Abstract
Ipomoea staphylina Rome & Schult, entrenched in ethnomedicinal practices, is recognised for its efficacy in treating stomach disorders. Traditionally used in Dharmapuri, Tamil Nadu for stomach ulcers, its matured stem bark latex is therapeutically relevant, especially for Helicobacter pylori (H. pylori) infections. This prompts scientific exploration into its antibacterial properties. The research validates the antibacterial efficacy of I. staphylina extracts against H. pylori, scrutinising the whole plant and matured stem through a comparative pharmacognostic analysis. Utilising herbal standardisation techniques, we confirm the heightened purity of the powder. Antimicrobial assessments show exceptional efficacy of DME (dried Ethanolic extract of I. staphylina) and HLS (hydro alcoholic extract of I. staphylina) extracts. Quercetin isolation by using advanced instrumentation (Nuclear magnetic resonance [NMR], High resolution mass spectrometry [HR-MS], High-performance thin-layer chromatography [HPTLC], Fourier transform infrared spectroscopy [FTIR]) ensures precise compound identification. This methodology guarantees an exhaustive analysis, confirming purity and identifying bioactive components. Standardisation underscores the elevated purity of I. staphylina, with phytochemical screening revealing a predominant presence of phenolics and flavonoids. Antibacterial investigations highlight significant activity, particularly with DME and HLS extracts. These findings substantiate I. staphylina’s medicinal significance, especially its matured stem latex, as a promising treatment for H. pylori-induced stomach ulcers, affirming traditional use by Dharmapuri villagers.
Keywords: Pharmacognostical Evaluations, Phytochemical Screening, Fluorescence Analysis, HPTLC Analysis, Quercetin
Highlights
Research confirms the antibacterial efficacy of Ipomoea staphylina extracts against H. pylori. Study involves pharmacognostic analysis of the whole plant and matured stem.
Phytochemical findings: High presence of phenolics and flavonoids in the extracts. Advanced instrumentation ensures precise identification of quercetin.
Medicinal significance and antimicrobial efficacy: DME and HLS extracts show exceptional antibacterial activity. Findings support the use of Ipomoea staphylina’s matured stem latex as a treatment for H. pylori-induced stomach ulcers.
INTRODUCTION
Herbal plants have been utilised for centuries in the treatment of various diseases (Balasubramaniam et al. 2020; Süntar 2020). Medicinal plants and their isolated compounds have (Maqbool et al. 2019) played a significant role in both conventional and alternative medicine for thousands of years (Maqbool et al. 2019; Süntar 2020). For instance, Cinchona bark has been traditionally used in South America for the treatment of malaria (Tolkushin et al. 2020). Notably, many well-known medications, including Curcumin, Digitoxin, Caffeine and Atropine, are derived from plants (Maqbool et al. 2019). The World Health Organisation (WHO) estimates that around 21,000 plant species have been utilised globally for medicinal purposes (Sundaramoorthy et al. 2021). Herbal medicines form the cornerstone of various alternative treatments that have gained popularity in recent years (Maqbool et al. 2019; Süntar 2020). Moreover, plant-based medications have a profound impact on drug development and design (Gomathi et al. 2012). The increased utilisation of herbal-based products can be attributed to their efficacy, affordability, reduced toxicity and eco-friendliness (Zhou et al. 2007). In fact, approximately 60% to 75% of cancer and infectious disease medications are derived from natural sources (Newman et al. 2000; Song et al. 2014). Given their ability to interact with proteins, herbs are considered valuable in drug design, contributing to the creation of novel medications (Newman et al. 2000; Song et al. 2014).
Ipomoea staphylina Rome & Schult, a plant deeply entrenched in ethnomedicinal practices, boasts a spectrum of biological properties particularly recognised for its efficacy in addressing stomach disorders (Dias et al. 2012; Narra & Kandavara 2014), the matured stem bark latex of this plant has been traditionally employed by communities in Dharmapuri, Tamil Nadu, for the treatment of stomach ulcers. The therapeutic relevance is noteworthy, especially considering that stomach ulcers are often attributed to Helicobacter pylori (H. pylori) bacterial infections (Majumdar & Looi 2024). This traditional application underscores the potential of I. staphylina as a valuable resource in managing gastric ailments, providing a rationale for scientific exploration into its antibacterial properties against H. pylori. Most commonly in human H. pylori is the main reason for the stomach ulcer (Youssefi et al. 2021). H. pylori is a gram-positive bacterium that infects the stomach (Kishikawa et al. 2020; Youssefi et al. 2021). H. pylori infection typically occurs during childhood (Kishikawa et al. 2020; Lee 2019; Majumdar & Bebb 2019). The bacteria can be transmitted through contaminated water, food or contact with the saliva, vomit or faeces of an infected person. H. pylori colonise the stomach lining, causing inflammation and weakening the protective mucous layer (Alexander et al. 2021). This can lead to the development of peptic ulcers, including gastric ulcers (in the stomach) and duodenal ulcers (in the upper part of the small intestine (Alexander et al. 2021; Kishikawa et al. 2020; Lee 2019; Majumdar & Bebb 2019; Youssefi et al. 2021). We can control stomach ulcers by controlling this bacterial growth. The principal objective of this research is to assess the antibacterial efficacy of I. staphylina against H. pylori, thereby substantiating its traditional use for anti-ulcer purposes in the Dharmapuri village. Additionally, the study aims to elucidate the traditional practices associated with I. staphylina in treating stomach ulcers within the Dharmapuri village community.
I. staphylina is an important climber known for its use in the treatment of respiratory problems, as an anthelmintic, purgative, for bronchitis, and for stomach ailments (Narra & Kandavara 2014; Raghavendra 2013; Ramesh, Deepa, et al. 2022; Ramesh, Rajeshkumar, et al. 2022). This plant exhibits a range of pharmacological effects, including antimicrobial, anti-inflammatory, antioxidant, anti-diabetic and anti-mutagenic properties (Raghavendra 2013). We have done the quantitative phytochemical screening of the plant extract, employing standardised methodologies (Krishnaveni et al. 1984; Soni & Sosa 2013; Yilmaz 2020). This analysis provided valuable insights into the composition and concentration of secondary metabolites inherent to the extract. Concurrently, the assessment of antioxidant potential was pursued using the DPPH (2,2-diphenyl-1-picrylhydrazyl) assay, recognised for its efficacy in gauging radical scavenging activity (Isildak et al. 2022; Sahidin et al. 2022; Sihag et al. 2022). The resulting data showcased a commendable percentage of radical scavenging activity, underscoring the extract’s promising antioxidant capabilities. These findings collectively contribute to a deeper understanding of the extract’s chemical composition and potential applications in various fields. To confirm the presence of secondary metabolites in the plant extract, crude drugs underwent High Performance Thin Layer Chromatography (HPTLC) analysis with standard drugs (Saxena et al. 2021). HPTLC was employed to identify and authenticate the phytochemicals and markers present in the I. staphylina plant extract (Gunjal Sanket & Dighe 2022; Mir et al. 2020). Through comprehensive antibacterial evaluations, the research endeavours to contribute empirical evidence supporting the plant’s potential therapeutic applications, aligning traditional knowledge with contemporary scientific scrutiny in traditional medicine.
Ethnopharmacology
I. staphylina, a plant of significant therapeutic importance, has been utilised for the treatment of various disorders, including purgation, stomach disorders, pain, rheumatism and inflammation (Firdous & Koneri 2014). In the region of Dharmapuri, the matured stem latex of I. staphylina has been traditionally employed to address stomach ulcers. Similarly, the residents of Gingee Hills have utilised leaf latex for the treatment of foot cracks (Muralidharan & Narasimhan 2013). Notably, the roots of I. staphylina have been used as an antidote for snake bites by the Irula and Palliyar tribes (Sarvalingam et al. 2014). Additionally, villagers in Karandamalai have administered a leaf decoction to alleviate stomach problems (Kottaimuthu 2008). The Chenchus tribes have employed leaf extract as a treatment for piles (Kumar T D & Pullaiah 1999). These traditional uses underscore the diverse therapeutic potential of I. staphylina in various cultural settings.
MATERIALS AND METHOD
Chemical and Reagents
Solvents including methanol, ethanol, ethyl acetate and petroleum ether were procured from SD Fine Chemicals (India). Additionally, CHCl3, NaOH, KOH, HCl, H2SO4, HNO3, Na2CO3, acetic acid and DPPH were obtained from Avra Chemicals (India). Reagents required for qualitative phytochemical testing, such as neutral FeCl3, alkaline reagent, Mayers solution, Benedict’s reagent, Salkowski reagent, Fehling’s solution and Folin-Ciocalteu reagent, were sourced from HiMedia (India).
Plant Collection
Plant parts were collected from the Dharmapuri region (Tamil Nadu, Latitudes N11°47′ and 12°33′ and Longitudes E77°02′ and 78°40′) during the winter season of December 2020. The soil in which the plant was grown is characterised as clay. To ensure accurate identification, the plant specimen has been authenticated by the Botanical Survey of India, Southern Region Coimbatore (BSI/SRC/5/23/2022/Tech/429).
Plant Extraction
This research investigation focuses on two specific components of I. staphylina, specifically the stem and leaves and matured stem, as targets for study. Fresh plant parts were subjected to extraction using two distinct methods: hot continuous percolation (Soxhlet) and immersion. Additionally, the Soxhlet method was followed for the extraction of bioactive compounds from the dried parts (dried under sunshade) of I. staphylina (Bag & Mumtaz 2013; López-Bascón & De Castro 2020). These chosen extraction techniques were carefully selected to ensure optimal recovery of valuable constituents from the plant material, facilitating subsequent analysis and characterisation.
Fresh Part Extraction
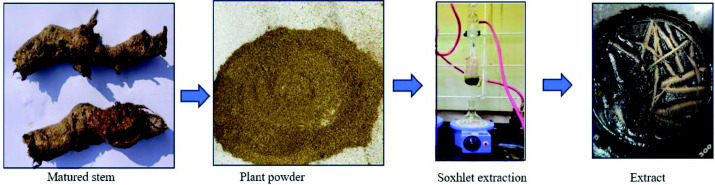
Fig. 1 shows the fresh plant part extraction of I. staphylina. Fresh plant parts were meticulously washed with distilled water to remove any impurities, followed by pulverisation using an electronic blender. The resulting blend was subjected to extraction using two distinct methods: Soxhlet and immersion (Bag & Mumtaz 2013; López-Bascón & De Castro 2020). In the Soxhlet method, solvents such as petroleum ether, ethanol and hydroalcohol (a mixture of 80% methanol and 20% water) were used. Conversely, for the immersion method, distilled water served as the solvent. Following the extraction process, the solvents were evaporated using a Rotavapor R-100 apparatus. The resulting dried crude extract was carefully collected in an airtight container and stored under refrigeration conditions. These standardised procedures ensure the preservation and stability of the extracted compounds for further analysis and experimentation.
Figure 1.
Fresh part extraction matured stem of I. staphylina.
Dry Part Extraction
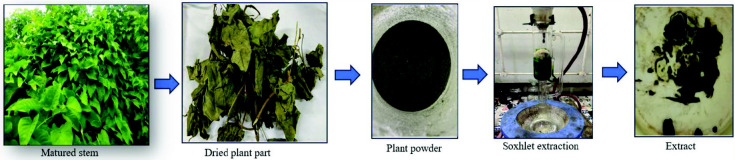
Fig. 2 shows the extraction process of dried plant parts of I. staphylina. The staphylina plant parts (matured stem and stem and leaf) were carefully dried under shade at room temperature for 10 days, ensuring the preservation of their chemical constituents. Subsequently, the dried parts were finely pulverised using an electronic blender, resulting in a homogeneous powder. The extraction process was conducted using the Soxhlet method, employing solvents such as petroleum ether, ethanol and hydroalcohol (a mixture comprising 80% methanol and 20% water) (Bag & Mumtaz 2013; López-Bascón & De Castro 2020). This extraction method facilitated the efficient extraction of bioactive compounds from the plant material. Following the extraction, the solvents were evaporated using a Rotavapor R-100 apparatus. The resulting dried crude extract was carefully collected and stored in an airtight container, ensuring protection from moisture and other degrading factors. To maintain the stability and integrity of the extracted compounds, the container was stored in a refrigerator, providing optimal storage conditions (Jeyadevi et al. 2019). These meticulous steps in sample preparation and storage guarantee the quality and usability of the extracted material for further scientific analysis and investigation.
Figure 2.
Dried part extraction stem and leaf of I. staphylina.
Physicochemical Standardisation
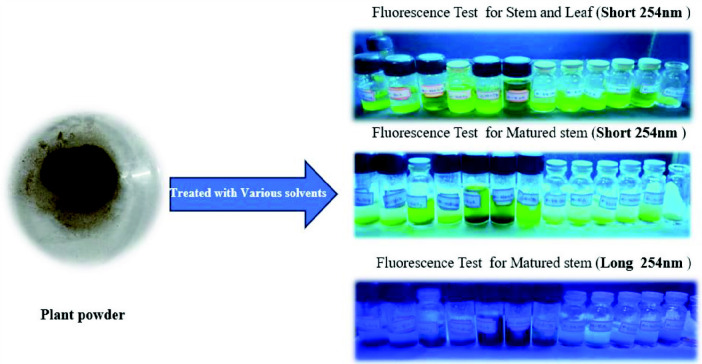
Fig. 3 shows the florescence test of I. staphylina plant powder. Before commencing any research work on a specific herb, it is essential to conduct a comprehensive assessment of its physicochemical parameters (Snehalatha & Rasmi 2021). In order to standardise the herbal powder, various pharmacognostic characteristics were evaluated, including ash value, loss on drying, acid-insoluble ash, water-soluble ash and fluorescence analysis (Khanal et al. 2018; Khandelwal 2008; Snehalatha & Rasmi 2021). These physicochemical standardisations were performed according to established protocols and guidelines. By examining these pharmacognostic characteristics, it becomes possible to identify potential adulterants and impurities present in the herbal drug, ensuring the purity and quality of the material under investigation. These evaluations serve as crucial preliminary steps in the research process, providing valuable insights into the composition and integrity of the herb being studied.
Figure 3.
Fluorescence analysis of I. staphylina.
EXPERIMENTAL SECTION
Pharmacognostic Evaluations
Prior to initiating any research, the standardisation of herbal drugs is imperative (Khandelwal 2008). In the case of I. staphylina herbal powder, pharmacognostic evaluations were conducted to ensure its quality and authenticity. These evaluations encompassed essential parameters, including loss on drying, ash value, water-soluble ash and acid-insoluble ash, the results of which are presented in Table 1 (Khanal et al. 2018; Khandelwal 2008). These rigorous tests serve the purpose of safeguarding against potential adulterants and maintaining the integrity of the herbal drug. Furthermore, fluorescence analysis was employed as a critical parameter to assess the purity and quality of the powdered drug material. These comprehensive assessments collectively contribute to the standardisation process, guaranteeing the reliability and effectiveness of the herbal drug under investigation.
Table 1.
Pharmacognostic evaluation of I. staphylina powder.
| S. No | Parameters | Matured stem (%) | Stem and leaf (%) |
|---|---|---|---|
| 1 | Loss on drying | 10.32 | 14.32 |
| 2 | Ash value | 4.40 | 8.27 |
| 3 | Water soluble ash | - | 2.60 |
| 4 | Acid insoluble ash | - | - |
Fluorescence studies employ estimations of fluorescence intensity as well as identify any fluorescent phytochemical compounds present in the plant.
Fluorescence Analysis
Fluorescence analysis of the powdered drugs was conducted following a standardised procedure (Das et al. 2021; Khanal et al. 2018; Khandelwal 2008). To perform the analysis, various solvents including water, methanol, ethanol, ethyl acetate, petroleum ether, chloroform, 10% sodium hydroxide, 10% potassium hydroxide, hydrochloric acid (4N), sulphuric acid (4N), nitric acid (4N) and acetic acid (4N) were individually mixed with the desired herb powder. After allowing sufficient time for interaction, the fluorescence of the plant powder was examined under both ultraviolet (UV) and visible light. The obtained fluorescence patterns provide valuable insights into the unique fluorescent characteristics of the matured stem, stem and leaf powders, as depicted in Tables 2 and 3, respectively. This fluorescence analysis serves as an important tool for the characterisation and assessment of the quality of the herbal drug, contributing to its standardisation and reliable utilisation in further research endeavours (Amir et al. 2019; Bashir et al. 2019; Jain et al. 2021; Misra et al. 2018; Ved et al. 2022).
Table 2.
Fluorescence analysis of matured stem powder of I. staphylina.
| S.No | Solvents | Visible | Long 365 nm | Short 254 nm |
|---|---|---|---|---|
| 1 | Powder | Brown | Brown | Light green |
| 2 | Water | Brown | Brown | Light green |
| 3 | Methanol | Brown | Brown | Light green |
| 4 | Ethanol | Brown | Brown | Light green |
| 5 | Ethyl acetate | Brown | Green | Light green |
| 6 | Petroleum ether | Brown | Light green | Light green |
| 7 | CHCl3 | Brown | Green | Green |
| 8 | 10%NaOH | Dark brown | Brownish green | Brownish green |
| 9 | 10%KOH | Dark brown | Brownish green | Brownish green |
| 10 | HCl (4N) | Brown | Brown | Light green |
| 11 | H2SO4 (4N) | Brown | Brown | Light green |
| 12 | HNO3 (4N) | Dark brown | Green | Green |
| 13 | Acetic acid (4N) | Brown | Brown | Light green |
Table 3.
Fluorescence analysis of stem and leaf powders of I. staphylina.
| S.No | Solvents | Visible | Long 365 nm | Short 254 nm |
|---|---|---|---|---|
| 1 | Powder | Brown | Brown | Light green |
| 2 | Water | Brown | Brown | Green |
| 3 | Methanol | Green | Dark green | Green |
| 4 | Ethanol | Light green | Green | Light green |
| 5 | Ethyl acetate | Light green | Green | Light green |
| 6 | Petroleum ether | Brown | Light green | Light green |
| 7 | CHCl3 | Brown | Light green | Green |
| 8 | 10%NaOH | Dark brown | Light green | Light green |
| 9 | 10%KOH | Dark brown | Light green | Light green |
| 10 | HCl (4N) | Brown | Light green | Light green |
| 11 | H2SO4 (4N) | Brown | Light green | Light green |
| 12 | HNO3 (4N) | Brown | Green | Light green |
| 13 | Acetic acid (4N) | Brown | Light green | Green |
Qualitative Phytochemical Screening
The extracted crude samples underwent a qualitative phytochemical investigation to identify the presence of various phytoconstituents, including alkaloids, tannins, flavonoids, phenols, saponins, proteins, sterols, terpenoids, carbohydrates, and fats and oils, utilising established standard methods (Amir et al. 2019; Banerjee & Firdous 2020; Bashir et al. 2019; Das et al. 2021; Harborne 1998; Jain et al. 2021; Khanal et al. 2018; Khandelwal 2008; Misra et al. 2018; Tiwari & Patel 2012; Ved et al. 2022). The results of the preliminary phytochemical screening of the freshly dried parts of I. staphylina are presented in Tables 4 and 5.
Table 4.
Fresh plant parts phytochemical screening report.
| Secondary metabolites | Name of the test | MS | SL | MSS | SLS | EMS | ESL | HMS | HLS |
|---|---|---|---|---|---|---|---|---|---|
| Phenols | Neutral FeCl3 test | − | − | ++ | + | + | + | + | + |
| Flavonoids | Alkaline reagent test | +++ | +++ | + | + | +++ | ++ | +++ | +++ |
| Alkaloids | Mayers test | + | ++ | − | − | ++ | + | + | + |
| Carbohydrates | Benedict’s test | − | − | − | − | − | ++ | − | + |
| Sterols | Salkowski test | + | + | + | + | + | + | − | + |
| Proteins | Biuret test | − | − | − | − | − | − | − | − |
| Saponins | Foam test | +++ | +++ | − | − | − | − | + | ++ |
| Terpenoids | + | + | + | + | + | + | + | + | |
| Tannins | − | − | − | + | − | + | − | − | |
| Fat and oils | − | − | ++ | ++ | ++ | ++ | − | − |
Note: − Negative, + Present, ++ Moderate, +++ Strongly present. MS = fresh matured stem water extract; SL = fresh stem and leaf water extract; SLS = fresh stem and leaf pet-ether extract; MSS = fresh matured stem pet-ether extract; EMS = fresh matured stem ethanol extract; ESL = fresh stem and leaf ethanol extract; HLS = fresh stem and leaf hydroalcoholic extract; HMS, fresh matured stem hydroalcoholic extract
Table 5.
Dried plant parts phytochemical screening report.
| Secondary metabolites | Name of the test | DSP | DMP | DSE | DME | DSH | DMH |
|---|---|---|---|---|---|---|---|
| Phenols | Neutral FeCl3 test | + | + | − | − | + | + |
| Gela | ++ | ++ | + | + | − | + | |
| Flavonoids | Alkaline reagent test | + | + | + | + | + | + |
| Alkaloids | Mayers test | ++ | ++ | + | ++ | + | + |
| Carbohydrates | Benedict’s test | − | − | + | ++ | ++ | +++ |
| Sterols | Salkowski test | + | +++ | ++ | +++ | ++ | ++ |
| Proteins | Biuret test | − | − | − | − | − | − |
| Saponins | Foam test | − | − | − | + | ++ | ++ |
| Terpenoids | Copper acetate test | + | + | + | + | ++ | ++ |
| Tannins | − | − | + | + | + | + | |
| Fat and oils | + | + | + | + | − | − |
Notes: − Negative, + Present, ++ Moderate, +++ Strongly present. DMP = dried matured stem pet-ether extract; DSP = dried stem and leaf pet-ether extract; DSE = dried stem and leaf ethanol extract; DME = dried matured stem ethanol extract; DSH = dried stem and leaf hydroalcoholic extract; DMH = dried matured stem hydroalcoholic extract.
Quantitative Phytochemical Screening
The quantitative phytochemical screening was done by following standard procedure and using spectrophotometer (Isildak et al. 2022; Krishnaveni et al. 1984; Soni & Sosa 2013; Yilmaz 2020).
Estimation of Phenols
Phenols were quantified using the FC (Folin-Ciocalteu reagent) technique, slightly modified in accordance with the procedures outlined in the reference. Initially, a 100 μL aliquot was drawn from a 10 mg/mL stock solution, with subsequent adjustment of the volume in each test tube to 3.0 mL through the addition of distilled water. The experimental protocol encompassed the sequential addition of 0.5 mL of Folin-Ciocalteau reagent and 2 mL of 20% Na2CO3 solution into the tubes. The resulting mixture was subjected to precise boiling within a water bath for a duration of 1 min. After this, the tubes underwent a cooling phase, following which absorbance measurements were taken at a wavelength of 650 nm, employing a spectrophotometer and utilising a reagent blank as a reference standard. Notably, gallic acid functioned as the standard substance throughout this investigation. To prepare the gallic acid solution, 1 mg of the compound was dissolved in 1 mL of ethanol. Subsequently, aliquots of this solution, measuring 50 μL, 100 μL, 150 μL, 200 μL and 250 μL, were meticulously extracted for subsequent analytical procedures.
Estimation of Flavonoids
Total flavonoid content was determined using the aluminium chloride colorimetric assay. A reaction mixture containing 1 mg of extract and 1 mL of distilled water was prepared. Sequential addition of 0.30 mL of 5% sodium nitrite, followed by 0.3 mL of 10% aluminium chloride after 5 min, was carried out. After another 5 min, 2 mL of 1M sodium hydroxide was added and the solution was diluted to 10 mL with distilled water. Reference standard solutions of Quercetin (20 μg/mL–100 μg/mL) were prepared similarly. Absorbance was measured at 510 nm against a reagent blank using a UV/Visible spectrophotometer. Total flavonoid content was expressed as μg QE/mg of extract.
Estimation of Tannins
The determination of tannin content in the sample was executed utilising the Folin-Ciocalteu method. This approach relies on colorimetric assessment, wherein the formation of a blue colour arises from the reduction of phosphotungstomolybdic acid by tannin-like compounds under alkaline conditions. To proceed, a solution containing 1 mg of extract or standard tannic acid (ranging from 50 μL to 250 μL) was prepared, achieving a final volume of 7.5 mL with distilled water. Subsequently, 0.5 mL of Folin-Ciocalteu reagent and 1 mL of 35% sodium carbonate solution were introduced. Further volume adjustments were made to reach a total of 10 mL using distilled water, following which absorbance measurements were taken at 700 nm. This method facilitated the precise quantification of tannin content in the sample
Estimation of Carbohydrates
Total carbohydrate quantification employed the phenol sulphuric acid method with spectrophotometric analysis (Krishnaveni et al. 1984). Each sample (100 μL) and standard underwent a controlled three-hour incubation in a water bath with 5 mL of 2.5 N HCl, followed by neutralisation with solid sodium carbonate. After volume adjustment (100 mL) and centrifugation, a series of test tubes received working standard volumes (50 μL–250 μL), phenol solution (1 mL) and 96% H2SO4 (5 mL). Following agitation and a 20-min water bath incubation, absorbance at 490 nm was measured. Carbohydrate content was quantified using glucose as the standard reference.
Antioxidant Activity
The investigation into the scavenging capacity of free radicals within the crude extracts was executed utilising a spectrophotometric methodology, with minor adaptations in accordance with the protocol stipulated by the reference (Isildak et al. 2022; Sahidin et al. 2022; Sihag et al. 2022). The samples and the established standard underwent meticulous preparation at varying concentrations of 50 mg/mL, 100 mg/mL, 150 mg/mL, 200 mg/mL and 250 mg/mL, all dissolved in a methanolic medium. A solution of 0.2 mmol/L concentration of DPPH was meticulously diluted in 50 mL of methanol, subsequently subjected to an incubation period of 30 min under controlled room temperature conditions, following which the absorbance was quantified at a specific wavelength of 517 nm. For precise quantification, the Microsoft Excel platform was employed to compute the percentage values indicative of the Radical Scavenging Activity (RSA), employing the formula mentioned below. Notably, Rutin was utilised as the reference standard throughout the experimental endeavour.
The RSA% inhibition was mathematically defined by:
| (1) |
HPTLC Analysis
Sample preparation
All extracts were weighed at a concentration of 5 mg/mL and dissolved in specific solvents. The resulting solutions were subjected to sonication for 5 min and then filtered using Whatman filter paper to remove any particulate matter. Precoated TLC (thin-layer chromatography) aluminium sheets with silica gel 60 F 254 (Merck) were utilised for the analysis. The samples were applied to the TLC plates using a Linomat 5 sample applicator, ensuring a band length of 5 mm and a speed of 150 nL/sec. This meticulous procedure ensures precise and uniform application of the extracts onto the TLC plates, enabling accurate separation and identification of the individual components present in the extracts (Kumar A et al. 2010; Swaroop et al. 2005; Tiwari & Patel 2012).
Mobile phase
Following the elution process optimising with various solvent combinations, a suitable mobile phase was established by employing a mixture of toluene, ethyl acetate, formic acid and glacial acetic acid in a ratio of (2:6:1:1). For the preparation of samples and standards, 1 mg of the compound was dissolved in 10 mL of the respective solvent. The application of these solutions was carefully performed by marking spots on precoated TLC aluminum sheets coated with silica gel 60 F 254 (Merck). These highly specific and controlled procedures facilitate the precise separation and analysis of the compounds present in the samples, ensuring accurate results and reliable identification of individual components.
Chromatogram and scanning
Chromatogram development was conducted using a twin trough glass chamber for a duration of 20 min, with a distance of 80 mm, employing a mobile phase consisting of toluene, ethyl acetate, formic acid and glacial acetic acid in a ratio of (2:6:1:1). Following the development process, the air-dried TLC plates were subjected to examination under UV light. Subsequently, scanning of the plates was performed using a CAMAG HPTLC Densitometer (Scanner) equipped with Deuterium light and CAMAG winCATS software, using wavelengths of 254 nm and 366 nm. The obtained results from the HPTLC analysis are presented in Figs. 2 and 3, providing valuable insights into the separation and detection of the analysed compounds.
Column chromatography
To isolate quercetin, the ethanolic extract of Ipomoea staphylina (ESL) was chosen based on the quantity and the results obtained from high-performance thin-layer chromatography (HPTLC). For the precise analysis of 10 g of the plant extract, column chromatography was performed using a stationary phase of silica gel (60–120 mesh) (Davies & Johnson 2007; Johnston et al. 2013; Reid & Sarker 2012). The mobile phase used in column chromatography was similar to the one used in HPTLC (composed of a mixture of ethyl acetate, formic acid, glacial acetic acid and water in a ratio of 20:2:2:1). The yellow-coloured spot, corresponding to quercetin, was isolated and verified using thin-layer chromatography (TLC) alongside a standard sample, confirming their identical retention factor (Rf) values. The resulting product was then dried and prepared for further analysis and characterisation (Meena & Patni 2008; Sambandam et al. 2016; Zahoor et al. 2018).
Characterisation
FTIR and NMR spectrum analysis
The isolated compound was subjected to Fourier-transform infrared spectroscopy (FTIR) analysis to verify the presence of specific functional groups and obtain information about its molecular structure. The FTIR results provided confirmation of the functional groups present in quercetin (Sambandam et al. 2016).
Additionally, the isolated compounds underwent Nuclear Magnetic Resonance (NMR) spectroscopy to determine the precise positioning of the proton and carbon binding sites (Gülşen et al. 2007). The analysis was performed using a Bruker 400 Mz instrument, and the obtained results were processed using Topspin software version 3.6.2. The NMR spectra, depicting the chemical shifts and peak patterns, are presented in Figs. 5 and 6, allowing for a detailed characterisation of the isolated compounds (Gülşen et al. 2007).
Figure 5.
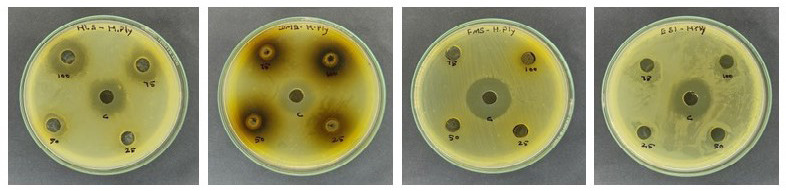
Antibacterial activity of I. staphylina.
Figure 6.
Antioxidant activity of (RSA%) dried extracts.
HRMS spectrum
High-resolution mass spectrometry (HRMS) was employed to determine the molecular weight of the isolated compound (Parvez et al. 2021). The presence of a molecular ion peak was confirmed based on the data obtained from the HRMS analysis. The molecular weight of the isolated compound was determined using an HRMS Model Name: Waters-Xevo G2-XS-QToF mass spectrometer.
Antibacterial activity
Antibacterial activities of the tested sample were evaluated using well diffusion method on Blood Agar. The bacterial strains H. pylori were used as references for the antibacterial assay (Balouiri et al. 2016; Gonelimali et al. 2018; Wylie et al. 2022). Blood Agar plates were inoculated with bacterial strain under aseptic conditions was spread plated by glass L-rod with 100 μL grown culture. Then, squire (10 mm) compound coated thin film were attached on each plate, after that followed by incubation at 37°C for 24 h. After the incubation period, the zone of inhibition was measured and reported in millimeters (mm). The extracts were dissolved in distilled water and Chloramphenicol was used as control (Balouiri et al. 2016; Gonelimali et al. 2018; Wylie et al. 2022).
RESULTS AND DISCUSSION
Pharmacognostical Evaluation
The moisture content in mature stems and leaves was found to be 10.32% and 14.32%, respectively, based on loss on drying. These findings led us to conclude that this particular plant contains some minerals because the matured stem’s ash value was 4.4% and the stem and leaf’s ash value was 8.27%. For the standardisation of herbal medication, each of these factors is crucial.
Qualitative Phytochemical Screening
According to the phytochemical analysis, flavonoids were found to be abundant in all dry powder extracts and highly abundant in fresh plant extracts. Phenols were present in most fresh plant part extracts, except for water extracts (MS, SL), while they were present in all dry powder extracts. Sterols were predominantly present in extracts from both fresh and dry plant parts, except for HMS extract. Alkaloids were detected in all extracts, both from fresh and dry plant parts, except for MSS and SLS. Furthermore, the phytochemical screening revealed that the extracts of Ipomoea plants contain a wide range of secondary metabolites, including tannins, terpenoids, carbohydrates, saponins, and fats and oils. The detailed results can be found in Tables 4 and 5.
Quantitative Phytochemical Analysis
Estimation of secondary metabolites
Total phenolic content (TPC) of various crude extracts is quantified in terms of gallic acid equivalents (GAE), Flavonoids with various plant extracts quantified in terms of Quercetin equivalents (QE), tannins quantified in terms of Tannic acid equivalents (TE) and carbohydrate quantified in terms of Glucose equivalents (GE). All the quantitative estimation was tabulated in Table 6.
Table 6.
Quantitative estimation of plant extracts.
| S. No | Sample code | Total phenolics (mg/g) | Total flavonoids (mg/g) | Total tannins (mg/g) | Total carbohydrates (mg/g) |
|---|---|---|---|---|---|
| 1 | SLS | 113.2 | 242.3 | - | - |
| 2 | ESL | 226.3 | 569.4 | - | 401.9 |
| 3 | HLS | 317.5 | 256.2 | - | 325.6 |
| 4 | MSS | - | 196.0 | - | - |
| 5 | EMS | 330.1 | 128.1 | 163.0 | - |
| 6 | HMS | - | 43.5 | - | - |
| 7 | DSP | - | 256.6 | - | - |
| 8 | DSE | - | 282.7 | - | 87.7 |
| 9 | DSH | 110.4 | - | - | 670.4 |
| 10 | DMP | 28.5 | 497.6 | 170.7 | - |
| 11 | DME | 36.6 | 567.3 | - | 783.5 |
| 12 | DMH | - | 115.3 | - | - |
Significant differences were observed among the various stem extracts under investigation. Notably, the ethanolic matured stem extract (EMS) displayed the highest TPC, registering at 330.1 mg of GAE per gram. Meanwhile, HLS extract showcased a slightly lower TPC of 317 mg GAE/g. In terms of Quercetin content, it was found that all extracts, except for the DSH extract, contained minimal amounts of this compound. Notably, the Ethanolic Stem Extract (ESL) stood out by exhibiting the highest Quercetin content among all extracts, measuring at 569.4 mg of QE per gram. It is noteworthy that the presence of tannins was exclusively identified in the EMS and DMP extracts, with tannin concentrations recorded at 163.0 mg and 170 mg of TE per gram, respectively. All other extracts lacked tannin content. Furthermore, the DME was distinguished by its elevated Carbohydrate content, measuring at 783.5 mg of GE per gram. These findings underscore the diverse chemical compositions present within the examined extracts.
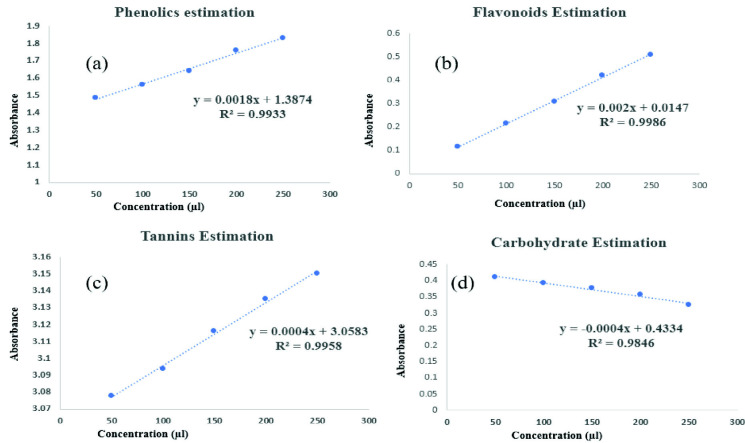
Fig. 4 shows the standard correlation graph of phenolics, flavonoids, tannins and carbohydrates estimations.
Figure 4.
(a) Standard correlation graph of phenolics estimations; (b) standard correlation graph of flavonoids estimations; (c) standard correlation graph of tannins estimations; (d) standard correlation graph of carbohydrates estimations.
Antibacterial Activity
Table 7 shows the antibacterial activity of I. staphylina extracts against H. pylori.
Table 7.
Antibacterial activity results.
| S.No | Sample name |
H. pylori (Zone of inhibition (mm) Concentration (μL) |
|||
|---|---|---|---|---|---|
|
| |||||
| 25 μL | 50 μL | 75 μL | 100 μL | ||
| 1 | Chloramphenicol | 16 | 15 | 20 | 21 |
| 2 | HLS | - | 12 | 15 | 18 |
| 3 | DME | 14 | 15 | 16 | 17 |
| 4 | EMS | - | - | - | - |
| 5 | ESL | - | - | - | - |
In contrast to the control represented by chloramphenicol at a concentration of 100 μL, the HLS sample manifests a significant zone of inhibition, measuring 18 mm. Even at a lower concentration of 50 μL, a substantial inhibitory zone of 12 mm is observed. The DME extract, at 25 μL, exhibits a zone of inhibition measuring 14 mm, while at 100 μL, it demonstrates an expanded inhibitory zone of 17 mm. These findings underscore the efficacy of these extracts, particularly against Gram-negative bacteria such as H. pylori, hinting at a potential influence on cellular replication mechanisms. This antibacterial activity suggests promising avenues for further investigation and potential applications in combating bacterial infections (Fig. 5).
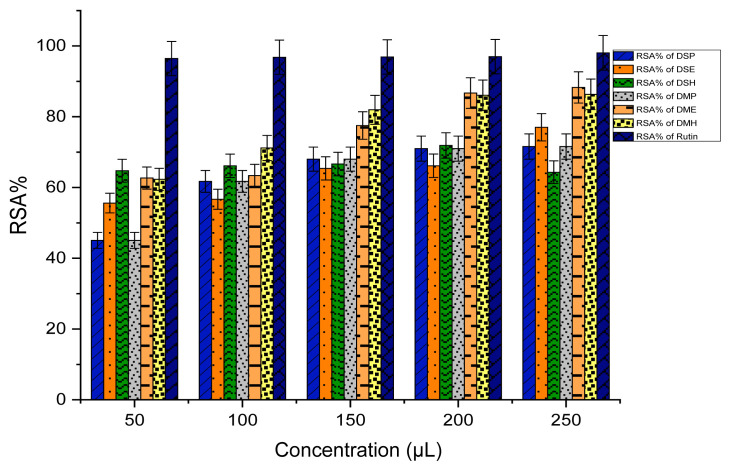
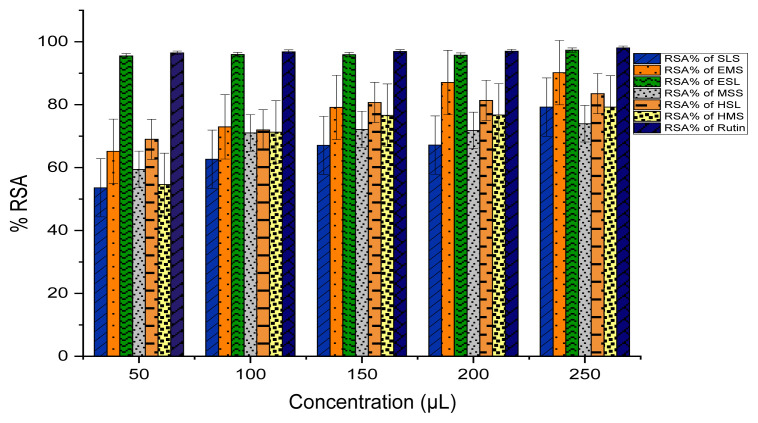
Antioxidant Assay
DPPH stands as a stable free radical commonly employed in evaluating the capacity of extracts to function as radical scavengers or hydrogen donors, thereby assessing their antioxidant ability. The quantified outcomes of DPPH scavenging for extracts are succinctly presented, where Rutin serves as the standard compound, and these results are visually represented in Figs. 6 and 7. The efficacy of various extracts in attenuating the DPPH radical is conveyed through the metric of inhibitory percentage. Noteworthy is the pronounced DPPH radical inhibition showcased by the crude ESL, and EMS extracts, denoting an elevated level of activity. Moreover, the extracts DME and DMH exhibit a commendable yet moderate efficacy in comparison to the standard. All remaining extracts also exhibit favourable scavenging activity against the DPPH radical.
Figure 7.
Antioxidant activity of (RSA%) fresh extracts.
HPTLC Analysis
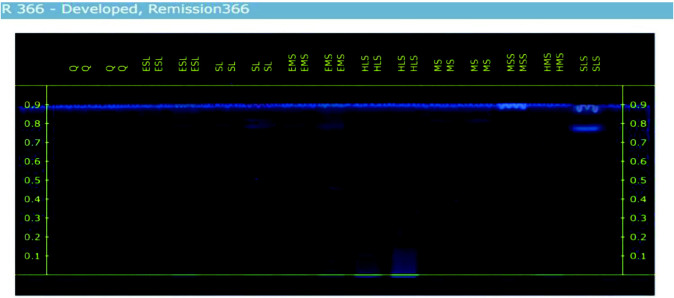
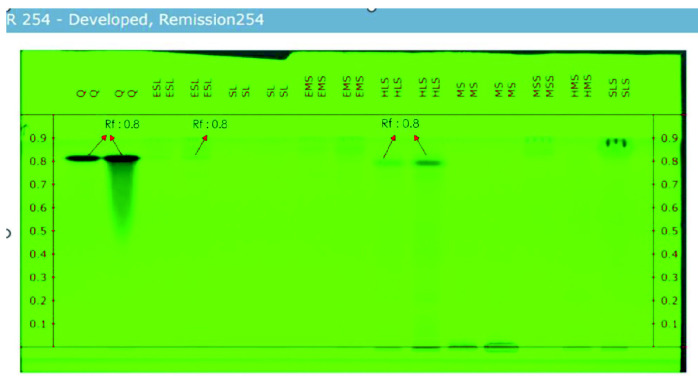
The HPTLC results indicate that among the tested extracts, EMS, HLS and EMS have the lowest concentration of Quercetin. Additionally, the preliminary phytochemical screening supports the strong presence of flavonoids in EMS, HLS and EMS. The Rf values of these three extracts match the reference Rf value (as shown in Table 8), confirming the presence of quercetin, a flavonoid. However, the HPTLC results reveal that quercetin is only present in trace amounts in all samples, except for SL and MS. Figs. 8 and 9 shows the HPTLC results of I. staphylina. Fig 8 shows UV remission 366 nm, and Fig 9 UV remission shows 254 nm.
Table 8.
HPTLC results.
| S. No | Compound | No. of peaks | Start Rf | Max Rf | End Rf | Area | Percentage (%) |
|---|---|---|---|---|---|---|---|
| 1 | Quercetin 2 μL | 1 | 0.787 | 0.826 | 0.858 | 0.02430 | 100 |
| 2 | Quercetin 5 μL | 1 | 0.787 | 0.833 | 0.854 | 0.01799 | 100 |
| 3 | ESL 2 μL | 1 | 0.804 | 0.828 | 0.854 | 0.00101 | 100 |
| 4 | ESL 5 μL | 1 | 0.813 | 0.828 | 0.854 | 100 | |
| 5 | SL 2 μL | - | - | - | - | - | - |
| 6 | SL 5 μL | 1 | 0.822 | 0.836 | 0.851 | 0.00022 | 100 |
| 7 | EMS 2 μL | - | - | - | - | - | - |
| 8 | EMS 5 μL | 2 | 0.796 | 0.813 | 0.822 | 0.00015 | 38.06 |
| 0.825 | 0.849 | 0.857 | 0.00025 | 61.94 | |||
| 9 | HLS 2 μL | 1 | 0.787 | 0.806 | 0.833 | 0.00600 | 100 |
| 10 | HLS 5 μL | 1 | 0.787 | 0.807 | 0.860 | 0.00858 | 100 |
| 11 | MS 2 μL | - | - | - | - | - | - |
| 12 | MS 5 μL | - | - | - | - | - | - |
| 13 | MSS 5 μL | 1 | 0.819 | 0.844 | 0.861 | 0.00142 | 100 |
| 14 | HMS 5 μL | 1 | 0.787 | 0.806 | 0.824 | 0.00052 | 100 |
| 15 | SLS 5 μL | 1 | 0.813 | 0.838 | 0.851 | 0.00102 | 100 |
Notes: ESL = fresh stem and leaf ethanol extract; SL = fresh stem and leaf water extract; EMS = fresh matured stem ethanol extract; HLS = fresh stem and leaf hydroalcoholic extract; MS = fresh matured stem water extract; MSS = fresh matured stem pet-ether extract; HMS = fresh matured stem hydroalcoholic extract; SLS = fresh stem and leaf pet-ether extract. The bold numbers represent the standard Rf values, while other bold numbers indicate matches with these standard Rf values.
Figure 8.
UV remission 366 of I. staphylina crude extracts.
Figure 9.
UV Remission 254 nm of I. staphylina crude extracts.
Based on the HPTLC data, the Rf values of the standard quercetin (2 μL) were determined to be 0.787, 0.836 and 0.858, while for the 5 μL it showed 0.787, 0.826 and 0.858, respectively. Most of the samples exhibited very similar Rf values, indicating a comparable composition. Notably, the ESL (5 μL) sample displayed an end Rf value that precisely matched the end Rf value of quercetin (5 μL), suggesting that the ESL (5 μL) contains only a minimal quantity of quercetin. Furthermore, the beginning Rf values of HLS (2 μL and 5 μL) and HMS (2 μL) precisely matched the Rf value of quercetin, indicating that both samples contain only a small amount of quercetin. However, the results of the HPTLC analysis revealed that quercetin is present in trace amounts in all samples, except for SL and MS.
FTIR Analysis
Fig. 10 shows the FTIR spectrum of Q-ESL and HLS. The FTIR spectra results detected from the isolated compound (Q-ESL) confirm the OH phenolic stretch and hydroxyl stretch at 3408 cm−1 and 3283 cm−1, respectively. Stretching of aryl C=O at 1666 cm−1 C-C aromatic band shows at 1610 cm−1 and aromatic C=C vibration shows at 1519 cm−1, O-H bands of the phenolic group observe at 1379 cm−1, C-H bond starching observe at 1319 cm−1, C-O stretching shows at 1254 cm−1 and 1200 cm−1, aromatic C-H bending appeared at 944 cm−1, 820 cm−1, 639 cm−1, 600 cm−1 and 490 cm−1. The presented results of FTIR spectra conclude the conformation of the Quercetin functional groups.
Figure 10.
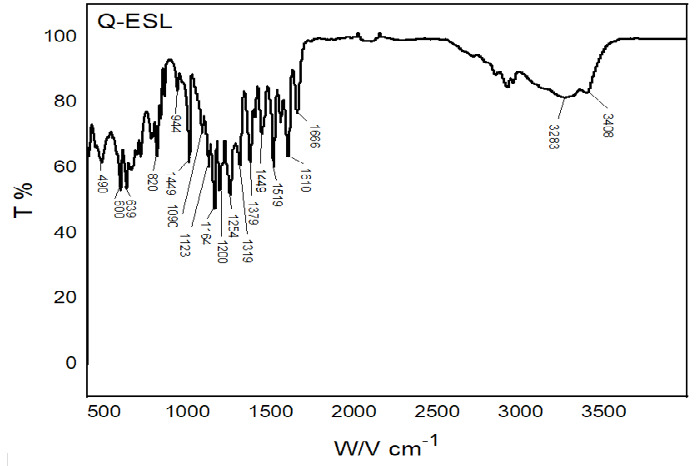
FTIR spectrum of Q-ESL and HLS.
NMR Spectrum Analysis
NMR spectroscopy was employed to investigate the proton and carbon bonding in the isolated compound. The NMR analysis provided valuable insights into the structural characteristics of the compound, confirming the presence of quercetin. The obtained NMR results, which offer detailed information about the proton and carbon of Isolated compound is depicted in Figs. 9 and 10.
The 1H NMR spectrum of Quercetin (400MHz, DMSO-d6): In Fig. 11, the phenyl OH proton shows at the range of 12.450 ppm (s, H), OH proton at 9.357 ppm (s, 2H). The other two OH protons which have the same environment appeared at the range of 7.643 ppm (s, 2H). Aromatic protons appeared in the range of 7.546 ppm (d, J = 4.2Hz, 1H), 6.896 ppm (d, J = 4.2Hz, 1H), 6.422 ppm (s, H), 6.186 ppm (s, 2H). As per the result aromatic protons appeared in the 6.184 ppm–7.643 ppm region and the phenyl OH appeared in the 12.457 ppm and 9.381 ppm which strongly confirms the quercetin structure.
Figure 11.
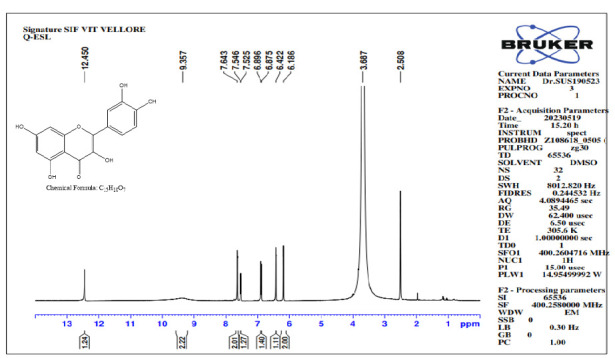
Proton NMR spectra of Q-ESL.
Fig.12 shows the 13C NMR spectrum of Quercetin. The 13C NMR spectrum of Quercetin (100 MHz, DMSO-d6, δ) δ 176.25, 164.29, 161.01, 159.59, 148.11, 147.27, 145.47, 136.13, 122.39, 120.54, 116.04, 115.43, 103.43, 98.66, 93.88. The 13C spectra show that 176.25 ppm is a carbonyl carbon peak and the other attached carbons showed 164.29 ppm–156.59 ppm. The respective peaks of 148.11 ppm–122.39 ppm are carbons attached to the OH functional group. All the aromatic carbons appeared at 116.04 ppm–93.88 ppm. The advanced NMR spectrum results confirm the structure of Quercetin.
Figure 12.
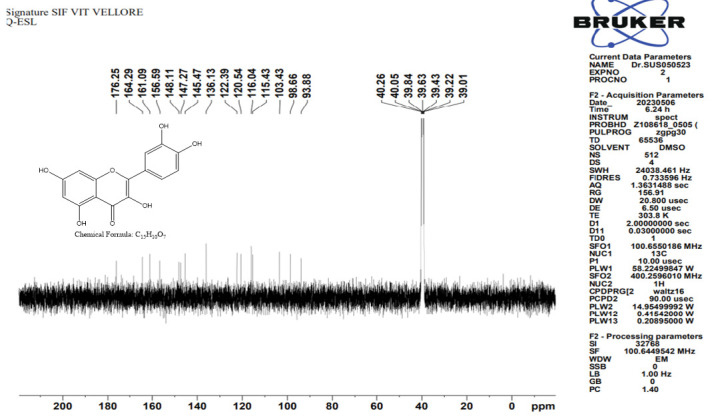
C13 Spectra of Q-ESL.
HR-MS Spectroscopy
HR-MS spectroscopy was performed to conclude the molecular weight of the isolated compound. The spectrum confirms the molecular ion peak [M + H]+ at a range of 303.04 which is exactly similar to the Quercetin molecular weight (see Fig. 13).
Figure 13.
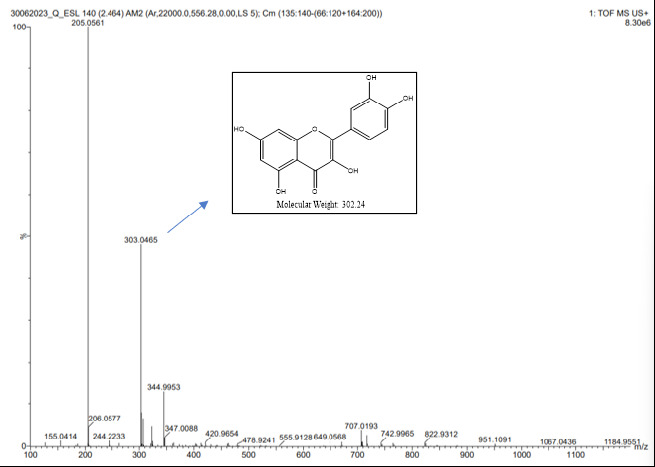
HRMS spectra of Q-ESL.
The phytochemical screening of the extract derived from I. staphylina yielded compelling findings, highlighting its rich repertoire of secondary metabolites (Banerjee & Firdous 2020; Bashir et al. 2019; Harborne 1998). The comprehensive analysis unveiled the presence of various prominent classes of secondary metabolites, including phenols, alkaloids, carbohydrates, sterols, saponins, terpenoids, tannins, fats and oils, and flavonoids. These extensive phytochemical screenings, coupled with subsequent pharmacognostic investigations, accentuate the profound significance of I. staphylina as a valuable source of bioactive compounds.
Employing HPTLC, the presence of the flavonoid quercetin was effectively detected, consolidating its status as a constituent within the extract (Jain et al. 2021; Ved et al. 2022). The isolation of the pure bioactive component, quercetin, from the ethanolic extract of I. staphylina was accomplished using advanced instrumentation methodologies, facilitating its successful extraction and characterisation for further scientific exploration and potential applications.
The main objective of this research is to conduct a comprehensive comparison of crude extracts through pharmacognostical analysis and evaluate the antibacterial activity of matured stem extracts against H. pylori bacteria. Various parts of I. staphylina underwent rigorous pharmacognostical studies, leading to successful extraction with different solvents. Tables 1 and 2 present the outcomes of pharmacognostic evaluations and standardisation, showcasing the purity of I. staphylina. Qualitative and quantitative phytochemical analyses revealed the presence of crucial secondary metabolites, including phenols, alkaloids and flavonoids. Herbal drug quality was ensured through stringent tests assessing parameters like loss on drying and ash value, along with fluorescence analysis. Additionally, antioxidant potential was assessed using the DPPH assay, yielding promising results. HPTLC confirmed the presence of quercetin, which was isolated via column chromatography and further characterised using advanced techniques such as NMR, FTIR and HRMS (Davies & Johnson 2007; Meena & Patni 2008; Reid & Sarker 2012).
The primary objective of this research is to scrutinise the antibacterial efficacy against H. pylori, the causative agent of stomach ulcers. The focus is to substantiate, through scientific means, the traditional utilisation of staphylina for addressing stomach disorders and ulcers. Antibacterial assays reveal noteworthy effectiveness in HLS and DME ethanolic extracts derived from matured stems, as evidenced by substantial zones of inhibition against H. pylori. Concurrently, these extracts showcase elevated levels of secondary metabolites, with a pivotal bioactive compound isolated from the ESL extract. These secondary metabolites have the ability to disrupt the membrane of the test pathogens thereby leading to the apoptosis of the test pathogens (Baazeem et al. 2021; Odiba et al. 2014). These findings underscore the potential application of I. staphylina in treating stomach ulcers induced by H. pylori. Furthermore, this research provides scientific validation for the traditional use of I. staphylina in Dharmapuri for stomach ulcer management.
CONCLUSION
In conclusion, this study has aimed to evaluate the antibacterial efficacy against H. pylori, the causative agent of stomach ulcers, with a specific focus on substantiating the traditional use of staphylina in addressing stomach disorders. The ethanolic extracts from matured stem extract demonstrated significant antibacterial activity, as indicated by substantial zones of inhibition against H. pylori. Moreover, these extracts exhibited heightened levels of secondary metabolites, including a crucial bioactive compound isolated from the I. staphylina plant extract. The identified secondary metabolites possess the capacity to disrupt the pathogen’s membrane, leading to the apoptosis of H. pylori. These findings emphasise the potential therapeutic application of I. staphylina in managing stomach ulcers induced by H. pylori. Additionally, the research contributes scientific validation for the traditional use of I. staphylina in Dharmapuri for the management of stomach ulcers, bridging traditional knowledge with empirical evidence.
ACKNOWLEDGEMENTS
The authors wish to express our sincere gratitude to the Vellore Institute of Technology for supporting the study. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
COMPETING INTERESTS: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
AUTHORS’ CONTRIBUTIONS: Lakshmanan Narayanan: Conceptualisation, investigation, formal analysis, methodology, data curation, writing–original draft, writing. Suseem S R: Formal analysis, investigation, writing–review and editing. All authors approved the final version of the manuscript after they had evaluated the findings.
REFERENCES
- Alexander SM, Retnakumar RJ, Chouhan D, Devi TNB, Dharmaseelan S, Devadas K, Thapa N, Tamang JP, Lamtha SC, Chattopadhyay S. Helicobacter pylori in human stomach: The inconsistencies in clinical outcomes and the probable causes. Frontiers in Microbiology. 2021;12:713955. doi: 10.3389/fmicb.2021.713955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir M, Ahmad N, Sarfaroz M, Ahmad W, Ahmad S, Mujeeb M, Pottoo FH. Tamarindus indica fruit: Pharmacognostical standardization, detection of contaminant, and in vitro antioxidant activity. Journal of Pharmacy and Bioallied Sciences. 2019;11(4):355. doi: 10.4103/jpbs.JPBS_46_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baazeem A, Almanea A, Manikandan P, Alorabi M, Vijayaraghavan P, Abdel-Hadi A. In vitro antibacterial, antifungal, nematocidal and growth promotingactivities of Trichoderma hamatum FB10 and its secondary metabolites. Journal of Fungi. 2021;7(5):331. doi: 10.3390/jof7050331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bag AK, Mumtaz SMF. Hepatoprotective and nephroprotective activity of hydroalcoholic extract of Ipomoea staphylina leaves. Bangladesh Journal of Pharmacology. 2013;8(3):263–268. doi: 10.3329/bjp.v8i3.14845. [DOI] [Google Scholar]
- Balasubramaniam G, Sekar M, Badami S. Pharmacognostical, physicochemical and phytochemical evaluation of Strobilanthes kunthianus (Acanthaceae) Pharmacognosy Journal. 2020;12(4):731–741. doi: 10.5530/pj.2020.12.106. [DOI] [Google Scholar]
- Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: A review. Journal of Pharmaceutical Analysis. 2016;6(2):71–79. doi: 10.1016/j.jpha.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A, Firdous SM. In vitro antimutagenic activity of Ipomoea staphylina. Bangladesh Journal of Pharmacology. 2020;15(1):41–43. doi: 10.3329/bjp.v15i1.43446. [DOI] [Google Scholar]
- Bashir A, Farooq S, Bhat ZA. Pharmacognostic standardization and phytochemical evaluation of Ailanthus altissima (Mill.) Swingle leaves. Journal of Drug Delivery and Therapeutics. 2019;9(2):179–183. [Google Scholar]
- Das PK, Vaghela JS, Badore N. Pharmacognostical, phytochemical and fluorescence analysis of the plant Bougainvillea spectabilis (Willd.) Research Journal of Pharmacy and Technology. 2021;14(7):3733–3738. doi: 10.52711/0974-360X.2021.00646. [DOI] [Google Scholar]
- Davies DR, Johnson TM. Isolation of three components from spearmint oil: An exercise in column and thin-layer chromatography. Journal of Chemical Education. 2007;84(2):318. doi: 10.1021/ed084p318. [DOI] [Google Scholar]
- Dias DA, Urban S, Roessner U. A historical overview of natural products in drug discovery. Metabolites. 2012;2(2):303–336. doi: 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firdous SM, Koneri R. Antidiabetic and antioxidant activities of Ipomoea staphylina leaves in streptozotocin (STZ) induced diabetic mice. Journal of PharmaSciTech. 2014;3(2):77–84. [Google Scholar]
- Gomathi D, Kalaiselvi M, Ravikumar G, Sophia D, Gopalakrishnan VK, Uma C. Secondary metabolite credentials of Evolvulus alsinoides by high performance thin layer chromatography (HPTLC) Journal of Biomedical Research. 2012;26(4):295–302. doi: 10.7555/JBR.26.20110128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonelimali FD, Lin J, Miao W, Xuan J, Charles F, Chen M, Hatab SR. Antimicrobial properties and mechanism of action of some plant extracts against food pathogens and spoilage microorganisms. Frontiers in Microbiology. 2018;9:1639. doi: 10.3389/fmicb.2018.01639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gülşen A, Makris DP, Kefalas P. Biomimetic oxidation of quercetin: Isolation of a naturally occurring quercetin heterodimer and evaluation of its in vitro antioxidant properties. Food Research International. 2007;40(1):7–14. doi: 10.1016/j.foodres.2006.07.009. [DOI] [Google Scholar]
- Gunjal Sanket B, Dighe PR. Analysis of herbal drugs by HPTLC: A review. Asian Journal of Pharmaceutical Research and Development. 2022;10(2):125–128. doi: 10.22270/ajprd.v10i2.1056. [DOI] [Google Scholar]
- Harborn AJ. Phytochemical methods a guide to modern techniques of plant analysis. Springer Science and Business Media; 1998. [Google Scholar]
- Isildak Ö, Yildiz I, Genc N. A new potentiometric PVC membrane sensor for the determination of DPPH radical scavenging activity of plant extracts. Food Chemistry. 2022;373:131420. doi: 10.1016/j.foodchem.2021.131420. [DOI] [PubMed] [Google Scholar]
- Jain PK, Jain S, Sharma S, Paliwal S, Singh G. Evaluation of anti-diabetic and antihypertensive activity of Phoenix sylvestris (L.) Roxb leaves extract and quantification of biomarker Quercetin by HPTLC. Phytomedicine Plus. 2021;1(4):100136. doi: 10.1016/j.phyplu.2021.100136. [DOI] [Google Scholar]
- Jeyadevi R, Ananth DA, Sivasudha T. Hepatoprotective and antioxidant activity of Ipomoea staphylina Linn. Clinical Phytoscience. 2019;5(1):1–11. doi: 10.1186/s40816-019-0112-4. [DOI] [Google Scholar]
- Johnston A, Scaggs J, Mallory C, Haskett A, Warner D, Brown E, Hammond K, McCormick MM, McDougal OM. A green approach to separate spinach pigments by column chromatography. Journal of Chemical Education. 2013;90(6):796–798. doi: 10.1021/ed300315z. [DOI] [Google Scholar]
- Khanal C, Swar S, Tandulkar U. Handbook of pharmacognosy. Kathmandu, Nepal: Natural Product Research Laboratory; 2018. [Google Scholar]
- Khandelwal K. Practical pharmacognosy. Pune, India: Pragati Books Pvt. Ltd; 2008. [Google Scholar]
- Kishikawa H, Ojiro K, Nakamura K, Katayama T, Arahata K, Takarabe S, Miura S, Kanai T, Nishida J. Previous Helicobacter pylori infection-induced atrophic gastritis: A distinct disease entity in an understudied population without a history of eradication. Helicobacter. 2020;25(1):e12669. doi: 10.1111/hel.12669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottaimuthu R. Ethnobotany of the Valaiyans of Karandamalai, Dindigul District, Tamil Nadu, India. Ethnobotanical Leaflets. 2008;2008(1):24. [Google Scholar]
- Krishnaveni S, Balasubramanian T, Sadasivam S. Sugar distribution in sweet stalk sorghum. Food Chemistry. 1984;15(3):229–232. doi: 10.1016/0308-8146(84)90007-4. [DOI] [Google Scholar]
- Kumar A, Lakshman K, Jayaveera KN, Tripathi SM, Satish KV. Estimation of gallic acid, rutin and quercetin in Terminalia chebula by HPTLC. Jordan Journal of Pharmaceutical Sciences. 2010;3(1):63–68. [Google Scholar]
- Kumar TD, Pullaiah T. Ethno-medico-botany of chenchus of Mahaboobnagar District, Andhra Pradesh. Ancient Science of Life. 1999;19(1–2):31. [PMC free article] [PubMed] [Google Scholar]
- Lee S-Y. Helicobacter pylori infection and the Kyoto classification of gastritis. The Korean Journal of Helicobacter and Upper Gastrointestinal Research. 2019;19(2):81–87. doi: 10.7704/kjhugr.2019.19.2.81. [DOI] [Google Scholar]
- López-Bascón MA, De Castro MDL. Soxhlet extraction. In: Poole CF, editor. Liquid-phase extraction. Elsevier; 2020. pp. 327–354. [DOI] [Google Scholar]
- Majumdar D, Bebb J. Helicobacter pylori infection and peptic ulcers. Medicine. 2019;47(5):292–300. doi: 10.1016/j.mpmed.2019.02.008. [DOI] [Google Scholar]
- Majumdar D, Looi S. Helicobacter pylori infection and peptic ulcers. Medicine. 2024;52(3):152–160. doi: 10.1016/j.mpmed.2023.12.006. [DOI] [Google Scholar]
- Maqbool M, Dar MA, Gani I, Mir SA, Khan M. Herbal medicines as an alternative source of therapy: A review. World Journal of Pharmacy and Pharmaceutical Sciences. 2019;3:374–380. [Google Scholar]
- Meena MC, Patni V. Isolation and identification of flavonoid “quercetin” from Citrullus colocynthis (Linn.) Schrad. Asian Journal of Experimental Sciences. 2008;22(1):137–142. [Google Scholar]
- Mir PA, Dar MA, Bader GN. Pharmacognostical standardization, phytochemical investigation, and anthelminthic activity of Arisaema propinquum Schott rhizomes. Pharmacognosy Research. 2020;12(2):181– 185. doi: 10.4103/pr.pr_106_19. [DOI] [Google Scholar]
- Misra D, Mandal M, Ghosh NN, Mandal V. Pharmacognostic standardization of an ethnomedicinal aquatic herb, Monochoria hastata (L.) Solms for its antibacterial potentiality. Pharmacognosy Journal. 2018;10(3):533–540. doi: 10.5530/pj.2018.3.87. [DOI] [Google Scholar]
- Muralidharan R, Narasimhan D. Plants used for topical application from Gingee hills, Tamil Nadu, India. Current Botany. 2013;3(4):49–52. [Google Scholar]
- Narra P, Kandavara P. Evaluation of cytotoxic, antimicrobial and antiinflammatory properties from the latex of Ipomea staphylina. Journal of Microbiology, Biotechnology and Food Sciences. 2014;3(5):350–352. [Google Scholar]
- Newman DJ, Cragg GM, Snader KM. The influence of natural products upon drug discovery. Natural Product Reports. 2000;17(3):215–234. doi: 10.1039/a902202c. [DOI] [PubMed] [Google Scholar]
- Odiba J, Musa A, Hassan H, Yahay S, Okolo E. Antimicrobial activity of isolated stigmast-5-en-3-β-ol (β-sitosterol) from honeybee propolis from North-Western, Nigeria. International Journal of Pharma Sciences and Research. 2014;5(12):908–918. [Google Scholar]
- Parvez MK, Ahmed S, Al-Dosari MS, Abdelwahid MAS, Arbab AH, Al-Rehaily AJ, Al-Oqail MM. Novel anti-hepatitis B virus activity of Euphorbia schimperi and its quercetin and kaempferol derivatives. ACS Omega. 2021;6(43):29100–29110. doi: 10.1021/acsomega.1c04320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra M. Isolation, characterization of phytoconstituents and pharmacological screening of Ipomoea staphylina. Asian Journal of Pharmaceutical and Clinical Research. 2013;6(1):30–33. [Google Scholar]
- Ramesh M, Deepa C, Kumar LR, Sanjay MR, Siengchin S. Life-cycle and environmental impact assessments on processing of plant fibres and its biocomposites: A critical review. Journal of Industrial Textiles. 2022;51(4):5518S–5542S. doi: 10.1177/1528083720924730. [DOI] [Google Scholar]
- Ramesh M, Rajeshkumar L, Deepa C, Tamil Selvan M, Kushvaha V, Asrofi M. Impact of silane treatment on characterization of Ipomoea staphylina Plant fiber reinforced epoxy composites. Journal of Natural Fibers. 2022;19(13):5888–5899. doi: 10.1080/15440478.2021.1902896. [DOI] [Google Scholar]
- Reid RG, Sarker SD. Isolation of natural products by low-pressure column chromatography. Natural Products Isolation. 2012;864:155–187. doi: 10.1007/978-1-61779-624-1_7. [DOI] [PubMed] [Google Scholar]
- Sahidin I, Rahim AR, Arba M, Yodha AWM, Rahmatika NS, Sabandar CW, Manggau MA, Khalid RM, Al Muqarrabun LMR, Rosandy AR. Radical scavenging and antimicrobial activities of diarylheptanoids and steroids from Etlingera calophrys rhizome. Sustainable Chemistry and Pharmacy. 2022;29:100767. doi: 10.1016/j.scp.2022.100767. [DOI] [Google Scholar]
- Sambandam B, Thiyagarajan D, Ayyaswamy A, Raman P. Extraction and isolation of flavonoid quercetin from the leaves of Trigonella foenum-graecum and their anti-oxidant activity. International Journal of Pharmacy and Pharmaceutical Sciences. 2016;8(6):120–124. [Google Scholar]
- Sarvalingam A, Rajendran A, Sivalingam R. Wild edible plant resources used by the Irulas of the Maruthamalai Hills, Southern Western Ghats, Coimbatore, Tamil Nadu. Indian Journal of Natural Products and Resources. 2014;5(2):198–201. [Google Scholar]
- Saxena HO, Parihar S, Pawar G, Choubey SK, Dhar P. Phytochemical screening and HPTLC fingerprinting of different parts of Solanum indicum L.: A dashmool species. Journal of Pharmacognosy and Phytochemistry. 2021;10(1):1935–1941. doi: 10.22271/phyto.2021.v10.i1aa.13633. [DOI] [Google Scholar]
- Sihag S, Pal A, Saharan V. Antioxidant properties and free radicals scavenging activities of pomegranate (Punica granatum L.) peels: An in-vitro study. Biocatalysis and Agricultural Biotechnology. 2022;42:102368. doi: 10.1016/j.bcab.2022.102368. [DOI] [Google Scholar]
- Snehalatha VR, Rasmi AR. Phytochemical evaluation and pharmacognostic standardization of Syzygium palghatense endemic to Western Ghats. Future Journal of Pharmaceutical Sciences. 2021;7:1–13. doi: 10.1186/s43094-021-00282-8. [DOI] [Google Scholar]
- Song Y, Sun H, Zhang A, Yan G, Han Y, Wang X. Plant-derived natural products as leads to anti-cancer drugs. Journal of Medicinal Plant and Herbal Therapy Research. 2014;2:6–15. [Google Scholar]
- Soni A, Sosa S. Phytochemical analysis and free radical scavenging potential of herbal and medicinal plant extracts. Journal of Pharmacognosy and Phytochemistry. 2013;2(4):22–29. [Google Scholar]
- Sundaramoorthy B, Mandal AK, Soman V, Sampangiramulu SDM, Narayana SKK, Ramachandran S, Parameswaran S, Ganesan K. Standardization of Kirāmpu kutinīr decoction and extract granules using pharmacognostic, physicochemical and HPTLC studies. International Journal of Pharmaceutical Investigation. 2021;11(4):362–367. doi: 10.5530/ijpi.2021.4.65. [DOI] [Google Scholar]
- Süntar I. Importance of ethnopharmacological studies in drug discovery: Role of medicinal plants. Phytochemistry Reviews. 2020;19(5):1199–1209. doi: 10.1007/s11101-019-09629-9. [DOI] [Google Scholar]
- Swaroop A, Gupta AP, Sinha AK. Simultaneous determination of quercetin, rutin and coumaric acid in flowers of Rhododendron arboreum by HPTLC. Chromatographia. 2005;62:649–652. doi: 10.1365/s10337-005-0669-6. [DOI] [Google Scholar]
- Tiwari P, Patel RK. Development and validation of HPTLC method for quantification of quercetin and rutin in Drakshasava. Asian Journal of Research in Chemistry. 2012;5(5):681–686. [Google Scholar]
- Tolkushin AG, Luchinin EA, Kholovnya-Voloskova ME, Zavyalov AA. History of aminoquinoline preparations: From cinchona bark to chloroquine and hydroxychloroquinon. Problemy Sotsial’noi Gigieny, Zdravookhraneniia i Istorii Meditsiny. 2020;28(Special issue):11181122. doi: 10.32687/0869-866X-2020-28-s2-1118-1122. [DOI] [PubMed] [Google Scholar]
- Ved A, Gupta S, Singh N, Shukla KS, Prakash O, Gaur N. Pharmacognostical standardization, isolation of phytoconstituents (β-sitosterol), HPTLC analysis of extracts of Operculina turpethum (Linn.) roots and evaluation of cytotoxic, in vitro, and in vivo anti-inflammatory activities. Letters in Organic Chemistry. 2022;19(8):636–650. doi: 10.2174/1570178618666210413143914. [DOI] [Google Scholar]
- Wylie MR, Windham IH, Blum FC, Wu H, Merrell DS. In vitro antibacterial activity of nimbolide against Helicobacter pylori. Journal of Ethnopharmacology. 2022;285:114828. doi: 10.1016/j.jep.2021.114828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz MA. Simultaneous quantitative screening of 53 phytochemicals in 33 species of medicinal and aromatic plants: A detailed, robust and comprehensive LC-MS/MS method validation. Industrial Crops and Products. 2020;149:112347. doi: 10.1016/j.indcrop.2020.112347. [DOI] [Google Scholar]
- Youssefi M, Tafaghodi M, Farsiani H, Ghazvini K, Keikha M. Helicobacter pylori infection and autoimmune diseases: Is there an association with systemic lupus erythematosus, rheumatoid arthritis, autoimmune atrophy gastritis and autoimmune pancreatitis? A systematic review and meta-analysis study. Journal of Microbiology, Immunology and Infection. 2021;54(3):359–369. doi: 10.1016/j.jmii.2020.08.011. [DOI] [PubMed] [Google Scholar]
- Zahoor M, Shafiq S, Ullah H, Sadiq A, Ullah F. Isolation of quercetin and mandelic acid from Aesculus indica fruit and their biological activities. BMC Biochemistry. 2018;19(1):1–14. doi: 10.1186/s12858-018-0095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S-F, Zhou Z-W, Li C-G, Chen X, Yu X, Xue CC, Herington A. Identification of drugs that interact with herbs in drug development. Drug Discovery Today. 2007;12(15–16):664–673. doi: 10.1016/j.drudis.2007.06.004. [DOI] [PubMed] [Google Scholar]