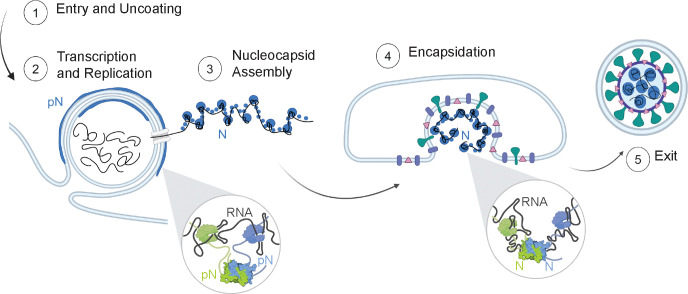
Figure 6: Model of N protein form and membrane-associated state during the viral lifecycle.
Inside the cell (1), the vast majority of N is found in its phosphorylated form, localized to the surface of viral replication organelles (2). Phosphorylation promotes linker-linker interactions across N dimers that weaken interaction between N and RNA (inset, left). This looser protein-RNA interaction network within condensates results in a relatively low viscosity and elasticity that may facilitate the molecular diffusion needed during RNA transcription and replication. A second population of N that is destined to form new virions binds to new viral genomic RNA (gRNA) and condenses RNA into small spherical complexes (3). This unmodified protein binds tightly to gRNA through both specific and nonspecific interactions (inset, right). These assemblies have high viscosity and elasticity that may support a protective function of N towards gRNA. The gRNA and N capsid is engulfed by the ERGIC membrane, facilitated by N and M interactions (4). New virions exit the infected cells (5).