Abstract
The antiinflammatory cytokine IL-10 inhibits the production of multiple, diverse inflammatory mediators from activated macrophages and dendritic cells, a process requiring STAT3 activation. However, the mechanisms involved in the broad inhibitory effects of IL-10 are controversial. I eliminated the contribution of the major confounding variable to understanding the antiinflammatory response, the 3′ UTR region of inflammatory mediator genes, through knock-in mutation and analysis of the effects of IL-10 on transcription rate of inflammatory genes. IL-10 activates STAT3 to act indirectly by selectively inhibiting gene transcription independent of general effects on NF-κB or posttranscriptional mRNA processing through a process that reduces the overall transcriptional rate of specific genes.
Keywords: cytokine, inflammation, macrophage
Early studies established that IL-10 has profound effects on activated macrophages and antigen-presenting cells (1-3). We now understand that the inhibitory response activated by IL-10 primarily functions to limit cytokine and chemokine production from macrophages and dendritic cells activated by agonists of Toll-like receptors (TLRs), such as LPS and bacterial lipoproteins. At the whole-animal level, extensive genetic and pharmacologic studies have established that IL-10 plays an essential, nonredundant role in limiting chronic and acute inflammation (4, 5). At the molecular level, however, the IL-10-activated pathways that control this process are controversial. Indeed, the pathways and mechanisms involved have been debated in dozens of primary publications and reviewed by others in depth (4-7). Understanding the IL-10-regulated pathways is significant for the future development of antiinflammatory agents because it should be possible to pharmacologically harness IL-10's inhibitory effects to either agonize or antagonize inflammation depending on the desired clinical application. For example, in life-threatening sepsis, endogenous IL-10 is insufficient to block the massive systemic cytokine release that occurs after TLR triggering: Exacerbation of the IL-10 pathway could provide an intervention to alleviate septic shock.
Two major insights into IL-10 signal transduction have recently been made. First, STAT3 has been shown to be essential for all known aspects of the antiinflammatory pathway (8-10). Second, at the mRNA level, IL-10 antagonizes a subset of genes activated by TLR signaling, accounting for 20-25% of mRNAs induced after LPS stimulation (10). Included in this population of genes are defined IL-10 targets, such as TNF-α, IL-6, IL-12p40, and numerous chemokine mRNAs. Thus, most LPS-induced genes are unaffected by IL-10 treatment, suggesting that IL-10 cannot affect a basic process common to all gene expression, such as inhibitory effects on a common basal transcription factor. For one target, IL-12p40, IL-10 inhibits expression at a step involving transcription through blocking polymerase II recruitment (6). Despite these advances, progress in understanding IL-10 signaling is presently retarded by two major controversies. The first concerns the mode of action of the IL-10-induced antiinflammatory response: Is the target transcription, posttranscriptional mRNA processing, or combinations of these effects (7)? The second controversy concerns the direct versus indirect actions of STAT3 in the IL-10 pathway. A previous study (2) proposed that IL-10 directs new gene expression whose products could execute the antiinflammatory response. This hypothesis has been difficult to confirm because of the powerful mRNA stabilizing and destabilizing effects of protein synthesis inhibitors on key IL-10 targets such as TNF-α (11-13). An alternate and attractive model is that STAT3 acts directly and specifically to inhibit gene expression, for example by titrating transcription factors away from active inflammatory promoters.
To resolve these issues, I performed a series of quantitative studies to dissect IL-10's antiinflammatory mechanism. The results show that IL-10 primarily targets transcription and that STAT3 acts indirectly.
Experimental Procedures
Generation of Tnfa 3′ UTR::Gapdh 3′ UTR Mice. Left and right arms of the targeting vector were generated by long-range PCR using Herculase enhanced DNA polymerase (Stratagene). These fragments were combined with the Gapdh 3′ UTR and loxP sites to flank the pgk-neo cassette in pGT-N38-Asc I (14). The final construct also included two missense mutations at codons encoding conserved glycine residues 199 and 200 at the trimer interface of TNF-α. W9 ES cells were selected as described in ref. 14, and correct integrants were detected by Southern blotting with MluI digestion of genomic DNA. Correctly targeted ES clones were then transiently transfected with pCMV-cre- and G418-sensitive clones isolated and tested for the correct alleles by PCR with primers 296 and 298 that amplify 1.2 kb (wild-type allele) and 0.6 kb (recombined allele). Correct clones were karyotyped and injected into C57BL/6 blastocysts to obtain chimeric mice. Chimeric males were bred with C57BL/6 females to obtain germline transmission of the recombined allele. Mice with two copies of the Tnfa 3′ UTR::Gapdh 3′ UTR allele were detected by using primers 298, 580, and 582 (Table 1, which is published as supporting information on the PNAS web site). Bone marrow-derived macrophages (BMDMs) were obtained from these animals and cultured and stimulated as described in refs. 10 and 15.
Real-Time, Primary-Transcript (PT) RT-PCR. Total RNA was isolated from IL-10-/- BMDMs by using TRIzol as described in ref. 10. RNA quality was first assessed by Northern blotting with probes for TNF-α, IL-1α, or GAPDH (Fig. 1a). RNA was resuspended at a final concentration of 0.45 μg/μl in 100 μl and treated with RNase-free DNase (Promega) for 45 min using 6 units of enzyme. RNA was phenol/chloroform-extracted and resuspended to a final concentration of 0.5 μg/μl, and 2 μg was reverse-transcribed by using SuperScript II (Invitrogen) according to the manufacturer's instructions. Identical samples from each time point were processed in the absence of reverse transcriptase and served as controls for genomic DNA contamination. cDNA was diluted to a final volume of 80 μl, and 1 μl was subjected to preliminary RT-PCRs using primer pairs designed for PT RT-PCR. An example is shown in Fig. 5, which is published as supporting information on the PNAS web site, using IL-12p40 primers as described by Smale and colleagues (6). All primers sequences are listed in Table 1. Relative amounts of each PT were then assayed using SYBR green detection and real-time RT-PCR. Each primer pair was first assessed for the ability to amplify each amplicon under standard real-time RT-PCR conditions (i.e., two-cycle PCR) using mouse genomic DNA as a standard. Note that the IL-12p40 primer pairs could not be adapted to SYBR green RT-PCR. All samples were processed in duplicate, and relative quantities were determined against a diluted standard of genomic DNA.
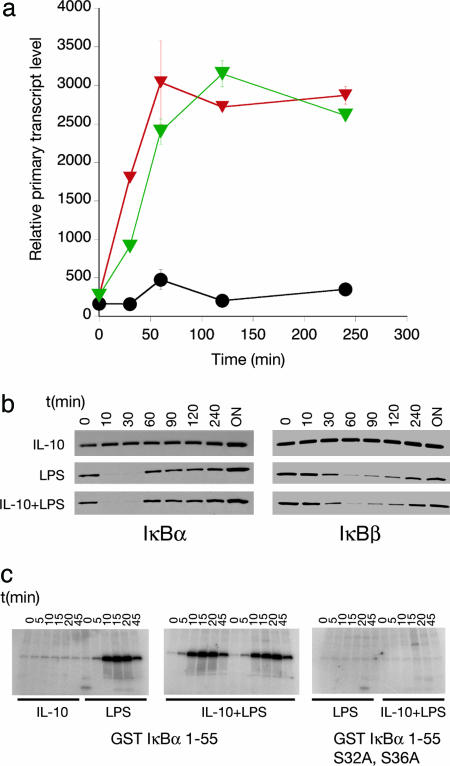
Fig. 1.
IL-10 inhibits the rate of transcription at LPS-induced inflammatory genes. (a) Total RNA was isolated from stimulated macrophages and subjected to denaturing agarose gel electrophoresis to assess RNA quality followed by Northern blotting using probes specific for IL-10 targets whose expression levels have been previously shown to decrease after costimulation with LPS and IL-10. Shown is the same Northern blot probed with cDNA probes for TNF-α or IL-1α. Note that IL-10 strongly inhibits total mRNA levels. (b) PT RT-PCR assays were performed for the target genes shown by using normalized DNase-treated RNA samples from IL-10-/- BMDMs stimulated with 10 ng/ml IL-10 (black triangles), 100 ng/ml LPS (red triangles), or LPS and IL-10 (green triangles) for 0, 30, 60, 120, and 240 min. RNA was reverse-transcribed in the presence (Upper) or absence (Lower) of reverse transcriptase (RT) and subjected to SYBR green real-time PT RT-PCR. Results are representative of three independent experiments.
Conventional RT-PCR. Total RNA samples were processed for quantitative RT-PCR by using procedures described refs. 10 and 15. Briefly, total RNAs were first separated by denaturing electrophoresis and analyzed by Northern blotting. Two microliters of each RNA was reverse-transcribed as described above and subjected to quantitative RT-PCR analysis using primer and FAM-labeled probes for TNF-α and β-actin (10, 15).
Immunoblotting, IκB Kinase (IKK) Assays, and Transient Transfection. Immunoblotting for IκB proteins was performed as described in ref. 16. IKK assays were performed by using recombinant GST-IκB-α fusion proteins (a gift from Neal Silverman, University of Massachusetts, Amherst) according to procedures described by Mercurio et al. (17). Transient transfection of RAW macrophages with a κB-dependent reporter plasmid was performed as described in ref. 18.
Results and Discussion
I first tested whether IL-10 inhibits the transcription of a subset of inflammatory target genes by using PT RT-PCR, an assay system that can be used to estimate the transcription rate of genes over time by measuring the accumulation of newly transcribed, unspliced, or partially spliced RNAs (6). PT levels of individual inflammatory mediator RNAs were estimated by quantitative real-time PCR after stimulation of IL-10-/- BMDMs with LPS or LPS and IL-10. IL-10-/- BMDMs were used because normal BMDMs produce large amounts of IL-10 after LPS stimulation, which contributes to background variability when measuring the effects of exogenously added IL-10 (10). Because IL-10-/- BMDMs respond normally to exogenous IL-10, the use of these cells increases the robustness of the assay (10). Quantitative real-time PCR experiments were performed to estimate the transcriptional rate of IL-10 targets, TNF-α, IL-1α, IL-6, and the chemokine KC. In each case, IL-10 blocked the LPS-induced transcriptional rate over time (Fig. 1b). Therefore, the primary effect of IL-10 is to target a transcriptional mechanism common to a subset of TLR-induced genes.
Previous studies have proposed that IL-10 can inhibit NF-κB activation and that this leads to the antiinflammatory effects (19-21). In contrast, other groups, including my own, have suggested that IL-10 has negligible effects on NF-κB activity (4, 7, 10). The experimental system described above allows the critical evaluation of the role of NF-κB inhibition in IL-10 signaling by probing the effects of IL-10 on known NF-κB-dependent genes. I therefore tested whether IL-10 blocked the LPS-mediated induction of IκBα, a gene whose transcription requires the rapid recruitment and activity of NF-κB (22). IL-10 had no effects of the induction of the IκBα PT (Fig. 2a). This finding was supported by immunblotting analysis for the resynthesis of IκBα and IKK assays (Fig. 2 b and c). Finally, transfection of a reporter plasmid into macrophages driven by four consensus κB sites showed normal induction after LPS treatment regardless of the presence of IL-10 (Fig. 6, which is published as supporting information on the PNAS web site). Taken together, these data confirm that IL-10 does not control the antiinflammatory response through a general block in NF-κB activity.
Fig. 2.
Analysis of the effects of IL-10 on IκB and IKK activity. (a) PT RT-PCR analysis of the effects of IL-10 on LPS-mediated induction of IκBα transcription. IL-10-/- BMDMs stimulated with 10 ng/ml IL-10 (black circles), 100 ng/ml LPS (green triangles) or LPS and IL-10 (red triangles) for 0, 30, 60, 120, and 240 min. RNA was reverse-transcribed and subjected to SYBR green real-time PT RT-PCR. (b) Immunoblotting analysis of IκBα and IκBβ levels in primary macrophages after stimulation with LPS and IL-10. Note that IκB proteins levels drop after proteosome-mediated destruction of each protein followed by resynthesis. (c) (Left and Center) IKK activity assays showing that IL-10 does not affect the ability of immunoprecipitated IKK to phosphorylate a GST-IκBα 1-55 purified recombinant fusion protein. (Right) The same assay using a mutant form of the substrate.
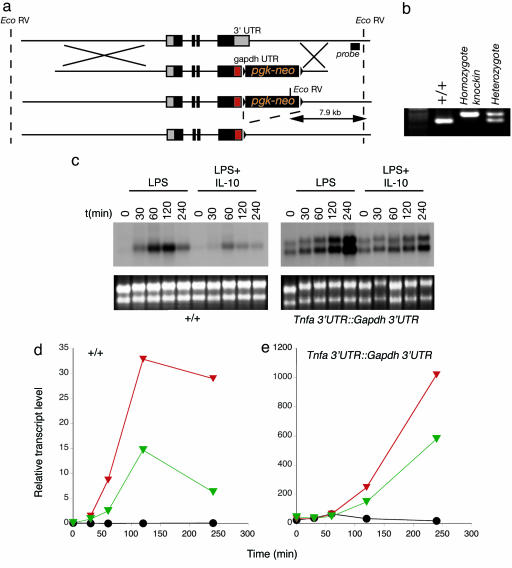
To confirm the PT findings described above using an independent genetic-based approach, I replaced the 3′ UTR of the Tnfa gene with the 3′ UTR of the Gapdh gene to eliminate the confounding effects of the 3′ UTR on measuring mRNA levels (Fig. 3 a and b). Chimeric mice bearing one copy of this allele died before weaning from excessive TNF-α toxicity. I therefore introduced two missense mutations into the region encoding the trimer interface to produce a protein I anticipated to be non-functional. Chimeric mice were readily obtained by using this strategy and were used to generate homozygous mice bearing two copies of the Tnfa 3′ UTR::Gapdh 3′ UTR allele. Mice were generated in Mendelian ratios and used as a source of primary BMDMs. As expected, resting levels of the TNF-α mRNA were elevated because of the stabilizing effects of the Gapdh 3′ UTR (Fig. 3c). LPS treatment further induced steady state levels of the stabilized TNF-α mRNA. Most significantly, IL-10 inhibited LPS-mediated induction of the stabilized TNF-α mRNA, consistent with the findings reported in Fig. 1. Although the kinetics and quantity of the stabilized TNF-α mRNA are changed relative to the wild-type TNF-α mRNA because of the posttranscriptional effects of the Gapdh 3′UTR, these results and those shown in Fig. 1 confirm that IL-10 regulates TNF-α mRNA levels independently of posttranscriptional effects that control mRNA stability and degradation.
Fig. 3.
Construction and analysis of mice in which the Tnfa locus has been modified to replace the endogenous 3′ UTR with the Gapdh 3′ UTR. (a) Schematic diagram of the modified locus after targeting and subsequent cre-lox-mediated elimination of the pgk-neo cassette. (b) PCR analysis of mice carrying the wild-type or Tnfa 3′ UTR::Gapdh 3′ UTR alleles. (c) Northern blotting analysis using a TNF-α cDNA probe of macrophages from wild-type or Tnfa 3′ UTR::Gapdh 3′ UTR mice treated with LPS or LPS and IL-10 under conditions described in the legend to Fig. 1. Note that the modification of the Tnfa locus causes two transcripts to be made. Equivalent total RNA levels are shown by ethidium bromide staining. (d and e) Representative conventional real-time RT-PCR analysis from RNA samples isolated from stimulated BMDMs from wild type for Tnfa 3′ UTR::Gapdh 3′ UTR mice. BMDMs were stimulated with IL-10 (black circles), LPS (red triangles), or LPS and IL-10 (green triangles) as described in the legend to Fig. 1. Note the scale difference between the two genotypes reflected the higher levels of the stabilized TNF-α mRNA in BMDMs from Tnfa 3′ UTR::Gapdh 3′ UTR mice.
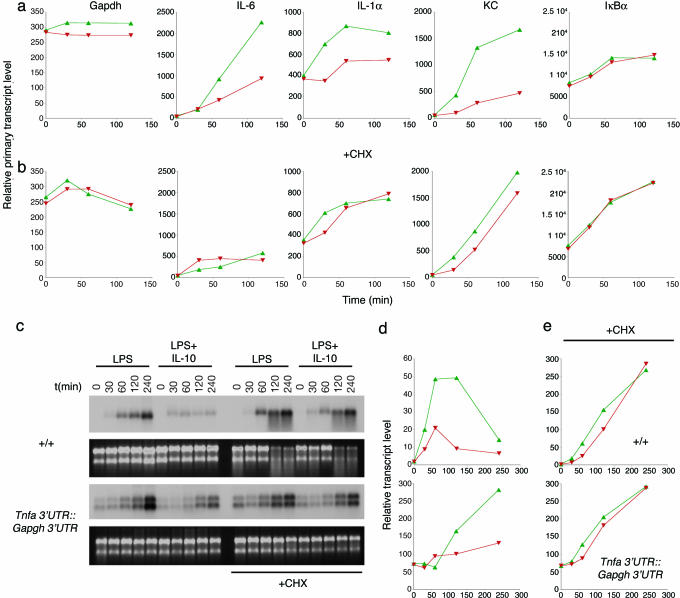
I next addressed the controversial question of the role of new protein synthesis in the antiinflammatory effect. The 3′UTR regions of cytokine and chemokine mRNAs cause mRNA stabilization and often superinduction after costimulation with TLR agonists and protein synthesis inhibitors (2, 11-13, 23-26). The effect of protein synthesis inhibitors on mRNA stabilization confounds the ability to determine whether IL-10 directly acts through STAT3 or whether new protein synthesis is required. For example, cycloheximide superinduces TNF-α mRNA levels because the labile proteins that normally degrade the TNF-α mRNA through the AU-rich 3′UTR are no longer synthesized (Fig. 7, which is published as supporting information on the PNAS web site). PT RT-PCR experiments were performed but this time in the presence or absence of cycloheximide (Fig. 4 a and b). A shorter time scale was used because cycloheximide is toxic to BMDMs after 2 h. The results confirmed that the effects of IL-10 on the transcription rate of immediate targets (IL-1α, KC) require new protein synthesis. For targets like IL-6, no conclusion could be drawn because new protein synthesis is required for the LPS-mediated induction of transcription (Fig. 4b, IL-6). These findings were then extended to the Tnfa 3′ UTR::Gapdh 3′ UTR model, of which I could ask whether cycloheximide treatment blocked the ability of IL-10 to inhibit LPS-induced TNF-α mRNA levels. The results (Fig. 4 c-e) confirm that IL-10 fails to block LPS-mediated induction of the modified TNF-α mRNA, independently confirming that new protein synthesis is essential for the execution of the antiinflammatory effects of IL-10.
Fig. 4.
The IL-10-mediated antiinflammatory effect requires new protein synthesis. (a and b) PT RT-PCR analysis of IL-10 targets in the absence (a) or presence (b) of 10 μg/ml cycloheximide (CHX). Total RNA was isolated from IL-10-/- macrophages stimulated as described in Fig. 1 (LPS, green triangles; LPS and IL-10, red triangles) and subjected to PT RT-PCR. Note that induction of IL-6 by LPS requires new protein synthesis and that the requirement for new protein synthesis by IL-10 to block this gene cannot be determined. Data are representative of two independent experiments performed under identical conditions. (c) Northern blotting analysis of the effects of cycloheximide on IL-10-mediated inhibition of TNF-α mRNA in Tnfa 3′ UTR::Gapdh 3′ UTR BMDMs. Data are representative of three independent experiments. (d and e) Quantitative analysis by RT-PCR of the effects of IL-10 on Tnfa 3′ UTR::Gapdh 3′ UTR mRNA levels. Shown is conventional RT-PCR analysis for TNF-α using a FAM-labeled probe. BMDMs were stimulated with LPS (green triangles) or LPS and IL-10 (red triangles) as described in the legend to Fig. 1. Data were normalized by using β-actin as a standard. Data are representative of three independent experiments.
I demonstrate here that IL-10's antiinflammatory effects are mediated through indirect actions of STAT3 on the transcriptional rate of target inflammatory genes. Previous studies in this area have produced contradictory results primarily because the assays systems used could not discriminate between the effects of transcription and the posttranscriptional control of mRNA levels (7). I have used two complementary approaches to address this problem. I deleted the entire mRNA stability control region from the Tnfa gene and replaced it with the Gapdh 3′ UTR that is not subject to the highly complex mechanisms that contribute to TNF-α mRNA stability. Macrophages from mice with the Tnfa 3′ UTR::Gapdh 3′ UTR allele showed elevated baseline TNF-α mRNA levels as expected. These results are consistent with another mutation in the Tnfa 3′UTR, where a single AU-rich element was deleted, resulting in stabilized mRNA levels and excessive TNF-α production (27). LPS stimulation increased the TNF-α mRNA levels proportionally compared with control BMDMs. However, in the absence of the endogenous 3′UTR, IL-10 was still capable of reducing the total mRNA levels suggesting that effects of IL-10 are independent of the 3′ UTR. This finding was supported by detailed examination of the PTs of known IL-10 targets; each of which showed an IL-10-dependent decrease after LPS and IL-10 treatment. Because I was estimating the rate of transcription before posttranscriptional processing events, these results show that the major effect of IL-10 is to target transcription, supporting previous findings concerning IL-12p40 inhibition by IL-10 (6, 28). These studies do not exclude, however, secondary effects of IL-10 mediated through the mRNA UTRs after IL-10's effects on transcription lower the total mRNA level to thresholds where posttranscriptional processing can alter mRNA levels further.
The results do not support a model in which IL-10 blocks general NF-κB activation. I could find no evidence that IL-10 inhibited the NF-κB-dependent induction of IκBα PTs, IκBα or IκBβ protein resynthesis, total κB-dependent reporter activity after LPS-mediated degradation, or the effects of LPS-induced IKK activity. Because IκBα is a canonical NF-κB-responsive gene (22), these data and others reported in the literature are contrary to claims that NF-κB activation is a key component of IL-10-mediated antiinflammatory effects. I cannot, however, exclude the possibility that NF-κB activity is controlled by IL-10 at individual promoters that are inhibited by IL-10 after TLR-dependent transcriptional induction.
Finally, I resolved the long-standing issue of whether new protein synthesis is required for the inhibitory effects of IL-10. By using the Tnfa 3′ UTR::Gapdh 3′ UTR mouse model and PT RT-PCR, I found that the actions of IL-10 are indirect and require the synthesis of intermediary proteins. Thus, STAT3, activated by IL-10 and essential for all IL-10's known inhibitory functions, must act to target genes(s) activated after TLR stimulation. Several groups, including my own, have searched for IL-10-induced genes that can execute the antiinflammatory response (10, 29, 30). Although several candidates have been tested, none has yet been found that would fulfill the criteria of a STAT3-induced transcriptional inhibitor that can selectively control transcription at inflammatory promoters. For example, Bcl-3 is induced by IL-10 and appears to have some effects on inhibiting TNF-α levels (30). However, the activity of Bcl-3 was specific for TNF-α and not IL-6. Furthermore, Bcl-3-/- macrophages were reported to lack the elevated cytokine and chemokine production that occurs in IL-10-/- and STAT3-deficient macrophages (30), a phenotype that would be expected from an antiinflammatory mediator genetically downstream of STAT3. The key, therefore, to understanding IL-10's actions will be to isolate the STAT3-induced gene(s) whose product(s) acts at multiple promoters to reduce transcription. I propose four models to account for the IL-10-mediated antiinflammatory effect: IL-10 could induce (i) the expression of a transcriptional repressor(s), (ii) proteins that modify chromatin environments to selectively silence active inflammatory promoters, (iii) the expression of genes products that posttranslationally modify the activity of transcription factors at specific promoters (e.g., NF-κB), or (iv) the degradation of key transcription factors at active promoters, as has been found for NF-κB (31).
Supplementary Material
Acknowledgments
I thank Charles Sherr and Doug Hilton for frank advice concerning aspects of this project and Neal Silverman for the gift of recombinant GST-IκBα substrates. This work was supported by National Institutes of Health Grant AI062921 and Cancer Center Support CORE Grant P30 CA 21765 and by the Sandler Program for Asthma Research and the American Lebanese Syrian Associated Charities.
Author contributions: P.J.M. designed research; P.J.M. performed research; P.J.M. contributed new reagents/analytic tools; P.J.M. analyzed data; and P.J.M. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: TLR, Toll-like receptor; PT, primary transcript; BMDM, bone marrow-derived macrophages; IKK, IκB kinase.
References
- 1.Bogdan, C., Vodovotz, Y. & Nathan, C. (1991) J. Exp. Med. 174, 1549-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bogdan, C., Paik, J., Vodovotz, Y. & Nathan, C. (1992) J. Biol. Chem. 267, 23301-23308. [PubMed] [Google Scholar]
- 3.Fiorentino, D. F., Zlotnik, A., Vieira, P., Mosmann, T. R., Howard, M., Moore, K. W. & O'Garra, A. (1991) J. Immunol. 146, 3444-3451. [PubMed] [Google Scholar]
- 4.Donnelly, R. P., Dickensheets, H. & Finbloom, D. S. (1999) J. Interferon Cytokine Res. 19, 563-573. [DOI] [PubMed] [Google Scholar]
- 5.Moore, K. W., de Waal Malefyt, R., Coffman, R. L. & O'Garra, A. (2001) Annu. Rev. Immunol. 19, 683-765. [DOI] [PubMed] [Google Scholar]
- 6.Zhou, L., Nazarian, A. A. & Smale, S. T. (2004) Mol. Cell. Biol. 24, 2385-2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams, L. M., Ricchetti, G., Sarma, U., Smallie, T. & Foxwell, B. M. (2004) Immunology 113, 281-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeda, K., Clausen, B. E., Kaisho, T., Tsujimura, T., Terada, N., Forster, I. & Akira, S. (1999) Immunity 10, 39-49. [DOI] [PubMed] [Google Scholar]
- 9.Williams, L., Bradley, L., Smith, A. & Foxwell, B. (2004) J. Immunol. 172, 567-576. [DOI] [PubMed] [Google Scholar]
- 10.Lang, R., Patel, D., Morris, J. J., Rutschman, R. L. & Murray, P. J. (2002) J. Immunol. 169, 2253-2263. [DOI] [PubMed] [Google Scholar]
- 11.Han, J. H., Beutler, B. & Huez, G. (1991) Biochim. Biophys. Acta 1090, 22-28. [DOI] [PubMed] [Google Scholar]
- 12.Han, J., Huez, G. & Beutler, B. (1991) J. Immunol. 146, 1843-1848. [PubMed] [Google Scholar]
- 13.Beutler, B., Krochin, N., Milsark, I. W., Luedke, C. & Cerami, A. (1986) Science 232, 977-980. [DOI] [PubMed] [Google Scholar]
- 14.Wilson, C. J., Guglielmo, C., Moua, N. D., Tudor, M., Grosveld, G., Young, R. A. & Murray, P. J. (2001) Anal. Biochem. 296, 270-278. [DOI] [PubMed] [Google Scholar]
- 15.Lang, R., Rutschman, R. L., Greaves, D. R. & Murray, P. J. (2002) J. Immunol. 168, 3402-3411. [DOI] [PubMed] [Google Scholar]
- 16.Lang, R., Pauleau, A.-L., Parganas, E., Takahashi, Y., Mages, J., Ihle, J. N., Rutschman, R. & Murray, P. J. (2003) Nat. Immunol. 4, 546-550. [DOI] [PubMed] [Google Scholar]
- 17.Mercurio, F., Zhu, H., Murray, B. W., Shevchenko, A., Bennett, B. L., Li, J., Young, D. B., Barbosa, M., Mann, M., Manning, A. & Rao, A. (1997) Science 278, 860-866. [DOI] [PubMed] [Google Scholar]
- 18.Pauleau, A.-L., Rutschman, R., Lang, R., Pernis, A., Watowich, S. S. & Murray, P. J. (2004) J. Immunol. 172, 7565-7573. [DOI] [PubMed] [Google Scholar]
- 19.Schottelius, A. J., Mayo, M. W., Sartor, R. B. & Baldwin, A. S., Jr. (1999) J. Biol. Chem. 274, 31868-31874. [DOI] [PubMed] [Google Scholar]
- 20.Wang, P., Wu, P., Siegel, M. I., Egan, R. W. & Billah, M. M. (1995) J. Biol. Chem. 270, 9558-9563. [DOI] [PubMed] [Google Scholar]
- 21.Lentsch, A. B., Shanley, T. P., Sarma, V. & Ward, P. A. (1997) J. Clin. Invest. 100, 2443-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saccani, S., Pantano, S. & Natoli, G. (2001) J. Exp. Med. 193, 1351-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Biswas, R., Datta, S., Gupta, J. D., Novotny, M., Tebo, J. & Hamilton, T. A. (2003) J. Immunol. 170, 6202-6208. [DOI] [PubMed] [Google Scholar]
- 24.Caput, D., Beutler, B., Hartog, K., Thayer, R., Brown-Shimer, S. & Cerami, A. (1986) Proc. Natl. Acad. Sci. USA 83, 1670-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denys, A., Udalova, I. A., Smith, C., Williams, L. M., Ciesielski, C. J., Campbell, J., Andrews, C., Kwaitkowski, D. & Foxwell, B. M. (2002) J. Immunol. 168, 4837-4845. [DOI] [PubMed] [Google Scholar]
- 26.Tebo, J., Der, S., Frevel, M., Khabar, K. S., Williams, B. R. & Hamilton, T. A. (2003) J. Biol. Chem. 278, 12085-12093. [DOI] [PubMed] [Google Scholar]
- 27.Kontoyiannis, D., Pasparakis, M., Pizarro, T. T., Cominelli, F. & Kollias, G. (1999) Immunity 10, 387-398. [DOI] [PubMed] [Google Scholar]
- 28.Aste-Amezaga, M., Ma, X., Sartori, A. & Trinchieri, G. (1998) J. Immunol. 160, 5936-5944. [PubMed] [Google Scholar]
- 29.Williams, L., Jarai, G., Smith, A. & Finan, P. (2002) J. Leukocyte Biol. 72, 800-809. [PubMed] [Google Scholar]
- 30.Kuwata, H., Watanabe, Y., Miyoshi, H., Yamamoto, M., Kaisho, T., Takeda, K. & Akira, S. (2003) Blood 102, 4123-4129. [DOI] [PubMed] [Google Scholar]
- 31.Saccani, S., Marazzi, I., Beg, A. A. & Natoli, G. (2004) J. Exp. Med. 200, 107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.