Abstract
Human immunodeficiency virus (HIV)-specific cytotoxic T lymphocytes (CTL) mediate immunologic selection pressure by both cytolytic and noncytolytic mechanisms. Non cytolytic mechanisms include the release of β-chemokines blocking entry of R5 HIV-1 strains. In addition, CD8+ cells inhibit X4 virus isolates via release of as yet poorly characterized soluble factors. To further characterize these factors, we performed detailed analysis of CTL as well as bulk CD8+ T lymphocytes from six HIV-1-infected individuals and from six HIV-1-seronegative individuals. Kinetic studies revealed that secreted suppressive activities of HIV-1-specific CTL and bulk CD8+ T lymphocytes from all HIV-1-infected persons are significantly higher than that of supernatants from seronegative controls. The suppressive activity could be blocked by monensin and brefeldin A, was heat labile, and appeared in a pattern different from that of secretion of chemokines (MDC, I-309, MIP-1α, MIP-1β, and RANTES), cytokines (gamma interferon, tumor necrosis factor alpha, and granulocyte-macrophage colony-stimulating factor), and interleukins (interleukin-13 and interleukin-16). This suppression activity was characterized by molecular size exclusion centrifugation and involves a suppressive activity of >50 kDa which could be bound to heparin and a nonbinding inhibitory activity of <50 kDa. Our data provide a functional link between CD8+ cells and CTL in the noncytolytic inhibition of HIV-1 and suggest that suppression of X4 virus is mediated through proteins. The sizes of the proteins, their affinity for heparin, and the pattern of release indicate that these molecules are not chemokines.
Cytotoxic T lymphocytes (CTL) inhibit human immunodeficiency virus type 1 (HIV-1) replication by both cytolytic and noncytolytic mechanisms (35, 37, 38). Soluble inhibitory factors produced by CD8+ cells have been shown to inhibit HIV-1 replication and may play a critical role in vivo as an antiviral host defense (13, 32). These inhibitory factors include β-chemokines (3, 9, 14, 29), a subclass of cytokines with chemotactic properties that act to block viral entry through the coreceptor CCR5, which is utilized by R5 strains of virus (13, 22, 31, 32), and less well characterized X4-suppressive soluble factor(s) produced by CD8+ cells (19, 23–25, 33, 35, 37, 38).
Whereas the role of β-chemokines in inhibiting R5 strains of HIV-1 has been well established, soluble factors produced by CD8+ cells that inhibit X4 strains of virus are less well defined. Stromal-derived factor 1 (SDF-1), a ligand for the coreceptor CXCR4, at high concentrations (1,000 ng/ml) can achieve significant inhibition (3, 28, 36) which can be increased by N-terminal modification (36). However, the production of SDF-1 by CD8+ cells has not been demonstrated (17), and other ligands for CXCR4 have not been found. The chemokine I-309 is another ligand able to block T-cell-tropic HIV-1 strains that can utilize CCR8 as a coreceptor but has no influence in cell systems where CXCR4 is used as the coreceptor (14). Another chemokine, MDC, blocked macrophage- and T-cell-tropic viruses in some but not all studies through an unknown mechanism (29) but also has not been shown to be produced by HIV-1 antigen-specific CTL. Additionally, interleukin-16 (IL-16) has been suggested to have antiviral activity (1).
Another incompletely defined substance or group of substances produced by CD8+ cells has been termed CD8+ cell antiviral factors (CAF) (35). CAF inhibits replication of X4 strains of HIV-1 at the level of viral transcription by suppressing long terminal repeat-driven expression of viral proteins (6, 24, 33). However, its identity as well as the phenotype of cells that produce it and the physiologic stimulus for its release still elude characterization. The fact that it has been preferentially observed in persons who are HIV-1 infected suggests that it may be an antigen-specific response, and at least some reports indicate that a factor with similar properties can be produced by HIV-1-specific CTL in an antigen-specific manner (38). However, no studies have reported a detailed comparison of CD8+ cells from both seropositive and seronegative persons, as well as HIV-1-specific CTL.
In this study, we have performed a detailed characterization of the cytokines and chemokines produced when HIV-specific CTL are triggered. We report the release of MDC, I-309, and IL-16 by HIV-1-specific CTL as well as CD8+ cells from HIV-1-seropositive persons. In addition, we show that these factors along with the β-chemokines do not account for all of the noncytolytic inhibition mediated by HIV-1-specific CTL.
MATERIALS AND METHODS
Subjects.
Peripheral blood mononuclear cells (PBMC) were obtained from six HIV-1-infected subjects. All had plasma HIV-1 loads of <400 RNA copies per ml and CD4+ cell counts of >500 per μl in the absence of therapy. Control blood samples were obtained from six HIV-1-seronegative, healthy donors. HIV-specific CTL clones were obtained from persons with established HIV-1 infections (4).
Bulk CD8+ cells and HIV-1-specific CTL clones.
Polyclonal CD8+ cells that were 90 to 99% CD3+ and CD8+ positive were generated by fluorescence-activated cell sorting (FACS) from the six seronegative and the six HIV-1-seropositive persons by positive selection with anti-CD8 antibody-coated immunomagnetic beads (PerSeptive Biosystems, Framingham, Mass.) as described elsewhere (7). HIV-1-specific CTL clones were obtained by cloning of stimulated PBMC at limiting dilution and characterized for epitope specificity and HLA restriction as previously described (15, 16, 34). The HIV-1-specific CTL clones included 115N2 (designated p24/HLA-Cw8), specific for a Cw8-restricted HIV-1 p24 epitope (amino acids [aa] 305 to 313; RAEQASQEV), 15160-XH66 (designated Gag/HLA-B14), specific for a B14-restricted Gag epitope (aa 298 to 306; DRFYKTLRA), 15160-CX74 (designated Nef/HLA-Cw8), specific for a Cw8-restricted Nef epitope (aa 82 to 91; KAAVDLSHFL), 15160-D75 (designated gp41/HLA-B14), specific for a B14-restricted gp41 epitope (aa 589 to 597 ERYLKDQQL), and 53B14 (designated Env/HLA-B7), specific for a B7-restricted Env epitope (aa 848 to 856; IPRRIRGL). Bulk CD8+ cell lines from seropositive and seronegative persons were established by incubating purified CD8+ cells (2 × 106) with 2 × 107 irradiated allogeneic feeder cells (PBMC) and 0.25 μg of phytohemagglutinin (PHA; Murex, Dartford, United Kingdom) per ml for 3 days. Cells were maintained in RPMI 1640 (Sigma, St. Louis, Mo.) supplemented with 10% heat-inactivated fetal calf serum (Sigma), 10 mM HEPES, 2 mM glutamine, 100 U penicillin per ml, 10 μg of streptomycin per ml, and 50 U of IL-2 per ml (R10-50). After 2 weeks, 0.5 × 106 cells/ml were stimulated by using CD3- cross-linking in a 1:4 ratio of cells to goat anti-mouse antibody-coated beads (PerSeptive Biosystems) saturated with a mouse anti-human 12F6 CD3 antibody (38) (2 μg of antibody/106 cells). The supernatant fluid was harvested at the designated time points by centrifugation at 3,000 × g for 10 min.
Assay for inhibition of HIV-IIIIB replication.
H9 cells (HLA A1, B6, Bw62, Cw3) were acutely infected with HIV-1IIIB at a multiplicity of infection of 10−2 50% tissue culture infective dose per ml and resuspended in R20. The cells were then plated in 2 ml R20 at 5 × 105 cells/ml in a 24-well plate. CD8+ cell supernatants were tested at a final dilution of 1:2. H9 cell supernatant (1 ml) was removed every 3 days and replaced with medium supplemented with CD8+ cell supernatant or cytokines. After 9 days, the concentration of p24 was measured with an HIV-1 p24 enzyme-linked immunosorbent assay (ELISA) kit (NEM Life Science, Boston, Mass.), and the percentage inhibition was calculated against the medium control.
Flow cytometric analysis.
FACS analysis was performed after 2 weeks of propagation for CD8+ cells, at the time cells were used in the CD3 cross-linking assays. Cells were stained using directly conjugated dye-labelled antibodies as follows: CD28/CD3/CD8/CD38, CD45-RA/CD8/HLA-DR/CD45-RO, CD62-L/CD3/CD8, CD25/CD3/CD8, and CD44/CD3/CD8, using antibodies purchased from PharMingen (San Diego, Calif.). Isotype-matched controls (immunoglobulin G1 [IgG1]-fluorescein isothiocyanate [FITC], IgG1-phycoerythrin, IgG1-peridinin chlorophyll protein, and IgG1-allophycocyanin) were also obtained from PharMingen. CXCR4 expression on H9 cells was determined using CXCR4-FITC-conjugated antibodies and an IgG2a-FITC control from PharMingen.
Characterization of soluble factors.
Molecular weights for the suppressive activities of harvested cell supernatants were determined after heparin binding (5-ml HiTrap heparin-Sepharose column, Amersham Pharmacia, Piscataway, N.J.) by Centricon centrifugation. The heparin-nonbound fraction was obtained by washing the column with phosphate-buffered saline (PBS), after which the heparin-bound fraction was eluted with 2 M NaCl in PBS. Heparin-bound or unbound fractions were then filtered through 20-ml Centricon membranes, (Millipore, Bedford, Mass.) with molecular exclusion sizes of 100, 50, 8, and 3 kDa sequentially, concentrated to 100 μl, and washed twice with a 200-fold volume of PBS. An equimolar to 350 mM NaCl suppressive heparin-bound fraction was obtained by loading 20 to 80 ml of supernatant onto the heparin column, washing with 20 ml of PBS, and running a 20-min PBS–1 M NaCl-PBS gradient at a flow rate of 500 μl/min. A 40-kDa heparin-bound Superdex suppressive fraction was obtained by concentrating the 350 mM heparin-bound fraction on a 50-kDa cutoff Centricon membrane and fractionating 200 μg of this concentrate on a Superdex-200 column (3.2 by 300 mm; Pharmacia) at a flow rate of 40 μl/min in PBS. Molecular mass of the heparin-bound Superdex fraction with the highest HIV-1 suppression was calibrated using the following calibration proteins (Sigma): thymus globulin (669 kDa), ferritin (440 kDa), catalase (232 kDa), aldolase (158 kDa), albumin (67 kDa), ovalbumin (45 kDa), and chymotrypsin (25 kDa). ELISAs were performed according to the manufacturer's instructions, using paired antibodies from R&D Systems, Inc. (Minneapolis, Minn.) and horseradish peroxidase-avidin D (Vector, Burlingame, Calif.). For the MDC ELISA, a monoclonal capture antibody (R&D) was used, whereas for the second antibody a polyclonal donkey anti-MDC antibody (ICOS, Bothell, Wash.) was used. The peroxidase enzyme function was coupled here with a chicken anti-donkey antibody (ICOS). The detection limit for all ELISAs was below 15 pg/ml except for SDF-1, where the detection limit was 2 ng/ml. Neutralizing antibodies for MDC, IL-16, I-309, and polyclonal control antibody (PeproTech, Rocky Hill, N.J.) were used alone or combined with a 4°C overnight preincubation of supernatant before use in the inhibition test. The neutralizing antibodies were used at a concentration of 5 μg/ml, where according the manufacturer they were able to neutralize approximately 50% of chemokine (100 ng/ml). Protein concentration was determined by the bicinchoninic acid method (Pierce, Rockford, Ill.). Proteinase treatment using proteinase K immobilized on beads (Sigma) was performed with 1 U/ml for supernatants at 37°C for 12 h, after which the solution was filtered. Heat treatment was performed at 60°C for 30 min. To block protein secretion, cells were treated with 2 μM monensin and 3.5 μM brefeldin A (both from Sigma) for 4 h during anti-CD3 cross-linking activation. To block protein synthesis, cycloheximide (Sigma) at 8.8 μM was used for 2 h at 37°C. Cells were then washed twice and stimulated with anti-CD3 for 4 h at 37°C. Supernatants of monensin-, brefeldin A-, and cycloheximide-treated cells were then used in inhibition tests. Cytokines used were from PeproTech Inc.; MDC(−2) is a modified form of MDC missing 2 aa at its N terminus. Protein purity and molecular weight were estimated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining (11) with a 15% slab gel, using a low-range protein molecular weight marker (Bio-Rad, Hercules, Calif.). The samples were treated with 2.5% 2-mercaptoethanol. For Western blot analysis, protein samples were treated with 2.5% 2-mercaptoethanol and separated by SDS-PAGE with a 15% slab gel. Immediately following separation by SDS-PAGE, the gel was treated with transfer buffer and blotted onto nitrocellulose paper (11). After blocking with 5% bovine serum albumin in PBS and 0.05% Tween 20 (Fisher Scientific, Springfield, N.Y.), the nitrocellulose paper was incubated with a 1/1,000 dilution of anti-MDC, anti-IL-16, and anti-I-309 rabbit antibodies (PeproTech) alone or combined for 16 h at 4°C in PBS, 0.05% Tween 20, and 0.1% bovine serum albumin. The nitrocellulose paper was washed and treated with 1/10,000-diluted anti-rabbit antibodies labeled with horseradish peroxidase (Vector). The membrane was washed in PBS, and Western blot chemiluminescence reagent (NEM) was added. The emitted light from the oxidative degradation of luminol was captured on Kodak X-Omat autoradiography film (Kodak, Rochester, N.Y.). For the Western blots we used a prestained broad-range protein molecular weight marker (Bio-Rad).
Ca2+ flux in leukocytes.
Ca2+ flux experiments were performed as described previously (12) 1 week after stimulation with 0.25 μg of PHA per ml, using positively selected CD4+ cells obtained using anti-CD4 antibody coated on immunomagnetic beads (PerSeptive Biosystems) or log-phase-growing H9 cells. Purified cells were loaded with 5.0 μM fura-2 acetoxymethyl ester (Molecular Probes, Eugene, Oreg.) for 60 min at 37°C in the dark at 107 cells/ml in Dulbecco modified Eagle medium supplemented with 1% heat-inactivated fetal bovine serum. Loaded cells were washed twice and resuspended in a buffer containing 145 mM NaCl, 4 mM KCl, 1 mM NaHPO4, 0.8 mM MgCl2, 1.8 mM CaCl2, 25 mM HEPES, and 22 mM glucose. Two milliliters of cells (106 cells/ml) was placed in a continuously stirred cuvette at 37°C in a dual-wavelength excitation source fluorimeter (Photon Technology Inc., South Brunswick, N.J.). Changes in cytosolic free calcium were determined after addition of the equimolar to 350 mM NaCl heparin-bound fraction, the 40-kDa heparin-bound Superdex fraction, and SDF-1α (Peprotech) by monitoring the excitation fluorescence intensity emitted at 510 nm in response to sequential excitation at 340 and 380 nm. The data are presented as the relative ratio of fluorescence at 340 and 380 nm.
Statistical analysis.
Fisher's exact test was used to determine significance. Standard error is shown as error bars.
RESULTS
Release of suppressive soluble factor(s) by HIV-1-specific CTL, as well as bulk CD8+ cells of HIV-1 seropositive and seronegative individuals.
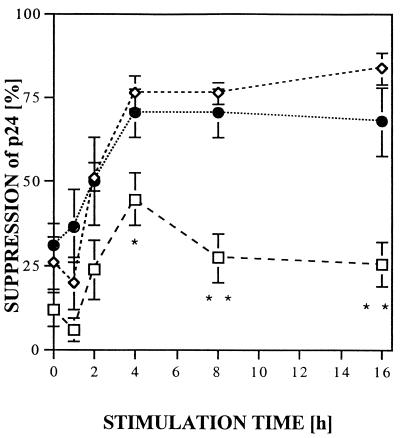
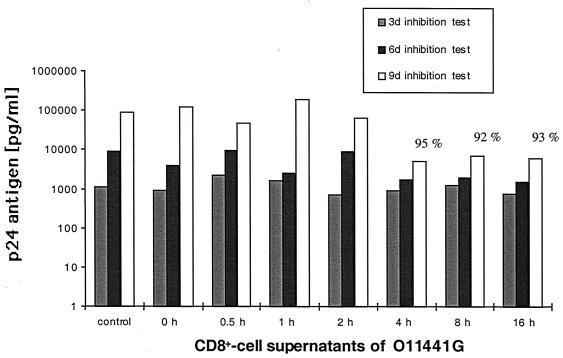
CD8+ cells produce soluble factors that inhibit X4 virus replication (6, 19, 23–25, 33, 35, 37, 38), but no kinetic studies have been reported as to when the suppressive activity is produced or whether it is derived from HIV-1-specific CTL. HIV-1-specific CTL clones as well as bulk CD8+ cells, from HIV-1-seropositive and seronegative individuals, that had been expanded in vitro in the presence of IL-2 were stimulated by anti-CD3 cross-linking for 1 to 16 h (Fig. 1). Cell-free supernatants were obtained by 10-min 3,000 × g centrifugation, diluted 1:2, and added to freshly HIV-1IIIB-infected H9 cells, and p24 production was monitored over a 9-day period. Virus production was weak at day 3 but readily apparent by day 6 and maximal at day 9, with levels of infection in the controls showing p24 levels of >100 ng/ml. Figure 1 compares relative levels of p24 HIV antigen suppression in assays using inhibition assay supernatant fluid harvested on day 9. Maximal inhibition of p24 was detected in CD8+ cell supernatants harvested 4 h after anti-CD3 stimulation (Fig. 1), averaging 44% (range, 35 to 66%) for seronegative CD8+ cells, with 76% (range, 50 to 95%) for HIV-seropositive CD8+ cells and 70% (range, 59 to 92%) for HIV-1-specific CTL. The range of inhibition at 4 h was similar at 16 h. Whereas the supernatants of the bulk CD8+ T cells from seronegative individuals showed a significantly lower level of inhibition starting at 4 h compared to CTL clones (P = 0.039) and bulk CD8+ cells from seropositive individuals (P = 0.001), no significant differences in the release of the suppressive soluble factor(s) were found between the CTL clones and the bulk CD8+ T cells from seropositive individuals at any of the six time points analyzed. The level of inhibition over the time points tested for the seronegative bulk CD8+ cells ranged from 30 to 60% of that produced by CD8+ cells and CTL from seropositive persons. Inhibition was not due to an antiproliferative effect on the H9 cells used in the inhibition assay as measured by total cell counts in the log phase of cell growth at days 3 to 6 in a control experiment without virus (data not shown). The maximal inhibition (95%) by the supernatants was found when 4-h supernatants of bulk CD8+ cells of seropositive individuals were analyzed at day 9 in the infection assay used to quantitate level of inhibition, compared to 15 and 81% suppression at days 3 and 6, respectively (Fig. 2). These results indicate that CD8+ cells from HIV-1-infected persons have an enhanced ability to suppress X4 strains of HIV-1 compared to cells from seronegative persons and that this activity is similar in magnitude and kinetics to that produced by stimulation of HIV-1-specific CTL.
FIG. 1.
Differential suppressive activity of CD8+ T cells of HIV-1-seronegative individuals (squares), CTL clones (diamonds), and asymptomatic HIV-1-seropositive individuals (circles). Supernatants of CD3-stimulated CD8+ T cells were collected after 2, 4, 8, and 16 h, diluted 1:2 in R20, and added to H9 cells acutely infected by HIVIIIB; 1 ml of H9 supernatant was removed when 1 ml of 1:2-diluted supernatants was added at days 3 and 6. The supernatant of the inhibition test was collected and tested for p24 production after 9 days in culture. Percentage of inhibition was calculated against an untreated control. The asterisks indicate a statistically significant difference of cells from HIV-1-seronegative individuals (n = 6) against both cells from HIV-1-seropositive individuals (n = 6) and CTL (n = 5) (∗, P < 0.05; ∗∗, P < 0.01: Fisher's exact test). Error bars of at least five independent experiments are shown.
FIG. 2.
Levels of HIV-1 p24 antigen after an inhibition test with supernatants from CD8+ cells of an HIV-1-infected long-term nonprogressor. The CD8+ cells were stimulated for a different period of time (0 to 16 h); supernatants were collected, and acutely infected H9 cells were incubated for 3, 6, or 9 days (3d, 6d, or 9d; see Materials and Methods). Percentage shows the highest inhibition of p24 antigen suppression against the medium control found at day 9. Time zero denotes 4-h supernatant without CD3 activation.
Phenotypic characterization of CD8+ cells from HIV-1-seropositive and seronegative persons.
In response to viral infection, unprimed naive CD8+ T cells clonally respond and differentiate into memory- and effector-type virus-specific T cells that are phenotypically distinct (2, 18, 21, 27, 39). To determine if phenotypes among the sources of CD8+ cells used are associated with the observed differences in release of the suppressive factor(s), we examined the prevalence of surface makers expressed at the time the cells were used for the anti-CD3 activation. For these studies, fresh CD8+ cells were obtained from HIV-1-seropositive and seronegative persons by positive selection with an anti-CD8 monoclonal antibody coated on immunomagnetic beads. These CD8+ cells and HIV-1-specific CTL clones were simulated with PHA and irradiated allogeneic feeder cells, propagated for 2 weeks, and stained for FACS analysis. The bulk CD8+ cells of seronegative individuals showed a significantly higher percentage of CD62-L cells than either the cells derived from seropositive individuals (P = 0.011) or CTL (P = 0.0004). The percentage of naive cells in seronegative persons as measured by the CD45-RA antibody was also higher but not significantly different from that for either cells from seropositive individuals (P = 0.165) or CTL (P = 0.096). The CD8+ cells from seronegative individuals are in the same state of activation as CD8+ cells from seropositive individuals and CTL, as measured by CD38 and CD44 activation (Table 1). No other significant differences between CTL and CD8+ cells could be found. Our data suggest that the decreased suppression mediated by CD8+ cell supernatants from seronegative persons is associated with a higher percentage of naive cells.
TABLE 1.
Cells stained by surface marker and significance of difference of bulk CD8+ cells of seronegative individuals compared to each bulk CD8+ cells of seropositive individuals and HIV-1-specific CTL
| Marker | % Stained
|
P | ||
|---|---|---|---|---|
| Seronegative CD8+ cells (n = 6) | Seropositive CD8+ cells (n = 3) | CTL (n = 4) | ||
| CD25 | 31.7 ± 3.5 | 45.3 ± 20.1 | 29.1 ± 12.8 | NSa |
| CD28 | 6.1 ± 0.5 | 7.2 ± 5.1 | 2.0 ± 0.3 | NS |
| CD38 | 87.5 ± 3.6 | 82.4 ± 14.9 | 93.6 ± 2.8 | NS |
| CD44 | 22.9 ± 5.2 | 30.6 ± 16.2 | 36.6 ± 14.3 | NS |
| CD45-RA | 22.3 ± 9.2 | 1.4 ± 0.5 | 0.6 ± 0.4 | NS |
| CD45-RO | 74.9 ± 1.7 | 82.2 ± 14.3 | 90.3 ± 9.0 | NS |
| HLA-DR | 60.2 ± 5.0 | 60.4 ± 25.8 | 27.5 ± 16.3 | NS |
| CD62-L | 11.7 ± 1.5 | 3.3 ± 1.7 | 0.9 ± 0.3 | <0.05 |
NS, not significant (P > 0.05).
Release of cytokines and chemokines by bulk CD8+ lymphocytes of HIV-1-seropositive and seronegative individuals and by HIV-1-specific CTL.
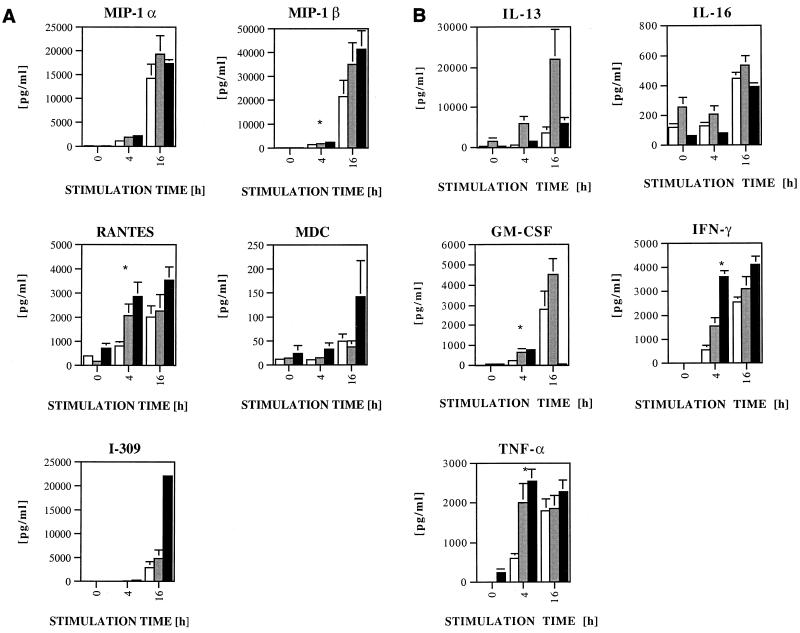
Having demonstrated that X4 virus-inhibitory factors are produced in greater amounts by CD8+ cells and CTL from HIV-seropositive persons, we next compared the magnitude and kinetics of inhibition to that of other CD8+ cell factors known to inhibit either R5 or X4 viruses. The supernatants of HIV-1-specific CTL clones, bulk CD8+ cells from HIV-1 seropositive persons, and bulk CD8+ cells from seronegative individuals were assayed for their secretion of cytokines after 0, 4, and 16 h of anti-CD3 stimulation (Fig. 3).
FIG. 3.
Secretion of β-chemokines (A) and other cytokines (B) of CD8+ T cells of HIV-1-seronegative individuals (open squares), asymptomatic HIV-1-seropositive individuals (gray squares), and CTL clones (black squares). Supernatants of CD3-stimulated CD8+ T cells were collected after 0, 4, and 16 h; time zero denotes supernatant of 4-h incubation without anti-CD3 stimulation. Supernatants were tested for cytokine concentrations by ELISA (see Materials and Methods). The asterisks indicate a statistically significant difference of cells from seronegative individuals against both cells from seropositive individuals and CTL clones (P < 0.05; Fisher's exact test). All error bars for at least five independent experiments are calculated, but some are too small to show.
The time course and magnitude of release of cytokines after CD3 cross-linking demonstrated significant differences when bulk CD8+ cells of seronegative individuals were compared to CTL and bulk CD8+ cells of seropositive individuals. No significant differences for MIP-1α, MDC, I-309, IL-13, or IL-16 were found (Fig. 3). Conversely, cells from seropositive individuals compared to seronegative persons produced, after 4 h of activation, more MIP-1β (P = 0.021), RANTES (P = 0.034), gamma interferon (IFN-γ) (P = 0.028), tumor necrosis factor alpha (TNF-α) (P = 0.017), and granulocyte-macrophage colony-stimulating factor (GM-CSF) (P = 0.034). CTL supernatants also produced significantly higher levels after 4 h for MIP-1β (P = 0.036), RANTES (P = 0.006), IFN-γ (P = 0.0001), TNF-α (P = 0.0001), and GM-CSF (P = 0.001) compared to the levels produced by seronegative bulk CD8+ cells. None of the individual factors assayed displayed a pattern of release similar to that seen for the X4 virus-suppressive factor(s), which was characterized by significant differences at 4 and 16 h of stimulation in seropositive compared to seronegative persons. As for differences between CTL clones and bulk CD8+ cells of seropositive individuals, CTL clones released three to five times more MDC, I-309, and GM-CSF than bulk CD8+ cells of seropositive individuals, whereas for bulk CD8+ cells of seropositive individuals, a four fold greater release of IL-13 was seen. This suggests that the factors affecting X4 virus replication have a pattern of release different from that of the chemokines MIP-1α, MIP-1β, RANTES, IL-13 (9, 26), MDC, I-309, and IL-16 (1, 14, 29). Additionally, the mechanism of action seems to be independent from the cytokines TNF-α and IFN-γ, known to influence HIV replication (10), based on the finding that the levels of these cytokines are similar in all groups at 16 h following stimulation, yet suppression is observed only in supernatants derived from cells of HIV-1-seropositive persons.
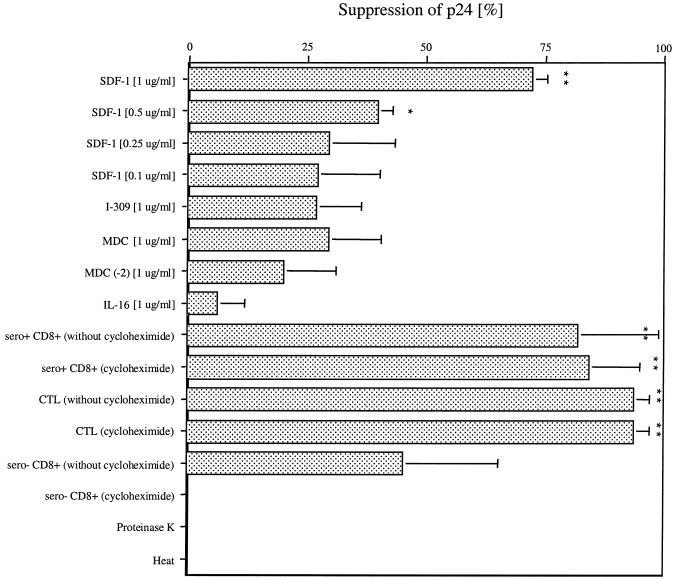
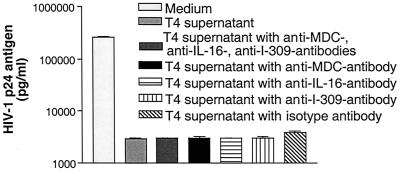
The conclusion that the X4 virus-suppressive factor is distinct from known cytokines, chemokines, and interleukins was also supported by direct inhibition assays using recombinant forms of these compounds. The amounts of chemokines released (I-309, MDC, and IL-16) were too low to be responsible for the observed inhibition. Even when used in amounts 45 to 25,000 times higher than measured, significant (greater than 50%) inhibition could never be achieved (Fig. 4). To further exclude the possibility that these proteins are responsible for the suppression tested, we used neutralizing antibodies against I-309, MDC, and IL-16 at a concentration of 5 μg/ml. None of the neutralizing antibodies alone or in combination inhibited the suppressive activity in the supernatants used (Fig. 5), excluding these factors as responsible for the suppressive activity measured. The only factor tested that showed significant inhibition was SDF-1α starting at a concentration of 0.5 μg/ml and 50% inhibitory dose (ID50) of 81 nM (0.63 μg/ml). However, no detectable SDF-1 was present in the stimulated CD8+ cell and CTL supernatants by ELISA, consistent with the findings of others (17).
FIG. 4.
Inhibition of p24 antigen after addition of cytokines (SDF-1 [1, 0.5, 0.25, and 0.1 μg/ml], I-309 [1 μg/ml], MDC [1 μg/ml], MDC(−2) [1 μg/ml], and IL-16 [1 μg/ml]), cycloheximide, proteinase K, and heat treatment. At days 3 and 6, 1 ml of the 2-ml H9 cell supernatant was removed and replaced with 1 ml of fresh R20 with new chemokines or with supernatants. At day 9, p24 antigen was measured. Here error bars for at least three independent experiments are shown. The asterisks indicate a statistically significant difference from the control (∗, P < 0.05; ∗∗, P < 0.01; Fisher's exact test).
FIG. 5.
Neutralization studies with antibodies against MDC, I-309, and IL-16. Supernatants after 4 h of stimulation were preincubated overnight at 4°C with anti-MDC, anti-I-309, and anti-IL-16 antibodies (5 μg/ml) alone or combined. The supernatants were diluted 1:2 when added to the inhibition test (see Materials and Methods). At day 9, p24 antigen of the supernatant fluids of the inhibition assays was measured. The experiment represents the average of three independent experiments. The controls include the isotype antibodies.
Characterization of suppressive activity.
Having shown that the suppressive activity released is different from cytokines, chemokines, and interleukins, we next evaluated the stability of this activity. The suppressive activity was 100% degradable with proteinase K and heat treatment (Fig. 4). Additionally, cells were treated with cycloheximide to determine if the inhibitory substance is preformed within cells. The bulk CD8+ cells of the seropositive individuals and CTL clones both demonstrated that the inhibitory activity was preformed, in that it was not inhibited with cycloheximide. In contrast, CD8+ cells from seronegative persons no longer produced inhibitory factors after cycloheximide treatment (Fig. 4). To analyze the exocytotic pathway, we incubated the cells with monensin and brefeldin A and found that the suppressive activity was blocked by >90% with these compounds. At the same time, treatment with monensin and brefeldin A resulted in greater than 70% inhibition of secretion of MIP-1α, MIP-1β, TNF-α, and IFN-γ (Table 2). These results indicate that the suppressive activity is secreted in a manner similar to these proteins.
TABLE 2.
Decrease of production of RANTES, MIP-1α, MIP-1β, TNF-α, and IFN-γ and of HIV-1IIIB suppression after treatment of CTL with monensin and brefeldin Aa
| Treatment | Decrease of concn (%) of:
|
Decrease of suppression (%) of HIV-1IIIB | ||||
|---|---|---|---|---|---|---|
| RANTES | MIP-1α | MIP-1β | TNF-α | IFN-γ | ||
| Monensin | 0 | 71.6 | 70.0 | 73.1 | 99.5 | 91.5 |
| Brefeldin A | 0 | 98.0 | 98.1 | 94.5 | >99.9 | 97.5 |
CTL clone 15160D75 at 5 × 105 cells/ml was activated by CD3 cross-linking and treated with monensin (2 μM) or with brefeldin A (3.5 μM). Chemokine concentration or inhibition tests with supernatants were performed after 4 h of stimulation.
Using supernatants derived 4 h following stimulation with anti-CD3 of bulk CD8+ cells from seropositive individuals or HIV-1-specific CTL, active fractions were obtained and used for further purifications. The protein amount necessary to block 50% of the virus (ID50) was found to be 5 mg/ml using these supernatants (1:2 dilution) derived 4 h after stimulation with anti-CD3 of bulk CD8+ cells of seropositive individuals or HIV-1-specific CTL.
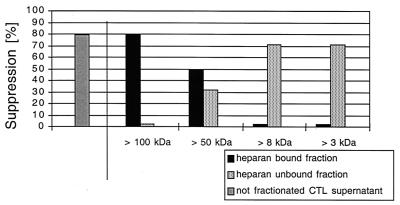
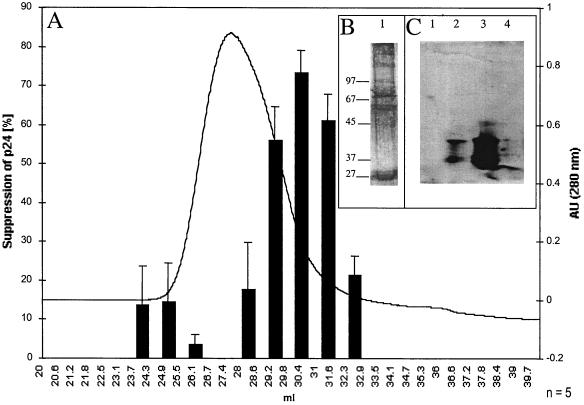
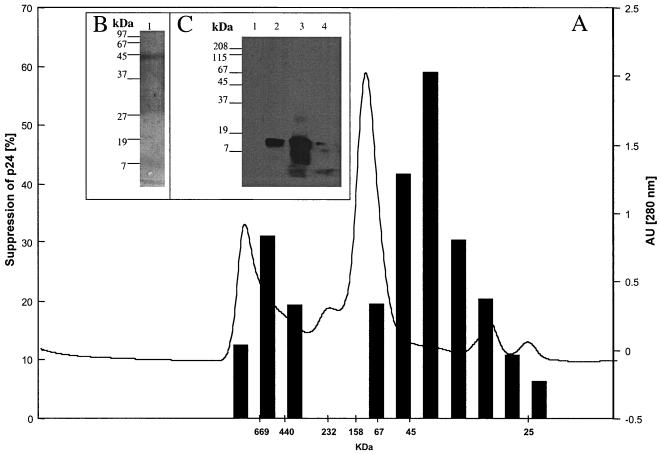
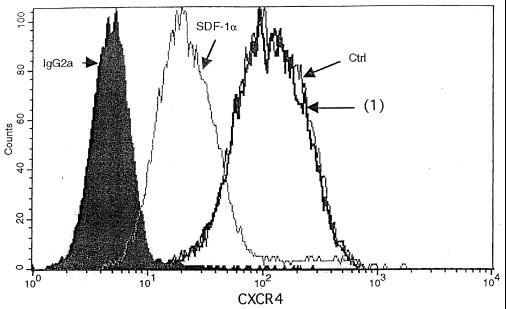
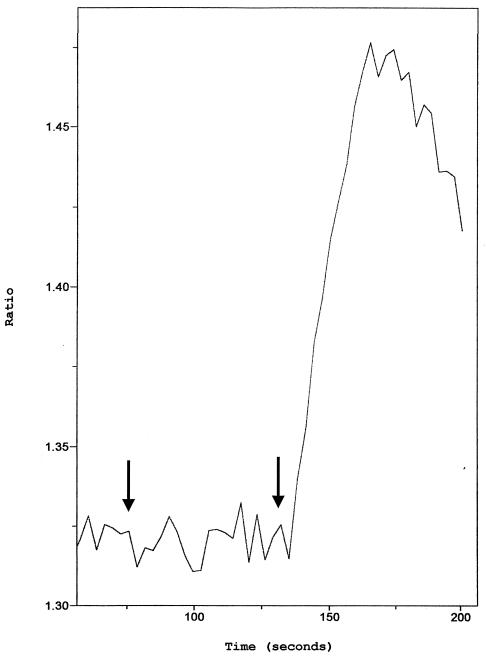
We next further characterized the binding properties and size of the suppressive activity. Supernatants were separated into heparin binding and nonbinding fractions. The nonbinding fraction was collected after washing of the heparin-Sepharose column with PBS. The binding fraction then was eluted with 2 M NaCl in PBS. Both eluates where then filtered sequentially through Centricon membrane with different exclusion sizes (100, 50, 8, and 3 kDa). Approximately half of the suppressive activity of the 4-h supernatants isolated by Centricon centrifugation is found in the <50-kDa heparin-unbound fraction, with an ID50 of 770 μg/ml (1:30 dilution), which is seven times lower than that of the starting material. The remaining suppressive activity is due to proteins of >50 kDa by Centricon centrifugation which bound to heparin (Fig. 6), with a 15-fold lower ID50 than the initial supernatant at 330 μg/ml (1:30 dilution) (Table 3). In a Western blot analysis with the heparin-bound fraction, MDC, IL-16, and I-309 were not detectable (Fig. 7C), demonstrating that these molecules did not contribute to the measured inhibition. Using the 350 mM heparin-bound fraction of 4-h supernatants of bulk CD8+ cells of seropositive individuals or HIV-1-specific CTL, we could increase the purification factor to 215 with an ID50 of 23.5 μg/ml at a 1:30 dilution; using the 40-kDa heparin-bound Superdex fraction, we found at a 1:75 dilution an ID50 of 5.5 μg/ml and a purification factor of 909 (Table 3). The strength of inhibition of the 40-kDa heparin-bound Superdex fraction was associated with the prevalence of a 43-kDa main protein as measured by SDS-PAGE (Fig. 8). In a Western blot analysis with the 40-kDa Superdex eluate, MDC, IL-16, and I-309 were not detectable (Fig. 8C) in assays using half of the total protein amount used for the 9-day inhibition test, where the fractions were 75 times diluted again indicating that these molecules are not responsible for the measured inhibition. Our data thus suggest that there are either two suppressive activities or one that is in two different configurations after CD3 activation. These can be differentiated by heparin binding and size. We then assessed for down-regulation of the CXCR4 receptor and for Ca2+ flux, characteristics seen for activities of chemokines, which can bind to heparin. We found no chemokine-like activity: using either the 350 mM heparin-bound fraction or the 40-kDa heparin-bound Superdex fraction, no down-regulation of the CXCR4 receptor (Fig. 9) or Ca2+-flux (Fig. 10) was observed.
FIG. 6.
Differential suppressive activity of fractions after HiTrap heparan sulfate chromatography of supernatant of 4-h anti-CD3-stimulated HIV-1-specific CTL clone 15160-D75. A 5-ml heparin column (Pharmacia) was loaded with 10 ml of supernatant and washed with 20 ml of PBS. The heparin-unbound fraction was filtered and concentrated to 100 μl sequentially through Centricon membranes with exclusion sizes of 100, 50, 8, and 3 kDa. The heparin-bound fraction was then obtained by washing the heparin column with 20 ml of 2 M NaCl in PBS, followed by the above-described size exclusion centrifugation steps. The supernatants on top of the Centricon membranes were concentrated to 100 μl, washed twice with a 200-fold times volume of PBS, and tested for activity. The controls include the buffer conditions.
TABLE 3.
ID50 and purification factors for each purification step, using 4-h supernatant of anti-CD3-stimulated bulk CD8+ cells of seropositive individuals or HIV-1-specific CTL
| Sample | ID50 (μg/ml) | Purification factor |
|---|---|---|
| Supernatant | 5,000 | 1 |
| Total heparan sulfate unbound | 770 | 7 |
| Total heparan sulfate bound | 330 | 15 |
| 350 mM heparan sulfate bound | 23.3 | 215 |
| 40-kDa heparan sulfate-bound Superdex | 5.5 | 909 |
FIG. 7.
HIV-1-suppressive activity of the heparin-bound eluates (A), silver-stained SDS-polyacrylamide gel of peak active suppressive fraction (B), and Western blot of peak active suppressive fraction, using a combination of antibodies against IL-16, MDC, and I-309 (C). (A) Heparin-bound eluates were diluted 1:30 in the inhibition test (see Materials and Methods). At day 9, HIV-1 p24 antigen was measured and compared against a buffer-treated control. (B) Lane 1, 20 μl of fraction with peak suppression was subjected to SDS-PAGE and silver stained. (C) Lane 1, Western blot of 20 μl of fraction with peak suppression (1/5 of total amount used for the 9-day inhibition test) incubated with the combined antibodies; lane 2, Western blot of the IL-16 protein (100 ng) incubated with combined antibodies; lane 3, Western blot of MDC protein (100 ng) incubated with combined antibodies; lane 4, Western blot of the I-309 protein (100 ng) incubated with combined antibodies. In control experiments, the Western blots were incubated alone with antibodies against IL-16, MDC, and I-309. AU, arbitrary units.
FIG. 8.
HIV-1-suppressive activity of the heparin-bound Superdex eluates (A) silver-stained SDS-polyacrylamide gel of peak active suppressive fraction (B), and Western blot of peak active suppressive fraction, using a combination of antibodies against IL-16, MDC, and I-309 (C). (A) Superdex eluates were diluted 1:75 in the inhibition test (see Materials and Methods). At day 9, HIV-1 p24 antigen was measured and compared against a buffer-treated control. (B) Lane 1, 20 μl of fraction with peak suppression was subjected to SDS-PAGE and silver stained. (C) Lane 1, Western blot of 20 μl of fraction with peak suppression (1/2 of total protein amount used for the 9-day inhibition test) incubated with the combined antibodies; lane 2, Western blot of the IL-16 protein (100 ng) incubated with combined antibodies; lane 3, Western blot of MDC protein (100 ng) incubated with combined antibodies; lane 4, Western blot of the I-309 protein (100 ng) incubated with combined antibodies. In control experiments, the Western blots were incubated alone with antibodies against IL-16, MDC, and I-309. AU, arbitrary units.
FIG. 9.
CXCR4 down-regulation by the heparin-bound suppressive fraction. CD4+ cells (106/ml) were incubated either with R10-50 (Ctrl), 15 to 30 μg of the 350 mM heparin-bound fraction [(1)], or SDF-1α (250 ng/ml), incubated for 45 min at 37°C, and then stained with monoclonal CXCR4-FITC antibody or control antibody (IgG2a). Staining was measured by FACS analysis. The data are representative of three or more experiments for primary CD4+ cells and H9 cells, using the 350 mM heparin-bound or the 40-kDa heparin-bound Superdex fraction (0.5 to 6 μg of protein).
FIG. 10.
Ca2+ flux induced by heparin-bound fraction versus SDF-1α. Ca2+ flux was monitored by the ratio of fluorescence of fura-2-loaded primary CD4+ cells. Arrows indicate the time (at 50 s) of adding 15 to 30 μg of the 350 mM heparin-bound fraction which was concentrated on a 50-kDa-cutoff Centricon membrane to 100 μl and washed twice with a 200-fold volume of PBS. SDF-1α (50 ng/ml) was added at 130 s to CD4+ cells (106/ml). The data are representative of three or more experiments for primary CD4+ cells and H9 cells, using the 350 mM heparin-bound or the 40-kDa heparin-bound Superdex fraction (0.5 to 6 μg of protein).
DISCUSSION
HIV-1-specific CTL exert potent antiviral effects that are mediated by distinct cytotoxic and noncytotoxic mechanisms. Whereas the role of β-chemokines in inhibiting R5 strains of HIV-1 is well established, soluble factors produced by CD8+ cells that inhibit X4 strains of virus are less well defined. In addition, there are few studies that address the relationship between these two effector mechanisms in the inhibition of X4 strains of the virus. Here we show that the noncytolytic, X4 virus-specific antiviral properties of HIV-1-specific CTL and CD8+ cells from seropositive persons have similar characteristics. Both exist in a preformed state within the cells, and both have similar initial kinetics of release following stimulation of cells. In addition, antiviral suppression mediated by both appears to be due to a secreted protein, since it can be inhibited by proteinase K treatment and is heat labile. The fact that the magnitude and kinetics of suppression are significantly different from those observed with CD8+ cells from uninfected persons underscores that this noncytolytic suppression is induced by HIV infection.
Our data indicate that the lower amounts of naive CD8+ cells are responsible for the detected higher release of X4-suppressive factors of HIV-1-seropositive individuals and HIV-specific CTL compared to CD8+ cells of seronegative individuals (Fig. 1; Table 1) and that the suppressive factor(s) suppresses HIV-1 replication in highly infected CD4+ cells (Fig. 2). Additionally, our data directly examine the properties of the inhibitory activity and indicate that the suppressive factor(s) is not likely a cytokine, for a number of reasons. The kinetics of release of the antiviral activity is distinct from the pattern of secretion of cytokines, chemokines, and interleukins. In our in vitro system we found that none of the known X4-suppressive factors (IL-16, MDC, I-309, and SDF-1) (1, 3, 14, 28, 29) displayed a pattern of release similar to that of the X4-suppressive factor(s) here described. Significant differences in release were observed at both 4 and 16 h after stimulation, comparing CD8+ cells of seronegative individuals with CD8+ cells of seropositive individuals and HIV-1-specific CTL clones (Fig. 3). We also tested for cytokines (IFN-γ, TNF-α, and GM-CSF), interleukins (IL-13 and IL-16), and suppressive chemokines (MIP-1α, MIP-1β, and RANTES) known to inhibit R5 viruses (9), and we found that none of these factors showed a pattern of secretion similar to that of these suppressive factors. Nevertheless, we do show that HIV-1-specific CTL release IL-16, MDC, and I-309, and this can occur in picogram to nanogram amounts (Fig. 3A). Additionally, these molecules used as recombinant proteins were not able to significantly suppress X4 HIV-1 even in high concentrations (Fig. 4). Here we show that of the chemokines tested, only SDF-1 was able to suppress X4 HIV-1 (Fig. 4), but this molecule was not detectable by ELISA. Additionally, SDF-1 RNA expression was not found with an SDF-1-specific DNA probe (data not shown), which is consistent with findings of others (17). Not only was the pattern of secretion of the tested cytokines and chemokines different, but the amounts produced for IL-16, MDC, and I-309 were not substantial enough to explain the measured suppressive activity. To exclude biologically active molecules of these chemokines not distinguished by the ELISA used but possibly responsible for the inhibition measured, we performed studies with neutralizing antibodies against IL-16, MDC, and I-309. None of these antibodies alone or in combination decreased the suppressive activity in the supernatants used, indicating that these molecules are not responsible for the suppressive activity (Fig. 5). Additionally, Western blots of the heparin-bound fraction and the 40-kDa heparin-bound Superdex fraction with anti-IL-16, anti-MDC, and anti-I-309 antibodies showed no evidence of these chemokines (Fig. 7C and Fig. 8C).
Although the above data suggest that the factor is not a known cytokine or chemokine, a number of experiments support the conclusion that the suppressive factor is a preformed secreted protein. The suppressive activity was found to be 100% degradable by proteinase K and heat (Fig. 4). In this respect, it appeared distinct from the 30 to 40-kDa CD8+ CAF (6, 35; J. A. Levy, personal communication), which has been reported to be heat stable. Additionally, the secretion of the soluble factor(s) was not significantly suppressed with cycloheximide in HIV-seropositive bulk CD8+ cells (P = 0.062) and CTL clones (P = 0.882), whereas it was totally abolished from CD8+ cells of seronegative individuals (P = 0.010). Thus, de novo synthesis was necessary to achieve measurable inhibition in supernatants from seronegative CD8+ cells after 4 h of stimulation, but this was not characteristic for seropositive persons (Fig. 4). Monensin and brefeldin A treatment decreased the suppression activity, indicating that factor release involves the exocytotic pathway. Additionally, monensin and brefeldin A treatment showed that the suppressive activity was not part of the RANTES-glycoaminoglycan complex (5) because RANTES was not blocked by monensin and brefeldin A treatment (Table 2).
Although we have not precisely identified the active fraction mediating antiviral suppressive activity, our data should facilitate future studies to further elucidate the contributing components. Our data indicate that there is at least one factor with two distinct configurations which differ in size and heparin binding properties. Approximately half of the total suppressive activity is within a heparin-bound fraction that contains proteins with molecular sizes of >50 kDa as determined by Centricon centrifugation. Additionally, the 350 mM heparin-bound fraction could not down-regulate CXCR4 (Fig. 9). This excludes a mechanism of inhibition for X4 viruses seen for the chemokines (3, 29). Also, the 350 mM heparin-bound fraction and the 40-kDa heparin-bound Superdex fraction did not induce a Ca2+ flux (Fig. 10). Additionally, the correlation between inhibition seen from the Superdex eluates and the prevalence of a 43-kDa main protein as measured by SDS-PAGE indicates that the chemokines are not responsible for the inhibition. Chemokines are typically much smaller (<10 kDa) and would be expected to bind to heparin and to induce a Ca2+ flux (12). A second fraction with suppressive activity did not bind to heparin and had proteins smaller than 50 kDa but larger than 3 kDa, also as determined by Centricon centrifugation (Fig. 6). Other studies of CD8+ cell noncytotoxic suppression have not examined the ability to bind to heparin, and so this finding cannot be compared to other published studies. Additionally, the suppressive factor(s) described here may be different from others described in the literature because a different stimulation approach was used compared to the conventional methods with anti-CD3/anti-CD28 and/or PHA and IL-2 stimulation and collection of supernatants 3 to 8 days later. Further experiments will be required to fully define the factors described here, determine their mechanism of inhibition, and establish at which step in the viral life cycle the CD8+ cell factor(s) is active (6, 25, 33). These data also need to be examined in the context of other studies of non cytolytic inhibition where a CD8+ CAF was reported to be released by baboon CD8+ cells (23) or Epstein-Barr virus-specific CD8+ cells (19).
In summary, our data provide a functional link between CTL and CD8+ cell-derived virus-suppressive factors. We hypothesize that the noncytotoxic activity may be particularly important at the level of the local microenvironment, where it may serve an important function in inhibiting the spread of infectious virus.
ACKNOWLEDGMENTS
We thank David Chantry of ICOS Co. for the gift of polyclonal MDC antibodies.
This research was funded in parts by grants from the Defense Advanced Research Projects Agency (MDA-972-97-1-00144), the Deutsche Forschungsgemeinschaft, and the NIH (AI30914, AI28568, and AI46999).
REFERENCES
- 1.Baier M, Werner A, Bannert N, Metzner K, Kurth R. HIV suppression by interleukin-16. Nature. 1995;378:563. doi: 10.1038/378563a0. [DOI] [PubMed] [Google Scholar]
- 2.Bass H Z, Fahey J L, Nishanian P, Detels R, Cumberland W, Kemeny M, Plaeger S. Relation of impaired lymphocyte proliferative function to other major human immunodeficiency virus type 1-induced immunological changes. Clin Diagn Lab Immunol. 1997;4:64–69. doi: 10.1128/cdli.4.1.64-69.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 4.Brander C, Goulder P J R. The evolving field of HIV CTL epitope mapping: new approaches to the identification of novel epitopes. In: Korber B T M, Brander C, Walker B D, Koup R A, Moore J, Haynes B, Meyers G, editors. HIV molecular immunology database. Vol. 1. Los Alamos, N. Mex: Los Alamos National Laboratory: Theoretical Biology and Biophysics; 2000. pp. 1–19. [Google Scholar]
- 5.Burns J M, Lewis G K, DeVico A L. Soluble complexes of regulated upon activation, normal T cells expressed and secreted (RANTES) and glycosaminoglycans suppress HIV-1 infection but do not induce Ca(2+) signaling. Proc Natl Acad Sci USA. 1999;96:14499–14504. doi: 10.1073/pnas.96.25.14499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen C H, Weinhold K J, Bartlett J A, Bolognesi D P, Greenberg M L. CD8+ T lymphocyte-mediated inhibition of HIV-1 long terminal repeat transcription: a novel antiviral mechanism. AIDS Res Hum Retroviruses. 1993;9:1079–1086. doi: 10.1089/aid.1993.9.1079. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Rinaldo C, Gupta P. A semiquantitative assay for CD8+ T-cell-mediated suppression of human immunodeficiency virus type 1 infection. Clin Diagn Lab Immunol. 1997;4:4–10. doi: 10.1128/cdli.4.1.4-10.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The beta-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 9.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 10.Emilie D, Fior R, Jarrousse B, Marfaing-Koka A, Merrien D, Devergne O, Crevon M C, Maillot M C, Galanaud P. Cytokines in HIV infection. Int J Immunopharmacol. 1994;16:391–396. doi: 10.1016/0192-0561(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 11.Euhus D M, Gupta R K, Morton D L. Characterization of a 90-100 kDa tumor-associated antigen in the sera of melanoma patients. Int J Cancer. 1990;45:1065–1070. doi: 10.1002/ijc.2910450615. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Zepeda E A, Combadiere C, Rothenberg M E, Sarafi M N, Lavigne F, Hamid Q, Murphy P M, Luster A D. Human monocyte chemoattractant protein (MCP)-4 is a novel CC chemokine with activities on monocytes, eosinophils, and basophils induced in allergic and nonallergic inflammation that signals through the CC chemokine receptors (CCR)-2 and -3. J Immunol. 1996;157:5613–5626. [PubMed] [Google Scholar]
- 13.Garzino-Demo A, Moss R B, Margolick J B, Cleghorn F, Sill A, Blattner W A, Cocchi F, Carlo D J, DeVico A L, Gallo R C. Spontaneous and antigen-induced production of HIV-inhibitory beta-chemokines are associated with AIDS-free status. Proc Natl Acad Sci USA. 1999;96:11986–11991. doi: 10.1073/pnas.96.21.11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horuk R, Hesselgesser J, Zhou Y, Faulds D, Halks-Miller M, Harvey S, Taub D, Samson M, Parmentier M, Rucker J, Doranz B J, Doms R W. The CC chemokine I-309 inhibits CCR8-dependent infection by diverse HIV-1 strains. J Biol Chem. 1998;273:386–391. doi: 10.1074/jbc.273.1.386. [DOI] [PubMed] [Google Scholar]
- 15.Johnson R P, Trocha A, Yang L, Mazzara G P, Panicali D L, Buchanan T M, Walker B D. HIV-1 gag-specific cytotoxic T lymphocytes recognize multiple highly conserved epitopes. Fine specificity of the gag-specific response defined by using unstimulated peripheral blood mononuclear cells and cloned effector cells. J Immunol. 1991;147:1512–1521. [PubMed] [Google Scholar]
- 16.Johnson V A, Walker B D. HIV-infected cell fusion assay1. In: Aldovini A, Walker B D, editors. Techniques in HIV research. New York, N.Y: Stockton Press; 1990. pp. 92–94. [Google Scholar]
- 17.Lacey S F, McDanal C B, Horuk R, Greenberg M L. The CXC chemokine stromal cell-derived factor 1 is not responsible for CD8+ T cell suppression of syncytia-inducing strains of HIV-1. Proc Natl Acad Sci USA. 1997;94:9842–9847. doi: 10.1073/pnas.94.18.9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landay A L, Mackewicz C E, Levy J A. An activated CD8+ T cell phenotype correlates with anti-HIV activity and asymptomatic clinical status. Clin Immunol Immunopathol. 1993;69:106–116. doi: 10.1006/clin.1993.1157. [DOI] [PubMed] [Google Scholar]
- 19.Le Borgne S, Fevrier M, Callebaut C, Lee S P, Riviere Y. CD8+ cell antiviral factor activity is not restricted to human immunodeficiency virus (HIV)-specific T cells and can block HIV replication after initiation of reverse transcription. J Virol. 2000;74:4456–4464. doi: 10.1128/jvi.74.10.4456-4464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levy J A, Mackewicz C E, Barker E. Controlling HIV pathogenesis: the role of the noncytotoxic anti-HIV response of CD8+ T cells. Immunol Today. 1996;17:217–224. doi: 10.1016/0167-5699(96)10011-6. [DOI] [PubMed] [Google Scholar]
- 21.Lieberman J, Trimble L A, Friedman R S, Lisziewicz J, Lori F, Shankar P, Jessen H. Expansion of CD57 and CD62L-CD45RA+ CD8 T lymphocytes correlates with reduced viral plasma RNA after primary HIV infection. AIDS. 1999;13:891–899. doi: 10.1097/00002030-199905280-00004. [DOI] [PubMed] [Google Scholar]
- 22.Liu R, Paxton W A, Choe S, Ceradini D, Martin S R, Horuk R, MacDonald M E, Stuhlmann H, Koup R A, Landau N R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 23.Locher C P, Blackbourn D J, Levy J A. Suppression of human immunodeficiency virus type 1 replication by a soluble factor produced by CD8+ lymphocytes from HIV-2-infected baboons. Immunol Lett. 1999;66:151–157. doi: 10.1016/s0165-2478(98)00175-8. [DOI] [PubMed] [Google Scholar]
- 24.Mackewicz C E, Blackbourn D J, Levy J A. CD8+ T cells suppress human immunodeficiency virus replication by inhibiting viral transcription. Proc Natl Acad Sci USA. 1995;92:2308–2312. doi: 10.1073/pnas.92.6.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackewicz C E, Patterson B K, Lee S A, Levy J A. CD8(+) cell noncytotoxic anti-human immunodeficiency virus response inhibits expression of viral RNA but not reverse transcription or provirus integration. J Gen Virol. 2000;81:1261–1264. doi: 10.1099/0022-1317-81-5-1261. [DOI] [PubMed] [Google Scholar]
- 26.Montaner L J, Doyle A G, Collin M, Herbein G, Illei P, James W, Minty A, Caput D, Ferrara P, Gordon S. Interleukin 13 inhibits human immunodeficiency virus type 1 production in primary blood-derived human macrophages in vitro. J Exp Med. 1993;178:743–747. doi: 10.1084/jem.178.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murali-Krishan K, Ahmed K. Cutting edge: naïve T cells masquerading as memory cells. J Immunol. 2000;1165:1733–1737. doi: 10.4049/jimmunol.165.4.1733. [DOI] [PubMed] [Google Scholar]
- 28.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J L, Arenzana-Seisdedos F, Schwartz O, Heard J M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 29.Pal R, Garzino-Demo A, Markham P D, Burns J, Brown M, Gallo R C, DeVico A L. Inhibition of HIV-1 infection by the beta-chemokine MDC. Science. 1997;278:695–698. doi: 10.1126/science.278.5338.695. [DOI] [PubMed] [Google Scholar]
- 30.Roos M T, van Lier R A, Hamann D, Knol G J, Verhoofstad I, van Baarle D, Miedema F, Schellekens P T. Changes in the composition of circulating CD8+ T cell subsets during acute Epstein-Barr and human immunodeficiency virus infections in humans. J Infect Dis. 2000;182:451–458. doi: 10.1086/315737. [DOI] [PubMed] [Google Scholar]
- 31.Samson M, Libert F, Doranz B J, Rucker J, Liesnard C, Farber C M, Saragosti S, Lapoumeroulie C, Cognaux J, Forceille C, Muyldermans G, Verhofstede C, Burtonboy G, Georges M, Imai T, Rana S, Yi Y, Smyth R J, Collman R G, Doms R W, Vassart G, Parmentier M. Resistance to HIV-1 infection in caucasian individuals bearing mutant alleles of the CCR-5 chemokine receptor gene. Nature. 1996;382:722–725. doi: 10.1038/382722a0. [DOI] [PubMed] [Google Scholar]
- 32.Scarlatti G, Tresoldi E, Bjorndal A, Fredriksson R, Colognesi C, Deng H K, Malnati M S, Plebani A, Siccardi A G, Littman D R, Fenyo E M, Lusso P. In vivo evolution of HIV-1 co-receptor usage and sensitivity to chemokine-mediated suppression. Nat Med. 1997;3:1259–1265. doi: 10.1038/nm1197-1259. [DOI] [PubMed] [Google Scholar]
- 33.Tomaras G D, Lacey S F, McDanal C B, Ferrari G, Weinhold K J, Greenberg M L. CD8+ T cell-mediated suppressive activity inhibits HIV-1 after virus entry with kinetics indicating effects on virus gene expression. Proc Natl Acad Sci USA. 2000;97:3503–3508. doi: 10.1073/pnas.070521097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walker B D, Flexner C, Birch-Limberger K, Fisher L, Paradis T J, Aldovini A, Young R, Moss B, Schooley R T. Long-term culture and fine specificity of human cytotoxic T-lymphocyte clones reactive with human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1989;86:9514–9518. doi: 10.1073/pnas.86.23.9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker C M, Moody D J, Stites D P, Levy J A. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science. 1986;234:1563–1566. doi: 10.1126/science.2431484. [DOI] [PubMed] [Google Scholar]
- 36.Yang O O, Swanberg S L, Lu Z, Dziejman M, McCoy J, Luster A D, Walker B D, Herrmann S H. Enhanced inhibition of human immunodeficiency virus type 1 by Met-stromal-derived factor 1β correlates with down-modulation of CXCR4. J Virol. 1999;73:4582–4586. doi: 10.1128/jvi.73.6.4582-4589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang O O, Walker B. CD8+ cells in human immunodeficiency virus type 1 pathogenesis: cytolytic and noncytolytic inhibition of viral replication. Adv Immunol. 1997;6:273–297. doi: 10.1016/s0065-2776(08)60600-8. [DOI] [PubMed] [Google Scholar]
- 38.Yang O O, Kalams S A, Trocha A, Cao H, Luster A, Johnson R P, Walker B D. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J Virol. 1997;71:3120–3128. doi: 10.1128/jvi.71.4.3120-3128.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zola H, Koh L Y, Mantzioris B X, Rhodes D. Patients with HIV infection have a reduced proportion of lymphocytes expressing the IL-2 receptor p55 chain (TAC, CD25) Clin Immunol Immunopathol. 1991;59:16–25. doi: 10.1016/0090-1229(91)90078-o. [DOI] [PubMed] [Google Scholar]