Abstract
The innovative fusion of essential oils with hydrogel engineering offers an optimistic perspective for the design and development of next-generation materials incorporating natural bioactive compounds. This review provides a comprehensive overview of the latest advances in the use of hydrogels containing essential oils for biomedical, dental, cosmetic, food, food packaging, and restoration of cultural heritage applications. Polymeric sources, methods of obtaining, cross-linking techniques, and functional properties of hydrogels are discussed. The unique characteristics of polymer hydrogels containing bioactive agents are highlighted. These include biocompatibility, nontoxicity, effective antibacterial activity, control of the sustained and prolonged release of active substances, optimal porosity, and outstanding cytocompatibility. Additionally, the specific characteristics and distinctive properties of essential oils are explored, along with their extraction and encapsulation methods. The advantages and disadvantages of these methods are also discussed. We have considered limitations due to volatility, solubility, environmental factors, and stability. The importance of loading essential oils in hydrogels, their stability, and biological activity is analyzed. This review highlights through an in-depth analysis, the recent innovations, challenges, and future prospects of hydrogels encapsulated with essential oils and their potential for multiple applications including biomedicine, dentistry, cosmetics, food, food packaging, and cultural heritage conservation.
Keywords: hydrogels, essential oils, encapsulation, biomedical applications, cosmetics, dentistry, active food packaging, restoration of cultural heritage
1. Introduction
Hydrogels form a unique category, being among the most modern multifunctional materials that can be applied in numerous fields. As a result, they have captured the interest of many scientists in various research fields. Hydrogels are essentially 3D cross-linked networks formed of hydrophilic polymeric materials that can retain large volumes of water and fluids [1]. They can be formulated from both synthetic polymers and biopolymers. Hydrogels based on natural biodegradable polymers, such as polysaccharides, polypeptides, and proteins have many advantages over synthetic ones and have gained particular importance lately [2]. One of the advantages is the porous macromolecular structure, which can be easily adjusted so that the hydrogels can incorporate different bioactive compounds and then release them in a controlled manner.
Due to their special properties, the so-called intelligent hydrogels have the ability to swell in an aqueous environment, show sensitivity to temperature, light, pH variations and other stimuli, self-healing, and shape memory [3].
Through different strategies, such as molecular design, cross-linking techniques, or the incorporation of different bioactive compounds, the properties and functions of hydrogels can be adapted for a wide variety of applications. They are widely used in the biomedical field for wound healing and tissue engineering, as well as drug delivery systems, pharmaceutical products, the food industry, cosmetics, hygiene products, and dentistry, etc.
Similarly to the remarkable class of hydrogels, essential oils (EOs) have shown an extraordinary increase in scientific interest in recent years, providing huge potential for a diverse range of modern applications, including cutting-edge fields such as nanotechnology, bioengineering, and biomedicine. Extracted from aromatic plants, EOs are hydrophobic products of high concentration and contain molecules with low molecular weight. The extraordinary therapeutic potential of EOs is due to the biological activity of their volatile chemical components (terpenoids, terpenes, and other aromatic compounds) and non-volatile (hydrocarbons, fatty acids, sterols, carotenoids, waxes, and flavonoids) [4].
This review brings a new perspective, scrutinizing the potential to incorporate different essential oils into hydrogels in order to develop efficient delivery systems for bioactive molecules of natural origin. Through this versatile delivery method, new products with improved bioactive activity can find use for many types of applications to provide both cost-effectiveness and high efficiency.
At present, the global production of essential oils is driven by the strong demand for consumption, from natural options to synthetic antioxidants, due to remarkable biological properties, including antimicrobial, antioxidant, anti-inflammatory, antiviral, and antitumor effects [5]. These distinctive qualities pave the way for promising opportunities in utilizing EOs across different fields.
As potent curative agents, EOs offer a viable alternative to synthetic drugs, particularly for their antimicrobial effectiveness against a wide range of pathogenic microorganisms. They are most commonly applied in the biomedical and pharmaceutical sectors.
Since ancient times, the sensory and pharmacological activities of essential oils have been studied for their use in preventive and curative treatments, particularly in cosmetics and countless personal care products.
In the food industry, EOs are used on a large scale, as a beneficial mechanism to combat undesirable microorganisms in food products. The natural bioactive capacities of EOs, such as antimicrobial and antioxidant properties, together with their aroma, flavor, or spicy taste, make them suitable for use as preservatives in the food industry.
EOs can be incorporated into different food systems to increase the shelf life of food while maintaining its quality. They can also be encapsulated in the form of edible coatings or films, to mitigate microbial development on the surface and protect the environment from synthetic packaging.
However, EOs cannot be applied directly, in their raw form, because they are unstable volatile compounds, fragile, and of very high concentration. They require special post-obtaining conditions so that the original chemical profile is not modified by different environmental conditions (light, heat, oxidation) [6].
Consequently, EOs can be encapsulated in different hydrogel matrices to prolong their effective biological activity and ensure their release in a controlled and sustained manner.
Hydrogels are versatile platforms with an adjustable structure, of a non-toxic nature that can be used safely [7]. The high-water content, the sustainable nature of the constituent polymers, and the ability to incorporate EOs give hydrogels favorable biocompatibility and key structural characteristics, making them suitable for a wide range of implementation [8].
This review aims to showcase significant research and findings through a detailed analysis of the main applications of essential oil-embedded hydrogels. Therefore, this review article provides a comprehensive overview of recent studies on obtaining hydrogels, and EOs, as well as their incorporation into hydrogel matrices, for a multitude of uses. These include fields such as biomedicine, dentistry, cosmetics, functional food products, food packaging, and even the preservation of stone cultural heritage.
2. Preparation of Hydrogels
Hydrogels are systems made up of polymers and solvents obtained in the form of 3D cross-linked networks. Cross-linking can be formed (i) physically, (ii) chemically or (iii) by ionizing radiation [9].
The main advantage of hydrogels obtained by the physical method is the absence of crosslinking agents which eliminates the risk of possible residual toxicity [9,10].
However, a notable disadvantage is that physically cross-linked hydrogels are reversible. Between the polymer chains, there are temporary bonds that appear in response to composition, pH, or temperature changes. Moreover, these hydrogels show weak mechanical and viscoelastic properties. Depending on the size of the polymer particles and the nature of the solvent in the 3D network, there may be hydrogen bonds, van der Walls bonds, electrostatic, hydrophobic interactions, or between-polymer chains with local crystallite generation [9].
Chemically obtained hydrogels are created by covalent cross-linking between the existing polymer chains. This leads to a stable or irreversible bond. Chemical cross-linking can be produced using various methods, including addition polymerization, photopolymerization, volume condensation, plasma or electromagnetic radiation, or interpenetration [10].
In general, a cross-linking agent with a multifunctional role is used, which unites the monomeric units and leads to the development of the polymer chain [10]. Chemically cross-linked hydrogels are advantageous due to their resistance to degradation and improved mechanical and viscoelastic properties compared to physically cross-linked ones [9]. On the other hand, hydrogels, obtained by anionic or cationic polymerization have as their main disadvantage the sensitivity to water, respectively, the limitation to non-polar monomers [9].
In the case of hydrogels made by radiation, they can be obtained at ambient temperature and physiological pH, even without a crosslinking agent, which makes them suitable to be applied in a wide range of biomedical, food, or cosmetic applications [11].
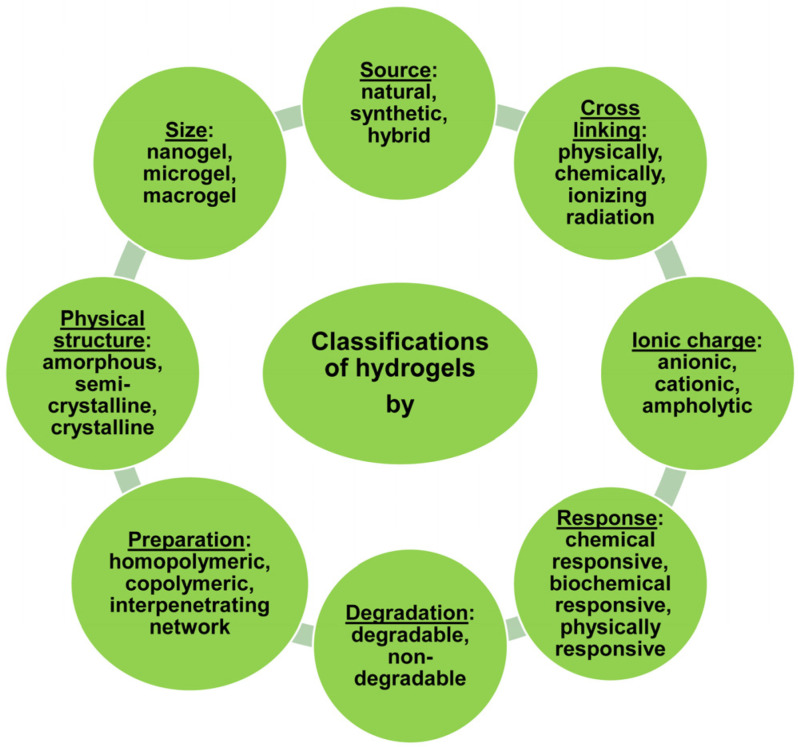
Hydrogels can be categorized according to various characteristics, providing deeper insight into their properties and potential uses (Figure 1). These characteristics include [9]:
-
➢
According to source: natural, synthetic, or hybrid.
-
➢
According to polymer structure: linear, branched, or cross-linked.
-
➢
According to physical appearance: macroporous, microporous, or nanoporous.
-
➢
According to charge: neutral, anionic (negatively charged), cationic (positively charged), or amphoteric, meaning they contain both positive and negative charges.
-
➢
According to responsiveness to stimuli: Hydrogels can also be classified by their sensitivity to external stimuli such as temperature, pH, light, or electric fields. Called “smart hydrogels,” these materials can undergo reversible changes in their structure or properties when subjected to these environmental influences.
-
➢
According to water content: superabsorbent hydrogels, and less moisture.
-
➢
According to degradability: biodegradable, and non-biodegradable.
Figure 1.
Classifications of hydrogels.
Polymers play an important role in the matrix of hydrogels, greatly influencing their properties. They can be divided into two main categories: natural polymers and synthetic polymers. Biodegradable polymeric materials, both natural and synthetic, have received more interest lately, due to the importance of environmentally friendly products and the possibility of their application in many fields such as biomedicine, pharmaceuticals, or agriculture [12].
The creation of advanced materials formulated from bioavailable and renewable raw materials, including waste, has been increasingly promoted, in agreement with the 12 principles of Green Chemistry and the achievement of the Sustainable Development Goals provided for the UN 2030 Agenda.
Natural polymers are polymer molecules of biological origin that can be obtained from different sources such as animals, bacteria, microorganisms (algae and fungi), or plants. The chemical structures of natural polymers are composed of monomers of amino acids, nucleotides, esters, or monosaccharides, that are covalently coupled to form peptides, polyphenols, polyesters, or polysaccharides [13].
Because they are similar to the components of the extracellular matrix (ECM), natural polymers have a reduced toxicity, with a low risk of causing adverse reactions. This biocompatibility has determined their widespread use in numerous biomedical, pharmaceutical, and cosmetic applications, as additives in textile products, and in food or agriculture [14].
The most used natural polymers include sodium alginate, starch, gelatin, chitosan, collagen, hyaluronic acid, κ-carrageenan, cellulose, gum arabic, silk, fibrin, and bacterial polyesters [15,16].
Synthetic polymers are created artificially in laboratories and can be mainly classified as thermoplastic and thermosetting polymers and elastomers. They are very often found in multiple fields, such as packaging and construction, as plastic materials, fibers, elastomers, or adhesives. As synthetic polymers, we can mention polyvinyl alcohol, poly(lactic acid), polyvinylpyrrolidone, poly(ε-caprolactone), polyurethane, polyethylene glycol, polyethylene oxide, poly(L-lactide-co-caprolactone), carboxymethyl cellulose and poly(vinylidene fluoride) [17]. Among them, some synthetic polymers are biocompatible and biodegradable, such as poly(lactic acid), carboxymethyl cellulose, poly(acrylic acid), poly(vinyl alcohol), or polyethylene glycol. Some of them have shown antitumor, antibiotic, antiviral, or antithrombotic activities and are often used as drug carriers, implants, diagnostic imaging agents, or as bio-ink in 3D printing for various biological scaffolds [18,19].
A new approach in hydrogel engineering is the design of complex systems through which hybrid hydrogels are obtained that incorporate both natural and synthetic polymers, but also other functional components [20]. The hybrid hydrogels that have been developed are capable of integrating nano- or microstructures, allowing for targeted action, controlled transport, and an adjustable release profile.
Hybrid nanogels have the ability to respond faster than macroscopic ones to environmental variations, proving their usefulness especially in biomedical applications such as therapies for tissue engineering, transport and delivery of chemicals in cancer therapy, or in optical detection [21].
3. Methods of Obtaining Essential Oils
Since ancient times, people have used EOs because they were believed to contain essential components that are necessary for healing and prolonging life. Alchemists referred to them as the “quintessence of plants”.
Essential oils are aromatic oil liquids in the form of complex natural mixtures of different polar and non-polar compounds, made from natural raw material of plant origin. The main chemical constituents of essential oils are volatile, lipophilic, and odoriferous substances that are commonly found in different parts of plants (leaves, flowers, fruits, or stems), giving them specific properties [22]. Since the beginning, aromatic plants have been used empirically as spices in kitchens, in perfumes, cosmetics, and aromatherapy, for preventive, curative, or therapeutic purposes. With the advent of distillation centers, the methods of obtaining essential oils have advanced and the therapeutic benefits of EO have been scientifically evaluated [23].
The different production techniques involve (i) hot distillation, with water or steam, (ii) cold or dry distillation, and (iii) through various mechanical processes. While the yield of obtaining pure essential oils from aromatic plants is very low, their price is commensurate with their natural bioactivity and implicit pharmaceutical and therapeutic benefits.
Over time, it has been proven that essential oils contain over 300 different aromatic components, showing an extremely varied therapeutic potential [24]. In practice, these capabilities of essential oils have been continuously explored and harnessed on a large scale for a wide range of multiple applications that have been successfully integrated into a variety of industries and contexts. The chemical composition of essential oils can vary, even for the same species, depending on certain parameters such as climatic factors, soil characteristics, harvesting conditions, post-harvest treatment, or the extraction methods used [24].
The uniqueness and importance of EOs are given by their medicinal and bioactive compounds, as well as by the other valuable constituents. EOs show significant variability in their composition, both in terms of quality and quantity. Since this variability is heavily influenced including on the extraction method used, it is crucial to identify optimal, and especially non-toxic extraction techniques.
This chapter will offer a concise summary of the main extraction methods, including both traditional and modern approaches, which are continuously being refined for improvement.
The main compounds discovered in EOs are terpenoid derivatives (80%) and phenylpropanoids (which give the specific spicy smell and aroma) [25].
There are several ways by which EOs are extracted from aromatic plants. The selection of an extraction method is influenced by the plant’s texture and characteristics, the specific essential oils being targeted, and the intended application of the final product. Every method demonstrates its own advantages and drawbacks.
Some classical EO extraction methods, also called conventional, have been practiced for hundreds of years and include steam distillation, water distillation, combined water and steam distillation, cohobation (or repeated distillation), maceration, cold pressing, and enfleurage. The limitations of these methods are mainly represented by low extraction yields, thermal degradation, or the need to use high mechanical power [23].
Other more recent methods, also called alternatives, try to demonstrate their efficiency in operation, to be ecological and viable from an economic point of view. Among these can be listed extraction with solvents, supercritical CO2, or resins, and fractional distillation, percolation, and the phytonic process (uses a new solvent based on hydrofluorocarbon 134) [23]. The main benefits of supercritical extraction with CO2 are its low cost and non-corrosive nature, which enables the production of thermally unstable EOs at an industrial level with a high yield. In addition, it is an ecological way by which safe EOs can be produced to be used in various applications in the food industry.
New extraction methods use “green concepts” to extract valuable components from aromatic plants. They act in accordance with the United Nations 2030 strategy, pursuing sustainable developments by reducing waste, using discarded by-products, recycling them, and reducing the carbon footprint in processing, which will have a positive impact towards a cleaner environment. The global EO market has been continuously growing, reaching USD 7.51 billion in 2018, and is expected to grow at a CAGR of over 9% between 2019 and 2026 [26].
Innovative techniques that respect these green concepts include ultrasound-assisted extraction of bioactive compounds, microwave-assisted extraction of essential oils, high-pressure liquid extraction, sub- and supercritical fluid extraction, pulsed electric fields, and high-voltage electric discharges [27].
Essential oils are categorized into three main categories which include (i) terpenes, (ii) terpenoids, and (iii) phenylpropanoids in their chemical composition. Terpene constituents can be classified into two primary groups: (i) components that have a hydrocarbon structure (such as monoterpenes diterpenes and sesquiterpenes) and (ii) those that are oxygenated, including acids, aldehydes, alcohols, esters, ketones, lactones, oxides, and phenols [28]. Among the most common terpenes, we can distinguish limonene, sabinene, α-pinene and p-cymene, for example in thyme and oregano, but also in lemon, grapefruit, eucalyptus, and rosemary EOs. Carvacrol, citronellal, carvone, and thymol are all terpenoids that are present in EOs like mint, lavender, tea tree, chamomile, or geranium. Clove, jasmine, rose, or pepper EOs contain phenylpropanoids that can be identified as cinnamaldehyde, eugenol, safrole, and vanillin. EOs also includes other components derived from amino acids, such as alanine, leucine, isoleucine, methionine, and valine [25].
The primary drawbacks of using EOs include their volatility, high sensitivity, poor stability, high sensitivity, and vulnerability to degradation at processing temperatures. To address these challenges, the encapsulation of EO in polymeric matrices improves their bioactivity, stability, and water solubility, and enables long-term sustained delivery across various applications [29].
4. Encapsulation of Essential Oils in Hydrogels
Most essential oils cannot be applied through direct contact with biological systems, because they can be irritating or even toxic in certain cases. Incorporating essential oils directly into hydrophilic matrices is not beneficial due to their hydrophobic nature, which diminishes the inherent bioactivity of their components, leading to the use of high concentrations to be functional. To preserve their biological activity over an extended period, particularly for biomedical applications, EOs should be encapsulated in various systems, such as lipidic nanoparticles, liposomes, films, emulsion gels, oil-in-water emulsions, or spray-dried microparticles [30].
Through encapsulation, risks due to possible toxicity are reduced, ensuring a safe delivery system. Moreover, this method increases the biological activities and efficiency of particularly volatile EOs through better absorption. In addition, encapsulation has often been widely used as a way of protecting essential oils, prolonging their active biocapacity and efficient delivery, which offers the possibility of their implementation in the medical, pharmaceutical, cosmetic, and food fields [31].
These benefits can be achieved by employing different techniques to encapsulate es- essential oils with diverse bioactivities [26,32]: (i) chemical; (ii) physico-chemical; (iii) mechanical; (iv) ultrasound-assisted emulsification; and (v) electrostatic extrusion.
An example is thyme EO, which is often used due to its therapeutic properties. Thyme (Thymus vulgaris L.) is a plant native to the Mediterranean area with both dietary and medicinal uses. It contains many polyphenolic compounds of biological interest, such as carvacrol, 5-isopropyl-2-methylphenol, and a p-cymene derivative with a characteristic smell, with antioxidant, antimicrobial, antidiabetic, anti-inflammatory, immunomodulatory, and anticancer bioactivities [33,34]. Thyme EO was encapsulated in the first step in sodium caseinate nanomicelles by a physical method [35]. Then, in the second step, these nanomicelles were introduced into the preparation of a hydrogel. This formulation aimed to improve the stability and protect the bioactivity of thyme EO. Finally, a gelatin nanocomposite hydrogel as a drug delivery platform was obtained, having antibacterial potential for wound healing both in vitro and in vivo [35].
The ionic gelation method, achieved by ionic bonding between alginate and some divalent cations, led to the creation of a biocompatible hydrogel material in the form of alginate microspheres that encapsulated thyme and calendula EO [36]. The study investigated the loading capacity, the encapsulation efficiency of EO, and the dissolution of microspheres under simulated digestion conditions.
Lipid matrices offer a suitable and stable environment for incorporating EOs and ensuring their controlled release [37]. In particular, solid lipid nanoparticles have aroused special interest for the encapsulation of bioactive compounds due to their large surface area and the potential to facilitate the protection of bioactive constituents in ambient conditions.
An interesting method for obtaining lipid matrices is homogenization at high shear followed by the ultrasonication method [37]. Thus, chitosan and polyvinyl alcohol hydrogels containing solid lipid nanoparticles loaded with EOs of Origanum vulgare and Thymus vulgaris were formulated and investigated as alternatives to synthetic fungicides. The materials made with EOs content have demonstrated abilities to reduce the infestation with phytopathogenic fungi responsible for the degradation of perishable fruits [37].
A new work investigated the capabilities of Perilla frutescens (L.), the annual aromatic plant cultivated and used for thousands of years in traditional medicine or as food. In the first step, microcapsule powders of Perilla frutescens (L.) [38] EOs were prepared by the spray drying method of a wall material (octenyl succinic anhydride starch). In the next step, they were further encapsulated with sodium alginate and chitosan by the polyelectrolyte complex coacervate method, obtaining stable hydrogel balls for aqueous and acidic food formulations with a complete and prolonged release of the encapsulated EOs [38].
Hydrophobic clove EO was loaded in situ into a hydrophilic chitosan polymer matrix to obtain functional coatings as food packaging [32]. By using this method, bioactive materials were obtained without the need for crosslinking agents.
The electrostatic extrusion technique that was applied to encapsulate fennel EO in an alginate polymer matrix, together with the incorporation of a whey protein followed by freeze-drying, is an original approach to improve the encapsulation efficiency and loading capacity [39]. The encapsulated EO maintained its qualitative appearance by keeping 58.95% of the volatile compounds [39].
5. Applications of Hydrogel Materials Enriched with Essential Oil
The exploration and application of the bioactivities of essential oils as natural phytotherapeutic agents in various biomedical fields arose from the need to develop alternative therapeutic approaches to traditional synthetic treatments.
Hydrogels are ideal host matrices for some limitations of EOs, such as volatility, high sensitivity to environmental factors, and lower stability [26]. Together, EOs and hydrogels are biocompatible and biodegradable materials, which demonstrate remarkable physicochemical properties and antibacterial, antioxidant, anti-inflammatory, and anti-cancer activities [40]. The porous 3D structure of hydrogels facilitates the incorporation of essential oils through hydrophobic interactions, enabling their sustained and controlled release in response to various stimuli such as hydrolytic and enzymatic activity, pH changes, or temperature variations [41].
The beneficial combination of essential oils with the engineering of hydrogels can be an advanced approach to the design and development of the next generation of hybrid biomedical systems that embed natural therapeutic compounds.
5.1. Biomedical Applications
5.1.1. Topical or Transdermal Delivery Systems
Natural polysaccharides are among the most widely used biopolymers in biomedical applications due to their biocompatibility, bioactivity, biodegradability, and exceptional rheological and biomucoadhesive properties. These attributes make them ideal for developing a wide variety of topical formulations, for wound healing, or as effective and inexpensive drug delivery systems [42]. Additionally, marine polysaccharides enhance hydrogel formation capabilities, making them particularly effective for skin applications in treating various dermatological conditions.
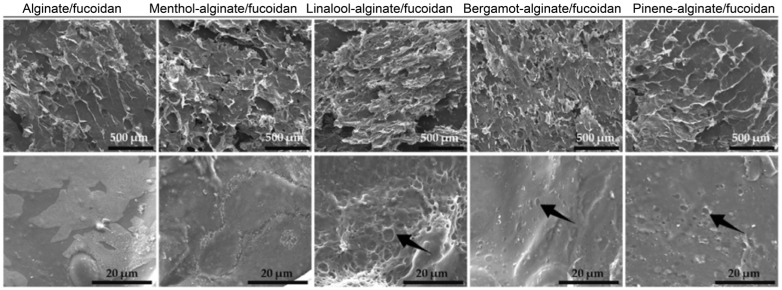
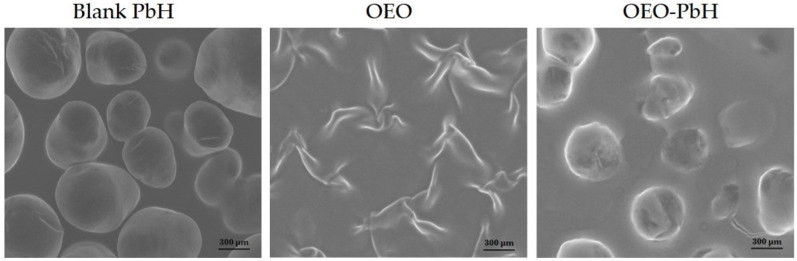
Alginate and fucoidan hydrogels loaded with menthol, L-linalool, bergamot oil, and β-pinene essential oils have been developed to improve skin permeability [43]. The aim of the study was to evaluate the way in which these EOs influence the penetration of the active ingredients through the skin, and the effect of the composition, in order to create effective formulations for topical or transdermal administration [43]. The porous morphology of the prepared hydrogels, presented in Figure 2, could be due to the lyophilized oil droplets, which can lead to these structures. Menthol, a cyclic monoterpene, is widely recognized for its ability to improve skin permeability by disrupting intercellular lipids in the stratum corneum. Bergamot EO (Citrus bergamia) is mainly composed of limonene, linalyl acetate, and linalool with anti-inflammatory properties and b-pinene, a bicyclic monoterpene, with antioxidant, anti-inflammatory, and analgesic effects. Combining the activities of EOs like menthol, L-linalool, bergamot oil, and β-pinene can indeed be a powerful strategy for overcoming the skin barrier and treating inflammation. Each of these essential oils has unique properties that, when combined, can work synergistically to enhance skin permeability and provide anti-inflammatory benefits [43].
Figure 2.
SEM images of the hydrogel samples [43].
5.1.2. Antimicrobial and Anti-Inflammatory Activity
Oral candidiasis is a fungal infection primarily produced by Candida species for which there is a rather limited antifungal treatment. This condition is particularly challenging to manage due to the limited availability of effective antifungal treatments and the potential for these treatments to cause adverse effects and contribute to the development of antifungal resistance.
Encapsulating biocides within hydrogels is an effective strategy for targeted delivery, offering controlled release and enhanced therapeutic effects. Specifically, using methylcellulose-based hydrogels incorporated with Melissa officinalis EO can enhance antimicrobial efficacy while maintaining biocompatibility with biological tissues [44]. The hydrogel formulation based on methylcellulose with Melissa officinalis EO demonstrated both antimicrobial activity and antifungal potential, making it an effective treatment for inhibiting oral candidiasis [44].
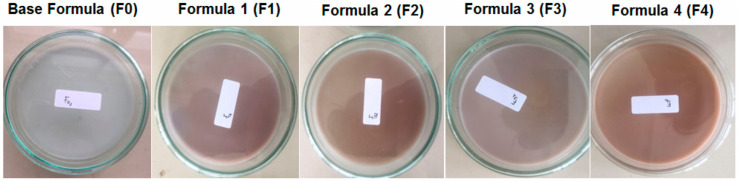
A complex study focused on the development of hydrogel films made from a combination of polyvinyl alcohol (PVA), corn starch, patchouli oil, and silver nanoparticles (Figure 3) [45]. These materials were chosen for their bioactive properties, particularly their effectiveness against Staphylococcus aureus and Staphylococcus epidermidis, both of which are common bacteria responsible for various infections, including skin and soft tissue infections. The nanoparticles were prepared by green synthesis, in the presence of both aqueous and methanolic extracts from patchouli plants (Pogostemon cablin Benth). The use of cross-linked polymeric hydrogel films with glutaraldehyde and containing biosynthesized silver nanoparticles with phytochemicals presents an advanced approach to developing antimicrobial materials [45].
Figure 3.
Photo of hydrogel films based on polyvinyl alcohol–cornstarch–patchouli oil and Ag nanoparticles [45].
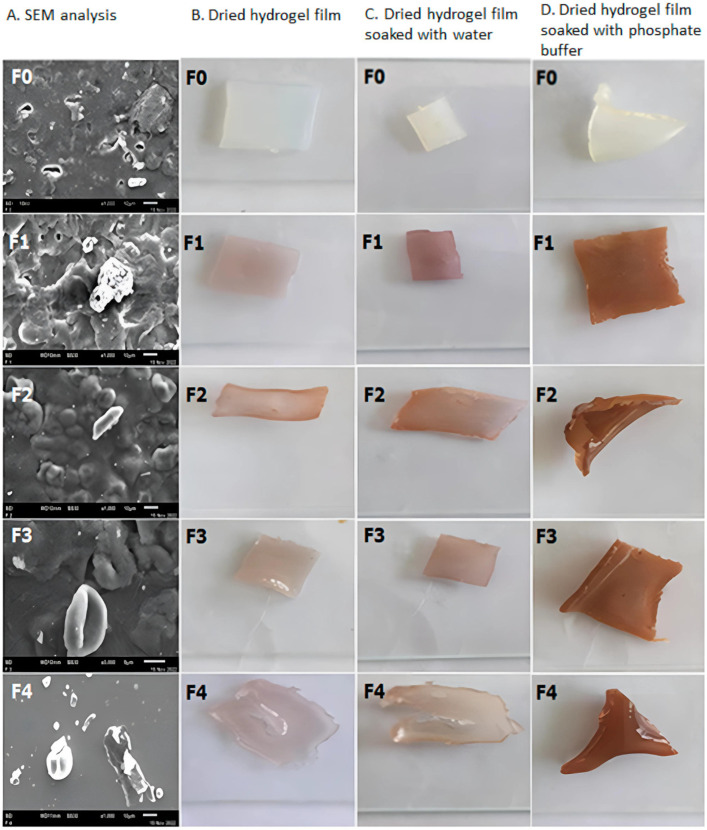
Figure 4 shows SEM and photo images during the swelling experiments.
Figure 4.
SEM images of hydrogel films based on polyvinyl alcohol/corn starch/patchouli oil (A) with Ag nanoparticles (samples F0–F4); (B) Dry samples; (C) Dry samples soaked in water; (D) Dry samples soaked with phosphate buffer [45].
Acne Vulgaris is a common inflammatory skin condition that affects many young individuals and often persists into adulthood. Traditional acne treatments, which mainly rely on antibiotics, have shown limited effectiveness and frequently disrupt the balance of the skin microbiome. Recent research suggests that essential oils and herbs could offer promising benefits for treating acne, a long-lasting inflammatory condition that can lead to scarring [46].
Thyme EO has excellent antibacterial and antioxidant properties that are suitable for inflammatory skin conditions such as acne [47]. Obtained by steam distillation of the flowering stems, Thyme EO contains thymol (37–55%) and 0.5-carvacrol (0.5–5.5%). These biocomponents have antibacterial activity, easily penetrating the lipid layer. Although Thyme EO is recognized for its insolubility in water, high volatility, and tendency to degrade rapidly when exposed to air, light, or high temperatures for long periods, the extraordinary potential of this plant has been explored in numerous studies.
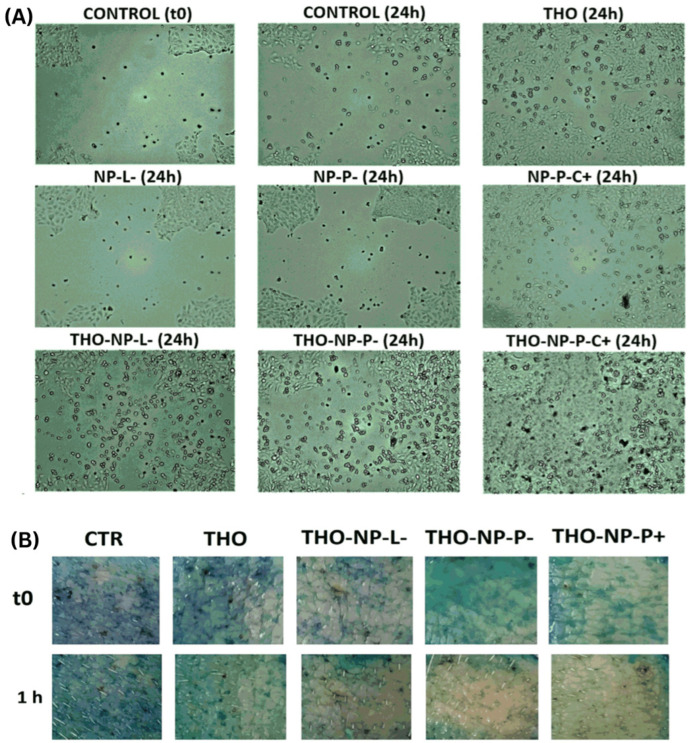
In a recent research, Thyme EO was encapsulated in biodegradable nanoparticles of poly-(D,L)-(lactic-co-glycolic acid) for skin and pharmaceutical applications [47]. Through functionalization, the nanosystems remained stable for a period of 6 months, by cold storage. In vitro, ex vivo, and in vivo evaluations on human volunteers indicated that Thyme EO demonstrated excellent antioxidant activity and healing of skin inflammation without leaving acne scars (Figure 5A,B) [47].
Figure 5.
Wound healing activity: (A) for in vitro scratch assay in HaCaT cell lines. Images were taken before (Control t0) and 24 h after incubation: untreated samples (control and 24 h) and treated samples, respectively thyme oil (THO) and functionalized hydrogels and their corresponding empty NPs. (B) Skin surface showed a reduction in methylene blue following ex vivo antioxidant activity of the samples [47].
5.1.3. Wound Dressing Applications
Numerous formulations of composite hydrogels have been studied as intricate systems composed of biopolymers, incorporating various bioactive elements from essential oils. These platforms, exhibiting synergistic properties, are being explored for use as advanced wound dressings with enhanced therapeutic potential [48].
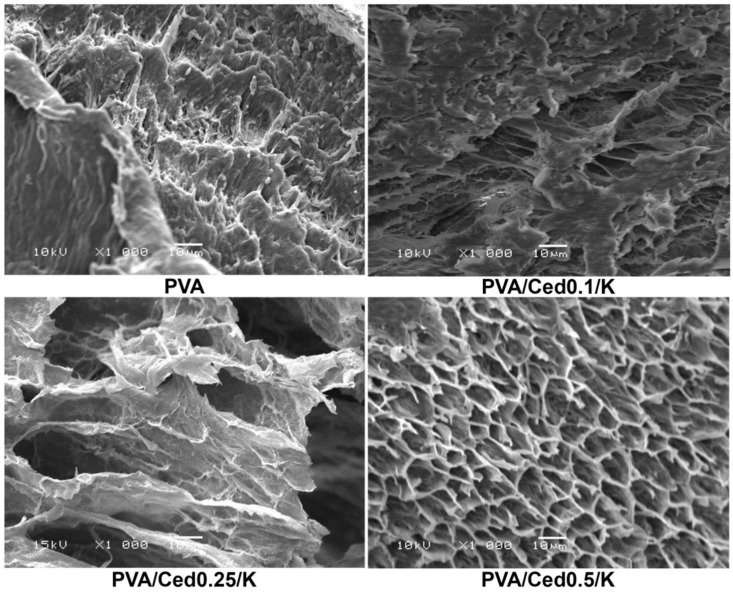
Cedarwood EO obtained from several types of conifers (e.g., Cedrus sp. and Juniperus sp.) is a mixture of safe organic chemicals with pesticidal and preservative properties. In order to develop effective hemostatic and antibacterial dressings for treating wounds, composite porous sponges were designed [49]. Polyvinyl alcohol was physically cross-linked with kaolin and incorporated cedar essential oil, through a freeze–thaw approach, yielding sponge hydrogels with distinct lamellar architectures. The addition of cedar and kaolin in the formulation improved the pore sizes and structure of the resulting sponges (Figure 6). Studies have shown the biocompatibility of these sponges, improved antibacterial activity against Bacillus cereus and Escherichia coli, and high free radical scavenging capacity and hemostatic performance [49].
Figure 6.
SEM micrographs at 1000× magnifications showing the microstructure in cross-sectional of composite sponges [49].
Clove (Syzygium aromaticum L. Myrtaceae) EO possesses significant biological activities beneficial to human health, such as antimicrobial, antioxidant, and insecticidal properties. Consequently, it has attracted considerable attention for its widespread use in the medical world, perfume, cosmetic, flavoring, and food industries [50]. It can be extracted by (i) hydrodistillation, (ii) steam distillation, (iii) ultrasound-assisted extraction, (iv) microwave-assisted extraction, (v) cold pressing, or (vi) supercritical fluid extraction. The extraction methods used determine the concentration of primary volatile compounds in clove essential oil and organic clove extracts. It contains mostly eugenol (at least 50%), respectively, eugenyl acetate, β-caryophyllene, and α-humulene (10–40%).
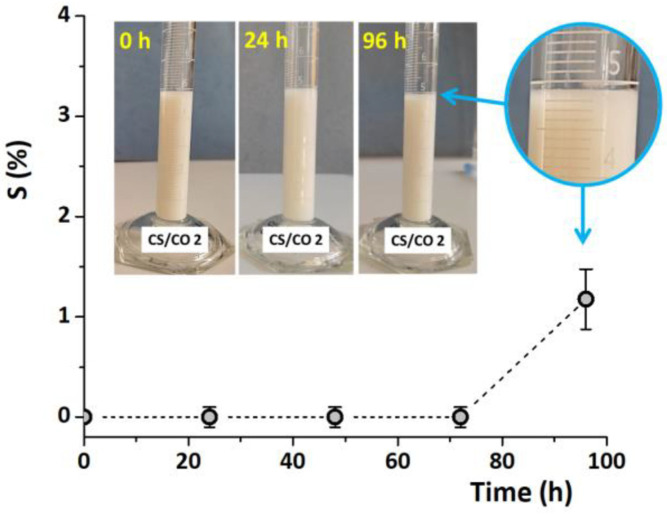
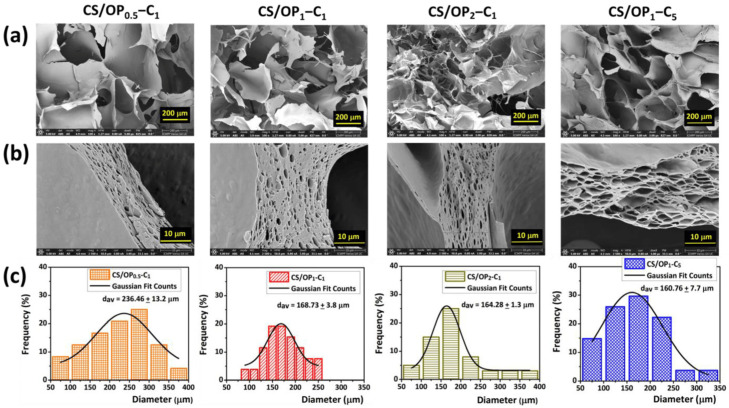
The development of different materials for biomedical applications has been in continuous growth lately [51]. Thus, the biological capabilities of clove EO were used in a very interesting recent study for the generation of hydrogels as wound dressings. The hydrogels were loaded with cloves EO by combining covalent and physical cross-linking methods. In the first step, EO was emulsified and stabilized in a chitosan-based solution, which was further strengthened by covalent cross-linking of the Schiff base with another polysaccharide, namely oxidized pullulan (Figure 7). In the next step, several freeze–thaw cycles were performed to stabilize the cloves EO in the physically cross-linked polymer walls. The hydrogels formed with a sponge-like porous structure (Figure 8) exhibited outstanding elasticity [52].
Figure 7.
Photos of the stable chitosan emulsion with clove EO content (after 0–4 days) kept in the dark and ambient conditions [52].
Figure 8.
Cross-sectional SEM images of chitosan and oxidized pullulan-based hydrogels loaded with clove EO: overview (a), wall detail (b), and pore size distribution with corresponding diagrams (c) [52].
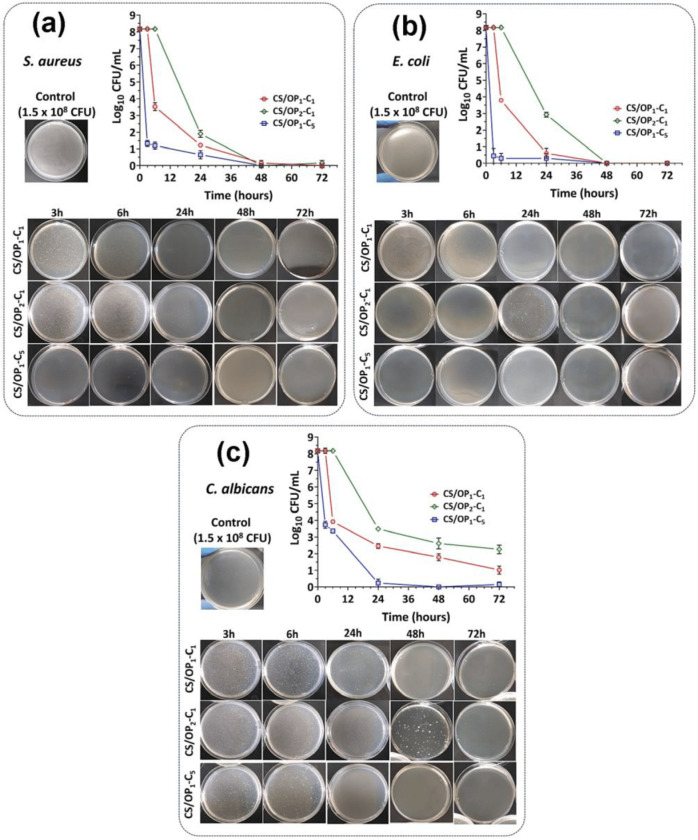
The antibacterial activity of hydrogels containing clove essential oil was evaluated by the time-kill method, for different incubation time intervals, against three bacterial strains and demonstrated antibacterial and antifungal effectiveness against S. aureus and E. coli (Figure 9) [52].
Figure 9.
Antibacterial effect of clove oil-loaded hydrogels evaluated by the time-kill method, after 3–72 h of incubation with S. aureus (a), E. coli (b), and C. albicans (c) [52].
A novel gelatin nanocomposite hydrogel formulation encapsulated thyme essential oil in sodium caseinate nanomicelles formulated as a gelatin nanocomposite hydrogel which has been investigated as a drug delivery platform for in vitro antibacterial and in vivo wound healing potential [35]. The evaluation tests of the biocompatible and hemocompatible hydrogel showed a sustained in vitro release profile of EOs, with a strong antibacterial effect. In addition, the wound-healing potential of the nanocomposite was investigated in vivo, demonstrating a significant wound reduction in the group of animals it was tested on, after only 18 days. Antibacterial hydrogel may be a promising active and biocompatible platform for sustained delivery of thyme essential oil [35].
Origanum vulgare L. (oregano) has been used since ancient times all over the world, as a culinary ingredient, spice, or preservative and in curative treatments, being carminative, tonic, stimulant, and diaphoretic. Numerous studies have reported the main characteristics of this common plant, which demonstrate good analgesic, antimicrobial, antifungal, antiviral, antioxidant, and anti-inflammatory activities. In addition, it helps to easily penetrate the skin for transdermal drug administration [53]. In local applications, it is useful in antiaging treatments, due to its antioxidant and anti-inflammatory properties which provides protection against free radicals of various reactive oxygen species [54,55]. It has a wealth of volatile and non-volatile components such as flavonoids, phenolic acids, and tannins, mainly phenolic monoterpenes such as carvacrol and thymol, with a variable chemical profile depending on the species and the geographical area [56].
Recent research used oregano EO in an innovative hydrogel formulation based on polymeric micelles (Figure 10) [57]. The release and permeation profile of the EO, the in vivo effects on biocompatibility, and the impact of the hydrogel on in ovo-angiogenesis were evaluated. It should be noted that the study avoided animal testing and a chick chorioallantoic membrane was used. The results showed a sustained release of EO, having a potential anti-angiogenic effect. This hydrogel with oregano EO content could be a natural therapeutic alternative in skin pathologies, such as fibroepithelial polyps [57].
Figure 10.
SEM micrographs of polymeric hydrogel (control), oregano essential oil, and micelle-based hydrogel sample containing oregano EO [57].
The bioactive and curative phytotherapeutic potential of essential oils has been exploited in a multitude of applications for wound healing [58,59].
Peppermint (Mentha × piperita) EO is widely used in the cosmetic industry for its aromatic fragrance. Its main constituents are menthol, menthone, and menthol acetate, and as secondary components, it contains bitter substances, caffeic acid, flavonoids, tannins, 1,8-eucalyptol, and propanone [60]. The pine needles EO mainly contains α-terpineol, linalool, and limonene, but also anethole, caryophyllene, and eugenol [61]. Fennel EO predominantly contains (E)-anethole, but also α-phellandrene and fenchone, methyl chavicol, p-cymene, and β-phellandrene [62].
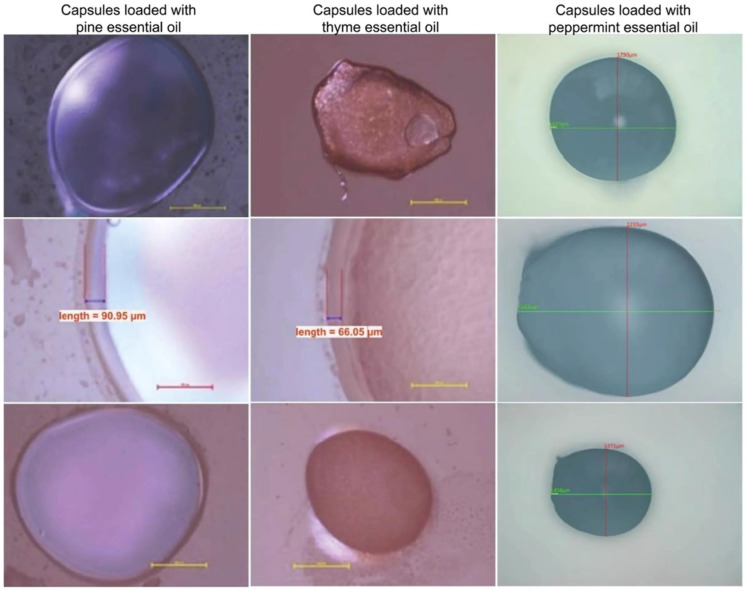
The healing capacities of four types of EOs have been used advantageously by encapsulating them in microcapsules in the first step, and then by incorporating them in polymer matrices in the form of films to develop dressings for wounds [63]. Polyvinyl alcohol, polyvinyl pyrrolidone, and hydroxypropyl methylcellulose were selected as polymeric materials. Poly(ethylene glycol) and glycerol were used as plasticizers, together with Zn stearate as a stabilizer, and vitamins A and E for the antioxidant effect. EOs of mint, thyme, pine, and fennel were loaded into the polymer matrices as active substances with antimicrobial effects. The different types and compositions of EOs and polymer components affect the shape and aspect of the microcapsules, which can be visibly observed (Figure 11). The results of the investigations showed that the samples made with EO content presented good inhibitory activity and antimicrobial properties against Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Pseudomonas aeruginosa, and Candida albicans [63].
Figure 11.
Optical microscopy images of polymer capsules of different shapes loaded with pine, thyme, and mint EO [63].
5.1.4. Chemotherapeutic
The alarming increase in the number of cancer cases in recent years highlights the pressing need for intensified efforts to improve therapeutic protocols [64,65]. Breast cancer, brain cancer, or invasive skin cancer affect millions of people every year and cause suffering and death all over the world [66,67]. With the multitude of different cancer treatment protocols, both surgical interventions and targeted therapies (chemo-, radio-, hormone-), the increase in the survival of cancer patients has been almost constant in recent years [68]. From here it is obvious the major urgency with which new therapies, combinatory, and targeted strategies are approached for a synergistic effect that will prolong the survival time and decrease the mortality rate [69,70].
Owing to some serious side effects of currently used anticancer chemotherapeutic methods or agents, there is a growing trend to use herbal medicine and its phytocompound derivatives [71]. It is important to use them both as ideal therapeutic alternatives and alongside chemotherapy treatments for many types of cancer [72,73,74].
Research is in a continuous dynamic and is actively focused on the discovery of new “green” pharmacological components for chemotherapies that offer potent potential activity with minimal side effects. A study aimed at obtaining new synergistic therapeutic agents (antimicrobial, antioxidant, and anticancer) was carried out by nanoencapsulation of clove essential oil in a nanogel based on squid chitosan and another phytochemical component, namely ρ-coumaric acid [75]. The in vitro evaluation of the nanogel encapsulated with clove essential oil indicated chemotherapeutic effects and potential for the prevention or therapy of pathologies induced by oxidative stress, microbial infection, or breast and skin cancer [75].
5.1.5. Carrier for Drug Delivery
Hydrogel delivery systems are excellent therapeutic tools for multiple clinical uses [76]. The adjustable 3D structure of hydrogels allows the inclusion of small molecules, macromolecules, or growth factors and they have the ability to protect drugs susceptible to degradation. It also ensures precise spatial and temporal control over the release of therapeutic factors and degradability [77].
Bioactive molecule delivery systems are designed and developed in the form of films, pearls, and nanogels. In order to create a smart drug carrier with intestinal release activity, alginate hydrogel beads containing essential oils were made [78]. Glycyrrhizic acid, licorice root extract, and Thymus EO were loaded into ß-cyclodextrin. By co-encapsulating them with alginate, active alginate hydrogel beads were obtained. Studying the release of EO from alginate beads in simulated gastric fluid and simulated intestinal fluids indicated a high release rate of both EOs [78].
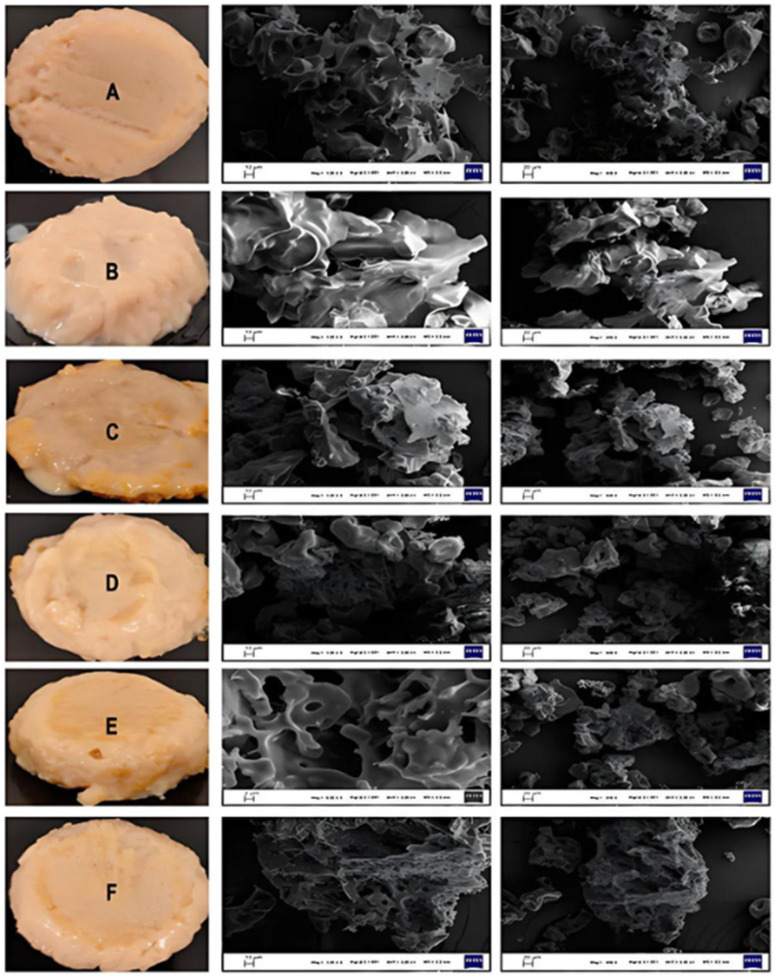
The characteristics of a natural hydrogel nanoliposome hybrid system were evaluated for the controllable release of thyme essential oil in the gastrointestinal tract [79]. Hydrogels based on pea protein and gum Arabic indicated the need for intermediates such as maltodextrin for stabilization. Figure 12 shows different photo and SEM images of different formulations, with and without nanoliposomes and nanoliposome–maltodextrin complexes [79].
Figure 12.
Photo and SEM images of different hydrogel formulations, with and without EO, nanoliposomes, and nanoliposome/maltodextrin complexes. (A): control hydrogel; (B): hydrogel with EO; (C): hydrogel with lecithin and EO (14.23%); (D): hydrogel with lecithin, maltodextrin and EO (20%); (E): hydrogel with lecithin, maltodextrin and EO (25%); (F): hydrogel with lecithin, maltodextrin, and EO (33%) [79].
5.1.6. Burn Healing
Burns or scalds to the skin are particularly serious injuries, sometimes life-threatening, as they can disrupt the body’s essential functions. This disruption is primarily due to the loss of water, electrolytes, and proteins, because of the wounds [80].
Burns need emergency medical care along with very strict infection control and surveillance measures to increase the rate of healing and survival. The appearance of multi-resistant organisms to antibiotics or some treatments, such as dressings or ointments inappropriate for the degree of burn, can lead to invasive infections [81].
Polysaccharide-based hydrogel dressings are more advantageous materials for the treatment of burns, compared to traditional textile dressings, due to the easy application and removal and rapid coverage of the wounds together with the surrounding areas, the good capacity to absorb exudate, and the comfort given by the improvement quickness of pain. In addition, the transparency of most hydrogels allows for easier management of lesions.
Numerous studies have reported the results of the use of polymeric hydrogels with different EOs content in the care of wounds and burns [82,83,84]. New formulations of hydrogel materials based on polyvinyl alcohol and gelatin enriched with ginger extract have been proposed as dressings for burn wound healing. The hydrogels demonstrated comparable wound healing efficacy to the commercial dressing on rabbit back burn wounds in vivo. In addition, they showed significantly higher wound healing activity than the control group, as evidenced by intensive collagen development observed in histopathological analysis [85]. A new study obtained and tested materials for the treatment of burns by designing dressings based on physically cross-linked carboxymethyl chitosan and carbomer 940 hydrogels. EOs of eucalyptus, ginger, and cumin were selected and loaded into them [86]. The hydrogel containing eucalyptus EO showed favorable antibacterial activities against S. aureus and E. coli. Moreover, experiments performed in vivo on mice demonstrated that hydrogel with eucalyptus EO improved wound healing in burn models and considerably promoted the regeneration of the dermis and epidermis. The histological analysis highlighted the decrease in the values of IL-6, TNF-α, and the increase in the values of the factors TGF-β, VEGF, and EGF, specific to the burn wound tissue area [86].
5.2. Dental Applications
Traditionally, plants, herbal extracts, and essential oils have been successfully used in dentistry to clean teeth and dental caries [87]. People traditionally crafted toothbrushes using natural bristles from twigs selected from medicinal plants, which were rich in oils. Fir, clove, bay, eucalyptus, juniper, neem, or oak were used, with a rich content of volatile oils that acted to stimulate blood circulation, and with tannins for contraction and cleaning of the gums [88]. They also used poppies or cranberries, rich in vitamins, to keep their gums healthy. It has been observed that aloe vera plants, marigolds, and grapefruit seeds have beneficial and anti-inflammatory effects in the oral cavity [89]. They inhibit the growth of aerobic or anaerobic bacteria and act to reduce gingival bleeding and gingivitis [90].
Phytochemicals provide a potential strategy in the prevention and treatment of dental caries, inflammation, and other oral infections and could be a powerful substitute for antibiotics [91,92].
A promising strategy for the prevention and treatment of dental caries, inflammation, and other oral infections is the use of phytochemicals both in current care products and in oral treatments [87]. These natural compounds could serve as a powerful alternative to antibiotics [93].
Extensive recent research has developed hydrogels with incorporated essential oils for the therapy of periodontitis [94]. These materials are described as dental drugs that could be used as photosensitizers in photodynamic therapy for the treatment of periodontitis. Oregano®, Frankincense®, and the Thieves® blend were incorporated as EOs, with a content of cloves, lemon, cinnamon bark, eucalyptus radiata, and rosemary extract. The main constituents identified from the mixture of selected and used oils included eugenol, pinene, limonene, carvacrol, and cymene [94].
5.3. Cosmetics Applications
Essential oils are integral to the formulation of care products and cosmetics, offering a wide range of benefits thanks to their rich and diverse composition of biocompounds [95,96]. Moreover, hydrogels combined with various chemical compounds can be incorporated into cosmetic formulations, offering multiple topical applications for both skin and hair [97,98].
Hydrogels for cosmetic preparations can be obtained from biopolymers of natural origin, such as alginate, collagen, gelatin, hyaluronic acid, chitosan, xanthan gum, pectin, starch, or cellulose [99,100]. These biopolymers themselves possess bioactivities advantageous to cosmetics. Thus, new cosmetic products were designed and made in the form of gels, microcapsules, or masks, both for skin and hair, with excellent hydration, softening, and elasticity performances, supporting and actively promoting anti-aging. Also, superabsorbent hydrogels have been developed in comfortable hygiene products, capable of absorbing fluids.
The combination of hydrogels with essential oils is a successful mixture, particularly useful and advantageous as cosmetic preparations or beauty and care products [101,102,103].
Recently, a study was reported that aimed at the design and creation of new cosmeceutical materials based on hydrogels with improved biological properties [104]. In the first step, Camellia oleifera EO was loaded into chitosan nanoparticles by emulsification and then ionic gelation. Then, hydrogels based on poly(vinyl alcohol), silk sericin, and gelatin were prepared, in which chitosan nanoparticles were embedded. Materials that showed tyrosinase inhibition and antioxidant activity could be useful in cosmeceutical applications, such as facial masks [104].
5.4. Food Applications
Hydrogel-based formulations with incorporated EOs have numerous applications in the food industry [105].
Hydrogels for food application should be categorized as follows: (i) Delivery; (ii) Packaging; (iii) Coating; (iv) Fat replacer; and (v) Texturizing. A growing field of research centers on hydrogel beads, which act as carriers for nano- or microparticles. These systems are highly effective for targeted drug delivery and can also be used as food supplements, including dietary additives, probiotics, or food components for special medical purposes [105,106]. These types of materials, which can be administered orally, have exploited the biological origin of natural polymers, especially polysaccharides and proteins, their specific biodegradability, and pH sensitivity [107]. A considerable amount of research has been devoted to the development of hydrogels for the encapsulation of food-grade components, such as vitamins, natural extracts, and essential oils [108].
Hydrogel beads show great potential for improving the bioavailability and performance of some compounds from the range of nutraceuticals, including EO, offering them protection against chemical degradation [109]. The granules are made through an accessible technique, in two steps: (i) obtaining the particles enriched with biopolymeric materials and nutraceutical content, and (ii) crosslinking the biopolymeric materials. The first step can be achieved by injection, phase separation, shearing, or templating, and the second step can be achieved by degree changes in the solvent quality, the incorporation of counter-ions or enzymes, or by heating–cooling cycles [110].
Recently, substantial amounts of waste from coffee pulp, generated during the extraction of essential oils, were analyzed [111]. These wastes were used to extract two different pectin fractions (highly methoxylated and low methoxylated). Pectins have been studied for their performance as EO carrier systems. The pectin fractions formed two systems of hydrogel beads, with or without chitosan, to encapsulate the EO of roasted coffee or green coffee. The two systems were analyzed in terms of their antioxidant activity and EO release profile for potential food applications. On the one hand, the highly methoxylated pectin obtained from Coffea arabica presented better EO encapsulation performances. On the other hand, surprisingly, the EOs obtained from roasted coffee showed superior antioxidant activity compared to that obtained from green coffee [111].
Food packaging serves as a passive barrier, shielding products from environmental factors, extending their shelf life by preventing contamination, and ensuring safe transportation and storage. Active packaging incorporated with essential oils allows interaction between food and the external environment, helping to regulate temperature, moisture levels, and microbial control, which ultimately enhances the quality and extends the shelf life of the food [112]. The upcoming chapter will focus on the key concerns and challenges faced by the food packaging industry. It will explore how essential oils influence the microstructure of packaging materials and examine their specific properties.
A new direction regarding the applications of hydrogels with EO content is represented by edible coatings that extend the shelf life of some perishable foods, by delaying oxidation and reducing the amount of packaging. EOs are known to be excellent natural antimicrobial and antibacterial agents [113]. Some contaminants such as gram-negative bacteria (E. coli) can cause serious diseases by contaminating food such as milk and meat or gram-positive ones (S. aureus and B. cereus), which cause the contamination of fruits or food products with starch content.
The richness of volatile compounds contained in EO, such as phenolics, determines the use of oils in edible films or coatings for flavoring, packaging, or preservation of food products. Different gels incorporating EO such as basil leaves, clove, cypress, fennel, lavender, oregano, pine, rosemary, thyme, and verbena have been used to inhibit lipid oxidation and microorganism growth in coatings for fish fillets, cheese, fruits, or vegetables [114,115,116,117,118].
Studies on two types of gelatin hydrogels containing rosemary and orange EO microdroplets prepared by simple emulsification in the presence of Tween®80 surfactant showed interesting conclusions [119]. The mechanical and antibacterial properties of these gels against some food contaminants such as E. coli, S. aureus, and B. cereus. indicated adequate characteristics as edible coatings of perishable foods, in order to preserve foods such as meat [119].
A novel area of research that has gained attention recently is the replacement of animal fats through the immobilization of oils within hydrogels [120]. Healthier meat products are a direction imposed both by worldwide recommendations and by consumer demands. Traditional products should be adapted to the nutritional characteristics recommended by specialists, by reformulating them. Therefore, a recent approach to improving the health of meat products is the use of healthy oils (vegetable or marine) as fat substitutes. Also, it is important to develop food products that are low in fat, but which retain their functional qualities, such as mayonnaise or ice cream [121]. However, it is a great challenge for researchers to keep the specific bioactivities of hydrogels with incorporated essential oils and use them as fat substitutes or as materials with specific textures. Future scientific discoveries based on nanotechnology will also develop such products.
5.5. Food Packaging Applications
Active packaging is representing intelligent materials that improve the preservation of food, especially perishable ones, extend the shelf life, and ensure safety by interacting with the food product through its various components [122].
Currently, there is a multitude of advantageous active packaging for applications in different fields. In the food industry, but also in the beverage industry, there is the most active packaging, due to the very high demand for increased shelf life, freshness, and safety. The pharmaceutical industry, medical technology, agriculture, and courier and delivery services are just a few other areas where there is a demand for these types of modern packaging.
Active packaging is created based on the active components of biopolymers or with different biocompounds incorporated into them [123].
For instance, biopolymeric hydrogels with essential oils incorporated as antimicrobial substances are advantageous systems for obtaining active food packaging. In addition to monitoring the condition and ensuring food safety, minimizing the risk of contamination, increasing the shelf life, or obtaining more durable packaging, smart packaging can help reduce food waste. In this context, adding EOs to packaging is a natural alternative that can replace chemical additives [124,125].
Cinnamon EO is a natural bacteriostatic agent, with potential applications in the field of food preservation [126]. In general, it has found many uses in culinary and medicinal applications. It contains numerous chemical constituents, of which, depending on the different species of Cinnamomum trees or shrubs, the most important are the compounds (E)-cinnamaldehyde, linalool, β-caryophyllene, eucalyptol, and eugenol [126]. Apart from the specific spicy taste and cinnamon flavor that is due to the cinnamaldehyde compound, the wide variety of components of cinnamon EO have antimicrobial, antioxidant, antifungal, and antidiabetic biological properties [127,128].
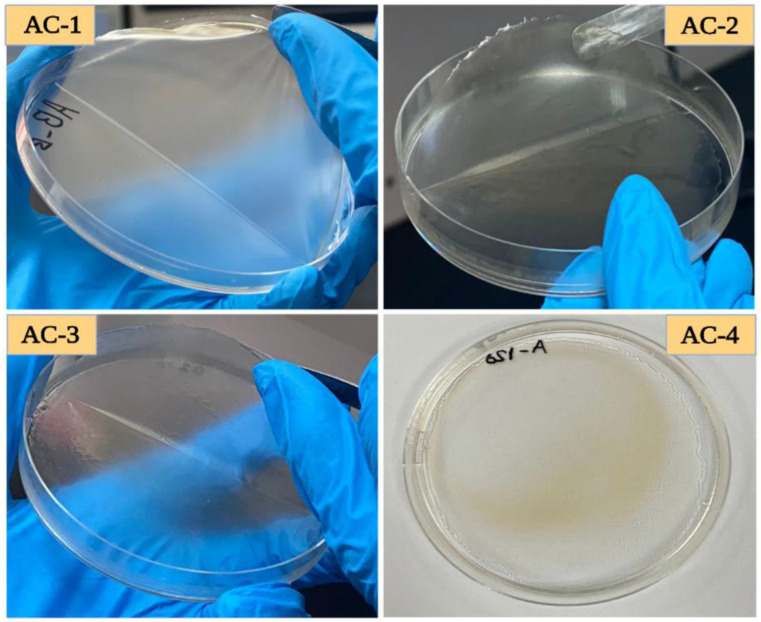
Sodium alginate and acacia gum hydrogels loaded with cinnamon EO were prepared as edible films and analyzed in terms of view of physicochemical characteristics [129]. The antioxidant capacity of the films was improved with increasing cinnamon EO concentration, making them promising candidates for use as active food packaging materials. Figure 13 shows the composite films prepared with different concentrations of cinnamon EO between 0–30 μL EO [129].
Figure 13.
Photo of the composite films based on sodium alginate and acacia gum with different concentrations of cinnamon EO: (AC1—without EO) (AC2—15 μL EO) (AC3—20 μL EO) (AC4—30 μL EO) [129].
Recently, the phytochemical components of the essential oil obtained from the Artemisia dracunculus plant, widely distributed geographically, were identified and evaluated [130]. Artemisia dracunculus EO was valorized by incorporating different amounts into hydrogel matrices based on polyvinyl alcohol and agar. The results of the antimicrobial tests indicated sustained antimicrobial activity against nine pathogenic strains (four Gram-positive and five Gram-negative). The incorporation of Artemisa dracunculus EO in these hydrogel models can lead to practical applications in the area of food technology, as an active and biodegradable alternative to classic packaging [130].
In another study, a quantity of powdered starch was obtained in the first stage from the use of residual biomass, then in the second stage, it was introduced into a formulation, to prepare cryogels and hydrogels [131]. The materials prepared by absorption or cross-linking by the Schiff-base reaction were loaded with diacetyl and mint EO. The prepared materials showed a good ability to adsorb water and deliver antimicrobial substances, being advantageous for possible fresh food packaging applications [131].
Furthermore, new antibacterial hydrogels were prepared by the method of freeze–thaw cycles [127]. Inclusion complexes methyl-β-cyclodextrin and thyme oil were incorporated into a polyvinyl alcohol matrix with polysaccharide content, respectively, dendrobium and guar gum, in various ratios. These materials presented very good mechanical performance, as well as antimicrobial and antioxidant activities favorable for the preservation of chicken breast, extending the shelf life by four days. These results indicate the potential of the materials for possible active packaging applications [132].
5.6. Restoration of Stone Cultural Heritage
Stone monuments in the sphere of cultural heritage suffer from biological damage. The variation in environmental conditions determines the growth of phototrophic microorganisms on stone surfaces, in the form of biofilms [133,134,135]. These microorganisms are made of microbial aggregates that act mechanically and produce micro-decohesion of the substrates [136]. In addition, biofilms, together with atmospheric pollutants, promote chemical corrosion, pigmentation, or discoloration of stone surfaces [137].
The classic restoration of stone surfaces uses both physical and chemical methods [138]. However, mechanical brushing can damage the surface of the monument, and chemical treatment can lead to a selection of resistant microbial species or can be harmful to the environment or the operators [139,140].
An innovative and eco-sustainable restoration technique is the use of essential oils with natural biocidal action, embedded in hydrogels, as alternatives to chemical treatments for the restoration of cultural heritage [141,142,143,144].
EOs of lavender and thyme were encapsulated in alginate hydrogel in order to create an easy-to-use and non-invasive restoration method [136]. The vitality of cyanobacterial biofilms was discouraged by applying hydrogel for different periods of time. The results of the tests indicated that the best inhibitory effect on the photosynthetic activity of microorganisms was shown by thyme oil rich in thymol, for a concentration of 0.1% (v/v) it was shown by thyme oil rich in thymol. It retained an effective antimicrobial action against cyanobacteria. Notably, the developed protocol allowed the use of a very small amount of essential oil as a green biocide [136].
In another study, thyme EO was also used in the preparation of poly(vinyl)alcohol and borax-based hydrogels, together with a double-layered hydroxide of ZnAl intercalated with sodium alginate, and silver nanoparticles or a mix of silver–silver chloride nanoparticles [145]. The hydrogels were thus formulated to mechanically remove the biopatina from two types of biodamaged stones: Carrara marble and St. Margarethen. The hydrogel with thyme EO content worked effectively for cleaning stones with porous structures and different compositions, damaged by the natural environment [145].
A real case study reported the results of in situ application of a sodium alginate hydrogel containing thyme EO. The experiment followed the restoration of three selected parts of Fortunato Depero’s mosaic located in a neighborhood in Rome (Italy) [146]. The material was prepared by a simple method and easily applied on large and vertical surfaces. The images taken before and after application demonstrated that a single treatment was enough to completely eliminate the microbial patina. The hydrogel loaded with thyme EO as a natural biocide showed a very good biocide performance [146].
In summary, Table 1 outlines the key characteristics of various essential oils discussed in this review, highlighting the main bioactive compounds, the extraction methods used, pharmacological features and potential applications. The brief summary of essential oil-enriched hydrogel applications introduced in this manuscript can be found in Table 2.
Table 1.
General characteristics of essential oils incorporated in hydrogels.
| Plants | Essential Oils | Main Constituents | Extraction Procedure | Pharmacological Properties | Applications | Reference |
|---|---|---|---|---|---|---|
| Cinnamomum zeylanicum | Cinnamon oil | cinnamaldehyde | Steam distillation and Soxhlex extraction | Antimicrobial, antibiotic, antioxidant | Food packaging materials, food preservation | [129,147,148,149] |
| Lavandula angustifolia | Lavandin essential oils | Terpenes (e.g., linalool, linalyl acetate, terpinen-4-ol) and terpenoids (e.g., eucalyptol) |
Steam distillation | Antioxidants, antibacterial, anxiolytics, analgesics, and anti-inflammatories | Wound healing, Microparticles as delivery system |
[48,150,151,152,153] |
| Cymbopogon (spp.) | Lemongrass essential oils | Terpenes and Terpenoids (Terpinen-4-ol, α-Terpineol (neral, isoneral, geranial, isogeranial, geraniol, geranyl acetate, citronellal, citronellol, germacrene-D, and elemol) | Steam distillation | Antifungal, antibacterial, antiviral, anticancer, and antioxidant | Pharmaceutical, cosmetics, and food preservations industries | [154,155] |
| Melaleuca alternifolia | Tea tree essential oils | Terpenes (e.g., terpinen-4-ol, 1,8-cineole) | Steam distillation | Antimicrobial and anti-inflammatory | Beads for food preservation | [156,157,158] |
| Mentha piperita | Peppermint essential oils | Menthol, menthone, neomenthol and iso-menthone | Steam distillation, hydrodistillation, microwave-assisted extraction, supercritical fluid extraction, ultrasonic-assisted extraction and countercurrent extraction | Anti-inflammatory, antibacterial, antiviral, scolicidal, immunomodulatory, antitumor, neuroprotective, antifatigue and antioxidant; hypoglycemic and hypolipidemic effects, gastrointestinal and dermatological diseases | Patches, wound dressing | [63,159,160,161,162] |
|
Ocimum basilicum (L.) |
Basil essential oils | Eugenol, e α-Pinene, β-Pinene, Methyl chavicol, 1,8 cineole, L-linalool, Ocimene, Borneol, Geraneol, B-Caryphyllone, and n-Cinnamate |
Hydrodistillation | Carminative, galactogogue, stomachic and antispasmodic tonic, vermifuge, | Food packaging, antiperspirant in agriculture |
[163,164,165,166] |
| Thymus vulgaris (L.) | Thyme essential oils | Carvacrol, 5-isopropyl-2-methylphenol, and a p-cymene | Hydrodistillation, steam distillation | Antioxidant, antimicrobial, antidiabetic, anti-inflammatory, immunomodulatory and anticancer bioactivities | Wound healing, wound dressing; beads as delivery systems |
[33,35,36,47,63,79,167] |
Table 2.
Applications of essential oils incorporated in hydrogels.
| Method of Preparations | Materials | Encapsulated Essential Oils | Applications | References | |
|---|---|---|---|---|---|
| Biomedical applications | Physical crosslinking | Sodium alginate/Fucoidan | Menthol, L-linalool, bergamot oil, and β-pinene | Topical or transdermal administration | [43] |
| Physical crosslinking | Methylcellulose (10% (w/v)) | Melissa officinalis EO | Treatment of oral candidiasis. | [44] | |
| Chemical crosslinking | Polyvinyl Alcohol/Corn Starch Hydrogel Films loaded with Silver Nanoparticles | Patchouli EO | Antimicrobial materials (against Staphylococcus aureus and Staphylococcus epidermidis) | [45] | |
| Solvent displacement method | Poly-(D,L)-(lactic-co-glycolic acid) | Thyme EO | Inflammatory skin disorders | [47] | |
| Physical crosslinking | Polyvinyl alcohol/kaolin | Cedar EO | Wound dressing | [49] | |
| Covalent and physical crosslinking | Chitosan/oxidized pullulan | Clove EO | Wound dressings | [52] | |
| Cold gelation process | Polymeric-Micelles-Based Hydrogels (Pluronic F127–20%w/w; and Pluronic L 31—1%w/w) | Oregano EO | Cutaneous application | [57] | |
| Chemical crosslinking; casting method | Polyvinyl alcohol/polyvinyl pyrrolidone; hydroxypropyl methyl cellulose; sodium alginate; polyethylene glycol; glycerol; Zn stearate; vitamin A and E | Fennel, pine, mint and thyme EO | Wound dressings | [63] | |
| Chemical crosslinking | Ultrasound-assisted deacetylated chitosan/ρ-coumaric acid | Clove EO | Chemotherapeutic/chemopreventive agent | [75] | |
| Thin-film dispersion technique/heat-induced gelation. | Pea protein (30%) and gum Arabic (1.5%); Soybean lecithin; maltodextrin and gum Arabic | Thyme EO | Delivery of bioactive compounds (food packing material, tissue engineering or drug delivery) | [79] | |
| Physical crosslinking | Carboxymethyl chitosan/carbomer 940 | Eucalyptus, ginger, and cumin EO | Burn dressing material for skin burn repair | [86] | |
| Dental applications | Physical crosslinking | Xanthan gum/Glycerin/Lyophilized Whey/Polyvinylpyrrolidone/PEG 400 | Oregano®, Frankincense®, Thieves®, Frankincense® EO | Therapy of periodontitis | [94] |
| Cosmetics applications | Ionic gelation | Poly(vinyl alcohol), silk sericin, and gelatin/chitosan nanoparticles | Camellia oleifera EO | Cosmetic product (facial masks) | [104] |
| Food applications | Emulsification/ionic crosslinking | Pectin and pectin/chitosan hydrogel beads | Green and roasted coffee EO | Systems for the delivery and controlled release of essential oils; food applications | [111] |
| Food packaging applications | Gelation/casting | Sodium alginate/acacia gum | Cinnamon EO | Hydrogel-based films as active food packaging materials | [129] |
| Restorations of the stone cultural heritage | Preparation directly in situ | Sodium alginate | Thyme EO | Biocides for restoration in a real case of study, i.e., the mosaic Le Professioni e le Arti of Fortunato Depero | [146] |
6. Challenges and Perspectives
The advancement of polymeric hydrogels presents emerging opportunities for their use across various fields, thanks to their biocompatibility, simple gelation process, ease of application, and the potential for functionalization. Hydrogels have the ability to alter their volume, phase, and structure when exposed to specific external stimuli, making them versatile for use in a wide range of sectors. However, the limited mechanical rigidity commonly found in some biocompatible hydrogels presents a significant challenge that needs to be addressed, particularly when dealing with rapid dynamic changes or when considering structural uniformity and long-term stability.
Integrating EO into the hydrogel matrix can enhance their biological activities, shield them from degradation, and serve as a platform for creating innovative biotechnological products. Furthermore, encapsulation seeks to address certain limitations of EOs, such as their volatility, reduced stability, and high sensitivity to environmental conditions. The controlled release of bioactive compounds from EOs encapsulated within hydrogels is crucial for effectively delivering these compounds to their target. On the other hand, a huge difficulty, especially in medical applications, is the very high qualitative and quantitative variability of the EO composition. This is determined by intrinsic factors that influence each other and are due to the varieties and age of the plants, the type of soil, the climate, or the time of harvesting, but also by some extrinsic factors such as the extraction methods used.
However, to enhance their effectiveness as targeted delivery systems of EOs, further research is required to assess their safety across various applications, ranging from biomedical to food industries.
The limitations related to the insufficient data on the stability, safety, and long-term bioactivity of these materials are emphasized. Moreover, the limited number of in vivo studies, particularly in the medical field, could delay their commercialization in the pharmaceutical and biomedical sectors.
7. Conclusions
This review aims to provide an overview of polymeric hydrogels containing essential oils, emphasizing their vast potential for applications in various fields. Hydrogels are valuable as delivery systems because of their ability to be biocompatible, biodegradable, and provide controlled release of plant-derived bioactive ingredients. Hydrogel structures, known for their remarkable swelling, gelling, and bioactive loading capabilities, play a vital role in the creation of functional materials. Most essential oils are accepted, credited, and appreciated as valuable bioactive ingredients, capable of performing multiple pharmacological functions, such as anticancer, antiseptic, antiviral, and antioxidant activities. Results from the cited literature suggest that hydrogels containing essential oils are ecological, sustainable materials, with improved biological properties, demonstrating effective antibacterial, antifungal, anticancer, and anti-inflammatory activities. Nevertheless, challenges remain in this field, including the need for standardization and the absence of cost-effective methods for scaling up production on a larger scale. Additionally, issues concerning stability and toxicity require thorough investigation. The advancement of essential oil-enriched hydrogel materials presents a growing opportunity to be applied in a wide range of fields, including biomedicine, cosmetics, dentistry, the food industry, and even heritage conservation.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflicts of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bayles A.V., Pleij T., Hofmann M., Hauf F., Tervoort T., Vermant J. Structuring hydrogel cross-link density using hierarchical filament 3D printing. ACS Appl. Mater. Interfaces. 2022;14:15667–15677. doi: 10.1021/acsami.2c02069. [DOI] [PubMed] [Google Scholar]
- 2.Vasile C., Pamfil D., Stoleru E., Baican M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules. 2020;25:1539. doi: 10.3390/molecules25071539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Löwenberg C., Balk M., Wischke C., Behl M., Lendlein A. Shape-memory hydrogels: Evolution of structural principles to enable shape switching of hydrophilic polymer networks. Acc. Chem. Res. 2017;50:723–732. doi: 10.1021/acs.accounts.6b00584. [DOI] [PubMed] [Google Scholar]
- 4.Unalan I., Boccaccini A.R. Essential oils in biomedical applications: Recent progress and future opportunities. Curr. Opin. Biomed. Eng. 2021;17:100261. doi: 10.1016/j.cobme.2021.100261. [DOI] [Google Scholar]
- 5.Yan M.-R., Wang C.-H., Cruz Flores N.H., Su Y.-Y. Targeting Open Market with Strategic Business Innovations: A Case Study of Growth Dynamics in Essential Oil and Aromatherapy Industry. J. Open Innov. Technol. Mark. Complex. 2019;5:7. doi: 10.3390/joitmc5010007. [DOI] [Google Scholar]
- 6.Mkaddem Mounira G. Recent Advances and New Applications. IntechOpen; Rijeka, Croatia: 2024. Essential Oils and Their Bioactive Molecules. [DOI] [Google Scholar]
- 7.Chelu M., Moreno J.C., Atkinson I., Cusu J.P., Rusu A., Bratan V., Aricov L., Anastasescu M., Seciu-Grama A.M., Musuc A.M. Green synthesis of bioinspired chitosan-ZnO-based polysaccharide gums hydrogels with propolis extract as novel func-tional natural biomaterials. Int. J. Biol. Macromol. 2022;211:410–424. doi: 10.1016/j.ijbiomac.2022.05.070. [DOI] [PubMed] [Google Scholar]
- 8.Majcher M.J., Hoare T. Applications of Hydrogels. In: Jafar Mazumder M., Sheardown H., Al-Ahmed A., editors. Functional Biopolymers. Springer; Cham, Switzerland: 2019. (Polymers and Polymeric Composites: A Reference Series). [DOI] [Google Scholar]
- 9.Chelu M., Musuc A.M. Polymer Gels: Classification and Recent Developments in Biomedical Applications. Gels. 2023;9:161. doi: 10.3390/gels9020161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sapuła P., Bialik-Wąs K., Malarz K. Are Natural Compounds a Promising Alternative to Synthetic Cross-Linking Agents in the Preparation of Hydrogels? Pharmaceutics. 2023;15:253. doi: 10.3390/pharmaceutics15010253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed M.S., Islam M., Hasan M.K., Nam K.-W. A Comprehensive Review of Radiation-Induced Hydrogels: Synthesis, Properties, and Multidimensional Applications. Gels. 2024;10:381. doi: 10.3390/gels10060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chelu M., Musuc A.M. Advanced Biomedical Applications of Multifunctional Natural and Synthetic Biomaterials. Processes. 2023;11:2696. doi: 10.3390/pr11092696. [DOI] [Google Scholar]
- 13.Yosri N., Khalifa S.A., Attia N.F., Du M., Yin L., Abolibda T.Z., Zhai K., Guo Z., El-Seedi H.R. Advancing sustainability in the green engineering of nanocomposites based on marine-derived polymers and their applications: A comprehensive review. Int. J. Biol. Macromol. 2024;274:133249. doi: 10.1016/j.ijbiomac.2024.133249. [DOI] [PubMed] [Google Scholar]
- 14.Rodrigues D.B., Reis R.L., Pirraco R.P. How are natural-based polymers shaping the future of cancer immunotherapy—A review. Polym. Rev. 2024;64:371–406. doi: 10.1080/15583724.2023.2234462. [DOI] [Google Scholar]
- 15.Monia T. Sustainable natural biopolymers for biomedical applications. J. Thermoplast. Compos. Mater. 2024;37:2505–2524. doi: 10.1177/08927057231214468. [DOI] [Google Scholar]
- 16.Manivannan R.K., Sharma N., Kumar V., Jayaraj I., Vimal S., Umesh M. A comprehensive review on natural macro-molecular biopolymers for biomedical applications: Recent advancements, current challenges, and future outlooks. Carbohydr. Polym. Technol. Appl. 2024;8:100536. doi: 10.1016/j.carpta.2024.100536. [DOI] [Google Scholar]
- 17.Buriti B.M.A.d.B., Figueiredo P.L.B., Passos M.F., da Silva J.K.R. Polymer-Based Wound Dressings Loaded with Essential Oil for the Treatment of Wounds: A Review. Pharmaceuticals. 2024;17:897. doi: 10.3390/ph17070897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satchanska G., Davidova S., Petrov P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers. 2024;16:1159. doi: 10.3390/polym16081159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harini A., Sofini S.P.S., Balasubramanian D., Girigoswami A., Girigoswami K. Biomedical applications of natural and synthetic polymer-based nanocomposites. J. Biomater. Sci. Polym. Ed. 2023;35:269–294. doi: 10.1080/09205063.2023.2283910. [DOI] [PubMed] [Google Scholar]
- 20.Cai M.-H., Chen X.-Y., Fu L.-Q., Du W.-L., Yang X., Mou X.-Z., Hu P.-Y. Design and Development of Hybrid Hydrogels for Biomedical Applications: Recent Trends in Anti-cancer Drug Delivery and Tissue Engineering. Front. Bioeng. Biotechnol. 2021;9:630943. doi: 10.3389/fbioe.2021.630943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katopodi T., Petanidis S., Floros G., Porpodis K., Kosmidis C. Hybrid Nanogel Drug Delivery Systems: Transforming the Tumor Microenvironment through Tumor Tissue Editing. Cells. 2024;13:908. doi: 10.3390/cells13110908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sadgrove N.J., Padilla-González G.F., Phumthum M. Fundamental Chemistry of Essen-tial Oils and Volatile Organic Compounds, Methods of Analysis and Authentication. Plants. 2022;11:789. doi: 10.3390/plants11060789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stratakos A.C., Koidis A. Chapter 4—Methods for Extracting Essential Oils. In: Preedy V.R., editor. Essential Oils in Food Preservation, Flavor and Safety. Academic Press; Cambridge, MA, USA: 2016. pp. 31–38. [DOI] [Google Scholar]
- 24.Dhifi W., Bellili S., Jazi S., Bahloul N., Mnif W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines. 2016;3:25. doi: 10.3390/medicines3040025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masyita A., Mustika Sari R., Dwi Astuti A., Yasir B., Rahma Rumata N., Emran T.B., Nainu F., Simal-Gandara J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X. 2022;13:100217. doi: 10.1016/j.fochx.2022.100217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yammine J., Chihib N.E., Gharsallaoui A., Ismail A., Karam L. Advances in essential oils encapsulation: Development, characterization and release mechanisms. Polym. Bull. 2024;81:3837–3882. doi: 10.1007/s00289-023-04916-0. [DOI] [Google Scholar]
- 27.Sousa V.I., Parente J.F., Marques J.F., Forte M.A., Tavares C.J. Microencapsulation of Essential Oils: A Review. Polymers. 2022;14:1730. doi: 10.3390/polym14091730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moghaddam M., Mehdizadeh L. Soft Chemistry and Food Fermentation. Academic Press; Cambridge, MA, USA: 2017. Chapter 13—Chemistry of Essential Oils and Factors Influencing Their Constituents; pp. 379–419. [DOI] [Google Scholar]
- 29.Lammari N., Louaer O., Meniai A.H., Elaissari A. Encapsulation of Essential Oils via Nanoprecipitation Process: Overview, Progress, Challenges and Prospects. Pharmaceutics. 2020;12:431. doi: 10.3390/pharmaceutics12050431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Syed I., Garg S., Sarkar P. Polymeric Gels. Woodhead Publishing; Cambridge, UK: 2018. Entrapment of essential oils in hydrogels for biomedical applications; pp. 125–141. [DOI] [Google Scholar]
- 31.El Asbahani A., Miladi K., Badri W., Sala M., Aït Addi E.H., Casabianca H., El Mousadik A., Hartmann D., Jilale A., Renaud F.N.R., et al. Essential oils: From extraction to encapsulation. Int. J. Pharm. 2015;483:220–243. doi: 10.1016/j.ijpharm.2014.12.069. [DOI] [PubMed] [Google Scholar]
- 32.Stoleru E., Dumitriu R.P., Ailiesei G.-L., Yilmaz C., Brebu M. Synthesis of Bioactive Materials by In Situ One-Step Direct Loading of Syzygium aromaticum Essential Oil into Chitosan-Based Hydrogels. Gels. 2022;8:225. doi: 10.3390/gels8040225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spyrou S., Bellou M.G., Papanikolaou A., Nakou K., Kontogianni V.G., Chatzikonstan-tinou A.V., Stamatis H. Evaluation of Antioxidant, Antibacterial and Enzyme-Inhibitory Properties of Dittany and Thyme Extracts and Their Application in Hydrogel Preparation. BioChem. 2024;4:166–188. doi: 10.3390/biochem4030009. [DOI] [Google Scholar]
- 34.Mostaghimi M., Majdinasab M., Hosseini S.M.H. Characterization of alginate hydrogel beads loaded with thyme and clove essential oils nanoemulsions. J. Polym. Environ. 2022;30:1647–1661. doi: 10.1007/s10924-021-02298-w. [DOI] [Google Scholar]
- 35.Alsakhawy S.A., Baghdadi H.H., El-Shenawy M.A., Sabra S.A., El-Hosseiny L.S. Encapsulation of thymus vulgaris essential oil in caseinate/gelatin nano-composite hydrogel: In vitro antibacterial activity and in vivo wound healing potential. Int. J. Pharm. 2022;628:122280. doi: 10.1016/j.ijpharm.2022.122280. [DOI] [PubMed] [Google Scholar]
- 36.Çakır C., Gürkan E.H. Enhancing therapeutic effects alginate microencapsulation of thyme and calendula oils using ionic gelation for controlled drug delivery. J. Biomater. Sci. Polym. Ed. 2024:1–29. doi: 10.1080/09205063.2024.2386220. [DOI] [PubMed] [Google Scholar]
- 37.Fincheira P., Espinoza J., Levío-Raimán M., Vera J., Tortella G., Brito A.M.M., Seabra A.B., Diez M.D., Quiroz A., Rubilar O. Formulation of essential oils-loaded solid lipid nanoparticles-based chitosan/PVA hydrogels to control the growth of Botrytis cinerea and Penicillium expansum. Int. J. Biol. Macromol. 2024;270:132218. doi: 10.1016/j.ijbiomac.2024.132218. [DOI] [PubMed] [Google Scholar]
- 38.Zhao Y., Li H., Wang Y., Zhang Z., Wang Q. Preparation, characterization and release kinetics of a multilayer encapsulated Perilla frutescens L. essential oil hydrogel bead. Int. J. Biol. Macromol. 2023;249:124776. doi: 10.1016/j.ijbiomac.2023.124776. [DOI] [PubMed] [Google Scholar]
- 39.Dobroslavić E., Cegledi E., Robić K., Elez Garofulić I., Dragović-Uzelac V., Repajić M. Encapsulation of Fennel Essential Oil in Calcium Alginate Microbeads via Electrostatic Extrusion. Appl. Sci. 2024;14:3522. doi: 10.3390/app14083522. [DOI] [Google Scholar]
- 40.Chelu M., Musuc A.M. Biomaterials in Microencapsulation. IntechOpen; London, UK: 2024. Biomaterials-Based Hydrogels for Therapeutic Applications. [DOI] [Google Scholar]
- 41.De France K.J., Xu F., Hoare T. Structured Macroporous Hydrogels: Progress, Challenges, and Opportunities. Adv. Healthc. Mater. 2018;7:1700927. doi: 10.1002/adhm.201700927. [DOI] [PubMed] [Google Scholar]
- 42.Chelu M., Calderon Moreno J.M., Musuc A.M., Popa M. Natural Regenerative Hydrogels for Wound Healing. Gels. 2024;10:547. doi: 10.3390/gels10090547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barbosa A.I., Lima S.A.C., Yousef I., Reis S. Evaluating the Skin Interactions and Permeation of Alginate/Fucoidan Hydrogels Per Se and Associated with Different Essential Oils. Pharmaceutics. 2023;15:190. doi: 10.3390/pharmaceutics15010190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Serra E., Saubade F., Ligorio C., Whitehead K., Sloan A., Williams D.W., Hidalgo-Bastida A., Verran J., Malic S. Methylcellulose Hydrogel with Melissa officinalis Essential Oil as a Potential Treatment for Oral Candidiasis. Microorganisms. 2020;8:215. doi: 10.3390/microorganisms8020215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khairan K., Hasan M., Idroes R., Diah M. Fabrication and Evaluation of Polyvinyl Alcohol/Corn Starch/Patchouli Oil Hydrogel Films Loaded with Silver Nanoparticles Biosynthesized in Pogostemon cablin Benth Leaves’ Extract. Molecules. 2023;28:2020. doi: 10.3390/molecules28052020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chelu M., Musuc A.M., Aricov L., Ozon E.A., Iosageanu A., Stefan L.M., Prelipcean A.-M., Popa M., Moreno J.C. Antibacterial Aloe vera Based Biocompatible Hydrogel for Use in Dermatological Applications. Int. J. Mol. Sci. 2023;24:3893. doi: 10.3390/ijms24043893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Folle C., Díaz-Garrido N., Mallandrich M., Suñer-Carbó J., Sánchez-López E., Halbaut L., Marqués A.M., Espina M., Badia J., Baldoma L., et al. Hydrogel of Thyme-Oil-PLGA Nanoparticles Designed for Skin Inflammation Treatment. Gels. 2024;10:149. doi: 10.3390/gels10020149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rusu A.G., Niță L.E., Roșca I., Croitoriu A., Ghilan A., Mititelu-Tarțău L., Grigoraș A.V., Crețu B.-E.-B., Chiriac A.P. Alginate-Based Hydrogels Enriched with Lavender Essential Oil: Evaluation of Physicochemical Properties, Antimicrobial Activity, and In Vivo Biocompatibility. Pharmaceutics. 2023;15:2608. doi: 10.3390/pharmaceutics15112608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamer T.M., Sabet M.M., Alhalili Z.A.H., Ismail A.M., Mohy-Eldin M.S., Hassan M.A. Influence of Cedar Essential Oil on Physical and Biological Properties of Hemostatic, Antibacterial, and Antioxidant Polyvinyl Alcohol/Cedar Oil/Kaolin Composite Hydrogels. Pharmaceutics. 2022;14:2649. doi: 10.3390/pharmaceutics14122649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cortés-Rojas D.F., de Souza C.R.F., Oliveira W.P. Clove (Syzygium aromaticum): A precious spice. Asian Pac. J. Trop. Biomed. 2014;4:90–96. doi: 10.1016/S2221-1691(14)60215-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chelu M., Popa M., Ozon E.A., Pandele Cusu J., Anastasescu M., Surdu V.A., Calderon Moreno J., Musuc A.M. High-Content Aloe vera Based Hydrogels: Physicochemical and Pharmaceutical Properties. Polymers. 2023;15:1312. doi: 10.3390/polym15051312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Suflet D.M., Constantin M., Pelin I.M., Popescu I., Rimbu C.M., Horhogea C.E., Fundueanu G. Chitosan–Oxidized Pullulan Hydrogels Loaded with Essential Clove Oil: Synthesis, Characterization, Antioxidant and Antimicrobial Properties. Gels. 2024;10:227. doi: 10.3390/gels10040227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Edris A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: A review. Phytother. Res. 2007;21:308–323. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 54.Laothaweerungsawat N., Sirithunyalug J., Chaiyana W. Chemical Compositions and Anti-Skin-Ageing Activities of Origanum vulgare L. Essential Oil from Tropical and Mediterranean Region. Molecules. 2020;25:1101. doi: 10.3390/molecules25051101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jianu C., Lukinich-Gruia A.T., Rădulescu M., Mioc M., Mioc A., Șoica C., Constantin A.T., David I., Bujancă G., Radu R.G. Essential Oil of Origanum vulgare var. aureum L. from Western Romania: Chemical Analysis, In Vitro and In Silico Screening of Its Antioxidant Activity. Appl. Sci. 2023;13:5076. doi: 10.3390/app13085076. [DOI] [Google Scholar]
- 56.Alekseeva M., Zagorcheva T., Atanassov I., Rusanov K. Origanum vulgare L.—A Review on Genetic Diversity, Cultivation, Biological Activities and Perspectives for Molecular Breeding. Bulg. J. Agric. Sci. 2020;26:1183–1197. [Google Scholar]
- 57.Avram Ș., Bora L., Vlaia L.L., Muț A.M., Olteanu G.-E., Olariu I., Magyari-Pavel I.Z., Minda D., Diaconeasa Z., Sfirloaga P., et al. Cutaneous Polymeric-Micelles-Based Hydrogel Containing Origanum vulgare L. Essential Oil: In Vitro Release and Permeation, Angiogenesis, and Safety Profile In Ovo. Pharmaceuticals. 2023;16:940. doi: 10.3390/ph16070940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cruz Sánchez E., García M.T., Pereira J., Oliveira F., Craveiro R., Paiva A., Gracia I., García-Vargas J.M., Duarte A.R.C. Alginate–Chitosan Membranes for the Encapsulation of Lavender Essential Oil and Development of Biomedical Applications Related to Wound Healing. Molecules. 2023;28:3689. doi: 10.3390/molecules28093689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nawaz A., Farid A., Safdar M., Latif M.S., Ghazanfar S., Akhtar N., Al Jaouni S.K., Selim S., Khan M.W. Formulation Development and Ex-Vivo Permeability of Curcumin Hydrogels under the Influence of Natural Chemical Enhancers. Gels. 2022;8:384. doi: 10.3390/gels8060384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liang J., Zhang Y., Chi P., Liu H., Jing Z., Cao H., Du Y., Zhao Y., Qin X., Zhang W., et al. Essential oils: Chemical constituents, potential neuropharmacological effects and aromatherapy—A review. Pharmacol. Res. Mod. Chin. Med. 2023;6:100210. doi: 10.1016/j.prmcm.2022.100210. [DOI] [Google Scholar]
- 61.Zeng W.C., Zhang Z., Gao H., Jia L.R., He Q. Chemical composition, antioxidant, and antimicrobial activities of essential oil from pine needle (Cedrus deodara) J. Food Sci. 2012;77:C824–C829. doi: 10.1111/j.1750-3841.2012.02767.x. [DOI] [PubMed] [Google Scholar]
- 62.Šunić L., Ilić Z.S., Stanojević L., Milenković L., Stanojević J., Kovač R., Milenković A., Cvetković D. Comparison of the Essential Oil Content, Constituents and Antioxidant Activity from Different Plant Parts during Development Stages of Wild Fennel (Foeniculum vulgare Mill.) Horticulturae. 2023;9:364. doi: 10.3390/horticulturae9030364. [DOI] [Google Scholar]
- 63.Gheorghita D., Grosu E., Robu A., Ditu L.M., Deleanu I.M., Gradisteanu Pircalabioru G., Raiciu A.-D., Bita A.-I., Antoniac A., Antoniac V.I. Essential Oils as Antimicrobial Active Substances in Wound Dressings. Materials. 2022;15:6923. doi: 10.3390/ma15196923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agelaki S., Boukovinas I., Athanasiadis I., Trimis G., Dimitriadis I., Poughias L., Morais E., Sabale U., Bencina G., Athanasopoulos C. A systematic literature review of the human papillomavirus prevalence in locally and regionally advanced and recur-rent/metastatic head and neck cancers through the last decade: The “ALARM” study. Cancer Med. 2024;13:e6916. doi: 10.1002/cam4.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siegel R.L., Giaquinto A.N., Jemal A. Cancer statistics. CA A Cancer J. Clin. 2024;74(2):203. doi: 10.3322/caac.21820. [DOI] [PubMed] [Google Scholar]
- 66.Alsabbagh R., Ahmed M., Alqudah M.A.Y., Hamoudi R., Harati R. Insights into the Molecular Mechanisms Mediating Extravasation in Brain Metastasis of Breast Cancer, Melanoma, and Lung Cancer. Cancers. 2023;15:2258. doi: 10.3390/cancers15082258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Waks A.G., Winer E.P. Breast cancer treatment: A review. JAMA. 2019;321:288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 68.Ali S., Li J., Pei Y., Khurram R., Rehman K.u., Rasool A.B. State-of-the-Art Challenges and Perspectives in Multi-Organ Cancer Diagnosis via Deep Learning-Based Methods. Cancers. 2021;13:5546. doi: 10.3390/cancers13215546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burz C., Pop V., Silaghi C., Lupan I., Samasca G. Prognosis and Treatment of Gastric Cancer: A 2024 Update. Cancers. 2024;16:1708. doi: 10.3390/cancers16091708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chunarkar-Patil P., Kaleem M., Mishra R., Ray S., Ahmad A., Verma D., Bhayye S., Dubey R., Singh H.N., Kumar S. Anticancer Drug Discovery Based on Natural Products: From Computational Approaches to Clinical Studies. Biomedicines. 2024;12:201. doi: 10.3390/biomedicines12010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tauro S., Dhokchawle B., Mohite P., Nahar D., Nadar S., Coutinho E. Natural Anticancer agents: Their therapeutic potential, challenges and Promising outcomes. Curr. Med. Chem. 2024;31:848–870. doi: 10.2174/0929867330666230502113150. [DOI] [PubMed] [Google Scholar]
- 72.Bajpai P., Usmani S., Kumar R., Prakash O. Recent advances in anticancer ap-proach of traditional medicinal plants: A novel strategy for cancer chemotherapy. Intell. Pharm. 2024;2:291–304. doi: 10.1016/j.ipha.2024.02.001. [DOI] [Google Scholar]
- 73.Jampilek J., Kralova K. Anticancer Applications of Essential Oils Formulated into Lipid-Based Delivery Nanosystems. Pharmaceutics. 2022;14:2681. doi: 10.3390/pharmaceutics14122681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Angelini P., Tirillini B., Akhtar M.S., Dimitriu L., Bricchi E., Bertuzzi G., Venanzoni R. Anticancer Plants: Natural Products and Biotechnological Implements. Volume 2. Springer; Singapore: 2018. Essential oil with anticancer activity: An overview; pp. 207–231. [DOI] [Google Scholar]
- 75.Kamal I., Khedr A.I., Alfaifi M.Y., Elbehairi S.E.I., Elshaarawy R.F., Saad A.S. Chemo-therapeutic and chemopreventive potentials of ρ-coumaric acid–Squid chitosan nanogel loaded with Syzygium aromaticum essential oil. Int. J. Biol. Macromol. 2021;188:523–533. doi: 10.1016/j.ijbiomac.2021.08.038. [DOI] [PubMed] [Google Scholar]
- 76.Liu B., Chen K. Advances in Hydrogel-Based Drug Delivery Systems. Gels. 2024;10:262. doi: 10.3390/gels10040262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Idumah C.I., Nwuzor I.C., Odera S.R., Timothy U.J., Ngenegbo U., Tanjung F.A. Recent advances in polymeric hydrogel nanoarchitectures for drug delivery applications. Int. J. Polym. Mater. Polym. Biomater. 2024;73:1–32. doi: 10.1080/00914037.2022.2120875. [DOI] [Google Scholar]
- 78.Shabkhiz M.A., Pirouzifard M.K., Pirsa S., Mahdavinia G.R. Alginate hydrogel beads containing Thymus daenensis essential oils/Glycyrrhizic acid loaded in β-cyclodextrin. Investigation of structural, antioxidant/antimicrobial properties and release assessment. J. Mol. Liq. 2021;344:117738. doi: 10.1016/j.molliq.2021.117738. [DOI] [Google Scholar]
- 79.Basyigit B. Designing Nanoliposome-in-Natural Hydrogel Hybrid System for Controlla-ble Release of Essential Oil in Gastrointestinal Tract: A Novel Vehicle. Foods. 2023;12:2242. doi: 10.3390/foods12112242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mogosanu G.D., Grumezescu A.M. Natural and synthetic polymers for wounds and burn dressing. Int. J. Pharm. 2014;463:127–136. doi: 10.1016/j.ijpharm.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 81.George B., Bhatia N., Suchithra T.V. Burgeoning hydrogel technology in burn wound care: A comprehensive meta-analysis. Eur. Polym. J. 2021;157:110640. doi: 10.1016/j.eurpolymj.2021.110640. [DOI] [Google Scholar]
- 82.Alven S., Peter S., Aderibigbe B.A. Polymer-Based Hydrogels Enriched with Essential Oils: A Promising Approach for the Treatment of Infected Wounds. Polymers. 2022;14:3772. doi: 10.3390/polym14183772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goh M., Du M., Peng W.R., Saw P.E., Chen Z. Advancing burn wound treatment: Exploring hydrogel as a transdermal drug delivery system. Drug Deliv. 2024;31:2300945. doi: 10.1080/10717544.2023.2300945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jiji S., Udhayakumar S., Rose C., Muralidharan C., Kadirvelu K. Thymol enriched bacterial cellulose hydrogel as effective material for third degree burn wound repair. Int. J. Biol. Macromol. 2019;122:452–460. doi: 10.1016/j.ijbiomac.2018.10.192. [DOI] [PubMed] [Google Scholar]
- 85.Khan B.A., Ullah S., Khan M.K., Uzair B., Menaa F., Braga V.A. Fabrication, Physical Characterizations, and In Vitro, In Vivo Evaluation of Ginger Extract-Loaded Gelatin/Poly(Vinyl Alcohol) Hydrogel Films against Burn Wound Healing in Animal Model. AAPS PharmSciTech. 2020;21:323. doi: 10.1208/s12249-020-01866-y. [DOI] [PubMed] [Google Scholar]
- 86.Wang H., Liu Y., Cai K., Zhang B., Tang S., Zhang W., Liu W. Antibacterial polysaccharide-based hydrogel dressing containing plant essential oil for burn wound healing. Burn. Trauma. 2021;9:tkab041. doi: 10.1093/burnst/tkab041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dalir Abdolahinia E., Hajisadeghi S., Moayedi Banan Z., Dadgar E., Delaramifar A., Izadian S., Simin Sharifi S., Maleki Dizaj S. Potential applications of medicinal herbs and phytochemicals in oral and dental health: Status quo and future perspectives. Oral Dis. 2023;29:2468–2482. doi: 10.1111/odi.14276. [DOI] [PubMed] [Google Scholar]
- 88.Kumar G., Jalaluddin M., Rout P., Mohanty R., Dileep C.L. Emerging trends of herbal care in dentistry. J. Clin. Diagn. Res. 2013;7:1827–1829. doi: 10.7860/JCDR/2013/6339.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chelu M., Musuc A.M., Popa M., Calderon Moreno J. Aloe vera-Based Hydrogels for Wound Healing: Properties and Therapeutic Effects. Gels. 2023;9:539. doi: 10.3390/gels9070539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Jo Y.H., Cho J.H., Park D.H., Yoon H.I., Han S.H., Yilmaz B. Antimicrobial activity, surface properties, and cytotoxicity of microencapsulated phytochemicals incorporated into three-dimensionally printable dental polymers. J. Dent. 2024;141:104820. doi: 10.1016/j.jdent.2023.104820. [DOI] [PubMed] [Google Scholar]
- 91.Wong Y.Y., Chow Y.L. Exploring the potential of spice-derived phytochemicals as alternative antimicrobial agents. eFood. 2024;5:e126. doi: 10.1002/efd2.126. [DOI] [Google Scholar]
- 92.Tzimas K., Antoniadou M., Varzakas T., Voidarou C. Plant-Derived Compounds: A Promising Tool for Dental Caries Prevention. Curr. Issues Mol. Biol. 2024;46:5257–5290. doi: 10.3390/cimb46060315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pandhi S., Kumar A. Odontonutraceuticals: Phytochemicals for Oral Health Care. In: Mishra P., Mishra R.R., Adetunji C.O., editors. Innovations in Food Technology. Springer; Singapore: 2020. [DOI] [Google Scholar]
- 94.Muresan S.M.C., Dreanca A., Repciuc C., Dejescu C., Rotar O., Pop R.A., Pantea S., Pall E., Ciotlaus I., Sarosi C., et al. Dental Hydrogels with Essential Oils with Potential Activity in Periodontitis. Appl. Sci. 2023;13:1787. doi: 10.3390/app13031787. [DOI] [Google Scholar]
- 95.Achagar R., Ait-Touchente Z., El Ati R., Boujdi K., Thoume A., Abdou A., Touzani R. A Comprehensive Review of Essential Oil–Nanotechnology Synergy for Advanced Dermo-cosmetic Delivery. Cosmetics. 2024;11:48. doi: 10.3390/cosmetics11020048. [DOI] [Google Scholar]
- 96.Rubio L., Pita A., Garcia-Jares C., Lores M. Natural Extracts and Essential Oils as Ingredients in Cosmetics: Search for Potential Phytomarkers and Allergen Survey. Cosmetics. 2024;11:84. doi: 10.3390/cosmetics11030084. [DOI] [Google Scholar]
- 97.Tocai A.-C., Memete A.R., Ganea M., Vicaș L.G., Gligor O.D., Vicas S.I. The Formulation of Dermato-Cosmetic Products Using Sanguisorba minor Scop. Extract with Powerful Antioxidant Capacities. Cosmetics. 2024;11:8. doi: 10.3390/cosmetics11010008. [DOI] [Google Scholar]
- 98.Verger A., Kichou H., Huang N., Perse X., Ardeza I.M., Pradel C., Goncalves Martins Da Conceicao R., Atanasova B., Legrand F.-X., Munnier E., et al. Effects of Hydrophilic Natural Deep Eutectic Solvents on the Rheological, Textural, and Sensory Properties of Carboxymethylcellulose-Based Cosmetic Hydrogels. ACS Sustain. Chem. Eng. 2024;12:7187–7199. doi: 10.1021/acssuschemeng.4c01866. [DOI] [Google Scholar]
- 99.Chelu M., Musuc A.M. Natural Biological Macromolecules for Designing Hydrogels as Health Care and Anti-aging Solutions. Eng. Proc. 2023;56:158. doi: 10.3390/ASEC2023-16519. [DOI] [Google Scholar]
- 100.Zagórska-Dziok M., Sobczak M. Hydrogel-Based Active Substance Release Systems for Cosmetology and Dermatology Application: A Review. Pharmaceutics. 2020;12:396. doi: 10.3390/pharmaceutics12050396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Morais F.P., Simões R.M.S., Curto J.M.R. Biopolymeric Delivery Systems for Cosmetic Applications Using Chlorella vulgaris Algae and Tea Tree Essential Oil. Polymers. 2020;12:2689. doi: 10.3390/polym12112689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sosnowska K., Tomczykowa M., Winnicka K., Kalemba D., Tomczyk M. In vivo evaluation of the antipsoriatic effect of hydrogel with lavandin essential oil and its main components after topical application. Acta Pol. Pharm. 2022;79:757–770. doi: 10.32383/appdr/160162. [DOI] [Google Scholar]
- 103.Yap X.F., Saw S.H., Lim V., Tan C.X. Plant Essential Oil Nanoemulgel as a Cosmeceutical Ingredient: A Review. Cosmetics. 2024;11:116. doi: 10.3390/cosmetics11040116. [DOI] [Google Scholar]
- 104.Kaolaor A., Kiti K., Pankongadisak P., Suwantong O. Camellia Oleifera oil-loaded chitosan nanoparticles embedded in hydrogels as cosmeceutical products with improved biological properties and sustained drug release. Int. J. Biol. Macromol. 2024;275:133560. doi: 10.1016/j.ijbiomac.2024.133560. [DOI] [PubMed] [Google Scholar]
- 105.de Vos P., Faas M.M., Spasojevic M., Sikkema J. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. Int. Dairy J. 2010;20:292–302. doi: 10.1016/j.idairyj.2009.11.008. [DOI] [Google Scholar]
- 106.Črnivec I.G.O., Ulrih N.P. Biopolymer Nanostructures for Food Encapsulation Purposes. Academic Press; Cambridge, MA, USA: 2019. Nano-hydrogels of alginate for encapsulation of food ingredients; pp. 335–380. [DOI] [Google Scholar]
- 107.Chambin O., Dupuis G., Champion D., Voilley A., Pourcelot Y. Colon-specific drug delivery: Influence of solution reticulation properties upon pectin beads performance. Int. J. Pharm. 2006;321:86–93. doi: 10.1016/j.ijpharm.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 108.Tavares L., Noreña C.P.Z., Barros H.L., Smaoui S., Lima P.S., Marques de Oliveira M. Rheological and structural trends on encapsulation of bioactive compounds of essential oils: A global systematic review of recent research. Food Hydrocoll. 2022;129:107628. doi: 10.1016/j.foodhyd.2022.107628. [DOI] [Google Scholar]
- 109.Popa E.G., Gomes M.E., Reis R.L. Cell delivery systems using alginate–carrageenan hy-drogel beads and fibers for regenerative medicine applications. Biomacromolecules. 2011;12:3952–3961. doi: 10.1021/bm200965x. [DOI] [PubMed] [Google Scholar]
- 110.McClements D.J. Recent progress in hydrogel delivery systems for improving nutraceutical bioavailability. Food Hydrocoll. 2017;68:238–245. doi: 10.1016/j.foodhyd.2016.05.037. [DOI] [Google Scholar]
- 111.Reichembach L.H., de Oliveira Petkowicz C.L., Guerrero P., de la Caba K. Pectin and pectin/chitosan hydrogel beads as coffee essential oils carrier systems. Food Hydrocoll. 2024;151:109814. doi: 10.1016/j.foodhyd.2024.109814. [DOI] [Google Scholar]
- 112.Carpena M., Nuñez-Estevez B., Soria-Lopez A., Garcia-Oliveira P., Prieto M.A. Essential Oils and Their Application on Active Packaging Systems: A Review. Resources. 2021;10:7. doi: 10.3390/resources10010007. [DOI] [Google Scholar]
- 113.Gómez-Estaca J., De Lacey A.L., López-Caballero M.E., Gómez-Guillén M.D.C., Montero P. Biodegradable gelatin–chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiol. 2010;27:889–896. doi: 10.1016/j.fm.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 114.Ahmad M., Benjakul S., Sumpavapol P., Nirmal N.P. Quality changes of sea bass slices wrapped with gelatin film incorporated with lemongrass essential oil. Int. J. Food Microbiol. 2012;155:171–178. doi: 10.1016/j.ijfoodmicro.2012.01.027. [DOI] [PubMed] [Google Scholar]
- 115.Kanelaki A., Zampouni K., Mourtzinos I., Katsanidis E. Hydrogels, Oleogels and Bigels as Edible Coatings of Sardine Fillets and Delivery Systems of Rosemary Extract. Gels. 2022;8:660. doi: 10.3390/gels8100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Iacovino S., Cofelice M., Sorrentino E., Cuomo F., Messia M.C., Lopez F. Alginate-Based Emulsions and Hydrogels for Extending the Shelf Life of Banana Fruit. Gels. 2024;10:245. doi: 10.3390/gels10040245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bandyopadhyay S., Saha N., Zandraa O., Pummerová M., Sáha P. Essential Oil Based PVP-CMC-BC-GG Functional Hydrogel Sachet for ‘Cheese’: Its Shelf Life Confirmed with Anthocyanin (Isolated from Red Cabbage) Bio Stickers. Foods. 2020;9:307. doi: 10.3390/foods9030307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Erceg T., Šovljanski O., Stupar A., Ugarković J., Aćimović M., Pezo L., Tomić A., Todosijević M. A comprehensive approach to chi-tosan-gelatine edible coating with β-cyclodextrin/lemongrass essential oil inclusion complex—Characterization and food application. Int. J. Biol. Macromol. 2023;228:400–410. doi: 10.1016/j.ijbiomac.2022.12.132. [DOI] [PubMed] [Google Scholar]
- 119.Goudoulas T.B., Vanderhaeghen S., Germann N. Micro-dispersed essential oils loaded gelatin hydrogels with antibacterial activity. LWT. 2022;154:112797. doi: 10.1016/j.lwt.2021.112797. [DOI] [Google Scholar]
- 120.Stefanowska K., Bucher M., Reichert C.L., Sip A., Woźniak M., Schmid M., Dobrucka R., Ratajczak I. Chitosan-based films with nanocellulose and propolis as active packaging materials. Ind. Crops Prod. 2024;219:119112. doi: 10.1016/j.indcrop.2024.119112. [DOI] [Google Scholar]
- 121.Domínguez R., Munekata P.E.S., Pateiro M., López-Fernández O., Manuel Lorenzo J. Immobilization of oils using hydrogels as strategy to replace animal fats and improve the healthiness of meat products. Curr. Opin. Food Sci. 2021;37:135–144. doi: 10.1016/j.cofs.2020.10.005. [DOI] [Google Scholar]
- 122.Nath P.C., Debnath S., Sridhar K., Inbaraj B.S., Nayak P.K., Sharma M. A Comprehensive Review of Food Hydrogels: Principles, Formation Mechanisms, Microstructure, and Its Applications. Gels. 2023;9:1. doi: 10.3390/gels9010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Pascuta M.S., Vodnar D.C. Nanocarriers for Sustainable Active Packaging: An Overview during and Post COVID-19. Coatings. 2022;12:102. doi: 10.3390/coatings12010102. [DOI] [Google Scholar]
- 124.Tomić A., Šovljanski O., Erceg T. Insight on Incorporation of Essential Oils as Antimicrobial Substances in Biopolymer-Based Active Packaging. Antibiotics. 2023;12:1473. doi: 10.3390/antibiotics12091473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Alonso P., Fernández-Pastor S., Guerrero A. Application of Cinnamon Essential Oil in Active Food Packaging: A Review. Appl. Sci. 2024;14:6554. doi: 10.3390/app14156554. [DOI] [Google Scholar]
- 126.Behbahani A.B., Falah F., Lavi Arab F., Vasiee M., Tabatabaee Yazdi F. Chemical composition and antioxidant, antimicrobial, and antiproliferative activities of Cinnamomum zeylanicum bark essential oil. Evid. Based Complement. Altern. Med. 2020;2020:5190603. doi: 10.1155/2020/5190603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Abdelwahab S.I., Mariod A.A., Taha M.M.E., Zaman F.Q., Abdelmageed A.H.A., Khamis S., Sivasothy Y., Awang K. Chemical composition and antioxidant properties of the essential oil of Cinnamomum altissimum Kosterm (Lauraceae) Arab. J. Chem. 2017;10:131–135. doi: 10.1016/j.arabjc.2014.02.001. [DOI] [Google Scholar]
- 128.Hou F., Chen X., Yi F., Song L., Zhan S., Han X., Zhang L., Li F., Wang X., Liu Z. Antibacterial and antibiofilm properties of cinnamon essential oil on Pseudomonas tolaasii and application of potato starch/CEO active pads in preservation of mushroom (Agaricus bisporus) Food Control. 2024;165:110705. doi: 10.1016/j.foodcont.2024.110705. [DOI] [Google Scholar]
- 129.Bhatia S., Al-Harrasi A., Shah Y.A., Altoubi H.W.K., Kotta S., Sharma P., Anwer M.K., Kaithavalappil D.S., Koca E., Aydemir L.Y. Fabrication, Characterization, and Antioxidant Potential of Sodium Alginate/Acacia Gum Hydrogel-Based Films Loaded with Cinnamon Essential Oil. Gels. 2023;9:337. doi: 10.3390/gels9040337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Rîmbu C.M., Serbezeanu D., Vlad-Bubulac T., Suflet D.M., Motrescu I., Lungoci C., Robu T., Vrînceanu N., Grecu M., Cozma A.P., et al. Antimicrobial Activity of Artemisia dracunculus Oil-Loaded Agarose/Poly(Vinyl Alcohol) Hydrogel for Bio-Applications. Gels. 2024;10:26. doi: 10.3390/gels10010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Boccia A.C., Pulvirenti A., Cerruti P., Silvetti T., Brasca M. Antimicrobial starch-based cryogels and hydrogels for dual-active food packaging applications. Carbohydr. Polym. 2024;342:122340. doi: 10.1016/j.carbpol.2024.122340. [DOI] [PubMed] [Google Scholar]
- 132.Chen M., Hu Z., Zheng H., Wang J., Xu X. Antimicrobial polysaccharide hydrogels embedded with methyl-β-cyclodextrin/thyme oil inclusion complexes for exceptional mechanical performance and chilled chicken breast preservation. Int. J. Biol. Macromol. 2024;267:131586. doi: 10.1016/j.ijbiomac.2024.131586. [DOI] [PubMed] [Google Scholar]
- 133.Li Q., Zhang B., Yang X., Ge Q. Deterioration-associated microbiome of stone monuments: Structure, variation, and assembly. Appl. Environ. Microbiol. 2018;84:e02680-17. doi: 10.1128/AEM.02680-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vázquez-Nion D., Silva B., Prieto B. Influence of the properties of granitic rocks on their bioreceptivity to subaerial phototrophic biofilms. Sci. Total Environ. 2018;610:44–54. doi: 10.1016/j.scitotenv.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 135.Gaylarde C. Influence of Environment on Microbial Colonization of Historic Stone Buildings with Emphasis on Cyanobacteria. Heritage. 2020;3:1469–1482. doi: 10.3390/heritage3040081. [DOI] [Google Scholar]
- 136.Ranaldi R., Rugnini L., Gabriele F., Spreti N., Casieri C., Di Marco G., Gismondi A., Bruno L. Plant essential oils suspended into hydrogel: Development of an easy-to-use protocol for the restoration of stone cultural heritage. Int. Biodeterior. Biodegrad. 2022;172:105436. doi: 10.1016/j.ibiod.2022.105436. [DOI] [Google Scholar]
- 137.Pinna D. Microbial Growth and its Effects on Inorganic Heritage Materials. In: Joseph E., editor. Microorganisms in the Deterioration and Preservation of Cultural Heritage. Springer; Cham, Switzerland: 2021. [DOI] [Google Scholar]
- 138.Toreno G., Isola D., Meloni P., Carcangiu G., Selbmann L., Onofri S., Caneva G., Zucconi L. Biological colonization on stone monuments: A new low impact cleaning method. J. Cult. Herit. 2018;30:100–109. doi: 10.1016/j.culher.2017.09.004. [DOI] [Google Scholar]
- 139.Cappitelli F., Cattò C., Villa F. The Control of Cultural Heritage Microbial Deterioration. Microorganisms. 2020;8:1542. doi: 10.3390/microorganisms8101542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sanmartín P., Rodríguez A., Aguiar U. Medium-term field evaluation of several widely used cleaning-restoration techniques applied to algal biofilm formed on a granite-built historical monument. Int. Biodeterior. Biodegrad. 2020;147:104870. doi: 10.1016/j.ibiod.2019.104870. [DOI] [Google Scholar]
- 141.Genova C., Fuentes E., Favero G., Prieto B. Evaluation of the Cleaning Effect of Natural-Based Biocides: Application on Different Phototropic Biofilms Colonizing the Same Granite Wall. Coatings. 2023;13:520. doi: 10.3390/coatings13030520. [DOI] [Google Scholar]
- 142.Gabriele F., Ranaldi R., Bruno L., Casieri C., Rugnini L., Spreti N. Biodeterioration of stone monuments: Studies on the influence of bioreceptivity on cyanobacterial biofilm growth and on the biocidal efficacy of essential oils in natural hydrogel. Sci. Total Environ. 2023;870:161901. doi: 10.1016/j.scitotenv.2023.161901. [DOI] [PubMed] [Google Scholar]
- 143.Russo R., Palla F. Plant Essential Oils as Biocides in Sustainable Strategies for the Conservation of Cultural Heritage. Sustainability. 2023;15:8522. doi: 10.3390/su15118522. [DOI] [Google Scholar]
- 144.Privitera A., Tuti S., Laverdura U.P., Duranti L., Di Bartolomeo E., Taddei A.R., Sodo A. One-step nanoencapsulation of essential oils and their application in hybrid coatings: A sustainable long-lasting treatment of stone materials against biodeterioration. Prog. Org. Coat. 2024;196:108759. doi: 10.1016/j.porgcoat.2024.108759. [DOI] [Google Scholar]
- 145.Boccalon E., Nocchetti M., Pica M., Romani A., Sterflinger K. Hydrogels: A ‘stepping stone’ towards new cleaning strategies for biodeteriorated surfaces. J. Cult. Herit. 2021;47:1–11. doi: 10.1016/j.culher.2020.07.008. [DOI] [Google Scholar]
- 146.Bruno L., Casieri C., Francesco Gabriele F., Ranaldi R., Rugnini L., Spreti N. In situ application of alginate hydrogels containing oxidant or natural biocides on Fortunato Depero’s mosaic (Rome, Italy) Int. Biodeterior. Biodegrad. 2023;183:105641. doi: 10.1016/j.ibiod.2023.105641. [DOI] [Google Scholar]
- 147.Wong Y.C., Ahmad-Mudzaqqir M.Y., Wan-Nurdiyana W.A. Extraction of essential oil from cinnamon (Cinnamomum zeylanicum) Orient. J. Chem. 2014;30:37. doi: 10.13005/ojc/300105. [DOI] [Google Scholar]
- 148.Liu X., Jia J., Duan S., Zhou X., Xiang A., Lian Z., Ge F. Zein/MCM-41 nanocomposite film incorporated with cinnamon essential oil loaded by modified supercritical CO2 impregnation for long-term antibacterial packaging. Pharmaceutics. 2020;12:169. doi: 10.3390/pharmaceutics12020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang Y., Yuan C., Liu Y., Cui B. Fabrication of kappa–carrageenan hydrogels with cinnamon essential oil/hydroxypropyl–β–cyclodextrin composite: Evaluation of physicochemical properties, release kinetics and antimicrobial activity. Int. J. Biol. Macromol. 2021;170:593–601. doi: 10.1016/j.ijbiomac.2020.12.176. [DOI] [PubMed] [Google Scholar]
- 150.Lesage-Meessen L., Bou M., Sigoillot J.C., Faulds C.B., Lomascolo A. Essential oils and distilled straws of lavender and lavandin: A review of current use and potential application in white biotechnology. Appl. Microbiol. Biotechnol. 2015;99:3375–3385. doi: 10.1007/s00253-015-6511-7. [DOI] [PubMed] [Google Scholar]
- 151.Pokajewicz K., Biało’n M., Svydenko L., Fedin R., Hudz N. Chemical composition of the essential oil of the new cultivars of Lavandula angustifolia Mill. Bred in Ukraine. Molecules. 2021;26:5681. doi: 10.3390/molecules26185681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cardia G.F.E., de Souza Silva-Comar F.M., da Rocha E.M.T., Silva-Filho S.E., Zagotto M., Uchida N.S., do Amaral V., Bersani-Amado C.A., Cuman R.K.N. Pharmacological, medicinal and toxicological properties of lavender essential oil: A review. Research. Soc. Dev. 2021;10:e23310514933. doi: 10.33448/rsd-v10i5.14933. [DOI] [Google Scholar]
- 153.Deng X., Chen J., Chen W. Hydrogel particles as a controlled release delivery system for lavender essential oil using pH triggers. Colloids Surf. A Physicochem. Eng. Asp. 2020;603:125134. doi: 10.1016/j.colsurfa.2020.125134. [DOI] [Google Scholar]
- 154.Schaneberg B.T., Khan I.A. Comparison of extraction methods for marker compounds in the essential oil of lemon grass by GC. J. Agric. Food Chem. 2002;50:1345–1349. doi: 10.1021/jf011078h. [DOI] [PubMed] [Google Scholar]
- 155.Mukarram M., Choudhary S., Khan M.A., Poltronieri P., Khan M.M.A., Ali J., Kurjak D., Shahid M. Lemongrass Essential Oil Components with Antimicrobial and Anticancer Activities. Antioxidants. 2022;11:20. doi: 10.3390/antiox11010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Carson C.F., Hammer K.A., Riley T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006;19:50–62. doi: 10.1128/CMR.19.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Castro J.I., Valencia-Llano C.H., Zapata M.E.V., Restrepo Y.J., Hernandez J.H.M., Navia-Porras D.P., Valencia Y., Valencia C., Grande-Tovar C.D. Chitosan/polyvinyl alcohol/tea tree essential oil composite films for biomedical applications. Polymers. 2021;13:3753. doi: 10.3390/polym13213753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li Z., Jiang L., Wang Y., Li M., Liu T., Liu Y. Chitosan–gellan gum polyelectrolyte hydrogel beads containing tea tree oil microcapsules: Preparation, characterization and application. Food Hydrocoll. 2024;157:110464. doi: 10.1016/j.foodhyd.2024.110464. [DOI] [Google Scholar]
- 159.Gomes D.S., Costa A., Pereira A.M., Casal M., Machado R. Biocomposites of silk-elastin and essential oil from Mentha piperita display antibacterial activity. ACS Omega. 2022;7:6568–6578. doi: 10.1021/acsomega.1c05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Messaoudi M., Rebiai A., Sawicka B., Atanassova M., Ouakouak H., Larkem I., Egbuna C., Awuchi C.G., Boubekeur S., Ferhat M.A., et al. Effect of Extraction Methods on Polyphenols, Flavonoids, Mineral Elements, and Biological Activities of Essential Oil and Extracts of Mentha pulegium L. Molecules. 2022;27:11. doi: 10.3390/molecules27010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zhao H., Ren S., Yang H., Tang S., Guo C., Liu M., Tao Q., Xu H. Peppermint essential oil: Its phytochemistry, biological activity, pharmacological effect and application. Biomed. Pharmacother. 2022;154:113559. doi: 10.1016/j.biopha.2022.113559. [DOI] [PubMed] [Google Scholar]
- 162.Monfared-Hajishirkiaee R., Ehtesabi H., Latifi H. Peppermint essential oil and ZnO nanoparticles: A green and effective combination for a cooling bilayer patch with antibacterial activity. J. Environ. Chem. Eng. 2024;12:112833. doi: 10.1016/j.jece.2024.112833. [DOI] [Google Scholar]
- 163.Mahcene Z., Khelil A., Hasni S., Akman P.K., Bozkurt F., Birech K., Goudjil M.B., Tornuk F. Development and characterization of sodium alginate-based active edible films incorporated with essential oils of some medicinal plants. Int. J. Biol. Macromol. 2020;145:124–132. doi: 10.1016/j.ijbiomac.2019.12.093. [DOI] [PubMed] [Google Scholar]
- 164.Torpol K., Sriwattana S., Sangsuwan J., Wiriyacharee P., Prinyawiwatkul W. Optimising chitosan–pectin hydrogel beads containing combined garlic and holy basil essential oils and their application as antimicrobial inhibitor. Int. J. Food Sci. Technol. 2019;54:2064–2074. doi: 10.1111/ijfs.14107. [DOI] [Google Scholar]
- 165.Khater R.M. Effect of hydrogel and antitranspirants treatments on the productivity of sweet basil (Ocimum basilicum L.) plant. Egypt. J. Desert Res. 2015;65:193–214. doi: 10.21608/ejdr.2015.5950. [DOI] [Google Scholar]
- 166.Shahrajabian M.H., Sun W., Cheng Q. Chemical components and pharmacological benefits of Basil (Ocimum basilicum): A review. Int. J. Food Prop. 2020;23:1961–1970. doi: 10.1080/10942912.2020.1828456. [DOI] [Google Scholar]
- 167.Kowalonek J., Stachowiak N., Bolczak K., Richert A. Physicochemical and antibacterial properties of alginate films containing tansy (Tanacetum vulgare L.) essential oil. Polymers. 2023;15:260. doi: 10.3390/polym15020260. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.