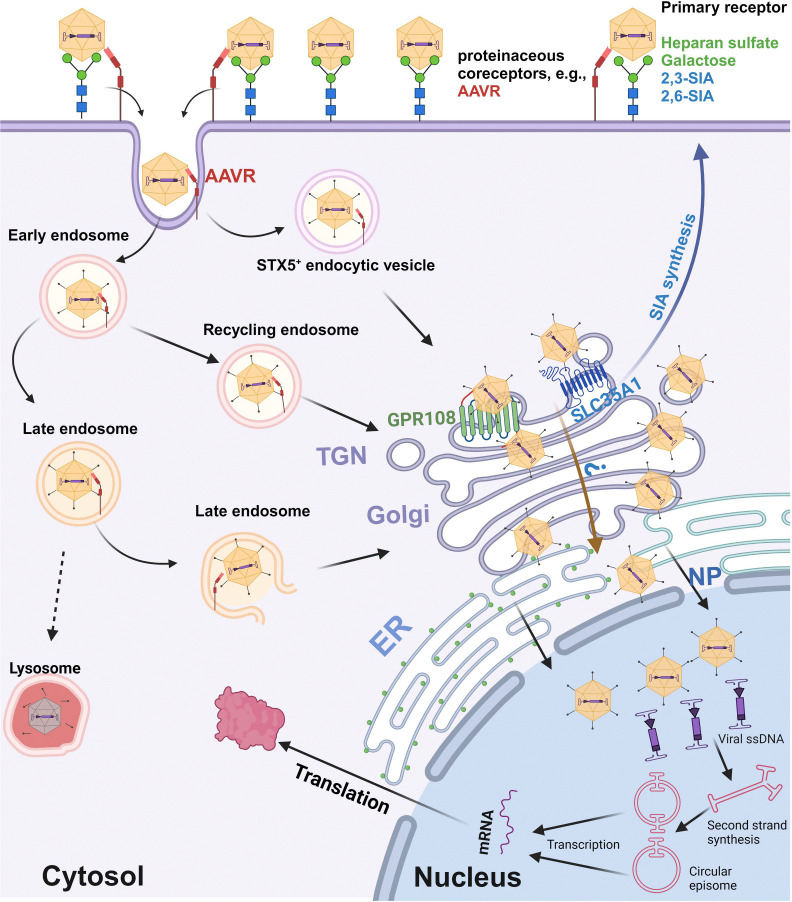
Figure 9. A model of SLC35A1 function in rAAV transduction.
AAV cell entry is initiated by interacting with specific glycan on the cell surface (primary attachment receptor) (15,16,18,19,21,23) and a proteinaceous receptor, e.g., AAVR (KIAA0319L) (26,28). Several intracellular trafficking pathways have been proposed based on AAV2 studies. Post endocytosis or internalization, AAV traffics through Rab7+ late endosomes, Rab11+ recycling endosomes (64), and the STX5+ endocytic vesicle (65), to the TGN (66–68), where GPR108 localized (35), as well as SLC35A1. We hypothesize that SLC35A1, which transports CMP-SIA from the cytosol into the Golgi apparatus lumen (41), mediates AAV transport from the cytosol into lumen of the Golgi apparatus in a GPR108-dependent (AAV2-type) or independent (AAV5-type) manner AAVs, which likely facilitates vector nuclear import. Then, AAV traffics through the Golgi apparatus to the nuclear membrane and enters the nucleus through the nuclear pore (NP), or routes to a nonproductive pathway, e.g., proteasome, for degradation (not shown). In the nucleus, AAV releases the ssDNA genome, which is converted to dsDNA intermediates (1). The dsDNA further undergoes intra/intermolecular recombination of the inverted terminal repeats (ITRs) to form either linear or circular episomes that are transcribed to produce mRNA. Created in BioRender.