Abstract
Transgenic plants expressing insecticidal proteins from the bacterium Bacillus thuringiensis (Bt) were grown on over 13 million ha in the United States and 22.4 million ha worldwide in 2004. Preventing or slowing the evolution of resistance by insects (“resistance management”) is critical for the sustainable use of Bt crops. Plants containing two dissimilar Bt toxin genes in the same plant (“pyramided”) have the potential to delay insect resistance. However, the advantage of pyramided Bt plants for resistance management may be compromised if they share similar toxins with single-gene plants that are deployed simultaneously. We tested this hypothesis using a unique model system composed of broccoli plants transformed to express different Cry toxins (Cry1Ac, Cry1C, or both) and a synthetic population of the diamondback moth (Plutella xylostella) carrying genes for resistance to Cry1Ac and Cry1C at frequencies of ≈0.10 and 0.34, respectively. After 24–26 generations of selection in the greenhouse, the concurrent use of one- and two-gene plants resulted in control failure of both types of Bt plants. When only two-gene plants were used in the selection, no or few insects survived on one- or two-gene Bt plants, indicating that concurrent use of transgenic plants expressing a single and two Bt genes will select for resistance to two-gene plants more rapidly than the use of two-gene plants alone. The results of this experiment agree with the predictions of a Mendelian deterministic simulation model and have important implications for the regulation and deployment of pyramided Bt plants.
Keywords: resistance management
Transgenic plants expressing insecticidal proteins from the bacterium Bacillus thuringiensis (Bt) were grown on over 13 million ha in the United States and 22.4 million ha worldwide in 2004 (1, 2). These crops have provided economic benefits to growers and reduced the use of other insecticides (2, 3). After 8 years of extensive use of Bt crops in the field, there have been no reports of product failure or increased resistance in insect pests (4, 5). However, there remains concern that the efficacy of Bt crops will be short-lived because of the evolution of resistance in targeted pests. The issue of insect resistance management has generated more data, meetings, and public comment than any other issue related to the re-registration of Bt crops by the U.S. Environmental Protection Agency (4).
The “high dose/refuge” strategy is the most widely used tactic to delay resistance (6–8). However, theoretical models (7, 9, 10) and our recent experimental data (11) indicate that plants containing two dissimilar Bt toxin genes (“pyramided”) have the potential to significantly delay the evolution of insect resistance compared with single-gene Bt crops. Pyramided cotton plants (“Bollgard II”) with two genes derived from Bt (Cry1Ac and Cry2Ab2) were approved for commercial use in Australia and the United States in 2002 (12, 13), and several companies are developing new cotton and corn varieties with pyramided Bt genes. However, there is concern that the benefits of pyramided Bt genes for resistance management may be negated if one-gene plants sharing similar Bt toxins continue to be deployed (6, 10). Newly developed pyramided varieties of Bt cotton and corn currently contain the same or similar genes as one-gene (Cry1Ac for Bt cotton, Cry1Ab for Bt corn) plants already marketed. If market forces result in a complicated landscape mix of one- and two-gene Bt plants, the impact of the pyramided Bt plants on slowing resistance evolution could be undermined (10). For example, a modeling study suggested that Cry2A resistance evolution in a cotton pest was maximized when Bt cotton varieties expressing one- and two-genes were both available, and that the overall durability of Bollgard II would be greater if it is deployed alone, compared with a sequential or mosaic deployment with Bollgard (Cry1Ac alone) (12). However, the risk of pest adaptation to pyramided Bt plants used in conjunction with one-gene plants has not been quantified empirically.
Resistance to foliar sprays of Bt has evolved under field conditions in the diamondback moth (Plutella xylostella) (14, 15) and, more recently, in the cabbage looper (Trichoplusia ni) in commercial greenhouses (16). Furthermore, laboratory populations of Bt-resistant P. xylostella, derived from field populations exposed to different Bt foliar sprays, have survived on transgenic crucifers expressing a high level of Cry1Ac (17–19) and Cry1C (20, 21) proteins. An autosomal recessive gene in P. xylostella has been shown to confer high levels of resistance to four Bt toxins, Cry1Aa, Cry1Ab, Cry1Ac, and Cry1F (22), consistent with results from our own strains of P. xylostella (23). Similarly, resistance to Cry1C in our strains was also autosomally inherited and recessive when tested with Cry1C transgenic broccoli (20) and did not show cross-resistance to Cry1A protoxins (Cry1Aa, Cry1Ab, and Cry1Ac) (24). Cry1C resistance appears to be controlled by more than one autosomal recessive gene based on inheritance and biochemical studies (20, 21). Subsequent mapping studies have demonstrated that the genes for Cry1C resistance in our colony are located in two linkage groups, suggesting that two genes are responsible for Cry1C resistance, whereas the gene for Cry1A resistance is located in a third linkage group (25).
The objective of this study was to determine whether the concurrent use of one- and two-gene plants will select for resistance more rapidly than the use of two-gene plants alone. We used a unique model system (11), composed of broccoli plants transformed to express different Cry toxins (Cry1Ac, Cry1C, or both) and populations of P. xylostella carrying resistance to each of the Bt Cry toxins, to conduct a selection experiment in the greenhouse. We then compared the experimental data with the predicted results from a theoretical Mendelian deterministic simulation model modified from Roush (9).
Materials and Methods
Insects. Three strains of P. xylostella were used. The susceptible Geneva 88 strain (S), the Cry1Ac-resistant strain (Cry1Ac-R), and the Cry1C-resistant strain (Cry1C-R), as described in refs. 11 and 21, were used to develop a hybrid population for the cage tests. The hybrid population was created by releasing 40 F1(S × Cry1Ac-R) moths and 132 F1(S × Cry1C-R) moths into a cage containing 28 moths of the S strain. The total number of moths in the cage was 200 with a 1:1 ratio for female and male moths from each strain. Eggs were collected from the cage and put on artificial diet to rear F1 larvae. About 1,000 F1–F3 moths were used to produce F2–F4 eggs of the synthetic population. F4 pupae were used in the selection experiment. The expected allele frequency of the synthetic population was 0.10 for Cry1A resistance and 0.33 for Cry1C resistance (i.e., the expected allele frequency for any locus that independently contributes to Cry1C resistance). The mean survival of F4 larvae was 1.7% on leaf disks of Cry1Ac broccoli plants, 1.3% on Cry1C plants, 0% on two-gene Bt plants, and 100% on non-Bt plants. Based on the survival rate of the homozygous resistant genotypes on Bt plants, the actual initial allele frequency at the start of the experiment was estimated to be 0.13 for Cry1Ac resistance (square root of 1.7% for monogenic inheritance) and 0.34 for Cry1C resistance (fourth root of 1.3% for two-gene inheritance).
Transgenic Broccoli Plants Expressing Bt Toxins. Three types of transgenic broccoli (Brassica oleracea L.) plants producing high levels of Cry1Ac, Cry1C, or both toxins were used in the cage study (17, 26, 27). The cry1Ac and cry1C progeny were verified by screening the plants with susceptible P. xylostella neonates when plants were 4 to 5 weeks old (11). The cry1Ac plants killed 100% of the neonates of F1 heterozygotes (S × Cry1Ac-R), whereas cry1C plants killed 100% of all instars of F1 heterozygotes (S × Cry1C-R) (21), indicating high levels of expression in each type of Bt plant. Broccoli plants that expressed both cry1Ac and cry1C genes were produced by sexual crosses between the two types of transgenic broccoli, and characterized for Bt protein production and control of S, Cry1A-R, and Cry1C-R P. xylostella strains (27). Analysis by ELISA showed that Cry1Ac and Cry1C proteins were produced in the hybrids and in their F1 progeny at levels comparable to the original single-gene parental lines (620–801 and 941–1,380 ng/mg fresh weight, respectively) (27). Two-gene plants were propagated in vitro via leaf explants (28). Because a small proportion of the plants obtained through clonal propagation had lower expression of Cry1C (28), levels of protein were verified by screening the plants with Cry1Ac-R and Cry1C-R P. xylostella neonates and the plants with low expression were eliminated.
Experimental Design. The selection experiment was conducted in greenhouses at Cornell University's New York State Agricultural Experiment Station under conditions similar to those previously reported (11, 29). Each cage was 1.8 m long × 0.9 m wide × 1.7 m high and constructed of nylon netting. Three treatments were included in the experiment: (i) 45% Cry1Ac and 45% two-gene plants plus 10% refuge; (ii) 45% Cry1C and 45% two-gene plants plus 10% refuge; and (iii) 90% two-gene plants plus 10% refuge.
There were three replicates (cages) for each treatment and 20 plants total in each cage (18 Bt plants plus 2 non-Bt refuge plants). Adults, but not larvae, could easily move between the different broccoli types, which were separated by a nylon netting barrier (0.9 m high). This arrangement simulated adjoining fields of one- and two-gene plants where there would be frequent interfield movement of adults but negligible movement of larvae. Five hundred F4 pupae of the synthetic population of P. xylostella were released into each cage. Non-Bt refuge plants were replaced each insect generation (≈20 days) or when most of the leaves of a plant were severely defoliated within a generation to allow most of the larvae to survive until pupation. Defoliated plants were cut at the base and placed onto replacement plants so that larvae on them were not lost. One- and two-gene Bt plants were replaced about every 60 days (three insect generations) up to generation 19. After this point, two-gene plants in most replicates of treatments 1 and 2 were replaced about every 20 days because of control failure. One replicate of treatment 2 was terminated after generation 20 because the two-gene plants were completely defoliated. No insecticide was used in the cages. The experiment was terminated after 26 generations. The temperature in the greenhouse ranged from 20°C to 27°C during the course of the experiment.
Data Collection. The number of P. xylostella larvae older than first instar (primarily third or fourth instar) and pupae on broccoli plants was counted every three to four generations when larval and pupal densities peaked. To test for resistance, ≈50 pupae from non-Bt refuge plants were collected from each cage every two to three generations (starting with the 11th generation) and used to obtain eggs. Survival of second instars derived from each cage was then tested on broccoli leaf disks expressing Cry1A, Cry1C, or both toxins in 30-ml plastic cups. Nontransgenic broccoli was used as a control. For each cage, a total of 100–200 larvae were tested on each type of Bt broccoli (in 10 replicates) and 50 larvae on non-Bt control broccoli (in 5 replicates). Survival was determined after 3 days at 27 ± 1°C. For the survivors on Cry1A or Cry1C broccoli after 3 days, 95–98% could pupate normally when they were reared continuously on the same type of Bt broccoli (unpublished data). After the end of the experiment, at least 200 pupae were collected from non-Bt plants in one replicate of treatment 3 that had the fewest survivors on Bt plants. To confirm the existence of resistance alleles in this cage, progeny larvae from the pupae were tested for resistance as described above but with 1,000 larvae (500 larvae for each of the two replicates) on each of the three types of Bt plants and 50 larvae on non-Bt control.
Statistical Analysis. SAS programs were used for analysis of variance (30). Data were transformed by using the arcsine square-root value for proportion of survival, or the log (x + 1) for insect density data, before each analysis of variance was performed. Treatment means were compared and separated by Tukey's honestly significant difference at P = 0.05 (30).
Modeling Studies. To determine whether the results of our experimental model system can be applied to a broader range of Bt crop/pest systems, a Mendelian deterministic simulation model revised from Roush (9) was developed to assess the risk of pest adaptation to Bt plants with one or two genes. The model assumed that there was one locus for resistance to Cry1Ac and two to Cry1C without linkage between the three loci (25). The initial frequencies used in the model were 0.1 for Cry1A resistance and 0.34 for Cry1C resistance, the same as the actual frequencies of resistance alleles used in the experiment. Moths were assumed to mate at random and produce eggs in densities proportional to their probabilities according to the Hardy–Weinberg equation, with the initial resistance frequency entered separately for each locus. An adjustable proportion of the larvae was assigned to refuges (e.g., 10%) where no selection occurs. The remaining larvae were assigned in adjustable proportions to plants with one or two toxins and survived or died on plants depending on their genotypes. Only homozygous Cry1A-R and Cry1C-R genotypes could survive on Cry1Ac or Cry1C plants, respectively, and only genotypes homozygous for both Cry1A-R and Cry1C-R genes could survive on two toxin plants (11). It was further assumed that larvae do not move between transgenic and nontransgenic hosts, a situation similar to what occurred in our setup. The model was based on discrete generations but also tracked population density on both transgenic and nontransgenic host plants. Population growth was density-independent and was entered as a fixed value, 10-fold per generation, to offset the 90% losses of susceptible insects on transgenic plants and maintain a roughly stable population size. The model was written in Fortran. Outputs of the model were the predicted resistance frequencies (percent homozygous resistant individuals based on the resistance allele frequencies) to Cry1A, Cry1C, or both toxins. The experimental results were considered to agree with the model if the observed evolution of resistance to Cry1A, Cry1C, or both toxins in different treatments occurred in the same general pattern and time frame as the model predicted.
Results
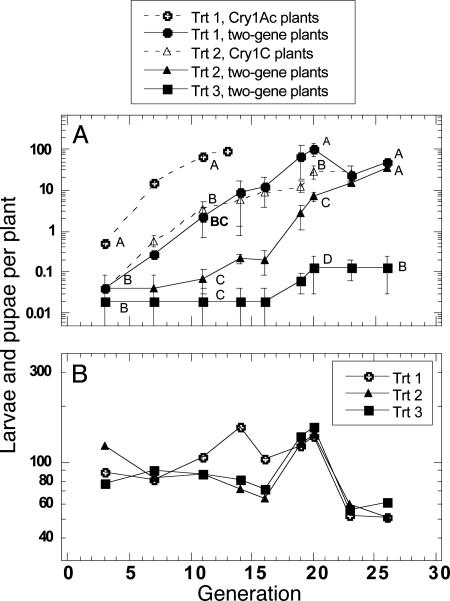
Population Density of P. xylostella. In cages containing both one- and two-gene plants (treatments 1 and 2), there were significantly more P. xylostella larvae and pupae on two-gene plants after 20–26 generations of selection compared with cages in treatment 3, where the only transgenic plants were two-gene plants (Fig. 1A).
Fig. 1.
Density of Cry1Ac/Cry1C-resistant diamondback moth on Bt plants (A) and non-Bt refuge plants (B) in greenhouse cages. Trt 1, 45% Cry1Ac and 45% two-gene plants plus 10% refuge; Trt 2, 45% Cry1C and 45% two-gene plants plus 10% refuge; Trt 3, 90% two-gene plants plus 10% refuge. Data were based on two replicates in treatment 1 after 20 generations when one replicate was terminated because of control failure. Means (±SEM) followed by the same letter within the same generation are not significantly different (P > 0.05).
In treatment 1, Cry1Ac plants were completely defoliated between generations 12 and 13 and were replaced by two-gene plants thereafter. Control failure and high insect densities were observed on two-gene broccoli plants in one replicate by generation 19 and in all other replicates by generation 26. In treatment 2, Cry1C plants were completely defoliated between generations 22 and 24 and were replaced by two-gene plants thereafter. Control failure and high insect densities were observed on two-gene broccoli plants by generation 26. In treatment 3, the density of P. xylostella on two-gene plants reached a maximum of 0, 0.13, and 0.33 larvae per plant, respectively, in the three replicates by the end of the experiment, and defoliation was minimal.
The density of P. xylostella on non-Bt refuge plants varied from ≈50–140 larvae and pupae per plant between generations and treatments (Fig. 1B), indicating that oviposition was relatively stable throughout the experiment and that there were insects available to reinfest transgenic plants in all treatments and cages.
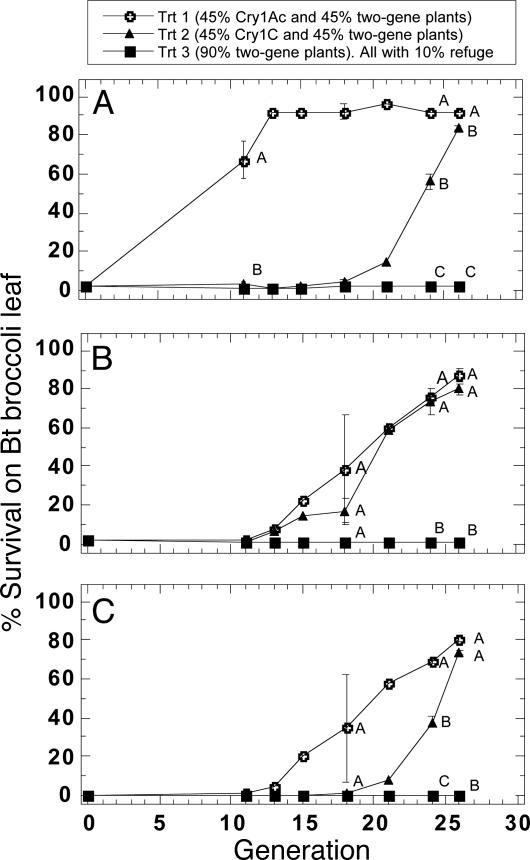
Resistance Evolution in P. xylostella Larvae. Resistance to Cry1Ac. Mean survival of P. xylostella on Cry1Ac broccoli was over 50% after 11 generations of selections in treatment 1, significantly higher than the survival of larvae in treatments 2 and 3 (Fig. 2A). Survival of larvae on Cry1Ac broccoli in treatment 2 did not reach 50% until the 24th generation of selection. Survival of larvae in treatment 3 did not exceed 5% in any replicate for the duration of the experiment.
Fig. 2.
Evolution of resistance by P. xylostella to plants producing Cry1Ac (A), Cry1C (B), or both toxins (C) under three different selection regimes in greenhouse cages. Means (±SEM) followed by the same letter within the same generation are not significantly different (P > 0.05).
Resistance to Cry1C. Mean survival of P. xylostella larvae on Cry1C broccoli exceeded 50% after 21 generations of selections in both treatments 1 and 2, whereas larval survival in treatment 3 was almost nil (Fig. 2B).
Resistance to Cry1Ac/Cry1C. A marked increase in resistance to two-gene plants was observed in one replicate of treatment 1 after 13 generations of selection. After 24 generations of selection, the mean survival of larvae on two-gene plants was over 60% and 30% in treatments 1 and 2, respectively, both of which were significantly higher than in treatment 3 (0%) (Fig. 2C).
After termination of the experiment at generation 26, the progeny larvae from the cage (replicate) in treatment 3 with the fewest numbers of larvae and pupae surviving on two-gene Bt plants showed 1.3% survival on Cry1Ac broccoli (n = 1,000) and 0.5% on Cry1C broccoli (n = 1,000), indicating that resistance alleles for both Cry1A-R and Cry1C-R were present in the cage. As expected, there was no survival on plants with both toxins. The survival of susceptible larvae was zero on Cry1Ac or Cry1C plants tested under the same conditions.
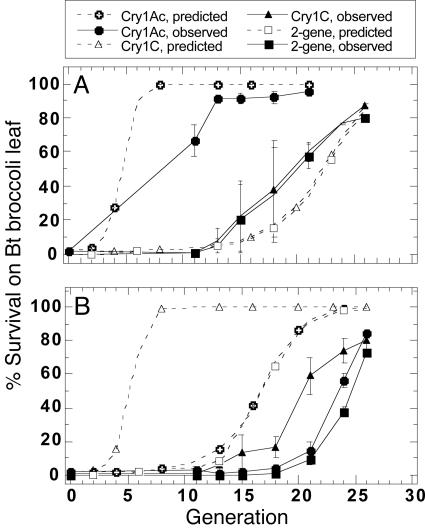
Predicted and Observed Results. The genetic simulation model predicted rapid resistance evolution to Cry1Ac, Cry1C, and both toxins with control failure for Bt plants by generation 26 in treatments 1 (Fig. 3A) and 2 (Fig. 3B) and no evident resistance evolution in treatment 3 (predicted data not shown because of overlaps for all points close to zero; observed data shown in Fig. 2). In particular, the model predicted for treatment 1 (Fig. 3A) that resistance will evolve first to the toxin that is being used singly in ≈10–12 generations. Resistance then evolves to the remaining effective toxin and therefore also to the pyramided plants, again in ≈10–15 generations (that is, once resistance has evolved to one toxin, selection acts effectively solely on the second toxin).
Fig. 3.
Comparison between the model predictions and observed results of resistance evolution to Cry1Ac/Cry1C of diamondback larvae in three treatments (Trt.). (A) Trt. 1, 45% Cry1Ac and 45% two-gene plants plus 10% refuge. (B) Trt. 2, 45% Cry1C and 45% two-gene plants plus 10% refuge. Trt. 3 (plot not shown), 90% two-gene plants plus 10% refuge (all points close to zero, observed data are shown in Fig. 2).
Treatment 2 was more perplexing, because although the resistance also evolved sequentially, resistance to Cry1C evolved even more slowly than expected for resistance under control of two genes. Perhaps most surprising is that treatment 2 suggests that a pyramided crop could fail rather rapidly after resistance evolved to the single toxin (Cry1C) plants. Survival on Cry1C broccoli in treatment 2 did not exceed 50% until generation 21, considerably later than the model predicted (generation 6), but subsequent resistance to Cry1Ac in this case did not take another dozen generations but only about four (Fig. 3B). We cannot be certain as to why this happened, but resistance to Cry1Ac and the consequent loss of effectiveness of the pyramided plants evolved closer to predictions than for Cry1C, at about generations 24 (observed) and 17 (predicted). From the model, it appears that the resistance alleles for Cry1Ac were increasing during the slow selection for Cry1C, at frequencies too low for us to measure experimentally, but were primed for rapid increase.
The model did not accurately predict trends in population densities (data not shown), most likely reflecting that the population dynamics in the cages was more complicated than we could adequately model. Varying population growth rates had little effect on rates of change in resistance frequency.
Discussion
We used a model system of Bt broccoli plants and P. xylostella (11) to examine the evolution of insect resistance under different deployment strategies for one- and two-gene Bt plants. In our experiment, there was high expression of all Bt toxins (Cry1Ac and/or Cry1C) in transgenic plants and no linkage or cross-resistance between the recessive Cry1Ac and Cry1C resistance in P. xylostella (11). The results of the greenhouse experiment indicated that concurrent use of one-gene and pyramided two-gene plants will select for resistance to pyramided Bt plants more rapidly than the use of two-gene plants alone, if the two-gene plants produce a similar toxin as the single-gene plants. The experimental data agreed in general with the predictions of a Mendelian deterministic simulation model in that resistance will evolve in sequential fashion, first to the single toxin and then to the remaining toxin in the pyramid.
Results from our previous tests demonstrated that pyramided plants expressing two dissimilar Bt toxin genes have the potential to delay the evolution of insect resistance to Bt crops more effectively than single-toxin plants (11). However, in that experiment, we did not observe a high level of resistance to Cry1C plants when they were used sequentially after Cry1Ac plants during the limited number of generations under selection, probably because the initial allele frequency (0.2) was not high enough for polygenic Cry1C resistance to develop since few individuals survived on Cry1C Bt broccoli. By using a higher initial allele frequency of Cry1C resistance (0.34) and a longer period of selection in the present experiment, a high level of resistance to Cry1C plants was observed after 21 generations of selection in treatment 2 (Fig. 2B). These results lend additional support to the conclusions drawn from our previous experiment (11) regarding the advantage of gene pyramiding for resistance management. Our results also show that an insect population can develop resistance to pyramided Bt plants expressing two dissimilar toxins.
As in our previous study (11), the initial allele frequency used in the present experiment was much higher than what is expected for most insects targeted by Bt crops (generally 0.001 or less) (7). Because of the relatively small population in a cage (n = 500 initially; n < 4,000 eggs laid after F1 and before control failure), it was necessary to use relatively higher initial resistance allele frequencies to obtain at least some survivors on either Cry1A or Cry1C Bt broccoli and thereby to assure that resistance would evolve in some treatments in the experiments. Otherwise, one might erroneously conclude that resistance could not evolve at all, when it surely could where such genotypes were present in a much larger field population. Results from the model indicated that the benefits of pyramiding and the use of two-gene plants alone are much better when initial resistance allele frequencies are lower than 0.001, an effect shown earlier for transgenic crops (9) and even earlier for mixtures of insecticides (31).
Models can serve as useful tools to evaluate the relative effectiveness of different resistance management strategies (6). The results of the greenhouse experiment were in close agreement with the predictions of the theoretical model for Treatments 1 and 3. However, resistance to Cry1C broccoli in Treatment 2 occurred several generations later (e.g., 15) than the model predicted. One reason for this apparent discrepancy may be the fitness costs that are associated with Cry1C resistance in our colony (J.-Z.Z., unpublished data). Incorporation of additional parameters such as fitness costs into the model may improve the predictive capacity of the model and its transferability to field situations. Our main conclusion, and one that should be of interest to regulators and seed companies, is that we found an overall general fit of the experimental data to the theoretical model predictions for the sequential evolution of resistance.
Because the concurrent use of single- and two-gene Bt plants can offer exposed populations a “stepping stone” to develop resistance to both toxins, it is important that regulatory decisions regarding the registration of plants with pyramided genes also consider the registration or de-registration of single-gene Bt plants. From a resistance management perspective, pyramided Bt plants should not be deployed simultaneously with single-gene plants if they share similar Bt toxins. In Australia, pyramided Bt cotton (Bollgard II) has been commercially available since 2002. The use of both one- and two-gene varieties was permitted for the first 2 years after the introduction of Bollgard II, but now only 2-gene varieties are allowed (2, 32, 33). The rapid phase-out of one-gene varieties was intended to minimize pest exposure to the single Bt toxin and thus to reduce the risk of resistance to pyramided plants. As a result, the original 30% cap on Bt cotton acreage has now been removed for Bollgard II, although a 20% non-Bt refuge is still required (2, 32). In the United States, plants with a single Bt gene currently remain the dominant Bt varieties. Our results indicate that the introduction of pyramided plants with currently deployed single-gene plants could enhance resistance evolution to the otherwise more durable pyramided plants if they are used simultaneously in the same area. Our previous data (11) and this study suggest that it would be advantageous from a resistance management stand-point for regulatory agencies to consider de-regulating single-gene plants as soon as pyramided plants are available. In addition to providing superior resistance management, pyramided plants may provide improved pest control (12, 34) and require a smaller refuge (9).
Although our experimental results are clear, we realize that the model system used in this study may differ in several respects from the crop/pest systems for which Bt plants are currently marketed. For example, Bt cotton does not deliver a high dose to Helicoverpa spp., one of its target pests. In addition, although resistance to Cry1Ac has been detected in P. xylostella worldwide (35), there have been no documented cases of increased insect resistance in Bt cotton (Cry1Ac) or Bt corn (Cry1Ab) in the field since they were first released commercially in 1996 (5). Thus, the results of the greenhouse experiment may not reflect all of the possible dynamics of resistance development in the field or for all Bt crop/insect systems. Developing the most appropriate regulation strategies for Bollgard II or other pyramided Bt cotton or corn varieties in the United States will require further study. However, because of the lack of insects with resistance to one or multiple Bt proteins in corn and cotton, respectively, our system remains a unique tool to study insect resistance management to pyramided Bt plants and can provide insights about the risk of using single and pyramided Bt plants simultaneously.
Our results from this and a previous study (11) have demonstrated empirically that pyramided two-Bt-gene plants are more effective at delaying resistance, but the delays in resistance will only be fully realized if they are not used in conjunction with one-gene plants sharing similar Bt proteins.
Acknowledgments
We thank F. Gould and B. E. Tabashnik for helpful comments and suggestions and Y. M. Cheung for technical assistance. This work was supported by U.S. Department of Agriculture Biotechnology Risk Assessment Program Grant 2001-33522-11279.
Author contributions: J.-Z.Z., R.T.R., E.D.E., and A.M.S. designed research; J.-Z.Z., J.C., and H.L.C. performed research; J.-Z.Z., J.C., H.L.C., S.L.B., R.T.R., E.D.E., and A.M.S. analyzed data; and J.-Z.Z., J.C., S.L.B., R.T.R., E.D.E., and A.M.S. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: Bt, Bacillus thuringiensis; Cry1Ac-R, Cry1Ac-resistant strain; Cry1C-R, Cry1C-resistant strain.
References
- 1.National Agriculture Statistics Service, U.S. Department of Agriculture (2004) http://usda.mannlib.cornell.edu/reports/nassr/field/pcp-bba/acrg0604.pdf.
- 2.James, C. (2004) ISAAA Briefs (International Service for the Acquisition of Agro-Biotech Applications, Ithaca, NY), No. 32.
- 3.Shelton, A. M., Zhao, J.-Z. & Roush, R. T. (2002) Annu. Rev. Entomol. 47, 845-881. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Environmental Protection Agency (2001) www.epa.gov/pesticides/biopesticides/pips/bt_brad.htm. [PubMed]
- 5.Tabashnik, B. E., Carrière, Y., Dennehy, T. J., Morin, S., Sisterson, M. S., Roush, R. T., Shelton, A. M. & Zhao, J.-Z. (2003) J. Econ. Entomol. 96, 1031-1038. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Environmental Protection Agency (1998) www.epa.gov/scipoly/sap/1998/february/finalfeb.pdf. [PubMed]
- 7.Gould, F. (1998) Annu. Rev. Entomol. 43, 701-726. [DOI] [PubMed] [Google Scholar]
- 8.Shelton, A. M., Tang, J. D., Roush, R. T., Metz, T. D. & Earle, E. D. (2000) Nat. Biotechnol. 18, 339-342. [DOI] [PubMed] [Google Scholar]
- 9.Roush, R. T. (1998) Philos. Trans. R. Soc. London B 353, 1777-1786. [Google Scholar]
- 10.Gould, F. (2003) Nat. Biotechnol. 21, 1450-1451. [DOI] [PubMed] [Google Scholar]
- 11.Zhao, J.-Z., Cao, J., Li, Y. X., Collins, H. L., Roush, R. T., Earle, E. D. & Shelton, A. M. (2003) Nat. Biotechnol. 21, 1493-1497. [DOI] [PubMed] [Google Scholar]
- 12.U.S. Environmental Protection Agency (2002) www.epa.gov/pesticides/biopesticides/ingredients/factsheets/factsheet_006487.htm. [PubMed]
- 13.Office of the Gene Technology Regulator (2002), www.ogtr.gov.au/ir/dir012.htm.
- 14.Tabashnik, B. E., Cushing, N. L., Finson, N. & Johnson, M. W. (1990) J. Econ. Entomol. 83, 1671-1676. [Google Scholar]
- 15.Shelton, A. M., Robertson, J. L., Tang, J. D., Perez, C., Eigenbrode, S. D., Preisler, H. K., Wilsey, W. T. & Cooley, R. J. (1993) J. Econ. Entomol. 86, 697-705. [Google Scholar]
- 16.Janmaat, A. F. & Myers, J. (2003) Proc. R. Soc. London Ser. B 270, 2263-2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metz, T. D., Roush, R. T., Tang, J. D., Shelton, A. M. & Earle, E. D. (1995) Mol. Breeding 1, 309-317. [Google Scholar]
- 18.Ramachandran, S., Buntin, G. D., All, J. N., Tabashnik, B. E., Raymer, P. L., Adang, M. J., Pulliam, D. A. & Stewart, C. N., Jr. (1998) J. Econ. Entomol. 91, 1239-1244. [Google Scholar]
- 19.Tang, J. D., Collins, H. L., Roush, R. T., Metz, T. D., Earle, E. D. & Shelton, A. M. (1999) J. Econ. Entomol. 92, 47-55. [DOI] [PubMed] [Google Scholar]
- 20.Zhao, J.-Z., Collins, H. L., Tang, J. D., Cao, J., Earle, E. D., Herrero, S., Escriche, B., Ferré, J., Roush, R. T. & Shelton, A. M. (2000) Appl. Environ. Microbiol. 66, 3784-3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao, J.-Z., Li, Y. X., Collins, H. L. & Shelton, A. M. (2002) J. Econ. Entomol. 95, 14-21. [DOI] [PubMed] [Google Scholar]
- 22.Tabashnik, B. E., Liu, Y.-B., Finson, N., Masson, L. & Heckel, D. G. (1997) Proc. Natl. Acad. Sci. USA 94, 1640-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang, J. D., Gilboa, S., Roush, R. T. & Shelton, A. M. (1997) J. Econ. Entomol. 90, 732-741. [Google Scholar]
- 24.Zhao, J.-Z., Li, Y. X., Collins, H. L., Cao, J., Earle, E. D. & Shelton, A. M. (2001) J. Econ. Entomol. 94, 1547-1552. [DOI] [PubMed] [Google Scholar]
- 25.Baxter, S., Zhao, J.-Z., Gahan, L. J., Shelton, A. M., Tabashnik, B. E. & Heckel, D. G. (2005) Insect Mol. Biol. 14, in press. [DOI] [PubMed]
- 26.Cao, J., Tang, J. D., Shelton, A. M. & Earle, E. D. (1999) Mol. Breeding 5, 131-141. [Google Scholar]
- 27.Cao, J., Zhao, J.-Z., Tang, J. D., Shelton, A. M. & Earle, E. D. (2002) Theor. Appl. Genet. 105, 258-264. [DOI] [PubMed] [Google Scholar]
- 28.Cao, J. & Earle, E. D. (2003) Plant Cell Rep. 21, 789-796. [DOI] [PubMed] [Google Scholar]
- 29.Tang, J. D., Collins, H. L., Metz, T. D., Earle, E. D., Zhao, J.-Z., Roush, R. T. & Shelton, A. M. (2001) J. Econ. Entomol. 94, 240-247. [DOI] [PubMed] [Google Scholar]
- 30.SAS Institute (1985) SAS User's Guide, Statistics (SAS Institute, Cary, NC), 5th Ed.
- 31.Mani, G. S. (1985) Genetics 109, 761-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CSIRO (2003) www.csiro.au/proprietaryDocuments/PI_info_bollgard.pdf.
- 33.Bates, S. L., Zhao, J.-Z., Roush, R. T. & Shelton, A. M. (2005) Nat. Biotechnol. 22, 57-62. [DOI] [PubMed] [Google Scholar]
- 34.Jackson, R. E., Bradley, J. R., Jr., Van Duyn, J. W. & Gould, F. (2004) J. Econ. Entomol. 97, 1719-1725. [DOI] [PubMed] [Google Scholar]
- 35.Ferré, J. & Van Rie, J. (2002) Annu. Rev. Entomol. 47, 501-533. [DOI] [PubMed] [Google Scholar]