Abstract
Hypermutation in immunoglobulin genes produces a high frequency of substitutions of all four bases, which are likely generated by low-fidelity DNA polymerases. Indeed, humans deficient for DNA polymerase (pol) η have decreased substitutions of A·T base pairs in variable and switch regions. To study the role of pol η in a genetically tractable system, we created mice lacking pol η. B cells from Polh-/- mice produced normal amounts of IgG, indicating that pol η does not affect class switch recombination. Similar to their human counterparts, variable and switch regions from Polh-/- mice had fewer substitutions of A·T base pairs and correspondingly more mutations of C·G base pairs, which firmly establishes a central role for pol η in hypermutation. Notably, the location and types of substitutions differ markedly from those in Msh6-/- clones, which also have fewer A·T mutations. The data suggest that pol η preferentially synthesizes a repair patch on the nontranscribed strand, whereas MSH6 functions to generate the patch.
Keywords: class switch recombination, somatic hypermutation, low-fidelity DNA polymerase, mismatch repair
Immunoglobulin (Ig) genes undergo a high frequency of somatic hypermutation during B cell activation. The mutations, which are predominantly nucleotide substitutions, are introduced into ≈2-kb regions of DNA containing variable (V) region genes and switch (S) regions that precede each constant region gene. Mutations in V gene exons can generate high-affinity antibodies that bind to antigen, whereas mutations in S region introns are linked to class switch recombination that produces different isotypes (reviewed in refs. 1-3). Hypermutation and switching are both initiated by the activation-induced cytidine deaminase protein (4, 5), which deaminates dC to dU in DNA (6-8). The dU lesion likely generates mutation in two phases, as originally proposed by Neuberger and colleagues (6). In phase 1, the dU lesion could generate mutations of C bases, or of G bases if C is deaminated on the complementary strand. For example, dU could remain in the DNA and be copied by a high-fidelity DNA polymerase (pol) to produce transitions of C·G. Alternatively, dU could be removed by uracil DNA glycosylase, and the abasic site could be copied or repaired by a low-fidelity pol to produce transitions and transversions of C·G.
In phase 2, the dU lesion could initiate a repair patch and generate mutations of neighboring bases, including A and T. In this pathway, the MSH2-MSH6 heterodimer (9-11) could bind to the U·G mispair (12, 13), an endonuclease could nick the DNA, and exonuclease 1 (14) would create a gap near the original dU lesion. The gap would then be filled in by a low-fidelity pol, and the types of substitutions would reflect the specificity of the pol. A plethora of pols have been studied for their role in hypermutation in animals and cells: β (15), δ (16), ζ (17, 18), η (19-21), ι (22, 23), κ (24, 25), λ (26), μ (26, 27), and Rev1 (28, 29). In animals, only pol η affects the spectra of mutations, because humans with xeroderma pigmentosum variant (XP-V) disease, who are deficient for the pol (30), have fewer substitutions of A·T pairs (19-21). Because the spectrum of mutations by pol η in vitro mimics the hypermutation pattern (31), pol η may be involved during the filling of gaps generated by an exonuclease.
However, the human XP-V studies are subject to two caveats: (i) the defective proteins carry a variety of natural mutations that inactivate the pol; and (ii) they are present on a heterogeneous background, and other proteins may have evolved to compensate for the lack of pol η. To clarify the role of pol η in somatic mutagenesis, we created homozygous Polh-/- mice and studied hypermutation and class switch recombination in their B cells. The data from murine pol η-deficient cells is consistent with the data from human XP-V cells, which rigorously establishes the involvement of pol η in hypermutation. Furthermore, this genetically tractable model now creates an opportunity to identify other proteins that work with pol η in the pathway.
Materials and Methods
Mice. Mice deficient for pol η were produced on a C57BL/6 background (F.H., unpublished work). Briefly, a neomycin-resistance cassette was inserted into exon 8 of the murine pol η gene; the insertion gives rise to a stop codon within the Neo gene. Because the potential truncated protein would lack part of the pol catalytic domains, it would not have pol activity or the C-terminal region to interact with proliferating cell nuclear antigen and nuclear localization. Preliminary experiments showed that no truncated protein was detectable by Western blotting. C57BL/6 mice for experiments were purchased from The Jackson Laboratory. All animal protocols were approved by the Animal Ethics Committees (Osaka University and National Institute on Aging, National Institutes of Health, Baltimore). Mice were used at 5-6 months of age. The mice were immunized with 100 μg of keyhole limpet hemocyanin (Calbiochem) in adjuvant (Ribi Immunochem) and boosted with keyhole limpet hemocyanin after 3 weeks. Spleens and Peyer's patches were removed 4 days after the second injection.
Flow Cytometry. Spleen cells were treated with ammonium chloride potassium lysing buffer (Quality Biological, Gaithersburg, MD) to lyse red blood cells. Cells were stained with combinations of a tricolor-labeled antibody to CD3 (Caltag, Burlingame, CA), fluorescein-labeled antibody to CD4 (Caltag), fluorescein-labeled peanut agglutinin (PNA) (EY Laboratories), phycoerythrin-labeled antibody to B220 (BD Biosciences Pharmingen), and phycoerythrin-labeled antibody to CD8 (Caltag). Flow cytometry analyses were gated on live cells as determined from forward and side scatter analysis.
Heavy Chain Class Analyses. For in vitro switching, resting splenic B cells were purified as described in ref. 11. Cells were labeled with 1 μM CFSE (Molecular Probes) and stimulated with either 50 μg/ml Escherichia coli LPS serotype O111:B4 (Sigma-Aldrich) or LPS plus 50 ng/ml mouse IL-4 (R&D Systems). After 4 days in culture, the cells were stained with 1 μM propidium iodide and either fluorescein-conjugated anti-mouse IgG3 or IgG1 (BD Biosciences Pharmingen). For the CFSE analysis, cells were stained with propidium iodide and allophycocyanin-conjugated rat anti-mouse IgG1 monoclonal antibody (BD Biosciences Pharmingen).
Hypermutation Analyses. Cells from the Peyer's patches of two or three immunized mice were stained with phycoerythrin-labeled antibody to B220 and fluorescein-labeled PNA. The cells were isolated by flow cytometry, and DNA was prepared from B220+PNA+ cells. For V regions, the 492-bp intron region downstream of JH4 from rearranged VHJ558 genes was sequenced by using forward nested primers described in ref. 23, and the following reverse primers: first reverse (nucleotides 2906-2926 of GenBank/EMBL/DDJB under accession no. J00440), 5′-GTGTTCCTTTGAAAGCTGGAC-3′; and second reverse (nucleotides 2827-2847) with a BamHI site in italics, 5′-cgcggatccGATGCCTTTCTCCCTTGACTC-3′. For Sμ regions, a 561-bp region upstream of the core Sμ region (32) was sequenced by using primers described in ref. 11. PCR products were digested with restriction enzymes, cloned into pBluescript, and sequenced by LARK Sequencing Technologies, Houston.
Results
Types of Lymphocytes. Percentages of splenic B and T cells were determined for pol η-deficient mice and are presented in Fig. 1. The number of total CD3 T cells and B220 B cells (Fig. 1 A), and the ratio of CD4 T helper and CD8 T effector cells (Fig. 1B) is similar in C57BL/6 and Polh-/- mice. These results indicate that the lack of pol η does not alter lymphocyte development. To determine whether B cells can be stimulated by endogenous antigens in the gut, flow cytometry was performed on cells from Peyer's patches. As seen in Fig. 1C, identical levels of B220+PNA+ activated cells were observed in wild type and pol η-deficient strains, suggesting that the Ig receptors have diversified adequately to respond to environmental antigens.
Fig. 1.
Normal B and T populations in Polh-/- mice as determined by flow cytometry. The percentage of cells in each population is shown in the quadrants and boxes. (A) Spleen cells analyzed for total B cells (B220+) and T cells (CD3+). (B) Spleen cells analyzed for CD4+ and CD8+ T cell populations. (C) Peyer's patch cells analyzed for activated B cells in germinal centers (B220+PNA+).
Class Switch Recombination. To test whether pol η influences heavy chain class switching, B cells were stimulated in vitro with LPS to produce IgG3 and with LPS plus IL-4 to produce IgG1. The data in Fig. 2A show that B cells from C57BL/6 and Polh-/- mice had identical levels of membrane IgG3 and IgG1. The cells were then gated for specific division times by CFSE staining, and IgG1 expression was measured at each division. As shown in Fig. 2B, cells from the third through seventh division expressed similar amounts of IgG1. These results indicate that the lack of pol η does not affect class switch recombination.
Fig. 2.
Normal isotypes produced by Polh-/- B cells after in vitro stimulation. (A) Flow cytometry: spleen cells were stimulated with LPS or LPS plus IL-4 for 4 days and stained with propidium iodide (PI) to detect live cells and antibodies to IgG isotypes. The percentage of cells that switched to IgG3 or IgG1 is shown in the lower right quadrant. (B) CSFE staining: cells stimulated with LPS plus IL-4 were labeled with CSFE and stained with PI and anti-IgG1. The percentage of IgG1-positive cells is shown for each division; white bars, C57BL/6; black bars, Polh-/-. Error bars, SE for three experiments.
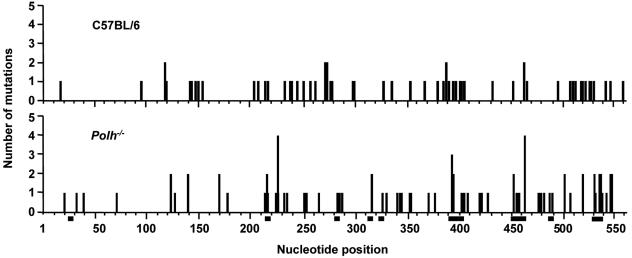
Mutations in JH4 Introns. To examine hypermutation in V regions, we sequenced a 492-bp intron region downstream of rearranged JH4 genes. Only clones with unique VDJ junctions and mutations were analyzed. Approximately 43 clones from B220+PNA+ cells from Peyer's patches were analyzed for each genotype, and the data are presented in Fig. 3 and Table 1. C57BL/6 clones had a frequency of 6 × 10-3 mutations per bp with 130 substitutions and one deletion (1 bp), whereas Polh-/- clones had a frequency of 7.6 × 10-3 mutations per bp with 163 substitutions and two deletions (1 and 12 bp) (Fig. 3A). The types of mutations were recorded from the nontranscribed strand and are presented in Table 1. For Polh-/- clones, there was a marked decrease in mutations of A and T bases and an increase in mutations of C and G compared with C57BL/6 clones. The data are summarized in Fig. 3B and show fewer mutations of A than T and more mutations of C than G, compared with wild type clones. The location of mutations in these clones is plotted in Fig. 4 and shows that the distribution is similar among the two groups. There was no obvious clustering of mutations in WGCW (W = A or T) motifs in the presence or absence of pol η.
Fig. 3.
Fewer A·T substitutions in JH4 introns from Polh-/- clones. (A) Total number of clones analyzed is shown in the center of each circle. Segments represent the proportion of clones that contain the indicated number of mutations. Frequencies are given in mutations per bp. (B) Total mutations are grouped for each of the four nucleotides.
Table 1. Substitutions in JH4 introns.
| Frequency, %
|
||
|---|---|---|
| Substitution | C57BL/6 (130 mutations) | Polh (163 mutations) |
| A to: G | 21 | 5 |
| T | 14 | 4 |
| C | 3 | 3 |
| T to: C | 15 | 6 |
| A | 5 | 1 |
| G | 3 | 2 |
| C to: T | 14 | 36 |
| A | 4 | 6 |
| G | 1 | 5 |
| G to: A | 14 | 20 |
| T | 3 | 4 |
| C | 3 | 8 |
Mutations are shown from the nontranscribed strand. Values are corrected to represent a sequence with equal amounts of the four nucleotides.
Fig. 4.
Distribution of substitutions in the JH4 intron. Nucleotides are numbered from the first base after the JH4 coding sequence, with vertical bars showing the number of mutations at each position. Squares below the abscissa depict WGCW motifs.
Mutations in Sμ Regions. Hypermutation also occurs at a high frequency in a 561-bp region located upstream of the Sμ core region (32). For this region, only clones with unique mutational patterns were considered, so that the data would not be skewed by duplicate sequences. The analysis of ≈70 clones from B220+PNA+ cells from the Peyer's patches of each strain is presented in Fig. 5 and Table 2. C57BL/6 clones (11) had a frequency of 1.3 × 10-3 mutations per bp with 63 substitutions and two deletions (5 and 15 bp), and Polh-/- clones had a frequency of 2.7 × 10-3 mutations per bp with 88 substitutions and 2 deletions (1 bp each) and 1 insertion (1 bp) (Fig. 5A). The types of substitutions are presented in Table 2 and Fig. 5B and show a striking decrease in mutations at A·T pairs and concomitant rise in mutations at C·G pairs in the Polh-/- clones. In particular, there were more mutations of C than G, compared with the wild-type clones. The distribution of mutations in the Sμ region is presented in Fig. 6, and was similar in the two genotypes.
Fig. 5.
Reduced A·T mutations in Sμ from Polh-/- clones. (A) The proportions of clones containing mutations are shown. Frequencies are given in mutations per bp. (B) Total mutations are grouped for each of the four nucleotides.
Table 2. Substitutions in Sμ regions.
| Frequency, %
|
||
|---|---|---|
| Substitution | C57BL/6 (63 mutations) | Polh−/− (88 mutations) |
| A to: G | 8 | 4 |
| T | 9 | 1 |
| C | 3 | 1 |
| T to: C | 11 | 4 |
| A | 8 | 2 |
| G | 3 | 3 |
| C to: T | 28 | 34 |
| A | 4 | 9 |
| G | 4 | 15 |
| G to: A | 16 | 17 |
| T | 4 | 2 |
| C | 2 | 8 |
Values are corrected for nucleotide composition of the sequence.
Fig. 6.
Location of mutations in the Sμ region. A region upstream of the Sμ core was sequenced. Squares below the abscissa represent WGCW motifs.
Discussion
The high frequency of substitutions in antibody genes strongly implies that they are caused by error-prone pols. We previously reported that humans with XP-V disease, who lack pol η, have significantly fewer mutations of A·T base pairs in V and S regions (19, 21). However, further studies of this intriguing enzyme are limited by the lack of an experimental model that can be both genetically and biochemically manipulated. To this end, we created mice deficient for pol η, and studied the pattern of hypermutation. Several conclusions about the role of pol η that were suggested in the human studies can now be verified by using this murine model system.
Pol η Does Not Affect Class Switch Recombination. Humans with XP-V have normal frequencies of switching to various IgG isotypes (20, 21). However, these data from peripheral blood lymphocytes may be skewed toward long-lived memory populations. A more rigorous test of the ability to switch heavy chain classes is to analyze IgG levels after mitogen-induced activation in synchronized short-term cultures. Under these conditions, we found that B cells from pol η-deficient and wild-type mice indeed had similar levels of surface IgG. Although pol η affects hypermutation in S regions, it apparently does not affect recombination, presumably because the enzymatic activity of pol η does not create nicks or double-strand breaks in the DNA. Conversely, other proteins, such as H2AX and ATM, do not affect hypermutation in S regions, but are intimately involved in class switching (32-34). Thus, although both hypermutation and class switch recombination are initiated by activation-induced cytidine deaminase, the resolution of the dU lesions clearly involves separate pathways to produce substitutions or recombination.
Pol η Generates Mutations of A·T Base Pairs. In the absence of pol η, the number of mutations of A·T pairs declined, whereas those of C·G pairs increased in both the JH4 intron and Sμ regions. Regarding the A·T mutations in the JH4 intron from Polh-/- clones, there was less of a bias for mutations of A compared with T nucleotides as recorded from the nontranscribed strand, which was also observed in XP-V clones (35). Because pol η inserts more mutations opposite template T than template A in vitro (36), it should generate more substitutions of A on the newly synthesized strand. Thus, pol η could produce the well-known A·T strand polarity in V genes by using the transcribed strand as a template and synthesizing bases preferentially on the nontranscribed strand. Regarding the C·G mutations in Polh-/- clones, there was more of a bias for mutations of C compared with G in both V and S regions. The prevalence of C mutations was greatest in the Sμ region, which was also noted in XP-V clones (21). DNA in switch regions can form stable R-loops (37), which would expose C on the nontranscribed strand to activation-induced cytidine deaminase activity. In the absence of pol η, mutations would occur primarily at deaminated cytosines, which would be more numerous on the nontranscribed strand. Pol η may also contribute to some mutations of C·G pairs, because it can synthesize several bases in a gap and has the potential to generate mispairs opposite all four nucleotides. Other roles for pol η to synthesize mutations at A·T base pairs have been proposed, such as its putative role as a reverse transcriptase (38) or its potential to incorporate dUTP opposite dA (39). However, the ability of the enzyme to catalyze error-prone synthesis on DNA is most consistent with its biochemical properties, which are stimulation of activity by MSH2-MSH6 (13) and a preference to generate mutations of A more than of T during strand synthesis (36).
Separate Roles for MSH2-MSH6 and Pol η in Hypermutation. The mismatch repair heterodimer MSH2-MSH6 and DNA pol η have been linked in the hypermutation pathway based on (i) their similar genetic phenotypes of fewer A·T mutations in gene-deficient mice (9-11, 40-42) and (ii) their biochemical interactions showing stimulation of pol η synthesis by MSH2-MSH6 (13). However, their disparate enzymatic activities suggest that they will produce different signatures of mutation. Indeed, as summarized in Table 3, there are major differences in heavy chain class switching and hypermutation between mice lacking the MSH6 partner of the duplex and DNA pol η.
Table 3. Comparison of class switch recombination and hypermutation in Polh−/− and Msh6−/− clones.
| Frequency, %
|
|||
|---|---|---|---|
| lg genes | C57BL/6 (190 mutations) | Polh−/− (255 mutations) | Msh6−/−* (98 mutations) |
| lgG cells† | 23 | 24 | 9 |
| Mutations in WGCW | 15 | 22 | 68 |
| A·T mutations | 53 | 20 | 16 |
| C·G transitions | 34 | 54 | 75 |
| C·G transversions | 13 | 26 | 9 |
Data are a summary of mutations from JH4 and Sμ introns and are corrected for base composition of the respective regions.
Data from ref. 11.
Cells with surface lgG1 after culture for 4 days with LPS plus IL-4.
First, class switch recombination is impaired in Msh6-/- B cells (10, 11) but not in Polh-/- B cells (Fig. 2). This finding implies that MSH6 is involved in the process that creates DNA strand breaks, perhaps through recruiting an endonuclease, whereas the enzymatic activity of pol η does not introduce breaks. Second, the distribution of mutations is strikingly different in the two genotypes. Approximately 68% of the mutations in JH4 introns and Sμ regions from Msh6-/- clones are in WGCW motifs (11), compared with only 20% from Polh-/- and wild-type clones (Figs. 4 and 6). The distribution suggests that activation-induced cytidine deaminase initially recognizes and deaminates C in the WGCW motif before processive deamination of other cytosines (43). Preferential mutation in WGCW motifs has also been observed by others (44, 45) and may represent entry sites for activation-induced cytidine deaminase into Ig DNA. MSH2-MSH6 would bind to the U·G mispair in WGCW and would attract an endonuclease and exonuclease 1 to generate a gap. MSH2-MSH6 may then recruit and stimulate pol η to insert bases downstream of the U·G mispair. In the absence of MSH2 or MSH6, there would be no gap, and mutations would be focused at WGCW sites. In the absence of pol η, another polymerase(s) may synthesize in the gap to disperse the mutations downstream of WGCW.
Third, although both pol η- and MSH6-deficient mice have fewer A·T and more C·G mutations, the types of substitutions of C·G differ between the strains. In Polh-/- clones, two-thirds of C·G mutations are transitions and one-third are transversions, whereas in Msh6-/- clones, the majority of C·G mutations are transitions. Transitions of C·G are a hallmark of mutation in Ung-/- mice (8), and likely reflect the bypass of dU by a high-fidelity pol, producing C·G to T·A transitions. In the absence of MSH2-MSH6, the dU may not be efficiently removed by uracil DNA glycosylase, resulting in a phenotype similar to Ung-/- clones. The possibility that MSH2-MSH6 stimulates uracil DNA glycosylase activity could be tested biochemically.
Acknowledgments
We thank J. Chrest and C. Morris for flow cytometry, S. Poosala and N. Hitt for assistance in importing mice, and N. P. Weng for T cell reagents. We also thank Roger Woodgate for continued discussions and manuscript editing.
Author contributions: S.A.M., R.P.W., F.H., and P.J.G. designed research; S.A.M., W.W.Y., R.P.W., T.O., Y.K., M.Y., C.M., and P.J.G. performed research; T.O., Y.K., M.Y., C.M., and F.H. contributed new reagents/analytic tools; S.A.M., F.H., and P.J.G. analyzed data; and P.J.G. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: pol, DNA polymerase; V, variable; S, switch; XP-V, xeroderma pigmentosum variant; PNA, peanut agglutinin.
Note. An analysis of hypermutation in an independently generated mouse deficient for pol η has been recently reported by Delbos et al. (46).
References
- 1.Chaudhuri, J. & Alt, F. A. (2004) Nat. Rev. Immunol. 4, 541-552. [DOI] [PubMed] [Google Scholar]
- 2.Goodman, M. F. & Scharff, M. D. (2005) J. Exp. Med. 201, 493-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pham, P., Bransteitter, R. & Goodman, M. F. (2005) Biochemistry 44, 2703-2715. [DOI] [PubMed] [Google Scholar]
- 4.Muramatsu, M., Kinoshita, K., Faragasan, S., Yamada, S., Shinkai, Y. & Honjo, T. (2000) Cell 102, 553-563. [DOI] [PubMed] [Google Scholar]
- 5.Revy, P., Muto, T., Levy, Y., Geissmann, F., Piebani, A., Sanal, O., Catalan, N., Forveille, M., Dufourcq-Lagelouse, R., Gennery, A., et al. (2000) Cell 102, 565-575. [DOI] [PubMed] [Google Scholar]
- 6.Petersen-Mahrt, S. K., Harris, R. S. & Neuberger, M. S. (2002) Nature 418, 99-103. [DOI] [PubMed] [Google Scholar]
- 7.Di Noia, J. & Neuberger, M. S. (2002) Nature 419, 43-48. [DOI] [PubMed] [Google Scholar]
- 8.Rada, C., Williams, G. T., Nilsen, H., Barnes, D. E., Lindahl, T. & Neuberger, M. S. (2002) Curr. Biol. 12, 1748-1755. [DOI] [PubMed] [Google Scholar]
- 9.Wiesendanger, M., Kneitz, B., Edelmann, W. & Scharff, M. D. (2000) J. Exp. Med. 191, 579-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li, Z., Scherer, S. J., Ronai, D., Iglesias-Ussel, M. D., Peled, J. U., Bardwell, P. D., Zhuang, M., Lee, K., Martin, A., Edelmann, W., et al. (2004) J. Exp. Med. 200, 47-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martomo, S. A., Yang, W. W. & Gearhart, P. J. (2004) J. Exp. Med. 200, 61-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu, L., Wu, J., Qiu, L., Jennings, C. D. & Li, G. M. (2002) J. Nutr. Biochem. 13, 355-363. [DOI] [PubMed] [Google Scholar]
- 13.Wilson, T. M., Vaisman, A., Martomo, S. A., Sullivan, P., Lan, L., Hanaoka, F., Yasui, A., Woodgate, R. & Gearhart, P. J. (2005) J. Exp. Med. 201, 637-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bardwell, P. D., Woo, C. J., Wei, K., Li, Z., Martin, A., Sack, S. Z., Parris, T., Edelmann, W. & Scharff, M. D. (2004) Nat. Immunol. 5, 224-229. [DOI] [PubMed] [Google Scholar]
- 15.Esposito, G., Texido, G., Betz, U. A. K., Gu, H., Müller, W., Klein, U. & Rajewsky, K. (2000) Proc. Natl. Acad. Sci. USA 97, 1166-1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Longacre, A., Sun, T., Goldsby, T. E., Preston, B. D. & Storb, U. (2003) Int. Immunol. 15, 477-481. [DOI] [PubMed] [Google Scholar]
- 17.Zan, H., Komori, A., Li, Z., Cerutti, A., Schaffer, A., Flajnik, M. F., Diaz, M. & Casali, P. (2001) Immunity 14, 643-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diaz, M., Verkoczy, L. K., Flajnik, M. F. & Klinman, N. R. (2001) J. Immunol. 167, 327-335. [DOI] [PubMed] [Google Scholar]
- 19.Zeng, X., Winter, D. B., Kasmer, C., Kraemer, K. H., Lehmann, A. R. & Gearhart, P. J. (2001) Nat. Immunol. 2, 537-541. [DOI] [PubMed] [Google Scholar]
- 20.Faili, A., Aoufouchi, S., Weller, S., Vuillier, F., Stary, A., Sarasin, A., Reynaud, C. A. & Weill, J. C. (2004) J. Exp. Med. 199, 265-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng, X., Negrete, G. A., Kasmer, C., Yang, W. W. & Gearhart, P. J. (2004) J. Exp. Med. 199, 917-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faili, A., Aoufouchi, S., Flatter, E., Guéranger, Q., Reynaud, C. A. & Weill, J. C. (2002) Nature 419, 944-947. [DOI] [PubMed] [Google Scholar]
- 23.McDonald, J. P., Frank, E. G., Plosky, B. S., Rogozin, I. B., Masutani, C., Hanaoka, F., Woodgate, R. & Gearhart, P. J. (2003) J. Exp. Med. 198, 635-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schenten, D., Gerlach, V. L., Guo, C., Velasco-Miguel, S., Hladik, C. L., White, C. L., Friedberg, E. C., Rajewsky, K. & Esposito, G. (2002) Eur. J. Immunol. 32, 3152-3160. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu, T., Shinkai, Y., Ogi, T., Ohmori, H. & Azuma, T. (2003) Immunol. Lett. 86, 265-270. [DOI] [PubMed] [Google Scholar]
- 26.Bertocci, B., de Smet, A., Flatter, E., Dahan, A., Bories, J. C., Landreau, C., Weill, J. C. & Reynaud, C. A. (2002) J. Immunol. 168, 3702-3706. [DOI] [PubMed] [Google Scholar]
- 27.Ruiz, J. F., Lucas, D., Garcia-Palomero, E., Saez, A. I., González, M. A., Piris, M. A., Bernad, A. & Blanco, L. (2004) Nucleic Acids Res. 32, 5861-5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simpson, L. J. & Sale, J. E. (2003) EMBO J. 22, 1654-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jansen, J. G., Tsaalbi-Shtylik, A., Langerak, P., Calléja, F., Meijers, C. M., Jacobs, H. & de Wind, N. (2005) Nucleic Acids Res. 33, 356-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masutani, C., Kusumoto, R., Yamada, A., Dohmae, N., Yokoi, M., Yuasa, M., Araki, M., Iwai, S., Takio, K. & Hanaoka, F. (1999) Nature 399, 700-704. [DOI] [PubMed] [Google Scholar]
- 31.Rogozin, I. B., Pavlov, Y. I., Bebenek, K., Matsuda, T. & Kunkel, T. A. (2001) Nat. Immunol. 2, 530-536. [DOI] [PubMed] [Google Scholar]
- 32.Reina-San-Martin, B., Defilippantonio, S., Hanitsch, L., Masilamani, R. F., Nussenzweig, A. & Nussenzweig, M. C. (2003) J. Exp. Med. 197, 1767-1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reina-San-Martin, B., Chen, H. T., Nussenzweig, A. & Nussenzweig, M. C. (2004) J. Exp. Med. 200, 1103-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lumsden, J. M., McCarty, T., Petiniot, L. K., Shen, R., Barlow, C., Wynn, T. A., Morse, H. C., III, Gearhart, P. J., Wynshaw-Boris, A., Max, E. E., et al. (2004) J. Exp. Med. 200, 1111-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayorov, V. I., Rogozin, I. B., Adkison, L. R. & Gearhart, P. J. (2005) J. Immunol., in press. [DOI] [PubMed]
- 36.Matsuda, T., Bebenek, K., Masutani, C., Rogozin, I. B., Hanaoka, F. & Kunkel, T. A. (2001) J. Mol. Biol. 312, 335-346. [DOI] [PubMed] [Google Scholar]
- 37.Yu, K., Chedin, F., Hsieh, C. L., Wilson, T. E. & Lieber, M. R. (2003) Nat. Immunol. 4, 442-451. [DOI] [PubMed] [Google Scholar]
- 38.Steele, E. J. (2004) DNA Repair (Amsterdam) 3, 687-692. [DOI] [PubMed] [Google Scholar]
- 39.Neuberger, M. S., DiNoia, J. M., Beale, R. C. L., Williams, G. T., Yang, Z. & Rada, C. (2005) Nat. Rev. Immunol. 5, 171-178. [DOI] [PubMed] [Google Scholar]
- 40.Phung, Q. H., Winter, D. B., Cranston, A., Tarone, R. E., Bohr, V. A., Fishel, R. & Gearhart, P. J. (1998) J. Exp. Med. 187, 1745-1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Frey, S., Bertocci, B., Delbos, F., Quint, L., Weill, J. C. & Reynaud, C. A. (1998) Immunity 9, 127-134. [DOI] [PubMed] [Google Scholar]
- 42.Rada, C., Ehrenstein, M. R., Neuberger, M. S. & Milstein, C. (1998) Immunity 9, 135-141. [DOI] [PubMed] [Google Scholar]
- 43.Pham, P., Bransteitter, R., Petruska, J. & Goodman, M. F. (2003) Nature 424, 103-107. [DOI] [PubMed] [Google Scholar]
- 44.Beale, R. C. L., Petersen-Mahrt, S. K., Watt, I. N., Harris, R. S., Rada, C. & Neuberger, M. S. (2004) J. Mol. Biol. 337, 585-596. [DOI] [PubMed] [Google Scholar]
- 45.Zarrin, A. A., Alt, F. W., Chaudhuri, J., Stokes, N., Kaushal, D., Du Pasquier, L. & Tian, M. (2004) Nat. Immunol. 5, 1275-1281. [DOI] [PubMed] [Google Scholar]
- 46.Delbos, F., De Smet, A., Faili, A., Aoufouchi, S., Weill, J. C. & Reynaud, C. A. (2005) J. Exp. Med. 201, 1191-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]