Abstract
Activation of the receptors for leukemia inhibitory factor (LIF) and IL-11 is essential for embryo attachment and decidualization in mice. Both receptors induce activation of the Stat family of signal transducers via the Jak/Stat pathway. Here, we aimed to establish whether activation of Stat3 in maternal endometrium is essential for successful implantation. Functional blockade of Stat3 before implantation, by injection into the uterine lumen of a cell-permeable Stat3 peptide inhibitor, reduced embryo implantation specifically by 70% (P < 0.001). Stat3 is phosphorylated in the luminal epithelium (LE) in response to LIF, and this phosphorylation was significantly reduced both in vitro and in vivo by the Stat3 inhibitor. The inhibitor also blocked induction by LIF of several LIF-regulated genes in the LE including Irg1, which has been shown previously to be essential for implantation. Successful implantation is therefore dependent on phosphorylation and activation of Stat3 in the endometrium before implantation. This finding provides a target for contraceptive development, based on selective blockade of signal transduction pathways essential for implantation. This study demonstrates that cell-permeable peptide inhibitors can be used effectively to target intracellular signaling pathways in the uterine LE.
Successful implantation depends on the differentiation of the endometrium to a receptive state through the actions of estrogen and progesterone. The receptive state is transient and in mice lasts 24 h, beginning early on day 4 of pregnancy (1). Embryos entering the uterus when it is not receptive fail to attach. Implantation begins with the apposition of trophoblast cells of the hatched blastocyst and the apical surface of the luminal epithelium (LE) of the endometrium. At the same time, stromal cells beneath the site of attachment proliferate and differentiate in the process of decidualization (2). This is essential to support subsequent development of the embryo and placenta. The action of steroids on the endometrium is known to be mediated by several essential cytokines and growth factors in preparation for implantation. These include leukemia inhibitory factor (LIF), IL-11, and calcitonin, which activate intracellular signaling pathways through receptors on the cell surface to control cell proliferation and differentiation (3–5). However, the exact molecular mechanisms by which steroids and cytokines act together to render the uterus permissive for attachment are not understood.
LIF was the first cytokine shown to be essential for implantation. In mice it is expressed in the endometrial glands for a short time on day 4 of pregnancy just before the onset of implantation (6). This expression is initiated by the nidatory estrogen peak on the morning of day 4 and results in secretion of LIF into the uterine lumen (7). Female mice lacking LIF produce normal blastocysts which attach to the LE, but implantation fails to proceed beyond the stage of apposition (3). Decidualization does not begin, and the uterus of these mice is resistant to artificial decidualizing stimuli. A single intrauterine injection of LIF at this time is sufficient to restore implantation (7).
The LIF receptor consists of two transmembrane proteins, LIF receptor α (LIFRα), which confers ligand specificity, and gp130, which is a component of several receptors of the IL-6 family of ligands (8). When LIF expression is maximal on day 4 of pregnancy in the glands, LIFRα is expressed primarily in the LE of the endometrium (9). Taken together, these results suggest that the action of LIF on the LE is essential to render the endometrium receptive. LIF is also up-regulated in the endometrium at the time of implantation in several other species including humans and non-human primates (10–12). Blockade of LIF's action by using antibodies to the receptor partially blocks implantation in macaques, suggesting that LIF may also be important in implantation in other species (11). Similarly, mice that lack the IL-11 receptor (IL-11R) also show implantation failure. Normally, IL-11R expression is induced in stromal cells immediately below the implantation site and corresponds closely to the expanding decidual zone. In IL-11R-deficient mice, embryo attachment occurs and decidualization begins but is not sustained, resulting in embryo necrosis after day 8 of pregnancy (4).
Ligand binding to either the LIFRα or IL-11R results in receptor dimerization with gp130 and activation of several signal transduction cascades. These include the mitogen-activated protein kinase (MAPK), protein kinase C (PKC), and the Jak/Stat pathway (13). Activation of Janus kinases (Jaks) causes phosphorylation of a family of transcription factors called signal transducers and activators of transcription (Stat) (14, 15). Stat proteins dimerize and translocate to the nucleus upon phosphorylation, where they act to regulate gene transcription (16). Incubation of mouse uterine epithelium with LIF has been shown to cause activation and nuclear translocation of Stat3 but does not activate the MAPK pathway (9). Mice with a mutation in the gp130 cytoplasmic domain, which selectively abolishes Jak/Stat signaling, are infertile because of implantation failure (17). This is despite the fact that in these animals the other signal transduction pathways activated by the LIF receptor are intact. These data imply that it is signaling via the Jak/Stat pathway that is primarily responsible for the onset of receptivity in response to LIF (9).
There is evidence that the Jak/Stat pathway is also important for receptivity in human endometrium. As in mice, receptivity develops in response to the action of progesterone on estrogen-primed endometrium (18). Administration of a single dose of the antiprogestin RU486 in vivo after ovulation renders the endometrium nonreceptive to the implanting embryo (19, 20). This treatment is widely available as an emergency postcoital contraceptive. We have recently shown that exposure of human secretory-phase endometrium to RU486 results in the rapid down-regulation of Jak1 mRNA levels (21). Thus, in human endometrium, alterations in the Jak/Stat pathway are correlated with loss of receptivity.
The involvement of Jak/Stat signaling in both murine and human implantation suggests a potential target for novel nonsteroidal contraception. The mouse data indicate that Stat activation through LIFR/gp130 is necessary but do not reveal which of the six different Stat signaling molecules are essential. This study used a selective inhibitor of Stat3 activation to determine whether activation of the Stat3 signal transducer is required for implantation. Introduction of a cell-permeable Stat3 peptide inhibitor into the uterine lumen on day 3 of pregnancy dramatically reduced the implantation rate. The Stat3 inhibitor blocked Stat3 phosphorylation in the LE in response to LIF, both in vitro and in vivo. LE isolated after in vivo administration of inhibitor before implantation showed reduced expression levels of LIF regulated genes, including Irg1, which is critical for implantation. These results suggest that Stat3 phosphorylation and subsequent activation of gene transcription in the LE is essential to implantation. Inhibition of this signal transduction pathway results in reduced implantation and provides an approach for the development of nonsteroidal postcoital contraception.
Materials and Methods
Animals. Virgin 8-wk-old female MF1 mice (Harlan) were housed at 22–24°C in rooms with a controlled light schedule. Females were paired with CBA/BL6 males and checked the following morning for vaginal plugs. The day of vaginal plug was recorded as day 1 of pregnancy. All animal care and experimental protocols were approved by the animal ethics committee of the University of Cambridge and the Home Office of the United Kingdom government.
Intrauterine Injection of a Stat3 Inhibitor. After detection of a vaginal plug, each female was treated by injection into the uterine lumen of either a cell-permeable Stat3 peptide inhibitor (Calbiochem) or a control peptide (Perbio Science, Cramlington, U.K.). The control peptide is identical in sequence to the inhibitor except that the tyrosine residue, which is essential for the inhibitory action, is not phosphorylated. Animals underwent midventral laparotomy on day 3 of pregnancy between 1600 and 1800 hours. Then, 25 μl of PBS containing 50 μg (final concentration 1 mM) of the peptide inhibitor was injected into the lumen of each uterine horn (n = 10). Control mice were injected with control peptide at an identical concentration (n = 10) or with vehicle (PBS, n = 10). On day 8 of pregnancy, the number of implantation sites was counted in each uterine horn. The dose of inhibitor used was determined from in vitro data that showed a maximal effect at 1 mM concentration (22, 23).
Embryo Toxicity Assay. Embryos (16-cell morulae) were collected by flushing uteri and oviducts of superovulated MF1 mice on day 3 of pregnancy. Recipient 8-week-old MF1 females were mated with vasectomized males. Embryos were pooled and randomized into control- and inhibitor-treated groups. Embryos were incubated for 1 h at 37°C under a humid air environment in M2 medium (Sigma) and 4% BSA with or without 0.5 mg/ml Stat3 peptide inhibitor. All embryos were rinsed and transferred to M16 medium (Sigma)/4% BSA for overnight culture at 37°C and 5% CO2 under oil (Sigma). Embryos were then scored on day 4 and sorted on the basis of morphology. Blastocysts and fluid-accumulating embryos from both groups were transferred at midday of day 4 to recipient females, five to six embryos per horn, and implantation rates were assessed 4 days later.
Isolation of the LE. Isolation of LE from uteri of mice was performed as described in ref. 24. Briefly, uteri from MF1 mice were digested in a solution of 0.5% dispase (Roche) in calcium- and magnesium-free Hank's balanced salt solution (Invitrogen) for 3 h at room temperature, followed by gentle mechanical separation of LE and stroma. For LIF treatment, LE were incubated in serum-free OptiMEM (GIBCO) for 3 h, followed by the addition of 50 ng/ml LIF (Chemicon) for 15 min, and washed in PBS, and RNA or protein was then isolated.
Immunoblotting. Tissues were lysed by using a modified RIPA lysis buffer [50 mM Tris, pH 7.4/1% Nonidet P-40/0.25% sodium-deoxycholate/150 mM NaCl/1 mM EDTA/1 mM EGTA/10% glycerol/100 mM sodium pyrophosphate/1 mM sodium decavana-date/1 mM sodium fluoride/1× complete protease inhibitor mixture (Roche)]. Protein concentrations were determined by using the BCA protein assay reagent kit (Pierce) and separated on 8% NuPAGE/Novex gels (Invitrogen). Proteins were transferred to Invitrolon PVDF membranes (Invitrogen) and blocked in 5% nonfat dry milk powder and 0.05% Tween 20. Membranes were incubated with an antibody specific to P-Tyr-Stat3 (Tyr-705) (9131, Cell Signaling Technology, Beverly, MA), and proteins were detected by using an anti-rabbit horseradish peroxidase secondary antibody (DAKO) with the ECL plus detection system (Amersham Pharmacia Biosciences). Proteins were quantified by scanning at a 100-μm resolution on a Storm 860 scanner (Molecular Dynamics/Amersham Pharmacia Biosciences) in blue f luorescence/chemifluorescence mode. Membranes were stripped in 100 mM 2-mercaptoethanol, 2% SDS, and 62.5 mM Tris at 50°C for 30 min. Membranes were then incubated after blocking with an anti-Stat3 antibody (9132, Cell Signaling Technology) and processed as above.
RNA Extraction and Real-Time PCR. Tissue was homogenized in TRIzol reagent (Invitrogen), and total RNA was purified on GenElute columns (Sigma). The relative expression of selected genes was compared between inhibitor-treated and control tissues by performing real-time RT-PCR with an ABI PRISM 7700 sequence detection system (TaqMan) according to the manufacturer's instructions (Applied Biosystems). The sequences for primers and probes for osteoblast-specific factor 2 (Osf2), immune response gene 1 (Irg1), insulin-like growth factor binding protein 3 (Igfbp3), and cochlin were as described in ref. 25. For arachidonate 15-lipoxygenase (Alox15) and ribosomal 18S RNA, prevalidated probes were purchased (Assays-on-Demand, Applied Biosystems). Primers for amphiregulin were designed by using primer express v.5.0 software (Applied Biosystems): gcgcgctcagtgctgtt (forward primer), tcattgagctccaaagcagct (reverse primer), and ctgctggtcttaggctcaggccattatg (probe). The amphiregulin probe was labeled with 5′ FAM and 3′ TAMRA. Standard curves were generated by serial dilution of a preparation of total RNA isolated from whole-mouse uterus. Data are expressed in arbitrary units relative to the level of the same transcript in this standard RNA. cDNA was produced from each sample of LE by reverse transcription, using 0.5 μg of total RNA with 200 units of SuperScript III (Invitrogen) according to the manufacturer's instructions. The values obtained were normalized against those from the control ribosomal 18S RNA to account for differing amounts of starting material.
Statistical Analysis. The statistical analysis of the data from the intrauterine injections of Stat3 peptide inhibitor on implantation was performed by using Welch's alternate t test. Embryo toxicity data were analyzed by using Fisher's exact test. Levels of Stat3 phosphorylation and gene expression in treated and control LE obtained from the Western blots and real-time PCR, respectively, were compared by using a nonparametric Wilcoxon paired test.
Results
Administration of Stat3 Inhibitor Reduces Implantation Rates. To test the hypothesis that Stat3 phosphorylation is essential for implantation, the effects of inhibiting Stat3 phosphorylation in the uterus with a membrane-permeable peptide were studied. Either control peptide or Stat3 inhibitor was injected into the uterine lumen on day 3 of pregnancy. This is ≈24 h before implantation commences. The number of implanted embryos in each horn was counted on days 8–10 of pregnancy. The uterine horns appeared normal except for slight swelling at the injection site. Ovulation was confirmed in all animals by the presence of corpora lutea in the ovary. The mean number of implanted embryos per uterine horn in the control group was 3.7 (control peptide) and 3.9 (PBS; data not shown), compared with 1.1 in the inhibitor-treated group (Fig. 1). This reduction of 70% was statistically significant (P < 0.001).
Fig. 1.
Effect of intrauterine injection of Stat3 inhibitor on implantation rates. Pregnant mice were injected on the evening of day 3 in both horns with either control peptide (controls, n = 10) or Stat3 peptide inhibitor (n = 10), and the number of implanted embryos per uterine horn was counted on day 8. The mean number of implanted embryos in the control group was 3.7 compared with 1.1 in the inhibitor-treated group, indicated by the line (P < 0.001) corresponding to a reduction in implantation rate of 70%.
Stat3 Inhibitor Has No Effect on Preimplantation Embryo Development. To determine whether the reduction in implantation rates observed with Stat3 inhibitor could be attributed to a direct effect on embryos, an embryo toxicity assay was performed. Approximately 100 embryos were collected at the 16-cell stage from normal MF1 mice on day 3 of pregnancy. This corresponds to the time of intrauterine injection used in Fig. 1. Embryos were exposed to Stat3 inhibitor, or vehicle (PBS) diluted in embryo culture medium, for 1 h, then washed and cultured overnight. The number of embryos reaching the blastocyst stage on day 4 was 59% and 84% in the control cultures and 64% and 89% in the inhibitor treated cultures in replicate experiments. Treated blastocysts were then transferred to recipient females that had been mated with vasectomized males, and implantation rates were determined on day 8 of pregnancy. The rate of implantation was 46% and 55% in the control group and in the inhibitor-treated group was 54% and 60%, respectively, in replicate experiments. Therefore, exposure to the Stat3 inhibitor had no effect either on the development of the embryos to the blastocyst stage or on their ability to implant.
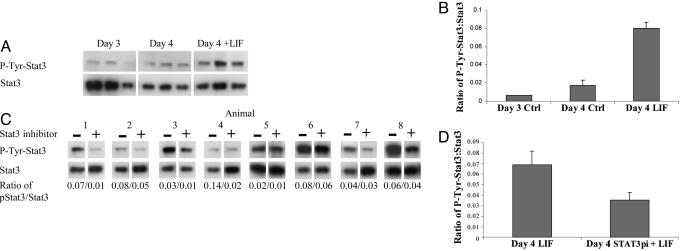
Response of LE to LIF Stimulation. We hypothesized that Stat3 phosphorylation in the LE is an obligatory step for LIF-induced uterine receptivity. We therefore sought to determine whether the Stat3 inhibitor was effective in blocking the responses induced in the LE by LIF. LE isolated on day 4 of pregnancy was allowed to recover for 3 h in vitro. LE from one horn was then stimulated with 50 ng/ml LIF in PBS for 15 min, whereas the LE from the other horn was treated with PBS alone. Western blotting and densitometry was used to quantitate levels of phospho-Stat3 and Stat3 in the LIF- and PBS-treated samples (Fig. 2 A and B). LIF treatment resulted in a 5-fold increase in the level of phospho-Stat3, relative to total Stat3 compared with the PBS treated day 4 samples, where levels were comparable to those seen in freshly isolated unstimulated LE from day 3. To determine whether the Stat3 inhibitor was effective, the experiment was repeated in the presence of 1 mM inhibitor. LE was isolated on day 4 of pregnancy and divided into two with one-half incubated with the inhibitor for 1 h (n = 8). The two samples (with and without inhibitor) were then stimulated with LIF, and the levels of Stat3 and phospho-Stat3 were determined (Fig. 2 C and D). In samples treated with the Stat3 phosphorylation inhibitor, the ratio of phospho-Stat3 to total Stat3 after LIF treatment was reduced by an average of 49% (P < 0.007). LIF stimulation of LE had no effect on the level of Stat1 phosphorylation, nor was this altered by the presence of the Stat3 inhibitor (data not shown). These results indicate that Stat3 is activated by LIF in the LE on day 4 of pregnancy and that the inhibitor is specific in inactivating only Stat3.
Fig. 2.
Stat3 phosphorylation status and response to LIF in LE isolated on day 3 (noon) or day 4 (noon) of pregnancy. Day-4 LE isolated from one horn was treated with LIF after recovery in culture, and the other horn represented an untreated control. (A) Levels of Stat3 phosphorylation and total Stat3 in day 3, day 4 (control), and day 4 plus LIF LE were analyzed by Western blotting. Note that exposure to film was considerably longer for phospho-Stat3 than total Stat3. (B) After densitometry with the phosphoimager, the ratio of phospho-Stat3 to Stat3 was calculated. LIF treatment of day-4 LE dramatically increased levels of phosphorylated Stat3 (P < 0.005, n = 3). (C) Stat3 inhibitor reduced phosphorylation of Stat3 in the LE in response to LIF in vitro. LE was isolated on day 4 (noon), allowed to recover in culture for 2 h, and then incubated with or without Stat3 inhibitor for 1 h. It was then stimulated with LIF, and the levels of phosphorylated Stat3 and total Stat3 in control or inhibitor-treated LE were measured. The ratio of Stat3 phosphorylation to total Stat3 is given below each lane of paired samples (n = 8). (D) Mean ratio of phosphorylated Stat3 to total Stat3 after LIF stimulation of horns treated with or without Stat3 inhibitor. The inhibitor reduced phosphorylation of Stat3 after LIF stimulation by an average of 49% (P < 0.007).
Effect of Stat3 Inhibitor on LIF-Stimulated Gene Expression in Vitro. If Stat3 phosphorylation in response to LIF is reduced by Stat3 inhibitor, then those genes up-regulated by LIF should also show a reduced response to LIF stimulation. LE isolated on day 4 from each uterine horn was divided into two, allowed to recover, and then treated with or without 1 mM Stat3 inhibitor for 1 h. Both samples were then stimulated with LIF at 50 ng/ml and cultured for a further 4 h. The expression of four mRNAs known to be regulated by LIF and two not regulated by LIF was assessed by real-time RT-PCR. Expression of Irg1, cochlin, and amphiregulin was significantly lower in the inhibitor-treated group compared with controls, indicating a reduced response to LIF (Fig. 3). Igfbp3 expression levels did not significantly differ from controls after a 4-h stimulation. Two other genes whose expression in the LE on day 4 is not regulated by LIF were assessed to determine whether Stat3 inhibitor had nonspecific effects on gene expression. Expression of both Osf2 and Alox15 was unaltered by the inhibitor, suggesting that its effects on the other three LIF-regulated genes were specific.
Fig. 3.
Effect of Stat3 inhibitor on the expression of LIF-regulated genes in vitro. LE isolated from day-4 (noon) uterine horns were divided into two equal pieces and treated with or without 1 mM Stat3 peptide inhibitor. One hour later, samples were stimulated with LIF (50 ng/ml) and cultured for a further 4 h, and total RNA was extracted. The expression levels of LIF-regulated genes were determined by real-time PCR. The mean reduction compared with control between paired treatments (n = 8) is indicated as a percentage.
In Vivo Effects of Stat3 Inhibitor on Stat3 Phosphorylation. Because the Stat3 inhibitor effectively reduced Stat3 activation in the LE in response to LIF in vitro, a key question was whether intrauterine injection of Stat3 inhibitor could also render the LE unresponsive to LIF. Pregnant mice were given intrauterine injections of PBS (control) in one horn and Stat3 inhibitor in the other on day 3 (1800 hours). The dose and timing was the same as that used to inhibit implantation. LE was then isolated from each horn on day 4 (noon), allowed to recover in culture for 3 h, and then stimulated with LIF for 15 min to assess its ability to activate Stat3 phosphorylation. A total of 18 h after in vivo administration of the inhibitor, Stat3 phosphorylation in the LE in response to LIF was reduced by 32% compared with the control LE from the same animal (Fig. 4). This finding shows that intrauterine injection of Stat3 inhibitor is able to reduce the response of the LE to LIF on day 4 of pregnancy.
Fig. 4.
In vivo administration of Stat3 inhibitor reduces phosphorylation of Stat3 in the LE in response to LIF. Mice were given intrauterine injections of PBS (control) in one horn and Stat3 inhibitor (Stat3pi) in the other on day 3 of pregnancy. LE was isolated on day 4 (noon), allowed to recover in culture for 3 h, and then stimulated for 15 min with LIF to assess the response of Stat3. (A) Relative levels of phospho-Stat3 and total Stat3 in the samples were determined by Western blotting, followed by densitometry with the phosphoimager, and the ratio is shown below each sample. (B) Stat3 inhibitor reduced Stat3 phosphorylation after LIF stimulation by an average of 32% 18 h after in vivo administration of the inhibitor (P < 0.04).
In Vivo Effects of Stat3 Inhibitor on LIF-Regulated Gene Expression. Intrauterine injection of the Stat3 inhibitor reduced implantation by 70% and significantly inhibited phosphorylation of Stat3 in the LE in response to LIF. The inhibitor was also able to oppose the up-regulation in the LE by LIF of several LIF-responsive genes in vitro. To assess whether the inhibitor could directly affect expression of LIF-regulated genes in vivo, perhaps accounting for the reduced implantation rates, the expression of these genes was examined by RT-PCR. Pregnant mice were given injections of PBS into one horn and Stat3 inhibitor into the other horn at 1800 hours on day 3. LE was isolated 24 h later, and total RNA was extracted. In vivo levels of mRNAs encoding Igfbp3, amphiregulin, and cochlin were significantly reduced in the inhibitor-treated horns compared with the paired PBS-treated LE from the same animals (Fig. 5). Irg1 expression levels did not significantly differ from controls at this time point. Levels of Osf2 and Alox15 mRNA transcripts were not significantly different between horns.
Fig. 5.
Effect of Stat3 inhibitor on the expression of LIF-regulated genes in vivo. Mice were given intrauterine injections of PBS (control) in one horn and Stat3 peptide inhibitor in the other on day 3 of pregnancy. LE was isolated on day 4 (1600 hours), and total RNA was extracted. The expression levels of LIF-regulated genes were determined by real-time PCR. The mean reduction compared with control between paired treatments (n = 10) is indicated as a percentage.
Expression Levels of LIF-Regulated Genes in the LE During Early Pregnancy. Up-regulation by LIF of Irg1, cochlin, and amphiregulin expression in LE was inhibited by the Stat3 inhibitor in vitro, but there was no apparent effect on Igfbp3. In contrast, in vivo injection of the inhibitor reduced the expression in LE of Igfbp3, amphiregulin, and cochlin but not Irg1 in response to endogenous LIF. To investigate this discrepancy, we examined the expression profile of these LIF-regulated genes in the LE in vivo by real-time RT-PCR between days 3 and 5 of pregnancy. The transcripts showed very different responses to endogenous LIF, which is expressed on the morning of day 4. Expression of cochlin, amphiregulin, and Irg1 increase on the morning of day 4, but by the evening of day 4 Irg1 levels have rapidly declined and are not significantly different from day 3 (Fig. 6A). In contrast, Igfbp3 shows a much slower response, with levels only beginning to increase in the evening of day 4 (Fig. 6C). These different dynamic responses probably account for the differences between the in vitro and in vivo results. For example, LE isolated on day 4 was only stimulated with LIF in vitro for 4 h before transcript levels were measured (Fig. 3). Given the apparent slow response of Igfbp3 to LIF, Igfbp3 expression may not have begun to respond to LIF, so no apparent effect of the Stat3 inhibitor might be expected. Conversely, Irg1 levels in vivo appear to respond rapidly on the morning of day 4 to LIF but have dropped back to levels similar to those seen on day 3 by the afternoon. When the effect of Stat3 inhibitor on gene expression was examined in vivo, LE was isolated on the afternoon of day 4, by which Irg1 levels have declined, so no apparent effect of the inhibitor on Irg1 expression is seen. However the short 4-h exposure of LE to LIF given in vitro captures the rapid rise in expression of Irg1, which is inhibited by the Stat3 peptide.
Fig. 6.
Expression levels of LIF-regulated genes in the LE during early pregnancy. LE was isolated from day 3 (9 p.m., n = 5), day 4 (9 a.m., n = 5), day 4 (9 p.m., n = 4), day 5 (9 a.m., n = 4), and day 5 (9 p.m., n = 3). RNA was extracted, and expression levels were determined by real-time PCR. Mean expression values for each time point are indicated. Asterisks indicate time points where expression is different from the level at 9 p.m. on day 3 (P < 0.05).
Discussion
We chose to target the Jak/Stat signal transduction pathway because it is implicated in implantation in mouse, non-human primates, and humans. Our previous studies have shown that Jak1 mRNA expression is down-regulated in human endometrial explants treated with the antiprogestin RU486 (21). In mice, activation of the LIF receptor is required for blastocyst implantation, and IL-11R is required 1 day later for normal decidual response to the implanting blastocyst (3, 4). Both receptors utilize gp130, and ligand binding can result in the activation of several members of the Stat family including Stat1, Stat3, and Stat5 through Jak kinases (9, 26). Stat3 was selected as the specific target because treatment of LE from day 4 of pregnancy with LIF results in Stat3 phosphorylation, with no effect on Stat1 or Stat5, suggesting that Stat3 is the mediator of LIF's action on these cells (9). In vivo, nuclear localization of Stat3 occurs in LE on day 4, coinciding with the onset of receptivity, and is also seen on day 5 in stromal cells undergoing decidualization. We confirmed that exposure of LE to LIF does not result in Stat1 phosphorylation, nor did the Stat3 inhibitor have any effect on levels in LE of total or phosphorylated Stat1. The effects of the inhibitor did not therefore appear to be due to nonspecific actions on other members of the Stat family.
Functional blockade of the Jak/Stat pathway has been achieved both in vitro and in vivo by using a variety of compounds such as AG-490, curcumin, and curcubitacin I (27–29). Although these agents predominantly block Jak/Stat activity, they can affect other signaling pathways. Recently, more specific Stat3 peptide inhibitors have been developed by fusing the Stat3 SH2 binding domain to a membrane-translocating sequence, which allows delivery of the active peptide to cells in vitro (22, 30, 31). The peptide used in this study contains the Stat3 SH2 domain binding peptide PYLKTK, where Y represents phosphotyrosine. This peptide competitively inhibits Stat3 dimerization and disrupts Stat3 dimers in vitro by direct interaction with Stat3 monomers (22). The phosphorylation of the tyrosine residue in this SH2-binding region of STAT3 is critical for dimerization, and peptides with an unphosphorylated tyrosine residue do not inactivate Stat3 dimers. Therefore, a nonphosphotyrosine peptide of the same sequence is an ideal control to assess any nonspecific effects of the phosphorylated inhibitor. The peptide inhibitor had not previously been administered in vivo. In these experiments, the drug was delivered directly to the uterine lumen because the primary target was the cells of the LE. This also avoided possible systemic endocrine effects, for example on the ovary, that might affect implantation indirectly. Competitive inhibition of Stat3 activation by the Stat3 inhibitor significantly reduced the number of implantation sites compared with controls.
To exclude the possibility that the effect of the inhibitor on implantation may have been due to an effect on the embryo, embryos isolated on day 3 of pregnancy were exposed to Stat3 inhibitor for 1 h. Previous reports have shown that the peptide is able to inhibit Stat3 activation in cell lines after <1 h, and the effect persists for up to 24 h (22, 23). In mice, embryos develop in the fallopian tube for the first 3 days of pregnancy and enter the uterus itself late on day 3 or early on day 4. A direct effect of the inhibitor after injection into the uterus on day 3 seems unlikely because Stat3 expression has only been reported in the embryo from embryonic day 6.0 and Stat3-null embryos do implant, although they degenerate later in pregnancy (32, 33). Indeed, our embryo toxicity assays showed that the inhibitor had no effect on embryo development before implantation and did not affect the ability of the embryos to implant. This finding implies that the reduced implantation caused by the Stat3 inhibitor is due to a direct effect on uterine receptivity.
We were able to show that the Stat3 inhibitor reduced the ability of the LE to activate Stat3 in response to LIF. Incubation of LE isolated on day 4 of pregnancy with LIF resulted in increased levels of phospho-Stat3, without any change in levels of phospho-Stat1. This finding is in agreement with a previous report and provides a convenient system to assess the effects of Stat3 inhibitors on the response of the LE to LIF (9). Preincubation of LE with Stat3 inhibitor in vitro resulted in reduced phospho-Stat3 levels in response to LIF and reduced induction of LIF-responsive genes such as cochlin, amphiregulin, and Irg1. However, expression of Igfbp3 was not significantly altered by the presence of the inhibitor at this time point. More importantly, LE isolated on day 4 of pregnancy, 24 h after intrauterine injection of Stat3 inhibitor, also showed a reduced level of phospho-Stat3 in response to LIF. Levels of expression of three of four LIF-regulated genes that we tested were reduced in vivo. The effects of the Stat3 inhibitor on the response to LIF of the mRNAs for Igfbp3 and Irg1 differed between the in vivo and in vitro experiments. Analysis of expression levels in the LE from day 3 to day 5 of pregnancy revealed that Irg1 mRNA levels respond rapidly to LIF, whereas Igfbp3 responds more slowly. For the in vitro experiment, the 4-h exposure of LE to LIF was probably too short to induce Igfbp3 expression. After inhibitor injection in vivo, the isolation of the LE in the afternoon of day 4 was too late to assess the effect of the inhibitor on Irg1 responses because Irg1 mRNA expression had fallen naturally back to the levels seen before LIF stimulation. These altered responses to LIF probably account for the discrepancy in the inhibitor's effect on the targets in our in vitro and in vivo experiments. The reduction in levels of mRNA for LIF-responsive genes in the LE was specific, because expression of Osf2 and Alox15, which are expressed in the LE but are not LIF-regulated, was unchanged. The inhibitor of Stat3 phosphorylation was able to significantly reduce the response of the LE to endogenous LIF exposure in vivo, many hours after administration of the inhibitor. This finding is consistent with previous reports suggesting that Stat3 inhibitor persists and is active in cell lines for up to 48 h after initial exposure (22). If inhibition of Stat3 activation extended to day 5 of pregnancy and the inhibitor reached decidualizing cells immediately beneath the LE, then activation of Stat3 by IL-11R in these cells might also be affected. This potential effect was not explored directly in this study.
Cochlin, Igfbp3, amphiregulin, and Irg1 are coregulated by LIF and progesterone in the uterus (25, 34, 35). Only Irg1, however, is believed to be important for implantation because down-regulation by antisense oligonucleotides is associated with decreased implantation (36). We have now shown that up-regulation of these genes by LIF in the LE depends, at least in part, on the activation of Stat3. One possible mechanism is that Stat3 dimers bind to consensus Stat3 response elements (STATRE) in the promoters of these genes. However, when the genomic regions 5′ to the coding sequences of all four genes were searched by in silico analysis (alibaba 2.1, Biobase) for putative STATRE, none were identified, suggesting an alternate regulatory mechanism. Alternatively, activated Stat3 can function as a transcriptional coactivator without direct DNA binding through STATRE. For example, Stat3 can enhance the transactivation of steroid receptors in a hormone-dependent manner, thereby increasing sensitivity of these receptors for their ligands (37). In rat decidua, phosphorylated Stat3 binds directly to the progesterone receptor (PR) (38). We propose that a similar regulatory mechanism may function in LE on day 4 of pregnancy. Transactivation of genes such as Irg1, amphiregulin, cochlin, and Igfbp3 may be regulated by complexes containing Stat3 and PR. This observation could provide a mechanism to explain the finding that expression of these genes at normal physiological levels on day 4 requires the action of both progesterone and LIF (25).
The administration of a cell-permeable Stat3 peptide inhibitor directly into the uterine lumen of mice in early pregnancy has allowed us to define a critical role for Stat3 in the process of embryo implantation. Inhibition of Stat3 phosphorylation in the LE prevents activation by LIF of several LIF-regulated genes including Irg1, which is known to be essential for embryo attachment, and this results in implantation failure. We describe the use of a cell-permeable peptide to modulate reproduction in vivo by the inhibition of intracellular signaling. These results suggest an approach for the development of postcoital contraceptive agents, based on selective targeting of signal transduction pathways necessary for endometrial receptivity.
Acknowledgments
We thank Sue Kimber for advice regarding preparation of LE and Kathrine Abell for technical advice on Western blotting. This work was supported by the Rockefeller/World Health Organization implantation initiative. A.M.S. was supported by the Meres senior research studentship from St. John's College, Cambridge Unviersity. M.H.J. thanks the Wellcome Trust for financial support.
Author contributions: R.D.C. and A.M.S. designed research; R.D.C., M.H.J., E.A.C., and A.M.S. performed research; R.D.C., M.H.J., D.S.C.-J., S.K.S., and A.M.S. analyzed data; and R.D.C. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: LE, luminal epithelium; LIF, leukemia inhibitory factor.
References
- 1.Psychoyos, S. (1986) Ann. N.Y. Acad. Sci. 476, 36-42. [DOI] [PubMed] [Google Scholar]
- 2.Abrahamsohn, P. A. & Zorn, T. M. (1993) J. Exp. Zool. 266, 603-628. [DOI] [PubMed] [Google Scholar]
- 3.Stewart, C. L., Kaspar, P., Brunet, L. J., Bhatt, H., Gadi, I., Kontgen, F. & Abbondanzo, S. J. (1992) Nature 359, 76-79. [DOI] [PubMed] [Google Scholar]
- 4.Robb, L., Li, R., Hartley, L., Nandurkar, H. H., Koentgen, F. & Begley, G. (1998) Nat. Med. 4, 303-308. [DOI] [PubMed] [Google Scholar]
- 5.Zhu, L. J., Bagchi, M. K. & Bagchi, I. C. (1998) Endocrinology 139, 330-339. [DOI] [PubMed] [Google Scholar]
- 6.Bhatt, H., Brunet, L. J. & Stewart, C. L. (1991) Proc. Natl. Acad. Sci. USA 88, 11408-11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, J. R., Cheng, J. R., Shatzer, T., Sewell, L., Hernandez, L. & Stewart, C. L. (2000) Endocrinology 141, 4365-4372. [DOI] [PubMed] [Google Scholar]
- 8.Heinrich, P. C., Behrmann, I., Muller-Newen, G., Schaper, F. & Graeve, L. (1998) Biochem. J. 334, 297-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng, J.-G., Chen, J. R., Hernandez, L., Alvord, W. G. & Stewart, C. L. (2001) Proc. Natl. Acad. Sci. USA 98, 8680-8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charnock-Jones, D. S., Sharkey, A. M., Fenwick P. & Smith, S. K. (1994) J. Reprod. Fertil. 10, 421-426. [DOI] [PubMed] [Google Scholar]
- 11.Yue, Z. P., Yang, Z. M., Wei, P., Li, S. J., Wang, H. B., Tan, J. H. & Harper, M. J. (2000) Biol. Reprod. 63, 508-512. [DOI] [PubMed] [Google Scholar]
- 12.Kholkute, S. D., Katkam, R. R., Nandedkar, T. D. & Puri, C. P. (2000) Mol. Hum. Reprod. 6, 337-343. [DOI] [PubMed] [Google Scholar]
- 13.Auernhammer, C. J. & Melmed, S. (2000) Endocr. Rev. 21, 313-345. [DOI] [PubMed] [Google Scholar]
- 14.Schindler, C. & Darnell, J. E., Jr. (1995) Annu. Rev. Biochem. 64, 621-651. [DOI] [PubMed] [Google Scholar]
- 15.Ihle, J. N. (1996) Cell 84, 331-334. [DOI] [PubMed] [Google Scholar]
- 16.Zhang, X., Blenis, J., Li, H.-C., Schindler, C. & Chen-Kiang, S. (1995) Science 267, 1990-1994. [DOI] [PubMed] [Google Scholar]
- 17.Ernst, M., Inglese, M., Waring, P., Campbell, I. K., Bao, S., Clay, F. J., Alexander, W. S., Wicks, I. P., Tarlinton, D. M., Novak, U., et al. (2001) J. Exp. Med. 194, 189-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chabbert Buffet, N., Djakoure, C., Christin Maitre, S. & Bouchard, P. (1995) Front. Neuroendocrinol. 19, 151-186. [DOI] [PubMed] [Google Scholar]
- 19.Hegele-Hartung, C., Mootz, U. & Beier, H. M. (1992) Endocrinology 131, 2446-2460. [DOI] [PubMed] [Google Scholar]
- 20.Gemzell-Danielsson, K., Swahn, M. L., Svalander, P. & Bygdeman, M. (1993) Hum. Reprod. 8, 870-873. [DOI] [PubMed] [Google Scholar]
- 21.Catalano, R. D., Yanaihara, A., Evans, A. L., Rocha, D., Prentice, A., Saidi, S., Print, C. G., Charnock-Jones, D. S., Sharkey, A. M. & Smith, S. K. (2003) Mol. Hum. Reprod. 9, 465-473. [DOI] [PubMed] [Google Scholar]
- 22.Turkson, J., Ryan, D., Kim, J. S., Zhang, Y., Chen, Z., Haura, E., Laudano, A., Sebti, S., Hamilton, A. D. & Jove, R. (2001) J. Biol. Chem. 276, 45443-45455. [DOI] [PubMed] [Google Scholar]
- 23.Bharti, A. C., Donato, N. & Aggarwal, B. B. (2003) J. Immunol. 171, 3863-3871. [DOI] [PubMed] [Google Scholar]
- 24.Sidhu, S. S. & Kimber, S. J. (1999) Biol. Reprod. 60, 147-157. [DOI] [PubMed] [Google Scholar]
- 25.Sherwin, J. R. A., Freeman, T. C., Scott, L., Kimber, S., Smith, A. G., Chambers, I., Smith, S. K. & Sharkey, A. M. (2004) Mol. Endocrinol. 18, 2185-2195. [DOI] [PubMed] [Google Scholar]
- 26.Underhill-Day, N., McGovern, L. A., Karpovich, N., Mardon, H. J., Barton, V. A. & Heath, J. K. (2003) Endocrinology 144, 3406-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nielsen, M., Kaltoft, K., Nordahl, M., Ropke, C., Geisler, C., Mustelin, T., Dobson, P., Svejgaard, A. & Odum, N. (1997) Proc. Natl. Acad. Sci. USA 94, 6764-6769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Natarajan, C. & Bright, J. J. (2002) J. Immunol. 169, 6506-6513. [DOI] [PubMed] [Google Scholar]
- 29.Blaskovich, M. A., Sun, J., Cantor, A., Turkson, J., Jove, R. & Sebti, S. M. (2003) Cancer Res. 63, 1270-1279. [PubMed] [Google Scholar]
- 30.Ren, Z., Cabell, L. A., Schaefer, T. S. & McMurray, J. S. (2003) Bioorg. Med. Chem. Lett. 13, 633-636. [DOI] [PubMed] [Google Scholar]
- 31.Schwarze, S. R., Ho, A., Vocero-Akbani, A. & Dowdy, S. F. (1999) Science 285, 1569-1572. [DOI] [PubMed] [Google Scholar]
- 32.Duncan, S. A., Zhong, Z., Wen, Z. & Darnell, J. E., Jr. (1997) Dev. Dyn. 208, 190-198. [DOI] [PubMed] [Google Scholar]
- 33.Takeda, K., Noguchi, K., Shi, W., Tanaka, T., Matsumoto, M., Yoshida, N., Kishimoto, T. & Akira, S. (1997) Proc. Natl. Acad. Sci. USA 94, 3801-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodriguez, C. I., Cheng, J.-G., Liu, L. & Stewart, C. L. (2004) Endocrinology 145, 1410-1418. [DOI] [PubMed] [Google Scholar]
- 35.Cheon, Y.-P., Li, Q., Xu, X., Demayo, F. J., Bagchi, I. C. & Bagchi, M. K. (2002) Mol. Endocrinol. 16, 2853-2871. [DOI] [PubMed] [Google Scholar]
- 36.Cheon, Y.-P., Xu, X., Bagchi, M. K. & Bagchi, I. C. (2003) Endocrinology 144, 5623-5630. [DOI] [PubMed] [Google Scholar]
- 37.De Miguel, F., Lee, S. O., Onate, S. A. & Gao, A. C. (2003) Nucl. Recept. 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu, T. & Ogle, T. F. (2002) Biol. Reprod. 67, 114-118. [DOI] [PubMed] [Google Scholar]