Abstract
The carboxyl terminus of the hepatitis C virus (HCV) nonstructural protein 3 (NS3) possesses ATP-dependent RNA helicase activity. Based on the conserved sequence motifs and the crystal structures of the helicase domain, 17 mutants of the HCV NS3 helicase were generated. The ATP hydrolysis, RNA binding, and RNA unwinding activities of the mutant proteins were examined in vitro to determine the functional role of the mutated residues. The data revealed that Lys-210 in the Walker A motif and Asp-290, Glu-291, and His-293 in the Walker B motif were crucial to ATPase activity and that Thr-322 and Thr-324 in motif III and Arg-461 in motif VI significantly influenced ATPase activity. When the pairing between His-293 and Gln-460, referred to as gatekeepers, was replaced with the Asp-293/His-460 pair, which makes the NS3 helicase more like the DEAD helicase subgroup, ATPase activity was not restored. It thus indicated that the whole microenvironment surrounding the gatekeepers, rather than the residues per se, was important to the enzymatic activities. Arg-461 and Trp-501 are important residues for RNA binding, while Val-432 may only play a coadjutant role. The data demonstrated that RNA helicase activity was possibly abolished by the loss of ATPase activity or by reduced RNA binding activity. Nevertheless, a low threshold level of ATPase activity was found sufficient for helicase activity. Results in this study provide a valuable reference for efforts under way to develop anti-HCV therapeutic drugs targeting NS3.
Hepatitis C virus (HCV) is the major causative agent of parenterally transmitted non-A, non-B hepatitis (6, 8, 25). The infection easily becomes persistent and causes chronic hepatitis (17), which may lead to liver cirrhosis (38, 42) and hepatocellular carcinoma (9, 36). Alpha interferon and ribavirin combination therapy is the only effective therapy for chronic hepatitis C; however, less than 50% of patients respond to the treatment (2, 3, 5, 28). Therefore, new, more effective treatments for hepatitis C urgently need to be developed.
The HCV genome encodes a large polyprotein comprised of 3,008 to 3,037 amino acids (aa) (4). Nonstructural protein 3 (NS3) of HCV, ranging from aa 1027 to 1657 of the polyprotein, is a multifunctional protein. The N terminus of NS3 expresses a serine protease (27), while two-thirds of the C- terminal region of the protein expresses an RNA helicase (18, 20, 40, 41). The homologous NS3s in all flaviviruses and pestiviruses sequenced to date contain conserved sequence motifs (31), suggesting that the enzyme may be important in viral replication and may be a potential target for developing antiviral drugs.
Helicases are the enzymes that unwind duplex DNA or RNA at the expense of energy derived from nucleoside triphosphate (NTP) hydrolysis (13). A large number of putative RNA and DNA helicases from different organisms have been identified (14, 23). Sequence comparisons have classified the helicases into three superfamilies (SF): SFI, SFII, and SFIII (14, 19, 37). The HCV NS3 helicase belongs to SFII, which contains eight conserved motifs (14, 24). Motifs I (AxxGxGKS/T) and II (DExH), known as Walker motifs A and B, respectively (44), are responsible for binding the NTP-Mg2+ complex (46), while motif VI (QRxGRxGR) is thought to bind nucleic acids (11, 19) because it possesses many basic residues (notably Arg).
The crystal structure of HCV NS3 helicase has recently been uncovered (7, 22, 48). The reported structures shared similar global conformation, which consisted of three domains forming a Y-shaped configuration. The structure reported by Kim et al. is the only one based on a cocrystallization of the helicase with a sulfate ion and a (dU)8 oligonucleotide (22). Notably, Kim et al. predicted that conserved motif VI (QRRGRTGR) would not interact with the single-stranded DNA oligonucleotide as originally expected but would be involved in ATP hydrolysis. On the other hand, hydrophobic residue Val-432 and aromatic residue Trp-501 are associated with the (dU)8 oligonucleotide. They also noted that His-293 and Gln-460 from motifs II and VI, respectively, lie on opposite sides of the interdomain cleft and referred to them as “gatekeepers.” However, the importance of gatekeepers has not been biochemically determined yet.
To decipher the link between the biochemical role and three-dimensional structure of the important amino acid residues, this study investigated the activities of 17 point mutation clones of NS3.
MATERIALS AND METHODS
Site-directed mutagenesis.
The plasmid 5′-1175/pET21a (41), which contains the RNA helicase domain encompassing aa 1175 (nucleotide [nt] 3864) to 1657 (nt 5312) cloned at the BamHI and HindIII sites of the pET21a plasmid, was used as the target plasmid for site-directed mutagenesis. Mutants A204V, K210N, D290N, E291Q, C292A, H293A, C292A/H293D, and R461Q were constructed using the Transformer site-directed mutagenesis kit (Clontech) according to the manufacturer's instructions. Meanwhile, mutations were confirmed by DNA sequencing. Table 1 lists the sequences of the mutagenic primers. The remaining mutants E291A, H293K, H293Q, T322A, T324A, V432A, C292A/H293D/Q460H, W501A, and V432A/W501A were constructed using recombinant PCR, with the desired mutations introduced in the internal mutagenic primers (Table 1). The mutated forms of the BamHI (nt 3864)-EcoRI (nt 4578) DNA fragments (for mutants E291A, H293K, H293Q, T322A, and T324A) or the EcoRI (nt 4578)-StuI (nt 5034) DNA fragments (for mutants V432A and W501A) were then used in place of the corresponding regions of the parental 5′-1175/pET21a plasmid. Mutant Q460H/C292A/H293D was generated by substituting the mutated EcoRI-StuI fragment harboring the Q460H mutation for the corresponding region of the C292A/H293D plasmid, while mutant V432A/W501A was constructed by substituting the EcoRI-StuI DNA fragment harboring the W501A mutation for the same region of the V432A plasmid. To ensure that only the desired mutation was introduced, the PCR portions were sequenced with the dideoxy DNA sequencing method.
TABLE 1.
Sequence of primers used for site-directed mutagenesis and recombinant PCRa
| Mutant | Mutagenic or recombinant PCR primer |
|---|---|
| A204V | nt 4019 CCATCTACACGTTCCCACTGGC nt 4040 |
| K210N | nt 4038 GGCAGCGGCAACAGCACTAAGG nt 4059 |
| D290N | nt 4277 CATAATGTGTAATGAGTGCCAC nt 4298 |
| E291Q | nt 4280 AATGTGTGATCAGTGCCACTC nt 4300 |
| C292A | nt 4283 GTGTGATGAGGCCCACTCAACTG nt 4305 |
| H293A | nt 4286 TGATGAGTGCGCCTCAACTGAC nt 4307 |
| C292A/H293D | nt 4383 GTGTGATGAGGCCGACTCAACTG nt 4305 |
| R461Q | nt 4793 CTCGCAGCAGCGAGGCAG nt 4810 |
| BamHI | (Sense) AAGGATCC nt 3864 CATGTTGTGGGCATC nt 3878 |
| EcoRI | (Sense) AAAA nt 4573 GAATTCATGCTGTAGCA nt 4589 |
| (Antisense) AAAA nt 4578 GAATTCCGAGGGCCGA nt 4563 | |
| StuI | (Antisense) AAAA nt 5034 GGCCTGTGAAGACGCTC nt 5018 |
| E291A | (Sense) nt 4280 AATGTGTGATGCGTGCCACTC nt 4300 |
| (Antisense) nt 4300 GAGTGGCACGCATCACACATT nt 4280 | |
| H293K | (Sense) nt 4286 TGATGAGTGCAAGTCAACTGAC nt 4307 |
| (Antisense) nt 4307 GTCAGTTGACTTGCACTCATCA nt 4286 | |
| H293Q | (Sense) nt 4286 TGATGAGTGCCAGTCAACTGAC nt 4307 |
| (Antisense) nt 4307 GTCAGTTGACTGGCACTCATCA nt 4286 | |
| T322A | (Sense) nt 4373 CGTGCTCGCCGCCGCTACGC nt 4392 |
| (Antisense) nt 4392 GCGTAGCGGCGGCGAGCACG nt 4373 | |
| T324A | (Sense) nt 4380 GCCACCGCTGCGCCTCCGGG nt 4399 |
| (Antisense) nt 4399 CCCGGAGGCGCAGCGGTGGC nt 4380 | |
| V432A | (Sense) nt 4702 GTAACGTGTGCCACCCAGAC nt 4723 |
| (Antisense) nt 4723 GTCTGGGTGGCACACGTGTTAC nt 4702 | |
| W501A | (Sense) nt 4910 GGGCTGTGCTGCGTACGAGC nt 4929 |
| (Antisense) nt 4929 GCTCGTACGCAGCACAGCCC nt 4910 | |
| C292A/H293D/Q460H | (Sense) nt 4789 CACGCTCGCACCGGCGAGGC nt 4808 |
| (Antisense) nt 4808 GCCTCGCCGGTGCGAGCGTG nt 4789 |
The mutated nucleotides are shown in boldface. The start and stop sites of the NS3 sequences within the primer are indicated by four-digit numbers. Recombinant PCR primers are given beginning with BamHI.
Expression and purification of mutant proteins.
The mutant plasmids were transformed into Escherichia coli BL21(DE3). Expression of the plasmids was induced by adding 0.2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 30°C for 3 h. Purification of the proteins was performed by using an Ni-affinity column as previously described (41). The final eluted proteins were dialyzed in TNE buffer (20 mM Tris-HCl, pH 7.9, 50 mM NaCl, and 1 mM EDTA) and were concentrated using Centriprep-10 (Amicon). The purified proteins were quantified using a bicinchoninic acid protein assay reagent (Pierce).
ATPase activity assays.
ATPase activity was assessed by measuring the extent of [α-32P]ATP hydrolysis as previously described (41). The reaction mixture (10 μl) contained 20 mM HEPES-KOH (pH 7.0), 2 mM dithiothreitol, 1.5 mM MgCl2, 5 μCi of [α-32P]ATP (3,000 Ci/mmol; Amersham), 100 μg of poly(U)/ml, 0.05 μg of wild-type or mutant helicases, and various concentrations of cold ATP ranging from 0.067 mM to 0.8 mM. For kinetic analysis, the reaction was carried out at room temperature for just 5 min to constrain the reaction rate within the linear phase and was then halted by adding EDTA to 20 mM. Reaction products were analyzed by thin-layer chromatography. One-half microliter of the reaction mixture was spotted onto plastic-backed polyethyleneimine cellulose F sheets (Merck) and was developed by ascending chromatography in 0.375 M potassium phosphate (pH 3.5) buffer. The sheets were dried and exposed to a FUJIX imaging plate, and the conversion rate was quantified by phosphorimager analysis (FUJIX BAS1000).
ATP binding assays.
UV cross-linking (29, 49) was used to assess the ATP binding ability of those mutants whose ATPase activity was undetectable. However, this is a nonequilibrium method of measuring ATP binding. One microgram of wild-type or mutant helicase protein and 100 μg of poly(U)/ml were premixed in 10 μl of a buffer containing 20 mM HEPES-KOH, pH 7.5, and 5 mM Mg(CH3CO2)2. Then 6 μCi (2 pmol) of [α-32P]ATP (3,000 Ci/mmol; Amersham) and 181 pmol of cold ATP were added. The reaction mixture was incubated on ice and was then irradiated using a UV cross-linker (Stratagene) (254 nm) situated at a distance of 4 cm for 25 min. Samples were boiled in sample buffer (100 mM Tris-HCl, pH 6.8, 2% sodium dodecyl sulfate [SDS], 20% β-mercaptoethanol, 20% glycerol, 4 mM EDTA, and 0.01% bromophenol blue) for 5 min and were then separated by SDS–12.5% polyacrylamide gel electrophoresis (PAGE). The gels were stained with Coomassie brilliant blue, dried, and processed for autoradiography.
Preparation of partial dsRNA substrates.
The partial double-stranded RNA (dsRNA) substrates were prepared by transcribing in vitro a portion of the multiple cloning sequences of a pGEM vector (Promega) in both orientations and then annealing these two RNA strands (41). After in vitro transcription, the transcripts were combined at a molar ratio of released strand (labeled) to template strand (unlabeled) of approximately 1:10 in a solution containing 20 mM HEPES-KOH (pH 7.6), 0.5 M NaCl, 1 mM EDTA, and 0.1% SDS. The mixture was boiled for 10 min, transferred to 65°C for 30 min, and then incubated at 25°C overnight. The hybridized products were mixed with 5× RNA loading dye (0.1 M Tris-HCl [pH 7.4], 20 mM EDTA, 0.5% SDS, 0.1% bromophenol blue, 0.1% xylene cyanol, and 50% glycerol) and then subjected to electrophoresis on an 8% native polyacrylamide (acrylamide/bisacrylamide ratio, 30:1)–1× Tris-borate-EDTA gel. The duplex RNA band was localized by autoradiography. The gel slice was excised, pulverized, and extracted with 0.5 M ammonium acetate (pH 7.0)–0.1% SDS–10 mM EDTA for 2 h at 25°C. The eluted substrate was then extracted with phenol-chloroform, ethanol precipitated, and resuspended in storage buffer (20 mM HEPES [pH 7.6] and 0.1 mM EDTA). The annealed product contains both 5′ and 3′ overhang regions and an internal double-stranded region.
RNA binding assays.
The binding of the partial dsRNA substrate to the HCV NS3 helicase or to the mutant proteins was analyzed by gel mobility shift assay. One-half microgram of the wild-type or mutant helicase protein was incubated with 1.3 pmol of radiolabeled partial dsRNA substrate and 15 pmol of cold single release strand in 20 μl of a helicase reaction buffer containing nonhydrolyzable ATP-γS. The binding reaction was incubated at 37°C for 15 min and was then terminated by adding 5× RNA loading dye containing 0.5% Nonidet P-40 instead of SDS and was electrophoresed on a 4% polyacrylamide (acrylamide/bisacrylamide ratio, 80:1)–1/3× Tris-borate-EDTA gel containing 5% glycerol. The bound complexes were visualized by autoradiography (41).
RNA helicase assays.
Helicase assays were conducted by measuring the unwinding of the radiolabeled dsRNA substrate as previously described (41) except that, for kinetic analysis, the reaction was performed for just 5 min to constrain the reaction rate within the linear phase. The reaction mixture (20 μl) contained 20 mM HEPES-KOH (pH 7.0), 2 mM dithiothreitol, 1.5 mM MnCl2, 2.5 mM ATP, 0.1 mg of bovine serum albumin per ml, 2 U of RNasin, 0.1 μg of wild-type or mutant helicases, and various amounts of dsRNA substrate ranging from 0.66 pmol to 10.56 pmol. The reaction was carried out at 37°C for 5 min and was then terminated by adding 5 μl of 5× RNA loading dye. An aliquot (12.5 μl) of each reaction was loaded onto an 8% native polyacrylamide (acrylamide/bisacrylamide ratio, 30:1)–1× Tris-borate-EDTA gel and electrophoresed. The gel was dried and autoradiographed. The ratio of single-stranded products to double-stranded substrates was quantified by phosphorimager analysis (FUJIX BAS1000).
RESULTS
Mutation, expression, and purification of HCV helicase proteins.
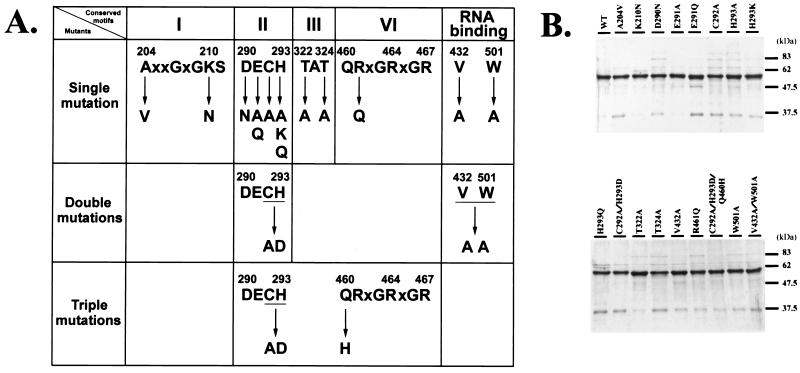
Like many other SFII helicases, the HCV NS3 helicase contains several conserved motifs. This work investigates the roles of conserved motifs I, II, III, and VI and the two possible RNA-interacting residues, Val-432 and Trp-501, in ATP hydrolysis, RNA binding, and RNA helicase activities. A total of 17 mutants were constructed using site-directed mutagenesis. Figure 1A illustrates the mutated positions and the amino acids replaced. The wild type and the mutant clones were expressed in an E. coli pET expression system, and the recombinant proteins were purified using the Ni-agarose affinity column. Figure 1B illustrates the SDS-PAGE results for 2 μg of each of the purified proteins. The estimated molecular mass of the wild-type NS3 helicase or its mutant proteins is approximately 56 kDa. The data revealed that all these proteins had similar purities (ranging from 80 to 88%).
FIG. 1.
Site-directed mutagenesis of the HCV NS3 helicase domain. (A) Summary of all mutant clones. The top line sequences represent conserved motifs I, II, III, and VI, respectively, of HCV NS3 helicase. The amino acid residue replacements are shown below the arrows. (B) Purities of mutant proteins. The wild-type and mutant proteins were expressed in E. coli and purified as described in Materials and Methods. Two micrograms of each of the purified proteins was resolved by SDS–12.5% PAGE and stained with Coomassie brilliant blue. WT denotes the wild-type helicase protein.
ATPase activity of helicase mutants.
ATPase activity was measured in the presence of poly(U) to stimulate the ATP hydrolysis activity of HCV NS3 (40, 41). Enzymatic activity was measured kinetically and expressed as kcat/Km, determined by varying the concentration of ATP substrate. All mutant proteins displayed a degree of reduced ATPase activity when compared to the wild-type protein (Table 2).
TABLE 2.
Summary of ATPase and RNA helicase activities of wild-type and mutant forms of HCV NS3 helicase
| Mutant | Mutated sequencea | ATPase activity
|
Helicase activity
|
||
|---|---|---|---|---|---|
| kcat/Km (mM−1min−1) | % of wild-type activity | kcat/Km (mM−1min−1) | % of wild-type activity | ||
| Motif I | |||||
| WT 5′-1175 | AxxGxGKS | 2,693.6 | 100 | 2,737.6 | 100 |
| A204V | VxxGxGKS | 2,491.7 | 93 | 1,966.1 | 72 |
| K210Nc | AxxGxGNS | UDb | 0 | UD | 0 |
| Motif II | |||||
| WT 5′-1175 | DECH | 2,693.6 | 100 | 2,737.6 | 100 |
| D290N | NECH | UD | 0 | UD | 0 |
| E291A | DACH | 2,104.5 | 78 | 2,212.7 | 81 |
| E291Q | DQCH | UD | 0 | UD | 0 |
| C292A | DEAH | 1,423.2 | 53 | 2,252.4 | 82 |
| H293A | DECA | 173.1 | 6 | 2,040.9 | 75 |
| H293K | DECK | 1,173.7 | 44 | 1,793.7 | 66 |
| H293Q | DECQ | 782.1 | 29 | 2,098.6 | 77 |
| C292A/H293D | DEAD | UD | 0 | UD | 0 |
| C292A/H293D/Q460H | DEAD/HRxGRxGR | UD | 0 | UD | 0 |
| Motif III | |||||
| WT 5′-1175 | TAT | 2,693.6 | 100 | 2,737.6 | 100 |
| T322A | AAT | 804.1 | 30 | 1,699.5 | 62 |
| T324A | TAA | 424.7 | 16 | 1,693.9 | 62 |
| Motif VI | |||||
| WT 5′-1175 | QRxGRxGR | 2,693.6 | 100 | 2,737.6 | 100 |
| R461Q | QQxGRxGR | 200.5 | 7 | UD | 0 |
| Nucleic acid interaction | |||||
| WT 5′-1175 | V432/W501 | 2,693.6 | 100 | 2,737.6 | 100 |
| V432A | A432 | 1,653.6 | 61 | 1,856.8 | 68 |
| W501A | A501 | 2,140.5 | 79 | UD | 0 |
| V432A + W501A | A432/A501 | 227.5 | 8 | UD | 0 |
Mutated residues are in boldface.
UD, undetectable.
The mutants that completely lose RNA helicase activity are K210N, D290N, E291Q, C292A/H293D, C292A/H293D/Q460H, R461Q, W501A, and V432A + W501A.
According to the data herein, mutations at motifs I and II (Walker motifs A and B, respectively) affected ATPase activity differently. While the A204V mutation did not alter ATPase activity, the K210N, D290N, and E291Q mutant proteins completely lost ATPase activity, suggesting that the Lys-210 residue of motif I (AxxGxGK210ST) and the Asp-290 and Glu-291 residues of motif II (D290E291CH) were crucial to ATPase activity. Yet, replacing Glu-291 (E291A) with Ala only slightly affected ATPase activity. Replacing the third residue of the DEC292H motif (C292A) with Ala reduced ATPase activity to nearly half that of the wild-type protein. The fourth residue of the DECH293 motif was also involved in ATP hydrolysis, as replacing His-293 (H293A or H293Q) with Ala or Gln reduced ATPase activity to 6 or 29%, respectively, whereas replacing His-293 (H293K) with Lys reduced it to 44% of wild-type protein activity. Double mutation at the third and fourth residues, C292A/H293D, further lowered the ATPase activity to undetectable levels. The results, collectively, indicate that all four residues of motif II are involved in ATP hydrolysis and that Asp-290, Glu-291, and His-293 are particularly crucial to ATPase activity.
As hypothesized, the gatekeepers are His-293 of the DECH293 motif and Gln-460 of the Q460RRGRTGR motif, found in the HCV NS3 helicase (22), or the Asp residue of the DEAD motif and the His residue of motif VI (HRIGRGGR), found in eIF4A (gatekeepers are underlined) (23). Since the double mutation clone C292A/H293D contained an Asp at position 293, a His was substituted for the Q460 therein to restore the gatekeeper relationship, yielding the triple mutation clone C292A/H293D/Q460H. The ATPase activity of this triple mutation clone was not restored, however (Table 2), thus demonstrating that gatekeepers cannot be replaced with those from different subgroups.
Motif III contains a conserved T322ATPP sequence and is thought to act as a hinge, connecting domains I and II. Substituting Ala for Thr-322 (T322A) or the Thr-324 residue (T324A) reduced ATPase activity dramatically (30 and 16%, respectively, of activity shown by the wild-type protein). Surprisingly, mutation of the Arg-461 residue of motif VI to Gln (R461Q) also significantly influenced ATPase activity (7% of that shown by the wild-type protein). Singly mutating the putative RNA-interacting residues (Val-432 or Trp-501) to Ala (V432A or W501) only slightly affected ATPase activity, but simultaneously mutating both residues (V432A/W501A) significantly reduced activity (8% of that shown by the wild-type protein).
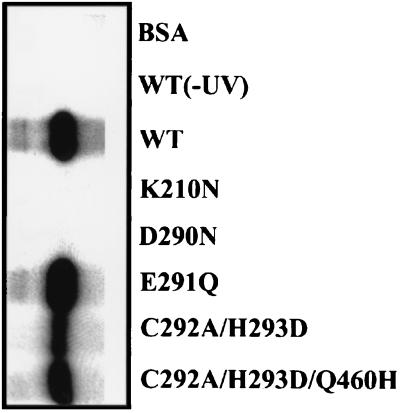
To further investigate why mutants K210N, D290N, E291Q, C292A/H293D, and C292A/H293D/Q460H displayed no ATPase activity, their ATP binding abilities were examined using a UV cross-linking method (29, 49). The purified proteins were incubated with poly(U) before adding the [α-32P]ATP and an excess amount, in relation to the enzyme, of cold ATP. The mixtures were subsequently cross-linked via UV light. Protein that displayed ATP binding activity would be covalently bound to ATP and became radiolabeled. The wild-type helicase protein was efficiently labeled, as illustrated in Fig. 2. No radioactivity was observed, however, if the incubation was not exposed to UV light or if the incubation used a control bovine serum albumin protein instead of the helicase protein, thus revealing a specific binding of ATP to the helicase protein. As for the mutant proteins, K210N and D290N completely lost their ATP binding ability, indicating their loss of ATPase activity, yet the other three mutant clones, C292A/H293D, C292A/H292D/Q460H, and E291Q, still retained various degrees of ATP binding ability. Other mechanisms must therefore exist, which act synergistically to account for the complete loss of ATPase activity.
FIG. 2.
ATP binding activity of the wild type and some mutant helicase proteins. One microgram of the wild-type or mutant helicase proteins was incubated with [α-32P]ATP in the presence of poly(U) and was UV cross-linked [WT(-UV)] as described in Materials and Methods. Samples were separated by SDS–12.5% PAGE. Following electrophoresis, the gels were stained with Coomassie brilliant blue, dried, and processed for autoradiography. WT, wild-type helicase protein; BSA, bovine serum albumin.
RNA binding activity of helicase mutants.
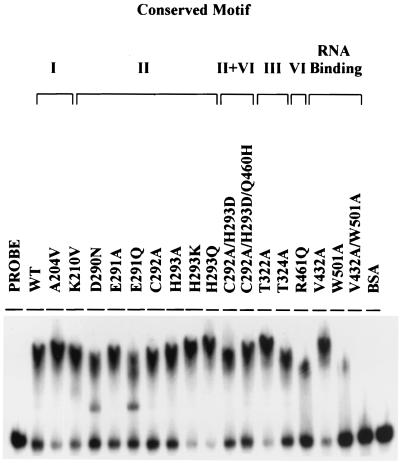
RNA binding activity was measured by gel mobility shift assay. As illustrated in Fig. 3, all mutant proteins but R461Q, W501A, and V432A/W501A bound RNA as efficiently as the wild-type protein did. Mutants A204V, K210N, H293K, H293Q, and T322A exhibited even slightly higher RNA binding activity than the wild-type protein. Notably, the V432A mutant exhibited virtually normal RNA binding activity. The W501A mutant retained only residual RNA binding activity, but the double mutation clone V432A/W501A almost completely lost RNA binding activity (Fig. 3). These results suggested that Trp-501 was crucial to RNA binding, whereas Val-432 might play an auxiliary role.
FIG. 3.
RNA binding activity of wild-type and mutant helicase proteins. The wild-type or mutant helicase proteins, cold release strand RNA, and labeled dsRNA substrate were incubated as described in Materials and Methods. The reaction mixtures were electrophoresed by native PAGE and were processed for autoradiography. WT denotes the wild-type helicase protein.
RNA helicase activity of helicase mutants.
The helicase activity of all mutant proteins was measured and expressed as kcat/Km, determined by varying the concentration of dsRNA substrate. Results given in Table 2 indicate that the helicase activity was completely abolished in two types of mutants: namely, those whose ATPase activity was completely lost (for example, K210N, D290N, E291Q, C292A/H293D, and C292A/H293D/Q460H) and those whose RNA binding activity was dramatically reduced (for example, R461Q, W501A, and V432A/W501A). The helicase activity of other mutants was only moderately affected, including mutants whose ATPase activity was severely reduced but not completely abolished (for example, H293A and T324A) (see below).
Relationship of ATPase activity and RNA helicase activity.
To correlate the ATPase activity and RNA helicase activity of these mutant proteins, both activities were translated into their ratios relative to wild-type protein activity (Table 2). The relationship between RNA helicase activity and ATPase activity is presented in Fig. 4. The unwinding of dsRNA is an ATP-dependent process, and ATP hydrolysis is a prerequisite for RNA helicase activity. Therefore, as anticipated, mutants with abolished ATPase activity (such as K210N, D290N, E291Q, C292A/H293D, and C292A/H293D/Q460H) would impede RNA helicase activity. Unexpectedly, some mutants (for example, H293A and T324A) which exhibited ATPase activity as low as 6 to 16% of that exhibited by the wild-type protein still displayed sufficient RNA helicase activity (75 and 62%, respectively) (Fig. 4). These analytical results suggest that a low ATPase activity threshold (e.g., 6%) is sufficient for the present RNA helicase assay. Accordingly, the loss of RNA helicase activity in mutants R461Q, W501A, and V432A/W501A is probably caused by reduced RNA binding ability or is a synergistic effect of both reduced ATPase and reduced RNA binding activities.
FIG. 4.
Relationship of ATPase activity and RNA helicase activity. Both ATPase activity and RNA helicase activity of all mutant proteins were translated into ratios relative to activity of the wild-type (WT) helicase protein (data shown in Table 2). The relationship was then plotted as the RNA helicase activity versus ATPase activity.
DISCUSSION
The structure of domains I and II of the HCV NS3 helicase displays a fold similar to that of domains 1A and 2A, respectively, of the PcrA DNA helicase, whose structure was recently solved by cocrystallizing the protein with a nonhydrolyzable ATP analog and a magnesium ion (43). In comparing the PcrA DNA helicase structure with those reported structures for the HCV NS3 helicase (7, 22, 48), some different aspects among these models are noted. The results of this study may thus provide clues to elucidating the relationship between the function and the three-dimensional structure of each critical amino acid residue.
Residues that influence ATP hydrolysis.
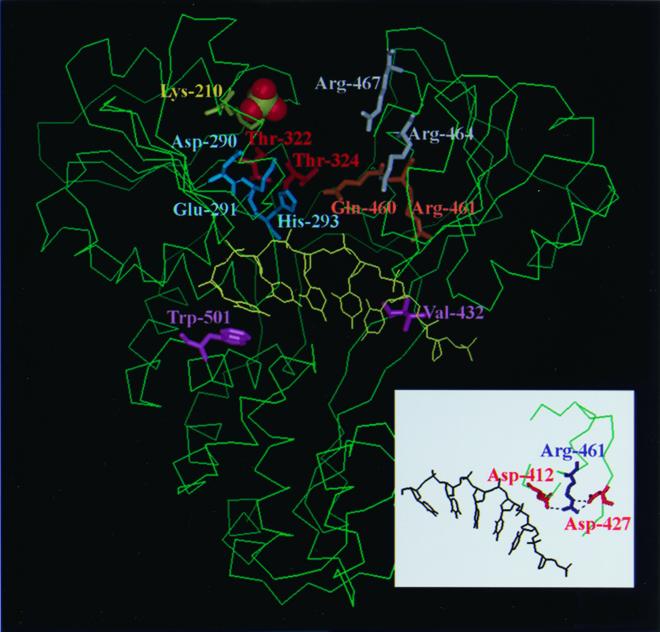
The amino acids in the conserved Walker A motif (Lys-210) and Walker B motif (Asp-290, Glu-291, and His-293) are crucial to ATP hydrolysis, as predicted. This investigation additionally identified other residues not contained in Walker A and B motifs but also vital to ATPase activity, for example, Gln-460, Thr-322, Thr-324, and Arg-461. To function in ATP hydrolysis, all of these residues except Arg-461 are conformationally proximal to NTP (Fig. 5).
FIG. 5.
Locations of the important amino acid residues in the three-dimensional conformation. The structure of HCV NS3 helicase was based on that determined by Kim et al. (22) using Protein Data Bank (PDB) accession code 1A1V. The important residues identified herein and the Arg-464/Arg-467 previously demonstrated (21, 22, 43) are emphasized by being represented in stick format, while the sulfate ion is represented in Corey-Pauling-Koltun (CPK) format. Residues His-293 and Gln-460 are located in domains I and II, respectively. It is hypothesized that the amine group of the histidine ring of His-293 formed a hydrogen bond with the carbonyl oxygen of the side chain of Gln-460. Residue Arg-461 is located in the interior of domain II. The inset indicates that the Arg-461 can form hydrogen bonds with Asp-412 and Asp-427, which are located at conserved motif V. These figures were drawn using InsightII.
Previous studies have demonstrated that the conserved lysine residue in Walker A (GxGKS) is involved in binding to the β phosphate of NTP (39, 43), while the conserved Asp-290 residue in Walker B (DECH), structurally proximal to the Walker A motif, can bind Mg2+ and assists in orienting the Mg2+-ATP substrate for ATP hydrolysis (1, 32, 47). Mutations of these two residues consequently completely impeded ATP binding and ATPase activities (Fig. 2 and Table 2).
The conserved Glu-291 residue of motif II (DExH) generally serves as a Lewis base, activating the attacking water molecule during the hydrolysis of ATP, as previously demonstrated in PcrA DNA helicase (43). The negatively charged carboxylate side chain of Glu-291 might accelerate the release of the hydrolysis product, Pi. Interestingly, replacing Glu-291 (E291Q) with Gln did not alter ATP binding activity but completely inhibited ATPase activity (Fig. 2 and Table 2). We reasoned that substituting Gln for Glu might cause loss of the Lewis base function or/and strengthen binding rather than releasing the hydrolysis product, Pi. Substitution of Ala for the Glu-291 (E291A), however, did not cause a significant reduction in ATPase activity. We suspect that water molecules will occupy the side chain position of the Glu-291 in mutant E291A and still serve as a Lewis base.
The side chains of Glu-291, His-293, and Thr-322 form a network of hydrogen bonds in the absence of substrate (48). Thus, when either residue was mutated (E291K, H293A, H293K, H293Q, C292A/H293D, and T322A) or the interactions between motifs I, II, and III were disrupted, the ATP binding and hydrolysis would then be affected. Moreover, the crystal structure (22) revealed a close proximity (∼4 Å) of His-293 in motif II and Gln-460 in motif VI (Fig. 5). This pair of residues was predicted to modulate the opening/closure switch of domains I and II upon ATP binding and served as gatekeepers (22). Another gatekeeper pair is the DEAD/HRxGR combination found in the DEAD helicase subgroup (for example, eIF4A) (14). We hypothesized that the amine group of the histidine ring of His-293 might form a hydrogen bond with the carbonyl oxygen of the side chain of Gln-460. Because the closed form of PcrA is stabilized by direct contact of the γ phosphate of ATP with Gln-254 and two Arg residues, Arg-287 and Arg-610 (43), which correspond to Arg-464 and Arg-467, respectively, in motif VI of the HCV NS3 helicase (Fig. 5), the interaction of His-293 and Gln-460 found in the latter might be predicted to facilitate the formation of hydrogen bonds between Arg-464/Arg-467 and ATP, thus producing a more stable closure conformation. However, even if Gln-460 of the double mutation clone C292A/H293D was further mutated to His (the triple mutation clone C292A/H293D/Q460H), such that the hydrogen bond might be restored between Asp-293 and His-460, ATPase activity was not restored (Table 2). Such a phenomenon was also observed in eIF4A and Prp2 helicase mutants, the gatekeepers of which were found to be not exchangeable between two different SFII subgroups (10, 34). All these results thus indicate that the network or microenvironment surrounding the key residues will contribute to the overall conformation, which is probably more important to the enzymatic activities.
The importance of Thr-322 and Thr-324 of motif III, which connects domains I and II, in ATPase activity most likely resulted from their roles in modulating the opening and closure of the ATP binding cleft between these two domains (7), whereas the role of Arg-461 is obscure. Because Arg-461 is located in the interior of domain II (Fig. 5) and forms hydrogen bonds with Asp-412 and Asp-427 (Fig. 5, inset) (22), it is unlikely that Arg-461 directly contacts ATP, but it is likelier that it holds a proper conformation for domain II. Thus, Arg-461 probably influences ATPase activity in an indirect manner. Finally, it was also observed herein that a single mutation at the RNA-interacting residues, Val-432 or Trp-501, did not markedly affect ATP hydrolysis activity, whereas simultaneous mutation at both residues severely reduced ATP hydrolysis activity (Table 2). Unlike mutant V432A or W501A, the V432A/W501A mutant almost totally lost RNA binding activity (Fig. 3). The experimental results thus suggest that the ATPase activity of HCV NS3 helicase is strongly stimulated by the presence of RNA, which agrees with previous analysis of the ATPase activity of the wild-type helicase protein in the presence or absence of polynucleotide (12, 16, 40, 41, 45).
Residues that influence RNA binding.
The crystal structures determined by Cho et al. and Yao et al. located the single-stranded RNA (ssRNA) in a channel formed by the interdomain cleft between domains I and II (7, 48), whereas that determined by Kim et al. located the oligonucleotide in a groove between the first two domains and the third (22). The latter thus resembles that of PcrA DNA helicase (43). The mutagenesis data described herein demonstrated that Trp-501 was crucial for RNA binding, thus supporting the model proposed by Kim et al. (22). But our data demonstrated that Val-432, one of the “bookends” for the ssRNA binding proposed by the model, might play only a minor role. Notably, however, simultaneous mutation at Val-432 and Trp-501 synergistically degraded RNA binding activity. The role of Val-432 determined in this study is also different from that reported by other groups using full-length NS3 (33, 35). The discrepancy is not clear but may be due to different proteins (helicase domain versus full-length NS3) and assay systems used in different laboratories.
Mutation of Arg-461 also reduced RNA binding activity, though not dramatically. This finding contradicts the results reported by Kim et al. (21) but is consistent with more recent results published by Kwong et al. (26). In support of our findings, the mutation at the same position of vaccinia virus NPH-II also decreased RNA binding (15). As discussed above, Arg-461 is located in the interior of domain II and forms a hydrogen bond with Asp-412 and Asp-427. Lin and Kim recently reported that Thr-411, right next to Asp-412, was one of the important residues to bind ssRNA (30). Thus, the decrease of RNA binding in the R461Q mutant may be indirectly caused by the interruption of the interaction between Arg-461 and Asp-412, which in turn leads to a conformational change in the polynucleotide binding channel and a loss of interaction between Thr-411 and ssRNA.
Factors that affect RNA helicase activity and the implications.
Unwinding of RNA is an ATP-dependent process which requires ATP binding, ATP hydrolysis, and RNA binding. It is reasonable to hypothesize that the mutants whose ATPase activity is abolished will also impede RNA helicase activity. Mutants K210N, D290N, E291Q, C292A/H293D, and C292A/H293D/Q460H indeed followed this rule (Table 2). Surprisingly, however, some mutants with fairly low ATPase activity (6 to 16% of the wild-type helicase) still exhibited sufficiently high RNA helicase activity (for example, H293A and T324A). Similar phenomena were observed in previous mutational studies (21, 30). These results thus suggest that the ATPase activity required for RNA unwinding may be minimal. On the contrary, reduction of RNA binding activity markedly influenced RNA helicase activity, e.g., R461Q and W501A (Table 2). Based on these findings, we suggest that interfering with RNA binding activity rather than interfering with ATPase activity should be considered more in designing antiviral therapeutics for hepatitis C.
ACKNOWLEDGMENTS
C.-L. Tai and W.-C. Pan contributed equally to this work.
We are indebted to Pei-Jer Chen and Ted Knoy for critical reading of the manuscript.
This work was supported in part by grants NSC 89-2315-B-002-014-MH and NSC 89-2321-B002-002 from National Science Council of the Republic of China and in part by financial support from the Liver Disease Prevention and Treatment Research Foundation.
REFERENCES
- 1.Black M E, Hruby D E. Site-directed mutagenesis of a conserved domain in vaccinia virus thymidine kinase. Evidence for a potential role in magnesium binding. J Biol Chem. 1992;267:6801–6806. [PubMed] [Google Scholar]
- 2.Brillanti S, Garson J, Foli M, Whitby K, Deaville R, Masci C, Miglioli M, Barbara L. A pilot study of combination therapy with ribavirin plus interferon alfa for interferon alfa-resistant chronic hepatitis C. Gastroenterology. 1994;107:812–817. doi: 10.1016/0016-5085(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 3.Brillanti S, Miglioli M, Barbara L. Combination antiviral therapy with ribavirin and interferon alfa in interferon alfa relapsers and non-responders: Italian experience. J Hepatol. 1995;23:13–15. [PubMed] [Google Scholar]
- 4.Chamberlain R W, Adams N, Saeed A A, Simmonds P, Elliott R M. Complete nucleotide sequence of a type 4 hepatitis C virus variant, the predominant genotype in the Middle East. J Gen Virol. 1997;78:1341–1347. doi: 10.1099/0022-1317-78-6-1341. [DOI] [PubMed] [Google Scholar]
- 5.Chemello L, Cavalletto L, Bernardinello E, Guido M, Pontisso P, Alberti A. The effect of interferon alfa and ribavirin combination therapy in naive patients with chronic hepatitis C. J Hepatol. 1995;23:8–12. [PubMed] [Google Scholar]
- 6.Chen D S, Kuo G C, Sung J L, Lai M Y, Sheu J C, Chen P J, Yang P M, Hsu H M, Chang M H, Chen C J, et al. Hepatitis C virus infection in an area hyperendemic for hepatitis B and chronic liver disease: the Taiwan experience. J Infect Dis. 1990;162:817–822. doi: 10.1093/infdis/162.4.817. [DOI] [PubMed] [Google Scholar]
- 7.Cho H S, Ha N C, Kang L W, Chung K M, Back S H, Jang S K, Oh B H. Crystal structure of RNA helicase from genotype 1b hepatitis C virus. A feasible mechanism of unwinding duplex RNA. J Biol Chem. 1998;273:15045–15052. doi: 10.1074/jbc.273.24.15045. [DOI] [PubMed] [Google Scholar]
- 8.Choo Q L, Kuo G, Weiner A J, Overby L R, Bradley D W, Houghton M. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 9.Di Bisceglie A M. Hepatitis C and hepatocellular carcinoma. Hepatology. 1997;26:34S–38S. doi: 10.1002/hep.510260706. [DOI] [PubMed] [Google Scholar]
- 10.Edwalds-Gilbert G, Kim D H, Kim S H, Tseng Y H, Yu Y, Lin R J. Dominant negative mutants of the yeast splicing factor Prp2 map to a putative cleft region in the helicase domain of DExD/H-box proteins. RNA. 2000;6:1106–1119. doi: 10.1017/s1355838200992483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuller-Pace F V. RNA helicase: modulators of RNA structure. Trends Cell Biol. 1994;4:271–274. doi: 10.1016/0962-8924(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 12.Gallinari P, Brennan D, Nardi C, Brunetti M, Tomei L, Steinkuhler C, De Francesco R. Multiple enzymatic activities associated with recombinant NS3 protein of hepatitis C virus. J Virol. 1998;72:6758–6769. doi: 10.1128/jvi.72.8.6758-6769.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gorbalenya A E, Koonin E V, Donchenko A P, Blinov V M. A novel superfamily of nucleoside triphosphate-binding motif containing proteins which are probably involved in duplex unwinding in DNA and RNA replication and recombination. FEBS Lett. 1988;235:16–24. doi: 10.1016/0014-5793(88)81226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gorbalenya A E, Koonin E V, Donchenko A P, Blinov V M. Two related superfamilies of putative helicases involved in replication, recombination, repair and expression of DNA and RNA genomes. Nucleic Acids Res. 1989;17:4713–4730. doi: 10.1093/nar/17.12.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gross C H, Shuman S. The QRxGRxGRxxxG motif of the vaccinia virus DExH box RNA helicase NPH-II is required for ATP hydrolysis and RNA unwinding but not for RNA binding. J Virol. 1996;70:1706–1713. doi: 10.1128/jvi.70.3.1706-1713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hesson T, Mannarino A, Cable M. Probing the relationship between RNA-stimulated ATPase and helicase activities of HCV NS3 using 2′-O-methyl RNA substrates. Biochemistry. 2000;39:2619–2625. doi: 10.1021/bi992127a. [DOI] [PubMed] [Google Scholar]
- 17.Hoofnagle J H. Hepatitis C: the clinical spectrum of disease. Hepatology. 1997;26:15S–20S. doi: 10.1002/hep.510260703. [DOI] [PubMed] [Google Scholar]
- 18.Jin L, Peterson D L. Expression, isolation, and characterization of the hepatitis C virus ATPase/RNA helicase. Arch Biochem Biophys. 1995;323:47–53. doi: 10.1006/abbi.1995.0008. [DOI] [PubMed] [Google Scholar]
- 19.Kadare G, Haenni A L. Virus-encoded RNA helicases. J Virol. 1997;71:2583–2590. doi: 10.1128/jvi.71.4.2583-2590.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim D W, Gwack Y, Han J H, Choe J. C-terminal domain of the hepatitis C virus NS3 protein contains an RNA helicase activity. Biochem Biophys Res Commun. 1995;215:160–166. doi: 10.1006/bbrc.1995.2447. [DOI] [PubMed] [Google Scholar]
- 21.Kim D W, Kim J, Gwack Y, Han J H, Choe J. Mutational analysis of the hepatitis C virus RNA helicase. J Virol. 1997;71:9400–9409. doi: 10.1128/jvi.71.12.9400-9409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim J L, Morgenstern K A, Griffith J P, Dwyer M D, Thomson J A, Murcko M A, Lin C, Caron P R. Hepatitis C virus NS3 RNA helicase domain with a bound oligonucleotide: the crystal structure provides insights into the mode of unwinding. Structure. 1998;6:89–100. doi: 10.1016/s0969-2126(98)00010-0. [DOI] [PubMed] [Google Scholar]
- 23.Koonin E V, Dolja V V. Evolution and taxonomy of positive-strand RNA viruses: implications of comparative analysis of amino acid sequences. Crit Rev Biochem Mol Biol. 1993;28:375–430. doi: 10.3109/10409239309078440. [DOI] [PubMed] [Google Scholar]
- 24.Korolev S, Yao N, Lohman T M, Weber P C, Waksman G. Comparisons between the structures of HCV and Rep helicases reveal structural similarities between SF1 and SF2 super-families of helicases. Protein Sci. 1998;7:605–610. doi: 10.1002/pro.5560070309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuo G, Choo Q L, Alter H J, Gitnick G L, Redeker A G, Purcell R H, Miyamura T, Dienstag J L, Alter M J, Stevens C E, et al. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science. 1989;244:362–364. doi: 10.1126/science.2496467. [DOI] [PubMed] [Google Scholar]
- 26.Kwong A D, Kim J L, Lin C. Structure and function of hepatitis C virus NS3 helicase. Curr Top Microbiol Immunol. 2000;242:171–196. doi: 10.1007/978-3-642-59605-6_9. [DOI] [PubMed] [Google Scholar]
- 27.Kwong A D, Kim J L, Rao G, Lipovsek D, Raybuck S A. Hepatitis C virus NS3/4A protease. Antivir Res. 1999;41:67–84. [PubMed] [Google Scholar]
- 28.Lai M Y, Kao J H, Yang P M, Wang J T, Chen P J, Chan K W, Chu J S, Chen D S. Long-term efficacy of ribavirin plus interferon alfa in the treatment of chronic hepatitis C. Gastroenterology. 1996;111:1307–1312. doi: 10.1053/gast.1996.v111.pm8898645. [DOI] [PubMed] [Google Scholar]
- 29.Laín S, Martín M T, Riechmann J L, García J A. Novel catalytic activity associated with positive-strand RNA virus infection: nucleic acid-stimulated ATPase activity of the plum pox potyvirus helicaselike protein. J Virol. 1991;65:1–6. doi: 10.1128/jvi.65.1.1-6.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin C, Kim J L. Structure-based mutagenesis study of hepatitis C virus NS3 helicase. J Virol. 1999;73:8798–8807. doi: 10.1128/jvi.73.10.8798-8807.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller R H, Purcell R H. Hepatitis C virus shares amino acid sequence similarity with pestiviruses and flaviviruses as well as members of two plant virus supergroups. Proc Natl Acad Sci USA. 1990;87:2057–2061. doi: 10.1073/pnas.87.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pai E F, Krengel U, Petsko G A, Goody R S, Kabsch W, Wittinghofer A. Refined crystal structure of the triphosphate conformation of H-ras p21 at 1.35 Å resolution: implications for the mechanism of GTP hydrolysis. EMBO J. 1990;9:2351–2359. doi: 10.1002/j.1460-2075.1990.tb07409.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paolini C, Lahm A, De Francesco R, Gallinari P. Mutational analysis of hepatitis C virus NS3-associated helicase. J Gen Virol. 2000;81:1649–1658. doi: 10.1099/0022-1317-81-7-1649. [DOI] [PubMed] [Google Scholar]
- 34.Pause A, Sonenberg N. Mutational analysis of a DEAD box RNA helicase: the mammalian translation initiation factor eIF-4A. EMBO J. 1992;11:2643–2654. doi: 10.1002/j.1460-2075.1992.tb05330.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Preugschat F, Danger D P, Carter III L H, Davis R G, Porter D J. Kinetic analysis of the effects of mutagenesis of W501 and V432 of the hepatitis C virus NS3 helicase domain on ATPase and strand-separating activity. Biochemistry. 2000;39:5174–5183. doi: 10.1021/bi9923860. [DOI] [PubMed] [Google Scholar]
- 36.Saito I, Miyamura T, Ohbayashi A, Harada H, Katayama T, Kikuchi S, Watanabe Y, Koi S, Onji M, Ohta Y, Choo Q L, Houghton M, Kuo G. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:6547–6549. doi: 10.1073/pnas.87.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmid S R, Linder P. D-E-A-D protein family of putative RNA helicases. Mol Microbiol. 1992;6:283–291. doi: 10.1111/j.1365-2958.1992.tb01470.x. [DOI] [PubMed] [Google Scholar]
- 38.Seeff L B. Natural history of hepatitis C. Hepatology. 1997;26:21S–28S. doi: 10.1002/hep.510260704. [DOI] [PubMed] [Google Scholar]
- 39.Subramanya H S, Bird L E, Brannigan J A, Wigley D B. Crystal structure of a DExx box DNA helicase. Nature. 1996;384:379–383. doi: 10.1038/384379a0. [DOI] [PubMed] [Google Scholar]
- 40.Suzich J A, Tamura J K, Palmer-Hill F, Warrener P, Grakoui A, Rice C M, Feinstone S M, Collett M S. Hepatitis C virus NS3 protein polynucleotide-stimulated nucleoside triphosphatase and comparison with the related pestivirus and flavivirus enzymes. J Virol. 1993;67:6152–6158. doi: 10.1128/jvi.67.10.6152-6158.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tai C-L, Chi W-K, Chen D-S, Hwang L-H. The helicase activity associated with hepatitis C virus nonstructural protein 3 (NS3) J Virol. 1996;70:8477–8484. doi: 10.1128/jvi.70.12.8477-8484.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tong M J, el-Farra N S, Reikes A R, Co R L. Clinical outcomes after transfusion-associated hepatitis C. N Engl J Med. 1995;332:1463–1466. doi: 10.1056/NEJM199506013322202. [DOI] [PubMed] [Google Scholar]
- 43.Velankar S S, Soultanas P, Dillingham M S, Subramanya H S, Wigley D B. Crystal structures of complexes of PcrA DNA helicase with a DNA substrate indicate an inchworm mechanism. Cell. 1999;97:75–84. doi: 10.1016/s0092-8674(00)80716-3. [DOI] [PubMed] [Google Scholar]
- 44.Walker J E, Saraste M, Runswick M J, Gay N J. Distantly related sequences in the alpha- and beta-subunits of ATP synthase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wardell A D, Errington W, Ciaramella G, Merson J, McGarvey M J. Characterization and mutational analysis of the helicase and NTPase activities of hepatitis C virus full-length NS3 protein. J Gen Virol. 1999;80:701–709. doi: 10.1099/0022-1317-80-3-701. [DOI] [PubMed] [Google Scholar]
- 46.Wittinghofer A, Pai E F. The structure of Ras protein: a model for a universal molecular switch. Trends Biochem Sci. 1991;16:382–387. doi: 10.1016/0968-0004(91)90156-p. [DOI] [PubMed] [Google Scholar]
- 47.Yan H G, Tsai M D. Mechanism of adenylate kinase. Demonstration of a functional relationship between aspartate 93 and Mg2+ by site-directed mutagenesis and proton, phosphorus-31, and magnesium-25 NMR. Biochemistry. 1991;30:5539–5546. doi: 10.1021/bi00236a029. [DOI] [PubMed] [Google Scholar]
- 48.Yao N, Hesson T, Cable M, Hong Z, Kwong A D, Le H V, Weber P C. Structure of the hepatitis C virus RNA helicase domain. Nat Struct Biol. 1997;4:463–467. doi: 10.1038/nsb0697-463. [DOI] [PubMed] [Google Scholar]
- 49.Yue V T, Schimmel P R. Direct and specific photochemical cross-linking of adenosine 5′-triphosphate to an aminoacyl-tRNA synthetase. Biochemistry. 1977;16:4678–4684. doi: 10.1021/bi00640a023. [DOI] [PubMed] [Google Scholar]