Abstract
The Sunda porcupine (Hystrix javanica, F.Cuvier, 1823) is a rodent-mammal species native to Indonesia and is utilised in traditional medicine for the treatment of various ailments. Some ethnic communities in Indonesia have traditional beliefs regarding Sunda porcupine’s quills, which are thought to relieve back pain and toothache. Despite this traditional knowledge, there is limited scientific research on the topic. The aim of this study was to identify active compound in an ethanolic crude extract of Sunda porcupine’s quills, and to evaluate its antioxidant and antimicrobial properties. The antioxidant activity was evaluated using 1,1-diphenyl-2-picrylhydrazyl (DPPH)-free radical scavenging assay while the antimicrobial activity was evaluated through microdilution resazurin assay. The total phenolic and flavonoid contents were also determined to support the antioxidant properties. The active compounds were identified using gas chromatography-mass spectrophotometer (GCMS) with the National Institute of Standards and Technology (NIST-11) library. The result showed that the extract possesses antioxidant properties (IC50 138.93 μg/mL) and antimicrobial properties against Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), Bacillus subtilis (B. subtilis), Pseudomonas aeruginosa (P. aeruginosa) and Candida albicans (C. albicans) (IC50 range 0.40 mg/mL–33.05 mg/mL). Total phenolic content (TPC) and total flavonoid content (TFC) were 27.29 ± 2.20 mgGAE/g and 27.09 ± 1.66 mgQE/g, respectively. A total of 24 active compounds from the crude extract were identified. As much as five compounds serve as antioxidant agents, including: butylated hydroxytoluene; eicosane; 1-iodo-hexadecane; methyl ester hexadecanoic acid; and L-(+)-ascorbic acid 2,6-dihexadecanoate. Furthermore, as much as 11 compounds serve as antimicrobial agents, including: tetradecane; pentadecane; 2-isopropyl-5-methyl-1-heptanol; hexadecane; butylated hydroxytoluene; eicosane; 1-iodo-hexadecane; methyl ester hexadecanoic acid; benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, methyl ester; L-(+)-ascorbic acid 2,6-dihexadecanoate; and octadecanoic acid. This study provides scientific validation for the use of the Sunda porcupine’s quills in traditional medicine and highlights the potential for further research in animal bioprospecting.
Keywords: Antioxidant, Antimicrobial, GCMS, Sunda Porcupine, Hystrix javanica
Highlights
The extract possesses antioxidant properties (DPPH IC50 138.93 μg/mL) and antimicrobial properties against E. coli, P aeruginosa, S. aureus, B. subtilis and C. albicans (IC50 range 0.40 mg/mL–33.05 mg/mL).
Total phenolic and total flavonoid content were 27.29 ± 2.20 mgGAE/g and 27.09 ± 1.66 mgQE/g.
A total of 24 active compounds from the crude extract were identified. As much as 5 compounds serve as antioxidant agents, including: butylated hydroxytoluene; eicosane; 1-iodo-hexadecane; methyl ester hexadecanoic acid; and L-(+)-ascorbic acid 2,6-dihexadecanoate. Furthermore, as much as 11 compounds serve as antimicrobial agents, including: tetradecane; pentadecane; 2-isopropyl-5-methyl-1-heptanol; hexadecane; butylated hydroxytoluene; eicosane; 1-iodo-hexadecane; methyl ester hexadecanoic acid; benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, methyl ester; L-(+)-ascorbic acid 2,6-dihexadecanoate; and octadecanoic acid.
INTRODUCTION
Animal-based medicine has been used for centuries in traditional medicine systems around the world. However, its use is not as widespread as that of plant-based medicine due to various factors such as cultural, religious, conservation and abundance considerations. The medicine can be derived from diverse range of animal materials, including animal metabolites, body parts or non-animal components such as bird nests, bee hives and cocoons. Inspired by ethnobiology and ethnomedicine, research into animal-based medicine has been conducted globally, including in Indonesia. Mardiastuti et al. (2021) documented the use of a variety of animal species in Indonesian traditional medicine, including 59 mammal species, 12 bird species, 37 reptile species and six amphibian species, with porcupines being one of the species used.
The porcupine is widely used in traditional medicine for centuries. Local people in Betung Kerihun National Park, West Kalimantan, Indonesia used porcupine in their traditional medicine (Putra et al. 2008). In Kalimantan (Borneo), porcupine’s quills are grinded into flour to treat acne and burned to relieve back pain (Inayah 2016; Krisyanto et al. 2019). Azliza et al. (2012) reported that the people in Ulu Kuang Village, Malaysia used the porcupine’s quills to treat asthma and breathlessness. In Java, some locals use the porcupine’s quills as a medicine for toothaches and ulcers (Inayah 2016; Krisyanto et al. 2019). Gomez (2021) reported that the porcupine’s quills are used for traditional medicine in Aceh, Bali and Kalimantan (Borneo). Another part of porcupine, bezoar stone, was reported to be used in traditional medicine in South East Asia and Europe to treat cancer, poisoning, fever and typhoid (Heinrich et al. 2020). Khan et al. (2019), reported that the porcupine’s bezoar stone is scientifically proven to have anticancer activity through in vivo and in vitro studies. However, these reported traditional medicine (ethnomedicine) of porcupine need scientific support to reveal the potency as medicinal candidate since the study is still limited.
One species of the porcupine that is thought to be used in traditional medicine is the Sunda porcupine (Hystrix javanica, F.Cuvier, 1823), which is reported to be found in certain regions of Indonesia including Java, Bali, Sumbawa, Flores, Lombok, Madura and Tonahdjampea (Van Weers 1979, 1983; Woods & Kilpatrick 2005; Aplin 2016). Recent research has investigated the potential of the Sunda porcupine in animal-based medicine. Prawira et al. (2020) reported the rapid wound healing in this species, while Gifardi et al. (2022) demonstrated that Sunda porcupine’s quills hexane extract could inhibit the growth of Staphylococcus aureus, a bacteria that infect the skin at certain concentration levels. Furthermore, Anita et al. (2017) reported that the tail meat of Sunda porcupine possesses aphrodisiac potency.
The exploration of active compounds from Sunda porcupine’s quills is of interest since its utilisation in traditional medicine and the lack of study in this area. This study aims to identify active compounds in Sunda porcupine’s quills ethanolic crude extract as well as to evaluate its antioxidant and antimicrobial properties. This research is expected to provide new knowledge and contribute to the discovery of traditional medicine as a potential source of drugs.
MATERIALS AND METHODS
Sample Extraction
Sunda porcupine’s quills were collected from the remains of physiological research samples and the simplisia were obtained by drying the quills in an oven (Isuzu model AT-S13, Japan) at 50°C for five days. The simplisia were grounded to a size of 60 mesh and had a water content of 9.1%. A maceration method was used to extract the active compound inside simplisia with a simplisia to solvent ratio of 1:30 using 70% ethanol (Merck, Germany). The crude extract was obtained by evaporating all solvent using a rotary evaporator (IKA model RV 10D S99, Germany) at 50°C. Then, it was stored at 4°C until further use (Budiman et al. 2021).
Determining Antioxidant Activity using DPPH-free Radicals Scavenging Assay
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) (Sigma Aldrich, Germany) free radical was used to measure the antioxidant activity of the Sunda porcupine’s quills crude extract. The procedure used was adapted from Handayani et al. (2022) and Aryal et al. (2019) with slight modifications. The sample was dissolved in methanol (Merck, Germany) at concentrations ranging from 0 μg/mL to 250 μg/mL. A mixture of 2 mL of the sample and 2 mL of 0.1 mM DPPH was incubated in the dark for 30 min. The absorbance of the sample was measured using a UV-Vis spectrophotometer (Thermo Fisher Scientific model Genesys 10-S, US) at a wavelength of 517 nm. The antioxidant activity was calculated using the following formula:
To determine the inhibitory concentration of 50% (IC50) value, the antioxidant activity score obtained from the DPPH assay was plotted against the concentration of the sample. The concentration of the sample that caused a 50% reduction of DPPH was determined from the graph as the IC50 value. Sample with lower IC50 value were considered more effective in neutralising free radicals.
Determining Total Phenolic Content (TPC)
A sample was first dissolved in distilled water to obtain a 1,000 μg/mL solution. A standard curve was created using gallic acid with a range of serial concentrations from 0 μg/mL to 200 μg/mL. A 0.2 mL sample or standard was added into 1.8 mL of distilled water and 0.2 mL of Folin-Ciocalteu reagent (Merck, Germany). The solution was homogenised and incubated for 6 min. After that, 2 mL of Na2CO3 7% (w/v) (Merck, Germany) was added to the solution, homogenised and incubated for 90 min. The absorbance was then measured at 750 nm using a UV-Vis spectrophotometer. TPC was calculated in milligram gallic acid equivalent per gram (mgGAE/g) sample (Maeng et al. 2017).
Determining Total Flavonoid Content (TFC)
The determination of TFC followed the Dowd method as described by Aryal et al. (2019). A sample solution of 1,000 μg/mL was prepared using distilled water. Quercetin (Sigma Aldrich, China) was used as a standard with serial concentrations ranging 0 μg/mL to 100 μg/mL. A 1 mL of prepared sample or standard was added to a mixture of 0.2 mL AlCl3 10% (w/v) (Merck, Germany) in methanol (Merck, Germany), 0.2 mL CH3COOK 1 M (Merck, Germany), and 5.6 mL distilled water. The solution was homogenised and incubated for 30 min. The absorbance was measured with a UV-Vis spectrophotometer at 415 nm. TFC was calculated in milligram quercetin equivalent per gram (mgQE/g) sample.
Determining Antimicrobial Activity using Resazurin Assay
The antimicrobial activity was assessed using microdilution method incorporated with resazurin (Sigma Aldrich, China) as the indicator of cell viability (EUCAST 2022; Sarker et al. 2007). The assay was conducted in 96-well plate against Escherichia coli (E. coli), Staphylococcus aureus (S. aureus), Bacillus subtilis (B. subtilis), Pseudomonas aeruginosa (P. aeruginosa) and Candida albicans (C. albicans). Prior to antimicrobial assay, the target microbes were grown in Luria Bertani (LB) broth (Himedia, India) and incubated overnight in an incubator
shaker (Bio-Shaker BR-300LF, Japan). The target microbes were then adjusted using McFarland turbidity standard 0.5 (Himedia, India) and diluted 1,000×, so that the final concentration of the cells was ± 1.5 × 105 CFU/mL. Antimicrobial assay of the extract was then conducted with concentration 100 mg/mL as the starting point and serially diluted to several concentrations followed by overnight incubation at 37°C. After the addition of 30 μL 0.1% resazurin, the cell suspension then incubated overnight and read under fluorescence with multimode reader Varioskan Lux (Thermo Fisher Scientific, US) with 530 excitation and 590 emission. The fluorescence data were used to determine the inhibition activity (%) using the following equation:
The IC50 was determined through a dose-response relationship using linear regression analysis, with the transformation of the concentration to a logarithmic scale.
Identification of Active Compound Using GCMS
The Sunda porcupine’s quills ethanolic crude extract was dissolved with dichloromethane (Merck, Germany) to make a 1,000 μg/mL solution. It was then filtered using a minisart syringe membrane 0.22 μm (Sartorius, Germany). The filtrate was injected into a gas chromatography-mass spectrophotometer (GCMS) instrument (Shimadzu GCMS-QP 2010 Ultra, Japan) equipped with an Rtx-5MS column (5% diphenyl: 95% dimethyl-polysiloxane) with length of 30 m and diameter of 0.25 mm. The mobile phase consisted of ultra-high purity helium on 30 kPa. The injector temperature was set at 200°C, the ion source at 230°C, and the interphase at 280°C, with a splitless injection mode. The oven temperature program was initiated at 60°C and increased to 150°C at a rate of 10°C/min and held for 3 min. The resulting chromatogram and m/z were compared with the National Institute of Standards and Technology (NIST-11) database to identify the active compound.
RESULTS
Antioxidant Activity, TPC and TFC
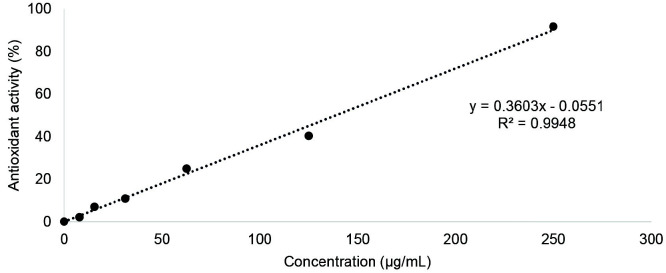
The antioxidant activity of Sunda porcupine’s ethanolic crude extract was assessed at varying concentrations, ranging from 0 μg/mL to 250 μg/mL. The curve of antioxidant activity against the sample concentration was determined with a regression equation of y = 0.3603× – 0.0551 and R2 = 99.48% as illustrated in Fig. 1. The IC50 of the extract’s antioxidant against DPPH free radical scavenging activity was determined to be 138.93 μg/mL.
Figure 1.
Antioxidant activity of Sunda porcupine’s ethanolic crude extract using DPPH free radical scavenging assay.
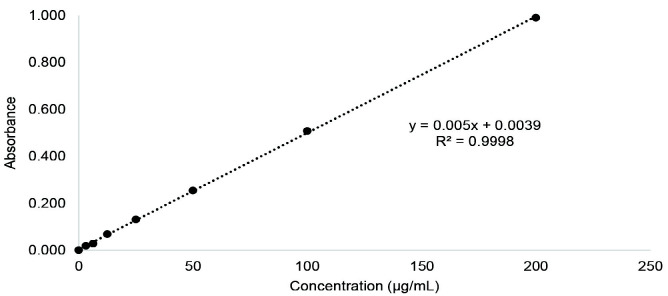
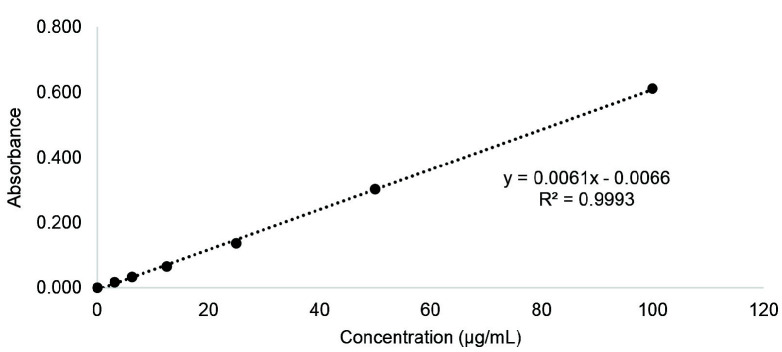
The standard curve of gallic acid and quercetin were used to determine the TPC and TFC as illustrated in Fig. 2. The linear regression equation of gallic acid and quercetin were derived as y = 0.005× + 0.0039 (R2 = 99.98%) and y = 0.0061× – 0.0066 (R2 = 99.93%), respectively. The TPC and TFC of the extract were calculated to be 27.29 ± 2.20 mgGAE/g sample and 27.09 ± 1.66 mgQE/g sample, respectively.
Figure 2.
Standard curve of (a) gallic acid and (b) quercetin.
Antimicrobial Activity
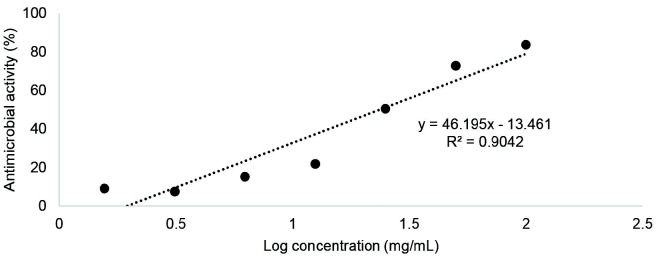
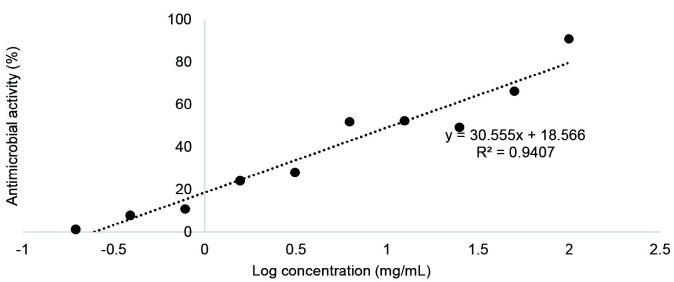
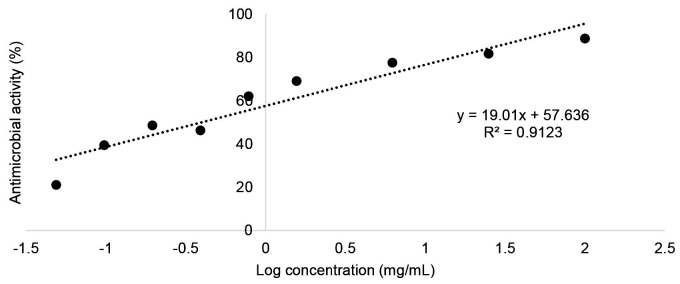
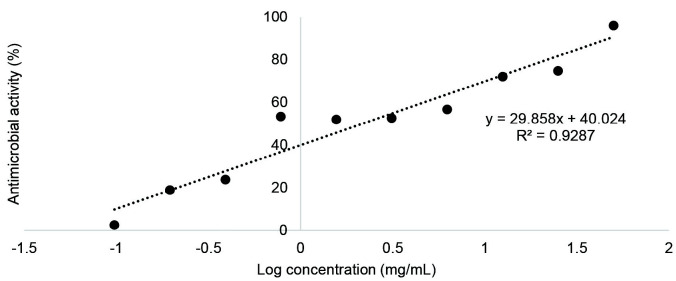
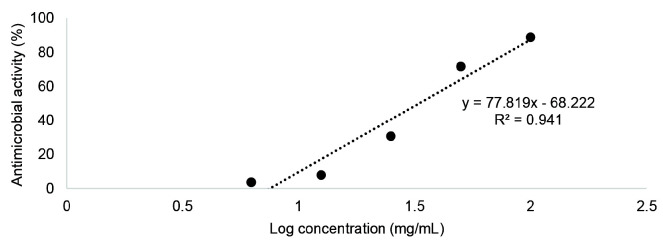
In the present study, the antimicrobial activity of Sunda porcupine’s quills ethanolic crude extract was evaluated for its antimicrobial activity against various microorganisms, including E. coli, P. aeruginosa, S. aureus, B. subtilis and C. albicans. The result is presented in Fig. 3, which shows the linear regression of antimicrobial activity against the log concentration of the extract for each microorganism.
Figure 3.
Antimicrobial activity of Sunda porcupine’s quills ethanolic crude extract against (a) E. coli, (b) P. aeruginosa, (c) S. aureus, (d) B. subtilis, and (e) C. albicans.
The data revealed that the extract possessed significant antimicrobial activity against all the tested microorganisms. The highest antimicrobial activity was observed against S. aureus with an IC50 of 0.40 mg/mL (Table 1), indicating that the extract could be a potential source of antibacterial agents, mainly to Gram positive bacteria.
Table 1.
The antimicrobial IC50 of Sunda porcupine’s quills ethanolic crude extract.
| Microorganism | Group | IC50 (mg/mL) |
|---|---|---|
| Escherichia coli | Gram negative | 23.65 |
| Pseudomonas aeruginosa | Gram negative | 10.68 |
| Staphylococcus aureus | Gram positive | 0.40 |
| Bacillus subtilis | Gram positive sporadic | 2.16 |
| Candida albicans | Fungi | 33.05 |
The linear regression equation for E. coli, P. aeruginosa, S. aureus, B. subtilis and C. albicans were y = 46.195× – 13.461 (R2 = 90.42%), y = 30.555× + 18.566 (R2 = 94.07%), y = 19.01× + 57.636 (R2 = 91.23%), y = 29.858× + 40.024 (R2 = 92.87%), and y = 77.819× + 68.222 (R2 = 94.10%) respectively with y-axis representing the percentage of antimicrobial activity and x-axis representing the log concentration of the extract (Fig. 3). These findings suggest that Sunda porcupine’s ethanolic crude extract could be a promising candidate for the development of novel antimicrobial agents.
Identification of Active Compound Using GCMS
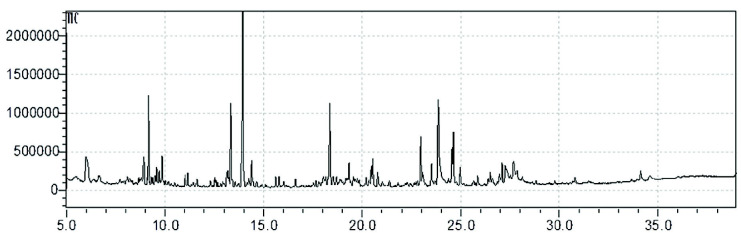
The GCMS analysis of the extract resulted in the chromatogram as shown in Fig. 4. After comparing the m/z data with the NIST-11 database, a total of 24 compounds were identified and are listed in Table 2. Among these compounds, six exhibit a relatively high intensity proportional to the percentage of area (more than 5%), including butylated hydroxytoluene (20.94%), L-(+)-ascorbic acid 2,6-dihexadecanoate (14.60%), eicosane (8.86%), 5-methyl-1-phenylbicyclo [3.2.0] heptane (8.21%), pentadecane (6.88%), and hexadecane (6.30%). The highest intensity to the percentage of area in chromatogram was found at retention time of 13.952 min and presumably represent butylated hydroxytoluene. It has been reported by Ayaz et al. (1980) and Lim et al. (1987) that it possesses antioxidant and antimicrobial properties. Furthermore, the second highest intensity was found at retention time of 23.860 min which presumably represent L-(+)-ascorbic acid 2,6-dihexadecanoate, also known as an antioxidant and antimicrobial agent (Igwe & Okwunodulu 2014; Hadi et al. 2016).
Figure 4.
Chromatogram of Sunda porcupine’s quills ethanolic crude extract.
Table 2.
The active compound identified by GCMS instrument with NIST-11 database.
| No. | Retention time (min) | Area (%) | Name | Molecular formula | Similarity (%) | Role(s) |
|---|---|---|---|---|---|---|
| 1 | 8.921 | 3.07 | Tetradecane | C14H30 | 75 | Antifungal and antibacterial (Ozdemir et al. 2004) |
| 2 | 9.167 | 6.88 | Pentadecane | C15H32 | 92 | Antimicrobial (Martinac et al.1987; Firdaus et al. 2019) |
| 3 | 9.570 | 1.30 | 2-Isopropyl-5-methyl-1-heptanol | C11H24O | 87 | Antimicrobial (Selvin et al. 2009) |
| 4 | 9.700 | 1.04 | 2-methyl-1-decanol | C11H24O | 87 | – |
| 5 | 9.855 | 2.22 | 2,6,11-trimethyl-dodecane | C15H32 | 91 | – |
| 6 | 11.024 | 1.08 | E-14-Hexadecenal | C16H30O | 84 | – |
| 7 | 13.199 | 1.33 | 2,6,11,15-tetramethyl-hexadecane | C20H42 | 82 | Flavouring agent (Pammi et al. 2021) |
| 8 | 13.346 | 6.30 | Hexadecane | C16H34 | 90 | Antifungal and antibacterial (Yogeswari et al. 2012; Akpuaka et al. 2013) |
| 9 | 13.952 | 20.94 | Butylated hydroxytoluene | C15H24O | 95 | Antimicrobial (Ayaz et al. 1980; Lim et al. 1987) |
| 10 | 18.020 | 1.15 | Dodecyl ester chloroacetic acid | C14H27ClO2 | 88 | – |
| 11 | 18.361 | 8.86 | Eicosane | C20H42 | 87 | Antifungal, antibacterial, inhibit foodborne pathogen, antitumour and cytotoxic effect (Hsouna et al. 2011; Yogeswari et al. 2012; Akpuaka et al. 2013; Kazemi 2015; Okechukwu 2020) |
| 12 | 18.538 | 1.23 | 1-iodo-hexadecane | C16H33I | 75 | Inhibitory effect on AD-like lesions, antimicrobial, antioxidant and anticancer (Kim et al. 2022) |
| 13 | 19.167 | 1.07 | 2-methoxycarbonyl-2-methylbrendane | C12H18O2 | 65 | – |
| 14 | 20.459 | 1.73 | Benzoic acid, 2-fluoro-5,6-dimethoxy | C9H9FO4 | 59 | – |
| 15 | 20.539 | 2.70 | (E)-2-Heptenedioic acid, 4-cyclopropyl-, dimethyl ester | C12H18O4 | 53 | – |
| 16 | 20.787 | 1.54 | Hexadecanal | C16H32O | 93 | – |
| 17 | 22.967 | 4.86 | Heptadecane, 2-methyl | C18H38 | 89 | – |
| 18 | 23.055 | 1.70 | Methyl ester hexadecanoic acid | C17H34O2 | 92 | Antioxidant, antifungal and antimicrobial (Hema et al. 2011; Astiti & Ramona 2021) |
| 19 | 23.509 | 1.76 | Benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, methyl ester | C18H28O3 | 91 | Antimicrobial (Ahmad et al. 2012; Akpuaka et al. 2013; Amrati et al. 2021) |
| 20 | 23.860 | 14.60 | L-(+)-ascorbic acid 2,6-dihexadecanoate | C38H68O8 | 86 | Antioxidant, antitumour, wound healing and antimicrobial properties (Igwe & Okwunodulu 2014; Hadi et al. 2016) |
| 21 | 24.550 | 3.51 | 5-methyl-1-phenylbicyclo[3.2.0] heptane | C14H18 | 69 | Antivirus (Poongulali & Sundararaman 2016) |
| 22 | 24.959 | 1.63 | Oxirane, heptadecyl | C19H38O | 93 | – |
| 23 | 27.667 | 3.48 | Octadecanoic acid | C18H36O2 | 83 | Antibacterial and antifungal (Akpuaka et al. 2013) |
|
| ||||||
| 24 | 27.845 | 1.32 | Tetracosane | C24H50 | 87 | Anticancer (Paudel et al. 2019) |
Note: (–) Unknown roles
DISCUSSION
The Sunda porcupine, an endemic mammal species of Indonesia, has a relatively wide distribution across several regions of the country, including Java, Bali, Sumbawa, Flores, Lombok, Madura and Tonahdjampea (Van Weers 1979, 1983; Woods & Kilpatrick 2005; Aplin 2016). This broad distribution has fostered a connection between the Sunda porcupine and the local people resulting traditional knowledge, including ethnobiology and ethnomedicine. This traditional knowledge is frequently not adequately documented and is instead passed down orally from generation to generation, leading to difficulties in accessing this information. Some indigenous communities in Indonesia are reported to use the Sunda porcupine’s quills for medicinal purposes such as treating acne, relieving backpain, curing ulcer and relieving toothache (Inayah 2016; Krisyanto et al. 2019). Furthermore, the Sunda porcupine’s quills is a unique skin derivate that provides an additional protective layer against harsh environment condition and acts as a defence tool against predators (Prawira et al. 2018). Therefore, it is plausible that the quills contain a certain compound that may be effective in combating environmental stress. The Sunda porcupine is a potential candidate from animal which can be used as medicinal sources since has filtered by its ethnomedicine from enormous natural resources. On the other hand, the use of Sunda porcupine is sustainable because the animal can reproduce easily. Female Sunda porcupine can breed twice a year which can give birth up to four young porcupines in one birthing period (Farida et al. 2019; Suyanto 2012). The Sunda porcupine was also reported to be successfully breed in captivity (Suyanto 2012). In captivity, the porcupine’s quills are a side-product since they shed periodically from the porcupine’s body. Therefore, the Sunda porcupine’s quills are sustainable for medicinal purposes because the porcupine is easily reproduced and the quills are shed periodically as a by-product in captivity.
The present study investigates the active compounds found inside the Sunda porcupine’s quills, specifically focusing on their antioxidant and antimicrobial properties. The quills were extracted using a 70% ethanol solvent via the maceration method. Ethanol was chosen as the solvent due to its safety profile in comparison to other organic solvents. The maceration process was selected to minimise the risk of damaging the active compounds through the application of heat. The extract in this study was identified using GCMS which successfully identified 24 active compounds as shown in Table 2. Most of these compounds were identified as biologically active, which aligns with previous studies.
Antioxidants are compounds that can help reduce oxidative stress, mainly grouped as endogenous and exogenous antioxidant. The endogenous antioxidants are antioxidant normally produced by human’s body such as reduced glutathione, catalase, superoxide dismutase, glutathione peroxidase, and reductase. The exogenous antioxidants are usually taken from the environment by foods or supplements including some vitamins like vitamins A, C and E (Adwas et al. 2019). The antioxidants work by scavenging or stimulating the breakdown of free radicals, both forms of antioxidants can help prevent the production of the free radicals (Maroof & Gan 2022). The antioxidant activity of Sunda porcupine’s quills ethanolic crude extract was determined using DPPH free radicals scavenging assay in various concentrations. Furthermore, the antioxidant activities with their respective concentrations were plotted in a linear regression to determine the antioxidant IC50. The antioxidant IC50 of the extract in present study was 138.93 μg/mL, indicating the concentration of the extract required to neutralise 50% of free radicals. A lower IC50 value indicates a smaller concentration of the sample needed to neutralise free radicals. The antioxidant properties are mostly caused by the content of phenolic and flavonoid compounds (Aryal et al. 2019; Maeng et al. 2017). The TPC and TFC of the extract was 27.29 ± 2.20 mgGAE/g and 27.09 ± 1.66 mgQE/g, respectively. The flavonoids are a part of phenolics. The score of TPC and TFC are close, indicating the phenolics content are mostly in the form of flavonoids.
Moreover, the antioxidant properties of the extract were in line with the identified compound obtained from GCMS analysis. There are five compounds with a total of 47.33%, proportional to the percentage of area in chromatogram responsible with antioxidant properties. These are butylated hydroxytoluene (20.94%), L-(+)-ascorbic acid 2,6-dihexadecanoate (14.60%), eicosane (8.86%), methyl ester hexadecanoic acid (1.70%), and 1-iodo-hexadecane (1.23%). Butylated hydroxytoluene was reported as an antioxidant to inhibit free radicals production for medicine and cosmetics (Ershov & Volod’kin 1962). The L-(+)-ascorbic acid 2,6-dihexadecanoateis a vitamin C derivative and it is important as a lipophilic antioxidant, antitumour, wound healing and antimicrobial properties (Igwe & Okwunodulu 2014; Hadi et al. 2016). Eicosane is a monoterpenic hydrocarbon and is reported to have antioxidant and anti-inflammatory properties by inhibiting the release of cytokines such as histamine, bradykinin, prostaglandins, thromboxanes and leukotrienes in rats (Kazemi 2015; Okechukwu 2020). Methyl ester hexadecanoic acid (methyl palmitate) is a fatty acid group with antioxidant, hypocholesterolaemia, and antiandrogenic properties (Hema et al. 2011; Astiti & Ramona 2021). The 1-iodo-hexadecane has been reported in some plant extract and possesses antioxidant properties (Kim et al. 2022).
The present study also investigates the antimicrobial properties of Sunda porcupine’s quills ethanolic crude extract, in addition to its antioxidant properties. Resazurin assay was used in this study to determine the antimicrobial activity of the extract against E. coli, P. aeruginosa, S. aureus, B. subtilis and C. albicans. The use of resazurin microdilution assay was selected since it can provide more accurate result through spectrophotometry, allowing for precise analysis signal readings. The antimicrobial activity was determined by measuring the resazurin readings after 24 h of incubation for bacteria and 72 h of incubation for yeast. Resazurin (7-hydroxy-3H-phenoxazin-3-one 10-oxide) is a blue dye that can be irreversibly reduced by oxidoreductase in active bacteria to a pink and highly red fluorescent substance called resorufin (Chakansin et al. 2022). This method is based on detection of microbial viability by observing the colour change of resazurin from blue to purple or pink (Jia et al. 2020). The test was considered positive if the well contents were blue in colour, indicating the extract inhibits the growth of microbial, and negative if the well contents were pink, indicating the microbial is still growing in the medium wells. The test results were valid if negative control wells (without extract) remained pink (Germ et al. 2019).
The antimicrobial IC50 values of Sunda porcupine quills extract against E. coli, P. aeruginosa, S. aureus, B. subtilis and C albicans was 23.65 mg/mL, 10.68 mg/mL, 0.40 mg/mL, 2.16 mg/mL and 33.05 mg/mL, respectively. These results indicate that the Sunda porcupine quills extract has a broad antimicrobial range as it can inhibit the growth of Gram-positive bacteria, Gram-negative bacteria, spore-forming bacteria and yeast. In particular, the S. aureus bacteria species was highly sensitive for antimicrobial activity. However, the antimicrobial assay was limited to several species in present study. The further research related to the antimicrobial assay with other microbial species may complete the potential of Sunda porcupine’s quills as an antimicrobial agent. Sunda porcupine’s quills ethanolic crude extract could inhibit the activity of S. aureus at the smallest concentration (0.4 mg/mL or 0.04% equivalent). The concentration of the Sunda porcupine’s quills ethanolic crude extract required to inhibit S. aureus in the present study was found to be significantly lower (0.04%) than reported by Gifardi et al. (2022) for the Sunda porcupine’s quills extracted using hexane solvent, which required at least a concentration of 25% for the inhibition of S. aureus growth. These findings suggest that the antimicrobial compounds in Sunda porcupine’s quills are more soluble in polar solvent such as ethanol 70%. Further research on the extraction and identification of the active compound in Sunda porcupine’s quills could lead to the development of novel and effective antimicrobial agents.
Furthermore, the antimicrobial properties of the Sunda porcupine ethanolic crude extract also appropriate with the GCMS analysis. As much as 11 identified compounds have been reported to possess antimicrobial properties. In total, it is about 70.12% of identified compound, proportional to the percentage of area in chromatogram, that possess antimicrobial properties. These compounds include butylated hydroxytoluene (20.94%), L-(+)-ascorbic acid 2,6-dihexadecanoate (14.60%), eicosane (8.86%), pentadecane (6.88%), hexadecane (6.30%), octadecanoic acid (3.48%), tetradecane (3.07%), benzenepropanoic acid, 3,5-bis(1,1-dimethylethyl)-4-hydroxy-, methyl ester (1.76%), methyl ester hexadecanoic acid (1.70%), 2-isopropyl-5-methyl-1-heptanol (1.30%) and 1-iodo-hexadecane (1.23%).
Butylated hydroxytoluene have been reported as antimicrobial to inhibit growth of some microorganism (Ayaz et al. 1980; Lim et al. 1987). The L-(+)-ascorbic acid 2,6-dihexadecanoate is an ascorbic acid derivate which is potential to prevent and treat common cold, gum diseases, acne, skin infection, tuberculosis, dysentery, and dental caries (Igwe & Okwunodulu 2014). Eicosane have been reported as antifungal, antibacterial, antitumour, inhibit foodborne pathogen, and has cytotoxic effect (Hsouna et al. 2011; Yogeswari et al. 2012; Akpuaka et al. 2013; Kazemi 2015; Okechukwu 2020). Pentadecane is reported as an antimicrobial compound by inhibited growth of E. coli and S. typhi that involves interaction with a cell’s electrical channel protein in silico analysis. Channel protein plays a role in pumping protons concerning the metabolism of amino acids of E. coli and S. typhi (Firdaus et al. 2019; Martinac et al. 1987). Octadecanoic acid or stearic acid is a fatty acid that displays antibacterial activity towards Gram-positive and Gram-negative bacteria (Abdalaziz et al. 2017; Casillas-Vargas et al. 2021). Hexadecane, tetradecane, 2-isopropyl-5-methyl-1-heptanol and 1-iodo-hexadecane has reported to possess antifungal and antimicrobial properties (Selvin et al. 2009; Yogeswari et al. 2012; Akpuaka et al. 2013; Kim et al. 2022). Benzenepropanoic acid was also reported to be effective against microbes such as E. coli, K. pneumoniae, P. aeruginosa and C. albicans (Amrati et al. 2021). Benzoic acid alone is known as a nonspecific antimicrobial agent with a wide spectrum of the activities against human pathogenic fungi and bacteria (Innocenti et al. 2009; Krátký et al. 2012). Methyl ester hexadecanoic acid was reported as antibacterial by disrupting bacterial cell wall and cell membrane (Astiti & Ramona 2021).
CONCLUSION
As much as 24 active compounds were identified from Sunda porcupine’s quills ethanolic crude extract using GCMS. The extract was investigated and showed antioxidant and antimicrobial properties. The IC50 of antioxidant was 138.93 μg/mL, while the IC50 of antimicrobial against E. coli, P aeruginosa, S. aureus, B. subtilis and C. albicans were 23.65 mg/mL, 10.68 mg/mL, 0.40 mg/mL, 2.16 mg/mL and 33.05 mg/mL, respectively. The antioxidant properties were also investigated through the determination of TPC and TFC with values of 27.29 ± 2.20 mgGAE/g and 27.09 ± 1.66 mgQE/g, respectively. There were five identified compounds thar serve as antioxidant and 11 identified compounds that serve as antimicrobial. The two highest intensities to the percentage of area in chromatogram were butylated hydroxytoluene (20.94% with RT = 13.952 min) and L-(+)-ascorbic acid 2,6-dihexadecanoate (14.60% with RT = 23.860 min). Both these compounds have been reported as antioxidant and antimicrobial agents. This study provides scientific validation for the use of the Sunda porcupine’s quills in traditional medicine and highlights the potential for further research in animal bioprospecting.
ACKNOWLEDGEMENTS
This research was supported by The Budget Implementation List of Indonesian Institute of Sciences Year 2021 (research grant number 46/A/DH/2021) and Faculty of Medicine, Universitas Muhammadiyah Prof. DR. HAMKA, Indonesia (Lemlit grant number 867/F.03.07/2022).
Footnotes
ETHICAL STATEMENT: This research is approved by The Committee of Ethical Clearance of Animal Use for Research Purposes in Indonesian Institute of Sciences (LIPI) with protocol number B-15897/IPH/KS.02.04/XII/2019.
AUTHORS’ CONTRIBUTIONS: Muhamad Arif Budiman: Conceptualisation, funding acquisition, and writing original draft, reviewing, and editing.
Pamungkas Rizki Ferdian: Conceptualisation, methodology, funding acquisition, and writing original draft, reviewing and editing.
Tri Hadi Handayani: Project administration, data collection, and writing original draft.
Rizki Rabeca Elfirta: Methodology, data collection, and writing original draft, reviewing and editing.
Masrukhin: Methodology, data collection, and writing original draft.
Herjuno Ari Nugroho: Data collection, data processing and visualisation, and writing original draft, reviewing and editing.
Ni Luh Putu Rischa Phadmachanty: Data collection, data processing and visualisation, and writing original draft.
Wartika Rosa Farida: Resources, supervision, and writing original draft, reviewing and editing.
Ardya Widyastuti: Project administration, data processing and visualisation, and writing original draft.
Dianita Dwi Sugiartanti: Methodology, data processing and visualisation, and writing original draft.
REFERENCES
- Abdalaziz MN, Ali MM, Gahallah MD, Garbi MI, Kabbashi AS. Evaluation of fixed oil, seed extracts of Carum carvi L. International Journal of Computational and Theoretical Chemistry. 2017;5(1):1–8. doi: 10.11648/j.ijctc.20170501.11. [DOI] [Google Scholar]
- Adwas AA, Elsayed ASI, Azab AE, Quwaydir FA. Oxidative stress and antioxidant mechanisms in human body. Journal of Applied Biotechnology and Bioengineering. 2019;6(1):43–47. doi: 10.15406/jabb.2019.06.00173. [DOI] [Google Scholar]
- Ahmad B, Khan I, Bashir S, Azam S. Chemical composition and antifungal, phytotoxic, brine shrimp cytotoxicity, insecticidal and antibacterial activities of the essential oils of Acacia modesta. Journal of Medicinal Plants Research. 2012;6(31):4653–4659. doi: 10.5897/JMPR12.016. [DOI] [Google Scholar]
- Akpuaka A, Ekwenchi MM, Dashak DA, Dildar A. Biological activities of characterized isolates of n-hexane extract of Azadirachta Indica A.Juss (neem) leaves. Nature and Science. 2013;11(5):141–147. [Google Scholar]
- Amrati FEZ, Bourhia M, Saghrouchni H, Slighoua M, Grafov A, Ullah R, Ezzeldin E, Mostafa GA, Bari A, Ibenmoussa S, et al. Caralluma europaea (Guss.) N.E.Br.: Anti-inflammatory, antifungal, and antibacterial activities against nosocomial antibiotic-resistant microbes of chemically characterized fractions. Molecules. 2021;26(3):636. doi: 10.3390/molecules26030636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anita S, Agusta A, Farida WR, Nugroho HA, Wulansari D. A preliminary study of aphrodisiac property from porcupine tail meat ethanol extract in male mice. Zoo Indonesia. 2017;26(1):52–58. [Google Scholar]
- Aplin K. Hystrix javanica The IUCN Red List of Threatened Species. 2016;2016:e.T10752A22231749. doi: 10.2305/IUCN.UK.2016-2.RLTS.T10752A22231749.en. [DOI] [Google Scholar]
- Aryal S, Baniya MK, Danekhu K, Kunwar P, Gurung R, Koirala N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from western Nepal. Plants. 2019;8(4):96. doi: 10.3390/plants8040096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astiti NPA, Ramona Y. GCMS analysis of active and applicable compounds in methanol extract of sweet star fruit (Averrhoa carambola L.) leaves. HAYATI Journal of Biosciences. 2021;28(1):12–22. doi: 10.4308/hjb.28.1.12. [DOI] [Google Scholar]
- Ayaz M, Luedecke LO, Branen AL. Antimicrobial effect of butylated hydroxyanisole and butylated hydroxytoluene on Staphylococcus aureus. Journal of Food Protection. 1980;43(1):4–6. doi: 10.4315/0362-028x-43.1.4. [DOI] [PubMed] [Google Scholar]
- Azliza MA, Ong HC, Vikineswary S, Noorlidah A, Haron NW. Ethno-medicinal resources used by the Temuan in Ulu Kuang Village. Studies on Ethno-Medicine. 2012;6(1):17–22. [Google Scholar]
- Budiman MA, Ferdian PR, Handayani TH, Nugroho HA, Elfirta RR, Farida WR. Screening of active compounds and LC50 toxicity assay of Sunda porcupine’s (Hystrix javanica f. Cuvier 1823) quills crude extract. Annales Bogorienses. 2021;25(2):73–81. [Google Scholar]
- Casillas-Vargas G, Ocasio-Malavé C, Medina S, Morales-Guzmán C, Del Valle RG, Carballeira NM, Sanabria-Ríos DJ. Antibacterial fatty acids: An update of possible mechanisms of action and implications in the development of the next-generation of antibacterial agents. Progress in Lipid Research. 2021;82:101093. doi: 10.1016/j.plipres.2021.101093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakansin C, Yostaworakul J, Warin C, Kulthong K, Boonrungsiman S. Resazurin rapid screening for antibacterial activities of organic and inorganic nanoparticles: potential, limitations and precautions. Analytical Biochemistry. 2022;637:114449. doi: 10.1016/j.ab.2021.114449. [DOI] [PubMed] [Google Scholar]
- Ershov VV, Volod’kin AA. Sterically hindered phenols. Bulletin of the Academy of Sciences of the USSR, Division of Chemical Science. 1962;11(12):2057–2060. doi: 10.1007/bf00911365. [DOI] [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing (EUCAST) EUCAST reading guide for broth microdilution (version 4.0) 2022. [accessed on 1 January 2023]. https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2022_manuals/Reading_guide_BMD_v_4.0_2022.pdf .
- Farida WR, Sari AP, Inayah N, dan Nugroho HA. Observations of behavioral development on common Porcupines (Hystrix brachyura) undergoing domestication. IOP Conference Series: Earth and Environmental Science; 2019. p. 012076. [DOI] [Google Scholar]
- Firdaus M, Kartikaningsih H, Sulifah U. Sargassum spp extract inhibits the growth of foodborne illness bacteria. AIP Conference Proceedings. 2019;2202(1):020083. doi: 10.1063/1.5141696. [DOI] [Google Scholar]
- Germ J, Poirel L, Kisek TC, Spik VC, Seme K, Premru MM, Zupanc TL, Nordmann P, Pirs M. Evaluation of resazurin-based rapid test to detect colistin resistance in Acinetobacter baumannii isolates. European Journal of Clinical Microbiology and Infectious Diseases. 2019;38(11):2159–2169. doi: 10.1007/s10096-019-03657-1. [DOI] [PubMed] [Google Scholar]
- Gifardi MD, Sutardi LN, Farida WR, Prawira AY, Agungpriyono S. Antibacterial activity of Sunda porcupine quill extract (Hystrix javanica) against Staphylococcus aureus. Biodiversitas. 2022;23(8):4355–4360. doi: 10.13057/biodiv/d230861. [DOI] [Google Scholar]
- Gomez L. The illegal hunting and exploitation of porcupines for meat and medicine in Indonesia. Nature Conservation. 2021;43:109–122. doi: 10.3897/natureconservation.43.62750. [DOI] [Google Scholar]
- Hadi MY, Mohammed GJ, Hameed IH. Analysis of bioactive chemical compounds of Nigella sativa using gas chromatography-mass spectrometry. Journal of Pharmacognosy and Phytotherapy. 2016;8(2):8–24. doi: 10.5897/JPP2015.0364. [DOI] [Google Scholar]
- Handayani TH, Budiman MA, Amalia RLR, Pribadi A, Elfirta RR, Ferdian PR. Aktivitas antioksidan, total fenolik, dan total flavonoid madu apis mellifera dari hutan akasia (Accacia crassicarpa) Riau, Indonesia dengan beberapa perlakuan pengeringan. Jurnal Biologi Indonesia. 2022;18(2):231–243. doi: 10.47349/jbi/18022022/231. [DOI] [Google Scholar]
- Heinrich S, Toomes A, Gomez L. Valuable stones: The trade in porcupine bezoars. Global Ecology and Conservation. 2020;24:e01204. doi: 10.1016/j.gecco.2020.e01204. [DOI] [Google Scholar]
- Hema R, Kumaravel S, Alagusundaram K. GC/MS determination of bioactive components of Murraya koenigii. Journal of American Science. 2011;7(1):80–83. [Google Scholar]
- Hsouna AB, Trigui M, Mansour RB, Jarraya RM, Damak M, Jaoua S. Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratonia siliqua essential oil with preservative effects against Listeria inoculated in minced beef meat. International Journal of Food Microbiology. 2011;148(1):66–72. doi: 10.1016/j.ijfoodmicro.2011.04.028. [DOI] [PubMed] [Google Scholar]
- Igwe OU, Okwunodulu FU. Investigation of bioactive phytochemical compounds from the chloroform extract of the leaves of Phyllanthus amarus by GC-MS technique. International Journal of Chemistry and Pharmaceutical Sciences. 2014;2(1):554–560. [Google Scholar]
- Inayah N. Potensi pengembangan landak (Hystrix sp.) sebagai produk komersial. Fauna Indonesia. 2016;15(2):37–43. [Google Scholar]
- Innocenti A, Hall RA, Schlicker C, Mühlschlegel FA, Supuran CT. Carbonic anhydrase inhibitors. Inhibition of the β-class enzymes from the fungal pathogens Candida albicans and Cryptococcus neoformans with aliphatic and aromatic carboxylates. Bioorganic and Medicinal Chemistry. 2009;17(7):2654–2657. doi: 10.1016/j.bmc.2009.02.058. [DOI] [PubMed] [Google Scholar]
- Jia H, Fang R, Lin J, Tian X, Zhao Y, Chen L, Cao J, Zhou T. Evaluation of resazurin-based assay for rapid detection of polymyxin-resistant gram-negative bacteria. BMC Microbiology. 2020;20(1):7. doi: 10.1186/s12866-019-1692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazemi M. Phenolic profile, antioxidant capacity and anti-inflammatory activity of Anethum graveolens L. essential oil. Natural Product Research. 2015;29(6):551–553. doi: 10.1080/14786419.2014.951934. [DOI] [PubMed] [Google Scholar]
- Khan AYF, Ahmed QU, Narayanamurthy V, Razali S, Asuhaimi FA, Saleh MSM, Johan MF, Khatib A, Seeni A, Wahab RA. Anticancer activity of grassy Hystrix brachyura bezoar and its mechanism of action: An in vitro and in vivo based study. Biomedicine and Pharmacotheraphy. 2019;114:108841. doi: 10.1016/j.biopha.2019.108841. [DOI] [PubMed] [Google Scholar]
- Kim DY, Won KJ, Hwang DI, Kim NY, Kim B, Lee HM. 1-Iodohexadecane alleviates 2,4-dinitrochlorobenzene-induced atopic dermatitis in mice: Possible involvements of the skin barrier and mast cell snare proteins. Molecules. 2022;27(5):1560. doi: 10.3390/molecules27051560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krátký M, Vinšová J, Buchta V. In vitro antibacterial and antifungal activity of salicylanilide benzoates. Scientific World Journal. 2012;98:42518–42522. doi: 10.1100/2012/290628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisyanto RD, Ardian H, Anwari MS. Kajian etnozoologi untuk pengobatan suku dayaksebaruk di Desa Setunggul Kecamatan Silat Hilir Kabupaten Kapuas Hulu. Jurnal Hutan Lestari. 2019;7(3):1282–1289. doi: 10.26418/jhl.v7i3.37405. [DOI] [Google Scholar]
- Lim CM, Kyung KH, Yoo YJ. Antimicrobial effects of butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT). Korean Journal of Food Science and Technology. 1987;19(1):54–60. [Google Scholar]
- Maeng JH, Shahbaz HM, Ameer K, Jo Y, Kwon JH. Optimization of microwave-assisted extraction of bioactive compounds from Coriolus versicolor mushroom using response surface methodology. Journal of Food Process Engineering. 2017;40(2):e12421. doi: 10.1111/jfpe.12421. [DOI] [Google Scholar]
- Mardiastuti A, Masy’ud B, Ginoga LN, Sastranegara H, Sutopo Short communication: Wildlife species used as traditional medicine by local people in Indonesia. Biodiversitas. 2021;22(1):329–337. doi: 10.13057/biodiv/d220140. [DOI] [Google Scholar]
- Maroof K, Gan SH. Bee products and diabetes mellitus. In: Boyacioglu D, editor. Bee products and their applications in the food and pharmaceutical industries. Cambridge, MA: Academic Press; 2022. pp. 63–114. [DOI] [Google Scholar]
- Martinac B, Buechner M, Delcour AH, Adler J, Kung C. Pressure-sensitive ion channel in Escherichia coli. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(8):2297–2301. doi: 10.1073/pnas.84.8.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okechukwu PN. Evaluation of anti-inflammatory, analgesic, antipyretic effect of eicosane, pentadecane, octacosane, and heneicosane. Asian Journal of Pharmaceutical and Clinical Research. 2020;13(4):29–35. doi: 10.22159/ajpcr.2020.v13i4.36196. [DOI] [Google Scholar]
- Ozdemir G, Karabay NU, Dalay MC, Pazarbasi B. Antibacterial activity of volatile component and various extracts of Spirulina platensis. Phytotherapy Research. 2004;18(9):754–757. doi: 10.1002/ptr.1541. [DOI] [PubMed] [Google Scholar]
- Pammi N, Bhukya KK, Lunavath RK, Bhukya B. Bioprospecting of palmyra palm (Borassus flabellifer) nectar: Unveiling the probiotic and therapeutic potential of the traditional rural drink. Frontiers in Microbiology. 2021;12:683996. doi: 10.3389/fmicb.2021.683996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel MR, Chand MB, Pant B, Pant B. Assessment of antioxidant and cytotoxic activities of extracts of Dendrobium crepidatum. Biomolecules. 2019;9(9):478. doi: 10.3390/biom9090478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poongulali S, Sundararaman M. Antimycobacterial, anticandidal and antioxidant properties of Terminalia catappa and analysis of their bioactive chemicals. International Journal of Pharmacy and Biological Sciences. 2016;6(2):69–83. [Google Scholar]
- Prawira AY, Hosaka YZ, Novelina S, Farida WR, Darusman HS, Agungpriyono S. Morphological evaluation of polysaccharide content and collagen composition during cutaneous wound healing in the Sunda porcupine (Hystrix javanica) Journal of Veterinary Medical Science. 2020;82(5):506–515. doi: 10.1292/jvms.19-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prawira AY, Novelina S, Darusman HS, Farida WR, Agungpriyono S. The dorsal skin structure contributes to the surface bacteria populations of Sunda Porcupine (Hystrix javanica) Anatomia, Histologia, Embryologia. 2018;47(6):591–598. doi: 10.1111/ahe.12401. [DOI] [PubMed] [Google Scholar]
- Putra YAE, Masy’ud B, Ulfah M. Diversity of medicinal animals in Betung Kerihun National Park, West Kalimantan, Indonesia. Media Konservasi. 2008;13(1):8–15. [Google Scholar]
- Sarker SD, Nahar L, Kumarasamy Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods. 2007;42(4):321–324. doi: 10.1016/j.ymeth.2007.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin J, Shanmughapriya S, Gandhimathi R, Kiran GS, Ravji TR, Natarajaseenivasan K, Hema TA. Optimization and production of novel antimicrobial agents from sponge associated marine actinomycetes Nocardiopsis dassonvillei MAD08. Applied Microbiology and Biotechnology. 2009;83(3):435–445. doi: 10.1007/s00253-009-1878-y. [DOI] [PubMed] [Google Scholar]
- Suyanto . Domestikasi landak Indonesia. Jakarta: LIPI press; 2012. [Google Scholar]
- Van Weers DJ. Specific distinction in Old World porcupines. Der Zoologische Garten Jena. 1983;53:226–232. [Google Scholar]
- Van Weers DJ. Notes on Southeast Asian Porcupines (Hystricidae, Rodentia). IV. On the taxonomy of the subgenus Acanthion F. Cuvier, 1823 with notes on the other taxa of the family. Beaufortia. 1979;29(356):215–272. [Google Scholar]
- Woods CA, Kilpatrick CW. Infraorder Hystricognathi. In: Wilson DE, Reeder DM, editors. Mammal species of the world. Baltimore, MD: Johns Hopkins University Press; 2005. pp. 1538–1600. [Google Scholar]
- Yogeswari S, Ramalakshmi S, Neelavathy R, Muthumary J. Identification and comparative studies of different volatile fractions from Monochaetia kansensis by GCMS. Global Journal of Pharmacology. 2012;6(2):65–71. [Google Scholar]