Abstract
Alzheimer’s disease (AD) disproportionately affects women, yet most preclinical research studies are male-centric. We performed lifespan analyses of male and female AD mouse models (APP/PS1 and APPNL-F/NL-F) and their shared genetic background control (C57BL/6). Survival curves support significant sex differences between within genotypes. Minimal longevity revealed increased age in male APP/PS1, and decreased age in APPNL-F/NL-F mice. Maximal longevity revealed an increased average age in males. Furthermore, median lifespan differed between sex and genotype. This study supports sexual dimorphic survival in two mouse models of AD, emphasizing the need to examine mechanisms and treatments in both sexes.
Keywords: Kaplan-Meier estimate, survival curve, amyloid-beta
INTRODUCTION
Alzheimer’s disease (AD) leads to progressive loss of memory and cognition, with aging considered the predominant risk factor. Although women have a longer lifespan, their risk of developing AD and the incidence of the disease is higher compared to age-matched men [1]. Women account for two thirds of AD cases in the United States, experience greater cognitive deterioration, and have broader dementia-related behavioral symptoms [2]. Despite this, the majority of preclinical AD research has historically been conducted in male mice. While recent research has examined sex-based mechanistic differences, survival rates have not been determined [3]. To address potential sex-based differences in healthspan in AD, we examined survival in two AD mouse models (APP/PS1 and APPNL-F/NL-F) and their shared genetic background control (C57BL/6). Our laboratory has shown sex differences in cognition and metabolism [4–12]; however, little is known about their biological aging differences that could be a contributing factor when making chronological aging comparisons.
Double transgenic APP/PS1 mice overexpress amyloid precursor protein (APP) and presenilin 1 (PS1) genes. Disease onset and severity is accelerated compared to some knock-in mouse models [13]. APP/PS1 mice express elevated soluble amyloid-β (Aβ)42 levels by 2-4 months of age that progress into plaque pathology [14, 15]. Changes in neurotransmission have been reported as early as 2-4 months, while cognitive decline and a visible accumulation of insoluble Aβ42 are observed at approximately 6-8 months of age in both male and female mice, with significant cognitive impairment by 10-12 months [14, 16]. APP/PS1 mouse models have also shown sex-related differences with earlier pathology and increased β-secretase processing observed in females [6, 7, 17].
APPNL-F/NL-F knock-in mice are a model of AD that express a humanized APP with both the Swedish and Iberian mutations. Differing from the APP/PS1 model, APPNL-F/NL-F mice express APP at wild-type levels to avoid an overexpression of APP [13] and are lacking PS mutations [18]. APPNL-F/NL-F mice have increased total Aβ42 production and eventual oligomerization and plaque formation characteristic of AD pathology [7]. Plaque deposition occurs in the APPNL-F/NL-F strain for both sexes as early as 6 months with behavioral changes occurring by 18 months of age [7, 18]. Sex differences have also been observed in APPNL-F/NL-F, but few studies have reported on these changes [5, 7, 18, 19]. Interestingly, certain behavioral aspects including reversal learning and attention deficits, in female APPNL-F/NL-F mice have no significant difference compared to males [18]. Male APPNL-F/NL-F have shown impaired spatial long-term memory during Morris Water Maze (MWM) tests, but do not show loss in recognition memory [5]. Another study shows 12-month-old APPNL-F/NL-F males performed significantly worse on the MWM task compared to age- and genotype- matched females [7].
Despite known sex differences in the human population with AD and recent insight into sexual dimorphism in mouse models of AD, little is known about the lifespan of the various mouse models of AD. In this study, we assessed sex differences in survival and longevity in APP/PS1 and APPNL-F/NL-F mice to underscore the disparate healthspan in relation to known amyloid pathology. The information presented in this manuscript will aid with future experimental design and provide a clearer understanding of lifespan and biological aging across the sexes of these two AD models.
MATERIALS AND METHODS
ANIMALS
Protocols for animal use were approved by the Institutional Animal Care and Use Committee at Southern Illinois University School of Medicine (Protocol #2022-055). Parental strains of C57BL/6 (RRID:IMSR JAX:000,664) and APP/PS1 (RRID:MMRRC_034832-JAX) originated from The Jackson Laboratory (Bar Harbor, ME), while APPNL-F/NL-F (RRID:IMSR_RBRC06343) founder breeders were obtained from Riken (Japan). Pregnant dams were housed individually with nesting material until after weaning pups. Post-weaning, mice were group housed based on sex and genotype on a 12:12 hr light:dark cycle, and food (LabDiet PMI Feeds Chow 5001) and water were available ad libitum. Genotypes were confirmed by tail tip DNA testing (TransnetYX, Inc; Cordova, TN). All mice were monitored from date of birth until date of natural death or at veterinarian’s request for moribund/health issues using TransnetYX Colony management software (Breeding Management Software; V1.0.205). Age was recorded by number of days alive. Mice with the following criteria were excluded: non-genotype strains, inconclusive or unknown genotype, experimental or breeding mice, unknown sex, unknown breeding origin, and those euthanized for reasons other than moribund issues at the request of the veterinarian.
DATA ANALYSIS
We incorporated a retrospective study design whereby data was retrieved from colony maintenance software (TransnetYX Colony). Kaplan-Meier survival distributions of percent survival probability with 95% confidence intervals were constructed based on number of days alive utilizing log-rank (Mantel-Cox) to assess significant differences between genotypes and sexes. Minimal and maximal longevity were calculated by averaging age across the youngest and oldest 20% survived, respectively. Averages of minimal and maximal longevity were compared using independent t-tests to assess significant age differences between genotypes and using one-way ANOVA (Tukey’s multiple comparisons) to analyze significant age differences between sexes. Median and interquartile range of survival times show survival days to 75, 50, and 25% of mice remaining for each respective genotype.
Significance for all tests were determined at p < 0.05. All data are presented as mean ± SEM and statistical analyses and figure construction were performed with GraphPad Prism (GraphPad Prism 9 Software, Inc., La Jolla, CA; RRID:SCR_002798).
RESULTS
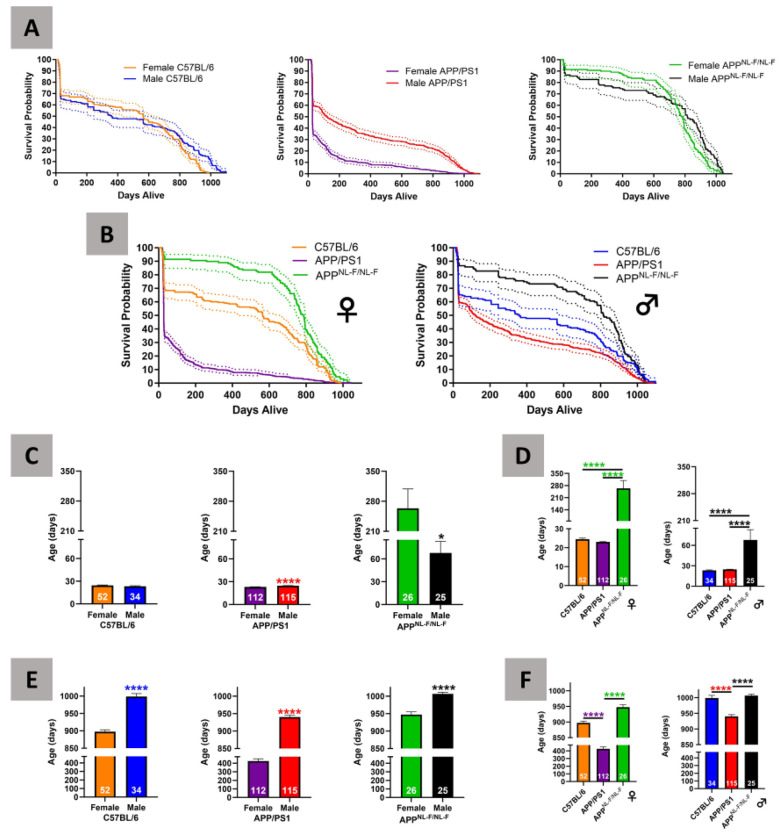
Survival analyses indicate significantly shorter lifespan for females compared to males for the C57BL/6 and APP/PS1 genotypes (C57BL/6: χ2 = 9.38, df = 1, p < 0.0001; APP/PS1: χ2 = 161.1, df = 1, p < 0.0001; Fig. 1A). APPNL-F/NL-F males and females have similar rates of survival (APPNL-F/NL-F: χ2 = 8.69, df = 1, p < 0.0001; Fig. 1A). Additionally, female APP/PS1 mice had the shortest survival rate compared to C57BL/6 and APPNL-F/NL-F mice, whereas female APPNL-F/NL-F mice had the longest survival rate (χ2 = 322.6, df = 2, p < 0.0001). C57BL/6 mice had the longest survival rate for males between the genotypes, whereas APPNL-F/NL-F mice had the shortest survival rate (χ2 = 43.66, df = 2, p < 0.0001) (Fig. 1B).
Figure 1 –
Survival and longevity analysis of C57BL/6, APP/PS1, and APPNL-F/NL-F mice
Kaplan Meier curve is shown as a solid line, 95% confidence limits are expressed as dotted lines. Survival curves comparing between sex (Fig. 1A) and between genotypes (Fig. 1B). Minimal longevity between sex (Fig. 1C) and genotype (Fig. 1D) comparisons for the shortest surviving 20%. Maximal longevity between sex (Fig. 1E) and genotype (Fig. 1F) comparisons for the oldest surviving 20%.
Female (n = 52, mean: 24.50 ± 0.55) and male (n = 34, mean: 23.26 ± 0.74) C57BL/6 mice had comparable minimal longevity (C57BL/6 t = 1.4, df = 84, p = 0.18; Fig. 1C). Male APP/PS1 mice (n = 115, mean: 24.67 ± 0.19) had significantly longer minimal longevity than genotype-matched females (n = 112, mean: 23.06 ± 0.19; APP/PS1 t = 5.9, df = 225, p < 0.0001; Fig. 1C). Female APPNL-F/NL-F mice (n = 26, mean: 263.70 ± 45.06) had significantly longer minimal longevity than males (n = 25, mean: 67.84 ± 15.06) of the same genotype (APPNL-F/NL-F t = 4.1, df = 49, p = 0.0002; Fig. 1C). Female and male APPNL-F/NL-F mice had the longest minimal longevity compared to sex-matched C57BL/6 or APP/PS1 mice [Females: F(2,187) = 91.6, p < 0.0001; Males: F(2,171) = 25.3, p < 0.0001; Fig. 1D], with no significant difference between same sex C57BL/6 and APP/PS1 mice.
Sex differences consisted of significantly shorter maximal longevity in female mice than male mice for all genotypes (C57BL/6: n = 52 and 34, mean: 897.4 ± 5.67 and 998.6 ± 8.55 respectively, t = 10.3, df = 84, p < 0.0001; APP/PS1: n = 112 and 115, mean: 425.50 ± 25.82 and 940.30 ± 5.53 respectively, t = 19.7, df = 225, p < 0.0001; APPNL-F/NL-F: n = 26 and 25, mean: 947.30 ± 7.97 and 1006 ± 4.98 respectively, t = 6.2, df = 49, p < 0.0001; Fig. 1E). Female and male APP/PS1 mice demonstrated the shortest maximal longevity among the three genotypes [Males: F(2, 171) = 25.6, p < 0.0001; Females: F(2,187) = 122.4, p < 0.0001; Fig. 1F]. There was no significant difference between either male or female C57BL/6 and APPNL-F/NL-F for maximal longevity (Fig. 1F).
C57BL/6 female mice show a higher median survival time at 564 days compared to males at 359 days (Table 1). Sex-related differences were dichotomous for APP/PS1 mice as males demonstrate a median survival time of 120 days compared to females with 28 days (Table 1). APPNL-F/NL-F male mice have a higher median survival time of 816 days compared to females at 782 days (Table 1).
Table 1 –
Median and interquartile range of survival times
| N | Female | Male | ||||
|---|---|---|---|---|---|---|
| C57BL/6 | APP/PS1 | APPNL-F/NL-F | C57BL/6 | APP/PS1 | APPNL-F/NL-F | |
| 257 | 562 | 128 | 172 | 573 | 126 | |
| Survival Days to: | ||||||
| 75% Remaining | 28 | 25 | 655 | 28 | 27 | 339 |
| 50% Remaining | 564 | 28 | 782 | 359 | 120 | 816 |
| 25% Remaining | 805 | 104 | 859 | 850 | 694 | 927 |
DISCUSSION
This study highlights sex differences in survival in mouse models of AD, which also supports differences in healthspan. Female APP/PS1 mice exhibited shorter lifespan than genotype-matched males in this study. This may be due to APP/PS1 female mice having higher soluble Aβ40 and Aβ42 by 4 months of age that progresses to greater plaque burden later in life than their age-matched male littermates [21] as well as increased microglial activation [22].
Female APPNL-F/NL-F mice had a higher minimal longevity but a lower maximal longevity than genotype-matched males. In another study, APPNL-F/NL-F female mice have shown a similar Aβ plaque burden to males at 2-5 months of age, however, female Aβ plaque burden was significantly increased at 12-16 months compared to males [23]. The observed sex-differences in this mouse strain could also be due to a disproportionate microglial response to Aβ in females, leading to faster disease progression [24]. The build-up of amyloid plaques due to an attenuated microglial clearance response may result in decreased rates of maximal survival for the APPNL-F/NL-F female mice.
Genotype differences were present in APP/PS1 and APPNL-F/NL-F mice compared to the C57BL/6 control. APP/PS1 mice feature an overexpression of wild-type APP, which can cause memory impairment with amyloid deposition also seen in APPNL-F/NL-F mice [18]. Interestingly, APPNL-F/NL-F female mice had the longest survival rate of the three genotypes assessed, possibly owing to this being a knock-in model with less severe pathology than APP/PS1. This is further supported by female APP/PS1 mice performing worse than APPNL-F/NL-F mice in spatial learning and memory tasks [7].
Mice with higher survival past the minimal longevity point in this study could be more resilient to the detrimental effects and accumulation of AD pathology. Male mice have higher rates of maximal longevity in this study, which may suggest a resilience of males in AD pathology that is not present in females. Conversely, the more aggressive pathology observed in female AD mice may accelerate their biological aging thus shortening their lifespan compared to males [21]. Overall, our results show that female APP/PS1 mice have higher attrition rates and a sharper decline than males, which parallels proposed sex-based differences in disease progression and pathology in humans with AD [25]. Female APPNL-F/NL-F mice show an initial sustained survival, but have a steeper, more dramatic decline in survival after midlife. Differences in survival in this group could be due to being a knock-in model with less severe disease pathology than transgenic strains [13].
Limitations for this study involve the reasoning behind sexually and genotypic dimorphism of survival rates. A possible explanation for the increased AD occurrence in elderly females as seen in older APP/PS1 female models is loss of reproductive hormones. Specifically, it has been shown that estrogen has neuroprotective effects in mitochondria exposed to Aβ [26]. The hippocampus, where AD pathology originates, has been shown to have high levels of estrogen receptors [23]. Estrogen decreases with age in both mice and humans [27, 28]. Female mice from the C57BL/6 genetic background control undergo reproductive senescence from 11 to 16 months of age [27]. As estrogen decreases with age in females, this could increase the incidence and hasten disease progression. Because males do not experience the same decrease in estrogen with age, this may explain why male AD mouse models in this study had greater maximal survival and why AD proportionately affects fewer men. Future studies will expand on the discrepancy in survival between sexes through further examination of estrogen present in female and male mice and how this decline may contribute to alterations in survival. Another limitation may be to explore the role of PS1 to understand why this leads to the least amount of survival for this group in males and females.
In conclusion, survival distributions indicated a significant difference between males and females for each genotype, and longer survival of males than females for the C57BL/6 and APP/PS1 groups. Same sex genotype comparisons support decreased survival distributions in APP/PS1 mice but increased in APPNL-F/NL-F mice for both sexes. Previous male-centric AD preclinical research may have contributed to the high failure rate of clinical trials to show disease modifying benefits, underlining the importance of including both sexes in animal research to increase translatability of findings to the human population.
FUNDING
This work was supported by the National Institutes of Health (R01 AG057767, R01 AG061937), the Dale and Deborah Smith Center for Alzheimer’s Research and Treatment, and the Kenneth Stark Endowment.
Abbreviations:
- AD
Alzheimer’s disease
- APP
amyloid precursor protein
- Aβ
amyloid-beta
- PS1
presenilin 1
Footnotes
CONFLICT OF INTEREST/ DISCLOSURE STATEMENT
The authors have no conflict of interest to report.
DATA AVAIALBILITY
The data to support the findings of this study are available from the corresponding author upon reasonable request
REFERENCES
- [1].Association As (2023) 2023 Alzheimer’s disease facts and figures. Alzheimers Dement 19, 1598–1695. [DOI] [PubMed] [Google Scholar]
- [2].Zhao L, Mao Z, Woody SK, Brinton RD (2016) Sex differences in metabolic aging of the brain: insights into female susceptibility to Alzheimer’s disease. Neurobiol Aging 42, 69–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Blenck CL, Harvey PA, Reckelhoff JF, Leinwand LA (2016) The Importance of Biological Sex and Estrogen in Rodent Models of Cardiovascular Health and Disease. Circ Res 118, 1294–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Britz J, Ojo E, Haque N, Dhukhwa A, Hascup ER, Hascup KN, Tischkau SA (2024) Sex-Dependent Effects of Chronic Circadian Disruption in AβPP/PS1 Mice. J Alzheimers Dis 97, 855–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Findley CA, McFadden SA, Hill T, Peck MR, Quinn K, Hascup KN, Hascup ER (2024) Sexual Dimorphism, Altered Hippocampal Glutamatergic Neurotransmission and Cognitive Impairment in APP Knock-In Mice. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].McFadden S, Sime LN, Cox MF, Findley CA, Peck MR, Quinn K, Fang Y, Bartke A, Hascup ER, Hascup KN (2023) Chronic, Mild Hypothermic Environmental Temperature Does Not Ameliorate Cognitive Deficits in an Alzheimer’s Disease Mouse. J Gerontol A Biol Sci Med Sci 78, 911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Britz J, Ojo E, Dhukhwa A, Saito T, Saido TC, Hascup ER, Hascup KN, Tischkau SA (2022) Assessing Sex-Specific Circadian, Metabolic, and Cognitive Phenotypes in the AβPP/PS1 and APPNL-F/NL-F Models of Alzheimer’s Disease. J Alzheimers Dis 85, 1077–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Fang Y, Peck MR, Quinn K, Chapman JE, Medina D, McFadden SA, Bartke A, Hascup KN, Hascup ER (2024) Senolytic Intervention Improves Cognition, Metabolism, and Adiposity in Female APP (NL-F/NL-F) Mice. bioRxiv. [DOI] [PubMed] [Google Scholar]
- [9].McFadden SA, Peck MR, Sime LN, Cox MF, Ikiz ED, Findley CA, Quinn K, Fang Y, Bartke A, Hascup ER, Hascup KN (2024) Thermotherapy has Sexually Dimorphic Responses in APP/PS1 Mice. bioRxiv. [DOI] [PubMed] [Google Scholar]
- [10].Fang Y, Medina D, Stockwell R, McFadden S, Quinn K, Peck MR, Bartke A, Hascup KN, Hascup ER (2023) Sexual dimorphic metabolic and cognitive responses of C57BL/6 mice to Fisetin or Dasatinib and quercetin cocktail oral treatment. Geroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cox MF, Hascup ER, Bartke A, Hascup KN (2022) Friend or Foe? Defining the Role of Glutamate in Aging and Alzheimer’s Disease. Front Aging 3, 929474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Findley CA, McFadden SA, Cox MF, Sime LN, Peck MR, Quinn K, Bartke A, Hascup KN, Hascup ER (2023) Prodromal Glutamatergic Modulation with Riluzole Impacts Glucose Homeostasis and Spatial Cognition in Alzheimer’s Disease Mice. J Alzheimers Dis 94, 371–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sasaguri H, Nilsson P, Hashimoto S, Nagata K, Saito T, De Strooper B, Hardy J, Vassar R, Winblad B, Saido TC (2017) APP mouse models for Alzheimer’s disease preclinical studies. Embo j 36, 2473–2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gulyaeva NV (2021) Hippocampal hyperglutamatergic signaling matters: Early targeting glutamate neurotransmission as a preventive strategy in Alzheimer’s disease: An Editorial Highlight for “Riluzole attenuates glutamatergic tone and cognitive decline in AβPP/PS1 mice” on page 513. J Neurochem 156, 399–402. [DOI] [PubMed] [Google Scholar]
- [15].Findley CA, Bartke A, Hascup KN, Hascup ER (2019) Amyloid Beta-Related Alterations to Glutamate Signaling Dynamics During Alzheimer’s Disease Progression. ASN Neuro 11, 1759091419855541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hascup KN, Findley CA, Sime LN, Hascup ER (2020) Hippocampal alterations in glutamatergic signaling during amyloid progression in AβPP/PS1 mice. Sci Rep 10, 14503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Jankowsky JL, Zheng H. (2017) Practical considerations for choosing a mouse model of Alzheimer’s disease. Molecular Neurodegeneration 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Masuda A, Kobayashi Y, Kogo N, Saito T, Saido TC, Itohara S (2016) Cognitive deficits in single App knock-in mouse models. Neurobiol Learn Mem 135, 73–82. [DOI] [PubMed] [Google Scholar]
- [19].Fang Y, Peck MR, Quinn K, Chapman JE, Medina D, McFadden SA, Bartke A, Hascup ER, Hascup KN (2024) Senolytic intervention improves cognition, metabolism, and adiposity in female APP(NL)(−F/NL-F) mice. Geroscience. [DOI] [PubMed] [Google Scholar]
- [20].Fang Y, Medina D, Stockwell R, McFadden S, Hascup ER, Hascup KN, Bartke A (2023) Resistance to mild cold stress is greater in both wild-type and long-lived GHR-KO female mice. Geroscience 45, 1081–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Wang J, Tanila H, Puoliväli J, Kadish I, Groen Tv (2003) Gender differences in the amount and deposition of amyloidβ in APPswe and PS1 double transgenic mice. Neurobiology of Disease 14, 318–327. [DOI] [PubMed] [Google Scholar]
- [22].Gallagher JJ, Minogue AM, Lynch MA (2013) Impaired Performance of Female APP/PS1 Mice in the Morris Water Maze Is Coupled with Increased Aß Accumulation and Microglial Activation. Neurodegenerative Diseases 11, 33–41. [DOI] [PubMed] [Google Scholar]
- [23].Rahmani R, Rambarack N, Singh J, Constanti A, Ali AB (2023) Age-Dependent Sex Differences in Perineuronal Nets in an APP Mouse Model of Alzheimer’s Disease Are Brain Region-Specific. International Journal of Molecular Sciences 24, 14917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sala Frigerio C, Wolfs L, Fattorelli N, Thrupp N, Voytyuk I, Schmidt I, Mancuso R, Chen WT, Woodbury ME, Srivastava G, Möller T, Hudry E, Das S, Saido T, Karran E, Hyman B, Perry VH, Fiers M, De Strooper B (2019) The Major Risk Factors for Alzheimer’s Disease: Age, Sex, and Genes Modulate the Microglia Response to Aβ Plaques. Cell Rep 27, 1293–1306.e1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li R, Singh M (2014) Sex differences in cognitive impairment and Alzheimer’s disease. Frontiers in Neuroendocrinology 35, 385–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Viña J, Lloret A (2010) Why women have more Alzheimer’s disease than men: gender and mitochondrial toxicity of amyloid-beta peptide. J Alzheimers Dis 20 Suppl 2, S527–533. [DOI] [PubMed] [Google Scholar]
- [27].Syed FA, Mödder UI, Roforth M, Hensen I, Fraser DG, Peterson JM, Oursler MJ, Khosla S (2010) Effects of chronic estrogen treatment on modulating age-related bone loss in female mice. J Bone Miner Res 25, 2438–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Carter AE, Merriam S (2023) Menopause. Med Clin North Am 107, 199–212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data to support the findings of this study are available from the corresponding author upon reasonable request