Abstract
Environmental exposure to a mixture of chemical xenobiotics acts as a double-edged sword, promoting or suppressing tumorigenesis and the development of breast cancer (BC). Before anything else, we are what we eat. In this review, we highlight both “the good” and “the bad” sides of the daily human diet and dietary patterns that could influence BC risk (BCR) and incidence. Thus, regularly eating new, diversified, colorful, clean, nutrient-rich, energy-boosting, and raw food, increases apoptosis and autophagy, antioxidation, cell cycle arrest, anti-inflammation, and the immune response against BC cells. Moreover, a healthy diet could lead to a reduction in or the inhibition of genomic instability, BC cell stemness, growth, proliferation, invasion, migration, and distant metastasis. We also emphasize that, in addition to beneficial compounds, our food is more and more contaminated by chemicals with harmful effects, which interact with each other and with endogenous proteins and lipids, resulting in synergistic or antagonistic effects. Thus, a healthy and diverse diet, combined with appropriate nutritional behaviors, can exert anti-carcinogenic effects and improve treatment efficacy, BC patient outcomes, and the overall quality of life of BC patients.
Keywords: breast cancer (BC), breast cancer risk (BCR), diet, nutrition, carcinogenic molecular pathways, bioactive compounds, food contaminants
1. Introduction
BC is the most diagnosed cancer worldwide, with over 2.3 million new cases and 685,000 deaths in 2020; BC diagnosis is predicted to increase to over 3 million new cases and one million deaths by 2040 [1]. Evidence suggests that environmental exposure to certain chemicals and lifestyle account for 70% to 90% of the risk factors for chronic diseases, whereas only 10% to 30% can be explained by a specific genomic landscape [2]. Both dietary compounds and daily nutrition-related habits may play key roles in preventing diseases [3] or, on the contrary, can exert carcinogenic effects, even at low levels of exposure [4]. The expression a “double-edged sword” refers to something that has both good and bad consequences. For example, food contaminants are usually chemical substances, as well as microbial or physical compounds present in edibles, which may be harmful to human health, with different levels of severity [5]. Moreover, food contaminants are generally environmental pollutants, which are not present naturally in raw food, with contamination occurring during the production, processing, distribution, storage, packaging, transportation, or preparation of food. On the contrary, a plethora of bioactive compounds are present in “functional food”, dietary supplements, and plant or animal nutraceuticals, which are known to exert beneficial effects on health [6].
Phytochemistry, as well as various omics fields, such as nutrigenomics, nutriproteomics, metabolomics, interactomics, and exposomics [7], offer a wide range of opportunities for the development of personalized diets for women at risk of developing BC [3]. It was shown that certain dietary compounds, mainly phytonutrients, can modulate gene expression, acting on the main cancer hallmarks: cell growth and proliferation, genome instability and mutagenesis, angiogenesis, metabolism reprogramming, anti-apoptosis, tumor-promoting inflammation, therapy resistance, invasion, and metastasis [8]. Plant-based diets may contribute to the inhibition of BC cell proliferation, differentiation, invasion, metastasis, angiogenesis, and anti-apoptotic mechanisms, by targeting numerous molecular pathways and promoting beneficial effects in terms of the prevention and treatment of BC [9]. Moreover, plant-based dietary bioactive compounds can modulate hormone-induced reactions, genetic and epigenetic regulation, and promote the activation of oxidative stress (OS) and endoplasmic reticulum stress, triggering redox reactions, as well as the aggressive behavior of tumor cells caused by the production of reactive oxygen species (ROS) [10]. Many types of food rich in bioactive compounds, such as polyphenolic compounds, carotenoids, terpenoids, and sulfur-containing compounds, have been recommended as chemopreventive and chemotherapeutic agents, including asparagus, broccoli, Brussels sprouts, kale, and other cruciferous vegetables, carrots, grapefruit, soy, spinach, and tomatoes, mainly for ER-negative BC [11]. On the other hand, a plethora of risk factors, such as alcohol consumption [12], added sugar in foods [13], and LDL cholesterol [14], stimulate inflammation, BC cell proliferation, invasion, migration, and metastasis. Moreover, nowadays, more than ever, more food is processed and contaminated with man-made chemicals that act as endocrine disruptors, accumulate in high-fat tissues, and stimulate BC initiation and progression.
There are dietary patterns, such as the typical Western diet (WD), characterized by a high intake of pre-packaged foods, refined grains, red and processed/ultra-processed meat, candy, cookies, high-sugar drinks, fried foods, high-saturated fat and high-fructose products, dairy products, alcohol, salt, sugar, sweeteners, and other additives [15]. Thus, the WD has been linked to the development of excess weight/obesity [16], the alteration of human gastrointestinal microbiota [17,18], and carcinogenesis [19]. In postmenopausal obese women, the risk of developing BC is increased [20], while gut microbiota dysbiosis plays a role in the development of BC through estrogen-dependent pathways and microbial-derived metabolites [21]. Conversely, the Mediterranean diet (MD) involves a plant-based dietary pattern, based on a high intake of olive oil and plant-based foods, such as vegetables, non-refined cereals, legumes, and nuts, and a moderate or low level of consumption of dairy and meat-based products, alcohol, and sweets [22]. Consequently, the MD could reduce BCR and enhance survival through its anti-inflammatory effects, antioxidant characteristics, and hormone–receptor interactions [22]. Kalam et al. (2023) showed that diet and diet-related behaviors can modulate carcinogenesis, cancer progression, treatment efficacy, and recurrence [23]. Post-diagnosis plant-based diets can also benefit prognosis in cancer patients [24], due to the bioactive compounds that occur in small quantities in foods and that may have beneficial effects on health [25]. In this review, we highlight “the good” and “the bad” sides of the daily human diet and dietary patterns that could influence BC risk (BCR) and incidence. We conclude that a balanced and personalized dietary structure, combined with appropriate nutritional behaviors, can improve treatment efficacy and BC patient outcomes.
2. The Good
Circadian nutritional behaviors have been associated with BC [26] and, when disrupted, become factors for BCR [27]. Moreover, eating more frequently, reducing evening energy intake, and fasting for longer intervals, can downregulate the biomarkers of inflammation, as well as BCR [28]. Breakfast is the first meal of the day, often considered to be “the most important meal of the day” [29]; evidence shows that a good quality breakfast and breakfast-based nutrients are beneficial for human health, in association with a lower body mass index (BMI), a higher level of satisfaction in life, and a higher intellectual performance [30,31,32,33,34,35,36,37]. However, Elahy et al. (2023) concluded that there are no associations between the frequency of breakfast meals or after-dinner snack habits and BCR in postmenopausal women [38]. It is known that cyclical fasting or fasting-mimicking diets enhance antitumor chemotherapy effects in TNBC models [39], as well as the activity of endocrine therapeutics in mouse models of hormone receptor-positive BC [40]. A systematic review conducted by Anemoulis et al. (2023) concluded that intermittent fasting reduces chemotherapy-induced DNA damage in BC patients [41]. These nutritional patterns combined with specific therapeutic drugs reduce circulating insulin growth factor 1 (IGF1), insulin and leptin, and inhibit AKT/mTOR signaling through the upregulation of early growth response protein 1 (EGR1) and the phosphatase and tensin homolog deleted on chromosome 10 (PTEN), thus promoting long-lasting tumor regression and reverting the acquired resistance to chemotherapy [40]. Thus, intermittent fasting, based on different patterns of time-restricted feeding behaviors, attenuates obesity-induced TNBC progression in cell and animal models, involving multiple pathways, such as those involved in EMT reduction, cell cycle disruption, reductions in systemic glucose and cholesterol levels, and the downregulation of inflammatory factors in the TME [42]. A multicase control study in Spain showed that having breakfast at a later time of day was associated with a non-significant increase in BCR [26].
Among the diverse dietary patterns, the Mediterranean diet, rich in antioxidants and anti-inflammatory compounds; the plant-based dietary pattern, rich in fibers, phytochemicals, lignans, carotenoids, vitamins C and E, folate, and phenolic acid; the prudent dietary pattern, rich in spices, plant-based oils, low-fat dairy, and seafood; the healthy dietary pattern, rich in fruits, vegetables, legumes, seeds, and nuts; the ketogenic dietary pattern, known as a high-fat and low-carb diet; the paleolithic dietary pattern, rich in vegetables, fruits, lean meats, fish, nuts, and seeds; and the dietary approaches used to stop hypertension, were all correlated with a lower risk of developing BC [43]. Diverse breakfast patterns have been geographically identified and are based on cereal or sweetened breads and milk, eggs, sweetened beverages, sandwiches, and fruit consumption [30]. In Romania, traditional food products are much more popular than foods purchased from chain stores [44], because local consumers are attracted by traditional and authentic gastronomy [45]. Moreover, the main basic food products consumed are meat and meat products traditionally based on pork/chicken, such as pork crackling, pork fat, grilled pork neck, pork loin, smoked pork knuckle, grilled meat rolls, cabbage rolls with ground pork and rice, chicken liver, deep-fried chicken, pastrami, and sausages, milk and/or milk products (sour cream, cheese), vegetables (fries, beans, mushrooms, smashed potatoes, cabbage, pickles, garlic), and fruits, bread/bakery and pastry products (mamaliga/polenta, noodles, ice cream, fried cheese doughnuts), and fish (mainly fried crap) or fish products [44]. Fast food consumption is more popular among children and adolescents [44]. There are studies that have suggested that certain foods are consumed in excess (products high saturated fat, cholesterol, salt, sugar, refined grains, and alcohol), while there is a deficiency in regard to the intake of nutritional factors (essential amino acids, polyunsaturated fatty acids, vitamins C, A, B, D, folic acid, calcium, and iron) [46].
These meals contain complex mixtures of natural and added chemical compounds that can exert double-edged sword effects on carcinogenic molecular pathways in breast tissue. For example, milk is a complex biofluid, rich in primary nutrients and is considered a complete and basic food that is consumed by billions of people worldwide [47]. Thus, pure cow’s milk and its derivative dairy products, such as cream, butter, cheese, and yogurt are a major sources of nutrition in the human diet [48]. Wajszczyk et al. (2021) showed that individual dairy products have a statistically significant, but bi-directional relationship with BCR, which was different for premenopausal and postmenopausal Polish women [49]. A cohort study conducted by these authors concluded that an increase in consumption of one serving of dairy products/week may significantly decrease the BCR, by 2%, for premenopausal women only, while cottage cheese consumption significantly reduced the BCR by 20%, following an increase of one serving/week, for postmenopausal women only [49]. A 5% decrease in BCR has been observed as a result of an increase in dairy consumption of one glass of milk/week in both strata of women during the menopause [49]. Arafat et al. (2023), after performing a systematic literature review, also concluded that dairy consumption was inversely associated with BCR, even when the effects of different types of dairy products and the dose–response relationship on BCR remain unknown [50]. More recently, Riseberg et al. (2024) showed that the overall amount of dairy consumption was not associated with BCR, but the results can vary according to the tumor subtype, and heterogeneity was observed in terms of the type of dairy food and the patient’s period of life [51]. Deschasaux-Tanguy et al. (2022) found no association between the consumption of dairy products or dairy desserts high in sugar and BCR [52].
Almost 70% of the fat in milk is saturated, 25% is monounsaturated, and 2.3% is polyunsaturated, with a variable omega-6/omega-3 ratio [53]. Moreover, α-linolenic acid (ALA), an omega-3 fatty acid, induces apoptosis and inhibits invasion, metastasis and angiogenesis, and arrests the cell cycle in human BC cells by inhibiting fatty acid synthase (FASN), which is usually overexpressed in various cancers [54,55]. Conjugated linoleic acid (CLA) is known as a group of isomers of linoleic acid (LA), of which cis-9 trans-11 (c9,t11-CLA) is the one that has the highest percentage, being produced through biohydrogenation by lactic acid bacteria in the rumen or by endogenous synthesis in the mammary gland [56]. Consequently, CLA is present in the milk, dairy, and meat products of ruminants, with dairy products being the principal source of CLA in the human diet [57]. Moreover, 3 g/day of CLA is the recommended intake, with beneficial effects on human health [58], in principal due to the antitumor effects of CLA [56]. Zeng et al. (2019) demonstrated that dietary intake of c9,t11-CLA enriched from butter reduces BC progression in vivo via the downregulation of the progesterone receptor (PR) and Ki-67 expression [57]. Milk proteins, which include 17–20% whey proteins (α-lactalbumin, β-lactoglobulin, glycoproteins, lactoferrin, immunoglobulins, peptide hormones, enzymes like lactoperoxidase, and serum albumin) and 78–80% casein (αs-1, αs-2, β-, and k-casein), as well as milk peptides, exert many biological proprieties, such as antibacterial, antiviral, antifungal, and antioxidant activities [59]. Using cow’s milk bottom-up proteomics, based on combinatorial SDS-PAGE profiling and trypsin digestion, followed by nanoHPLC-electrospray ionization tandem mass spectrometry (nLC-ESI-MS/MS), Vincent et al. (2016) identified 186 different major and minor proteins, including β2-microglobulin (β2-M), osteopontin (OPN), lipoprotein lipase (LPL), sulfhydryl oxidase (SOX), xanthine dehydrogenase/oxidase (XOR), β-1,4-galactosyltransferase 1 (Gal-T1), lactadherin/milk fat globule-EGF factor 8 (MFG-E8), lactotransferrin, mucins 1 and 15, α-1-acid glycoprotein, α-1B-glycoprotein, α-2-HS-glycoprotein, pancreatic secretory granule membrane major glycoprotein GP2, platelet glycoprotein 4, Zn-α-2-glycoprotein, milk glycosylation-dependent cell adhesion molecule 1/lactophorin (GlyCAM1), dystroglycan (DG), and peptidoglycan recognition protein 1 [48]. Some reports suggest that OPN may exhibit antitumorigenic characteristics in certain circumstances [60]. Orally administrated lactoferrin (LF), a natural proapoptotic iron-binding multifunctional glycoprotein from bovine milk, belonging to the transferrin family, can exert strong anticancer activities [61]. Pereira et al. (2016) showed that bovine LF preferentially induces apoptosis in the highly metastatic BC cell lines, Hs 578T and MDA-MB-231, but not in the less metastatic T47D or in the non-tumorigenic MCF10A cell lines [62]. The authors demonstrated that LF decreases the extracellular acidification rate and causes intracellular acidification in metastatic BC cells through the inhibition of plasmalemmal V-H+-ATPase, which transports protons across cellular membranes [62].
Milk is also an abundant source of extracellular vesicles (EVs); bovine milk-derived extracellular vesicles (MEVs) are able to sensitize TNBC cells into doxorubicin by targeting metabolism and STAT signaling pathways, thus reducing the abundance of many tumorigenic proteins associated with a worse prognosis and low overall survival in TNBC [63]. Samuel et al. (2021) showed that orally administrated MEVs survive the degrading conditions in the mouse gut and can be detected in various organs, while MEVs implanted in BC cells reduced the primary tumor burden, but accelerated metastasis in BC mouse models through the induction of EMT, providing context-based and opposing roles of MEVs as metastasis promoters and suppressors [64]. Ramezani et al. (2023) showed that bovine milk lactoferrin-loaded exosomes induce selective toxicity against BC cells compared to normal cells and that incorporating lactoferrin into exosomes could have an antitumorigenic role through inducing the overexpression of the proapoptotic BH3 interacting domain death agonist (BID) protein and diminishing the expression of the anti-apoptotic protein Bcl2 in the human MDA-MB-231 BC cell line, following exoLF treatment [65]. Shariatikia et al. (2017) showed that mare, donkey, cow, and camel milk, and their casein and whey proteins, have potent cytotoxic effects against the MCF7 BC cell line in a dose-dependent manner, while sheep and goat milk and their proteins did not exert any cytotoxic activity [59].
A comprehensive meta-analysis of prospective cohort studies concluded that fermented milk-derived products, including yogurt and sour milk, were associated with lower cancer mortalities [66]. However, another meta-analysis conducted by Chen et al. (2019) concluded that low-fat/skimmed milk, whole milk, and yogurt intake had no effect on BCR [67]. To sustain this idea, a pooled analysis of 21 cohort studies conducted by Wu et al. (2021) showed that dietary calcium consumption was not clearly associated with BCR, but higher yogurt and cottage/ricotta cheese intake were found to be inversely correlated with the risk of ER-negative BC [68]. Cheese is one of the fermented dairy products rich in proteins, minerals, organic acids, bioactive peptides, oligosaccharides, and vitamins, and also contains diverse non-pathogenic microorganisms that act as probiotics [69]. Kamal and Talib (2023) showed that a combination of the ketogenic diet, which is high in fat, low in carbohydrates, and sufficient in terms of proteins, and probiotics inhibits BC in mice through the downregulation of IGF-1 and immune system modulation [70]. Ryser et al. (2022) showed that a gram-negative bacterium, Morganella morganii, isolated from the outer layers of raclette-type cheese, influences the formation of biogenic amines in cheese, such as cadaverine and putrescine, which is undesirable, since their consumption can cause intoxication [71]. Interestingly, treatment of BC cell lines with cadaverine according to its serum reference range reverted ETM, decreased cell motility and invasion, and inhibited cell stemness by reducing mitochondrial oxidation [72]. Moreover, Ritota et al. (2022) emphasized that bioactive compounds, such as picrocrocin/safranal and crocin, from cow and ewe cheeses made with saffron, usually used to add a natural yellow to orange color to cheeses, exert antiproliferative effects in regard to MDA-MB-231 BC cells [73].
Nondairy/plant-based substitutes for cow’s milk and its derivatives, manufactured from soy, rice, coconut, and almonds, have gained in popularity in recent years, with adoption of vegetarian and vegan diets [74]. In the USA, the proportion of individuals reporting that they consumed soy milk was 1.54% in 2017–2020 [75]. Moreover, non-Hispanic Asian and Black ethnicities have significantly increased the consumption of soy milk [75]. Soy milk and its derivatives contain many natural isoflavones that are similar to estrogen hormones, known as phytoestrogens [76], so the beneficial effects of soy food intake remain controversial [77]. There is a hypothesis that suggests that soy isoflavones, genistein and daidzein, may stimulate the proliferation of ER+ BC cells, even at low concentrations [77]. However, genistein, a natural isoflavonoid, is also considered to be a potent anti-BC agent, usually present in high quantities in soybeans, inducing antiproliferative effects/arrest of the cell cycle and apoptosis, and preventing tumor angiogenesis [78]. In different brands of commercially available soy milk, the mean genistein content is 17.58 ± 8.38 µg/mL [76]. At high concentrations, genistein kills MCF7 BC cells [79] or delays TNBC tumor growth [80], inhibiting proliferation/differentiation and inducing apoptosis [81,82]. Genistein also inhibits angiogenesis [83], induces tamoxifen resistance and growth in ER+/HER2+ BC cells, and inhibits the growth of ER-/HER2+ BC cells [84]. At the molecular level, genistein suppresses the IGF-1R/p-AKT signalling pathway; decreases the Bcl-2/Bax ratio [81]; downregulates the NF-κB/Bcl-xL/TAp63 signaling pathway; induces the modification of key epigenetic cancer-associated genes, their enzymatic activities, genomic DNA, and histone methylation [80]; upregulates PI3K and MAPK signalling; and downregulates p27Kip1 levels in ER+/HER2+ BC cells [84]. In addition, daidzein, an isoflavone from fruits, nuts, soy beans, and soy-based products [85], inhibits BC cell proliferation, induces apoptosis [85,86], and inhibits TNF-α-induced migration and invasion [87]. The antineoplastic effects of daidzein are mediated by cell cycle arrest, the inhibition of cyclin D and CDK2/4, and increases in p21Cip1, p57Kip2 expression, and caspase-9 activity [85]. Moreover, daidzein generates ROS, disrupts mitochondrial function, decreases cyclin-D expression [86], inhibits TNF-α, suppresses Hedgehog/GLI1 signalling [87], upregulates Bax, downregulates Bcl-2, induces apoptosis, lowers the ERα/β ratio, and increases ROS production [88].
Meat has been a part of the human diet since the early stages of our existence, with archeological findings suggesting that food was cooked for the first time around 300,000–400,000 years ago (some 7000–14,000 generations ago) [89]. Even if meat consumption is correlated with an increased BCR, Lo et al. (2019) showed that poultry consumption may be associated with reduced risk, so substituting poultry for red meat could decrease BCR [90].
Grains are one of the most important foods consumed worldwide that contain bioactive compounds, mainly found in whole grain cereals, such as wheat, rye, oats, and barley [91,92]. A meta-analysis conducted by Xiao et al. (2018) concluded that high intake of whole grains might be inversely associated with BCR in case-control studies, but not in cohort studies [91]. However, carbohydrate-based foods with high glycemic index may influence BCR via the insulin growth factor axis [93]. The contamination of cereals and cereals-based products with mycotoxins, such as aflatoxins, has been associated with mutagenesis and carcinogenesis [94,95]. Aljazzar et al. (2022) showed that aflatoxin B1 (AFB1) causes significant toxicity in regard to MCF7 BC cells through oxidative stress (OS), as well as transcriptomic alterations in regard to drug-metabolizing enzymes, transporters, and antioxidant enzymes as a result of in xenobiotics [95]. Moreover, AFB1 upregulates pro-inflammatory markers, such as tumor necrosis factor alpha (TNFα), cytokine, and cyclooxygenase-2, correlated with the reduction in the mRNA expression of immunity-related genes, including interleukins 8 and 10 [95]. In BC, TNFα has a pro-tumorigenic role and correlates with increased proliferation, a higher malignancy grade, metastasis, and poor prognosis [96,97], due to the ability of TNFα to upregulate TAZ, a transcriptional co-activator that promotes BCSC self-renewal in human BC cell lines [98]. Dietary baker’s yeast, commonly used in baking bread and other bakery products, can enhance the apoptotic ability of paclitaxel against the human MCF7 BC cell line and the metastatic murine 4T1 cell line [99]. A systematic review conducted by Grudzinska et al. (2023) emphasized that dietary sprouts may play a role in the chemoprevention of BC [100]. Thus, germinated wheat flour reduces the growth of MCF7 and MDA-MB-231 human BC cell lines, upregulating apoptosis [101].
Eggs are also frequently consumed worldwide as a nutrient-rich food, due to their protein and peptide content [102]. Due to advancements in MS-based proteomics, 167 proteins were identified in the egg white proteome, which varies based upon the storage of the eggs at different temperatures over different time spans, early embryonic development, egg varieties, and stress conditions [103]. Ovalbumin and ovotransferrin have been detected as major egg proteins responsible for multiple bioactivities [103,104]. Thus, the native ovotransferrin (OTRF/OTF)/conalbumin, an iron-binding glycoprotein from the transferrin protein family, present both in avian plasma and egg white [105], is known to have anticancer activities, with negative effects on the proliferation of MCF7 BC cells by inducing apoptosis [104,106].
Nutritional supplements are also consumed at breakfast time [30]. For example, bee pollen is an excellent dietary supplement in regard to human nutrition [107]. Pollen, also known as bee bread (BB), contains various bioactive polyphenolic compounds, such as isoflavonoids (genistein, daidzein, glycitein, biochanin A, formononetin, puerarin, coumestrol, and equol) and flavonoids, like quercetin, kaempferol, apigenin, luteolin, myricetin, hesperetin, rutin, naringenin, catechin, epicatechin, epigallocatechin, and proanthocyanidins [108]. Genistein is the major isoflavone identified in bee pollen [109], interfering with several biological processes, pathways, and genes/proteins, including PTEN, PI3K, PIP3, AKT, mTOR, Bcl-2 Bax, caspase-3, cyclin B, VEGF, HIF1α, p21, and p16, EGFR/AKT/NF-κB, DNA methylation, the ER pathway, and MMP genes [78]. At a concentration of 370 µM, genistein revealed a cytotoxic effect on MCF7, T47D, and MDA-MB-231 BC cell lines, while at a concentration of 0.37 µM, no significant effect on BC cell viability was observed [110]. Synergically, doxorubicin (DOX), cisplatin, and BB, blocked the migration of MDA-MB-231 cells and suppressed the proapoptotic BID gene, overexpressing the anti-apoptotic Bcl-2 gene, reducing the toxicity of chemotherapeutic drugs on this TNBC cell line [111]. Another study found that the synergistic effect of BB with DOX led to the suppression of the proliferation of breast tumors in 4T1 tumor-bearing BALB/C mice and inhibited the oxidative damage of DOX, increasing the expression of apoptotic genes and proteins, the p53 level, as well as serum interferon-γ (IFN-γ), and reducing the estrogen level, Ki-67 and Bcl-2 proliferation biomarkers, nitric oxide, and pro-inflammatory cytokines [108].
Leafy vegetables can be effective in terms of “green chemoprevention” and treating BC [112]. Moreover, broccoli and broccoli sprouts contain many active biochemicals, such as sulforaphane (SFN), a natural organosulfur compound known to counteract the tumorigenic effects of chemical xenobiotics in food and the environment [112]. Kaboli et al. (2020) concluded that SFN reduces NF-κB activity, downregulates apoptosis inhibitors, decreases the activity of histone deacetylases leading to cell cycle arrest, as well as increases the sensitivity of BC cells to chemotherapy [113]. Moreover, SFN is involved in the modulation of gene expression and nuclear factor-erythroid factor 2-related factor 2 (NRF2) antioxidant signaling [113]. Wu et al. (2019) indicated that NRF2 signaling has a double-edged sword effect in regard to cell survival, because the NRF2/KEAP1 pathway exerts anticancer activities, but also activates pro-survival genes and promotes cancer cell proliferation [114]. Moreover, Surh (2021) emphasized that SFN blocks the T cell-mediated immune response necessary for tumor immune surveillance [115]. Recently, Zhang et al. (2022) showed that SFN derived from broccoli, kale, cabbage, cauliflower, garden cress, and mustard [112,113], suppresses the metastasis of TNBC cells by targeting the RAF/MEK/ERK pathway to inhibit the formation of actin stress fibers and TGF-β1-induced BC cell migration, invasion, and metastasis [116].
Evidence suggests that consuming a high amount of onions (Allium cepa) and garlic (Allium sativum) is protective against BC [117]. Allicin, the major active biocompound present in freshly crushed garlic [118], is also a bioactive organo-sulfur compound [119], able to induce cell cycle arrest and apoptosis in MCF7 and MDA-MB-231 BC cell lines through tumor-suppressor p53 signaling pathway activation [120]. Moreover, Shi et al. (2024) showed that a combinatorial treatment of allicin with doxorubicin (DOX) resulted in better effects in regard to inhibiting proliferation and increasing the apoptosis of MCF7 and MDA-MB-231 DOX-resistant BC cells, than the treatment with DOX or allicin alone [118]. Thus, allicin inactivates the NRF2/HO-1 signaling pathway and improves the DOX sensitivity of BC cells [118]. Onion contains allicin, quercetin, fisetin, and other organo-sulfur compounds, such as diallyl disulfide and diallyl trisulfide [121]. Onion, one of the most popular vegetables in the world, is a major source of quercetin [122,123], a flavonoid abundantly found in plants, vegetables, and fruits, mainly in cruciferous vegetables, grapes, apples, tomatoes, and blueberries [124]. Quercetin inhibits the proliferation, migration, and invasion of 4T1 BC cells, suppressing the IL-6/JAK2/STAT3 signaling pathway and promoting the cytotoxicity of tumor immune cells in the TME [125]. Moreover, quercetin induces apoptosis and suppresses cell proliferation in MCF7 and MDA-MB-231 BC cells, changing endonuclease-G (Endo-G) and the expression of caspases 3/8/9 [124].
It was also shown that lycopene has anticancer activities and that it could be considered as a potentially effective compound in BC prevention and treatment [126,127]. The regulation of oxidative and inflammatory processes, angiogenesis, the induction of apoptosis, the inhibition of cell proliferation and metastasis formation, as well as the modulation of gap junctional intercellular communication, growth factors, and signal transduction pathways, have been the most cited mechanisms in terms of lycopene action [127,128]. Lycopene is a red-colored carotenoid pigment found in tomatoes and tomatoes-based products, red fruits, red carrots, watermelons, red grapefruits, papayas, and apricots, which is known to enhance protection against cancer [127]. Takeshima et al. (2014) showed that lycopene induces ERK1/2 activation, cyclin D1 suppression, and p21 upregulation in MCF7 (ER/PR positive), SK-BR-3 (HER2+), and MDA-MB-468 (TNBC) cell lines [129]. Lycopene inhibits AKT phosphorylation and mTOR in TNBC cells, leading to the upregulation of proapoptotic Bax [129]. Unfortunately, chlorpyrifos (CPF) is an organophosphate insecticide extensively used in the production of tomatoes [130]. Ventura et al. (2019) demonstrated that the concentration of this xenobiotic in the environment alters mammary histology and the hormonal balance in chronically exposed rats, acting as a BCR factor [131]. These authors emphasized that CPF alters HDAC1 mRNA expression, which promotes mammary tumor development [131]. Moreover, CPF acts as an endocrine disruptor that promotes migration, invasion, and the stemness phenotype in 3D cultures of MCF7 and MDA-MB-231 BC cell lines and induces the activation of many BC-related pathways, such as EMT [132].
Resveratrol is a natural dietary polyphenol that affects the expression of several cytokines, caspases, MMPs, adhesion molecules, and growth factors. It modulates the activity of several signalling pathways, such as PI3K/AKT, NF-κB, and Notch, which play crucial roles in carcinogenesis [133,134]. Moreover, resveratrol induces Bax-dependent, but p53-independent, apoptosis in MDA-MB-231 BC cells [135]. Resveratrol exists in 70 types of plants and can inhibit the migration and metastasis of MDA-MB-231 human BC cells by reversing transforming growth factor (TGF)-β1-induced EMT [136]. In vitro, resveratrol can decrease the expression levels of MMP2 and MMP9, fibronectin, α-SMA, P-PI3K, SMAD2, SMAD3, P-SMAD2, P-SMAD3, vimentin, Snail1, and Slug, increasing the expression levels of E-cadherin [136]. In vivo, resveratrol inhibits lung metastasis in mice bearing MDA-MB-231 human BC xenografts [136].
Curcumin is a polyphenol derived from turmeric (Curcuma longa) that inhibits breast cancer stem cell (BCSC) properties and cell proliferation and promotes apoptosis in the MCF7 BC cell line [137,138]. This polyphenol inhibits the proliferation of BCSCs through the modulation of several signaling pathways, such as NF-kB signaling, which is known as an important curcumin-regulated pathway [139]. NF-kB signaling is involved in the maintenance of a variety of stem cells [140], including BCSCs, which overexpress components of the NF-kB signaling pathway and have high NF-kB activity levels [141]. Moreover, sonic hedgehog (Shh) and WNT/β-catenin signalling pathways are also crucial in maintaining the stemness of BCSCs, with curcumin decreasing the activity of BCSCs by inhibiting tumor sphere formation and decreasing BCSC biomarkers, such as CD44, ALDHA1, Nanog, OCT4, and SOX2, thus downregulating both the Shh and WNT/β-catenin signalling pathway activities, which results in BCSC inhibition [142]. In addition, nuclear factor erythroid 2-related factor 2 (NRF2) is known to regulate oxidative stress, being involved in the development of cancer stem cells and metastasis [143]. Many authors have demonstrated or reviewed how curcumin activates the NRF2 signaling pathway, inducing cellular protection against oxidative injury [144,145]. Moreover, curcumin has very little toxicity in terms of normal stem cells, but has numerous cytotoxic effects on CSCs, due to the suppression of IL-6, IL-8, and IL-1, which stimulate CSCs [146]. Overall, curcumin could function as a cytotoxic and anti-metastasis agent for BC [142]. These chemopreventive, anticancer, and cytotoxic proprieties are also modulated through the downregulation of oncogenic RAF-1, the suppression of telomerase, and the upregulation of TNF-α and IL-8 genes [138]. In MDA-MB-231 and Hs578T TNBC cells, curcumin inhibits motility and migration, downregulating the expression of the proteins involved in EMT, such as the mTOR and PI3K/AKT signalling pathways [147]. Curcumin also downregulates the mRNA expression of vimentin, fibronectin, and β-catenin, and upregulates E-cadherin mRNA expression levels [148].
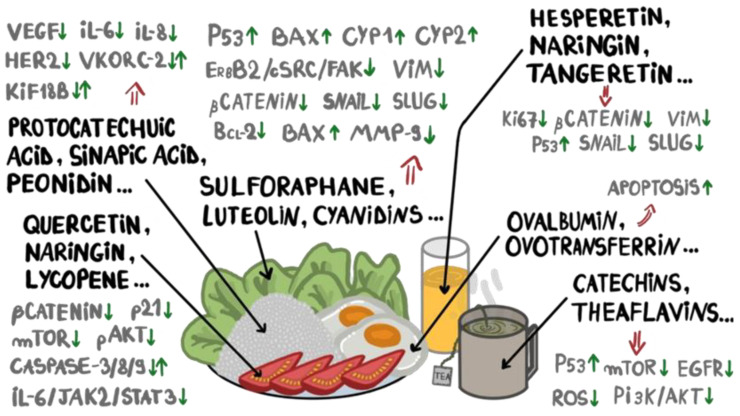
Black and green tea can have chemopreventive effects on BC development, with some authors suggesting that women with a family history of BC should drink about five cups of tea per day in order to decrease the BCR [149]. Flavan-3-ols are a subclass of flavonoids, consisting of polyphenolic phytochemicals found in a wide variety of food sources, especially in fruits and teas [150,151,152,153]. Catechins and theaflavins are both flavan-3-ols with significant bioactive properties, including anticancer, anti-mutagenic, antioxidative, and anti-inflammatory effects [154]. The beneficial effects, at the molecular level, of bioactive compounds that exert anti-BC potential are listed in Figure 1 and Table 1.
Figure 1.
Biomarkers and biological pathways involved in breast cancer initiation and progression, targeted by bioactive compounds present in a daily, diverse diet.
Table 1.
Potential anticarcinogenic roles of phytochemicals in regard to breast cancer.
| Bioactive Dietary Compounds | Food Sources | In Vitro and In Vivo Models | Effects on Molecular Biomarkers/Signaling Pathways | Effects on Biopathological Processes | Role in BC |
|---|---|---|---|---|---|
| Sulforaphane | Organo-sulfur compound obtained from broccoli/broccoli sprouts, kale, cabbage, cauliflower, garden cress, mustard [112,113] | MDA-MB-231 and MDA-MB-157 [116] | Targets MAPK/ERK [116]; downregulates NF-κB, AKT, and KEAP1; affects histone deacetylases involved in chromatin remodeling, and NRF2 antioxidant signaling [113] | Inhibits cell proliferation; causes apoptosis and cell cycle arrest; has antioxidant activities [113]; suppresses TGF-β1-induced migration, invasion, and metastasis of TNBC cells [116] | Chemoprotective [116], putative potential for BC treatment [113] |
| Allicin | Organo-sulfur compound from garlic (Allium sativum) [119] | MCF7 and HCC-70 [155], MCF7 and MDA-MB-231 [120] | Downregulates caspase 3/8/9 and Bcl-XL; upregulates NOXA, p21, and BAK expression [155]; induces p53 activation [120] | Decreases BC cell proliferation and viability and increases apoptosis, induces cell cycle arrest [120,155], improves DOX sensitivity [118] | Antitumor [155] |
| Quercetin | Flavonoid from fruits, vegetables (cruciferous vegetables, grapes, apples, tomatoes, blueberries), and herbal products (Hypericum perforatum, Sambucus nigra) [156] | 4T1 and xenograft mouse model [125]; MCF-7 and MDA-MB-231 [124] | Suppresses IL-6/JAK2/STAT3 pathway [125], modulates the expression of caspase-3/8/9 [124] | Suppresses TNBC progression (proliferation, migration, and invasion) [125], induces apoptosis [124] | Potential anti-BC agent [124]; potential adjuvant for immune therapy in TNBC [125] |
| Luteolin/luteolol/ digitoflavone |
Flavonoid from carrots, broccoli, celery, perilla mint leaves and seeds, apple skin, cabbages, parsley, onion leaves, thyme [157,158,159] | MDA-MB-231, BT549, and mouse model [157]; MDA-MB-231, MDA-MB-486, 4T1, BT549 [158]; MDA-MB-453 and MCF7 [160] |
Reverses EMT through the suppression of β-catenin and VIM; stimulates E-cadherin and claudin; downregulates N-cadherin, Snail, and Slug; reorganizes F-actin [157]; inactivates AKT/mTOR and downregulates MMP9 through H3K27Ac and H3K56Ac [158]; upregulates miR-203, inhibits Ras/Raf/MEK/ERK, downregulates Bcl-2, upregulates Bax, impedes TGFβ1-induced EMT, decreases VIM, ZEB1, and N-cadherin, and increases E-cadherin [160] |
Inhibits migration and invasion of TNBC cells [157], inhibits proliferation and metastasis, and promotes apoptosis of AR+ TNBC cells [158] | Chemopreventive and potential therapeutic agent for TNBC [157], including AR+ TNBC [158] |
| Hesperetin | Flavanone glycoside from Citrus fruits (oranges and lemons) [161] | MDA-MB-231 [161]; MCF7, including mammospheres [162,163,164] | Inhibits the Fyn/paxillin/RhoA signalling pathway [161]; activates the ASK1/JNK pathway, initiates the accumulation of ROS [162]; modulates the expression of p53, PPARG, and Notch1 [163]; downregulates CDK2/4 and cyclins, and upregulates p21Cip1 and p27Kip1, stimulates the binding of CDK4 to p21Cip1 [164] |
Inhibits the migration and invasion induced by TGF-β1 [161]; exerts cytotoxic and proapoptotic effects [162]; inhibits BCSCs, exerts cytotoxicity on mammospheres, inhibits mammospheres, colony formation and migration, modulates cell cycle and induces apoptosis [163]; suppresses proliferation and stops the cell cycle in G1 [164] |
Potential anti-BC agent, especially for TNBC [161] |
| Hesperidin | Flavanone from citrus fruits [165] | MCF7 cells [165], MDA-MB-231 [166], mammospheres [167], MDA-MB-231 [168], Wistar rats [169] |
Suppresses AKT and NF-kB signalling, inhibits PD-L1 [166], increases p53 [167], binds to MCL-1 receptor [168], attenuates Ki67 [169] | Suppresses cell proliferation [165], inhibits cell migration and growth [166], suppresses mammospheres and colony formation, induces apoptosis [167], exerts cytotoxic effects [168] |
Anti-BC activity [165,167], protective against DMBA-induced BC [169] |
| Naringenin | Flavanone glycoside from grapefruits, apples, onions, tea [170,171] | MDA-MB-231, Wistar rats induced with BC by DMBA [170], C57BL/6J mice induced with BC through a transplant of E0771 cells [172], Balb/c mice induced with BC through a transplant of transduced 4T1-Luc2 cells [173], MCF-7 [174] |
Modulates mitochondrial-mediated pathway, upregulates caspase-3/7 [170], increases AMPK, decreases cyclin D1 [172], inhibits PKC, inhibits secretion of TGF-β1, causing its intracellular accumulation [173], inhibits PI3K and MAPK [174] |
Inhibits cell proliferation and cell cycle, induces apoptosis, reduces the incidence of BC tumors [170], decreases cell viability in vivo, suppresses cell cycle progression [172], inhibits lung metastasis, increasing the survival rates of the mice [172], suppresses proliferation, impairs glucose uptake [174] |
Antineoplastic agent [170], putative therapeutic option for TGF-β1 modulation [173], antiproliferative agent [174] |
| Naringin | Flavanone glycoside from tomatoes, grapefruits, and other Citrus fruits [175] | MDA-MB-231, MDA-MB-468, BT-549 [175] | Increases p21, decreases survivin/BIRC5, suppresses β-catenin pathway [176] | Inhibits cell proliferation, stimulates apoptosis [176] | Potential treatment agent for BC [176] |
| Apigenin | Flavone from parsley, onions, chamomile, oranges, wheat sprouts [177], celery, green peppers [178], thyme [179] | MDA-MB-453 and BT-474 [177], SK-BR-3 [177,178], MCF-7 [177,180], HBL-100 [177], MCF7-T, MCF7-F [179], MDA-MB-231, A549, SK-Hep1, nude mice [181] | Depletes HER2/neu and disrupts HER2/neu-GRP94 complex [177], modulates CDK1, p21Cip1, and p53 [178], induces degradation of ERα and AIB1 [179], blocks PI3K/AKT pathway [177,181] and β4 integrin function, inhibits pAKT, inhibits cell motility, migration, and invasion [181], inhibits AKT/FOXM pathway, suppresses FOXM1, and modulates ER signalling [180] | Suppresses BC cell growth, induces apoptosis [177], inhibits proliferation [180], ref. [177], activates p53-induced apoptosis [177], inhibits growth of ERα+ BC cells [179], inhibits metastasis [181] | Potential anticancer treatment [177] |
| Tangeretin | Flavone from lemons, oranges [182], other Citrus fruits [183] | MCF7, MDA-MB-468, MDA-MB-231, nude mice injected with MDA-MB-231 cells [182,184], Sprague-Dawley rats induced with DMBA [185], Wistar rats induced with DMBA [183] | Inhibits STAT3 and SOX2 pathways, decreases STAT3-DNA binding, reduces STAT3 in BCSCs [182], induces CYP1A1/CYP1B1 activity [184], decreases ROS and pro-inflammatory factors, protects against LPO [185], upregulates p53/p21, suppresses MMP2/9 and VEGF, reduces PCNA and COX2 [183] | Inhibits proliferation [182,183,184] and metastasis [183], inhibits BCSC formation, induces apoptosis, inhibits mammospheres and colony formation [182], decreases tumorigenicity and OS levels, boosts antioxidant levels [185] | Anti-BC effects |
| Daidzein | Isoflavone from fruits, nuts, soy beans and soy-based products [85] | MCF-7 [85,86,186], MDA-MB-453 [85], T47D [186], MCF-10DCIS [87] |
Induces cell cycle arrest, inhibits cyclin D, CDK2/4, increases p21Cip1 and p57Kip2 expression, increases caspase-9 activity [85]; generates ROS, disrupts mitochondrial function [86]; inhibits TNF-α and suppresses hedgehog/Gli1 signalling [87]; upregulates Bax and downregulates Bcl-2, induces apoptosis and lowers ERα/β ratio and ROS outbursts [88] |
Inhibits cell proliferation, induces apoptosis [85,86]; inhibits migration and invasion [87] | Anti-BC potential [88] |
| Genistein | Phytoestrogenic soy (Glycine max)-derived compound [81] from soy nuts, soy powder, soy milk, tofu, miso, natto [187], lupin, fava beans, kudzu, and psoralea [80]; exerts tyrosine kinase-modulating activities [84] |
MCF-7 [79,81,82]; MCF7 and MDA-MB-435 transfected with human HER2 [84]; PDX mouse models for TNBC [80] | Suppresses IGF-1R/p-AKT and decreases Bcl-2/Bax [81]; downregulates NF-κB/Bcl-xL/TAp63, influences key epigenetic associated genes, genomic DNA, and histone methylation [80]; upregulates PI3K and MAPK signalling, downregulates p27 Kip1 levels in ER+/HER2+ BC cells [84] | High concentrations kill MCF7 BC cells [79] or delay TNBC tumor growth [80]; inhibits proliferation/differentiation, induces apoptosis [81,82]; inhibits angiogenesis [83]; induces tamoxifen resistance and growth in ER+/HER2+ BC cells and inhibits growth of ER-/HER2+ BC cells [84] | Exhibits anticancer effects on various cancers [188]; chemoprevention in terms of BC carcinogenesis is concentration-, exposure time-, and BC subtype-dependent |
| Genistin | A glucoside form of genistein, readily absorbed in the intestine, found in soy beans and soy-derived foods, some legumes, and vegetables [187,189] |
MCF-7, MDA-MB-231 [189] |
Docks to ERα, ERβ, lowers CA 15-3 levels [190]; induces negative regulation of ERα signalling pathway, suppresses expression of oncogenic biomarkers [189] | Stimulates cell cycle arrest and apoptosis, reduces BC cell growth, proliferation, and angiogenesis [189] | Chemoprevention and therapy in terms of ER+ BCs [189]; useful for potential new drug discovery for BC management and treatment [190] |
| Lycopene | Major carotenoid found in tomatoes, red fruits, red carrots, watermelons, grapefruits, papayas [127] | MCF7, SK-BR-3, MDA-MB-468 [129] |
Inhibits pAKT and mTOR signalling pathways, upregulates Bax [129] | Inhibits cell proliferation and cell cycle progression, initiates apoptosis [128] | Chemopreventive for TNBC [129] |
| Gallic acid | Hydroxybenzoic acid in fruits, vegetables, medicinal plants, such as grapes, gallnuts, pomegranates, hawthorn, tea leaves, capers [191,192,193], honey [194] | MCF7 [191], HCC1806 [195], MDA-MB-231 [196] | Suppresses PI3K/AKT/EGFR, nuclear accumulation of β-catenin [191,195], activates mitochondrial apoptosis pathways [195] | Inhibits survival of acidity-adapted BC cells and reduces metastatic characteristics induced by acidity [191], suppresses proliferation, promotes apoptosis [195] and ferroptosis [196] | Promising therapeutic agent for metastatic BC [191], antioxidant [193], suppresses TNBC progression [195] |
| Vanillic acid | Hydroxybenzoic acid in medicinal plants (e.g., Angelica sinensis), olives, cereals, whole grains, fruits, green tea, juices, berries, wines [197] | MCF7 [198] | Affects ROS pathway [198] | Generates ROS, promotes apoptosis [198] | Antiproliferative effects [198] |
| Protocatechuic acid | Hydroxybenzoic acid in olives (Olea europaea)/olive oil, hibiscus, white grape (Vitis vinifera) wine [199], purple rice bran extract [200], edible mushrooms (Hydnum repandum) [201], potatoes, onions, wheat [202] | MCF7 [199] | Reduces IL-6, IL-8, and suppresses VEGF [199] | Induces apoptosis and limits invasion and metastasis [199] | Potent anticancer agent [199], antioxidant [202] |
| Syringic acid | Hydroxybenzoic acid from olive oil, dates, grapes [203], foxtail millet bran (Setaria italica) [204] | MCF7, MDA-MB-231 [204] |
Downregulates GRP78/SERBP-1/SCD1 signalling axis [204] | Antiproliferative activities [203,204] | Anti-BC agent [204], antioxidant [203] |
| Ellagic acid | Hydroxybenzoic acid from fruits, seeds, nuts, pomegranates, raspberries, strawberries, black raspberries, almonds, and walnuts [205] | MCF7 [205] | Regulates TGF-β/SMAD3 signalling axis, inhibits CDK6, binds to ACTN4 and induces its degradation via ubiquitin–proteasome pathway, reduces VEGFR-2 [205] | Suppresses BC cell growth, migration, invasion, metastasis, stimulates apoptosis, inhibits angiogenesis [205] | Anti-BC activities [205] |
| Caffeic acid | Hydroxycinnamic acid from fruits, green and roasted coffee, vegetables, tea, oils, spices [206,207], honey, and propolis extracts [194,208] | MCF7 [206] | Stimulates p53 and p21 genes, inhibits CDK2 [206], inhibits DNA methylation [208] | Induces apoptosis, cytotoxic effects, morphological changes in BC cells [206] | Putative antitumor agent [206] |
| Cinnamic acid | Hydroxycinnamic acid from cinnamon, grapes, tea, cocoa, spinach, celery [209] | MDA-MB-231, HEK293 [209] | Increases TNF-α-TNFR1 apoptotic pathway and caspases 8/3 [209] | Increases apoptosis and DNA damage [209] | Anti-BC agent [209] |
| p-Coumaric acid | Hydroxycinnamic acid from whole cereal grains, fruit, vegetables, Brazilian green propolis extracts [210] | MCF7 [210], BT20, BT549, MDA-MB-231, MDA-MB-436 TNBC [208] | Inhibits iNOS, COX-2, IL-1β, TNF-α, suppresses p-IκB, ERK1/2, blocks NF-κB and MAPKs pathways [211] | Reduces cell viability/cytotoxic effects, reverts the epigenetic silencing of the tumor suppressor RASSF1A [208], supports anti-inflammatory and immunomodulatory mechanisms [211] | Putative antiproliferative/anticancer agent [210,212] |
| Ferulic acid | Hydroxycinnamic acid from plants: ferulic (Ferula foetida), angelica, jujube kernel, rice bran, wheat bran [213], nuts, seeds [214] | MDA-MB-231 [215], MCF7 [216] | Regulates EMT [215] | Decreases viability and proliferation, increases apoptosis via activation of caspase-8 and -9, suppresses migration and metastasis [215,216] | Antitumor agent [215], antioxidant agent that protects DNA from OS [214,217] |
| Sinapic acid | Hydroxycinnamic acid from citrus fruits (oranges, grapefruits, lemons), berries; herbs (canola, mustard seed, rapeseed); cereals, wheat, rice, spices, oil seeds, vegetables, vinegar, Salvia officinalis, Myristica fragrans [218] | MCF7, T47D, MDA-MB-468, SK-BR-3 [219] | Downregulation of VKORC1 and KIF18B [219] | Induces apoptosis [219] | Cytotoxic agent in regard to luminal A BC cell lines [219] |
| Rosmarinic acid | Hydroxycinnamic acid from medicinal plants, herbs, spices (Boraginaceae, Lamiaceae, Labiatae) [220] | MDA-MB-231, MDA-MB-468 TNBC [220] | Upregulates TNF, GADD45A, BNIP3, HRK, TNFRSF25, inhibits BIRC5/survivin, MARK4, hedgehog pathway and hippo signalling, decreases proliferation and migration via Bcl-2/BAX signalling pathway, inhibits NF-κB signalling [220,221] | Antiproliferation and migration/cell cycle arrest, apoptosis [220] |
Anti-BC agent, antioxidant [220] |
| Chlorogenic acid | Hydroxycinnamic acid from fruits (apples, plums), vegetables (potatoes, eggplants), olive oil, spices, wine, coffee beans [222,223,224], honey [194] | Subcutaneous tumor mouse model of 4T1 cells [222] | Inhibits NF-κB/EMT signalling pathways [222] | Induces apoptosis, inhibits pulmonary metastasis, and improves anti-BC immunity [222] | Potential candidate for therapy of BC [222] |
| Avenanthramides (AVN-A, B, C) | Phenolic alkaloids found in oats (Avena sativa, Poaceae) [225] | MDA-MB-231 [226] | Activates caspase 3/7 [226] | Activates apoptosis and senescence, blocks cell proliferation, inhibits EMT and metastasis [225] | Anticancer effects [225] |
| Cyanidins/cyanidin 3-O-glucoside | Water-soluble anthocyanins found in leaves, petals, flowers, red fruits, blackberries, cranberries, grapes, cherries, apples, raspberries, peaches, plums, beans, red cabbage, red onions, purple sweet potatoes, carrots, avocadoes, olives [227,228] | BT474, MDA-MB-231, MCF7 [228,229] | Increases the expression of p53, Bax, caspase 3, CYP1, CYP2, and decreases Bcl2 [228], blocks ERBB2/cSRC/FAK pathway [229] | Proapoptotic and cytotoxic effects [228], inhibits invasion and metastasis [230], anti-mutagenic and anticarcinogenic effects [231] | Anticancer agent [228] |
| Delphinidin | Polyphenolic natural pigment occurring in berries, eggplant, wine [232] | MDA-MB-231, BT474 [233] | Induces protective autophagy via suppression of mTOR and activation of AMPK pathway in HER2+ BC cells [233] | Inhibits proliferation [108], promotes apoptosis and autophagy [233], exerts anti-mutagenic and anticarcinogenic effects [231] |
Anticancer effects [233], antioxidant [232] |
| Malvidin/ malvidin-3-O-glucoside |
Abundant anthocyanin in red wine, red grapes (Vitis vinifera), the skin of colored fruits, blueberries (Vaccinium corymbosum), blackberries (Rubus sp.) bilberries (Vaccinium myrtillus), red raspberries (Rubus idaeus), black raspberries (Rubus occidentalis), cranberries (Vaccinium macrocarpon), strawberries (Fragaria ananassa) [234] | MCF7 | Increases p21, caspases 3/8/9, Bax/Bcl-2, inhibits NF-κB, PI3K, TNF-α, STAT3, MMP2/9, IL-6, WNT, Notch1, and cyclin D1 [234] | Induces cell cycle arrest, antioxidation, anti-inflammation, autophagy, and apoptosis; inhibits proliferation, metastasis/cell invasion [234] | Anticarcinogenic potential [234] |
| Peonidin | Anthocyanidin found in purple sweet potatoes (Ipomoea batatas) [235], pigmented rice (red, black, dark purple) [230] | In silico [235] | Inhibits the overexpression of HER2 protein [235] | Proapoptotic, antiproliferative, anti-metastasis role [230] | Anti-BC activity [235] |
| Resveratrol | Non-flavonoid polyphenol from blueberries, grapes, red wine, raspberries, mulberries, apples, pomegranates, soy beans, peanuts | MDA-MB-231 [135] | Modulates PI3K/AKT, NF-κB, and Notch signalling pathways [133,134] | Induces Bax-dependent, but p53-independent, apoptosis [135] | Chemopreventive and putative therapeutic agent [236] |
| Curcumin | Polyphenol derived from turmeric (Curcuma longa) | MCF7 [137,138], MDA-MB-231, and Hs 578T [147] | Modulates NF-κB [137]; downregulates oncogenic RAF-1, suppresses telomerase, upregulates TNF-α and IL-8 genes [138]; inhibits EMT through downregulation of mTOR and PI3K/AKT signaling [147] | Inhibits cell stemness [148], proliferation, and promotes apoptosis [137]; suppresses motility and metastasis in TNBC [147] | Chemopreventive agent [137], anticancer and cytotoxic properties [138]; potential therapeutic agent [147] |
| Epicatechin | Flavan-3-ol from green tea, cocoa, grapes, apricots, green algae [237] | 4T1 [237], [238], TNBC mice model [150], MCF-7 [239] [240], MDA-MB-468 [153], MDA-MB-231 [240] |
Increases Bax/Bcl-2 ratio, increases the expression of CDH1, MTSS1, PTEN, BMRS, FAT1, and SMAD4 [237], modulates the AMPK and Akt/mTOR pathways [238] |
Decreases cell growth [150,238], inhibits metastasis-associated proliferation, reduces migration [150], cytostatic effects at lower concentrations [239], inhibits proliferation [238], proapoptotic [153,240] | Antiproliferative agent, similar effects to doxorubicin in terms of tumor growth inhibition and survival rates [238], could be used as an inhibitor for BC progression (anti-metastatic, anti-migratory, anti-invasion) [150] |
| Catechins | Flavan-3-ols found in black grapes, strawberries, cider, red algae, green algae, red wines, kiwis, green tea, gooseberries [241,242] | 4T1 [241] | Downregulates EGFR, APP, Bcl-2, DNMT, HIF1a, and PSMB5; upregulates caspase 3 and GADD45b [241] | Suppresses proliferation, stimulates apoptosis [241] | Antiproliferative agent [241,242] |
| Epigallocatechin gallate | Flavan-3-ol from green and black tea, apples, cherries, red algae, other fruits and vegetables [151,208,242] | BT20, BT549, MDA-MB-436, MDA-MB-231, MCF7, T47D, Hs 578T, allograft Balb/c model [151,208] |
Downregulates mTOR, PI3K/AKT, p53/Bcl-2, EGFR, VEGF, STAT3, NF-κB, SCUBE2, TIMP3, DNMT, ERα; activates JNK, caspases 9/3 [151,208] | Decreases cell growth, increases apoptosis, prevents DNA damage and proliferation, inhibits invasion, reduces cell viability, has cytotoxic effects [208] | Antiproliferative and anti-invasion agent, hypomethylating agent [208] |
| Theaflavin | Antioxidant polyphenol found in black tea [152,243] |
T47D, MDA-MB-231 [244], MCF-7, ZR-75-1 [245] |
Upregulates Fas/caspase 8, downregulates pAKT/pBAD pathway [244]; increases p53, Bax, activates caspase 6/7/9, increases ROS, stimulates p53, downregulates MMP2 and MMP9, inhibits the translocation of NF-kB/p65 to the nucleus [245] | Induces apoptosis [244], probably in a p-53-dependent manner [152], reduces cell viability, inhibits cell migration, could induce p53 phosphorylation of the Ser15 residue [245] | Proapoptotic agent [244] |
| Theaflavin-3-gallate | Polyphenol from fruits and veggies | MCF-7, MCF-10A [246] | Downregulates HSP90, MMP9, VEGFA, and SPP1 genes [246] | Inhibits cell proliferation, no cytotoxic effects on non-malignant breast cells (MCF-10A), induces apoptosis by stopping the cell cycle in the G2/M phase, decreases migration and colony formation [246] | Potentiate other BC therapies [246] |
| Phlorizin | Bioactive chalcone found in Asteraceae, Ericaceae, Saxifragaceae, Proteaceae, Rosaceae, Rutaceae, Fabaceae, Lamiaceae, Plantaginaceae, Pyrus communis [247] | MDA-MB-231, T47D [248], MCF7 [249] | Inhibits ERα signalling pathway, increases apoptotic caspase 3 via p53 [249] | Stimulates apoptosis, induces cytotoxicity/genotoxicity [248,249], antioxidant, anti-inflammatory, affects the composition of gut microbiota and development [250] | Anti-BC potential [247] |
| Kaempferol | Flavonoid from plants, fruits, vegetables, onions, apples, berries, tea [124,251] | MCF7 [252,253,254], MDA-MB-231, xenograft models [124,255] | Suppresses cyclin D1, p21, TWIST, and p38 MAPK [252], downregulates SNAI2, PLAU, CSF1, inhibits IGF1/IGF1R-mediated EMT [255] | Induces apoptosis, inhibits growth, migration, and proliferation of BC cells [124,252,256] | Anticancer effects [124,252], potentiates sensitivity to chemotherapy drugs [253,254] |
3. The Bad
The pro-inflammatory diet, based on a high intake of red, processed meat and alcohol [257], was associated with an increased BCR [258], while a long-term anti-inflammatory diet can improve the survival of BC patients [259]. The Western dietary pattern, rich in hydrogenated fat, soft drinks, animal fat, fast food, refined cereals, sweets, and processed meat, and the unhealthy dietary pattern, rich in sugars, processed juices, soft drinks, potato chips and mayonnaise, desserts, solid oils, red and processed meat, and high salt intake, are also associated with increased BCR [43]. Eating breakfast at a later time was associated with increased BCR among premenopausal women [26] and skipping breakfast was associated with an elevated risk in terms of all-cause and cancer-related mortality [260] and seems to be a bad idea for patients with cancer [261]. Moreover, poor diets that include refined sugar, saturated and trans fats, as well as a low level of natural antioxidants and fiber intake, were linked to increased BCR through the modulation of inflammation-related pathways and biomarkers [262] that play a key role in BC initiation and progression [263]. To exemplify this, nuclear factor-kappa B (NF-kB) is a pro-inflammatory nuclear transcription factor and the activation of the NF-kB signaling pathway is common in BC [264]. Alcohol exposure activates the NF-kB pathway and enhances the transcription of NF-kB-targeted genes [12]. Moreover, Starek-Swiechowich et al. (2023) showed that alcohol, even at low concentrations, as well as its major metabolite, acetaldehyde, causes TNBC cell proliferation, migration, and invasion via the activation of p38 MAPK and JNK phosphorylation [12]. Kansestani et al. (2019) showed that high glucose intake increased MCF7 BC cell proliferation, viability, VEGF secretion, and Bcl-2 expression, decreasing apoptosis, and stimulating angiogenesis, due to the activation of the NF-kB pathway by increasing reactive oxygen species [265].
BC progression can be also affected by systemic nutrients [14]. For example, meat consumption is annually rising at a global level, because meat is an important source of animal-based proteins, with superior anabolic potential when compared with plant-derived proteins [266], but it also enhances the risk of several types of cancer and other chronic diseases [267]. Lo et al. (2019) emphasized that the increased consumption of red meat was associated with invasive BC risk, which was also correlated with certain meat-cooking practices [90]. Inoue-Choi et al. (2015) showed that a higher intake of processed meat was associated with a 27% higher risk of localized postmenopausal BC and a 19% higher risk of distant BC [268]. Moreover, higher nitrite intake from processed meat was positively associated with localized cancer, whereas heme iron intake from red meat was positively correlated with BCR overall and all cancer stages [268]. Kim and Shin (2021) showed that processed meat consumption increases the risk of hypercholesterolemia, hypertriglyceridemia, and dyslipidemia, whereas red meat consumption also increases the risk of hypercholesterolemia, hyper-LDL cholesterolemia, and dyslipidemia [269], which have been linked to BC incidence [270]. Brindisi et al. (2022) demonstrated that cholesterol activates the estrogen-related receptor alpha (ERRα) pathway, promoting EMT in MCF7 and MDA-MB-231 BC cells [271]. Moreover, these authors concluded that BC cells exposed to high cholesterol levels promoted an increase in macrophage infiltration with the induction of the M2 phenotype, known as the tumor growth promoter [272], angiogenesis, and the induction of the cancer-associated fibroblast phenotype [271]. Recently, Magalhães et al. (2024) demonstrated that a high-cholesterol diet promotes phenotypic changes in BC cells and their intravasation through the LDL–LDLR axis, contributing to BC progression and metastasis in vitro and in various animal models [14]. Moreover, LDL also increases the serine proteinase inhibitor, clade E member 2 (SERPINE2) expression, which is known to be overexpressed in invasive ductal carcinoma of the breast [273]. Krawczynska et al. (2024) showed that neutrophils exposed to a cholesterol metabolite become able to secrete extracellular vesicles that promote EMT and stem cell characteristics in BC cells through the activation of the WNT/β-catenin signaling pathway, influencing BC progression [274]. Additionally, the low HDL cholesterol level was related to an increased BCR [270]. However, a cohort study in Japanese women, conducted by Narii et al. (2023), showed that triglycerides were not associated with BCR, while HDL cholesterol was inversely associated with BCR only in women over 50 years old [275].
Several pieces of analyses suggest that a higher intake of fish can be associated with higher incidence rates of ER+ BC [276], while oily fish intake has been found to be negatively correlated with the incidence of total BC, mainly in the cases of ER- BC [277]. Other authors have confirmed the protective effect of omega-3 fatty acids as a result of fish consumption against BC in Asian patients [278]. However, there are serious risks involving the consumption of fish contaminated with toxins, such as methylmercury, polychlorinated biphenyls (PCBs), dioxins, pesticides, and plastic waste [279]. Thus, food of aquatic origin is an important source of human exposure to methylmercury [280] and a low level of exposure to mercury can induce cancer cell proliferation by the estrogen receptor (ER), extracellular signal-regulated kinases 1/2 (ERK1/2), c-JUN NH(2)-terminal kinase (JNK), NADPH-oxidase, and nuclear factor erythroid 2-related factor 2 (NRF2) signaling, combined with anti-apoptotic and pro-survival signaling, the accumulation of DNA modifications, and the inhibition of DNA repair machinery [281]. Microplastics also accumulate in aquatic organisms and trigger various endocrine and metabolic pathways [282]. A study performed by Park et al. (2023) showed that polypropylene microplastics enhance metastasis-related gene expression and cytokines in BC cells [283]. Fish absorb dioxins and PCBs from their environment and these toxins enter the human body through food, including the consumption of fatty fish [284]. Invasive BCR was positively associated with dioxin exposure [285].
The various transformations involving processed meat can lead to the formation of harmful and potentially carcinogenic compounds [286]. Thus, processed meat contains polycyclic aromatic hydrocarbons (PAHs), heterocyclic aromatic amines (HAAs), residues, such as nitrosamines, biogenic amines, and a wide variety of other contaminants, such as antibiotics and other veterinary medicines and growth promoters [287,288,289,290]. Low-dose PAH-enriched mixtures have been shown to upregulate aryl hydrocarbon receptor (AhR) expression and cytochrome P450 (CYP) activity in ER+ BC cells, thus increasing cell proliferation and stimulating the expression of anti-apoptotic proteins [291]. Furthermore, a cohort study concluded that PAH exposure during pregnancy could interact with tobacco smoke, thus impacting the breast tissue in mothers and daughters, potentially influencing BCR across many generations [292]. HAAs are formed during high-temperature meat cooking and are linked to BC initiation [293,294,295]. Moreover, aromatic amines are generally highly lipophilic and can accumulate in the fatty tissue of breasts [296]. Nitrosamines are potent carcinogens formed in processed meats, when nitrites and nitrates interact with amines during food processing [297]. Studies have shown a positive association between BC and the consumption of processed meats containing these carcinogens [298]. Research indicates that consuming processed meat more than once a week is linked with a higher risk of BC, especially in women who also consume alcohol [299,300,301]. Moreover, a meta-analysis of prospective studies found that processed meat intake is associated with a 6% increase in BC development [302], while another study revealed that the said consumption is linked to a 9% higher BCR [303].
Nevertheless, milk consumption is more often associated with benefits than harm, but milk intake can be associated with a higher risk of hormone-related cancers [304]. A comprehensive meta-analysis of prospective cohort studies concluded that a high amount of whole/high-fat milk consumption can be associated with higher cancer mortality [66]. The intake of milk and dairy products has been related to a higher risk of breast and prostate cancers, due to the positive association with systemic levels of insulin-like growth factor 1 (IGF-1), insulin and estrogen signaling [305,306], and the IGF signaling pathway, implicated in the regulation of breast cancer stem cells (BCSCs), EMT, local migration and invasion, angiogenesis, and chemotherapy resistance [307]. More than 400 fatty acids (FAs) have been detected in milk; however, saturated fatty acids (SFAs) are present in a greater concentration [308]. Recently, based on a systematic review and meta-analysis, Mei et al. (2024) emphasized that high total SFA levels have been correlated with increased BCR [309]. Additionally, Jiang et al. (2024) also studied the association between dietary intake of SFAs and BCR, emphasizing that this impact depends on the carbon chain lengths of SFAs, attributable to the dietary sources and biological activities of such compounds [310].
An increased level of intake of omega-3 fatty acids associated with a decrease in omega-6, resulting in a higher omega-3/omega-6 ratio, is inversely associated with BCR [311]. Linoleic acid (LA), an omega-6 acid, predominantly present in oil seeds (soy bean, sunflower, rapeseed, and cotton), as well as α-linolenic acid (ALA), an omega-3 fatty acid found in flaxseed and fresh forage, are the FA precursors of conjugated linoleic acid (CLA) synthesis [56]. Espinosa-Neira et al. (2011) previously demonstrated that LA, an essential and the major polyunsaturated fatty acid (PUFA) in most diets, induces an EMT-like process in the mammary epithelial cells, MCF10A [312]. The authors showed that LA promotes a decrease in E-cadherin expression, as well as in increase in Snail1, Snail2, Twist1, Twist2, Sip1, vimentin, and N-cadherin expression [312]. Moreover, LA induces focal adhesion kinase (FAK) and NF-κB activation, and promotes an increase in MMP2 and MMP9 secretion, cell migration, and invasion in MCF10A cells [312]. Serna-Marquez et al. (2017) demonstrated that LA induces AKT2 activation, invasion, increases NF-κB-DNA binding activity, miR34a upregulation, and miR9 downregulation in the MDA-MB-231 BC cell line [313].
Milk also contains a plethora of proteins that may develop malignant activities. Thus, lactadherin/milk fat globule-EGR factor 8 (MFG-E8) is a glycoprotein associated with the milk fat globule membrane that plays crucial roles in cell adhesion and the promotion of angiogenesis, thus overexpressed lactadherin has been associated with poor prognosis and low survival in BC and other types of malignancies [314]. MFG-E8 can be considered as a potential biomarker and therapeutic target for breast carcinoma, emphasizing the decreased expression in ER+ and HER2+ BCs, while highly expressed in TNBC cell lines and patient sera [315]. Consequently, MFG-E8 downregulation was associated with cell cycle arrest and cell apoptosis, also inhibiting the expression of MMPs and EMT-associated proteins [315]. Osteopontin (OPN), a secreted multifunctional phosphorylated protein [60] present in milk from cows, buffalos, sheep, goats, and yaks [316], has also been identified to be highly expressed in the tumor tissue and plasma of BC patients, including TNBC tissues and cells, in association with a poor clinical prognosis and reduced survival, while OPN downregulation inhibits BC skeletal metastasis in vitro [317,318]. Recently, Guo et al. (2024) showed that OPN promotes tumor growth and metastasis and GPX4-mediated anti-lipid peroxidation in TNBC by activating the PI3K/Akt/mTOR pathway, elevating cell proliferation, invasive and migratory abilities, tumor sphere formation and angiogenesis [318]. Jia et al. (2024) showed that cottage/ricotta cheese intake was causally associated with luminal A-like BC, while the risk of ER-negative BC decreased [69]. Moreover, cow’s milk is a rich source of growth factors, including transforming growth factor (TGF)-β [319], a pluripotent cytokine with a key role in EMT, invasion, migration, and apoptosis, which acts as a growth inhibitor in early BC and a growth promoter in advanced stages of the disease [320].
Beyond all these negative effects, milk and milk-based products may contain pesticides that act as endocrine disruptors, with carcinogenic potential [67]. For example, atrazine, a herbicide that can bioaccumulate over time, was detected over the permissible limit for human consumption in bovine milk samples obtained from dairy farms [321]. Atrazine promotes 4T1 TNBC cell proliferation and migration, suppresses local and systemic immune function, and upregulates the expression of matrix metalloproteinase MMP2, MMP7, and MMP9 [322]. Moreover, atrazine induced IL-4 overexpression, while IFN-γ and TNF-α were found to be decreased in TME and serum [322]. Dairy products, including raw milk, ultra-high temperature milk, pasteurized milk, pasteurized and traditional butter, and pasteurized and traditional cheese, may also contain cypermethrin, deltamethrin, and hexachlorobenzene, over the maximum limit set by the EU [323]. Cypermethrin is a synthetic pyrethroid frequently used in agriculture and households for insect control [324]. Flumethrin, another pyrethroid pesticide, induces genotoxicity in MCF7 BC cells, even at low concentrations [325]. Hexachlorobenzene (HCB) is an organochlorine compound, which is able to bioaccumulate in high-fat tissues, that binds to the aryl hydrocarbon receptor, activating the membrane and nuclear pathways involved in BC development, such as ERα signaling, and insulin-like growth factor-1, epidermal growth factor, and transforming growth factor beta 1 receptors [326]. Thus, HCB stimulates epithelial cell proliferation, migration, invasion, and angiogenesis [326]. Unfortunately, even in the case of vegan milk substitutes, such as soy milk and its derivatives, genetically modified (GM) glyphosate-tolerant soy beans (GT), which dominate the soy bean market throughout the world, introduce thousands of tons of herbicides into the food chain [327] and bioaccumulate glyphosate (GLY), as well as its major degradation product aminomethylphosphonic acid (AMPA), which may itself act as an endocrine disruptor, mimicking 17β-estradiol that promotes ERα activity in BC cells [328]. The most common crops associated with the use of GLY include soy bean (Glycine max), corn (Zea mays), canola (Brassica napus), sugar beet (Beta vulgaris), and wheat (Triticum aestivum) [329]. Both GLY and AMPA have been shown to act as potential endocrine disruptors, while also exhibiting cytotoxic effects on BC cell lines. A study performed on MCF-7 and MDA-MB-468 cells highlighted the effects of these compounds on well-known signaling pathways, concluding that GLY and AMPA can dysregulate hedgehog, TGF-β, NOTCH, JAK-STAT, WNT, RAS, MAPK, and PI3K-AKT pathways, also affecting DNA repair processes, the cell cycle, and apoptosis [330], or increasing BC cell proliferation rates [328].
A literature search conducted by Keum et al. (2015) concluded that consuming more than five eggs/week was significantly associated with an increased BCR compared with no egg consumption [102]. As well as dairy products, home-grown eggs could be exposed to pesticides, such as hexachlorocyclohexane, aldrin, and malathion, more than commercial eggs, due to the direct interactions between eggs and the polluted environment [331]. Hexachlorocyclohexane has been associated with an increased BCR [332].
Augustin et al. (2013) emphasized that BCR revealed strong positive associations with bread and pasta consumption in women [93]. Pogurschi et al. (2021) analyzed the presence of acrylamide, which forms in several heated products consumed almost daily, such as coffee beverages, potato chips, pasta, pizza bases, cereal flakes and breakfast cereals, pancakes, pretzels, bread and pastries [333,334]. A large cohort study suggested a positive association between dietary acrylamide and BCR, mainly in premenopausal women, while other authors showed that acrylamide consumption did not increase the BCR [335,336]. A hormonal mode of action for acrylamide has been hypothesized to decipher the tumorigenesis in mammary glands based on acrylamide-induced DNA adduction and its mutagenesis-related potential [337].
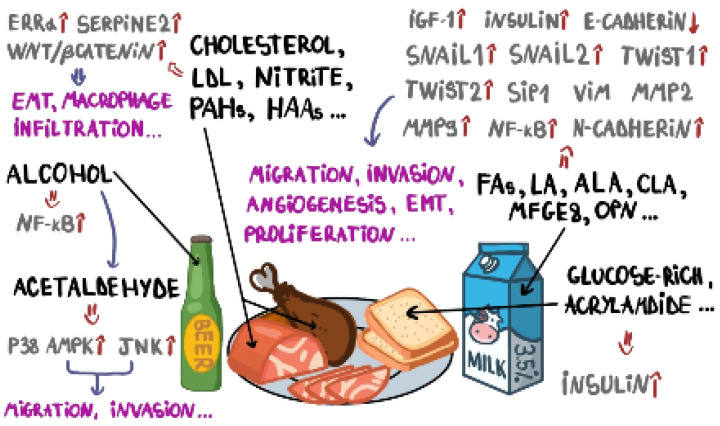
Recently, a study performed by Lara-Castor et al. (2024) found that the intake of sugar-sweetened beverages among children and adolescents in 185 countries increased by 23% from 1990 to 2018 [338]. The consumption of sugar-sweetened beverages was associated with a slightly higher BCR among lean women, but no significant increases in BCR were reported overall, as well as for the consumption of artificially sweetened beverages [339]. However, there is evidence based on large-scale population-based studies that suggests that there is a positive association between a higher intake of artificial sweeteners, such as aspartame and acesulfame-K, and increased BCR [340]. Other findings, based on a systematic review and meta-analysis, confirmed that there is no association between the exposure to artificial sweeteners and the incidence of BC [341]. However, many studies sustain a direct association between sugar-sweetened beverages and weight gain, being overweight, and obesity in children and adolescents [342], whereas obesity can increase the amount of circulating proinflammatory cytokines, promote tumor angiogenesis, and stimulate cancer cell stemness, increasing BC growth, invasion, and metastasis [343]. The harmful effects, at the molecular level, of dietary compounds that exert pro-tumorigenic effects in BC are illustrated in Figure 2.
Figure 2.
Biomarkers and molecular pathways involved in BC development, targeted by harmful compounds present in a daily diet.
4. Outlook
Evidence suggests that environmental exposure to chemicals and lifestyle account for 70% to 90% of the risk factors for chronic diseases, whereas only 10% to 30% can be explained by the specific genomic landscape [2]. In this review, we highlighted both “the good” and “the bad” sides of the daily human diet or dietary patterns and behaviors that influence BC risk (BCR) and incidence. Thus, pro-inflammatory diets, based on a high intake of red, processed meat, and alcohol, have been associated with increased BCR, while diets rich in antioxidants and anti-inflammatory compounds were all correlated with a lower risk of developing BC.
Milk, meat, eggs, and bread, including their derivatives, are complex foods, rich in nutrients, considered complete and basic ingredients in almost every meal and specific dietary pattern worldwide. However, milk, bread, eggs, and meat bi-directionally impact BCR. Moreover, milk contains proteins and lipids that can induce apoptosis and BC cell cycle arrest, inhibiting invasion, metastasis, and tumor angiogenesis. However, several milk proteins, such as osteopontin, may exhibit antitumorigenic characteristics, but only in certain circumstances. In addition, osteopontin can also promote tumor growth and metastasis in TNBC, by activating the PI3K/AKT/mTOR pathway, elevating BC cell proliferation, invasion, and migratory abilities, tumor sphere formation, and angiogenesis. Lactoferrin, a natural proapoptotic iron-binding multifunctional glycoprotein from bovine milk, can exert strong anticancer activities, inducing apoptosis in highly metastatic BC cell lines. Bovine milk-derived extracellular vesicles (EVs) are able to sensitize TNBC cells to doxorubicin, but many proteins from EVs provide context-based and opposing roles, acting both as BC metastasis promoters and suppressors. The intake of milk and dairy products has been related to a higher risk of breast and prostate cancers, due to their positive association with systemic levels of insulin-like growth factor 1 (IGF-1), insulin and estrogen signaling, which are implicated in the regulation of breast cancer stem cells (BCSCs), EMT, local migration and invasion, angiogenesis, and chemotherapy resistance. Linoleic acid (LA), an essential and the major polyunsaturated fatty acid (PUFA) in most diets, induces an EMT-like process in mammary epithelial cells. Lactadherin, a glycoprotein associated with the milk fat globule membrane, plays crucial roles in cell adhesion and the promotion of angiogenesis, so overexpressed lactadherin has been associated with a poor prognosis and a low survival rate in BC. Cottage/ricotta cheese intake is causally associated with luminal A-like BC and its consumption decreases the risk of ER-negative BC, while several additives derived for saffron and used to color cheese may exert antiproliferative effects on TNBC cells. Atrazine, a herbicide that can bioaccumulate over time, detected over the permissible limit for human consumption in bovine milk, promotes TNBC cells proliferation and migration, suppresses local and systemic immune function, and upregulates the expression of matrix metalloproteinases MMP2/7/9. Hexachlorobenzene (HCB), an organochlorine compound, is able to bioaccumulate in high-fat tissues, impacting ERα signaling, insulin-like growth factor-1, the epidermal growth factor, and transforming growth factor beta 1 receptors.
Meat consumption is annually rising at the global level, but red and processed meat are associated with invasive BC risk, which is also correlated with certain meat-cooking practices. High-cholesterol-based dietary patterns promote the intravasation of BC cells, as well as the progression of BC, due to the activation of ERα signaling pathway and the development an inflammatory TME. Food of aquatic origin is an important source of human exposure to methylmercury and other endocrine disruptors and even a low level of exposure can induce BC cell proliferation through the activation of ER, ERK1/2, JNK, NADPH-oxidase, and NRF2 signaling, combined with anti-apoptotic and pro-survival signaling, the accumulation of DNA modifications, and the inhibition of DNA repair machinery. Microplastics accumulated in aquatic organisms also trigger various endocrine and metabolic pathways. Low-dose polycyclic aromatic hydrocarbon (PAH)-enriched mixtures have been shown to increase BC cell proliferation and stimulate the expression of anti-apoptotic proteins. Moreover, PAH exposure during pregnancy impacts the breast tissue in mothers and daughters, potentially influencing BCR across many generations. Heterocyclic aromatic amines (HAAs) are formed during high-temperature meat cooking, are generally highly lipophilic, accumulate in the fatty tissue of the breasts, and were linked to BC initiation. Ethanol intake, alone or combined with red and processed meat, even at low concentrations, as well as its major metabolite, acetaldehyde, causes TNBC cell proliferation, migration and invasion, as well as high levels of glucose, which increases BC cell proliferation and viability, while decreasing apoptosis and stimulating tumor angiogenesis and ROS production.
Grains are one of the most important foods consumed worldwide, which contain bioactive compounds, but the contamination of cereals and cereals-based products with mycotoxins, such as aflatoxins, has been associated with mutagenesis and carcinogenesis. Common crops are associated with the use of glyphosate (GLY) and its major degradation product, aminomethylphosphonic acid (AMPA). Even in case of vegan milk or meat substitutes, such as soy-based derivatives, genetically modified glyphosate-tolerant soy beans (GT), which dominate the soy bean market throughout the world, introduce thousands of tons of herbicides into the food chain. GLY and AMPA can dysregulate hedgehog, TGF-β, NOTCH, JAK-STAT, WNT, RAS, MAPK, and PI3K-AKT pathways, also affecting DNA repair processes, the cell cycle, and apoptosis of BC cells. Acrylamide, which forms in several heated products consumed almost daily, such as coffee beverages, potato chips, pasta, pizza bases, cereal flakes and breakfast cereals, pancakes, pretzels, bread and pastries, has a hormonal mode of action, enhancing tumorigenesis in mammary glands based on acrylamide-induced DNA adduction and its mutagenesis-related potential. Ovotransferrin, an iron-binding glycoprotein present in both avian plasma and egg white, has anticancer activities, with negative effects on the proliferation of BC cells by inducing apoptosis. However, home-grown eggs could be exposed to pesticides, such as hexachlorocyclohexane, aldrin, and malathion, more than commercial eggs, due to the direct interactions between hens or eggs and the polluted environment. Many studies claim that there is a direct association between sugar-sweetened beverages and weight gain, being overweight, and obesity in children and adolescents, whereas obesity can increase the amount of circulating proinflammatory cytokines, promote tumor angiogenesis, and stimulate cancer cell stemness, increasing BC growth, invasion, and metastasis.
However, regularly eating new, diversified, colorful, clean, nutrient-rich, energy-boosting, and raw food, enables increased apoptosis and autophagy, antioxidation, cell cycle arrest, anti-inflammation, and immune response against BC cells, the reduction or inhibition of genomic instability, BC cell stemness, growth, proliferation, invasion, migration, and metastasis. Uncontrolled growth is a hallmark of cancer and is an important event during BC development and progression. Dietary compounds can induce cell cycle arrest through the upregulation of p21, and the downregulation of CDK2/4/6, survivin/BIRC5, PCNA, cyclin D1, MARK4, and RASSF1A. For example, survivin, also known as the BIRC5 protein, is overexpressed in TNBC [344], associated with a high proliferative capacity of tumor cells, so targeting survivin/BIRC5 may be an option for patients with TNBC. A diet containing tomatoes, Citrus fruits, medicinal plants, and spices, rich in naringin and Rosmarinic acid, could help the induction of BC cell cycle arrest. The development of cyclin-dependent kinase (CDK1/2/4/6) inhibitors with reduced toxicity is important for improving BC patient survival outcomes. Thus, hesperetin, apigenin, daidzein, ellagic, and caffeic acids, all present in herbal teas, Citrus fruits, nuts, berries, spices, vegetables, green and roasted coffee, oils, honey, and propolis extracts, downregulate CDK 1/2/4/6 that, as well as cyclins, play an important role in BC cell cycle progression [345]. The cyclin-dependent kinase inhibitor 1A (p21/CIP1) also has an important role in the cell cycle by inhibiting the activity of CDKs [346]. Allicin found in garlic, hesperidin from Citrus fruits, naringin, apigenin, tangerine, daidzein, caffeic acid, malvidin, an abundant anthocyanin found in red wine and colored fruits, such as all types of berries, and kaempferol, have the ability to induce cell cycle arrest. Microtubule-affinity regulating kinase 4 (MARK4) controls the early step in cell division and migration through the Hippo signaling pathway and is considered to be a potential drug target. Rosmarinic acid shows a significant binding affinity to MARK4, inhibiting its activity [347].
Inducing apoptosis is a key strategy to control excessive BC cell proliferation and natural products possess this property, stimulating proapoptotic mechanisms, including mitochondrial functions, PI3K/AKT, ROS, and MAPK-mediated pathways [348]. Thus, bioactive compounds increase ROS, caspases 3/7/8/9, the Bax/Bcl-2 ratio, BAK, NOXA, p53, and downregulate STAT3, pAKT, mTOR, JAK2, and survivin/BIRC5. Thus, all the bioactive compounds listed in Table 1 are able to induce apoptosis. Consequently, a plant-based diet and “eating the rainbow” is important for the induction of cellular death in BC. As the hallmark of BC neoplastic behavior, metastasis is a multistep process that includes cell migration and the colonization of distant niches into target organs. The epithelial–mesenchymal transition (EMT) confers BC cells with enhanced stem cells, and invasive and metastatic proprieties, so EMT blocking is crucial to avoid the spread of BC cells [349]. Thus, everyday plant-based meals contain the phytonutrients able to downregulate mesenchymal markers and EMT-associated pathways β-catenin, vimentin, N-cadherin, MMP 2/9, PI3K/AKT, mTOR, ERK1/2, Snail1, Slug, ZEB1, overexpressing epithelial biomarkers, such as claudin, E-cadherin, and PTEN. Dietary compounds also target antioxidation through activation of the NRF/KEAP1 signaling pathway with anti-BC effects, and anti-inflammation, through the downregulation of pro-inflammatory interleukins 1/6/8, NF-kB, TNF-α, COX2, and iNOS. Autophagy can be stimulated by the upregulation of p-AMPK and the downregulation of mTOR.
Eating more frequently, reducing evening energy intake, consuming early breakfast and dinner also reduce systemic inflammation and, consequently, BCR. It is important to carry out complex studies to understand, mainly at the molecular level, the bioavailability, absorption in the small intestine, distribution, metabolism, bioaccumulation, degradation, interaction with colonic microflora, elimination, and toxicity of harmful, as well as beneficial dietary compounds, on their own or in chemical mixtures, in order to translate the results from experiments involving BC cell lines, systemic reviews, meta-analysis, and cohort studies, into the most appropriate and personalized dietary patterns for each BC patient, approaching the disease in a holistic manner. Thus, we suggest that a balanced dietary structure combined with personalized nutritional behaviors can really improve treatment efficacy and BC patient outcomes.
5. Conclusions
Here, we highlighted both “the good” and “the bad” sides of daily human diet or dietary patterns and behaviors that influence BC risk and incidence, and provided an outlook on the major factors that can influence the onset and/or progression of BC. Environmental exposure to chemicals and lifestyle account for 70% to 90% of the risk factors for chronic diseases, whereas only 10% to 30% can be explained by the specific genomic landscape. So, regularly eating new food allows for increased apoptosis and autophagy, antioxidation, cell cycle arrest, anti-inflammation, and immune response against BC cells, the reduction or inhibition of genomic instability, BC cell stemness, growth, proliferation, invasion, migration, and metastasis. In addition, eating more frequently, reducing evening energy intake, consuming early breakfast and dinner also reduce systemic inflammation and, consequently, BC risk. Thus, a balanced and personalized dietary structure combined with appropriate nutritional behaviors can improve treatment efficacy and BC patient outcomes.
Acknowledgments
The authors thank the members of the Biochemistry and Proteomics Laboratories for the pleasant working environment. This project was supported in part by the National Cancer Institute of the National Institutes of Health under Award Number R15CA260126. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
AMPK—monophosphate-activated protein kinase; APP—amyloid-beta precursor; ASK1—apoptosis signal-regulating kinase 1; BAD—Bcl-2-associated death promoter; Bax—Bcl-2-associated X protein, apoptosis regulator; Bcl-2—B-cell lymphoma 2 protein; BCSCs—breast cancer stem cells; BNIP3—Bcl-2 interacting protein 3; CDKs—cyclin-dependent kinases; COX2—cyclooxygenase 2; CSF1—colony-stimulating factor 1; DNMT1—DNA (cytosine-5)-methyltransferase 1; EGF—epidermal growth factor; EGFR—epidermal growth factor receptor; EMT—epithelial-mesenchymal transition; ERα—estrogen receptor alpha; ERK1/2—extracellular signal-regulated protein kinase 1/2; GADD45b—growth arrest and DNA damage-inducible beta; GRP78—glucose regulated protein 78; HER2—epidermal growth factor receptor 2; HIF1—hypoxia-inducible factor 1; HRK—harakiri; IGF1—insulin growth factor 1; IGF1R—insulin-like growth factor-1 receptor; IκB—IkappaB kinase; IL-6—interleukin 6; iNOS—inducible nitric oxide synthase; JAK2—Janus kinase 2; JNK—c-Jun N-terminal kinase ; KIF18B—kinesin family member 18B; KEAP1—Kelch-like ECH-associated protein 1; MAPK—mitogen-activated protein kinase; MARK4—microtubule affinity-regulating kinase 4; MCL1—myeloid cell leukemia 1; MMP-2—matrix metalloproteinase 2; MMP-9—matrix metalloproteinase 9; mTOR—mammalian target of rapamycin; MTSS1—metastasis suppressor protein 1; NF-κB—nuclear factor kappa light chain enhancer of activated B cells; NOTCH1—neutrogenic locus notch homolog protein 1; NRF2—nuclear factor erythroid 2-related factor 2; PCNA—proliferating-cell nuclear antigen; PD-L1—programmed death-ligand 1; pAKT—phosphorylated serine/threonine-specific protein kinase; pERK-phosphorylated-extracellular signal-regulated kinase; PI3K –phosphatidylinositol-4,5-bisphosphate 3-kinase; PPARG—peroxisome proliferator-activated receptor gamma; PSMB5—proteasome subunit beta type-5; p27/Kip1—cyclin-dependent kinase inhibitor 1B; PDX—patient-derived xenograft; PLAU—plasminogen activator, urokinase; PTEN—phosphatase and TENsin homolog deleted on chromosome 10; RASSF1A—Ras association domain family protein 1 isoform A; ROS—reactive oxygen species; SCD—stearoyl-CoA 9-desaturase; SCUBE2—signal peptide, CUB domain and EGF-like domain containing 2; SERBP1—SERPINE1 mRNA-binding protein 1; SLUG—zinc finger protein SNAI2; STAT3—signal transducer and activator of transcription; TGF-β1—transforming growth factor beta 1; TIMP3—TIMP metallopeptidase inhibitor 3; TME—tumor microenvironment; TNBC—triple negative breast cancer; TNF—tumor necrosis factor; TNFRSF25—tumor necrosis factor receptor superfamily 25; VEGF—vascular endothelial growth factor; VIM—vimentin; VKORC1—vitamin K epoxide reductase complex subunit 1, WNT—wingless-related interaction site.
Author Contributions
Conceptualization, A.-N.N. and C.C.D.; literature search, A.-N.N., C.-L.J., T.M.J., K.W., N.N. and C.C.D.; writing—original draft preparation, A.-N.N., C.-L.J., T.M.J., K.W., N.N. and C.C.D.; writing—review and editing, A.-N.N., C.-L.J., T.M.J., K.W., N.N. and C.C.D.; project administration, C.C.D.; funding acquisition, C.C.D. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Funding Statement
This publication was supported in part by the National Cancer Institute of the National Institutes of Health under Award Number R15CA260126. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Arnold M., Morgan E., Rumgay H., Mafra A., Singh D., Laversanne M., Vignat J., Gralow J.R., Cardoso F., Siesling S., et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast. 2022;66:15–23. doi: 10.1016/j.breast.2022.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bucher M.L., Anderson F.L., Lai Y., Dicent J., Miller G.W., Zota A.R. Exposomics as a tool to investigate differences in health and disease by sex and gender. Exposome. 2023;3:osad003. doi: 10.1093/exposome/osad003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sellami M., Bragazzi N.L. Nutrigenomics and Breast Cancer: State-of-Art, Future Perspectives and Insights for Prevention. Nutrients. 2020;12:512. doi: 10.3390/nu12020512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kobets T., Smith B.P.C., Williams G.M. Food-Borne Chemical Carcinogens and the Evidence for Human Cancer Risk. Foods. 2022;11:2828. doi: 10.3390/foods11182828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rather I.A., Koh W.Y., Paek W.K., Lim J. The Sources of Chemical Contaminants in Food and Their Health Implications. Front. Pharmacol. 2017;8:830. doi: 10.3389/fphar.2017.00830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorrenti V., Burò I., Consoli V., Vanella L. Recent Advances in Health Benefits of Bioactive Compounds from Food Wastes and By-Products: Biochemical Aspects. Int. J. Mol. Sci. 2023;24:2019. doi: 10.3390/ijms24032019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neagu A.-N., Jayaweera T., Corrice L., Johnson K., Darie C.C. Breast Cancer Exposomics. Life. 2024;14:402. doi: 10.3390/life14030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022;12:31–46. doi: 10.1158/2159-8290.CD-21-1059. [DOI] [PubMed] [Google Scholar]
- 9.Bhattacharya T., Dutta S., Akter R., Rahman H., Karthika C., Nagaswarupa H.P., Murthy H.C.A., Fratila O., Brata R., Bungau S. Role of Phytonutrients in Nutrigenetics and Nutrigenomics Perspective in Curing Breast Cancer. Biomolecules. 2021;11:1176. doi: 10.3390/biom11081176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mecca M., Sichetti M., Giuseffi M., Giglio E., Sabato C., Sanseverino F., Marino G. Synergic Role of Dietary Bioactive Compounds in Breast Cancer Chemoprevention and Combination Therapies. Nutrients. 2024;16:1883. doi: 10.3390/nu16121883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan M., Zhang G., Bai W., Han X., Li C., Bian S. The Role of Bioactive Compounds in Natural Products Extracted from Plants in Cancer Treatment and Their Mechanisms Related to Anticancer Effects. Oxidative Med. Cell. Longev. 2022;2022:1429869. doi: 10.1155/2022/1429869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Starek-Świechowicz B., Budziszewska B., Starek A. Alcohol and breast cancer. Pharmacol. Rep. 2023;75:69–84. doi: 10.1007/s43440-022-00426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Epner M., Yang P., Wagner R.W., Cohen L. Understanding the Link between Sugar and Cancer: An Examination of the Preclinical and Clinical Evidence. Cancers. 2022;14:6042. doi: 10.3390/cancers14246042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magalhães A., Cesário V., Coutinho D., Matias I., Domingues G., Pinheiro C., Serafim T., Dias S. A high-cholesterol diet promotes the intravasation of breast tumor cells through an LDL–LDLR axis. Sci. Rep. 2024;14:9471. doi: 10.1038/s41598-024-59845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clemente-Suárez V.J., Beltrán-Velasco A.I., Redondo-Flórez L., Martín-Rodríguez A., Tornero-Aguilera J.F. Global Impacts of Western Diet and Its Effects on Metabolism and Health: A Narrative Review. Nutrients. 2023;15:2749. doi: 10.3390/nu15122749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malinowska A.M., Mlodzik-Czyzewska M.A., Chmurzynska A. Dietary patterns associated with obesity and overweight: When should misreporters be included in analysis? Nutrition. 2020;70:110605. doi: 10.1016/j.nut.2019.110605. [DOI] [PubMed] [Google Scholar]
- 17.López-Taboada I., González-Pardo H., Conejo N.M. Western Diet: Implications for Brain Function and Behavior. Front. Psychol. 2020;11:564413. doi: 10.3389/fpsyg.2020.564413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heras V.L., Melgar S., MacSharry J., Gahan C.G. The Influence of the Western Diet on Microbiota and Gastrointestinal Immunity. Annu. Rev. Food Sci. Technol. 2022;13:489–512. doi: 10.1146/annurev-food-052720-011032. [DOI] [PubMed] [Google Scholar]
- 19.Newsome R., Yang Y., Jobin C. Western diet influences on microbiome and carcinogenesis. Semin. Immunol. 2023;67:101756. doi: 10.1016/j.smim.2023.101756. [DOI] [PubMed] [Google Scholar]
- 20.Dehesh T., Fadaghi S., Seyedi M., Abolhadi E., Ilaghi M., Shams P., Ajam F., Mosleh-Shirazi M.A., Dehesh P. The relation between obesity and breast cancer risk in women by considering menstruation status and geographical variations: A systematic review and meta-analysis. BMC Women’s Health. 2023;23:392. doi: 10.1186/s12905-023-02543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruo S.W., Alkayyali T., Win M., Tara A., Joseph C., Kannan A., Srivastava K., Ochuba O., Sandhu J.K., Went T.R., et al. Role of Gut Microbiota Dysbiosis in Breast Cancer and Novel Approaches in Prevention, Diagnosis, and Treatment. Cureus. 2021;13:e17472. doi: 10.7759/cureus.17472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen G., Leary S., Niu J., Perry R., Papadaki A. The Role of the Mediterranean Diet in Breast Cancer Survivorship: A Systematic Review and Meta-Analysis of Observational Studies and Randomised Controlled Trials. Nutrients. 2023;15:2099. doi: 10.3390/nu15092099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalam F., James D.L., Li Y.R., Coleman M.F., A Kiesel V., Feliciano E.M.C., Hursting S.D., Sears D.D., Kleckner A.S. Intermittent fasting interventions to leverage metabolic and circadian mechanisms for cancer treatment and supportive care outcomes. JNCI Monogr. 2023;2023:84–103. doi: 10.1093/jncimonographs/lgad008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardt L., Mahamat-Saleh Y., Aune D., Schlesinger S. Plant-Based Diets and Cancer Prognosis: A Review of Recent Research. Curr. Nutr. Rep. 2022;11:695–716. doi: 10.1007/s13668-022-00440-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Teodoro A.J. Bioactive Compounds of Food: Their Role in the Prevention and Treatment of Diseases. Oxid. Med. Cell Longev. 2019;2019:3765986. doi: 10.1155/2019/3765986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palomar-Cros A., Harding B.N., Espinosa A., Papantoniou K., Pérez-Gómez B., Straif K., Ardanaz E., Villa T.F., Amiano P., Gómez-Acebo I., et al. Association of time of breakfast and nighttime fasting duration with breast cancer risk in the multicase-control study in Spain. Front. Nutr. 2022;9:941477. doi: 10.3389/fnut.2022.941477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D’cunha K., Park Y., Protani M.M., Reeves M.M. Circadian rhythm disrupting behaviours and cancer outcomes in breast cancer survivors: A systematic review. Breast Cancer Res. Treat. 2022;198:413–421. doi: 10.1007/s10549-022-06792-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marinac C.R., Sears D.D., Natarajan L., Gallo L.C., Breen C.I., Patterson R.E. Frequency and circadian timing of eating may influence biomarkers of inflammation and insulin resistance associated with breast cancer risk. PLoS ONE. 2015;10:e0136240. doi: 10.1371/journal.pone.0136240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spence C. Breakfast: The most important meal of the day? Int. J. Gastron. Food Sci. 2017;8:1–6. doi: 10.1016/j.ijgfs.2017.01.003. [DOI] [Google Scholar]
- 30.Gibney M.J., Barr S.I., Bellisle F., Drewnowski A., Fagt S., Livingstone B., Masset G., Moreiras G.V., Moreno L.A., Smith J., et al. Breakfast in Human Nutrition: The International Breakfast Research Initiative. Nutrients. 2018;10:559. doi: 10.3390/nu10050559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferrer-Cascales R., Sánchez-SanSegundo M., Ruiz-Robledillo N., Albaladejo-Blázquez N., Laguna-Pérez A., Zaragoza-Martí A. Eat or Skip Breakfast? The Important Role of Breakfast Quality for Health-Related Quality of Life, Stress and Depression in Spanish Adolescents. Int. J. Environ. Res. Public Health. 2018;15:1781. doi: 10.3390/ijerph15081781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.López-Gil J.F., Tully M.A., Cristi-Montero C., Brazo-Sayavera J., Gaya A.R., Calatayud J., López-Bueno R., Smith L. Is the frequency of breakfast consumption associated with life satisfaction in children and adolescents? A cross-sectional study with 154,151 participants from 42 countries. Nutr. J. 2024;23:78. doi: 10.1186/s12937-024-00979-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.A Betts J., Richardson J.D., A Chowdhury E., Holman G.D., Tsintzas K., Thompson D. The causal role of breakfast in energy balance and health: A randomized controlled trial in lean adults. Am. J. Clin. Nutr. 2014;100:539–547. doi: 10.3945/ajcn.114.083402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adolphus K., Lawton C.L., Dye L. The effects of breakfast on behavior and academic performance in children and adolescents. Front. Hum. Neurosci. 2013;7:425. doi: 10.3389/fnhum.2013.00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tolfrey K., Zakrzewski J.K. Breakfast, glycaemic index and health in young people. J. Sport Health Sci. 2012;1:149–159. doi: 10.1016/j.jshs.2012.09.001. [DOI] [Google Scholar]
- 36.Tang Z., Zhang N., Liu A., Luan D., Zhao Y., Song C., Ma G. The effects of breakfast on short-term cognitive function among Chinese white-collar workers: Protocol for a three-phase crossover study. BMC Public Health. 2017;17:92. doi: 10.1186/s12889-017-4017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellisle F., Hébel P., Salmon-Legagneur A., Vieux F. Breakfast Consumption in French Children, Adolescents, and Adults: A Nationally Representative Cross-Sectional Survey Examined in the Context of the International Breakfast Research Initiative. Nutrients. 2018;10:1056. doi: 10.3390/nu10081056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elahy V., Thomson C., Neuhouser M.L., Jiang L., Lee S., Pan K., Vitolins M., Chlebowski R., Lane D., Odegaard A.O. Frequency of Consuming Breakfast Meals and After-Dinner Snacks Is not Associated with Postmenopausal Breast Cancer Risk: Women’s Health Initiative Observational Study. J. Nutr. 2023;153:1089–1100. doi: 10.1016/j.tjnut.2023.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ligorio F., Fucà G., Vingiani A., Iannelli F., Lobefaro R., Ferraris C., Belfiore A., Scaperrotta G., Depretto C., Martinetti A., et al. 248P Targeting triple-negative breast cancer metabolism with neoadjuvant chemotherapy plus fasting-mimicking diet plus/minus metformin: The BREAKFAST trial. Ann. Oncol. 2023;34:S282–S283. doi: 10.1016/j.annonc.2023.09.446. [DOI] [Google Scholar]
- 40.Caffa I., Spagnolo V., Vernieri C., Valdemarin F., Becherini P., Wei M., Brandhorst S., Zucal C., Driehuis E., Ferrando L., et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature. 2020;583:620–624. doi: 10.1038/s41586-020-2502-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anemoulis M., Vlastos A., Kachtsidis V., Karras S.N. Intermittent Fasting in Breast Cancer: A Systematic Review and Critical Update of Available Studies. Nutrients. 2023;15:532. doi: 10.3390/nu15030532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Son D.-S., Done K.A., Son J., Izban M.G., Virgous C., Lee E.-S., Adunyah S.E. Intermittent Fasting Attenuates Obesity-Induced Triple-Negative Breast Cancer Progression by Disrupting Cell Cycle, Epithelial–Mesenchymal Transition, Immune Contexture, and Proinflammatory Signature. Nutrients. 2024;16:2101. doi: 10.3390/nu16132101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zamzam S., Said S., Yaghi J., Faisal F.S., Hassan D., Majeed S.A., Al Rajabi A., Tayyem R. Dietary Patterns Associated with Breast Cancer in the Middle East: A Scoping Review. Nutrients. 2024;16:579. doi: 10.3390/nu16050579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Soare I., Zugravu C.L., Zugravu G.A. Research on Consumer Perception Regarding Traditional Food Products of Romania. Foods. 2023;12:2723. doi: 10.3390/foods12142723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lequeux-Dincă A.-I., Preda M., Vijulie I. Authentic Romanian Gastronomy—A Landmark of Bucharest’s City Center. Tour. Hosp. 2024;5:251–275. doi: 10.3390/tourhosp5020017. [DOI] [Google Scholar]
- 46.Costandache M., Nenciu D.-S. The structure of Romanian’s food consumption and its implications on health condition and quality of life. Rev. Romana Stat. 2013;12:41–51. [Google Scholar]
- 47.Antunes I.C., Bexiga R., Pinto C., Roseiro L.C., Quaresma M.A.G. Cow’s Milk in Human Nutrition and the Emergence of Plant-Based Milk Alternatives. Foods. 2022;12:99. doi: 10.3390/foods12010099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vincent D., Ezernieks V., Elkins A., Nguyen N., Moate P.J., Cocks B.G., Rochfort S. Milk Bottom-Up Proteomics: Method Optimization. Front. Genet. 2016;6:360. doi: 10.3389/fgene.2015.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wajszczyk B., Charzewska J., Godlewski D., Zemła B., Nowakowska E., Kozaczka M., Chilimoniuk M., Pathak D.R. Consumption of Dairy Products and the Risk of Developing Breast Cancer in Polish Women. Nutrients. 2021;13:4420. doi: 10.3390/nu13124420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arafat H.M., Omar J., Shafii N., Naser I.A., Al Laham N.A., Muhamad R., Al-Astani T.A.D., Shaqaliah A.J., Shamallakh O.M., Shamallakh K.M., et al. The association between breast cancer and consumption of dairy products: A systematic review. Ann. Med. 2023;55:2198256. doi: 10.1080/07853890.2023.2198256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Riseberg E., Wu Y., Lam W.C., Eliassen A.H., Wang M., Zhang X., Willett W.C., A Smith-Warner S. Lifetime dairy product consumption and breast cancer risk: A prospective cohort study by tumor subtypes. Am. J. Clin. Nutr. 2024;119:302–313. doi: 10.1016/j.ajcnut.2023.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deschasaux-Tanguy M., Barrubés Piñol L., Sellem L., Debras C., Srour B., Chazelas E., Wendeu-Foyet G., Hercberg S., Galan P., Kesse-Guyot E., et al. Dairy product consumption and risk of cancer: A short report from the NutriNet-Santé prospective cohort study. Int. J. Cancer. 2022;150:1978–1986. doi: 10.1002/ijc.33935. [DOI] [PubMed] [Google Scholar]
- 53.Månsson H.L. Fatty acids in bovine milk fat. Food Nutr. Res. 2008;52:1821. doi: 10.3402/fnr.v52i0.1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang W., Guo X., Wang C., Alzhan A., Liu Z., Ma X., Shu Q. α-Linolenic acid induces apoptosis, inhibits the invasion and metastasis, and arrests cell cycle in human breast cancer cells by inhibiting fatty acid synthase. J. Funct. Foods. 2022;92:105041. doi: 10.1016/j.jff.2022.105041. [DOI] [Google Scholar]
- 55.Yan H., Zhang S., Yang L., Jiang M., Xin Y., Liao X., Li Y., Lu J. The Antitumor Effects of α-Linolenic Acid. J. Pers. Med. 2024;14:260. doi: 10.3390/jpm14030260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Balcazar I.C.A., Rivera L.D.G., Chavira J.S., Drouaillet B.E., Albarrán M.R., Martínez Y.B. Relationship between the Composition of Lipids in Forages and the Concentration of Conjugated Linoleic Acid in Cow’s Milk: A Review. Animals. 2022;12:1621. doi: 10.3390/ani12131621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zeng Y., Liu P., Yang X., Li H., Li H., Guo Y., Meng X., Liu X. The dietary c9,t11-conjugated linoleic acid enriched from butter reduces breast cancer progression in vivo. J. Food Biochem. 2020;44:e13163. doi: 10.1111/jfbc.13163. [DOI] [PubMed] [Google Scholar]
- 58.Nasrollahzadeh A., Tavani S.M., Arjeh E., Jafari S.M. Production of conjugated linoleic acid by lactic acid bacteria; important factors and optimum conditions. Food Chem. X. 2023;20:100942. doi: 10.1016/j.fochx.2023.100942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shariatikia M., Behbahani M., Mohabatkar H. Anticancer activity of cow, sheep, goat, mare, donkey and camel milks and their caseins and whey proteins and in silico comparison of the caseins. Mol. Biol. Res. Commun. 2017;6:57–64. [PMC free article] [PubMed] [Google Scholar]
- 60.Tan Y., Zhao L., Yang Y.-G., Liu W. The Role of Osteopontin in Tumor Progression Through Tumor-Associated Macrophages. Front. Oncol. 2022;12:953283. doi: 10.3389/fonc.2022.953283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Superti F. Lactoferrin from Bovine Milk: A Protective Companion for Life. Nutrients. 2020;12:2562. doi: 10.3390/nu12092562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pereira C.S., Guedes J.P., Gonçalves M., Loureiro L., Castro L., Gerós H., Rodrigues L.R., Côrte-Real M. Lactoferrin selectively triggers apoptosis in highly metastatic breast cancer cells through inhibition of plasmalemmal V-H+-ATPase. Oncotarget. 2016;7:62144–62158. doi: 10.18632/oncotarget.11394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sanwlani R., Kang T., Gummadi S., Nedeva C., Ang C., Mathivanan S. Bovine milk-derived extracellular vesicles enhance doxorubicin sensitivity in triple negative breast cancer cells by targeting metabolism and STAT signalling. Proteomics. 2023;23:e2200482. doi: 10.1002/pmic.202200482. [DOI] [PubMed] [Google Scholar]
- 64.Samuel M., Fonseka P., Sanwlani R., Gangoda L., Chee S.H., Keerthikumar S., Spurling A., Chitti S.V., Zanker D., Ang C.-S., et al. Oral administration of bovine milk-derived extracellular vesicles induces senescence in the primary tumor but accelerates cancer metastasis. Nat. Commun. 2021;12:3950. doi: 10.1038/s41467-021-24273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramezani R., Mohammadian M., Hosseini E.S., Zare M. The effect of bovine milk lactoferrin-loaded exosomes (exoLF) on human MDA-MB-231 breast cancer cell line. BMC Complement. Med. Ther. 2023;23:228. doi: 10.1186/s12906-023-04045-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin S., Je Y. Dairy Consumption and Total Cancer and Cancer-Specific Mortality: A Meta-Analysis of Prospective Cohort Studies. Adv. Nutr. Int. Rev. J. 2021;13:1063–1082. doi: 10.1093/advances/nmab135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen L., Li M., Li H. Milk and yogurt intake and breast cancer risk: A meta-analysis. Medicine. 2019;98:e14900. doi: 10.1097/MD.0000000000014900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wu Y., Huang R., Wang M., Bernstein L., Bethea T.N., Chen C., Chen Y., Eliassen A.H., Freedman N.D., Gaudet M.M., et al. Dairy foods, calcium, and risk of breast cancer overall and for subtypes defined by estrogen receptor status: A pooled analysis of 21 cohort studies. Am. J. Clin. Nutr. 2021;114:450–461. doi: 10.1093/ajcn/nqab097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jia Y., Feng C., Sun S., Li C., Li J., Yao P., Wei X., Lv W., Wang W., Zhang Y., et al. Cheese intake, probiotics and breast cancer: A Mendelian randomization analysis. J. Funct. Foods. 2024;119:106352. doi: 10.1016/j.jff.2024.106352. [DOI] [Google Scholar]
- 70.Kamal A.K.S., Talib W.H. Combination of ketogenic diet and probiotics inhibits breast cancer in mice by immune system modulation and reduction of Insulin growth factor-1. Pharmacia. 2023;70:1411–1422. doi: 10.3897/pharmacia.70.e111822. [DOI] [Google Scholar]
- 71.Ryser L.T., Arias-Roth E., Berthoud H., Delbès-Paus C., Chassard C., Bruggmann R., Irmler S. Cadaverine, putrescine, and histamine formation of Morganella morganii in raclette-type cheese. Int. Dairy J. 2022;129:105362. doi: 10.1016/j.idairyj.2022.105362. [DOI] [Google Scholar]
- 72.Kovács T., Mikó E., Vida A., Sebő É., Toth J., Csonka T., Boratkó A., Ujlaki G., Lente G., Kovács P., et al. Cadaverine, a metabolite of the microbiome, reduces breast cancer aggressiveness through trace amino acid receptors. Sci. Rep. 2019;9:1300. doi: 10.1038/s41598-018-37664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ritota M., Comitato R., Manzi P. Cow and Ewe Cheeses Made with Saffron: Characterization of Bioactive Compounds and Their Antiproliferative Effect in Cervical Adenocarcinoma (HeLa) and Breast Cancer (MDA-MB-231) Cells. Molecules. 2022;27:1995. doi: 10.3390/molecules27061995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Woodside J.V., Brennan S., Cantwell M. Are Soy-Milk Products Viable Alternatives to Cow’s Milk. In: Wilson T., Temple N.J., editors. Beverage Impacts on Health and Nutrition. 2nd ed. Springer International Publishing; Cham, Switzerland: 2016. pp. 151–162. [Google Scholar]
- 75.Storz M.A., Brommer M., Lombardo M., Rizzo G. Soy Milk Consumption in the United States of America: An NHANES Data Report. Nutrients. 2023;15:2532. doi: 10.3390/nu15112532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Freddo N., Nardi J., Bertol C.D., Dallegrave E., Leal M.B., Barreto F., Frizzo I.B., Rossato-Grando L.G. Isoflavone Quantitation in Soymilk: Genistein Content and Its Biological Effect. CyTA-J. Food. 2019;17:20–24. doi: 10.1080/19476337.2018.1544590. [DOI] [Google Scholar]
- 77.Poschner S., Maier-Salamon A., Zehl M., Wackerlig J., Dobusch D., Pachmann B., Sterlini K.L., Jäger W. The Impacts of Genistein and Daidzein on Estrogen Conjugations in Human Breast Cancer Cells: A Targeted Metabolomics Approach. Front. Pharmacol. 2017;8:699. doi: 10.3389/fphar.2017.00699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bhat S.S., Prasad S.K., Shivamallu C., Prasad K.S., Syed A., Reddy P., Cull C.A., Amachawadi R.G. Genistein: A Potent Anti-Breast Cancer Agent. Curr. Issues Mol. Biol. 2021;43:1502–1517. doi: 10.3390/cimb43030106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pawlicka M.A., Zmorzyński S., Popek-Marciniec S., Filip A.A. The Effects of Genistein at Different Concentrations on MCF-7 Breast Cancer Cells and BJ Dermal Fibroblasts. Int. J. Mol. Sci. 2022;23:12360. doi: 10.3390/ijms232012360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sharma M., Arora I., Chen M., Wu H., Crowley M.R., Tollefsbol T.O., Li Y. Therapeutic Effects of Dietary Soybean Genistein on Triple-Negative Breast Cancer via Regulation of Epigenetic Mechanisms. Nutrients. 2021;13:3944. doi: 10.3390/nu13113944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen J., Duan Y., Zhang X., Ye Y., Ge B., Chen J. Genistein induces apoptosis by the inactivation of the IGF-1R/p-Akt signaling pathway in MCF-7 human breast cancer cells. Food Funct. 2015;6:995–1000. doi: 10.1039/C4FO01141D. [DOI] [PubMed] [Google Scholar]
- 82.Choi E.J., Jung J.Y., Kim G.-H. Genistein inhibits the proliferation and differentiation of MCF-7 and 3T3-L1 cells via the regulation of ERα expression and induction of apoptosis. Exp. Ther. Med. 2014;8:454–458. doi: 10.3892/etm.2014.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Varinska L., Gal P., Mojzisova G., Mirossay L., Mojzis J. Soy and Breast Cancer: Focus on Angiogenesis. Int. J. Mol. Sci. 2015;16:11728–11749. doi: 10.3390/ijms160511728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang X., Yang S., McKimmey C., Liu B., Edgerton S.M., Bales W., Archer L.T., Thor A.D. Genistein induces enhanced growth promotion in ER-positive/erbB-2-overexpressing breast cancers by ER-erbB-2 cross talk and p27/kip1 downregulation. Carcinogenesis. 2010;31:695–702. doi: 10.1093/carcin/bgq007. [DOI] [PubMed] [Google Scholar]
- 85.Choi E.J., Kim G.-H. Daidzein causes cell cycle arrest at the G1 and G2/M phases in human breast cancer MCF-7 and MDA-MB-453 cells. Phytomedicine. 2008;15:683–690. doi: 10.1016/j.phymed.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 86.Jin S., Zhang Q.Y., Kang X.M., Wang J.X., Zhao W.H. Daidzein induces MCF-7 breast cancer cell apoptosis via the mitochondrial pathway. Ann. Oncol. 2009;21:263–268. doi: 10.1093/annonc/mdp499. [DOI] [PubMed] [Google Scholar]
- 87.Bao C., Namgung H., Lee J., Park H.-C., Ko J., Moon H., Ko H.W., Lee H.J. Daidzein Suppresses Tumor Necrosis Factor-α Induced Migration and Invasion by Inhibiting Hedgehog/Gli1 Signaling in HumanBreast Cancer Cells. J. Agric. Food Chem. 2014;62:3759–3767. doi: 10.1021/jf500231t. [DOI] [PubMed] [Google Scholar]
- 88.Kumar V., Chauhan S.S. Daidzein Induces Intrinsic Pathway of Apoptosis along with ER α/β Ratio Alteration and ROS Production. Asian Pac. J. Cancer Prev. 2021;22:603–610. doi: 10.31557/APJCP.2021.22.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Furness J.B., Bravo D.M. Humans as cucinivores: Comparisons with other species. J. Comp. Physiol. B. 2015;185:825–834. doi: 10.1007/s00360-015-0919-3. [DOI] [PubMed] [Google Scholar]
- 90.Lo J.J., Park Y.-M., Sinha R., Sandler D.P. Association between meat consumption and risk of breast cancer: Findings from the Sister Study. Int. J. Cancer. 2019;146:2156–2165. doi: 10.1002/ijc.32547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xiao Y., Ke Y., Wu S., Huang S., Li S., Lv Z., Yeoh E.-K., Lao X., Wong S., Kim J.H., et al. Association between whole grain intake and breast cancer risk: A systematic review and meta-analysis of observational studies. Nutr. J. 2018;17:87. doi: 10.1186/s12937-018-0394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pajari A.M., Freese R., Kariluoto S., Lampi A.M., Piironen V. Bioactive compounds in whole grains and their implications for health. In: Landberg R., Scheers N., editors. Whole Grains and Health. 2nd ed. John Wiley & Sons Ltd.; Chichester, UK: 2021. pp. 301–336. [DOI] [Google Scholar]
- 93.Augustin L.S.A., Malerba S., Lugo A., Franceschi S., Talamini R., Serraino D., Jenkins D.J.A., La Vecchia C. Associations of bread and pasta with the risk of cancer of the breast and colorectum. Ann. Oncol. 2013;24:3094–3099. doi: 10.1093/annonc/mdt383. [DOI] [PubMed] [Google Scholar]
- 94.Kumar P., Gupta A., Mahato D.K., Pandhi S., Pandey A.K., Kargwal R., Mishra S., Suhag R., Sharma N., Saurabh V., et al. Aflatoxins in Cereals and Cereal-Based Products: Occurrence, Toxicity, Impact on Human Health, and Their Detoxification and Management Strategies. Toxins. 2022;14:687. doi: 10.3390/toxins14100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aljazzar A., El-Ghareeb W.R., Darwish W.S., Abdel-Raheem S.M., Ibrahim A.M., Hegazy E.E., Mohamed E.A. Effects of aflatoxin B1 on human breast cancer (MCF-7) cells: Cytotoxicity, oxidative damage, metabolic, and immune-modulatory transcriptomic changes. Environ. Sci. Pollut. Res. 2022;30:13132–13140. doi: 10.1007/s11356-022-23032-6. [DOI] [PubMed] [Google Scholar]
- 96.Mercogliano M.F., Bruni S., Elizalde P.V., Schillaci R. Tumor Necrosis Factor α Blockade: An Opportunity to Tackle Breast Cancer. Front. Oncol. 2020;10:584. doi: 10.3389/fonc.2020.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cruceriu D., Baldasici O., Balacescu O., Berindan-Neagoe I. The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: Molecular insights and therapeutic approaches. Cell. Oncol. 2020;43:1–18. doi: 10.1007/s13402-019-00489-1. [DOI] [PubMed] [Google Scholar]
- 98.Liu W., Lu X., Shi P., Yang G., Zhou Z., Li W., Mao X., Jiang D., Chen C. TNF-α increases breast cancer stem-like cells through up-regulating TAZ expression via the non-canonical NF-κB pathway. Sci. Rep. 2020;10:1804. doi: 10.1038/s41598-020-58642-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Badr El-Din N.K., Mahmoud A.Z., Hassan T.A., Ghoneum M. Baker’s Yeast Sensitizes Metastatic Breast Cancer Cells to Paclitaxel In Vitro. Integr. Cancer Ther. 2018;17:542–550. doi: 10.1177/1534735417740630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Grudzińska M., Galanty A., Paśko P. Can edible sprouts be the element of effective chemopreventive strategy?—A systematic review of in vitro and in vivo study. Trends Food Sci. Technol. 2023;139:104130. doi: 10.1016/j.tifs.2023.104130. [DOI] [Google Scholar]
- 101.Cho K., Lee C.W., Ohm J. In Vitro Study on Effect of Germinated Wheat on Human Breast Cancer Cells. Cereal Chem. 2016;93:647–649. doi: 10.1094/CCHEM-04-16-0102-N. [DOI] [Google Scholar]
- 102.Keum N., Lee D.H., Marchand N., Oh H., Liu H., Aune D., Greenwood D.C., Giovannucci E.L. Egg intake and cancers of the breast, ovary and prostate: A dose-response meta-analysis of prospective observational studies. Br. J. Nutr. 2015;114:1099–1107. doi: 10.1017/S0007114515002135. [DOI] [PubMed] [Google Scholar]
- 103.Mann K. Chapter 16—Proteomics of Egg White. In: Colgrave M.L., editor. Proteomics in Food Science. Academic Press; Cambridge, MA, USA: 2017. pp. 261–276. [Google Scholar]
- 104.Rathnapala E.C.N., Ahn D.U., Abeyrathne S. Functional properties of ovotransferrin from chicken egg white and its derived peptides: A review. Food Sci. Biotechnol. 2021;30:619–630. doi: 10.1007/s10068-021-00901-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Giansanti F., Leboffe L., Angelucci F., Antonini G. The Nutraceutical Properties of Ovotransferrin and Its Potential Utilization as a Functional Food. Nutrients. 2015;7:9105–9115. doi: 10.3390/nu7115453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ibrahim H.R., Kiyono T. Novel Anticancer Activity of the Autocleaved Ovotransferrin against Human Colon and Breast Cancer Cells. J. Agric. Food Chem. 2009;57:11383–11390. doi: 10.1021/jf902638e. [DOI] [PubMed] [Google Scholar]
- 107.Kostić A., Milinčić D.D., Barać M.B., Shariati M.A., Tešić L., Pešić M.B. The Application of Pollen as a Functional Food and Feed Ingredient—The Present and Perspectives. Biomolecules. 2020;10:84. doi: 10.3390/biom10010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li J. Bee Pollen and Doxorubicin by Synergistic Effects Inhibit the Proliferation of Breast Tumors in 4T1 Tumor-bearing BALB/c Mice: A Biochemical, Immunohistochemical, and Molecular Approach. Pharmacogn. Mag. 2023;20:159–178. doi: 10.1177/09731296231203809. [DOI] [Google Scholar]
- 109.El Ghouizi A., Bakour M., Laaroussi H., Ousaaid D., El Menyiy N., Hano C., Lyoussi B. Bee Pollen as Functional Food: Insights into Its Composition and Therapeutic Properties. Antioxidants. 2023;12:557. doi: 10.3390/antiox12030557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jurzak M., Ramos P., Pilawa B., Bednarek I.A. Comparative EPR Studies on the Influence of Genistein on Free Radicals in Non-Irradiated and UV-Irradiated MCF7, T47D and MDA-MB-231 Breast Cancer Cells. Biomedicines. 2024;12:518. doi: 10.3390/biomedicines12030518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Caner A., Onal M.G., Silici S. The effect of bee bread (Perga) with chemotherapy on MDA-MB-231 cells. Mol. Biol. Rep. 2021;48:2299–2306. doi: 10.1007/s11033-021-06259-3. [DOI] [PubMed] [Google Scholar]
- 112.Nandini D., Rao R.S., Deepak B., Reddy P.B. Sulforaphane in broccoli: The green chemoprevention!! Role in cancer prevention and therapy. J. Oral Maxillofac. Pathol. 2020;24:405. doi: 10.4103/jomfp.JOMFP_126_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kaboli P.J., Khoshkbejari M.A., Mohammadi M., Abiri A., Mokhtarian R., Vazifemand R., Amanollahi S., Sani S.Y., Li M., Zhao Y., et al. Targets and mechanisms of sulforaphane derivatives obtained from cruciferous plants with special focus on breast cancer—Contradictory effects and future perspectives. Biomed. Pharmacother. 2019;121:109635. doi: 10.1016/j.biopha.2019.109635. [DOI] [PubMed] [Google Scholar]
- 114.Wu S., Lu H., Bai Y. Nrf2 in cancers: A double-edged sword. Cancer Med. 2019;8:2252–2267. doi: 10.1002/cam4.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Surh Y.-J. Nrf2 paradox: Can cancer patients eat broccoli? Food Front. 2021;2:25–28. doi: 10.1002/fft2.61. [DOI] [Google Scholar]
- 116.Zhang Y., Lu Q., Li N., Xu M., Miyamoto T., Liu J. Sulforaphane suppresses metastasis of triple-negative breast cancer cells by targeting the RAF/MEK/ERK pathway. npj Breast Cancer. 2022;8:40. doi: 10.1038/s41523-022-00402-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Desai G., Schelske-Santos M., Nazario C.M., Rosario-Rosado R.V., Mansilla-Rivera I., Ramírez-Marrero F., Nie J., Myneni A.A., Zhang Z.-F., Freudenheim J.L., et al. Onion and Garlic Intake and Breast Cancer, a Case-Control Study in Puerto Rico. Nutr. Cancer. 2019;72:791–800. doi: 10.1080/01635581.2019.1651349. [DOI] [PubMed] [Google Scholar]
- 118.Shi G., Li X., Wang W., Hou L., Yin L., Wang L. Allicin Overcomes Doxorubicin Resistance of Breast Cancer Cells by Targeting the Nrf2 Pathway. Cell Biochem. Biophys. 2024;82:659–667. doi: 10.1007/s12013-024-01215-x. [DOI] [PubMed] [Google Scholar]
- 119.Talib W.H., Baban M.M., Azzam A.O., Issa J.J., Ali A.Y., AlSuwais A.K., Allala S., AL Kury L.T. Allicin and Cancer Hallmarks. Molecules. 2024;29:1320. doi: 10.3390/molecules29061320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Maitisha G., Aimaiti M., An Z., Li X. Allicin induces cell cycle arrest and apoptosis of breast cancer cells in vitro via modulating the p53 pathway. Mol. Biol. Rep. 2021;48:7261–7272. doi: 10.1007/s11033-021-06722-1. [DOI] [PubMed] [Google Scholar]
- 121.Dorrigiv M., Zareiyan A., Hosseinzadeh H. Onion (Allium cepa) and its Main Constituents as Antidotes or Protective Agents against Natural or Chemical Toxicities: A Comprehensive Review. Iran. J. Pharm. Res. IJPR. 2021;20:3–26. doi: 10.22037/IJPR.2020.112773.13940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nishimura M., Muro T., Kobori M., Nishihira J. Effect of Daily Ingestion of Quercetin-Rich Onion Powder for 12 Weeks on Visceral Fat: A Randomised, Double-Blind, Placebo-Controlled, Parallel-Group Study. Nutrients. 2019;12:91. doi: 10.3390/nu12010091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Savitha S., Bhatkar N., Chakraborty S., Thorat B.N. Onion quercetin: As immune boosters, extraction, and effect of dehydration. Food Biosci. 2021;44:101457. doi: 10.1016/j.fbio.2021.101457. [DOI] [Google Scholar]
- 124.Dilek B., Meltem Ö. Quercetin suppresses cell proliferation using the apoptosis pathways in MCF-7 and MDA-MB-231 human breast carcinoma cells in monolayer and spheroid model cultures. S. Afr. J. Bot. 2023;162:259–270. doi: 10.1016/j.sajb.2023.09.017. [DOI] [Google Scholar]
- 125.Liao Y., Xie X., Zhang C., Zhong H., Shan L., Yu P., Xu L. Quercetin exerts anti-tumor immune mechanism by regulating IL-6/JAK2/STAT3 signaling pathway to deplete Treg cells. Toxicon. 2024;243:107747. doi: 10.1016/j.toxicon.2024.107747. [DOI] [PubMed] [Google Scholar]
- 126.Sesso H.D., Buring J.E., Zhang S.M., Norkus E.P., Gaziano J.M. Dietary and Plasma Lycopene and the Risk of Breast Cancer. Cancer Epidemiol. Biomark. Prev. 2005;14:1074–1081. doi: 10.1158/1055-9965.EPI-04-0683. [DOI] [PubMed] [Google Scholar]
- 127.Kapała A., Szlendak M., Motacka E. The Anti-Cancer Activity of Lycopene: A Systematic Review of Human and Animal Studies. Nutrients. 2022;14:5152. doi: 10.3390/nu14235152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ozkan G., Günal-Köroğlu D., Karadag A., Capanoglu E., Cardoso S.M., Al-Omari B., Calina D., Sharifi-Rad J., Cho W.C. A mechanistic updated overview on lycopene as potential anticancer agent. Biomed. Pharmacother. 2023;161:114428. doi: 10.1016/j.biopha.2023.114428. [DOI] [PubMed] [Google Scholar]
- 129.Takeshima M., Ono M., Higuchi T., Chen C., Hara T., Nakano S. Anti-proliferative and apoptosis-inducing activity of lycopene against three subtypes of human breast cancer cell lines. Cancer Sci. 2014;105:252–257. doi: 10.1111/cas.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ma P., Wang L., Xu L., Li J., Zhang X., Chen H. Rapid quantitative determination of chlorpyrifos pesticide residues in tomatoes by surface-enhanced Raman spectroscopy. Eur. Food Res. Technol. 2019;246:239–251. doi: 10.1007/s00217-019-03408-8. [DOI] [Google Scholar]
- 131.Ventura C., Zappia C.D., Lasagna M., Pavicic W., Richard S., Bolzan A.D., Monczor F., Nunez M., Cocca C. Effects of the pesticide chlorpyrifos on breast cancer disease. Implication of epigenetic mechanisms. J. Steroid Biochem. Mol. Biol. 2019;186:96–104. doi: 10.1016/j.jsbmb.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 132.Lasagna M., Ventura C., Hielpos M., Mardirosian M., Martín G., Miret N., Randi A., Núñez M., Cocca C. Endocrine disruptor chlorpyrifos promotes migration, invasion, and stemness phenotype in 3D cultures of breast cancer cells and induces a wide range of pathways involved in cancer progression. Environ. Res. 2021;204:111989. doi: 10.1016/j.envres.2021.111989. [DOI] [PubMed] [Google Scholar]
- 133.Ghafouri-Fard S., Bahroudi Z., Shoorei H., Hussen B.M., Talebi S.F., Baig S.G., Taheri M., Ayatollahi S.A. Disease-associated regulation of gene expression by resveratrol: Special focus on the PI3K/AKT signaling pathway. Cancer Cell Int. 2022;22:298. doi: 10.1186/s12935-022-02719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jang J.Y., Im E., Kim N.D. Mechanism of Resveratrol-Induced Programmed Cell Death and New Drug Discovery against Cancer: A Review. Int. J. Mol. Sci. 2022;23:13689. doi: 10.3390/ijms232213689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gogada R., Prabhu V., Amadori M., Scott R., Hashmi S., Chandra D. Resveratrol induces p53-independent, X-linked inhibitor of apoptosis protein (XIAP)-mediated Bax protein oligomerization on mitochondria to initiate cytochrome c release and caspase activation. J. Biol. Chem. 2011;286:28749–28760. doi: 10.1074/jbc.M110.202440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun Y., Zhou Q.-M., Lu Y.-Y., Zhang H., Chen Q.-L., Zhao M., Su S.-B. Resveratrol Inhibits the Migration and Metastasis of MDA-MB-231 Human Breast Cancer by Reversing TGF-β1-Induced Epithelial-Mesenchymal Transition. Molecules. 2019;24:1131. doi: 10.3390/molecules24061131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liu J.L., Pan Y.Y., Chen O., Luan Y., Xue X., Zhao J.J., Liu L., Jia H.Y. Curcumin inhibits MCF-7 cells by modulating the NF-κB signaling pathway. Oncol. Lett. 2017;14:5581–5584. doi: 10.3892/ol.2017.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fawzy R.M., Abdel-Aziz A.A., Bassiouny K., Fayed A.M. Phytocompounds-based therapeutic approach: Investigating curcumin and green tea extracts on MCF-7 breast cancer cell line. J. Genet. Eng. Biotechnol. 2024;22:100339. doi: 10.1016/j.jgeb.2023.100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Liu H.-T., Ho Y.-S. Anticancer effect of curcumin on breast cancer and stem cells. Food Sci. Hum. Wellness. 2018;7:134–137. doi: 10.1016/j.fshw.2018.06.001. [DOI] [Google Scholar]
- 140.Rinkenbaugh A.L., Baldwin A.S. The NF-κB Pathway and Cancer Stem Cells. Cells. 2016;5:16. doi: 10.3390/cells5020016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Vazquez-Santillan K., Melendez-Zajgla J., Jimenez-Hernandez L.E., Gaytan-Cervantes J., Muñoz-Galindo L., Piña-Sanchez P., Martinez-Ruiz G., Torres J., Garcia-Lopez P., Gonzalez-Torres C., et al. NF-kappaΒ-inducing kinase regulates stem cell phenotype in breast cancer. Sci. Rep. 2016;6:37340. doi: 10.1038/srep37340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li X., Wang X., Xie C., Zhu J., Meng Y., Chen Y., Li Y., Jiang Y., Yang X., Wang S., et al. Sonic hedgehog and Wnt/β-catenin pathways mediate curcumin inhibition of breast cancer stem cells. Anti-Cancer Drugs. 2018;29:208–215. doi: 10.1097/CAD.0000000000000584. [DOI] [PubMed] [Google Scholar]
- 143.Kumar H., Kumar R.M., Bhattacharjee D., Somanna P., Jain V. Role of Nrf2 Signaling Cascade in Breast Cancer: Strategies and Treatment. Front. Pharmacol. 2022;13:720076. doi: 10.3389/fphar.2022.720076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ashrafizadeh M. Curcumin Activates the Nrf2 Pathway and Induces Cellular Protection Against Oxidative Injury. Curr. Mol. Med. 2020;20:116–133. doi: 10.2174/1566524019666191016150757. [DOI] [PubMed] [Google Scholar]
- 145.Shahcheraghi S.H., Salemi F., Peirovi N., Ayatollahi J., Alam W., Khan H., Saso L. Nrf2 Regulation by Curcumin: Molecular Aspects for Therapeutic Prospects. Molecules. 2021;27:167. doi: 10.3390/molecules27010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sordillo P.P., Helson L. Curcumin and cancer stem cells: Curcumin has asymmetrical effects on cancer and normal stem cells. Anticancer. Res. 2015;35:599–614. [PubMed] [Google Scholar]
- 147.Chen Z.M., Lu P.M., Li M.M., Zhang Q.M., He T., Gan L. Curcumin suppresses metastasis of triple-negative breast cancer cells by modulating EMT signaling pathways: An integrated study of bioinformatics analysis. Medicine. 2024;103:e37264. doi: 10.1097/MD.0000000000037264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hu C., Li M., Guo T., Wang S., Huang W., Yang K., Liao Z., Wang J., Zhang F., Wang H. Anti-metastasis activity of curcumin against breast cancer via the inhibition of stem cell-like properties and EMT. Phytomedicine. 2018;58:152740. doi: 10.1016/j.phymed.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 149.Zhang D., Nichols H.B., Troester M., Cai J., Bensen J.T., Sandler D.P. Tea consumption and breast cancer risk in a cohort of women with family history of breast cancer. Int. J. Cancer. 2020;147:876–886. doi: 10.1002/ijc.32824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Pérez-Durán J., Luna A., Portilla A., Martínez P., Ceballos G., Ortíz-Flores M.Á., Solis-Paredes J.M., Nájera N. (-)-Epicatechin Inhibits Metastatic-Associated Proliferation, Migration, and Invasion of Murine Breast Cancer Cells In Vitro. Molecules. 2023;28:298. doi: 10.3390/molecules28176229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Al Mamun A., Sharker B., Bhuiyan M.R., Sadikuj Jaman M. Anticancer activity and therapeutic uses of catechins on breast, prostate and lung cancer: Future perspective and clinical proofs. J. Clin. Case Rep. Med. Images Health Sci. 2023;3:2023. doi: 10.55920/JCRMHS.2023.03.001118. [DOI] [Google Scholar]
- 152.O’neill E.J., Termini D., Albano A., Tsiani E. Anti-Cancer Properties of Theaflavins. Molecules. 2021;26:987. doi: 10.3390/molecules26040987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Thomas P., Dong J. (-)-Epicatechin acts as a potent agonist of the membrane androgen receptor, ZIP9 (SLC39A9), to promote apoptosis of breast and prostate cancer cells. J. Steroid Biochem. Mol. Biol. 2021;211:105906. doi: 10.1016/j.jsbmb.2021.105906. [DOI] [PubMed] [Google Scholar]
- 154.Sen A.K., Sen D.B., Zanwar A.S., Balaraman R., Shah U., Maheshwari R.A. Catechins and Theaflavins: An Overview on Therapeutic Application. J. Nat. Remedies. 2022;22:330–346. doi: 10.18311/jnr/2022/30181. [DOI] [Google Scholar]
- 155.Rosas-González V.C., Téllez-Bañuelos M.C., Hernández-Flores G., Bravo-Cuellar A., Aguilar-Lemarroy A., Jave-Suárez L.F., Haramati J., Solorzano-Ibarra F., Ortiz-Lazareno P.C. Differential effects of alliin and allicin on apoptosis and senescence in luminal A and triple-negative breast cancer: Caspase, Δψm, and pro-apoptotic gene involvement. Fundam. Clin. Pharmacol. 2020;34:671–686. doi: 10.1111/fcp.12559. [DOI] [PubMed] [Google Scholar]
- 156.Wach A., Pyrzyńska K., Biesaga M. Quercetin content in some food and herbal samples. Food Chem. 2005;100:699–704. doi: 10.1016/j.foodchem.2005.10.028. [DOI] [Google Scholar]
- 157.Lin D., Kuang G., Wan J., Zhang X., Li H., Gong X., Li H. Luteolin suppresses the metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via downregulation of β-catenin expression. Oncol. Rep. 2016;37:895–902. doi: 10.3892/or.2016.5311. [DOI] [PubMed] [Google Scholar]
- 158.Wu H.-T., Lin J., Liu Y.-E., Chen H.-F., Hsu K.-W., Lin S.-H., Peng K.-Y., Lin K.-J., Hsieh C.-C., Chen D.-R. Luteolin suppresses androgen receptor-positive triple-negative breast cancer cell proliferation and metastasis by epigenetic regulation of MMP9 expression via the AKT/mTOR signaling pathway. Phytomedicine. 2020;81:153437. doi: 10.1016/j.phymed.2020.153437. [DOI] [PubMed] [Google Scholar]
- 159.Ahmed S., Khan H., Fratantonio D., Hasan M.M., Sharifi S., Fathi N., Ullah H., Rastrelli L. Apoptosis induced by luteolin in breast cancer: Mechanistic and therapeutic perspectives. Phytomedicine. 2019;59:152883. doi: 10.1016/j.phymed.2019.152883. [DOI] [PubMed] [Google Scholar]
- 160.Gao G., Ge R., Li Y., Liu S. Luteolin exhibits anti-breast cancer property through up-regulating miR-203. Artif. Cells Nanomed. Biotechnol. 2019;47:3265–3271. doi: 10.1080/21691401.2019.1646749. [DOI] [PubMed] [Google Scholar]
- 161.Lu Q., Lai Y., Zhang H., Ren K., Liu W., An Y., Yao J., Fan H. Hesperetin Inhibits TGF-β1-Induced Migration and Invasion of Triple Negative Breast Cancer MDA-MB-231 Cells via Suppressing Fyn/Paxillin/RhoA Pathway. Integr. Cancer Ther. 2022;21:15347354221086900. doi: 10.1177/15347354221086900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Palit S., Kar S., Sharma G., Das P.K. Hesperetin Induces Apoptosis in Breast Carcinoma by Triggering Accumulation of ROS and Activation of ASK1/JNK Pathway. J. Cell. Physiol. 2015;230:1729–1739. doi: 10.1002/jcp.24818. [DOI] [PubMed] [Google Scholar]
- 163.Hermawan A., Ikawati M., Khumaira A., Putri H., Jenie R.I., Angraini S.M., Muflikhasari H.A. Bioinformatics and In Vitro Studies Reveal the Importance of p53, PPARG and Notch Signaling Pathway in Inhibition of Breast Cancer Stem Cells by Hesperetin. Adv. Pharm. Bull. 2021;11:351–360. doi: 10.34172/apb.2021.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Choi E.J. Hesperetin Induced G1-Phase Cell Cycle Arrest in Human Breast Cancer MCF-7 Cells: Involvement of CDK4 and p21. Nutr. Cancer. 2007;59:115–119. doi: 10.1080/01635580701419030. [DOI] [PubMed] [Google Scholar]
- 165.Lee C.J., Wilson L., Jordan M.A., Nguyen V., Tang J., Smiyun G. Hesperidin suppressed proliferations of both Human breast cancer and androgen-dependent prostate cancer cells. Phytotherapy Res. 2010;24:S15–S19. doi: 10.1002/ptr.2856. [DOI] [PubMed] [Google Scholar]
- 166.Kongtawelert P., Wudtiwai B., Shwe T.H., Pothacharoen P., Phitak T. Inhibitory Effect of Hesperidin on the Expression of Programmed Death Ligand (PD-L1) in Breast Cancer. Molecules. 2020;25:252. doi: 10.3390/molecules25020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hermawan A., Khumaira A., Ikawati M., Putri H., Jenie R.I., Angraini S.M., Muflikhasari H.A. Identification of key genes of hesperidin in inhibition of breast cancer stem cells by functional network analysis. Comput. Biol. Chem. 2020;90:107427. doi: 10.1016/j.compbiolchem.2020.107427. [DOI] [PubMed] [Google Scholar]
- 168.Taghizadeh M.S., Niazi A., Moghadam A., Afsharifar A. Experimental, molecular docking and molecular dynamic studies of natural products targeting overexpressed receptors in breast cancer. PLoS ONE. 2022;17:e0267961. doi: 10.1371/journal.pone.0267961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Patel P., Shah J. Protective effects of hesperidin through attenuation of Ki67 expression against DMBA-induced breast cancer in female rats. Life Sci. 2021;285:119957. doi: 10.1016/j.lfs.2021.119957. [DOI] [PubMed] [Google Scholar]
- 170.Zhao Z., Jin G., Ge Y., Guo Z. Naringenin inhibits migration of breast cancer cells via inflammatory and apoptosis cell signaling pathways. Inflammopharmacology. 2019;27:1021–1036. doi: 10.1007/s10787-018-00556-3. [DOI] [PubMed] [Google Scholar]
- 171.Sharma M., Dwivedi P., Rawat A.K.S., Dwivedi A.K. 3—Nutrition nutraceuticals: A proactive approach for healthcare. In: Grumezescu A.M., editor. Nutraceuticals. Academic Press; Cambridge, MA, USA: 2016. pp. 79–116. [Google Scholar]
- 172.Ke J.-Y., Banh T., Hsiao Y.-H., Cole R.M., Straka S.R., Yee L.D., Belury M.A. Citrus flavonoid naringenin reduces mammary tumor cell viability, adipose mass, and adipose inflammation in obese ovariectomized mice. Mol. Nutr. Food Res. 2017;61:1600934. doi: 10.1002/mnfr.201600934. [DOI] [PubMed] [Google Scholar]
- 173.Zhang F., Dong W., Zeng W., Zhang L., Zhang C., Qiu Y., Wang L., Yin X., Zhang C., Liang W. Naringenin prevents TGF-β1 secretion from breast cancer and suppresses pulmonary metastasis by inhibiting PKC activation. Breast Cancer Res. 2016;18:38. doi: 10.1186/s13058-016-0698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Harmon A.W., Patel Y.M. Naringenin Inhibits Glucose Uptake in MCF-7 Breast Cancer Cells: A Mechanism for Impaired Cellular Proliferation. Breast Cancer Res. Treat. 2004;85:103–110. doi: 10.1023/B:BREA.0000025397.56192.e2. [DOI] [PubMed] [Google Scholar]
- 175.Bharti S., Rani N., Krishnamurthy B., Arya D.S. Preclinical Evidence for the Pharmacological Actions of Naringin: A Review. Planta Medica. 2014;80:437–451. doi: 10.1055/s-0034-1368351. [DOI] [PubMed] [Google Scholar]
- 176.Li H., Yang B., Huang J., Xiang T., Yin X., Wan J., Luo F., Zhang L., Li H., Ren G. Naringin inhibits growth potential of human triple-negative breast cancer cells by targeting β-catenin signaling pathway. Toxicol. Lett. 2013;220:219–228. doi: 10.1016/j.toxlet.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 177.Way T.D., Kao M.C., Lin J.K. Apigenin induces apoptosis through proteasomal degradation of HER2/neu in HER2/neu-overexpressing breast cancer cells via the phosphatidylinositol 3-kinase/Akt-dependent pathway. J. Biol. Chem. 2004;279:4479–4489. doi: 10.1074/jbc.M305529200. [DOI] [PubMed] [Google Scholar]
- 178.Choi E.J., Kim G.H. Apigenin causes G(2)/M arrest associated with the modulation of p21(Cip1) and Cdc2 and activates p53-dependent apoptosis pathway in human breast cancer SK-BR-3 cells. J. Nutr. Biochem. 2009;20:285–290. doi: 10.1016/j.jnutbio.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 179.Long X., Fan M., Bigsby R.M., Nephew K.P. Apigenin inhibits antiestrogen-resistant breast cancer cell growth through estrogen receptor-α-dependent and estrogen receptor-α-independent mechanisms. Mol. Cancer Ther. 2008;7:2096–2108. doi: 10.1158/1535-7163.MCT-07-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pham T.H., Le Page Y., Percevault F., Ferrière F., Flouriot G., Pakdel F. Apigenin, a Partial Antagonist of the Estrogen Receptor (ER), Inhibits ER-Positive Breast Cancer Cell Proliferation through Akt/FOXM1 Signaling. Int. J. Mol. Sci. 2021;22:470. doi: 10.3390/ijms22010470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lee W.J., Chen W.K., Wang C.J., Lin W.L., Tseng T.H. Apigenin inhibits HGF-promoted invasive growth and metastasis involving blocking PI3K/Akt pathway and beta 4 integrin function in MDA-MB-231 breast cancer cells. Toxicol. Appl. Pharmacol. 2008;226:178–191. doi: 10.1016/j.taap.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 182.Ko Y.-C., Choi H.S., Liu R., Kim J.-H., Kim S.-L., Yun B.-S., Lee D.-S. Inhibitory Effects of Tangeretin, a Citrus Peel-Derived Flavonoid, on Breast Cancer Stem Cell Formation through Suppression of Stat3 Signaling. Molecules. 2020;25:2599. doi: 10.3390/molecules25112599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Arivazhagan L., Pillai S.S. Tangeretin, a citrus pentamethoxyflavone, exerts cytostatic effect via p53/p21 up-regulation and suppresses metastasis in 7,12-dimethylbenz(alpha)anthracene-induced rat mammary carcinoma. J. Nutr. Biochem. 2014;25:1140–1153. doi: 10.1016/j.jnutbio.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 184.Surichan S., Arroo R.R., Tsatsakis A.M., Androutsopoulos V.P. Tangeretin inhibits the proliferation of human breast cancer cells via CYP1A1/CYP1B1 enzyme induction and CYP1A1/CYP1B1-mediated metabolism to the product 4′ hydroxy tangeretin. Toxicol In Vitro. 2018;50:274–284. doi: 10.1016/j.tiv.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 185.Periyasamy K., Baskaran K., Ilakkia A., Vanitha K., Selvaraj S., Sakthisekaran D. Antitumor efficacy of tangeretin by targeting the oxidative stress mediated on 7,12-dimethylbenz(a) anthracene-induced proliferative breast cancer in Sprague-Dawley rats. Cancer Chemother. Pharmacol. 2015;75:263–272. doi: 10.1007/s00280-014-2629-z. [DOI] [PubMed] [Google Scholar]
- 186.Montalesi E., Cipolletti M., Cracco P., Fiocchetti M., Marino M. Divergent Effects of Daidzein and Its Metabolites on Estrogen-Induced Survival of Breast Cancer Cells. Cancers. 2020;12:167. doi: 10.3390/cancers12010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sharifi-Rad J., Quispe C., Imran M., Rauf A., Nadeem M., Gondal T.A., Ahmad B., Atif M., Mubarak M.S., Sytar O., et al. Genistein: An Integrative Overview of Its Mode of Action, Pharmacological Properties, and Health Benefits. Oxidative Med. Cell. Longev. 2021;2021:3268136. doi: 10.1155/2021/3268136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hsieh P.-L., Liao Y.-W., Hsieh C.-W., Chen P.-N., Yu C.-C. Soy Isoflavone Genistein Impedes Cancer Stemness and Mesenchymal Transition in Head and Neck Cancer through Activating miR-34a/RTCB Axis. Nutrients. 2020;12:1924. doi: 10.3390/nu12071924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hwang S.T., Yang M.H., Baek S.H., Um J.Y., Ahn K.S. Genistin attenuates cellular growth and promotes apoptotic cell death breast cancer cells through modulation of ERalpha signaling pathway. Life Sci. 2020;263:118594. doi: 10.1016/j.lfs.2020.118594. [DOI] [PubMed] [Google Scholar]
- 190.Elkhalifa A.E.O., Al-Shammari E., Kuddus M., Adnan M., Sachidanandan M., Awadelkareem A.M., Qattan M.Y., Khan M.I., Abduljabbar S.I., Baig M.S., et al. Structure-Based Multi-Targeted Molecular Docking and Dynamic Simulation of Soybean-Derived Isoflavone Genistin as a Potential Breast Cancer Signaling Proteins Inhibitor. Life. 2023;13:1739. doi: 10.3390/life13081739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hong R., Lim S.C., Lee T.B., Han S.I. Anticancer Effect of Gallic Acid on Acidity-Induced Invasion of MCF7 Breast Cancer Cells. Nutrients. 2023;15:3596. doi: 10.3390/nu15163596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yang K., Zhang L., Liao P., Xiao Z., Zhang F., Sindaye D., Xin Z., Tan C., Deng J., Yin Y., et al. Impact of Gallic Acid on Gut Health: Focus on the Gut Microbiome, Immune Response, and Mechanisms of Action. Front. Immunol. 2020;11:580208. doi: 10.3389/fimmu.2020.580208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Xiang Z., Guan H., Zhao X., Xie Q., Xie Z., Cai F., Dang R., Li M., Wang C. Dietary gallic acid as an antioxidant: A review of its food industry applications, health benefits, bioavailability, nano-delivery systems, and drug interactions. Food Res. Int. 2024;180:114068. doi: 10.1016/j.foodres.2024.114068. [DOI] [PubMed] [Google Scholar]
- 194.Cheung Y., Meenu M., Yu X., Xu B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019;22:290–308. doi: 10.1080/10942912.2019.1579835. [DOI] [Google Scholar]
- 195.Lin S., Qin H.-Z., Li Z.-Y., Zhu H., Long L., Xu L.-B. Gallic acid suppresses the progression of triple-negative breast cancer HCC1806 cells via modulating PI3K/AKT/EGFR and MAPK signaling pathways. Front. Pharmacol. 2022;13:1049117. doi: 10.3389/fphar.2022.1049117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Khorsandi K., Kianmehr Z., Hosseinmardi Z., Hosseinzadeh R. Anti-cancer effect of gallic acid in presence of low level laser irradiation: ROS production and induction of apoptosis and ferroptosis. Cancer Cell Int. 2020;20:18. doi: 10.1186/s12935-020-1100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Kaur J., Gulati M., Singh S.K., Kuppusamy G., Kapoor B., Mishra V., Gupta S., Arshad M.F., Porwal O., Jha N.K., et al. Discovering multifaceted role of vanillic acid beyond flavours: Nutraceutical and therapeutic potential. Trends Food Sci. Technol. 2022;122:187–200. doi: 10.1016/j.tifs.2022.02.023. [DOI] [Google Scholar]
- 198.Shanmugam M. Vanillic acid exhibits potent antiproliferative and free radical scavenging effects under in vitro conditions. Int. J. Nutr. Pharmacol. Neurol. Dis. 2023;13:188–198. [Google Scholar]
- 199.Yin M.-C., Lin C.-C., Wu H.-C., Tsao S.-M., Hsu C.-K. Apoptotic Effects of Protocatechuic Acid in Human Breast, Lung, Liver, Cervix, and Prostate Cancer Cells: Potential Mechanisms of Action. J. Agric. Food Chem. 2009;57:6468–6473. doi: 10.1021/jf9004466. [DOI] [PubMed] [Google Scholar]
- 200.Punvittayagul C., Luangsuphabool T., Wongpoomchai R. Protocatechuic acid as a potent anticarcinogenic compound in purple rice bran against diethylnitrosamine-initiated rat hepatocarcinogenesis. Sci. Rep. 2022;12:10548. doi: 10.1038/s41598-022-14888-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sułkowska-Ziaja K., Muszyńska B., Szewczyk A. Antioxidant components of selected indigenous edible mushrooms of the obsolete order Aphyllophorales. Rev. Iberoam. De Micol. 2015;32:99–102. doi: 10.1016/j.riam.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 202.Cadena-Iñiguez J., Santiago-Osorio E., Sánchez-Flores N., Salazar-Aguilar S., Soto-Hernández R.M., Riviello-Flores M.d.l.L., Macías-Zaragoza V.M., Aguiñiga-Sánchez I. The Cancer-Protective Potential of Protocatechuic Acid: A Narrative Review. Molecules. 2024;29:1439. doi: 10.3390/molecules29071439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Jha S., Prabakaran A., Sahoo R.K., Batheja S., Gupta U., Alexander A. Antiproliferative activity of syringic acid-loaded nanostructured lipid carriers against MCF-7 human breast carcinoma cells. J. Drug Deliv. Sci. Technol. 2024;98:105902. doi: 10.1016/j.jddst.2024.105902. [DOI] [Google Scholar]
- 204.Zhang L., La X., Tian J., Li H., Li A., Liu Y., Wu C., Li Z. The phytochemical vitexin and syringic acid derived from foxtail fillet bran inhibit breast cancer cells proliferation via GRP78/SREBP-1/SCD1 signaling axis. J. Funct. Foods. 2021;85:104620. doi: 10.1016/j.jff.2021.104620. [DOI] [Google Scholar]
- 205.Golmohammadi M., Zamanian M.Y., Jalal S.M., Noraldeen S.A.M., Ramírez-Coronel A.A., Oudaha K.H., Obaid R.F., Almulla A.F., Bazmandegan G., Kamiab Z. A comprehensive review on Ellagic acid in breast cancer treatment: From cellular effects to molecular mechanisms of action. Food Sci. Nutr. 2023;11:7458–7468. doi: 10.1002/fsn3.3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rezaei-Seresht H., Cheshomi H., Falanji F., Movahedi-Motlagh F., Hashemian M., Mireskandari E. Cytotoxic activity of caffeic acid and gallic acid against MCF-7 human breast cancer cells: An in silico and in vitro study. Avicenna J. Phytomedicine. 2019;9:574–586. doi: 10.22038/AJP.2019.13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Alam M., Ahmed S., Elasbali A.M., Adnan M., Alam S., Hassan I., Pasupuleti V.R. Therapeutic Implications of Caffeic Acid in Cancer and Neurological Diseases. Front. Oncol. 2022;12:860508. doi: 10.3389/fonc.2022.860508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Assumpção J.H.M., Takeda A.A.S., Sforcin J.M., Rainho C.A. Effects of Propolis and Phenolic Acids on Triple-Negative Breast Cancer Cell Lines: Potential Involvement of Epigenetic Mechanisms. Molecules. 2020;25:1289. doi: 10.3390/molecules25061289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Pal A., Tapadar P., Pal R. Exploring the Molecular Mechanism of Cinnamic Acid-Mediated Cytotoxicity in Triple Negative MDA-MB-231 Breast Cancer Cells. Anti-Cancer Agents Med. Chem. 2021;21:1141–1150. doi: 10.2174/1871520620666200807222248. [DOI] [PubMed] [Google Scholar]
- 210.Rodrigues D.M., Portapilla G.B., de Sicco G.S., da Silva I.F.R., de Albuquerque S., Bastos J.K., Campo V.L. Novel synthetic derivatives of cinnamic and p-coumaric acids with antiproliferative effect on breast MCF-7 tumor cells. Nat. Prod. Res. 2023;37:4210–4220. doi: 10.1080/14786419.2023.2177992. [DOI] [PubMed] [Google Scholar]
- 211.Zhao Y., Liu J. Anti-Inflammatory Effects of p-coumaric Acid in LPS-Stimulated RAW264.7 Cells: Involvement of NF-κB and MAPKs Pathways. Med. Chem. 2016;6:327–330. doi: 10.4172/2161-0444.1000365. [DOI] [Google Scholar]
- 212.Kaur J., Kaur R. p-Coumaric Acid: A Naturally Occurring Chemical with Potential Therapeutic Applications. Curr. Org. Chem. 2022;26:1333–1349. doi: 10.2174/1385272826666221012145959. [DOI] [Google Scholar]
- 213.Bao X., Li W., Jia R., Meng D., Zhang H., Xia L. Molecular mechanism of ferulic acid and its derivatives in tumor progression. Pharmacol. Rep. 2023;75:891–906. doi: 10.1007/s43440-023-00494-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Gupta A., Singh A.K., Loka M., Pandey A.K., Bishayee A. Chapter Eight—Ferulic acid-mediated modulation of apoptotic signaling pathways in cancer. In: Donev R., editor. Advances in Protein Chemistry and Structural Biology. Academic Press; Cambridge, MA, USA: 2021. pp. 215–257. [DOI] [PubMed] [Google Scholar]
- 215.Zhang X., Lin D., Jiang R., Li H., Wan J., Li H. Ferulic acid exerts antitumor activity and inhibits metastasis in breast cancer cells by regulating epithelial to mesenchymal transition. Oncol. Rep. 2016;36:271–278. doi: 10.3892/or.2016.4804. [DOI] [PubMed] [Google Scholar]
- 216.ElKhazendar M., Chalak J., El-Huneidi W., Vinod A., Abdel-Rahman W.M., Abu-Gharbieh E. Antiproliferative and proapoptotic activities of ferulic acid in breast and liver cancer cell lines. Trop. J. Pharm. Res. 2019;18:2571–2576. [Google Scholar]
- 217.Serafim T.L., Milhazes N., Borges F., Oliveira P.J. Chapter 74—Caffeic and Ferulic Acid Derivatives: Use in Breast Cancer. In: Preedy V.R., editor. Coffee in Health and Disease Prevention. Academic Press; San Diego, CA, USA: 2015. pp. 663–671. [Google Scholar]
- 218.Pandi A., Kalappan V.M. Pharmacological and therapeutic applications of Sinapic acid—An updated review. Mol. Biol. Rep. 2021;48:3733–3745. doi: 10.1007/s11033-021-06367-0. [DOI] [PubMed] [Google Scholar]
- 219.Dwivedi P.S.R., Shastry C.S. The cytotoxic potential of sinapic acid on luminal A breast cancer; a computational and experimental pharmacology approach. J. Biomol. Struct. Dyn. 2023:1–16. doi: 10.1080/07391102.2023.2274980. [DOI] [PubMed] [Google Scholar]
- 220.Konstantinou E.K., Panagiotopoulos A.A., Argyri K., Panoutsopoulos G.I., Dimitriou M., Gioxari A. Molecular Pathways of Rosmarinic Acid Anticancer Activity in Triple-Negative Breast Cancer Cells: A Literature Review. Nutrients. 2023;16:2. doi: 10.3390/nu16010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Messeha S.S., Zarmouh N.O., Asiri A., Soliman K.F. Rosmarinic acid-induced apoptosis and cell cycle arrest in triple-negative breast cancer cells. Eur. J. Pharmacol. 2020;885:173419. doi: 10.1016/j.ejphar.2020.173419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zeng A., Liang X., Zhu S., Liu C., Wang S., Zhang Q., Zhao J., Song L. Chlorogenic acid induces apoptosis, inhibits metastasis and improves antitumor immunity in breast cancer via the NF-κB signaling pathway. Oncol. Rep. 2021;45:717–727. doi: 10.3892/or.2020.7891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Veljkovic E., Xia W., Phillips B., Wong E.T., Ho J., Oviedo A. Nicotine and Other Tobacco Compounds in Neurodegenerative and Psychiatric Diseases. Academic Press; Cambridge, MA, USA: 2018. Chapter 10—Other Compounds From Tobacco With Potential Impact on Neurodegenerative Diseases; pp. 83–97. [Google Scholar]
- 224.Sakai E., Farhana F., Yamaguchi Y., Tsukuba T. Chapter 1—Potentials of natural antioxidants from plants as antiosteoporotic agents. In: Attaur R., editor. Studies in Natural Products Chemistry. Elsevier; Amsterdam, The Netherlands: 2022. pp. 1–28. [Google Scholar]
- 225.Turrini E., Maffei F., Milelli A., Calcabrini C., Fimognari C. Overview of the Anticancer Profile of Avenanthramides from Oat. Int. J. Mol. Sci. 2019;20:4536. doi: 10.3390/ijms20184536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Hastings J., Kenealey J. Avenanthramide-C reduces the viability of MDA-MB-231 breast cancer cells through an apoptotic mechanism. Cancer Cell Int. 2017;17:93. doi: 10.1186/s12935-017-0464-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Posadino A.M., Giordo R., Ramli I., Zayed H., Nasrallah G.K., Wehbe Z., Eid A.H., Gürer E.S., Kennedy J.F., Aldahish A.A., et al. An updated overview of cyanidins for chemoprevention and cancer therapy. Biomed. Pharmacother. 2023;163:114783. doi: 10.1016/j.biopha.2023.114783. [DOI] [PubMed] [Google Scholar]
- 228.Mirmalek S., Faraji S., Ranjbaran S., Aryan H., Arani H., Jangholi E., Marzouni H., Salimi-Tabatabaee S. Cyanidin 3-glycoside induced apoptosis in MCF-7 breast cancer cell line. Arch. Med Sci. 2020;16:1092–1098. doi: 10.5114/aoms.2020.93789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Xu M., Bower K.A., Wang S., Frank J.A., Chen G., Ding M., Wang S., Shi X., Ke Z., Luo J. Cyanidin-3-Glucoside inhibits ethanol-induced invasion of breast cancer cells overexpressing ErbB2. Mol. Cancer. 2010;9:285. doi: 10.1186/1476-4598-9-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Nafisah W., Nugraha A.P., Nugroho A., Sakinah A.I., Nusantara D.S., Philia J., Kurniawinata M.I., Aini W., Herlina V.T. Benefit of Asian pigmented rice bioactive compound and its implication in breast cancer: A systematic review [version 1; peer review: 2 approved, 1 approved with reservations, 1 not approved] F1000Research. 2023;12:371. doi: 10.12688/f1000research.130329.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Omenn G.S. What accounts for the association of vegetables and fruits with lower incidence of cancers and coronary heart disease? Ann. Epidemiol. 1995;5:333–335. doi: 10.1016/1047-2797(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 232.Husain A., Chanana H., Alam Khan S., Dhanalekshmi U.M., Ali M., Alghamdi A.A., Ahmad A. Chemistry and Pharmacological Actions of Delphinidin, a Dietary Purple Pigment in Anthocyanidin and Anthocyanin Forms. Front. Nutr. 2022;9:746881. doi: 10.3389/fnut.2022.746881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Chen J., Zhu Y., Zhang W., Peng X., Zhou J., Li F., Han B., Liu X., Ou Y., Yu X. Delphinidin induced protective autophagy via mTOR pathway suppression and AMPK pathway activation in HER-2 positive breast cancer cells. BMC Cancer. 2018;18:342. doi: 10.1186/s12885-018-4231-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Sood R., Sanjay, Choi H.-K., Lee H.-J. Potential anti-cancer properties of malvidin and its glycosides: Evidence from in vitro and in vivo studies. J. Funct. Foods. 2024;116:106191. doi: 10.1016/j.jff.2024.106191. [DOI] [Google Scholar]
- 235.Laksmiani N.P.L., I Widiastari M., Reynaldi K.R. The inhibitory activity of peonidin purple sweet potato in human epidermal receptor-2 receptor (her-2) expression by in silico study. J. Physics. Conf. Ser. 2018;1040:012010. doi: 10.1088/1742-6596/1040/1/012010. [DOI] [Google Scholar]
- 236.Sinha D., Sarkar N., Biswas J., Bishayee A. Resveratrol for breast cancer prevention and therapy: Preclinical evidence and molecular mechanisms. Semin. Cancer Biol. 2016;40–41:209–232. doi: 10.1016/j.semcancer.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 237.Khezrian A., Shojaeian A. Therapeutic Opportunities in Breast Cancer by Targeting Macrophage Migration Inhibitory Factor as a Pleiotropic Cytokine. Breast Cancer Basic Clin. Res. 2024;18:11782234241276310. doi: 10.1177/11782234241276310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Almaguer G., Ortiz-Vilchis P., Cordero P., Martinez-Vega R., Perez-Durán J., Meaney E., Villarreal F., Ceballos G., Nájera N. Anticancer potential of (−)-epicatechin in a triple-negative mammary gland model. J. Pharm. Pharmacol. 2021;73:1675–1682. doi: 10.1093/jpp/rgab133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kiran S., Patra A., Verma P., Purkait S., Chhabra G., Guttula P.K., Ghosh A. Restoration of Altered Oncogenic and Tumor Suppressor microRNA Expression in Breast Cancer and Colorectal Cancer Cell using Epicatechin. Curr. Mol. Pharmacol. 2023;16:915–926. doi: 10.2174/1874467216666230210091839. [DOI] [PubMed] [Google Scholar]
- 240.Pereyra-Vergara F., Olivares-Corichi I.M., Perez-Ruiz A.G., Luna-Arias J.P., García-Sánchez J.R. Apoptosis Induced by (−)-Epicatechin in Human Breast Cancer Cells is Mediated by Reactive Oxygen Species. Molecules. 2020;25:1020. doi: 10.3390/molecules25051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Ndacyayisenga J., Tolo F.M., Wamunyokoli F., Maina E.N. Effects of tea catechin extracts from BB35 and purple (TRFK 306) tea clones on the gene expression of Egfr, App, Bcl2, Dnmt, Casp3, Hif1a, Gadd45b and Psmb5 genes involved in triple negative breast cancer diseases: In silico and in vitro study. Inform. Med. Unlocked. 2024;46:101469. doi: 10.1016/j.imu.2024.101469. [DOI] [Google Scholar]
- 242.Delgado L., Fernandes I., González-Manzano S., de Freitas V., Mateus N., Santos-Buelga C. Anti-proliferative effects of quercetin and catechin metabolites. Food Funct. 2014;5:797–803. doi: 10.1039/c3fo60441a. [DOI] [PubMed] [Google Scholar]
- 243.Zhang L., Meng S., Yan B., Chen J., Zhou L., Shan L., Wang Y. Anti-Proliferative, Pro-Apoptotic, Anti-Migrative and Tumor-Inhibitory Effects and Pleiotropic Mechanism of Theaflavin on B16F10 Melanoma Cells. OncoTargets Ther. 2021;14:1291–1304. doi: 10.2147/OTT.S286350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Lahiry L., Saha B., Chakraborty J., Adhikary A., Mohanty S., Hossain D.M.S., Banerjee S., Das K., Sa G., Das T. Theaflavins target Fas/caspase-8 and Akt/pBad pathways to induce apoptosis in p53-mutated human breast cancer cells. Carcinogenesis. 2009;31:259–268. doi: 10.1093/carcin/bgp240. [DOI] [PubMed] [Google Scholar]
- 245.Adhikary A., Mohanty S., Lahiry L., Hossain D.M.S., Chakraborty S., Das T. Theaflavins retard human breast cancer cell migration by inhibiting NF-kappaB via p53-ROS cross-talk. FEBS Lett. 2010;584:7–14. doi: 10.1016/j.febslet.2009.10.081. [DOI] [PubMed] [Google Scholar]
- 246.Bhadresha K., Kumar S.P., Brahmbhatt J., Patel C., Pandya P., Jain N., Rawal R. Theaflavin-3-gallate, a natural antagonist for Hsp90: In-silico and in-vitro approach. Chem. Interact. 2022;353:109774. doi: 10.1016/j.cbi.2021.109774. [DOI] [PubMed] [Google Scholar]
- 247.Nahar L., Al-Groshi A., Kumar A., Sarker S.D. Arbutin: Occurrence in Plants, and Its Potential as an Anticancer Agent. Molecules. 2022;27:8786. doi: 10.3390/molecules27248786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Avelino-Flores M.d.C., Cruz-López M.D.C., Jimenez-Montejo F.E., Reyes-Leyva J. Cytotoxic Activity of the Methanolic Extract of Turnera diffusa Willd on Breast Cancer Cells. J. Med. Food. 2014;18:299–305. doi: 10.1089/jmf.2013.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Hazman Ö., Sarıova A., Bozkurt M.F., Ciğerci İ.H. The anticarcinogen activity of β-arbutin on MCF-7 cells: Stimulation of apoptosis through estrogen receptor-α signal pathway, inflammation and genotoxicity. Mol. Cell. Biochem. 2021;476:349–360. doi: 10.1007/s11010-020-03911-7. [DOI] [PubMed] [Google Scholar]
- 250.Ma J., Chen S., Li Y., Wu X., Song Z. Arbutin improves gut development and serum lipids via Lactobacillus intestinalis. Front. Nutr. 2022;9:948573. doi: 10.3389/fnut.2022.948573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Chinnikrishnan P., Ibrahim I.A.A., Alzahrani A.R., Shahzad N., Sivaprakasam P., Pandurangan A.K. The Role of Selective Flavonoids on Triple-Negative Breast Cancer: An Update. Separations. 2023;10:207. doi: 10.3390/separations10030207. [DOI] [Google Scholar]
- 252.Ranganathan S., Halagowder D., Sivasithambaram N.D. Quercetin Suppresses Twist to Induce Apoptosis in MCF-7 Breast Cancer Cells. PLoS ONE. 2015;10:e0141370. doi: 10.1371/journal.pone.0141370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Mawalizadeh F., Mohammadzadeh G., Khedri A., Rashidi M. Quercetin potentiates the chemosensitivity of MCF-7 breast cancer cells to 5-fluorouracil. Mol. Biol. Rep. 2021;48:7733–7742. doi: 10.1007/s11033-021-06782-3. [DOI] [PubMed] [Google Scholar]
- 254.Aljabr B.A., Zihlif M., Abu-Dahab R., Zalloum H. Effect of quercetin on doxorubicin cytotoxicity in sensitive and resistant human MCF7 breast cancer cell lines. Biomed. Rep. 2024;20:58. doi: 10.3892/br.2024.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Chen W.J., Tsai J.H., Hsu L.S., Lin C.L., Hong H.M., Pan M.H. Quercetin Blocks the Aggressive Phenotype of Triple Negative Breast Cancer by Inhibiting IGF1/IGF1R-Mediated EMT Program. J. Food Drug Anal. 2021;29:98–112. doi: 10.38212/2224-6614.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Maugeri A., Calderaro A., Patanè G.T., Navarra M., Barreca D., Cirmi S., Felice M.R. Targets Involved in the Anti-Cancer Activity of Quercetin in Breast, Colorectal and Liver Neoplasms. Int. J. Mol. Sci. 2023;24:2952. doi: 10.3390/ijms24032952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Adams C., Conigrave J.H., Lewohl J., Haber P., Morley K.C. Alcohol use disorder and circulating cytokines: A systematic review and meta-analysis. Brain, Behav. Immun. 2020;89:501–512. doi: 10.1016/j.bbi.2020.08.002. [DOI] [PubMed] [Google Scholar]
- 258.Gholamalizadeh M., Afsharfar M., Fathi S., Tajadod S., Mohseni G.K., Shekari S., Vahid F., Doaei S., Kachaei H.S., Majidi N., et al. Relationship between breast cancer and dietary inflammatory index; a case–control study. Clin. Nutr. ESPEN. 2022;51:353–358. doi: 10.1016/j.clnesp.2022.08.001. [DOI] [PubMed] [Google Scholar]
- 259.Wang K., Sun J.-Z., Wu Q.-X., Li Z.-Y., Li D.-X., Xiong Y.-F., Zhong G.-C., Shi Y., Li Q., Zheng J., et al. Long-term anti-inflammatory diet in relation to improved breast cancer prognosis: A prospective cohort study. npj Breast Cancer. 2020;6:36. doi: 10.1038/s41523-020-00179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Helo D., Appiah L., Bhende K.M., Byrd T.L., Appiah D. The association of skipping breakfast with cancer-related and all-cause mortality in a national cohort of United States adults. Cancer Causes Control. 2021;32:505–513. doi: 10.1007/s10552-021-01401-9. [DOI] [PubMed] [Google Scholar]
- 261.Fillon M. Skipping breakfast is a bad idea for patients with cancer. CA Cancer J. Clin. 2021;71:363–365. doi: 10.3322/caac.21691. [DOI] [PubMed] [Google Scholar]
- 262.Tapan T.K., Iyigun Z.E., Ilgun S., Ozmen V. Evaluation of the eating habits of breast cancer patients. Pak. J. Med. Sci. 2020;36:1562–1566. doi: 10.12669/pjms.36.7.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Danforth D.N. The Role of Chronic Inflammation in the Development of Breast Cancer. Cancers. 2021;13:3918. doi: 10.3390/cancers13153918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Wang W., Nag S.A., Zhang R. Targeting the NFκB Signaling Pathways for Breast Cancer Prevention and Therapy. Curr. Med. Chem. 2014;22:264–289. doi: 10.2174/0929867321666141106124315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kansestani A.N., Mansouri K., Hemmati S., Zare M.E., Moatafaei A. High Glucose-reduced Apoptosis in Human Breast Cancer Cells Is Mediated by Activation of NF-κB. Iran. J. Allergy Asthma Immunol. 2019;18:153–162. doi: 10.18502/ijaai.v18i2.918. [DOI] [PubMed] [Google Scholar]
- 266.Ford K.L., Arends J., Atherton P.J., Engelen M.P., Gonçalves T.J., Laviano A., Lobo D.N., Phillips S.M., Ravasco P., Deutz N.E., et al. The importance of protein sources to support muscle anabolism in cancer: An expert group opinion. Clin. Nutr. 2021;41:192–201. doi: 10.1016/j.clnu.2021.11.032. [DOI] [PubMed] [Google Scholar]
- 267.Godfray H.C.J., Aveyard P., Garnett T., Hall J.W., Key T.J., Lorimer J., Pierrehumbert R.T., Scarborough P., Springmann M., Jebb S.A. Meat consumption, health, and the environment. Science. 2018;361:eaam5324. doi: 10.1126/science.aam5324. [DOI] [PubMed] [Google Scholar]
- 268.Inoue-Choi M., Sinha R., Gierach G.L., Ward M.H. Red and processed meat, nitrite, and heme iron intakes and postmenopausal breast cancer risk in the NIH-AARP Diet and Health Study. Int. J. Cancer. 2016;138:1609–1618. doi: 10.1002/ijc.29901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Kim S.-A., Shin S. Red meat and processed meat consumption and the risk of dyslipidemia in Korean adults: A prospective cohort study based on the Health Examinees (HEXA) study. Nutr. Metab. Cardiovasc. Dis. 2021;31:1714–1727. doi: 10.1016/j.numecd.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 270.Nouri M., Mohsenpour M.A., Katsiki N., Ghobadi S., Jafari A., Faghih S., Banach M., Mazidi M. Effect of Serum Lipid Profile on the Risk of Breast Cancer: Systematic Review and Meta-Analysis of 1,628,871 Women. J. Clin. Med. 2022;11:4503. doi: 10.3390/jcm11154503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Brindisi M., Frattaruolo L., Fiorillo M., Dolce V., Sotgia F., Lisanti M.P., Cappello A.R. New insights into cholesterol-mediated ERRα activation in breast cancer progression and pro-tumoral microenvironment orchestration. FEBS J. 2022;290:1481–1501. doi: 10.1111/febs.16651. [DOI] [PubMed] [Google Scholar]
- 272.Strizova Z., Benesova I., Bartolini R., Novysedlak R., Cecrdlova E., Foley L.K., Striz I. M1/M2 macrophages and their overlaps—Myth or reality? Clin. Sci. 2023;137:1067–1093. doi: 10.1042/CS20220531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Yang Y., Xin X., Fu X., Xu D. Expression pattern of human SERPINE2 in a variety of human tumors. Oncol. Lett. 2018;15:4523–4530. doi: 10.3892/ol.2018.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Krawczynska N., Wang Y., Lim K., Das Gupta A., Lenczowski A., Abughazaleh M., Bendre S.V., Kockaya L.I., Schane C.P., Fei Y., et al. Neutrophils exposed to a cholesterol metabolite secrete extracellular vesicles that promote epithelial-mesenchymal transition and stemness in breast cancer cells. bioRxiv. 2024:bioRxiv:2024.08.02.606061. doi: 10.1101/2024.08.02.606061. [DOI] [Google Scholar]
- 275.Narii N., Zha L., Komatsu M., Kitamura T., Sobue T., Ogawa T. Cholesterol and breast cancer risk: A cohort study using health insurance claims and health checkup databases. Breast Cancer Res. Treat. 2023;199:315–322. doi: 10.1007/s10549-023-06917-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Tjønneland A., Stripp C., Christensen J., Thomsen B.L., Olsen A., Overvad K., Møller S. Fish Intake Is Positively Associated with Breast Cancer Incidence Rate. J. Nutr. 2003;133:3664–3669. doi: 10.1093/jn/133.11.3664. [DOI] [PubMed] [Google Scholar]
- 277.Wu S., Ma X., Zhang X., Shi C., Cao M., Yang C., Qi Y., Liu Y. Relationship between oily fish intake and breast cancer based on estrogen receptor status: A Mendelian randomization study. Breast Cancer Res. Treat. 2023;203:145–152. doi: 10.1007/s10549-023-07130-8. [DOI] [PubMed] [Google Scholar]
- 278.Nindrea R.D., Aryandono T., Lazuardi L., Dwiprahasto I. Protective Effect of Omega-3 Fatty Acids in Fish Consumption Against Breast Cancer in Asian Patients: A Meta-Analysis. Asian Pac. J. Cancer Prev. 2019;20:327–332. doi: 10.31557/APJCP.2019.20.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Abera B.D., Adimas M.A. Health benefits and health risks of contaminated fish consumption: Current research outputs, research approaches, and perspectives. Heliyon. 2024;10:e33905. doi: 10.1016/j.heliyon.2024.e33905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Brodziak-Dopierała B., Fischer A. Analysis of the Mercury Content in Fish for Human Consumption in Poland. Toxics. 2023;11:717. doi: 10.3390/toxics11080717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Skalny A.V., Aschner M., Sekacheva M.I., Santamaria A., Barbosa F., Ferrer B., Aaseth J., Paoliello M.M., Rocha J.B., Tinkov A.A. Mercury and cancer: Where are we now after two decades of research? Food Chem. Toxicol. 2022;164:113001. doi: 10.1016/j.fct.2022.113001. [DOI] [PubMed] [Google Scholar]
- 282.Yedier S., Yalçınkaya S.K., Bostancı D. Exposure to polypropylene microplastics via diet and water induces oxidative stress in Cyprinus carpio. Aquat. Toxicol. 2023;259:106540. doi: 10.1016/j.aquatox.2023.106540. [DOI] [PubMed] [Google Scholar]
- 283.Park J.H., Hong S., Kim O.-H., Kim C.-H., Kim J., Kim J.-W., Hong S., Lee H.J. Polypropylene microplastics promote metastatic features in human breast cancer. Sci. Rep. 2023;13:6252. doi: 10.1038/s41598-023-33393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Polak-Juszczak L., Waszak I., Szlinder-Richert J., Wójcik I. Levels, time trends, and distribution of dioxins and polychlorinated biphenyls in fishes from the Baltic Sea. Chemosphere. 2022;306:135614. doi: 10.1016/j.chemosphere.2022.135614. [DOI] [PubMed] [Google Scholar]
- 285.VoPham T., Bertrand K.A., Jones R.R., Deziel N.C., DuPré N.C., James P., Liu Y., Vieira V.M., Tamimi R.M., Hart J.E., et al. Dioxin exposure and breast cancer risk in a prospective cohort study. Environ. Res. 2020;186:109516. doi: 10.1016/j.envres.2020.109516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Meurillon M., Engel E. Encyclopedia of Meat Sciences. CRC Press; Boca Raton, FL, USA: 2022. Potential chemical hazards linked to meat processing. [Google Scholar]
- 287.Bulanda S., Janoszka B. Consumption of Thermally Processed Meat Containing Carcinogenic Compounds (Polycyclic Aromatic Hydrocarbons and Heterocyclic Aromatic Amines) versus a Risk of Some Cancers in Humans and the Possibility of Reducing Their Formation by Natural Food Additives—A Literature Review. Int. J. Environ. Res. Public Health. 2022;19:4781. doi: 10.3390/ijerph19084781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Biswas A.K., Jairath G., Mendiratta S.K., Kumar D., Bauer F. Encyclopedia of Meat Sciences (Third Edition) Elsevier; Amsterdam, The Netherlands: 2022. Residues associated with meat production and processing. [DOI] [Google Scholar]
- 289.Muthukumar M., Banerjee R., Naveena B.M. Potential chemical hazards associated with meat. In: Dikeman M., editor. Encyclopedia of Meat Sciences. 3rd ed. Elsevier; Oxford, UK: 2024. pp. 582–590. [DOI] [Google Scholar]
- 290.Reig M., Toldrá F. Chapter 11—Residues of harmful chemicals and their detection techniques. In: Biswas A.K., Mandal P.K., editors. Meat Quality Analysis. Academic Press; Cambridge, MA, USA: 2020. pp. 173–183. [Google Scholar]
- 291.Gearhart-Serna L.M., Davis J.B., Jolly M.K., Jayasundara N., Sauer S.J., Di Giulio R.T., Devi G.R. A polycyclic aromatic hydrocarbon-enriched environmental chemical mixture enhances AhR, antiapoptotic signaling and a proliferative phenotype in breast cancer cells. Carcinog. 2020;41:1648–1659. doi: 10.1093/carcin/bgaa047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Kehm R.D., Walter E.J., Oskar S., White M.L., Tehranifar P., Herbstman J.B., Perera F., Lilge L., Miller R.L., Terry M.B. Exposure to polycyclic aromatic hydrocarbons during pregnancy and breast tissue composition in adolescent daughters and their mothers: A prospective cohort study. Breast Cancer Res. 2022;24:47. doi: 10.1186/s13058-022-01546-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Sheikh I.A., Jiffri E.H., Kamal M.A., Ashraf G.M., Beg M.A. Lactoperoxidase, an Antimicrobial Milk Protein, as a Potential Activator of Carcinogenic Heterocyclic Amines in Breast Cancer. Anticancer. Res. 2017;37:6415–6420. doi: 10.21873/anticanres.12095. [DOI] [PubMed] [Google Scholar]
- 294.Rahman U.U., Sahar A., Khan M.I., Nadeem M. Production of heterocyclic aromatic amines in meat: Chemistry, health risks and inhibition. A review. LWT. 2014;59:229–233. doi: 10.1016/j.lwt.2014.06.005. [DOI] [Google Scholar]
- 295.Bellamri M., Walmsley S.J., Turesky R.J. Metabolism and biomarkers of heterocyclic aromatic amines in humans. Genes Environ. 2021;43:29. doi: 10.1186/s41021-021-00200-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Sheikh I.A., Beg M.A., Yasir M. Molecular Interactions of Carcinogenic Aromatic Amines, 4-Aminobiphenyl and 4,4′-Diaminobiphenyl, with Lactoperoxidase—Insight to Breast Cancer. Anticancer. Res. 2017;37:6245–6249. doi: 10.21873/anticanres.12075. [DOI] [PubMed] [Google Scholar]
- 297.Deveci G., Tek N.A. N-Nitrosamines: A potential hazard in processed meat products. J. Sci. Food Agric. 2024;104:2551–2560. doi: 10.1002/jsfa.13102. [DOI] [PubMed] [Google Scholar]
- 298.Nabizadeh S., Aeini K., Barzegar F., Arabameri M., Hosseini H., Kamankesh M., Mohammadi A. Volatile N-nitrosamines in processed meat products: An approach for monitoring dietary exposure, assessing human risk, and evaluating variable correlations by principal component analysis and heat map. Food Chem. Toxicol. 2024;188:114649. doi: 10.1016/j.fct.2024.114649. [DOI] [PubMed] [Google Scholar]
- 299.Diallo A., Deschasaux M., Latino-Martel P., Hercberg S., Galan P., Fassier P., Allès B., Guéraud F., Pierre F.H., Touvier M. Red and processed meat intake and cancer risk: Results from the prospective NutriNet-Santé cohort study. Int. J. Cancer. 2018;142:230–237. doi: 10.1002/ijc.31046. [DOI] [PubMed] [Google Scholar]
- 300.Cauchi J.P., Camilleri L., Scerri C. Environmental and lifestyle risk factors of breast cancer in Malta—A retrospective case-control study. EPMA J. 2016;7:20. doi: 10.1186/s13167-016-0069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Zhu P., Zhang Y., Zou S., Yu X., Song M., Lin M., Yang H. Consumption of processed meat and its interactions with alcohol drinking and polygentic risk scores on breast cancer risk: A cohort study in the UK biobank. medRxiv. 2022:medRxiv:2022.08.30.22279400. doi: 10.1101/2022.08.30.22279400. [DOI] [Google Scholar]
- 302.Farvid M.S., Sidahmed E., Spence N.D., Angua K.M., Rosner B.A., Barnett J.B. Consumption of red meat and processed meat and cancer incidence: A systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2021;36:937–951. doi: 10.1007/s10654-021-00741-9. [DOI] [PubMed] [Google Scholar]
- 303.Farvid M.S., Stern M.C., Norat T., Sasazuki S., Vineis P., Weijenberg M.P., Wolk A., Wu K., Stewart B.W., Cho E. Consumption of red and processed meat and breast cancer incidence: A systematic review and meta-analysis of prospective studies. Int. J. Cancer. 2018;143:2787–2799. doi: 10.1002/ijc.31848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Zhang X., Chen X., Xu Y., Yang J., Du L., Li K., Zhou Y. Milk consumption and multiple health outcomes: Umbrella review of systematic reviews and meta-analyses in humans. Nutr. Metab. 2021;18:7. doi: 10.1186/s12986-020-00527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Ventura E.R., Konigorski S., Rohrmann S., Schneider H., Stalla G.K., Pischon T., Linseisen J., Nimptsch K. Association of dietary intake of milk and dairy products with blood concentrations of insulin-like growth factor 1 (IGF-1) in Bavarian adults. Eur. J. Nutr. 2020;59:1413–1420. doi: 10.1007/s00394-019-01994-7. [DOI] [PubMed] [Google Scholar]
- 306.Melnik B.C., John S.M., Carrera-Bastos P., Cordain L., Leitzmann C., Weiskirchen R., Schmitz G. The Role of Cow’s Milk Consumption in Breast Cancer Initiation and Progression. Curr. Nutr. Rep. 2023;12:122–140. doi: 10.1007/s13668-023-00457-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Lee J.-S., Tocheny C.E., Shaw L.M. The Insulin-like Growth Factor Signaling Pathway in Breast Cancer: An Elusive Therapeutic Target. Life. 2022;12:1992. doi: 10.3390/life12121992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Reis L.G., Silva T.H.D. Effect of cow’s milk with different PUFA n-6: N-3 ratios on performance, serum lipid profile, and blood parameters of grower gilts. PLoS ONE. 2022;17:e0258629. doi: 10.1371/journal.pone.0258629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Mei J., Qian M., Hou Y., Liang M., Chen Y., Wang C., Zhang J. Association of saturated fatty acids with cancer risk: A systematic review and meta-analysis. Lipids Health Dis. 2024;23:32. doi: 10.1186/s12944-024-02025-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Jiang Y., Li L.-T., Hou S.-H., Chen L.-N., Zhang C.-X. Association between dietary intake of saturated fatty acid subgroups and breast cancer risk. Food Funct. 2024;15:2282–2294. doi: 10.1039/D3FO04279K. [DOI] [PubMed] [Google Scholar]
- 311.de Lorgeril M., Salen P. Helping women to good health: Breast cancer, omega-3/omega-6 lipids, and related lifestyle factors. BMC Med. 2014;12:54. doi: 10.1186/1741-7015-12-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Espinosa-Neira R., Mejia-Rangel J., Cortes-Reynosa P., Salazar E.P. Linoleic acid induces an EMT-like process in mammary epithelial cells MCF10A. Int. J. Biochem. Cell Biol. 2011;43:1782–1791. doi: 10.1016/j.biocel.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 313.Serna-Marquez N., Diaz-Aragon R., Reyes-Uribe E., Cortes-Reynosa P., Salazar E.P. Linoleic acid induces migration and invasion through FFAR4- and PI3K-/Akt-dependent pathway in MDA-MB-231 breast cancer cells. Med. Oncol. 2017;34:111. doi: 10.1007/s12032-017-0969-3. [DOI] [PubMed] [Google Scholar]
- 314.Durán-Jara E., Vera-Tobar T., Lobos-González L.D.L. Lactadherin: From a Well-Known Breast Tumor Marker to a Possible Player in Extracellular Vesicle-Mediated Cancer Progression. Int. J. Mol. Sci. 2022;23:3855. doi: 10.3390/ijms23073855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Yang Y., Li J., Song Q., Zhu K., Yu X., Tian Y., Zhang J. Reduction in milk fat globule-EGF factor 8 inhibits triple-negative breast cancer cell viability and migration. Oncol. Lett. 2019;17:3457–3465. doi: 10.3892/ol.2019.9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Sørensen E.S., Christensen B. Milk Osteopontin and Human Health. Nutrients. 2023;15:2423. doi: 10.3390/nu15112423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Kovacheva M., Zepp M., Schraad M., Berger S., Berger M.R. Conditional Knockdown of Osteopontin Inhibits Breast Cancer Skeletal Metastasis. Int. J. Mol. Sci. 2019;20:4918. doi: 10.3390/ijms20194918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Guo M., Liu M., Li W., Wang C., Zhang L., Zhang H. Osteopontin promotes tumor growth and metastasis and GPX4-mediated anti-lipid peroxidation in triple-negative breast cancer by activating the PI3k/Akt/mTOR pathway. J. Cancer Res. Clin. Oncol. 2024;150:155. doi: 10.1007/s00432-024-05658-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Panahipour L., Stähli A., Haiden N., Gruber R. TGF-β activity in cow milk and fermented milk products: An in vitro bioassay with oral fibroblasts. Arch. Oral Biol. 2018;95:15–21. doi: 10.1016/j.archoralbio.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 320.Shukla N., Naik A., Moryani K., Soni M., Shah J., Dave H. TGF-β at the crossroads of multiple prognosis in breast cancer, and beyond. Life Sci. 2022;310:121011. doi: 10.1016/j.lfs.2022.121011. [DOI] [PubMed] [Google Scholar]
- 321.Urseler N., Bachetti R., Biolé F., Morgante V., Morgante C. Atrazine pollution in groundwater and raw bovine milk: Water quality, bioaccumulation and human risk assessment. Sci. Total. Environ. 2022;852:158498. doi: 10.1016/j.scitotenv.2022.158498. [DOI] [PubMed] [Google Scholar]
- 322.Wang M., Chen J., Zhao S., Zheng J., He K., Liu W., Zhao W., Li J., Wang K., Wang Y., et al. Atrazine promotes breast cancer development by suppressing immune function and upregulating MMP expression. Ecotoxicol. Environ. Saf. 2023;253:114691. doi: 10.1016/j.ecoenv.2023.114691. [DOI] [PubMed] [Google Scholar]
- 323.Ranjbar F., Movassaghghazani M. Cypermethrin, deltamethrin, and hexachlorobenzene contents in milk and dairy products in Tehran, Iran. Int. J. Environ. Stud. 2023;80:1808–1819. doi: 10.1080/00207233.2023.2236361. [DOI] [Google Scholar]
- 324.El-Danasoury H.M., Khozimy A.M. Toxic Effects of Administration of Cypermethrin, Vitamin E, Zinc and Their Mixtures on the Hormonal Levels of Thyroid, Kidney Functions and some Biochemical Parameters in Male Mice. J. Appl. Plant Prot. 2020;9:39–49. [Google Scholar]
- 325.Kara-Ertekin S., Yazar S., Erkan M. In vitro toxicological assessment of flumethrin’s effects on MCF-7 breast cancer cells. Hum. Exp. Toxicol. 2021;40:2165–2177. doi: 10.1177/09603271211022789. [DOI] [PubMed] [Google Scholar]
- 326.Miret N.V., Pontillo C.A., Zárate L.V., de Pisarev D.K., Cocca C., Randi A.S. Impact of endocrine disruptor hexachlorobenzene on the mammary gland and breast cancer: The story thus far. Environ. Res. 2019;173:330–341. doi: 10.1016/j.envres.2019.03.054. [DOI] [PubMed] [Google Scholar]
- 327.Bøhn T., Millstone E. The Introduction of Thousands of Tonnes of Glyphosate in the food Chain—An Evaluation of Glyphosate Tolerant Soybeans. Foods. 2019;8:669. doi: 10.3390/foods8120669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Muñoz J.P., Araya-Osorio R., Mera-Adasme R., Calaf G.M. Glyphosate mimics 17β-estradiol effects promoting estrogen receptor alpha activity in breast cancer cells. Chemosphere. 2023;313:137201. doi: 10.1016/j.chemosphere.2022.137201. [DOI] [PubMed] [Google Scholar]
- 329.Ridley W.P., Sidhu R.S., Pyla P.D., Nemeth M.A., Breeze M.L., Astwood J.D. Comparison of the Nutritional Profile of Glyphosate-Tolerant Corn Event NK603 with That of Conventional Corn (Zea mays L.) J. Agric. Food Chem. 2002;50:7235–7243. doi: 10.1021/jf0205662. [DOI] [PubMed] [Google Scholar]
- 330.Stur E., Aristizabal-Pachon A.F., Peronni K.C., Agostini L.P., Waigel S., Chariker J., Miller D.M., Thomas S.D., Rezzoug F., Detogni R.S., et al. Glyphosate-based herbicides at low doses affect canonical pathways in estrogen positive and negative breast cancer cell lines. PLoS ONE. 2019;14:e0219610. doi: 10.1371/journal.pone.0219610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Alaboudi A.R., Osaili T.M., Alrwashdeh A. Pesticides (Hexachlorocyclohexane, Aldrin, and Malathion) Residues in Home-Grown Eggs: Prevalence, Distribution, and Effect of Storage and Heat Treatments. J. Food Sci. 2019;84:3383–3390. doi: 10.1111/1750-3841.14918. [DOI] [PubMed] [Google Scholar]
- 332.Liu H., Sun Y., Ran L., Li J., Shi Y., Mu C., Hao C. Endocrine-disrupting chemicals and breast cancer: A meta-analysis. Front. Oncol. 2023;13:1282651. doi: 10.3389/fonc.2023.1282651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Pogurschi E.N., Zugravu C.A., Ranga I.N., Trifunschi S., Munteanu M.F., Popa D.C., Tudorache M., Custura I. Determination of Acrylamide in Selected Foods from the Romanian Market. Foods. 2021;10:2110. doi: 10.3390/foods10092110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Mihalache O.A., Dall’asta C. The burden of disease due to dietary exposure to acrylamide in Italy: A risk assessment-based approach. Food Chem. Toxicol. 2024;188:114699. doi: 10.1016/j.fct.2024.114699. [DOI] [PubMed] [Google Scholar]
- 335.Bellicha A., Wendeu-Foyet G. Dietary exposure to acrylamide and breast cancer risk: Results from the NutriNet-Santé cohort. Am. J. Clin. Nutr. 2022;116:911–919. doi: 10.1093/ajcn/nqac167. [DOI] [PubMed] [Google Scholar]
- 336.Atabati H., Abouhamzeh B., Abdollahifar M.-A., Javadinia S.S., Bajestani S.G., Atamaleki A., Raoofi A., Fakhri Y., Oliveira C.A., Khaneghah A.M. The association between high oral intake of acrylamide and risk of breast cancer: An updated systematic review and meta-analysis. Trends Food Sci. Technol. 2020;100:155–163. doi: 10.1016/j.tifs.2020.04.006. [DOI] [Google Scholar]
- 337.Besaratinia A., Pfeifer G.P. A review of mechanisms of acrylamide carcinogenicity. Carcinogenesis. 2007;28:519–528. doi: 10.1093/carcin/bgm006. [DOI] [PubMed] [Google Scholar]
- 338.Lara-Castor L., Micha R., Cudhea F., Miller V., Shi P., Zhang J., Sharib J.R., Erndt-Marino J., Cash S.B., Barquera S., et al. Intake of sugar sweetened beverages among children and adolescents in 185 countries between 1990 and 2018: Population based study. BMJ. 2024;386:e079234. doi: 10.1136/bmj-2024-079234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Romanos-Nanclares A., Collins L.C., Hu F.B., Willett W.C., A Rosner B., Toledo E., Eliassen A.H. Sugar-Sweetened Beverages, Artificially Sweetened Beverages, and Breast Cancer Risk: Results From 2 Prospective US Cohorts. J. Nutr. 2021;151:2768–2779. doi: 10.1093/jn/nxab172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Debras C., Chazelas E., Srour B., Druesne-Pecollo N., Esseddik Y., Szabo de Edelenyi F., Agaësse C., De Sa A., Lutchia R., Gigandet S., et al. Artificial sweeteners and cancer risk: Results from the NutriNet-Santé population-based cohort study. PLoS Med. 2022;19:e1003950. doi: 10.1371/journal.pmed.1003950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Ye X., Zhang Y., He Y., Sheng M., Huang J., Lou W. Association between Consumption of Artificial Sweeteners and Breast Cancer Risk: A Systematic Review and Meta-Analysis of Observational Studies. Nutr. Cancer. 2023;75:795–804. doi: 10.1080/01635581.2023.2178957. [DOI] [PubMed] [Google Scholar]
- 342.Keller A., Bucher Della Torre S. Sugar-Sweetened Beverages and Obesity among Children and Adolescents: A Review of Systematic Literature Reviews. Child. Obes. 2015;11:338–346. doi: 10.1089/chi.2014.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Picon-Ruiz M., Morata-Tarifa C., Valle-Goffin J.J., Friedman E.R., Slingerland J.M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J. Clin. 2017;67:378–397. doi: 10.3322/caac.21405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Garlapati C., Joshi S., Bhattarai S., Krishnamurthy J., Turaga R.C., Nguyen T., Li X., Aneja R. PLK1 and AURKB phosphorylate survivin differentially to affect proliferation in racially distinct triple-negative breast cancer. Cell Death Dis. 2023;14:12. doi: 10.1038/s41419-022-05539-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Izadi S., Nikkhoo A., Hojjat-Farsangi M., Namdar A., Azizi G., Mohammadi H., Yousefi M., Jadidi-Niaragh F. CDK1 in Breast Cancer: Implications for Theranostic Potential. Anti-Cancer Agents Med. Chem. 2020;20:758–767. doi: 10.2174/1871520620666200203125712. [DOI] [PubMed] [Google Scholar]
- 346.Manousakis E., Miralles C.M., Esquerda M.G., Wright R.H.G. CDKN1A/p21 in Breast Cancer: Part of the Problem, or Part of the Solution? Int. J. Mol. Sci. 2023;24:17488. doi: 10.3390/ijms242417488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Anwar S., Shamsi A., Shahbaaz M., Queen A., Khan P., Hasan G.M., Islam A., Alajmi M.F., Hussain A., Ahmad F., et al. Rosmarinic Acid Exhibits Anticancer Effects via MARK4 Inhibition. Sci. Rep. 2020;10:10300. doi: 10.1038/s41598-020-65648-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Yuan L., Cai Y., Zhang L., Liu S., Li P., Li X. Promoting Apoptosis, a Promising Way to Treat Breast Cancer With Natural Products: A Comprehensive Review. Front. Pharmacol. 2022;12:801662. doi: 10.3389/fphar.2021.801662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Sun X., Wang M., Wang M., Yao L., Li X., Dong H., Li M., Li X., Liu X., Xu Y. Exploring the Metabolic Vulnerabilities of Epithelial–Mesenchymal Transition in Breast Cancer. Front. Cell Dev. Biol. 2020;8:655. doi: 10.3389/fcell.2020.00655. [DOI] [PMC free article] [PubMed] [Google Scholar]