Abstract
A major unknown in human immunodeficiency virus (HIV-1) vaccine design is the efficacy of antibodies in preventing mucosal transmission of R5 viruses. These viruses, which use CCR5 as a coreceptor, appear to have a selective advantage in transmission of HIV-1 in humans. Hence R5 viruses predominate during primary infection and persist throughout the course of disease in most infected people. Vaginal challenge of macaques with chimeric simian/human immunodeficiency viruses (SHIV) is perhaps one of the best available animal models for human HIV-1 infection. Passive transfer studies are widely used to establish the conditions for antibody protection against viral challenge. Here we show that passive intravenous transfer of the human neutralizing monoclonal antibody b12 provides dose-dependent protection to macaques vaginally challenged with the R5 virus SHIV162P4. Four of four monkeys given 25 mg of b12 per kg of body weight 6 h prior to challenge showed no evidence of viral infection (sterile protection). Two of four monkeys given 5 mg of b12/kg were similarly protected, whereas the other two showed significantly reduced and delayed plasma viremia compared to control animals. In contrast, all four monkeys treated with a dose of 1 mg/kg became infected with viremia levels close to those for control animals. Antibody b12 serum concentrations at the time of virus challenge corresponded to approximately 400 (25 mg/kg), 80 (5 mg/kg), and 16 (1 mg/kg) times the in vitro (90%) neutralization titers. Therefore, complete protection against mucosal challenge with an R5 SHIV required essentially complete neutralization of the infecting virus. This suggests that a vaccine based on antibody alone would need to sustain serum neutralizing antibody titers (90%) of the order of 1:400 to achieve sterile protection but that lower titers, around 1:100, could provide a significant benefit. The significance of such substerilizing neutralizing antibody titers in the context of a potent cellular immune response is an important area for further study.
Increasingly it is apparent that eliciting a T-cell response through vaccination is highly beneficial in terms of being able to control human immunodeficiency virus type 1 (HIV-1) replication following infection (2). Nevertheless, there is still great interest in eliciting a neutralizing antibody response that may synergize with the T-cell response or possibly even provide sterile protection on its own. Interestingly, studies of vaccination against a murine retrovirus show that the best protection is provided by a combination of specific B, CD4+ T, and CD8+ T cells (7). It was suggested that persistent infection with the retrovirus could be prevented only when antibody-producing cells were present (8).
Classically, antibody protection against viral challenge is investigated through passive transfer studies. In the case of HIV-1, this has been difficult for polyclonal antibody preparations because of the generally low titers of neutralizing antibody in serum elicited by natural infection or immunization. A few monoclonal antibodies have been generated that do effectively neutralize primary HIV-1 isolates, and these have been used in passive transfer studies. Thus, the neutralizing human monoclonal antibody b12 was shown to protect hu-PBL-SCID mice against challenge with two primary HIV-1 viruses (JR-CSF and AD6) (10). In each case, protection required concentrations of antibody in serum at the time of challenge that were sufficient to neutralize essentially all of the virus inoculum. A similar requirement for complete neutralization of the challenge virus was made in a study using macaques (38). Here the intravenous challenge virus was a simian/human immunodeficiency virus (SHIV) derived from a primary virus (SHIVDH12), and the infused antibody was derived from the plasma of chimpanzees infected with HIVDH12. The polyclonal antibody preparation had a very high neutralizing titer which was completely specific for the challenge virus (5). The neutralizing antibodies 2G12 and 2F5 in combination with a polyclonal human anti-HIV preparation (HIVIG) showed partial protection against intravenous challenge with the pathogenic SHIV89.6PD virus (24). When a similar study was performed using a vaginal challenge with SHIV89.6PD, there was some indication that protection was easier to achieve. For example, in contrast to the intravenous challenge study, partial protection was also observed with a single antibody (2G12) with only modest neutralizing activity against this challenge virus (26). Overall, however, most of the macaque data indicated that sterile protection required complete antibody neutralization of challenge virus. Similar conclusions were reached for HIV-1 challenge of hu-PBL-SCID mice (11, 29) or SHIV challenge of macaques (1) using viruses containing the env genes of T-cell-line-adapted viruses.
Vaginal challenge of macaques with a SHIV is probably one of the best animal models available for natural HIV-1 infection of humans. This is particularly the case for heterosexual transmission of HIV-1, which is the primary route of infection worldwide. The studies of Mascola and colleagues described above are therefore of particular value (26). One limitation of those studies is that they use a virus, SHIV89.6PD, that differs in its coreceptor usage from those viruses that are usually involved in human transmission or early systemic spread in the body. Thus, SHIV89.6PD expresses an envelope showing dual R5X4 tropism with a strong bias toward X4 usage. This is known since HIV-189.6 entry can be completely blocked by X4 inhibitors but is insensitive to a CCR5-specific inhibitor (12). However, viruses isolated during acute infection of both women and men usually (>90% of the time) have the R5 phenotype. Similarly, R5 viruses are generally involved in mother-to-infant transmission. Furthermore, CCR5-Δ32 homozygous individuals, who lack CCR5 expression, are strongly protected against HIV-1 infection (15, 20). Therefore it is clearly of considerable importance to understand the efficacy of antibody against mucosal challenge with an R5 virus in the macaque model. A further consideration is that the studies described above using SHIV89.6PD in macaques did not provide a complete titration of protection with antibody concentration. To address these issues, we investigated the ability of the neutralizing antibody b12 at various doses to protect against vaginal challenge with the R5 virus SHIV162P4.
SHIV162P4 is based on molecular clones of SIVmac239 and the well-established R5 HIV-1 primary isolate SF162 and was isolated following three sequential in vivo passages in rhesus macaques as described elsewhere (13, 22, 40). For the purposes of the antibody protection studies described here, the important features of SHIV162P4 are that it is an R5 virus and that it replicates to high titers following infection. However, the virus does display pathogenic behavior in that a proportion of animals show depressed CD4 counts and progress to AIDS-like symptoms.
The antibody chosen for the protection studies was the human monoclonal antibody immunoglobulin G1 (IgG1) b12 (4). This antibody shows broad neutralization of primary HIV-1 isolates and is directed to an epitope on gp120 overlapping the CD4 binding site (32, 34, 41). The antibody effectively neutralizes SHIV162P4 in vitro, with 90% neutralization occurring at a concentration of b12 of 1 to 2 μg/ml. Since concentrations in serum of up to the order of 500 to 1,000 μg/ml can be achieved in macaques via passive immunization, this potency ensures that a wide range of effective antibody concentrations can be studied in vivo.
The results show that the antibody b12 can completely protect macaques against vaginal challenge with an R5 virus at the highest dose used (25 mg/kg of body weight). The dose titration shows partial protection at an intermediate dose (5 mg/kg) and no protection at the lowest dose (1 mg/kg) used. Together with other protection experiments, the data emphasize that antibody alone provides sterile protection with complete virus neutralization independent of virus isolate, neutralizing antibody or a combination of antibodies, challenge route, or animal model used. However, the benefit in terms of a lower viremia may be apparent at lower effective antibody concentrations.
MATERIALS AND METHODS
Macaques.
Protocols for animals (female macaques, 4.5 to 8.5 kg) were reviewed and approved by the relevant Institutional Animal Care and Use Committees. The animals were housed in accordance with American Association for Accreditation of Laboratory Animal Care standards. For all procedures, animals were lightly anesthetized with 10 mg of ketamine HCl/kg. All macaques were experimentally naive and were negative for antibodies against HIV-1, SIV, and type D retrovirus at the start of the experiments.
Thirty days prior to virus challenge, the animals received 30 mg of medroxyprogesterone acetate (Depo-Provera; Upjohn, Kalamazoo, Mich.) by intramuscular injection (26). Antibody preparations were administered by intravenous injection 6 h prior to virus challenge. The challenge virus diluted in 1 ml of phosphate-buffered saline (PBS) was introduced atraumatically into the vagina with an 8 French pediatric feeding tube attached to a syringe barrel. Macaques were maintained in an immobilized state, with the perineum slightly elevated, for approximately 15 min post-viral challenge. The animals were monitored by assessment of routine hematology, CD4 and CD8 lymphocyte subset counts, blood chemistry, and plasma viral loads at regular intervals. Inguinal lymph nodes were biopsied at 7 and 21 days postchallenge. Vaginal fluids were collected weekly as described below.
Challenge virus.
The virus used in this study was SHIV162P passage 4, which has been described elsewhere (13, 40). Briefly, SHIVSF162 was constructed by replacing the tat, rev, and env genes of the pathogenic molecular clone SIVmac239 with corresponding regions from the R5, HIV-1 molecular clone SF162 (40). SHIV162P4 was then derived from SHIVSF162 by serial passaging of the latter virus in vivo; this involved three sequential blood-bone marrow transfusions into naive macaques, followed by virus reisolation (13). SHIV162P4 retains the R5 phenotype of HIV-1SF162 (13).
Neutralization assays.
Neutralization assays were performed using phytohemagglutinin (PHA)-activated rhesus peripheral blood mononuclear cells (PBMC) as target cells. All assays were performed with cells from a single rhesus macaque (no. 355); cells from this animal replicated SHIV162P efficiently. PBMC were isolated on Histopaque-1077 (Sigma, St. Louis, Mo.) and stimulated overnight with 8 μg of PHA (Sigma)/ml and 100 U of interleukin 2 (provided by Maurice Gately via the NIH AIDS Research and Reference Reagent Program)/ml. Antibody was incubated with 100 50% tissue culture infective doses (TCID50s) of SHIV for 1 h at 37°C in a volume of 100 μl in round-bottom microtiter plates. An equal volume of PHA-stimulated rhesus PBMC in medium (RPMI 1640 supplemented with 10% fetal bovine serum [FBS], 100 U of interleukin 2/ml, 2 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml) was then added. The plates were incubated for 3 days at 37°C, washed three times with RPMI 1640, and incubated in culture medium for an additional 4 days. The amount of SHIV present in each well was quantified using a p27 antigen enzyme-linked immunosorbent assay (ELISA) (Retro-tek; Zeptometrix, Buffalo, N.Y.) as recommended by the manufacturer.
Plasma viral loads.
Quantitative assays for the measurement of SIV RNA were performed at Bayer Diagnostics (Berkeley, Calif.) using a branched DNA signal amplification assay for SIV (23). The lower limit of the assay was 400 SHIV RNA copies per ml.
Antibodies.
IgG1 b12 is a human antibody (IgG1, κ) recognizing an epitope overlapping the CD4 binding site of gp120 (3, 4). Recombinant IgG1 was expressed in Chinese hamster ovary (CHO-K1) cells in glutamine-free Glasgow minimum essential medium supplemented with 10% dialyzed fetal bovine serum (Tissue Culture Biologicals, Tulare, Calif.), minimal essential medium nonessential amino acids (Gibco-BRL, Grand Island, N.Y.), 1 mM minimal essential medium sodium pyruvate (Gibco-BRL), 500 μM l-glutamic acid, 500 μM l-asparagine, 30 μM adenosine, 30 μM guanosine, 30 μM cytidine, 30 μM uridine, 10 μM thymidine (Sigma), 100-U/ml penicillin, 100-μg/ml streptomycin, and 50 μM l-methionine sulfoximine (Sigma) in a 3-liter spinner flask, purified using protein A affinity chromatography (Amersham Pharmacia Biotech, Uppsala, Sweden), and then dialyzed against PBS. Care was taken to minimize contamination with endotoxin, which was monitored using a quantitative chromagenic Limulus Amoebecyte Lysate assay (BioWhittaker, Walkersville, Md.) performed according to the manufacturer's recommendations. When detected, endotoxin was removed using polymyxin affinity column chromatography (Bio-Rad, Hercules, Calif.). Antibody used for the passive transfer experiments contained <1 IU of endotoxin/ml. The control IgG was purified from sera from normal human donors, and endotoxin levels were also below 1 IU/ml.
Vaginal antibody measurements.
The accurate determination of the actual concentrations of antibody in mucosal secretions was performed as described by Kozlowski et al. (16). Vaginal secretions were absorbed to cellulose wicks (1 by 15 mm) (Solan Weck-Cel surgical spears; Xomed Surgical Products, Jacksonville, Fla.). Prior to sample collection, each wick was placed in a sterile test tube and weighed. After insertion of a vaginal speculum, wicks were grasped with a forceps and gently placed into the posterior vaginal fornix. After 5 min, wicks were collected, returned to the test tube, weighed again, and frozen at −70°C. The weight of the absorbed vaginal fluid was calculated from the weight increase, assuming that the density of vaginal fluid is similar to that of water. This volume was used to determine the dilution factor for each sample in the following extraction procedure. The vaginal fluid was extracted from the wicks by adding 200 μl of PBS containing 1% fetal bovine serum and a protease inhibitor cocktail [200 μM 4-(2-aminoethyl)-benzenesulfonyl fluoride, 160 μM aprotonin, 10 μM bestatin, 3 μM trans-epoxysuccinyl-l-leucylamido-(4-guanidino) butane, 4 μM leupeptin, 2 μM pepstatin A (Calbiochem, La Jolla, Calif.)]. After incubation on ice for 30 min, the diluted vaginal fluid was collected by spinning it through a 0.45-μm-pore-size filter (Spin-X; Corning, Corning, N.Y.) for 10 min at 14,000 × g at 4°C. The clarified supernatant was then tested for antibody content, using an ELISA. Actual antibody concentrations in vaginal fluids were calculated by multiplying by the dilution factor as determined above. Relatively small amounts of vaginal fluid were collected (typically 50 to 200 μl). The samples were taken at 6 h, 7 days, and 14 days post-b12 infusion.
Antibody assays by ELISA.
We used two different ELISAs to determine b12 concentrations in serum and vaginal fluid. First we used a gp120 ELISA. Recombinant gp120JR-FL (kindly provided by Paul Maddon, Progenics Pharmaceuticals, Tarrytown, N.Y.) was coated to the wells of a microtiter plate (Corning) at a concentration of 2 μg/ml by incubation overnight at 4°C. The plates were washed four times with PBS–0.05% Tween 20 and blocked with 3% bovine serum albumin. Following washing, serial dilutions of serum or vaginal fluid samples were applied to the plate and incubated for 2 h at 37°C. A b12 antibody standard curve was run on each plate. After washing, goat anti-human IgG F(ab′)2 fragments coupled to alkaline phosphatase (Pierce, Rockville, Ill.) were added and incubated for 1 h at 37°C. The plates were washed, and bound conjugate was detected with p-nitrophenyl phosphate substrate (Sigma).
The second assay was an ELISA based on the B2.1 peptide. B2.1 is a homodimer of the peptide HERSYMFSDLENRCI-(biotinylated Orn)-KK (dimer molecular weight, 5,572.2; >95% pure and >90% dimer; synthesized by AnaSpec, San Jose, Calif.). This peptide, isolated by a random peptide library approach, binds the b12 antigen binding site with high specificity as described in detail elsewhere (43). B2.1 was coated to the wells of a microtiter plate at a concentration of 5 μg/ml by incubation overnight at 4°C. After four washes with PBS–0.05% Tween-20, the plates were blocked with 3% bovine serum albumin. Serial dilutions of serum or vaginal fluid samples were then applied to the plate and incubated for 4 h at 4°C. After washing, goat anti-human IgG F(ab′)2 fragments coupled to alkaline phosphatase (Pierce) were added and incubated for 1 h at room temperature before detection of bound antibody as described above.
RESULTS
Infection of rhesus macaques by SHIV162P4 by the vaginal route
SHIV162P4 is an HIV/SIV chimera containing the env, tat, rev, and vpu genes from the prototypical R5 primary isolate HIV-1SF162 in the context of the molecular clone of SIVmac239 (13, 40). Pathogenic variants of SHIV162P were isolated by inoculation of the molecular clone into two rhesus macaques followed by three sequential blood-bone marrow transfusions. The virus used in this study was obtained after the third passage and is designated SHIV162P4. This virus retains the R5 phenotype of the parental HIV-1SF162.
To establish the dose of SHIV162P4 required for infecting female rhesus macaques by the vaginal route, we titrated the virus in a challenge experiment. Three groups of three monkeys were pretreated with medroxyprogesterone acetate and challenged 30 days later with 600, 300, or 100 TCID50s of SHIV162P4. All animals became infected and had detectable plasma viremias two weeks after challenge (Table 1). Moreover, lymph node biopsies from all animals on day 18 tested positive for p27 antigen in a coculture assay (data not shown). All animals experienced high viral loads ranging from 5.1 to 7.1 logs between 2 and 4 weeks postexposure, which in three animals decreased to undetectable levels (<400 RNA copies per ml) at 2 to 3 months postexposure but which were sustained in others. There was no correlation between the challenge dose and the maintenance of an elevated viral load, which appears to be a phenomenon that is inherently variable from animal to animal. Three animals with high viral loads (one from each group) died on days 117, 174, and 439, respectively. Each of these animals had pathological lesions consistent with simian AIDS (Table 1). A fourth animal with an undetectable viral load died after 434 days of an unrelated cause and without pathological abnormalities. In the following experiments we used a TCID50 dose of 300 to ensure that the challenge was robust and that infection was guaranteed in the absence of antibody intervention.
TABLE 1.
Plasma viral loads after vaginal challenge with various doses of SHIV162P4
| Animal | Challenge dosea | Log viral load in plasma on day:
|
Day of death | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 7 | 11 | 14 | 21 | 28 | 60 | 90 | 321 | 376 | |||
| J440 | 600 | <2.6 | 4.0 | 5.3 | 6.6 | 6.2 | 6.0 | 6.5 | Day 174b | ||
| N083 | 600 | <2.6 | 4.8 | 5.9 | 6.3 | 5.7 | 4.1 | 3.0 | <2.6 | <2.6 | Day 434c |
| I806 | 600 | <2.6 | 3.6 | 5.1 | 6.6 | 5.2 | <2.6 | <2.6 | <2.6 | <2.6 | |
| N462 | 300 | <2.6 | 2.8 | 4.7 | 6.4 | 5.7 | Not done | <2.6 | <2.6 | <2.6 | |
| N321 | 300 | <2.6 | 6.2 | 7.1 | 6.9 | 6.4 | 5.5 | 7.2 | Day 117d | ||
| I624 | 300 | <2.6 | 4.2 | 5.6 | 5.7 | 5.3 | <2.6 | <2.6 | <2.6 | <2.6 | |
| M290 | 100 | <2.6 | <2.6 | 3.7 | 5.0 | 6.3 | <2.6 | 6.0 | <2.6 | <2.6 | |
| L492 | 100 | 2.8 | 5.4 | 6.8 | 6.6 | 4.7 | 3.0 | 3.0 | 4.8 | 5.0 | |
| H658 | 100 | <2.6 | 4.6 | 6.3 | 7.6 | 6.4 | <2.6 | 3.5 | 6.4 | 6.4 | Day 439e |
Challenge dose in TCID50s.
Found dead. Autopsy findings: thymic atrophy, amyloidosis, emaciated, pneumonia.
Found dead. Autopsy normal, suspect anesthetic death.
Euthanized because of severe weight loss. Autopsy findings: emaciated, pneumonia, colitis.
Euthanized. Autopsy findings: thymic atrophy, amyloidosis.
Protection against SHIV162P4 vaginal challenge by IgG1 b12.
Previous experiments, discussed above, have generally found that relatively high titers of neutralizing antibody in serum are required to protect animals against HIV-1 challenge. With these data in mind, we designed a study to determine the titers of neutralizing antibody required to protect rhesus macaques from vaginal challenge with an R5 virus.
IgG1 b12 is a broadly neutralizing antibody directed against an epitope overlapping the CD4 binding site on gp120. The primary isolate HIV-1SF162 is neutralized (concentration at which 90% of the organisms are inhibited [IC90]) by b12 at a concentration of 2 μg/ml in PHA-activated human PBMC-based neutralization assays. SHIV162P4, derived from HIV-1SF162, was neutralized by 90% at antibody concentrations of 6 and 2 μg/ml in assays using PHA-activated PBMC from humans and rhesus macaques, respectively. This indicates that cloning of the SF162 envelope into the SIV genetic background and in vivo passage of the virus to create SHIV162P4 did not significantly change the neutralization sensitivity to b12. Based on knowledge of the in vitro neutralization sensitivity of SHIV162P4 to b12 and what was learned from previous passive immunization studies, we hypothesized that complete protection of a macaque would require a concentration of b12 in serum in excess of 200 μg/ml. We therefore administered IgG1 b12 intravenously to groups of four monkeys in doses of 25, 5, and 1 mg/kg. A control group of two monkeys received a 25-mg/kg dose of normal human polyclonal IgG that lacked any antiviral activity in in vitro neutralization assays. The monkeys were challenged 6 h later with 300 TCID50s of SHIV162P4.
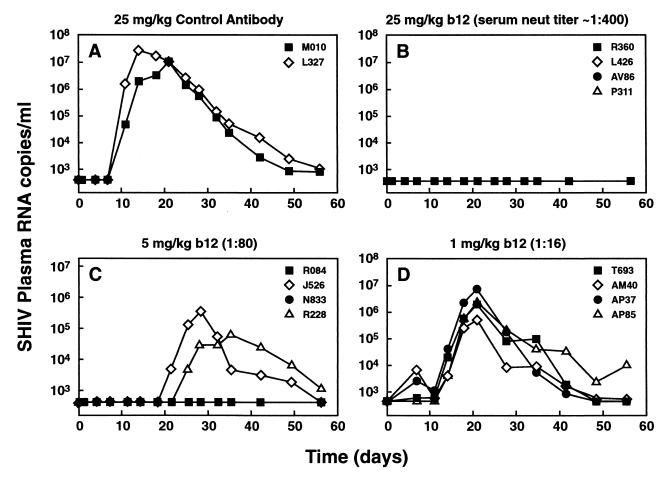
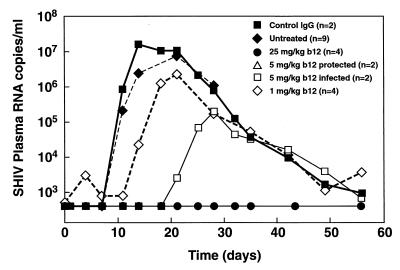
The two monkeys pretreated with the control antibody developed high levels of viremia starting on day 11, peaking at 1 × 107 to 2 × 107 RNA copies per ml of plasma between days 14 and 21 (Fig. 1), a profile very similar to that of the SHIV162P4-challenged monkeys in the virus titration experiment described above (Table 1; Fig. 2). In contrast, SHIV plasma RNA remained undetectable in all four monkeys pretreated with b12 at 25 mg/kg and also in two of the four monkeys pretreated with 5 mg of b12/kg. However, two of the four monkeys in the 5-mg/kg group did become infected, although the viral loads in these animals were strongly reduced compared to those for control animals and the peak viremia was delayed (Fig. 2). The monkeys pretreated with the lowest amount of b12 (1 mg/kg) all became infected with viral loads that were of similar magnitude to those of the control monkeys yet appeared slightly delayed in their onset (Fig. 2).
FIG. 1.
Temporal analysis of SHIV162P4 plasma viremia in macaques pretreated with different doses of antibody b12. Plasma viral loads are shown for animals treated with 25 mg of control antibody/kg (A), 25 mg of b12/kg (B), 5 mg of b12/kg (C), and 1 mg of b12/kg (D) following vaginal challenge with SHIV162P4. Each curve depicts an individual animal. The curves for R360, L426, AV86, and P311 are coincident. The curves for R084 and N833 are coincident. Neut, neutralizing.
FIG. 2.
Comparison of averaged plasma viral loads for macaques in different treatment groups. The mean viral loads were calculated for all the animals in each of the groups described in the legend to Fig. 1. In addition, we calculated the mean viral load of the nine untreated animals from the challenge virus titration experiment. Although the viral challenge dose in this experiment varied, calculating the mean was warranted since the onset as well as the peak viremias fell into a relatively narrow range (Table 1). The 5-mg/kg group was split into two subgroups corresponding to the two completely protected monkeys (curve is coincident with the 25-mg/kg curve) and the two monkeys in which viremia was delayed.
Coculture assays with PHA-activated rhesus PBMC were performed with PBMC from all monkeys. Results of the assays were in agreement with the plasma viral load data described above. All the cocultures with PBMC from the monkeys that developed detectable plasma RNA viral loads were positive for p27 antigen on day 14, except for the culture involving cells from animal R228 from the 5-mg/kg b12 group; this animal was the slowest to develop plasma viremia postinfection. PBMC cocultures for this monkey tested positive at a later time point (32 days postinfection). Cocultures with PBMC from the monkeys with undetectable viral loads in contrast remained negative for p27 antigen in ELISA during a 1-month culture period (data not shown). Finally, we tested the sera from the protected monkeys for anti-p27 antibody in ELISA. All four monkeys from the 25-mg/kg group and the two protected monkeys from the 5-mg/kg group tested negative for p27 antibody on day 42 and day 84 postinfection (data not shown). The absence of detectable SHIV RNA in plasma, p27 antigen in PBMC coculture assays, and anti-p27 in serum indicate a sterile protection in these monkeys.
Antibody and serum neutralization titers for b12-treated monkeys.
To verify the b12 concentrations and neutralization titers achieved at the time of challenge, we determined b12 concentrations by ELISA and performed rhesus PBMC-based neutralization assays with the SHIV162P4 challenge stock (Table 2). ELISAs were performed using recombinant gp120JR-FL and the peptide B2.1. The B2.1 peptide is a mimotope derived from a random-peptide library and is a highly specific probe for IgG1 b12 (43). The IgG1 b12 concentrations determined with both the gp120 and B2.1 ELISAs are in good agreement with each other and the neutralization data. The 90% neutralization titers achieved in the three groups of animals were approximately 1:400, 1:80, and 1:16 for the 25-, 5-, and 1-mg/kg doses, respectively (Table 2). The half-life of IgG1 b12 in plasma was about 1 week (data not shown).
TABLE 2.
Antibody concentration and neutralization titers achieved in plasma and vaginal fluidsa
| Antibody | Dose (mg/kg) | Animal ID | b12 concn in plasma at time of challenge (μg/ml)
|
Neutralization titer in plasma at time of challenge (IC90)b | b12 concn in vaginal fluid at time of challenge (μg/ml)
|
||
|---|---|---|---|---|---|---|---|
| gp120 ELISA | B2.1 ELISA | gp120 ELISA | B2.1 ELISA | ||||
| b12 | 25 | R360 | 710 | 840 | 1:400 | 30 | 13.5 |
| L426 | 725 | 600 | 1:400 | 20 | 25 | ||
| AV86 | 695 | 910 | 1:400 | 18.6 | 27.9 | ||
| P311 | 690 | 940 | 1:400 | 17.4 | 21.8 | ||
| b12 | 5 | R084 | 180 | 210 | 1:40 | 1.0 | 1.5 |
| J526 | 175 | 230 | 1:80 | 3.1 | 2.5 | ||
| N833 | 170 | 360 | 1:80 | 3.2 | 7.8 | ||
| R228 | 170 | 290 | 1:80 | 22.2 | 37 | ||
| b12 | 1 | T693 | 12 | 12 | 1:16 | ND | ND |
| AM40 | 20 | 20 | 1:16 | ND | ND | ||
| AP37 | 15 | 15 | 1:16 | ND | ND | ||
| AP85 | 15 | 18 | 1:32 | ND | ND | ||
| Control IgG | 25 | M010 | 0 | 0 | <1:8 | 0 | 0 |
| L327 | 0 | 0 | ≤1:8 | 0 | 0 | ||
Neutralization of SHIV162P4 by b12 (IC90) was achieved at a b12 concentration of 2 μg/ml. ND, not done.
All neutralization assays were performed with SHIV162P4 challenge virus in PHA-activated rhesus PBMC and determination of p27 antigen by ELISA.
Concentrations of antibody in vaginal fluid.
Concentration of IgG1 b12 in vaginal fluid were determined by gp120 and B2.1 ELISAs as shown in Table 2. The values shown are actual IgG1 b12 concentrations as we corrected for the dilution factor during sample preparation. The concentrations of IgG1 b12 in the vaginal fluids at the time of challenge of the animals treated with the two highest doses of b12 are 25- to 100-fold lower than the concentrations achieved in the plasma and range from 1 to 10 times the b12 IC90 against the challenge virus. Notably, a neutralization titer of about 1:10 in vaginal fluid in combination with a plasma neutralization titer of 1:80 may be insufficient for providing sterile immunity as shown by the infection of animal R228.
It is of interest that b12 concentrations in the vaginal fluid did not peak immediately postinfusion, as approximately 2- and 1.5-fold higher (mean) b12 concentrations were detected 7 and 14 days later. In contrast, b12 plasma concentrations, as expected, peaked immediately after (intravenous) administration (not shown). If vaginal antibody levels contribute to protection, then our studies may therefore slightly underestimate the protective effect corresponding to a given titer of neutralizing antibody in serum.
Absence of neutralization escape in the infected animals in the 5-mg/kg b12 treatment group.
Among the monkeys treated with 5 mg of b12/kg, two were completely protected whereas two others became infected with a strongly delayed and reduced plasma viremia. It is formally possible that these breakthrough infections occurred through the presence of b12 neutralization-resistant SHIV variants in the challenge stock or the generation of neutralization escape variants in vivo. Accordingly, virus was rescued and expanded by PBMC coculture from one infected monkey treated with 5 mg of b12/kg (R228) and one infected monkey from the control antibody group (M010). The PBMC used were isolated from blood drawn at the peak of viremia, i.e., day 14 for M010 and day 32 for R228. Neutralization assays demonstrated that sensitivities of both the M010- and the R228-rescued SHIV to neutralization by b12 remained equal to that of the challenge virus SHIV162P4 (data not shown).
DISCUSSION
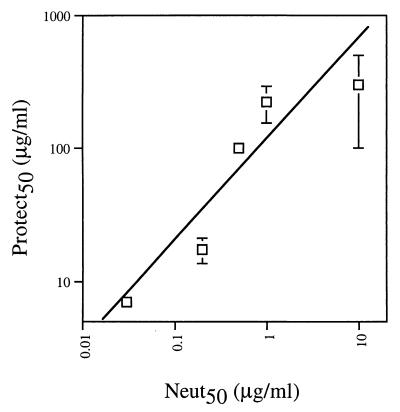
The principal goal of this study was to evaluate the ability of neutralizing antibody to protect against mucosal challenge by an R5 HIV-1. Using vaginal challenge in an SHIV/macaque model, the results strongly suggest that neutralizing antibody titers in the serum correlate with protection against HIV-1 infection via mucosal exposure. At serum neutralizing (90%) antibody titers on the order of 1:400, the human monoclonal antibody b12 provided sterile protection against vaginal challenge with the R5 chimeric virus SHIV162P4. At titers of 1:80, 50% of the animals were protected, whereas at titers of 1:16, all the animals became infected. Thus, sterile protection corresponds to antibody concentrations in serum that neutralize essentially all the virus in corresponding in vitro neutralization assays. This observation, initially made with the hu-PBL-SCID mouse model (10, 30), has now been made for b12 protection against HIV-1 in a number of systems, including different animal models, challenge viruses (including both primary and laboratory-adapted viruses), and routes of challenge. In Fig. 3, the relationship between b12 neutralization in vitro (antibody concentration at half-maximal neutralization) and protection in vivo (antibody serum concentration at half-maximal protection) is plotted. The linearity of the curve, with a slope of approximately 200, illustrates the predictive value of the neutralization titer for protection. The relatively high value of the slope indicates the stringent requirement for protection, i.e., antibody concentrations required for protection are 2 orders of magnitude greater than those required for neutralization. The graph therefore emphasizes a strong correlation between neutralization and protection independent of the animal model, challenge route, or HIV-1 isolate used.
FIG. 3.
The concentration of b12 resulting in half-maximal protection (Protect50) in animals as a function of the concentration resulting in half-maximal neutralization (Neut50) in vitro. The concentrations of b12 required to neutralize 50% of the challenge virus in vitro and completely protect 50% of animals against infection with the same virus were derived from this study and previous studies using hu-PBL-SCID mice (10, 29). The data points shown (left to right) correspond to HIV-1LAI (hu-PBL-SCID), HIV-1SF2 (hu-PBL-SCID), HIV-1JR-CSF (hu-PBL-SCID), SHIV162P4 (macaques), and HIV-1AD6 (hu-PBL-SCID). For experiments in which half-maximal protection was not determined exactly, an error bar is shown with a range indicating the lower and upper limits of the antibody concentration required.
Passive transfer studies using other neutralizing anti-HIV antibodies have generally conformed to our observations with b12 (1, 24, 26, 38). Mascola et al. (26), however, did report that the antibody 2G12 and combinations of this antibody with the antibody 2F5 and polyclonal immune IgG (HIVIG) were somewhat more effective in sterile protection against mucosal challenge with the chimeric virus SHIV89.6PD than against intravenous challenge. In particular, those studies showed a surprising efficacy of the antibody 2G12 in mucosal protection. The reasons for this efficacy are unclear, but understanding it is clearly important.
Generally, examination of the literature on passive transfer to naive animals for a wide variety of different viruses and model systems shows that titers of neutralizing antibody in serum around or greater than 1:100 are required for sterile protection (28). For instance, serum neutralizing antibody titers of 1:380 are required to sterilely protect cotton rats against respiratory syncytial virus challenge (33). Therefore, HIV-1 does not seem to be unusual in this regard, and indeed one could anticipate that in naive animals in the absence of other protective immunity, such high neutralizing antibody titers would be required. One distinguishing feature of HIV-1 is that protective neutralizing antibody titers using monoclonal antibodies correspond to relatively high antibody concentrations. This is because the anti-HIV monoclonal antibodies available are relatively modest in their neutralizing potency compared to those against some other viruses. Primary isolate neutralizing antibodies, such as b12, 2G12, and 2F5, typically show 90% neutralization at concentrations of 1 to 10 μg/ml, so that complete neutralization, which may require 100 times 90% neutralization values, corresponds to 100 μg/ml to 1 mg/ml. In contrast, neutralizing monoclonal antibodies against other viruses can show 90% neutralization at 0.1 μg/ml, corresponding to only about 10 μg/ml for complete protection (28). Since neutralization is correlated with affinity for the HIV-1 envelope trimer, at least for T-cell-line-adapted viruses (31, 34, 36), the modest neutralizing activity of anti-HIV-1 antibodies likely reflects a modest affinity for the trimer. In turn this may reflect the fact that the primary isolate-neutralizing anti-HIV-1 antibodies are cross-reactive and able to recognize a variety of closely related molecular shapes (i.e., envelope epitopes from a range of HIV-1 isolates) rather than a single uniquely defined molecular shape.
The vaginal model employed in the earlier studies of Mascola et al. (26) was very similar to the one employed here. A caveat of the model may be that a progesterone analog was used to thin the vaginal epithelium. HIV-1 and SIV infection can occur by direct transmission through the vaginal mucosa (14, 23, 27). However, the intact vaginal mucosa represents a formidable barrier against infection, since 100- to 1,000-fold-greater doses of SIVmac251 are required to establish infection compared to intravenous injection of virus (27, 39). The incidence of vaginal transmission of SIV can be enhanced by progesterone treatment, which produces a thinning of the vaginal epithelium (23). The increased infection rates after progesterone treatment then allow challenge of animals with a viral inoculum which is comparable to the dose of virus likely to be present in seminal fluid of HIV-1-infected individuals (10 to 1,000 TCID50 per ejaculate) (6, 42). However, the vaginal thinning may create conditions that are significantly different from those present during human transmission. For example, it has been pointed out that thinning may allow greater transudation of IgG into the vaginal fluid than normal (35) and that the hormonal treatment itself may influence the amount of antibody in the vaginal secretion (17–19, 21, 37). If protection were mediated by vaginal IgG this could lead to an overestimation of the efficacy of antibody under normal conditions. However, the concordance of the results reported here with those of intravenous challenge experiments using HIV-1 and of studies with other viruses suggests that such an overestimation is probably not occurring.
The early events involved in transmission of HIV-1 across mucosal surfaces are still a subject of great uncertainty. Dendritic cells may be among the first cells infected after virus penetration of the mucosal epithelium (14, 25). Interestingly, it has been shown previously that neutralizing antibodies can block the entry of HIV-1 into dendritic cells and, in addition, effectively interrupt the transmission of HIV-1 from dendritic cells to T cells (9). This may be crucial, although again we note that the data are consistent with protection occurring by neutralization of free virus by systemic antibody.
The major focus of our study was the ability of antibody to provide sterile protection from viral challenge. However, antibody may provide benefits at doses below those required for sterile protection. In the study of Mascola et al. (26) on SHIV89.6PD vaginal challenge, such doses produced lower viremias and a lesser depletion of CD4 cells. In this study, we also noted that an intermediate dose produced a lower and delayed viremia. At the lowest dose, however, which nevertheless produced a neutralizing titer in serum of about 1:16, viremia was approximately equal in magnitude and only slightly delayed relative to results with control animals. We have not investigated the longer-term effects of antibody on the potential disease course in this study.
In SHIV89.6PD infection, CD4 depletion is extremely rapid and pronounced in most animals; in some respects the infection is more aptly described as acute than persistent. In SHIV162P4 infection, on the other hand, CD4 depletion is observed most prominently in intestinal lymphocytes and is not well observed in circulating T cells (13). Disease can take a long time to develop, as illustrated in the cohort of animals used in the virus titration arm of this study. It will be interesting to see if any of the animals that were treated with 5 mg of b12/kg but became infected subsequently develop disease.
Finally, in a vaccinated individual, the immune system can call upon specific T and B cells as well as preexisting antibody to combat a pathogen. With a murine retrovirus infection, it has been shown that cellular and humoral responses in combination are required for viral clearance (7). Passive transfer studies have shown that antibody alone can protect against HIV-1/SHIV challenge but at levels that are probably not attainable by vaccination. A major question now, in our opinion, is whether antibody at lower levels can act in concert with cellular responses to prevent HIV-1 infection.
ACKNOWLEDGMENTS
We are very grateful to James Bradac and Alan Schultz for making possible access to the nonhuman primates used in this study. We thank Alexandra Trkola for her contributions to this work. We thank Howard Fox for providing blood from rhesus macaques and the TSRI General Clinical Research Center for providing human blood (grant number M01-RR00833). We are grateful to David Montefiori for helpful suggestions regarding the primary isolate neutralization assays in rhesus PBMC. We thank Meng Wang for performing neutralization assays and Paul Carney for assistance with antibody preparation. We gratefully acknowledge James Blanchard for facilitating the rhesus macaque experiments at the Tulane Regional Primate Research Center (contract number NO1-AI65300).
J.P.M. is an Elizabeth Glaser Scientist of the Pediatric AIDS Foundation and a Stavros S. Niarchos Scholar. The Department of Microbiology and Immunology at the Weill Medical College gratefully acknowledges the support of the William Randolph Hearst Foundation. This work was supported by NIH grants AI40377 (to P.W.H.I.P.), AI 41952 (to P.A.M.), HL59735 and AI36082 (to J.P.M.), and AI33292 and HL59727 (to D.R.B.).
REFERENCES
- 1.Baba T W, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini L A, Posner M R, Katinger H, Stiegler G, Bernacky B J, Rizvi T A, Schmidt R, Hill L R, Keeling M E, Lu Y, Wright J E, Chou T C, Ruprecht R M. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 2.Barouch D H, Santra S, Schmitz J E, Kuroda M J, Fu T M, Wagner W, Bilska M, Craiu A, Zheng X X, Krivulka G R, Beaudry K, Lifton M A, Nickerson C E, Trigona W L, Punt K, Freed D C, Guan L, Dubey S, Casimiro D, Simon A, Davies M E, Chastain M, Strom T B, Gelman R S, Montefiori D C, Lewis M G, Emini E A, Shiver J W, Letvin N L. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science. 2000;290:486–492. doi: 10.1126/science.290.5491.486. [DOI] [PubMed] [Google Scholar]
- 3.Burton D R, Barbas III C F, Persson M A A, Koenig S, Chanock R M, Lerner R A. A large array of human monoclonal antibodies to type 1 human immunodeficiency virus from combinatorial libraries of asymptomatic seropositive individuals. Proc Natl Acad Sci USA. 1991;88:10134–10137. doi: 10.1073/pnas.88.22.10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton D R, Pyati J, Koduri R, Sharp S J, Thornton G B, Parren P W H I, Sawyer L S W, Hendry R M, Dunlop N, Nara P L, Lamacchia M, Garratty E, Stiehm E R, Bryson Y J, Cao Y, Moore J P, Ho D D, Barbas C F. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science. 1994;266:1024–1027. doi: 10.1126/science.7973652. [DOI] [PubMed] [Google Scholar]
- 5.Cho M W, Lee M K, Chen C H, Matthews T, Martin M A. Identification of gp120 regions targeted by a highly potent neutralizing antiserum elicited in a chimpanzee inoculated with a primary human immunodeficiency virus type 1 isolate. J Virol. 2000;74:9749–9754. doi: 10.1128/jvi.74.20.9749-9754.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coombs R W, Speck C E, Hughes J P, Lee W, Sampolea R, Ross S O, Dragavon J, Peterson G, Hooton T M, Collier A C, Corey L, Koutsly L, Krieger J N. Association between culturable human immunodeficiency virus type I (HIV-1) in semen and HIV-1 RNA levels in semen and blood: evidence for compartmentalization of HIV-1 between semen and blood. J Infect Dis. 1998;177:320–330. doi: 10.1086/514213. [DOI] [PubMed] [Google Scholar]
- 7.Dittmer U, Brooks D M, Hasenkrug K J. Requirement for multiple lymphocyte subsets in protection by a live-attenuated vaccine against retroviral infection. Nat Med. 1999;5:189–193. doi: 10.1038/5550. [DOI] [PubMed] [Google Scholar]
- 8.Dittmer U, Race B, Hasenkrug K J. Kinetics of the development of protective immunity in mice vaccinated with a live attenuated retrovirus. J Virol. 1999;73:8435–8440. doi: 10.1128/jvi.73.10.8435-8440.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frankel S S, Steinman R M, Michael N L, Kim S R, Bhardwaj N, Pope M, Louder M K, Ehrenberg P K, Parren P W H I, Burton D R, Katinger H, VanCott T C, Robb M L, Birx D L, Mascola J R. Neutralizing monoclonal antibodies block human immunodeficiency virus type 1 infection of dendritic cells and transmission to T cells. J Virol. 1998;72:9788–9794. doi: 10.1128/jvi.72.12.9788-9794.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gauduin M C, Parren P W H I, Weir R, Barbas III C F, Burton D R, Koup R A. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat Med. 1997;3:1389–1393. doi: 10.1038/nm1297-1389. [DOI] [PubMed] [Google Scholar]
- 11.Gauduin M C, Safrit J T, Weir R, Fung M S, Koup R A. Pre- and post-exposure protection against human immunodeficiency virus type 1 infection mediated by a monoclonal antibody. J Infect Dis. 1995;171:1203–1209. doi: 10.1093/infdis/171.5.1203. [DOI] [PubMed] [Google Scholar]
- 12.Glushakova S, Yi Y, Ggrivel J C, Singh A, Schols D, De Clercq E, Collman R G, Margolis L. Preferential coreceptor utilization and cytopathicity by dual-tropic HIV-1 in human lymphoid tissue ex vivo. J Clin Investig. 1999;104:R7–R11. doi: 10.1172/JCI7403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harouse J M, Gettie A, Tan R C, Blanchard J, Cheng-Mayer C. Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science. 1999;284:816–819. doi: 10.1126/science.284.5415.816. [DOI] [PubMed] [Google Scholar]
- 14.Hu J, Gardner M B, Miller C J. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74:6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Y, Paxton W, Wolinsky S, Neumann A, Zhang L, He T, Kang S, Ceradini D, Jin Z, Yazdandakhsh K, Kunstman K, Erickson D, Dragon E, Landau N, Phair J, Ho D, Koup R. The role of a mutant CCR5 allele in HIV-1 transmission and disease progression. Nat Med. 1996;2:1240–1243. doi: 10.1038/nm1196-1240. [DOI] [PubMed] [Google Scholar]
- 16.Kozlowski P A, Cu-Uvin S, Neutra M R, Flanigan T P. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in the rectal and genital secretions of women. Infect Immun. 1997;65:1387–1394. doi: 10.1128/iai.65.4.1387-1394.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kutteh W H, Mestecky J. Secretory immunity in the female reproductive tract. Am J Reprod Immunol. 1994;31:40–46. doi: 10.1111/j.1600-0897.1994.tb00845.x. [DOI] [PubMed] [Google Scholar]
- 18.Kutteh W H, Moldoveanu Z, Mestecky J. Mucosal immunity in the female reproductive tract: correlation of immunoglobulins, cytokines, and reproductive hormones in human cervical mucus around the time of ovulation. AIDS Res Hum Retrovir. 1998;14(Suppl. 1):S51–S55. [PubMed] [Google Scholar]
- 19.Kutteh W H, Prince S J, Hammond K R, Kutteh C C, Mestecky J. Variations in immunoglobulins and IgA subclasses of human uterine cervical secretions around the time of ovulation. Clin Exp Immunol. 1996;104:538–542. doi: 10.1046/j.1365-2249.1996.36742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu R, Paxton W, Choe S, Ceradini D, Martin S, Koruk R, MacDonald M, Stuhlmann H, Koup R, Landau N. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 21.Lü F X, Ma Z, Rourke T, Srinivasan S, McChesney M, Miller C J. Immunoglobulin concentrations and antigen-specific antibody levels in cervicovaginal lavages of rhesus macaques are influenced by the stage of the menstrual cycle. Infect Immun. 1999;67:6321–6328. doi: 10.1128/iai.67.12.6321-6328.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luciw P A, Pratt-Lowe E, Shaw K E, Levy J A, Cheng-Mayer C. Persistent infection of rhesus macaques with T-cell-line-tropic and macrophage-tropic clones of simian/human immunodeficiency virus (SHIV) Proc Natl Acad Sci USA. 1995;92:7490–7494. doi: 10.1073/pnas.92.16.7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marx P A, Spira A I, Gettie A, Dailey P J, Veazey R S, Lackner A A, Mahoney C J, Miller C J, Claypool L E, Ho D D, Alexander N J. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2:1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 24.Mascola J R, Lewis M G, Stiegler G, Harris D, VanCott T C, Hayes D, Louder M K, Brown C, Sapan C V, Frankel S S, Lu Y, Robb M L, Katinger H, Birx D L. Protection of macaques against pathogenic SHIV-89.6PD by passive transfer of neutralizing antibodies. J Virol. 1999;73:4009–4018. doi: 10.1128/jvi.73.5.4009-4018.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mascola J R, Schlesinger Frankel S, Broliden K. HIV-1 entry at the mucosal surface: role of antibodies in protection. AIDS. 2000;14(Suppl. 3):S167–S174. [PubMed] [Google Scholar]
- 26.Mascola J R, Stiegler G, VanCott T C, Katinger H, Carpenter C B, Hanson C E, Beary H, Hayes D, Frankel S S, Birx D L, Lewis M G. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 27.Miller C J, Marthas M, Torten J, Alexander N J, Moore J P, Doncel G F, Hendrickx A G. Intravaginal inoculation of rhesus macaques with cell-free simian immunodeficiency virus results in persistent or transient viremia. J Virol. 1994;68:6391–6400. doi: 10.1128/jvi.68.10.6391-6400.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parren P W H I, Burton D R. The anti-viral activity of antibodies in vitro and in vivo. Adv Immunol. 2001;77:195–262. doi: 10.1016/S0065-2776(01)77018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parren P W H I, Ditzel H J, Gulizia R J, Binley J M, Barbas III C F, Burton D R, Mosier D E. Protection against HIV-1 infection in hu-PBL-SCID mice by passive immunization with a neutralizing human monoclonal antibody against the gp120 CD4-binding site. AIDS. 1995;9:F1–F6. doi: 10.1097/00002030-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Parren P W H I, Gauduin M C, Koup R A, Poignard P, Sattentau Q J, Fisicaro P, Burton D R. Relevance of the antibody response against human immunodeficiency virus type 1 envelope to vaccine design. Immunol Lett. 1997;58:125–132. doi: 10.1016/s0165-2478(97)00109-0. [DOI] [PubMed] [Google Scholar]
- 31.Parren P W H I, Mondor I, Naniche D, Ditzel H J, Klasse P J, Burton D R, Sattentau Q J. Neutralization of HIV-1 by antibody to gp120 is determined primarily by occupancy of sites on the virion irrespective of epitope specificity. J Virol. 1998;72:3512–3519. doi: 10.1128/jvi.72.5.3512-3519.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parren P W H I, Wang M, Trkola A, Binley J M, Purtscher M, Katinger H, Moore J P, Burton D R. Antibody neutralization-resistant primary isolates of human immunodeficiency virus type 1. J Virol. 1998;72:10270–10274. doi: 10.1128/jvi.72.12.10270-10274.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prince G A, Horswood R L, Chanock R M. Quantitative aspects of passive immunity to respiratory syncytial virus infection in infant cotton rats. J Virol. 1985;55:517–520. doi: 10.1128/jvi.55.3.517-520.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roben P, Moore J P, Thali M, Sodroski J, Barbas III C F, Burton D R. Recognition properties of a panel of human recombinant Fab fragments to the CD4 binding site of gp120 that show differing abilities to neutralize human immunodeficiency virus type 1. J Virol. 1994;68:4821–4828. doi: 10.1128/jvi.68.8.4821-4828.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robert-Guroff M. IgG surfaces as an important component in mucosal protection. Nat Med. 2000;6:129–130. doi: 10.1038/72206. [DOI] [PubMed] [Google Scholar]
- 36.Sattentau Q J, Moore J P. Human immunodeficiency virus type 1 neutralization is determined by epitope exposure on the gp120 oligomer. J Exp Med. 1995;182:185–196. doi: 10.1084/jem.182.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schumacher G F B, Yang S L. Cyclic changes of immunoglobulins and specific antibodies in human and rhesus monkey cervical mucus. In: Insler V, Bettendorf G, editors. The uterine cervix in reproduction. Stuttgart, Germany: Georg Thieme Verlag; 1977. pp. 187–203. [Google Scholar]
- 38.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho M W, Martin M A. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 39.Sodora D L, Gettie A, Miller C J, Marx P A. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res Hum Retrovir. 1998;14(Suppl. 1):S119–S123. [PubMed] [Google Scholar]
- 40.Tan R C, Harouse J M, Gettie A, Cheng-Meyer C. In vivo adaptation of SHIV(SF162): chimeric virus expressing a NSI, CCR5-specific envelope protein. J Med Primatol. 1999;28:164–168. doi: 10.1111/j.1600-0684.1999.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 41.Trkola A, Pomales A P, Yuan H, Korber B, Maddon P J, Allaway G, Katinger H, Barbas III C F, Burton D R, Ho D D, Moore J P. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vernazza P L, Gilliam B L, Dyer J, Fiscus S A, Eron J J, Frank A C, Cohen M S. Quantification of HIV in semen: correlation with antiviral treatment and immune status. AIDS. 1997;11:987–993. [PubMed] [Google Scholar]
- 43.Zwick M B, Bonnycastle L L C, Menendez A, Irving M B, Barbas III C F, Parren P W H I, Burton D R, Scott J K. Identification and characterization of a peptide that specifically binds the human, broadly neutralizing anti-human immunodeficiency virus type 1 antibody b12. J Virol. 2001;75:6692–6699. doi: 10.1128/JVI.75.14.6692-6699.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]