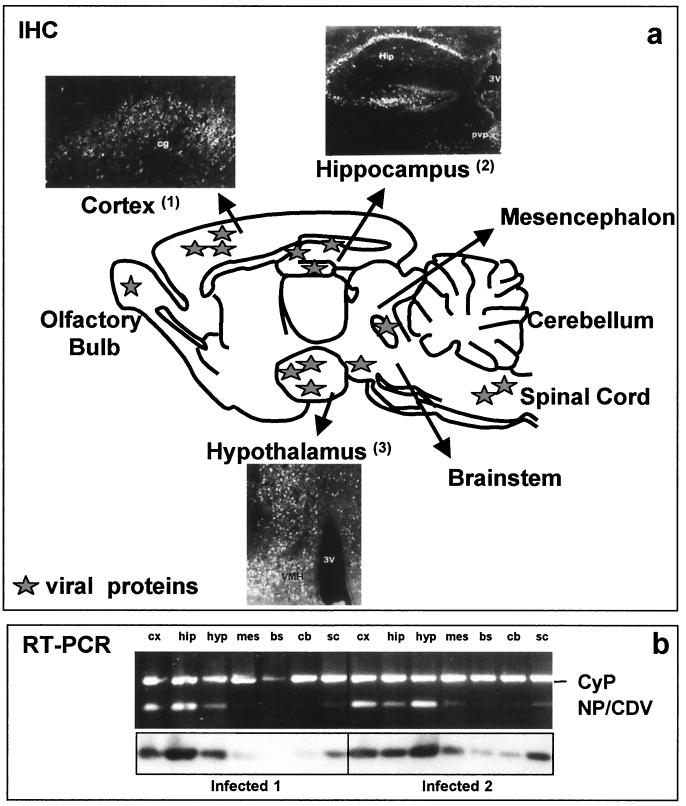
Using in situ hybridization, it was previously demonstrated that CDV replication predominantly occurs in a few brain structures, in particular the cortex (frontal, cingulum, and entorhinal), hypothalamus, hippocampus, spinal cord, and monoaminergic nuclei, such as the substantia nigra, raphe nuclei, and locus coeruleus, located in the mesencephalon or brain stem (7). This elective viral replication was also seen by using IHC (anti-CDV polyclonal antibodies) and, as shown in Fig. 1a, was mainly located in the neurons of cortical layers, pyramidal cells of the hippocampus, and hypothalamic nuclei.
FIG. 1.
(a) Schematic drawing showing selective expression patterns of viral products. Viral proteins were mainly found in the cortical layers of the frontal, entorhinal, and cingular (cg) cortexes, pyramidal cells of the hippocampus (Hip), and several periventricular hypothalamic nuclei. pvp, paraventricular thalamic pars posterior nuclei; 3V, third ventricle. (b) CDV replication in microdissected brain structures. Expression of NP-CDV (the most abundant viral transcript, according to the transcriptional CDV strategy [11]) was analyzed using RT-PCR, with amplicons being visualized using ethidium bromide staining (upper panel) and after Southern blot hybridization (lower panel). Cyclophilin (CyP) or G3PDH gene expression served as a loading control and served to quantify the signals, expressed as arbitrary units and calculated as the ratio of the NP-CDV value to the corresponding CyP or G3PDH value. Cortex, hippocampus, hypothalamus, and spinal cord exhibited the highest permissivity, whereas in the mesencephalon, brain stem, and cerebellum, the NP level was lower or undetectable. The cortex, hippocampus, and hypothalamus were consistently more permissive in several experiments. cx, cortex; mes, mesencephalon; hip, hippocampus; hyp, hypothalamus; bs, brain stem; cb, cerebellum; sc, spinal cord.
In the present study, the expression of the viral transcript NP-CDV was also measured using RT-PCR as a reliable index of the efficiency of viral replication in order to evaluate the impact of infection on MMP, TIMP, and cytokine expression. At the time point corresponding to clinical encephalitis and the highest mortality rate (14 dpi), previous analyses with two separate mice (Fig. 1b) showed that replication occurred predominantly in the cortex, hippocampus, hypothalamus, and spinal cord, whereas the mesencephalon, brain stem, and cerebellum were not infected or were only weakly infected. In the three separate series of infection experiments used for analysis of MMP, TIMP, and cytokine expression, NP-CDV transcripts were found in microdissected cerebral structures, with the greatest viral replication being seen in the cortex, hippocampus, hypothalamus, and spinal cord. NP-CDV transcript levels were somewhat variable from experiment to experiment since, in two experiments, the transcription level was almost threefold higher than that in the third experiment. Nevertheless, the pattern of viral expression was similar in all three experiments in that the maximal levels of viral transcripts were consistently found in the same brain structures and the hypothalamus always showed the highest levels of viral transcription. This differential CDV replication in the mouse brain underscores the region-specific permissivity of the brain.
Preferential upregulation of type IV collagenases/gelatinases MMP-2 and MMP-9 expression in the hippocampus.
There is evidence that metalloproteinases may be involved in neural impairment following bacterial or viral infection (20, 32). As our animal model of cerebral infection provides a suitable paradigm for such alterations, we analyzed collagenase/gelatinase expression in microdissected brain regions from infected and sham-inoculated mice using semiquantitative RT-PCR and RPA. When antibodies were available, we also analyzed MMP protein expression using IHC. The presence and proteolytic activity of type IV collagenases/gelatinases MMP-2 and MMP-9 was also determined by gel zymography and ISZ.
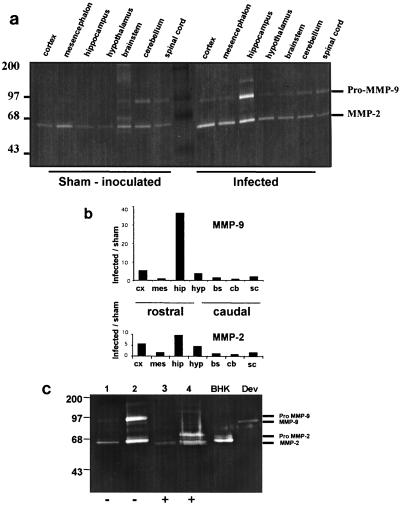
MMP-2 and MMP-9 proteins were detected by substrate gel zymography (Fig. 2a) on the basis of their gelatinolytic activity and molecular masses (proenzyme and active forms). To optimize analysis, brains from 3 infected and 3 sham-inoculated mice were cut into two hemispheres before microdissection and were pooled; one pool was used for protein purification and zymography analysis, and the other was used to measure transcript levels. Lysates from brain structures from sham-inoculated mice gave a band with weak gelatinolytic activity corresponding to the active form of MMP-2 (apparent molecular mass, 65 kDa) in all structures examined, whereas the 92-kDa gelatinolytic band, corresponding to the proenzyme form of MMP-9, was not detectable except at a very low levels in the brain stem, cerebellum, and spinal cord. This constitutive low level of expression of MMP-2 and MMP-9 was confirmed at the transcriptional level (data not shown). Lysates from infected mice showed only small changes in MMP-2 and MMP-9 expression in the caudal part of the brain (brain stem, cerebellum, and spinal cord), but elevated levels of pro-MMP-9 and MMP-2 were seen in the rostral part of the encephalon, the greatest increase being seen in the hippocampus (40- and 10-fold in pro-MMP-9 and MMP-2, respectively) (Fig. 2b). Zymography also revealed a clear band with a molecular mass of 130 kDa, possibly representing a complex of MMP-9 with neutrophil gelatinase-associated lipocalin. As shown in Fig. 2c, treatment of lysates of the hippocampus with APMA, which converts the proenzymes to the active forms, and the use of supernatant of PMA-treated BHK21 cell culture and TNF-α-treated neural cell line (Dev) lysates, which contain the MMP-2 and MMP-9 active forms, respectively, unambiguously confirmed active MMP-2 and pro-MMP-9 expression in the infected hippocampus. Upregulation of MMP-9 in the hippocampus was confirmed using an activity assay kit, which detects the overall potential enzymatic activity (data not shown). Surprisingly, MMP-2 and MMP-9 transcript levels, analyzed by RT-PCR, were only slightly increased or were unchanged (see Fig. 4) even in the same animals (used both for RNA and gelatinase analyses), indicating a discrepancy between transcriptional and translational expression.
FIG. 2.
Expression of gelatinases MMP-2 and MMP-9 in microdissected brain structures. (a) Gelatin zymography. To increase their concentration, the gelatinases were purified from brain structure lysates on gelatin-Sepharose beads, using the method of Zhang and Gottschall (67), and loaded on a 9% polyacrylamide gel containing gelatin (0.07%) and activated (20 h at 37°C), and then the gels were stained. Constitutive expression of active MMP-2 (65 kDa) was detected in all sham-inoculated structures, while faint MMP-9 proteolytic activity (92 kDa) was seen only in the mesencephalon, brain stem, cerebellum, and spinal cord. MMP-2 and pro-MMP-9 were markedly upregulated in infected brain structures, in particular in the rostral part of the brain. (b) Densitometric analysis. Data expressed as the ratio of infected/sham-inoculated normalized values (relative units) showed upregulation of MMP-2 and MMP-9 mainly in the hippocampus and, to a lesser extent, in the cortex and hypothalamus of infected mice. cx, cortex; mes, mesencephalon; hip, hippocampus; hyp, hypothalamus; bs, brain stem; cb, cerebellum; sc, spinal cord. (c) APMA treatment of hippocampal lysates. To determine if gelatinases are expressed as prozymogens or active enzymes, hippocampal lysates from sham-inoculated and infected mice at 14 dpi were treated with APMA and then electrophoresed as above. The zymograms for the untreated samples (lanes 1 and 2) showed two clear bands of respective apparent molecular masses of 65 and 92 kDa, presumably corresponding to active MMP-2 and pro-MMP-9, which were strongly upregulated in infected hippocampus (lane 2). In the APMA-treated samples (lanes 3 and 4), the 92-kDa product disappeared while a 75-kDa apparent molecular mass product became visible; the 65-kDa product corresponds to active MMP-2 that remained unchanged. Culture supernatant from PMA-treated BHK21 cells (containing the MMP-2 active forms) and TNF-α-treated DEV cells (containing the MMP-9 active form) were used as positive controls. Lanes 1 and 3, hippocampus from a sham-inoculated mouse; lanes 2 and 4, hippocampus from an infected mouse
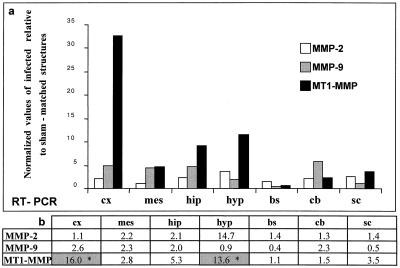
FIG. 4.
MMP-2, MMP-9, and MT1-MMP mRNA expression analyzed by RT-PCR and densitometry. Total RNAs (0.5 μg) extracted from microdissected brain structures from infected and sham-inoculated mice were subjected to RT-PCR. After electrophoresis on an agarose gel and electrotransfer, Southern blotting of the amplicons was performed. Hybridization of specific internal radiolabeled probes allowed the semiquantification of each PCR product. MMP expression was then analyzed by phosphorimaging densitometry. (a) The relative mRNA content for each amplicon was calculated as a fraction of the levels of the housekeeping gene G3PDH mRNA (normalized values), and the results were expressed as a ratio of levels in infected mice relative to those in sham-inoculated mice (relative units). Only slight MMP-2 and MMP-9 upregulation was seen in brain structures of CDV-infected mice, the difference not being significant in the Mann-Whitney test (b). In contrast, marked upregulation of MT1-MMP was seen in infected mice, mainly in the rostral brain (cortex, hippocampus, and hypothalamus), the difference being statistically significant in the cortex and hypothalamus (P < 0.05, indicated by asterisks in the shaded columns). cx, cortex; mes, mesencephalon; hip, hippocampus; hyp, hypothalamus; bs, brain stem; cb, cerebellum; sc, spinal cord.
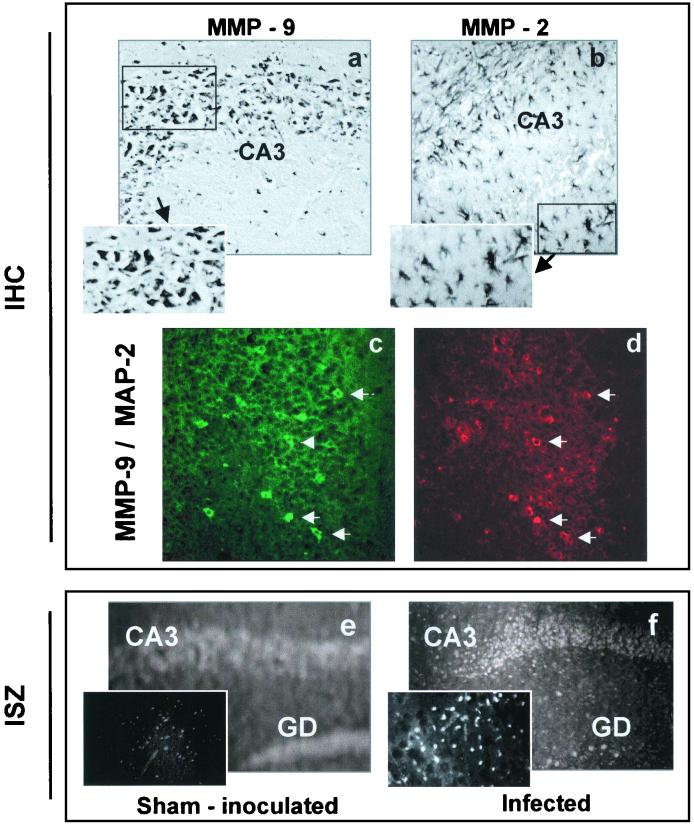
To identify the gelatinase-expressing cells in the hippocampus, we performed IHC. As determined by localization and size, MMP-9-immunoreactive cells mainly corresponded to neurons (Fig. 3a), this being confirmed by double-labeling experiments, which detected coexpression of MMP-9 and the neuronal marker MAP-2 in the same cell (Fig. 3c and d). Nevertheless, it should be noted that glial cells also may express MMP-9. MMP-2 was expressed almost exclusively in astrocytes (Fig. 3b). The histological localization of gelatinolytic activity determined by ISZ on cerebral sections showed weak diffuse constitutive proteolytic activity in sham-inoculated mice (Fig. 3e), whereas gelatinolytic activity was clearly increased in the hippocampus (CA3 and dentate gyrus) of infected mice, mainly at the neuronal level (Fig. 3f). This in situ gelatinase activity could be blocked by phenantroline (100 μM), a broad-spectrum MMP inhibitor (data not shown). Taken together, the gel zymography, IHC, and ISZ results indicated that upregulation of gelatinase expression took place predominantly in the hippocampus.
FIG. 3.
Immunodetection of gelatinases MMP-2 and MMP-9 and localization of gelatinolytic activity by ISZ. Immunodetection (IHC) of MMP-9 and MMP-2 using antibodies against the whole protein (reactive with both the proenzyme and enzyme forms) showed MMP-9 to be present in the hippocampus (a) at the level of the CA3 pyramidal layer. At the cellular level, MMP-9 was mainly found in neurons, identified by their size and localization (panel a and insert), as confirmed using double labeling. (c) MMP-9 (green), indicated by white arrows; (d) neuronal marker MAP-2 (red), indicated by white arrows. It is noteworthy that all the MAP-2-positive cells are not always MMP-9 positive. MMP-2 was mainly located in astrocyte-type cells (panel b and insert). For ISZ, the quenched fluorescent substrate (gelatin) was added directly to tissue sections, and enzymatic activity was detected by the unmasked fluorescence. Cellular gelatinolytic activity was faint and diffuse in brain sections from sham-inoculated mice (e) and was markedly enhanced in the cells of the pyramidal layers of the hippocampus from CDV-infected mice (f), which is also shown at a higher magnification (insert in panel f versus that in panel e). Magnifications, ×28 (a, b, e, f); ×40 (c, d, and inserts in panels a, b, e, and f).
Upregulation of MT1-MMP expression in the hypothalamus and cortex.
Since our data showed upregulated expression of gelatinases MMP-2 and MMP-9, we examined whether the expression of other MMPs, namely MMP-3, MMP-7, and MT1-MMP (also known as MMP-14 and which, as a complex with TIMP-2, can activate MMP-2 [62]), was modulated during viral infection of the brain. These analyses were carried out at the transcriptional level using both semiquantitative RT-PCR and RPA, which allow relative quantification using an internal standard and the simultaneous assessment of several mRNAs in the same sample without amplification, respectively.
For the RT-PCR analysis, the results of one representative series of infection experiments, corresponding to 3 infected and 3 sham-inoculated animals, are shown in Fig. 4a and are expressed as normalized values of infected relative to sham-matched brain structures. In infected mice, the expression of MMP-3 and MMP-7 was unchanged (data not shown) and there were only slight changes in MMP-2 and MMP-9 expression (Fig. 4a), but a clear increase in MT1-MMP expression was seen occurring mainly in the rostral part of the brain. Expression was increased in the frontal cortex (30-fold), hippocampus (10-fold), and hypothalamus (13-fold), but not in the brain stem, cerebellum, and spinal cord.
To obtain a more accurate assessment of changes in MT1-MMP expression during CDV infection, a nonparametrical statistical analysis (Mann-Whitney U test) was carried out on the results of three separate series of infection experiments. Despite some variability in the magnitude of the effects, the MT1-MMP transcript expression pattern was similar in all three series. As shown in Fig. 4b, significant upregulation of MT1-MMP was seen only in the cortex and hypothalamus of infected mice (P < 0.05). In addition, results obtained using individual infected (3) and sham-inoculated (5) hypothalami showed MT1-MMP upregulation (10 ± 1.13 versus 4.36 ± 1.04 relative units, parametrical t test, P < 0.05) but no change in MMP-9 expression. The RT-PCR results for the hypothalamus were confirmed by RPA, which showed that of the nine MMPs (MMP-2, -3, -7, -9, -10, -11, -12, -13, and MT1-MMP), only MT1-MMP was upregulated (data not shown) during the early phase of encephalitis, at which time intense viral replication occurs.
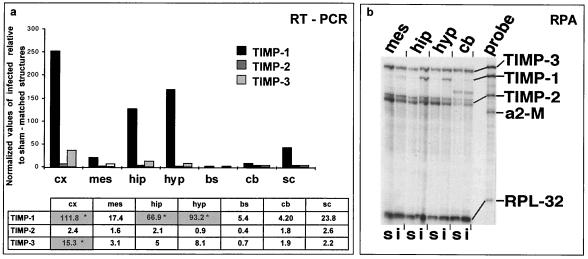
Upregulated expression of TIMP-1 mRNA in the cortex, hippocampus, and hypothalamus and of TIMP-3 mRNA in the cortex during brain CDV infection.
We also analyzed TIMP expression in order to evaluate the MMP/TIMP balance, which determines the proteolytic activity of the environment. Substantial amounts of TIMP-2 and TIMP-3 transcripts were seen in sham-inoculated mice, while TIMP-1 was only weakly expressed. In infected mice, the relative TIMP-1 and TIMP-3 mRNA contents were increased (Fig. 5a; results of one representative series of infection experiments are shown), while TIMP-2 expression was unchanged. TIMP-3 expression was increased in the cortex (30-fold), hippocampus (12-fold), and hypothalamus (7-fold), but no clear change was seen in the caudal part of the brain (brain stem, cerebellum, and spinal cord). Marked upregulation of TIMP-1 expression was seen in the rostral part of the brain, including the cortex, hippocampus, and hypothalamus (250-, 120-, and 170-fold increases, respectively), with smaller changes in the caudal encephalon, such as the spinal cord (50-fold) and mesencephalon (20-fold). Interestingly, the expression pattern of TIMP-1 paralleled that of MT1-MMP. Mann-Whitney U test performed on the three series of infection experiments showed that there was significant upregulation of TIMP-1 in the cortex, hippocampus, and hypothalamus and of TIMP-3 only in the cortex of infected mice. Moreover, results obtained in additional experiments using the hypothalamus of individual infected (3) and sham-inoculated (5) mice showed TIMP-1 upregulation in the infected mice (12.4 ± 2.1 versus 1.5 ± 0.5 relative units; parametrical t test, P < 0.001) but no change in TIMP-3 expression.
FIG. 5.
TIMP-1, TIMP-2, and TIMP-3 expression analyzed using semiquantitative RT-PCR and RPA. (a) RT-PCR showed dramatic upregulation of TIMP-1 in the cortex, hippocampus, hypothalamus, and to a lesser extent, in the spinal cord, with only slight or no variation in TIMP-2 expression. TIMP-3 was mainly up-expressed in the cortex and hippocampus and, to a lesser extent, in the hypothalamus and mesencephalon. The results of a typical experiment for a pool of structures from 3 infected mice and 3 sham-inoculated mice are shown. Statistical analysis of TIMP expression using the nonparametric Mann-Whitney test showed significant increases (P < 0.05, indicated by asterisks in the shaded columns) only in the rostral part of the CNS of infected mice for TIMP-1 (cortex, hippocampus, and hypothalamus) and TIMP-3 (cortex). (b) RPA analysis showed TIMP gene expression in the CDV-infected brain. Total RNA (6 μg) from the hippocampus, hypothalamus, mesencephalon, and cerebellum from sham-inoculated and CDV-infected mice was analyzed as described in Materials and Methods. s, sham-inoculated mice; i, infected mice; a2-M, α2-macroglobulin; RPL32-4A, internal loading control. The figure shows induction of TIMP-1 only in the hippocampus and hypothalamus and no variation in TIMP-2 and TIMP-3 expression in infected mice, whatever the structures. cx, cortex; mes, mesencephalon; hip, hippocampus; hyp, hypothalamus; bs, brain stem; cb, cerebellum; sc, spinal cord.
Analysis of TIMP gene expression using RPA performed, in contrast to RT-PCR, without any amplification procedure showed marked upregulation of TIMP-1 gene expression in the hippocampus and hypothalamus of infected mice compared to that of sham-inoculated mice (Fig. 5b), confirming the results described above.
To summarize, these experiments on the expression of several molecules in different brain structures allow us to classify brain structures into three types according to their MMP and TIMP expression in response to viral infection: (i) the cortex and hypothalamus, in which MT1-MMP and TIMP-1 mRNAs were mainly upregulated, with TIMP-3 expression also being increased in the cortex, (ii) the hippocampus, which exhibited the highest levels of MMP-2 and MMP-9 protein expression and TIMP-1 mRNA upregulation, and (iii) the mesencephalon and caudal structures, such as the brain stem, cerebellum, and spinal cord, which showed no significant modifications, despite being infected. The MMP and TIMP pattern of expression and gelatinolytic activity suggests an imbalance in favor of proteolysis in the cortex, hippocampus, and hypothalamus. Indeed, ECM proteolysis could be demonstrated by fibronectin deposits and neosynthesis in infected cortex and hypothalamus (preliminary results, not shown).
Inflammatory cytokine expression and infiltrated immune cells are observed as conspicuous features of the inflammatory state in CDV-infected brain areas.
As cytokines are potent regulators of MMP and TIMP transcription, we used RT-PCR to measure the expression of proinflammatory (TNF-α, IFN-γ, and IL-6) and anti-inflammatory (IL-4 and IL-10) cytokines in the same microdissected brain structures from the three series of infection experiments used for MMP and TIMP expression analyses. These results allowed us to evaluate the functional relevance of these molecules and their relationship to viral replication and also to delineate whether a pro- or anti-inflammatory environment is established during acute infection.
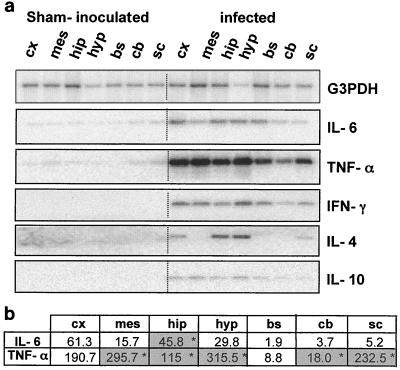
We have previously demonstrated by in situ RT-PCR that neuronal expression of TNF-α and IL-6 occurs in the hippocampus and hypothalamus of CDV-infected mice (4). Here, using a more sensitive and semiquantitative method of mRNA analysis (RT-PCR), we observed increased expression of TNF-α and IL-6 mRNAs in all infected brain structures except the brain stem and cerebellum compared with that of matched structures from sham-inoculated mice (Fig. 6a; results representative of one series of infection experiments). TNF-α and IL-6 mRNA expression was increased in the cortex (540- and 150-fold, respectively), hippocampus (780- and 45-fold), and hypothalamus (155- and 100-fold). Mann-Whitney U tests performed on the three series of infection experiments indicated that a wider range of structures showed upregulation of TNF-α expression than upregulation of IL-6 expression, seen only in the hippocampus. Thus, the same stimulus, i.e., brain viral infection, differentially regulates the expression of proinflammatory cytokines. Interestingly, IL-6 showed significant upregulation only in the hippocampus, the structure exhibiting MMP-2, MMP-9, and TIMP-1 dysregulation, and increased GFAP expression (data not shown).
FIG. 6.
Brain expression of inflammatory cytokines (IL-6, TNF-α, IFN-γ, IL-4, and IL-10). (a) Total RNAs (0.5 μg) extracted from brain structures were subjected to RT-PCR. Amplicons were electrophoresed on an agarose gel and electrotransferred. Hybridization of the Southern blots using radiolabeled specific internal probes allowed the semiquantification of each PCR product. The results indicate that IFN-γ was expressed in all infected brain structures, while IL-4 expression was restricted to the hippocampus and hypothalamus. IL-10 mRNA was almost undetectable. (b) Expression of cytokines (IL-6 and TNF-α) was calculated as a fraction of that of G3PDH (normalized values) and then was expressed as an infected/sham-inoculated ratio. Mann-Whitney U test was then carried out on the results of three separate series of infection experiments and showed a significant increase (P < 0.05, indicated by asterisks in the shaded columns) of IL-6 only in the hippocampus and of TNF-α in almost all infected brain structures, except the cortex and brain stem. cx, cortex; mes, mesencephalon; hip, hippocampus; hyp, hypothalamus; bs, brain stem; cb, cerebellum; sc, spinal cord.
As also shown in Fig. 6a, de novo synthesis of IFN-γ was detected in infected brain structures, suggesting infiltration of activated peripheral T cells or even activation of resident cells (5). The highly virus-permissive brain structures, i.e., the cortex, hippocampus, and hypothalamus, contained the highest levels of IFN-γ mRNA (1.50, 1.43, and 2.23 arbitrary units, respectively; relative to housekeeping gene expression; mean of results for three series of infection experiments). Low induction of anti-inflammatory cytokine expression also occurred in infected brain structures. More precisely, IL-4 mRNA expression was induced in the hippocampus and hypothalamus and, to a lesser extent, in the cortex, whereas IL-10 mRNA was found in all brain structures except the spinal cord, although IL-10 mRNA levels were very low, close to the threshold for phosphorimaging detection.
To summarize, according to the mouse Th1/Th2 paradigm, which depends on the amounts of proinflammatory cytokines and the IFNγ/IL-4 ratio, CDV-infected brain structures exhibited a Th1-like profile, but caution may be needed, as this T-cell subset differentiation nomenclature is not clearly established for the brain. Also, even though the level of anti-inflammatory response seen in the hippocampus and hypothalamus was low, it might exert a negative regulating action on Th1 immune responses.
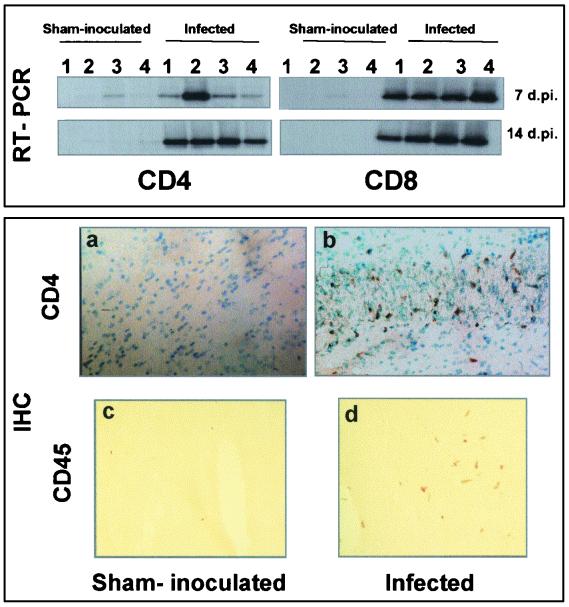
Cytokines can be produced by resident cells, as has already described (4), and/or by infiltrating immune cells. We therefore looked for the presence of CD4 and CD8 T cells by using both IHC and RT-PCR for specific markers of T infiltrating cells (Lyt-2 and L3T4). The presence of CD4 and CD8 T-cell markers, detected using RT-PCR, was seen in infected brain areas as early as 7 dpi (Fig. 7). This was corroborated by the presence of infiltrating CD4 T cells (Fig. 7b). Recruitment of CD45 (Fig. 7d) and CD11b (data not shown) expressing cells was also observed in the infected mouse brain. CDV brain infection therefore elicits an inflammatory response consistent with the high levels of cytokine expression and the presence of infiltrating cells of immune phenotype.
FIG. 7.
Expression of mouse cell surface molecules. (Upper figure) Expression of L3T4 and Lyt-2 (specific markers for CD4 and CD8 T cells, respectively) using the RT-PCR procedure, Southern blotting, and amplicon hybridization. Specific 3′ primers were used for the RT procedure (reverse primer; see Table 1). Analyses were carried out at 7 and 14 dpi. CD4 and CD8 markers could be detected as soon as 7 dpi (lane 1, hippocampus; lane 2, hypothalamus; lane 3, mesencephalon; lane 4, brain stem). (Lower figures) Immunodetection of the cell surface antigens CD4 and CD45. Fresh unfixed brain sections from sham-inoculated and infected mice at 14 dpi were fixed in cold ethanol and then incubated with antibodies against L3T4 (1:100) and CD45R (1:100). T-cell CD4 antigens were detected in the brains of infected mice, e.g., in the hippocampus (b; dark staining of DAB deposits, cells counterstained using methyl green). CD45 cell surface markers were diffusely expressed by infiltrating immune cells through the brain parenchyma (d). Weak or no staining was seen in the brains of sham-inoculated mice (a and c). Magnifications, ×28 (a and b) and ×70 (c and d)
Correlation analysis of the expression of MMPs, TIMPs, cytokines, and NP mRNAs.
To evaluate the regulatory links between viral replication and the expression of cytokines, MMPs, and TIMPs, we used Spearman's Rho test, in which a significant correlation between two molecules would suggest either that their expression is regulated by the same factors or that the expression of one regulates the transcription of the other. Among the several correlation tests performed (each variable being compared to another and the analysis being performed on 14 different molecules in 7 different brain structures from 14 mice, divided into two groups of 7), we highlight the values relating to the three structures, the cortex, hippocampus, and hypothalamus, which are highly permissive for viral replication and which are relevant in terms of MMP, TIMP, and cytokine expression.
The viral transcript (NP-CDV) was correlated with expression of MT1-MMP, TIMP-1, and TIMP-3 in the cortex, whereas in the hippocampus it was correlated only with TIMP-1 expression (Table 2), suggesting a relationship between viral burden and MMP/TIMP imbalance in these two structures.
TABLE 2.
Statistical analysis using the Spearman test: pairing cytokines, MT1-MMP, or TIMPs and the viral transcript (NP-CDV)a
| Variable paired with NP-CDV | Result forb:
|
||||||
|---|---|---|---|---|---|---|---|
| CX | MES | HIP | HYP | BS | CB | SC | |
| IL-6 | 0.829 | 0.729 | 0.943 | 0.500 | 0.314 | 0.257 | 0.543 |
| TNF-α | 0.829 | 0.943 | 0.943 | 0.771 | 0.600 | 0.886 | 0.886 |
| IFN-γ | 1 | 1 | 0.943 | 0.771 | 0.943 | 1 | 1 |
| IL-10 | 0.829 | 0.943 | 0.771 | 0.829 | 1 | 1 | 0.729 |
| IL-4 | 0.914 | 0.771 | 1 | 0.729 | 0.371 | 0.771 | 1 |
| MT1-MMP | 0.943 | 0.600 | 0.714 | 0.714 | 0.143 | 0.543 | 0.829 |
| TIMP-1 | 0.943 | 0.829 | 0.943 | 0.714 | 0.671 | 0.600 | 0.829 |
| TIMP-3 | 0.886 | 0.657 | 0.714 | 0.200 | −0.429 | 0.314 | 0.657 |
Correlation analysis was performed by pairing each variable with another, using the Spearman correlation coefficient (Rho), based on the ranks of the values. Significant correlations (P < 0.05) are indicated by boldface numbers. Relationships between NP-CDV expression and that of pro- and anti-inflammatory cytokines, MT1-MMP, TIMP-1, and TIMP-3 are shown. The analyses indicate a strong correlation between the expression of cytokines and the viral transcript in a wide range of structures and a poor correlation between the expression of MT1-MMP and TIMPs and the viral transcript.
CX, cortex; MES, mesencephalon; HIP, hippocampus; HYP, hypothalamus; BS, brain stem; CB, cerebellum; SC, spinal cord.
Moreover, there was a large correlation between the expression of the viral component and that of three proinflammatory cytokines: IFN-γ in all brain structures except the hypothalamus, TNF-α in all brain structures except the cortex and hypothalamus, and IL-6 only in the hippocampus. A correlation between inhibitory cytokine expression and that of the viral transcript was seen in far fewer brain structures (Table 2). These data show that during the acute encephalitis phase, high viral replication is more frequently associated with the expression of proinflammatory, rather than anti-inflammatory, cytokines. Surprisingly, no relationship was seen between the expression of pro- or anti-inflammatory cytokines and that of NP-CDV in the hypothalamus, despite the high level of CDV transcripts; this may reflect antagonism between pro- and anti-inflammatory cytokines (10).
There was a correlation between MT1-MMP and TIMP-1 mRNA levels in the cortex and hypothalamus (Table 3), suggesting a coordinated regulation of these two molecules via their own regulatory molecules.
TABLE 3.
Statistical analysis using the Spearman test: pairing pro- or anti-inflammatory cytokines and MMPs or TIMPsa
| Cytokine and variable | Result forb:
|
||||||
|---|---|---|---|---|---|---|---|
| CX | MES | HIP | HYP | BS | CB | SC | |
| MT1-MMP | |||||||
| IL-6 | 0.943 | 0.843 | 0.829 | 0.786 | 0.943 | 0.886 | 0.600 |
| TNF-α | 0.943 | 0.600 | 0.600 | 0.943 | 0.315 | 0.600 | 0.600 |
| IFN-γ | 0.943 | 0.600 | 0.657 | 0.943 | 0.257 | 0.543 | 0.829 |
| IL-4 | 0.857 | 0.714 | 0.714 | 0.986 | 0.543 | 0.429 | 0.829 |
| IL-10 | 0.771 | 0.371 | 0.257 | 0.886 | 0.143 | 0.543 | 0.329 |
| TIMP-1 | 1 | 0.829 | 0.829 | 0.943 | 0.557 | 0.771 | 1 |
| TIMP-1 | |||||||
| IL-6 | 0.943 | 0.929 | 1 | 0.929 | 0.614 | 0.714 | 0.600 |
| TNF-α | 0.943 | 0.886 | 0.829 | 0.886 | 0.443 | 0.543 | 0.600 |
| IFN-γ | 0.943 | 0.829 | 0.886 | 0.943 | 0.757 | 0.600 | 0.829 |
| IL-4 | 0.857 | 0.714 | 0.943 | 0.900 | 0.671 | 0.286 | 0.829 |
| TIMP-3 | 0.886 | 0.771 | 0.829 | 0.143 | −0.271 | 0.600 | 0.771 |
| TIMP-3 | |||||||
| IL-6 | 0.714 | 0.900 | 0.829 | 0.157 | −0.143 | 0.086 | 0.200 |
| IFN-γ | 0.886 | 0.657 | 0.657 | 0.429 | −0.143 | 0.314 | 0.657 |
Correlation analysis was performed by pairing each variable with another, using the Spearman correlation coefficient (Rho), based on the ranks of the values. Significant correlations (P < 0.05) are indicated by boldface numbers. Relationship between proinflammatory and anti-inflammatory cytokines and MT1-MMP, TIMP-1, or TIMP-3 is shown. The analyses indicate a correlation between the expression of proinflammatory cytokines and MT1-MMP and TIMP-1 in a wide range of structures and between the expression of anti-inflammatory cytokines and MT1-MMP and TIMP-1 in a restricted range of structures. The expression of TIMP-3 correlated only with that of proinflammatory cytokines in a restricted range of structures.
CX, cortex; MES, mesencephalon; HIP, hippocampus; HYP, hypothalamus; BS, brain stem; CB, cerebellum; SC, spinal cord.
In general, a correlation was also found between the expression of MT1-MMP or TIMP-1 and that of proinflammatory cytokines (TNF-α, IFN-γ, and IL-6) mainly in the cortex, hippocampus, and hypothalamus (Table 3), two exceptions being between IL-6 and MT1-MMP in the hypothalamus and between TNF-α and TIMP-1 in the hippocampus. TIMP-3 expression was correlated with IFN-γ expression only in the cortex.
The expression of anti-inflammatory cytokines showed a less frequent correlation with MT1-MMP expression (only in the hypothalamus for both IL-4 and Il-10) and with TIMP-1 expression (only with IL-4 in both the hippocampus and hypothalamus; see Table 3). In addition, no significant correlation at the transcriptional level was seen between MMP-2 and MMP-9 and pro- and anti-inflammatory cytokines, despite an increase in their protein levels or activity (data not shown).
These results point to an in vivo link between the expression of certain MMPs, TIMPs, and cytokines consistent with the regulatory role of cytokines and in agreement with previously published data (19, 21, 48, 49).
Taken together, these results indicate that a regulatory link between viral infection and proinflammatory cytokine levels is more likely than one with anti-inflammatory cytokine levels. On the other hand, it is likely that the relationship between MMP and TIMP expression and proinflammatory cytokine levels is stronger than that between MMP and TIMP expression and NP-CDV expression. It is noteworthy that viral replication leads to differential and regional cytokine, MMP, and TIMP responses in the brain.
DISCUSSION
There is increasing evidence for the involvement of MMPs in the pathogenesis of various CNS inflammatory diseases, including bacterial or viral meningitis (32, 34), and previous studies have clearly demonstrated a link between retroviral infection, production of virally induced inflammatory molecules (including cytokines), the clinical status of infected patients, and alteration of the MMP/TIMP balance (35). Therefore, the development of an in vivo model of CNS infection in mice using a highly neurovirulent strain of CDV, with different clinical manifestations (early encephalitis and late motor or metabolic pathologies) that are associated with active viral replication and persistence (3, 6, 7), gave the opportunity to investigate the in vivo effect of virus on the MMP/TIMP balance in the CNS. In the present study, we investigated the region-specific expression of MMPs and TIMPs in the CNS of CDV-infected mice during the acute phase of encephalitis in order to delineate possible regulatory links between MMP, TIMP, and cytokine transcript levels and those of the viral transcript encoding NP, the most abundant product of viral replication (11).
Three conclusions can be drawn from the present work: (i) the expression of certain MMPs and TIMPs is upregulated in a region-specific manner, occurring mainly in the rostral brain, i.e., the cortex, hippocampus, and hypothalamus, (ii) concomitant region-specific upregulation (TNF-α and IL-6) or induction (IFN-γ) of proinflammatory cytokines is also seen, and (iii) there is a strong relationship between viral replication in the CNS and inflammatory cytokine production and between the production of these cytokines and that of certain MMPs and TIMPs. These data strongly suggest that inflammatory cytokines induced by viral replication may be, at least in part, responsible for the modulation of MMP and TIMP expression. Since there was a strong correlation between the expression of NP-CDV and that of cytokines, one may hypothesize that CDV induces cytokine expression, which in turn modulates the expression of MMPs and TIMPs. This sequential hypothesis is supported by our preliminary data indicating that cytokine induction precedes the increase in MT1-MMP expression (data not shown).
Moreover, infected brain structures may present a special environment, possibly tailored by MMPs and TIMPs, which set in motion a local inflammatory response, also demonstrated by the presence of infiltrating cells.
Thus, brain structures in CDV-infected mice can be classified into three groups according to their responses: (i) structures in the caudal brain, such as the mesencephalon, brain stem, and cerebellum, in which MMP and TIMP expression remains unchanged, (ii) structures, such as the cortex and hypothalamus, showing a marked increase in MT1-MMP, TIMP-1 mRNA levels, with the cortex also showing TIMP-3 upregulation, and (iii) structures, such as the hippocampus, which respond preferentially to viral infection by increases in MMP-2 and MMP-9 protein levels or activity and TIMP-1 mRNA expression. These changes in the expression of MMP-2, MMP-9, MT1-MMP, TIMP-1, and TIMP-3 emphasize the potential role of these agents in neurological disorders, as previously described for HIV-associated neurological disease (13), HTLV-1-associated myelopathy (20), and MS or its animal model, experimental allergic encephalitis (EAE) (14, 31, 48). In contrast, TIMP-2 mRNA expression was unchanged in all structures studied.
Although the activity or protein levels of MMP-2 and MMP-9 were increased in infected mice, in particular in the hippocampus, their transcript levels were only slightly modified. This discrepancy between mRNA levels and enzyme activity suggests that the increased gelatinolytic activity mainly results from the activation of previously synthesized pro-gelatinases stored within cells, as suggested for some pro-collagenases (60). However, we cannot exclude the possibility that the increase in MMP-2 and MMP-9 protein levels reflects either a stable steady state of the mRNAs or posttranscriptional or posttranslational regulatory events. The fact that TIMP-2 levels were not changed argues for pro-MMP-2 activation by MT1-MMP, a membrane-bound protein which can be activated intracellularly (2, 51, 58, 62) and is therefore likely to be secreted in an active form. Note that the soluble catalytic domain of MT1-MMP cleaves the propeptide of MMP-2, thus initiating autoproteolytic activation. However, the question of how MMP-2 activation actually occurs in the infected hippocampus remains unanswered. Nevertheless, activated MMP-2 could in turn be responsible for activation of other pro-MMPs, explaining the large increased gelatinolytic activity in the hippocampus. Interestingly, after seizures following kainate injection, MMP-2 and MMP-9 upregulation is induced in the hippocampus, in which active gelatinases are associated with glial and/or microglial activation (55, 66). In our infectious model, we also found that the strongest glial activation, demonstrated by GFAP upregulation, occurred mainly in the hippocampus and was associated with increased in situ gelatinolytic activity. Astrocytes and neurons were the main source of MMP-2 and MMP-9, as reported elsewhere (1, 25, 55), and the increase in MMP protein levels during the inflammatory process may point to an activated state of infected neurons, already shown by the neuronal localization of IL-6 and TNF-α in the hippocampus of CDV-infected mice (4).
Upregulation of MMP-2, MMP-9, and MT1-MMP in brain structures of CDV-infected mice may lead to tissue remodeling during viral encephalitis. Indeed, in MS the localization of the gelatinase, MMP-9, in reactive astrocytes in demyelinating lesions strongly suggests its involvement in tissue degradation (14). MT1-MMP proteolytic activity can target ECM components, including denatured collagen (gelatin) or fibronectin (15, 52, 62). The specific overexpression of TIMP-1 in the cortex and hypothalamus and of TIMP-3 in the cortex raises the issue of the net proteolytic activity and tissue integrity. TIMPs are secreted proteins which inhibit MMP activity and are therefore indirectly involved in the maintenance of cell cytoarchitecture and ECM-dependent signaling (for a review see reference 17). The increased expression of TIMP-1 and TIMP-3 in the CDV-infected brain could counterbalance proteolysis, as suggested in a variety of neurological disorders. Thus, TIMP-1 induction has been demonstrated to occur during inflammatory reactions in lipopolysaccharide-induced endotoxemia (50). In EAE, the upregulation of TIMP-1 seen in the region surrounding the inflamed area presumably limits the proteolytic and inflammatory processes (49). However, in brain structures of CDV-infected mice, the increase in TIMP-1 and TIMP-3 (about 100- and 10-fold, respectively) was unable to fully inhibit gelatinolytic activity, especially in the cortex, hippocampus, and hypothalamus. The concomitant increase in TIMP-1 and GFAP expression in the hippocampus may indicate that TIMP-1 not only functions as an MMP inhibitor but also contributes to the proliferation of glial cells, since it is a potential inducer of cell proliferation in vitro (28, 29, 46, 68).
Concomitantly with the upregulation of MT1-MMP (cortex and hypothalamus), TIMP-1 (cortex, hippocampus, and hypothalamus), and TIMP-3 (cortex), infected brain structures showed high cytokine levels compared to those of sham-inoculated counterparts. More precisely, IFN-γ induction (all brain structures) and marked upregulation of TNF-α (mesencephalon, hippocampus, hypothalamus, brain stem, and spinal cord) and of IL-6 (hippocampus) were seen, contrasting with the weaker induction of IL-4 (hippocampus, hypothalamus, and spinal cord) and of IL-10 (all structures except the spinal cord). The preferential upregulation of Th1-like cytokines (TNF-α, IL-6, and IFN-γ) relative to Th2-like cytokines (IL-4 and IL-10) in brain structures during the active phase of CDV replication may reflect a host immune response, since Th1 cytokines, such as TNF-α and IFN-γ, are critical for resistance to a number of neurotropic viral infections (18). Such coordinated modulation of MMP and TIMP expression has also been described in a variety of Th1-mediated diseases of the CNS, such as MS (14) or HTLV-1 associated myelopathy (20).
The increased expression of MMPs and TIMPs in neural cells may be explained by the presence of binding sites for transcription factors in the promoters of the MMP-2, MMP-9, MT1-MMP, TIMP-1, and TIMP-3 genes, which might be activated, in part, by cytokines. Thus, the increased expression of MMP-2 and MMP-9, especially in the hippocampus, can be attributed to upregulation of IL-6 and TNF-α, which have been described as the main actors in MMP modulation (19, 26, 54). TIMP-1 upregulation in the cortex, hippocampus, and hypothalamus could be due to synergistic activation of AP-1 (30) and polyoma enhancer activator 3 sites in the TIMP-1 promoter. TIMP-1 upregulation may be mediated by TNF-α, levels of which were increased in the same structures as TIMP-1, acting by stimulation of early gene production (38), or may reflect activation of the IL-6–oncostatin M-responsive element located in the TIMP-1 promoter (9). This notion is supported by findings for GFAP–IL-6 and GFAP–TNF-α transgenic mice which show an induction of TIMP-1 expression in areas of transgene expression (49). The fact that TIMP-3 upregulation is correlated with TNF-α expression in the cortex is consistent with the results of in vitro work showing that TNF-α can upregulate TIMP-3 expression in neural cells (21). Similarly, a positive correlation between MT1-MMP and TNF-α expression in the cortex and hypothalamus is in agreement with previous observations that, in mice expressing a TNF-α transgene, MT1-MMP is upregulated, especially in the forebrain and hindbrain (49). The exact mechanism of transcriptional regulation is still unknown, since the MT1-MMP promoter has only very recently been described (39). Nevertheless, the possibility of the direct regulation of MMP promoters by viral proteins cannot be ruled out, as Epstein-Barr virus proteins have been shown to activate these elements (65), but to date such gene transactivators have not been characterized in the negative-stranded viruses.
The MMP/TIMP imbalance induced by the local inflammatory process in brain structures highly permissive to CDV infection may lead to impaired ECM proteolytic processes. Indeed, various MMPs have been shown to degrade multiple substrates, including myelin basic protein, type IV collagen, and fibronectin (12, 45, 53, 64). Our preliminary observations on fibronectin cleavage, neosynthesis, and deposits, mainly in the cortex and hypothalamus of CDV-infected mice, argue for such enhanced proteolysis. Activation of the latent form of proteinases, a necessary physiological event in maintaining ECM integrity, is a prerequisite for regeneration processes (33) and synaptic plasticity (37) in the adult brain. The crucial role of MMPs in inflammation (24) indicates that perturbation of the MMP/TIMP axis may be decisive in pathogenesis during the encephalitic phase of infection. Intense proteolytic activity resulting from inappropriate synthesis and activation of MMPs may perturb cell signaling and neurotransmission by altering the physical characteristics of the protein meshwork of the ECM and the extracellular space (ECS), with subsequent ECS modifications (volume and neurochemical broadcasting constants) (for a review see reference 47). For example, cellular swelling (also seen in the brain of mice acutely infected with CDV; our unpublished observations) consecutive to the loss of ECM components may lead to a reduced ECS volume and result in an increased concentration of neuroactive molecules, thus enhancing the risk of reaching the toxic threshold (59). This is of particular interest in the light of perturbations of neurotransmission levels (dopaminergic and peptidergic) seen in the late CDV-induced pathologies (3; personal observations).
In conclusion, our results show that viral infection can differentially alter the expression of MMPs and TIMPs correlated to inflammatory cytokine expression in a brain region-specific manner, underlining the importance of assessing the differential impact of virus infection on different brain regions to understand the virally induced neurological disorders and emphasizing the fact that parameters other than the stimulus itself have to be considered. The local microenvironment, especially the types and amounts of neurochemicals, ECM components, and receptors present in this milieu, may explain how the same stimulus can generate differential responses leading to an increased susceptibility of certain brain structures and specific cells.
ACKNOWLEDGMENTS
We are grateful to Tom Barkas for critical evaluation of the English.
This work was supported by grants from INSERM–INRA, ARSEP.
REFERENCES
- 1.Backstrom J R, Lim G P, Cullen M J, Tokes Z A. Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-beta peptide (1–40) J Neurosci. 1996;16:7910–7919. doi: 10.1523/JNEUROSCI.16-24-07910.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basbaum C B, Werb Z. Focalized proteolysis: spatial and temporal regulation of extracellular matrix degradation at the cell surface. Curr Opin Cell Biol. 1996;8:731–738. doi: 10.1016/s0955-0674(96)80116-5. [DOI] [PubMed] [Google Scholar]
- 3.Bencsik A, Akaoka H, Giraudon P, Belin M F, Bernard A. Inhibition of tyrosine hydroxylase expression within the substantia nigra of mice infected with canine distemper virus. J Neuropathol Exp Neurol. 1997;56:673–685. [PubMed] [Google Scholar]
- 4.Bencsik A, Malcus C, Akaoka H, Giraudon P, Belin M F, Bernard A. Selective induction of cytokines in mouse brain infected with canine distemper virus: structural, cellular and temporal expression. J Neuroimmunol. 1996;65:1–9. doi: 10.1016/0165-5728(95)00173-5. [DOI] [PubMed] [Google Scholar]
- 5.Bentivoglio M, Florenzano F, Peng Z C, Kristensson K, Aldskogius M, Olsson T, Aldskogius H. Neuronal IFN-gamma in tuberomammillary neurones. Co-induction of neuronal interferon-gamma and nitric oxide synthase in rat motor neurons after axotomy: a role in nerve repair or death? Neuroreport. 1994;5:2413–2416. [Google Scholar]
- 6.Bernard A, Cohen R, Khuth S T, Vedrine B, Verlaeten O, Akaoka H, Giraudon P, Belin M F. Alteration of the leptin network in late morbid obesity induced in mice by brain infection with canine distemper virus. J Virol. 1999;73:7317–7327. doi: 10.1128/jvi.73.9.7317-7327.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bernard A, Fevre-Montange M, Bencsik A, Giraudon P, Wild T F, Confavreux C, Belin M F. Brain structures selectively targeted by canine distemper virus in a mouse model infection. J Neuropathol Exp Neurol. 1993;52:471–480. doi: 10.1097/00005072-199309000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Bernard A, Wild T F, Tripier M F. Canine distemper infection in mice: characterization of a neuro-adapted virus strain and its long-term evolution in the mouse. J Gen Virol. 1983;64:1571–1579. doi: 10.1099/0022-1317-64-7-1571. [DOI] [PubMed] [Google Scholar]
- 9.Bugno M, Graeve L, Gatsios P, Koj A, Heinrich P C, Travis J, Kordula T. Identification of the interleukin-6/oncostatin M response element in the rat tissue inhibitor of metalloproteinases-1 (TIMP-1) promoter. Nucleic Acids Res. 1995;23:5041–5047. doi: 10.1093/nar/23.24.5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burger D, Dayer J M. Inhibitory cytokines and cytokine inhibitors. Neurology. 1995;45:S39–S43. doi: 10.1212/wnl.45.6_suppl_6.s39. [DOI] [PubMed] [Google Scholar]
- 11.Cattaneo R, Rebmann G, Baczko K, ter Meulen V, Billeter M A. Altered ratios of measles virus transcripts in diseased human brains. Virology. 1987;160:523–526. doi: 10.1016/0042-6822(87)90031-6. [DOI] [PubMed] [Google Scholar]
- 12.Chandler S, Coates R, Gearing A, Lury J, Wells G, Bone E. Matrix metalloproteinases degrade myelin basic protein. Neurosci Lett. 1995;201:223–226. doi: 10.1016/0304-3940(95)12173-0. [DOI] [PubMed] [Google Scholar]
- 13.Conant K, McArthur J C, Griffin D E, Sjulson L, Wahl L M, Irani D N. Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Ann Neurol. 1999;46:391–398. doi: 10.1002/1531-8249(199909)46:3<391::aid-ana15>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 14.Cuzner M L, Gveric D, Strand C, Loughlin A J, Paemen L, Opdenakker G, Newcombe J. The expression of tissue-type plasminogen activator, matrix metalloproteases and endogenous inhibitors in the central nervous system in multiple sclerosis: comparison of stages in lesion evolution. J Neuropathol Exp Neurol. 1996;55:1194–1204. doi: 10.1097/00005072-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 15.d'Ortho M P, Will H, Atkinson S, Butler G, Messent A, Gavrilovic J, Smith B, Timpl R, Zardi L, Murphy G. Membrane-type matrix metalloproteinases 1 and 2 exhibit broad-spectrum proteolytic capacities comparable to many matrix metalloproteinases. Eur J Biochem. 1997;250:751–757. doi: 10.1111/j.1432-1033.1997.00751.x. [DOI] [PubMed] [Google Scholar]
- 16.Dudov K P, Perry R P. The gene family encoding the mouse ribosomal protein L32 contains a uniquely expressed intron-containing gene and an unmutated processed gene. Cell. 1984;37:457–468. doi: 10.1016/0092-8674(84)90376-3. [DOI] [PubMed] [Google Scholar]
- 17.Edwards D R, Beaudry P P, Laing T D, Kowal V, Leco K J, Leco P A, Lim M S. The roles of tissue inhibitors of metalloproteinases in tissue remodelling and cell growth. Int J Obes Relat Metab Disord. 1996;20(Suppl.):S9–S15. [PubMed] [Google Scholar]
- 18.Finke D, Brinckmann U G, ter Meulen V, Liebert U G. Gamma interferon is a major mediator of antiviral defense in experimental measles virus-induced encephalitis. J Virol. 1995;69:5469–5474. doi: 10.1128/jvi.69.9.5469-5474.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giraudon P, Buart S, Bernard A, Belin M F. Cytokines secreted by glial cells infected with HTLV-I modulate the expression of matrix metalloproteinases (MMPs) and their natural inhibitor (TIMPs): possible involvement in neurodegenerative processes. Mol Psychiatry. 1997;2:107–110. doi: 10.1038/sj.mp.4000218. [DOI] [PubMed] [Google Scholar]
- 20.Giraudon P, Buart S, Bernard A, Thomasset N, Belin M F. Extracellular matrix-remodeling metalloproteinases and infection of the central nervous system with retrovirus human T-lymphotropic virus type I (HTLV-I) Prog Neurobiol. 1996;49:169–184. doi: 10.1016/0301-0082(96)00017-2. [DOI] [PubMed] [Google Scholar]
- 21.Giraudon P, Szymocha R, Buart S, Bernard A, Cartier L, Belin M F, Akaoka H. T lymphocytes activated by persistent viral infection differentially modify the expression of metalloproteinases and their endogenous inhibitors, TIMPs, in human astrocytes: relevance to HTLV-I-induced neurological disease. J Immunol. 2000;164:2718–2727. doi: 10.4049/jimmunol.164.5.2718. [DOI] [PubMed] [Google Scholar]
- 22.Giraudon P, Thomasset N, Bernard A, Verrier B, Belin M F. Induction of MMP9 (92 kDa gelatinase) activity and expression of tissue inhibitor of metalloproteinase-2 mRNA (TIMP-2) in primitive neuroectodermal cells infected with retrovirus HTLV-I. Eur J Neurosci. 1995;7:841–848. doi: 10.1111/j.1460-9568.1995.tb01071.x. [DOI] [PubMed] [Google Scholar]
- 23.Giraudon P, Vernant J C, Confavreux C, Belin M F, Desgranges C. Matrix metalloproteinase 9 (gelatinase B) in cerebrospinal fluid of HTLV-1 infected patients with tropical spastic paraparesis. Neurology. 1998;50:1920. doi: 10.1212/wnl.50.6.1920. [DOI] [PubMed] [Google Scholar]
- 24.Goetzl E J, Banda M J, Leppert D. Matrix metalloproteinases in immunity. J Immunol. 1996;156:1–4. [PubMed] [Google Scholar]
- 25.Gottschall P E, Deb S. Regulation of matrix metalloproteinase expressions in astrocytes, microglia and neurons. Neuroimmunomodulation. 1996;3:69–75. doi: 10.1159/000097229. [DOI] [PubMed] [Google Scholar]
- 26.Gottschall P E, Yu X. Cytokines regulate gelatinase A and B (matrix metalloproteinase 2 and 9) activity in cultured rat astrocytes. J Neurochem. 1995;64:1513–1520. doi: 10.1046/j.1471-4159.1995.64041513.x. [DOI] [PubMed] [Google Scholar]
- 27.Greene C E, Appel M J G, editors. Infectious diseases of the dog and cat. Philadelphia, Pa: W. B. Saunders Co.; 1999. pp. 9–22. [Google Scholar]
- 28.Hayakawa T. Tissue inhibitors of metalloproteinases and their cell growth-promoting activity. Cell Struct Funct. 1994;19:109–114. doi: 10.1247/csf.19.109. [DOI] [PubMed] [Google Scholar]
- 29.Hayakawa T, Yamashita K, Tanzawa K, Uchijima E, Iwata K. Growth-promoting activity of tissue inhibitor of metalloproteinases-1 (TIMP-1) for a wide range of cells. A possible new growth factor in serum. FEBS Lett. 1992;298:29–32. doi: 10.1016/0014-5793(92)80015-9. [DOI] [PubMed] [Google Scholar]
- 30.Jaworski J, Biedermann I W, Lapinska J, Szklarczyk A, Figiel I, Konopka D, Nowicka D, Filipkowski R K, Hetman M, Kowalczyk A, Kaczmarek L. Neuronal excitation-driven and AP-1-dependent activation of tissue inhibitor of metalloproteinases-1 gene expression in rodent hippocampus. J Biol Chem. 1999;274:28106–28112. doi: 10.1074/jbc.274.40.28106. [DOI] [PubMed] [Google Scholar]
- 31.Kieseier B C, Clements J M, Pischel H B, Wells G M, Miller K, Gearing A J, Hartung H P. Matrix metalloproteinases MMP-9 and MMP-7 are expressed in experimental autoimmune neuritis and the Guillain-Barre syndrome. Ann Neurol. 1998;43:427–434. doi: 10.1002/ana.410430404. [DOI] [PubMed] [Google Scholar]
- 32.Kieseier B C, Paul R, Koedel U, Seifert T, Clements J M, Gearing A J, Pfister H W, Hartung H P. Differential expression of matrix metalloproteinases in bacterial meningitis. Brain. 1999;122:1579–1587. doi: 10.1093/brain/122.8.1579. [DOI] [PubMed] [Google Scholar]
- 33.Kinoh H, Sato H, Tsunezuka Y, Takino T, Kawashima A, Okada Y, Seiki M. MT-MMP, the cell surface activator of proMMP-2 (pro-gelatinase A), is expressed with its substrate in mouse tissue during embryogenesis. J Cell Sci. 1996;109:953–959. doi: 10.1242/jcs.109.5.953. [DOI] [PubMed] [Google Scholar]
- 34.Kolb S A, Lahrtz F, Paul R, Leppert D, Nadal D, Pfister H W, Fontana A. Matrix metalloproteinases and tissue inhibitors of metalloproteinases in viral meningitis: upregulation of MMP-9 and TIMP-1 in cerebrospinal fluid. J Neuroimmunol. 1998;84:143–150. doi: 10.1016/s0165-5728(97)00247-6. [DOI] [PubMed] [Google Scholar]
- 35.Lezin A, Buart S, Smadja D, Akaoka H, Bourdonne O, Perret-Liaudet A, Cesaire R, Belin M F, Giraudon P. Tissue inhibitor of metalloproteinase 3, matrix metalloproteinase 9, and neopterin in the cerebrospinal fluid: preferential presence in HTLV type I-infected neurologic patients versus healthy virus carriers. AIDS Res Hum Retrovir. 2000;16:965–972. doi: 10.1089/08892220050058380. [DOI] [PubMed] [Google Scholar]
- 36.Lim G P, Backstrom J R, Cullen M J, Miller C A, Atkinson R D, Tokes Z A. Matrix metalloproteinases in the neocortex and spinal cord of amyotrophic lateral sclerosis patients. J Neurochem. 1996;67:251–259. doi: 10.1046/j.1471-4159.1996.67010251.x. [DOI] [PubMed] [Google Scholar]
- 37.Liu Y, Fields R D, Festoff B W, Nelson P G. Proteolytic action of thrombin is required for electrical activity-dependent synapse reduction. Proc Natl Acad Sci USA. 1994;91:10300–10304. doi: 10.1073/pnas.91.22.10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Logan S K, Garabedian M J, Campbell C E, Werb Z. Synergistic transcriptional activation of the tissue inhibitor of metalloproteinases-1 promoter via functional interaction of AP-1 and Ets-1 transcription factors. J Biol Chem. 1996;271:774–782. doi: 10.1074/jbc.271.2.774. [DOI] [PubMed] [Google Scholar]
- 39.Lohi J, Lehti K, Valtanen H, Parks W C, Keski-Oja J. Structural analysis and promoter characterization of the human membrane-type matrix metalloproteinase-1 (MT1-MMP) gene. Gene. 2000;242:75–86. doi: 10.1016/s0378-1119(99)00549-1. [DOI] [PubMed] [Google Scholar]
- 40.Lukashev M E, Werb Z. ECM signalling: orchestrating cell behaviour and misbehaviour. Trends Cell Biol. 1998;8:437–441. doi: 10.1016/s0962-8924(98)01362-2. [DOI] [PubMed] [Google Scholar]
- 41.Lukes A, Mun-Bryce S, Lukes M, Rosenberg G A. Extracellular matrix degradation by metalloproteinases and central nervous system diseases. Mol Neurobiol. 1999;19:267–284. doi: 10.1007/BF02821717. [DOI] [PubMed] [Google Scholar]
- 42.Maeda A, Sobel R A. Matrix metalloproteinases in the normal human central nervous system, microglial nodules, and multiple sclerosis lesions. J Neuropathol Exp Neurol. 1996;55:300–309. doi: 10.1097/00005072-199603000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Matrisian L M. The matrix-degrading metalloproteinases. Bioessays. 1992;14:455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- 44.Matrisian L M. Metalloproteinases and their inhibitors in matrix remodeling. Trends Genet. 1990;6:121–125. doi: 10.1016/0168-9525(90)90126-q. [DOI] [PubMed] [Google Scholar]
- 45.Murphy G, Gavrilovic J. Proteolysis and cell migration: creating a path? Curr Opin Cell Biol. 1999;11:614–621. doi: 10.1016/s0955-0674(99)00022-8. [DOI] [PubMed] [Google Scholar]
- 46.Nemeth J A, Rafe A, Steiner M, Goolsby C L. TIMP-2 growth-stimulatory activity: a concentration- and cell type-specific response in the presence of insulin. Exp Cell Res. 1996;224:110–115. doi: 10.1006/excr.1996.0117. [DOI] [PubMed] [Google Scholar]
- 47.Nicholson C, Sykova E. Extracellular space structure revealed by diffusion analysis. Trends Neurosci. 1998;21:207–215. doi: 10.1016/s0166-2236(98)01261-2. [DOI] [PubMed] [Google Scholar]
- 48.Pagenstecher A, Stalder A K, Campbell I L. RNAse protection assays for the simultaneous and semiquantitative analysis of multiple murine matrix metalloproteinase (MMP) and MMP inhibitor mRNAs. J Immunol Methods. 1997;206:1–9. doi: 10.1016/s0022-1759(97)00077-x. [DOI] [PubMed] [Google Scholar]
- 49.Pagenstecher A, Stalder A K, Kincaid C L, Shapiro S D, Campbell I L. Differential expression of matrix metalloproteinase and tissue inhibitor of matrix metalloproteinase genes in the mouse central nervous system in normal and inflammatory states. Am J Pathol. 1998;152:729–741. [PMC free article] [PubMed] [Google Scholar]
- 50.Pagenstecher A, Stalder A K, Kincaid C L, Volk B, Campbell I L. Regulation of matrix metalloproteinases and their inhibitor genes in lipopolysaccharide-induced endotoxemia in mice. Am J Pathol. 2000;157:197–210. doi: 10.1016/S0002-9440(10)64531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pei D, Weiss S J. Furin-dependent intracellular activation of the human stromelysin-3 zymogen. Nature. 1995;375:244–247. doi: 10.1038/375244a0. [DOI] [PubMed] [Google Scholar]
- 52.Pei D, Weiss S J. Transmembrane-deletion mutants of the membrane-type matrix metalloproteinase-1 process progelatinase A and express intrinsic matrix-degrading activity. J Biol Chem. 1996;271:9135–9140. doi: 10.1074/jbc.271.15.9135. [DOI] [PubMed] [Google Scholar]
- 53.Proost P, Van Damme J, Opdenakker G. Leukocyte gelatinase B cleavage releases encephalitogens from human myelin basic protein. Biochem Biophys Res Commun. 1993;192:1175–1181. doi: 10.1006/bbrc.1993.1540. [DOI] [PubMed] [Google Scholar]
- 54.Ries C, Petrides P E. Cytokine regulation of matrix metalloproteinase activity and its regulatory dysfunction in disease. Biol Chem Hoppe-Seyler. 1995;376:345–355. [PubMed] [Google Scholar]
- 55.Rivera S, Tremblay E, Timsit S, Canals O, Ben-Ari Y, Khrestchatisky M. Tissue inhibitor of metalloproteinases-1 (TIMP-1) is differentially induced in neurons and astrocytes after seizures: evidence for developmental, immediate early gene, and lesion response. J Neurosci. 1997;17:4223–4235. doi: 10.1523/JNEUROSCI.17-11-04223.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rozenblatt S, Eizenberg O, Ben-Levy R, Lavie V, Bellini W J. Sequence homology within the morbilliviruses. J Virol. 1985;53:684–690. doi: 10.1128/jvi.53.2.684-690.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sabath D E, Broome H E, Prystowsky M B. Glyceraldehyde-3-phosphate dehydrogenase mRNA is a major interleukin 2-induced transcript in a cloned T-helper lymphocyte. Gene. 1990;91:185–191. doi: 10.1016/0378-1119(90)90087-8. [DOI] [PubMed] [Google Scholar]
- 58.Sato H, Kinoshita T, Takino T, Nakayama K, Seiki M. Activation of a recombinant membrane type 1-matrix metalloproteinase (MT1-MMP) by furin and its interaction with tissue inhibitor of metalloproteinases (TIMP)-2. FEBS Lett. 1996;393:101–104. doi: 10.1016/0014-5793(96)00861-7. [DOI] [PubMed] [Google Scholar]
- 59.Sykova E, Mazel T, Simonova Z. Diffusion constraints and neuron-glia interaction during aging. Exp Gerontol. 1998;33:837–851. doi: 10.1016/s0531-5565(98)00038-2. [DOI] [PubMed] [Google Scholar]
- 60.Van der Zee E, Everts V, Beertsen W. Cytokine-induced endogenous procollagenase stored in the extracellular matrix of soft connective tissue results in a burst of collagen breakdown following its activation. J Periodontal Res. 1996;31:483–488. doi: 10.1111/j.1600-0765.1996.tb01413.x. [DOI] [PubMed] [Google Scholar]
- 61.Werb Z. ECM and cell surface proteolysis: regulating cellular ecology. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 62.Will H, Atkinson S J, Butler G S, Smith B, Murphy G. The soluble catalytic domain of membrane type 1 matrix metalloproteinase cleaves the propeptide of progelatinase A and initiates autoproteolytic activation. Regulation by TIMP-2 and TIMP-3. J Biol Chem. 1996;271:17119–17123. doi: 10.1074/jbc.271.29.17119. [DOI] [PubMed] [Google Scholar]
- 63.Yamanouchi K. Comparative aspects of pathogenicity of measles, canine distemper, and rinderpest viruses. Jpn J Med Sci Biol. 1980;33:41–66. doi: 10.7883/yoken1952.33.41. [DOI] [PubMed] [Google Scholar]
- 64.Yong V W, Krekoski C A, Forsyth P A, Bell R, Edwards D R. Matrix metalloproteinases and diseases of the CNS. Trends Neurosci. 1998;21:75–80. doi: 10.1016/s0166-2236(97)01169-7. [DOI] [PubMed] [Google Scholar]
- 65.Yoshizaki T, Sato H, Furukawa M, Pagano J S. The expression of matrix metalloproteinase 9 is enhanced by Epstein-Barr virus latent membrane protein 1. Proc Natl Acad Sci USA. 1998;95:3621–3626. doi: 10.1073/pnas.95.7.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang J W, Deb S, Gottschall P E. Regional and differential expression of gelatinases in rat brain after systemic kainic acid or bicuculline administration. Eur J Neurosci. 1998;10:3358–3368. doi: 10.1046/j.1460-9568.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 67.Zhang J W, Gottschall P E. Zymographic measurement of gelatinase activity in brain tissue after detergent extraction and affinity-support purification. J Neurosci Methods. 1997;76:15–20. doi: 10.1016/s0165-0270(97)00065-4. [DOI] [PubMed] [Google Scholar]
- 68.Zhao W Q, Li H, Yamashita K, Guo X K, Hoshino T, Yoshida S, Shinya T, Hayakawa T. Cell cycle-associated accumulation of tissue inhibitor of metalloproteinases-1 (TIMP-1) in the nuclei of human gingival fibroblasts. J Cell Sci. 1998;111:1147–1153. doi: 10.1242/jcs.111.9.1147. [DOI] [PubMed] [Google Scholar]