Abstract
Identification of the hydrolytic enzymes involved in follicle rupture during vertebrate ovulation remains a central challenge for research in reproductive biology. Here, we report a previously uncharacterized approach to this problem by using an in vitro ovulation system in the medaka, Oryzias latipes, which is a small freshwater teleost. We found that follicle rupture in the medaka ovary involves the cooperation of at least three matrix metalloproteinases (MMPs), together with the tissue inhibitor of metalloproteinase-2b protein. We determined the discrete roles of each of these proteins during follicle rupture. Our results indicated that gelatinase A induces the hydrolysis of type IV collagen constituting the basement membrane, membrane-type 2 MMP degrades type I collagen present in the theca cell layer, and MT1-MMP and the tissue inhibitor of metalloproteinase-2b are involved in the production and regulation of gelatinase A. These findings will help clarify the mechanism of follicle wall degradation during ovulation in mammalian species.
Keywords: medaka fish
Ovulation, which is triggered by a preovulatory surge of luteinizing hormone released from the pituitary gland, is a dynamic process that results in the liberation of a mature fertilizable ovum from the ovarian follicle. This event, which is known as follicle rupture, has been the subject of intensive investigation over the past century (1–5). Previous studies carried out primarily in mammalian species, including humans, have established that follicle rupture is accomplished by the dissolution of the granulosa cell basement membrane and fragmentation of the collagenous matrix at the apex of the follicular wall, thereby implicating the involvement of proteolytic enzymes. Indeed, a variety of proteases have been proposed as candidates for rupturing the follicle. It is generally believed that follicle rupture during ovulation takes place because of the actions of two proteolytic enzyme systems: the plasminogen activator/plasmin system (3, 6, 7) and the matrix metalloproteinase (MMP) system (8–11). However, it has been demonstrated that most mouse strains lacking individual proteases retain apparently normal reproductive ability, which is not consistent with this hypothesis (5, 12). A few MMP-deficient mice, including those lacking membranetype (MT)1-MMP (13), and a disintegrin and metalloproteinase domain-17 (14), die in utero or shortly after birth; thus, the roles played by these proteases in ovulation remain to be clarified. In addition, the involvement of cathepsin L, and a disintegrin and MMP domain with thrombospondin-like motifs (ADAMTS-1), has been suggested by studies of mice lacking progesterone receptors (15). Mice null for ADAMTS-1 have been shown to develop fewer mature follicles (16), although its relationship to follicle rupture is not clear. A recent study suggested that one function of ADAMTS-1 in ovulation is to cleave versican in the matrix of the expanded cumulus–oocyte complex (17). In short, the proteases that are essential for follicle rupture in ovulation have not yet been identified, despite much effort.
In all vertebrates, the growth and proliferation of oogonia, their development to the oocyte stage, and their eventual release from the ovaries are thought to be under similar endocrine regulation (18–20), although the basic ovarian plan has several morphological variants. When searching for the fundamental mechanisms that are common to vertebrate ovaries, the use of the medaka, Oryzias latipes, which is a small freshwater teleost, has several advantages because of its short generation time and the cyclic nature of ovarian activity in mature adults (21, 22): under a constant long photoperiod of 14-h light/10-h dark at 27°C, the medaka usually spawns daily within 1 h of the onset of light for several consecutive days. Thus, we can readily time the successive events of spawning, such as the completion of vitellogenesis, breakdown of the germinal vesicle, and ovulation (23). Moreover, by using this fish, the process of follicle rupture and oocyte extrusion can be observed in vitro in isolated intact follicles (24, 25). We therefore investigated vertebrate ovulatory processes in the medaka, with the particular aim of identifying the enzymes responsible for the proteolytic degradation of the follicle walls.
In this study, we initially designed an in vitro experimental system for the study of ovulation by using dissected ovarian follicles, and we then used this system to examine the effects of various protease inhibitors on the rate of ovulation. The finding that inhibitors of MMPs drastically suppressed in vitro ovulation prompted us to further explore the spatial and temporal expression patterns of most, if not all, of the MMP genes expressed in the follicular tissue of the medaka at both the mRNA and protein levels. We also examined the relevance of individual MMPs to the ovulatory process.
Materials and Methods
Medaka and in Vitro Ovulation. Mature female adults of the orangered variety of medaka were kept in indoor tanks under reproductive conditions (photoperiod, 10-h dark/14-h light; temperature, 27°C). Except where indicated, ovaries were removed at -6 or -3 h of ovulation and were placed in aseptic saline solution (26). Ovarian follicles were immediately isolated by using forceps under a dissecting microscope and were then transferred into 90% medium 199 solution (Earle's medium 199; Dainippon Seiyaku, Osaka), adjusted to pH 7.4 with NaHCO3. At least 20 follicles per culture dish were used in each experiment. The follicles were cultured at 26–27°C in 4 ml of culture medium by using a 35 × 10-mm tissue-culture dish. Ovulation was monitored every hour, and the number of oocytes that had successfully ovulated was counted. The ovulation rate was defined as the percentage of ovulated follicles at a given time. The process of ovulation is indicated in hours relative to the beginning of the light period, set at 0 h.
The follicles were incubated with various protease inhibitors, including the MMP inhibitors tumor necrosis factor-α protease inhibitor (TAPI)-1 (Peptide Institute, Osaka), TAPI-2 (Peptide Institute), and GM6001 (Chemicon), to assess their effects on in vitro ovulation. The concentrations of inhibitors were as follows: EDTA, 2 mM; o-phenanthroline, 1 mM; TAPI-1, 0.1 mM; TAPI-2, 0.1 mM; GM6001, 10 μM; diisopropyl fluorophospate, 0.2 mM; PMSF, 0.2 mM; benzamidine, 0.2 mM; antipain, 0.1 mM; chymostatin, 0.1 mM; leupeptin, 0.1 mM; E-64, 0.2 mM; iodoacetic acid, 50 μM; and pepstatin, 10 μM. For the experiments with antibodies, purified antibody fractions (100 μg) were included in the culture system. IgG fractions prepared from preimmune rabbit antiserum by means of a protein G-Sepharose column were used as controls. In some experiments, actinomycin D (Sigma), cycloheximide (Sigma), and EDTA were added for various periods of incubation.
Isolation of Oocytes and Follicle Layers from Ovulating Follicles. Follicles that were about to ovulate were isolated as described above. Before spontaneous in vitro ovulation, the oocytes were mechanically separated from the follicles by using forceps. A follicle devoid of its oocyte is composed of a single inner layer of granulosa cells and a single layer of outer theca cells separated by a basement membrane; the oocyte-free follicle thus prepared was referred to as the “follicle layer.” After ovulation, the oocytes and follicle layers were collected.
Preparations of Extracts and Culture Media for Immunoblotting. Except where indicated, whole ovaries, dissected follicles, isolated oocytes, and follicle layer tissues were homogenized in PBS and centrifuged at 13,000 × g for 10 min to obtain supernatant fractions and precipitates. The supernatants were used directly for blotting, whereas the precipitate fractions were further boiled in 1% SDS and centrifuged at 13,000 × g for 10 min, and then the resulting supernatants were used. The in vitro follicle culture media were concentrated before use. Samples or immune precipitates were analyzed by Western blot analysis. The primary antibodies were affinity-purified antibodies produced by using blot membranes or anti-human TIMP-2 monoclonal antibody (Santa Cruz Biotechnology).
Immunoprecipitation and Zymography. Ovarian follicle extracts were prepared as described above. Protein G-Sepharose beads were treated with buffer A (20 mM Tris·HCl, pH 8.0/0.15 M NaCl/0.05% Tween-20) containing 0.1% BSA for 30 min at 4°C. After washing the beads with buffer A, they were incubated with purified antibodies for 1 h at 4°C and washed with buffer B (50 mM Tris·HCl, pH 8.0/0.15 M NaCl/1% Triton X-100/0.1% SDS). The beads were then incubated at 4°C for 16 h with the extracts or in vitro ovulation culture media that had been added to the same volume of 2× buffer B. The beads were washed four times, incubated with SDS sample buffer without 2-mercaptoethanol for 6 h at room temperature, and analyzed by gelatin zymography.
Cloning, RT-PCR, in situ hybridization, recombinant protein preparation, enzyme assays, antibody generation, immunohistochemistry, and the medaka type I collagen isolation procedures are described in Supporting Methods, which is published as supporting information on the PNAS web site. Primers and strategies for cloning and protein synthesis are discussed in Tables 1–4, which are published as supporting information on the PNAS web site.
Results
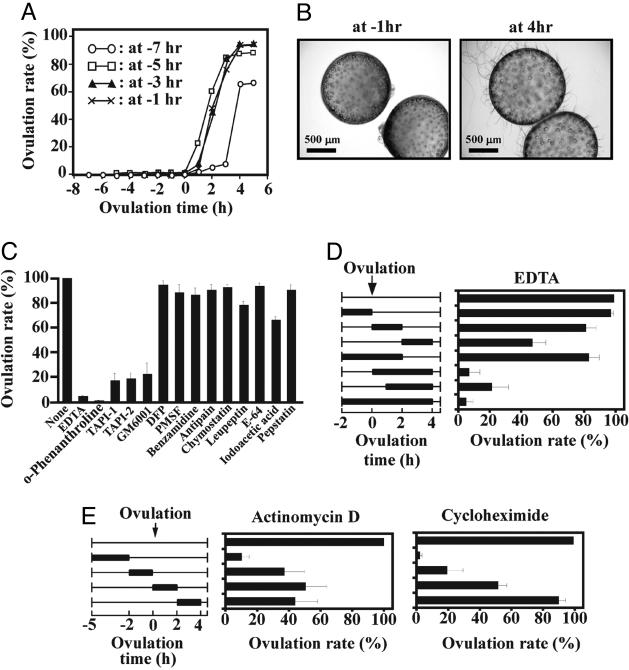
Metalloproteinase Inhibitors Inhibit in Vitro Ovulation. Follicles isolated at various time points from the ovaries of the medaka were cultured at 26–27°C to determine the rate of ovulation. Throughout the study, we defined the time of ovulation as the point at which the fish were expected to ovulate in vivo: this timing corresponded to the start of the light period and was set to 0 h. Follicles that were isolated 2–6 h before the predicted time of ovulation ovulated in vitro (Fig. 1A). When follicles were isolated 7 h before the predicted onset of ovulation (that is, at -7 h), the ovulation rate was reduced. Ovulated oocytes were readily recognized by the appearance of freed attaching filaments on their surface (Fig. 1B). Under these conditions, breakdown of the germinal vesicle occurred in almost all of the ovulated oocytes examined. Ovulated oocytes were successfully fertilized and could subsequently develop into adults; ≈75% of the fertilized oocytes developed normally to hatching (data not shown). Based on these observations, the in vitro ovulation experiments in this study were conducted by using follicles isolated 3 or 6 h before the onset of the light phase.
Fig. 1.
In vitro ovulation in medaka and the effects of various chemicals. (A) Follicles obtained from mature medaka ovaries at various preovulation times were incubated. The ovulation rates were determined, and the results are shown as the means of at least five experiments. (B) Follicles collected at -6 h were incubated. Photographs were taken at -1 and 4 h of ovulation. (C) Follicles collected at -3 h of ovulation were incubated with various protease inhibitors, and the ovulation rates were determined at 4 h. The results are shown as the means (±SD) of at least five experiments. (D) Follicles were collected at -3 h for in vitro ovulation. The timing of EDTA treatments (2 mM) is indicated by the thick bars (Left). The initiation and termination of the treatments were accomplished by rapid exchanges of the culture medium (Right). The ovulation rates were determined at 4 h of ovulation. The results are presented as the means (±SD) of at least five experiments. (E) Follicles were collected and incubated at -5 h. Actinomycin D (1 μg/ml) (Center) or cycloheximide (1 μg/ml) (Right) was added to the culture. (Left) The timing of the treatments is indicated by the thick bars. The other details remained as described above.
EDTA, o-phenanthroline, TAPI-1, TAPI-2, and GM6001, all of which have been shown to be inhibitors of MMPs, drastically reduced the rate of ovulation (Fig. 1C). Follicles cultured in the presence of the inhibitors had apparently normal morphology (data not shown). When EDTA was included in the tissue cultures between 0 and 4 h, ovulation was completely suppressed (Fig. 1D). In addition, ovulation was almost completely suppressed when actinomycin D or cycloheximide was added between -5 and 0 h (Fig. 1E). These results indicated that the synthesis of mRNA and protein is required for follicle rupture during ovulation. The results also indicate that MMPs play important roles in the proteolytic events associated with the ovulatory process in the medaka.
Medaka Ovarian Follicles Express at Least Seven Species of MMPs. To search for candidate MMPs that are involved in follicle rupture during ovulation in the fish, RT-PCR was performed with two degenerate oligonucleotide primers by using total RNA isolated from fish ovaries at the time of ovulation. Subcloning of the amplified cDNA fragments and subsequent analyses identified seven distinct medaka MMPs: gelatinase A (AB033754), gelatinase B (AB033755), stromelysin-3 (AB055705), MT1-MMP (AB185847), MT2-MMP (AB072928), MT3-MMP (AB072929) and MT5-MMP (AB047650). In situ hybridization analysis revealed that gelatinase A, MT1-MMP, MT3-MMP, MT5-MMP, and stromelysin-3 mRNA were expressed in small, growing oocytes, whereas gelatinase B and MT2-MMP mRNA were localized in the tissues ovulated follicles (Fig. 6, which is published as supporting information on the PNAS web site). The results regarding gelatinase A, gelatinase B, MT5-MMP, and stromelysin-3 were consistent with the findings of our previous reports (27–29).
Effects of the Medaka MMPs on Type I and Type IV Collagens. Active recombinant proteins of the seven MMPs were synthesized as fusion proteins, and the MT-MMPs were produced without cytoplasmic and transmembrane domains. All of the MMP preparations yielded a single protein band on SDS/PAGE under reducing conditions (Fig. 7A, which is published as supporting information on the PNAS web site). The gelatin-hydrolyzing activities of the MMPs were detected by using gelatin zymography (Fig. 7B).
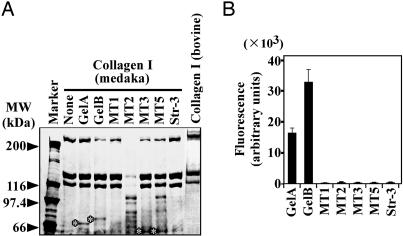
Follicle rupture during ovulation is thought to result from dissolution of the collagen fibers in the tunica albuginea and the theca externa, and the major species present in the connective tissue matrix are believed to be type I and type IV collagen. Therefore, we examined the effects of the medaka MMPs on medaka type I collagen. MT2-MMP degraded the collagen most rapidly (Fig. 2A), although hydrolysis was also observed with MT5-MMP. Medaka type I collagen was resistant to gelatinases A and B, MT1-MMP, MT3-MMP, and stromelysin-3. Because we were unable to test medaka type IV collagen because of the difficulty of obtaining this protein substrate in sufficient quantities, collagen of mammalian origin was used. Medaka gelatinases A and B were able to hydrolyze bovine type IV collagen (Fig. 2B).
Fig. 2.
Hydrolysis of collagens by recombinant medaka MMPs. (A) Type I collagen purified from the whole body of the medaka fish was incubated with purified MMPs, and degradation of the collagen was analyzed by SDS/PAGE (reducing conditions). The results of control experiments without enzymes, but with medaka collagen (None) and bovine type I collagen, are also shown. *, bands of recombinant enzymes that were added to the incubations. (B) FITC-labeled bovine type IV collagen was used as a substrate for the enzymatic activity of the purified MMPs.
We also tested whether gelatinases A and B could act as substrates for MT-MMPs. For this purpose, recombinant proenzymes of the gelatinases were prepared. Incubation of progelatinase A with active MT1-MMP, MT2-MMP, and MT3-MMP resulted in the conversion of the proenzyme (68 kDa) to the mature enzyme (52 kDa) through a 55-kDa intermediate (Fig. 7C). MT5-MMP did not show any detectable activity toward progelatinase A. None of the MT-MMPs tested were able to activate progelatinase B (data not shown).
Ovulating Follicles Express MMP Proteins in Distinct Temporal Patterns. To detect MMPs in the fish ovary, we prepared antibodies against the seven MMPs. Western blot analysis of the fish ovary extracts indicated that the fish ovary expresses gelatinases A and B, MT1-MMP, MT2-MMP, MT5-MMP, and stromelysin-3, whereas MT3-MMP was not detected at any of the levels of significance examined here (Fig. 8, which is published as supporting information on the PNAS web site). Immunohistochemical staining with antibodies for gelatinase A, MT1-MMP, MT5-MMP, and stromelysin-3 produced signals in the oocyte cytoplasm of small growing follicles (Fig. 9, which is published as supporting information on the PNAS web site). In contrast, anti-gelatinase B and anti-MT2-MMP antibodies revealed the presence of their respective antigens in the follicular tissue just after oocyte release. These results were consistent with those from the in situ hybridization experiments (Fig. 6). Although we attempted to localize MT3-MMP immunohistochemically in the ovary sections, no clear signals were observed. This finding provided further support for the view that MT3-MMP mRNA is not translated sufficiently in the oocyte to permit the detection of the corresponding protein.
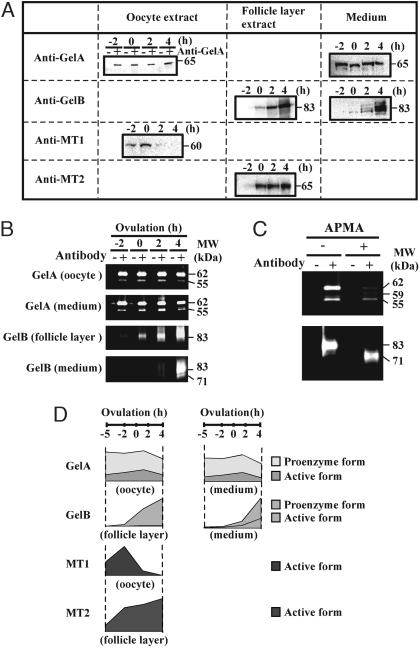
To gain a better understanding of the relationship between ovulation and MMPs, changes in the expression of the six MMPs during the course of in vitro ovulation were investigated by using immunoprecipitation/Western blotting or Western blotting with specific antibodies. Using the extracts of 20 isolated follicles that were ready to ovulate, immunoreactive materials were detected with antibodies to gelatinase A, gelatinase B, MT1-MMP, or MT2-MMP (Fig. 3A) but not with antibodies to MT5-MMP or stromelysin-3 (data not shown). Zymographic analyses of the immunoprecipitates revealed that the oocyte extract and the culture medium contained gelatinase A in two forms: 62- and 55-kDa proteins (Fig. 3B, Top and Top Middle). To clarify the relationship between these two forms of gelatinase A, we examined the effects of p-aminophenylmercuric acetate (APMA), which is a well known activator of secreted MMPs (30), on these proteins. Treatment with this compound resulted in a strong reduction in the 62-kDa band, with concomitant appearance of a 59-kDa band in the oocyte extract and culture medium (Fig. 3C Upper). In addition, the 55-kDa protein remained present after APMA treatment. These results demonstrated that the 62-, 59-, and 55-kDa polypeptides are the proprotein, intermediate protein, and mature active forms of gelatinase A, respectively.
Fig. 3.
Expression of MMPs in ovarian follicles. (A) Mature ovarian follicles were isolated at -6 h of ovulation and cultured in vitro. At the indicated times, the oocytes, follicular layers, and medium were obtained separately. The oocyte extracts were immunoprecipitated with rabbit anti-medaka gelatinase A antibodies, and the resulting precipitates were analyzed by Western blot analysis with rat anti-medaka gelatinase A antibodies. Extracts of the oocytes and follicle layers and the concentrated media were used directly for the Western blot analysis. The sizes (kilodaltons) of the proteins are indicated. (B) The oocyte extracts and media were immunoprecipitated with rabbit anti-gelatinase A antibodies, and the immunoprecipitated materials were analyzed by using gelatin zymography (Top and Top Middle). Extracts of the follicular layers and medium were immunoprecipitated with rabbit anti-gelatinase B antibodies, and the immunoprecipitated materials were analyzed by gelatin zymography (Bottom and Bottom Middle). (C) The in vitro follicle culture medium at 2 h of ovulation was concentrated, treated with 1 mM APMA at 4°C overnight, and immunoprecipitated with anti-gelatinase A antibodies. The immunoprecipitated materials were then subjected to gelatin zymographic analysis (Upper). Extracts of the follicular layers at 2 h of ovulation were treated with APMA, as described above, and were then immunoprecipitated with anti-gelatinase B antibodies. The immunoprecipitates were analyzed by using gelatin zymography (Lower). The sizes of the proteins are indicated. (D) The relative amounts of inactive proenzyme and active mature enzyme at each time point were calculated based on the results shown in A and B.
Immunoprecipitation followed by zymographic analyses for gelatinase B showed that the follicular layers expressed the 83-kDa polypeptide alone intracellularly, whereas the follicle culture medium contained an additional 71-kDa polypeptide (Fig. 3B). The results of APMA treatment confirmed that these were the proprotein and active mature enzymes, respectively (Fig. 3C).
Based on these results, changes in the intracellular and extracellular levels of proactive and mature active MMPs during the course of ovulation can be summarized as shown in Fig. 3D, assuming that the MT-MMPs that are detectable in the tissue extracts are predominantly in the active form. In fact, the sizes of the polypeptides detected by using antibodies specific for MT1-MMP and MT2-MMP were in good agreement with the theoretically predicted sizes. Because gelatinase A, gelatinase B, MT1-MMP, and MT2-MMP were present in an active enzymatic form in the follicles at the time of ovulation, these four MMPs may play a role in follicle rupture during ovulation.
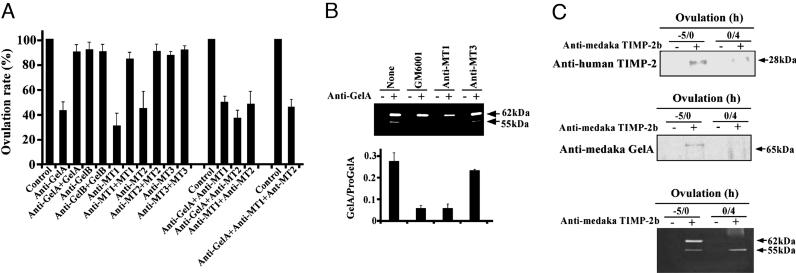
Specific Antibodies for Gelatinase A, MT1-MMP, and MT2-MMP Reduce the Rate of in Vitro Ovulation. We found that the addition of rabbit sera to the system caused the complete suppression of ovulation, regardless of whether antisera or nonimmune control sera were used (data not shown). This reaction was presumably due to protease inhibitors that were present in the sera in large quantities. Therefore, we purified IgGs and specific antibodies to isolate the serum protease inhibitors for further investigation. We confirmed that IgG fractions containing anti-MMP antibodies inhibited the enzyme activities of the respective antigens by >70% in vitro with purified recombinant MMPs and FITC-labeled porcine gelatin type I as a substrate (Fig. 10, which is published as supporting information on the PNAS web site). Incubation of the follicles with anti-gelatinase A, anti-MT1-MMP, and anti-MT2-MMP antibodies resulted in a marked reduction of ovulation (Fig. 4A). The addition of antibodies together with the respective antigens to the culture nullified the ovulation-suppressing activities of the antibodies. Anti-gelatinase B and anti-MT3-MMP antibodies were found to exert no effect on the rate of ovulation. The combination of two MMP antibodies inhibited in vitro ovulation, although the extent of inhibition was similar to that produced by only one type of antibody alone. This finding was also the case for the combined effect of three MMP antibodies. These results indicate that gelatinase A, MT1-MMP, and MT2-MMP play important roles during in vitro ovulation in the medaka.
Fig. 4.
Roles of MMPs and TIMP-2b in follicle rupture. (A) Follicles were incubated with preimmune rabbit IgG (control) or specific antibodies or specific antibodies plus antigens. The results are presented as the means (±SD) of five separate experiments. (B) Follicles isolated at -6 h were incubated with GM6001, anti-MT1-MMP, or anti-MT3-MMP antibodies. The medium collected at 0 h was immunoprecipitated with anti-gelatinase A antibodies, and the precipitates were analyzed by using gelatin zymography (Upper). Progelatinase A (62 kDa) and activated gelatinase A (55 kDa) are indicated. The intensities of the bands shown in Upper were quantified by densitometry, and the ratio of gelatinase A to progelatinase A in each treatment was determined (Lower). The results are presented as the means (±SD) of three experiments. (C) Follicles isolated at -6 h were incubated. Culture media derived from the preovulation period between -5 and 0 h (-5/0) and from the postovulation period between 0 and 4 h (0/4) were obtained. Immunoprecipitation was conducted by using anti-medaka TIMP-2b antibodies, and the precipitates were analyzed by Western blotting with mouse anti-human TIMP-2 monoclonal antibody (Top) or anti-gelatinase A antibody (Middle), or by gelatin zymography (Bottom).
Regulation of Gelatinase A Activity by MT1-MMP and TIMP-2b in Cultured Follicles. When follicles isolated at -6 h of ovulation were cultured in the presence of GM6001 or anti-MT1-MMP antibody for 5 h, the production of active gelatinase A was markedly reduced (Fig. 4B). The generation of active gelatinase A during the 5-h incubation period was not affected by the addition of anti-MT3-MMP antibody. The results revealed that MT1-MMP plays a role in the activation of progelatinase A in culture.
We next investigated how active gelatinase A generated during the preovulation period was secreted into the medium without leading to marked degradation of the basement membrane. One plausible explanation for the absence of degradation was that intrinsic MMP inhibitors might inhibit the activity of gelatinase A. To test this idea, we isolated three TIMP cDNA clones, TIMP-2a (AB185849), TIMP-2b (AB193468), and TIMP-3 (AB193469), from medaka ovary mRNAs. Among these clones, TIMP-2b mRNA was found to be the predominant transcript in fully grown oocytes (Fig. 11, which is published as supporting information on the PNAS web site). We therefore focused on the role of TIMP-2b in the regulation of gelatinase A activity. The oocyte extracts and the culture medium of ovulating follicles contained TIMP-2b with an Mr of 28 kDa (Fig. 4C, Top; see also Fig. 12, which is published as supporting information on the PNAS web site). The maximal TIMP-2b band intensity was detected in the culture medium at -2 h of ovulation, and the signals from subsequent samples examined at 2-h intervals became steadily weaker. These results thus confirmed that the TIMP-2b protein was present in ovulating follicles and was most actively secreted from the oocytes of in vitro-cultured follicles during the preovulation period. We further analyzed the anti-TIMP-2b immunoprecipitates of the culture medium by using Western blot analysis and gelatin zymography. A 65-kDa polypeptide was identified (under reducing conditions) by using anti-gelatinase-A antibody (Fig. 4C Middle), and two polypeptides of 62 and 55 kDa were detected (under nonreducing conditions) by examining the gelatinolytic activity (Fig. 4C Bottom). These results demonstrated the presence of gelatinase A (and progelatinase A) in complex with TIMP-2b in the in vitro ovulation culture medium.
In summary, our results demonstrated that TIMP-2b is expressed in ovulating follicles and reaches a maximum concentration during the preovulation period. These findings strongly suggest that this intrinsic inhibitor regulates gelatinase A activity at or around the time of ovulation.
Discussion
In 1916, Schochet (1) first noted that “the rupture of the Graafian follicle is due in part to the digestion of the theca folliculi by a proteolytic ferment or enzyme in the liquor folliculi.” Since then, intense efforts have been made to identify the proteases that are essential for follicle rupture during ovulation, primarily in mammalian species. Although the identification of proteases responsible for follicle rupture remains to be achieved, previous studies have strongly suggested that this process is accomplished by the cooperative effects of several MMPs and their inhibitors (4, 5). Our current findings strongly suggested that this result is indeed the case.
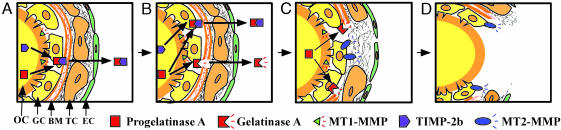
This study indicated that gelatinase A, MT1-MMP, and MT2-MMP play important roles in the process of follicle rupture during ovulation in the medaka. On the basis of our data, we can propose a mechanistic model of this process, implicating these MMPs together with the intrinsic inhibitor TIMP-2b (Fig. 5). Namely, in the follicle that is destined to ovulate, the synthesis of progelatinase A and MT1-MMP begins in the oocyte at some point before ovulation. At the same time, the oocyte produces TIMP-2b. When MT1-MMP has been activated intracellularly and is localized on the surface of the oocyte, this membrane-bound MMP may interact with secreted progelatinase A and TIMP-2b to form the progelatinase A/MT1-MMP/TIMP-2b complex, which is well documented as the unique mechanism for progelatinase A activation in mammals (30–32). Progelatinase A activation in the follicles of the medaka ovary appears to occur in a similar manner. The present model is supported by the finding that the activation of progelatinase A in the culture medium of ovarian follicles was noticeably inhibited by the addition of anti-MT1-MMP antibodies. The expression of furin, which is likely to be involved in the activation of MT1-MMP, has recently been reported in the oocytes of ovarian follicles of the medaka (33). Active gelatinase A produced in this manner may be inhibited until the time of ovulation by the formation of a complex with TIMP-2b; this inhibition is thought to be due to a presumed excess of the inhibitor compared with the amount of enzyme (Fig. 5A). At the time of ovulation, the transcription and translation of MT2-MMP and gelatinase B are initiated in the granulosa cells of the ovulating follicle. The former may undergo furin-catalyzed intracellular activation and may subsequently be distributed over the surface of the cells, whereas the latter is secreted from the same cells into the extracellular space in an inactive precursor form. Increased production of active gelatinase A and gelatinase B, together with a decrease in TIMP-2b at or around the time of ovulation, may reverse the molar ratio of MMP/TIMP-2b compared with that of the preovulation period, resulting in the release of MMP from inhibition by TIMP-2b. Unlike MT1-MMP and MT2-MMP, gelatinase A is able to degrade type VI collagen in vitro. Therefore, the basement membrane situated between the granulosa and theca cell layers might be a target substrate for this MMP (Fig. 5B). Although medaka gelatinase B was found to hydrolyze type IV collagen more rapidly than gelatinase A, the results of our in vitro ovulation experiments by using specific antibodies indicated that the former does not participate in basement-membrane degradation. Basement-membrane degradation resulting from the activity of gelatinase A allows the granulosa cells to come into contact with the ECM in the interstitial space, where the theca cells are localized. Because MT2-MMP hydrolyzed medaka collagen type I in vitro (Fig. 3) and because this type of collagen is thought to be a major ECM protein in this region, the MT2-MMP expressed on the surface membrane of the granulosa cells is thought to be responsible for the dissolution of the collagen (Fig. 5C). It should be noted that MT2-MMP may play an additional role in the activation of progelatinase A, because we demonstrated here that it was able to activate the precursor gelatinase in vitro. Follicle rupture during ovulation in the medaka does not require extensive ECM degradation throughout the layer surrounding the oocyte. As is the case in mammalian ovulation (2), hydrolysis of the ECM proteins at a single restricted site is expected to be sufficient for ovulation in the medaka. Once this local hydrolysis has occurred, the subsequent process appears to proceed spontaneously (Fig. 5D). Clearly, the mechanical force generated by the swelling of the ovulating oocyte facilitates ovulation. However, the mechanism and regulation of this restricted hydrolysis is still unknown. We should also note that externally added active recombinant MMPs, including gelatinase A and MT2-MMP, were found to have no accelerating effect on in vitro ovulation. This finding may indicate the importance of well regulated molecular interactions between the intrinsic MMPs, TIMP-2b, and ECM proteins in follicle rupture. In summary, upon ovulation, gelatinase A is responsible for the hydrolysis of type IV collagen that forms the basement membrane, whereas MT2-MMP degrades type I collagen present in the theca cell layer. Moreover, MT1-MMP and TIMP-2b are both involved in the activation of progelatinase A and the regulation of its activity.
Fig. 5.
A model of follicle rupture during ovulation in the medaka. (A) In the follicle, a few hours before ovulation, progelatinase A is activated by MT1-MMP on the surface of the oocyte, whereas activated gelatinase A is immediately inactivated by TIMP-2b. (B) At the time of ovulation, the hydrolysis of basement membrane type IV collagen is initiated by active gelatinase A at the follicle–ovarian surface contact site. (C) MT2-MMP, which is now expressed on the surface of the granulosa cells, can degrade the type I collagen that is present in the theca cell layer. (D) As a result, the oocyte is exposed at the contact site, leading to ovulation. BM, basement membrane; EC, epithelial cell; GC, granulosa cell; OC, oocyte; TC, theca cell.
We believe that this study identifies proteases that are critical for follicle rupture during ovulation in vertebrates. The use of similar experimental systems established for mammalian ovaries (34–37) will most likely aid in the determination of the enzymes that facilitate follicle rupture in mammals. The eventual identification of mammalian ovulatory proteases will provide information that is of potential clinical importance. Our current approach and findings are expected to be useful for future studies in this area.
Acknowledgments
We thank Dr. Junji Ohnishi (Hokkaido University, Sapporo, Japan) for his valuable comments and discussions. This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (14204079), research fellowships from the Japan Society for the Promotion of Science for Young Scientists, and the 21st Century Center of Excellence program.
Author contributions: K.O., M.M., and T.T. designed research; K.O., N.T., and M.S. performed research; K.O., N.T., M.S., M.M., and T.T. analyzed data; and T.T. wrote the paper.
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: APMA, p-aminophenylmercuric acetate; MMP, matrix metalloproteinase; MT, membrane-type; TIMP, tissue inhibitor of metalloproteinase.
Data deposition: The sequences reported in this paper have been deposited in the DNA Data Bank of Japan database (accession nos. AB185847, AB072928, AB072929, AB185849, AB193468, and AB193469).
References
- 1.Schochet, S. S. (1916) Anat. Rec. 10, 447-457. [Google Scholar]
- 2.Espey, L. L. (1967) Am. J. Physiol. 212, 1397-1401. [DOI] [PubMed] [Google Scholar]
- 3.Tsafriri, A. (1995) Adv. Exp. Med. Biol. 377, 121-140. [DOI] [PubMed] [Google Scholar]
- 4.Richards, J. (2001) Endocrinology 142, 2184-2193. [DOI] [PubMed] [Google Scholar]
- 5.Ny, T., Wahlberg, P. & Brändström, I. J. M. (2002) Mol. Cell. Endocrol. 187, 29-38. [DOI] [PubMed] [Google Scholar]
- 6.Beers, W. H. (1975) Cell 6, 379-386. [DOI] [PubMed] [Google Scholar]
- 7.Murdoch, W. J. & McDonnel, A. C. (2002) Reproduction 123, 743-750. [DOI] [PubMed] [Google Scholar]
- 8.Curry, T. E., Jr., Dean, D. D., Woessner, J. F., Jr., & LeMaire, W. J. (1985) Biol. Reprod. 33, 981-991. [DOI] [PubMed] [Google Scholar]
- 9.Liu, K., Wahlberg, P. & Ny, T. (1998) Endocrinology 139, 4735-4738. [DOI] [PubMed] [Google Scholar]
- 10.Hagglund, A. C., Ny, A., Leonardsson, G. & Ny, T. (1999) Endocrinology 140, 4351-4358. [DOI] [PubMed] [Google Scholar]
- 11.Jo, M. & Curry, T. E., Jr. (2004) Biol. Reprod. 71, 1796-1806. [DOI] [PubMed] [Google Scholar]
- 12.Pendás, A. M., Folgueras, A. R., Llano, E., Caterina, J., Frerard, F., Rodríguez, F., Astudillo, A., Noël, A., Birkedal-Hansen, H. & López-Otín, C. (2004) Mol. Cell. Biol. 24, 5304-5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holmbeck, K., Bianco, P., Caterina, J., Yamada, S., Kromer, M., Kuznetsov, S. A., Mankani, M., Robey, P. G., Poole, A. R., Piodoux, I., et al. (1999) Cell 99, 81-92. [DOI] [PubMed] [Google Scholar]
- 14.Peschon, J. J., Slack, J. L., Reddy, P., Stocking, K. L., Sunnarborg, S. W., Lee, D. C., Russell, W. E., Nelson, N., Kozlosky, C. J., Wolfson, M. F., et al. (1998) Science 282, 1281-1284.48. [DOI] [PubMed] [Google Scholar]
- 15.Robker, R. L., Russell, D. L., Espey, L. L., Lydon, J. P., O'Malley, B. W. & Richards, J. S. (2000) Proc. Natl. Acad. Sci. USA 97, 4689-4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shindo, T., Kurihara, H., Kuno, K., Yokoyama, H., Wada, T., Kurihara, Y., Imai, T., Wang, Y., Ogata, M, Nishimatsu, H., et al. (2000) J. Clin. Invest. 105, 1345-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russell, D. L., Doyle, K. M., Ochsner, S. A., Sandy, J. D. & Richards, J. S. (2003) J. Biol. Chem. 278, 42330-42338. [DOI] [PubMed] [Google Scholar]
- 18.Schroeder, P. C. & Talbot, P. (1985) Gamete Res. 11, 191-221. [Google Scholar]
- 19.Richards, J. (1994) Endocr. Rev. 15, 725-751. [DOI] [PubMed] [Google Scholar]
- 20.Nagahama, Y., Yoshikuni, M., Yamashita, M. & Tanaka, M. (1994) in Fish Physiology, eds. Sherwood, N. M. & Hew, C. L. (Academic, New York), pp. 393-439.
- 21.Ishikawa, Y. (2000) BioEssays 22, 487-495. [DOI] [PubMed] [Google Scholar]
- 22.Wittbrodt, J., Shima, A. & Schartl, M. (2002) Nat. Rev. Genet. 3, 53-64. [DOI] [PubMed] [Google Scholar]
- 23.Iwamatsu, T. (1978) J. Exp. Zool. 206, 355-364. [DOI] [PubMed] [Google Scholar]
- 24.Hirose, K. & Donaldson, E. (1972) Bull. Jpn. Soc. Sci. Fish. 38, 97-100. [Google Scholar]
- 25.Pendergrass, P. & Schroeder, P. (1976) J. Reprod. Fertil. 47, 229-233. [DOI] [PubMed] [Google Scholar]
- 26.Shibata, Y., Iwamatsu, T., Oba, Y., Kobayashi, D., Tanaka, M., Nagahama, Y., Suzuki, N. & Yoshikuni, M. (2000) J. Biol. Chem. 275, 8349-8354. [DOI] [PubMed] [Google Scholar]
- 27.Matsui, H., Ogiwara, K., Ohkura, R., Yamashita, M. & Takahashi, T. (2000) Eur. J. Biochem. 267, 4658-4667. [DOI] [PubMed] [Google Scholar]
- 28.Kimura, A., Shinohara, M., Ohkura, R. & Takahashi, T. (2001) Biochim. Biophys. Acta 1518, 115-123. [DOI] [PubMed] [Google Scholar]
- 29.Ogiwara, K., Matsui, H., Kimura, A. & Takahashi, T. (2002) Mol. Reprod. Dev. 61, 21-31. [DOI] [PubMed] [Google Scholar]
- 30.Nagase, H. (1997) Biol. Chem. 378, 151-160. [PubMed] [Google Scholar]
- 31.Sato, H., Takino, T., Okada, Y., Cao, J., Shinagawa, A., Yamamoto, E. & Seiki, M. (1994) Nature 370, 61-65. [DOI] [PubMed] [Google Scholar]
- 32.Brew, K., Dinakarpandian, D. & Nagase, H. (2000) Biochim. Biophys. Acta 1477, 267-283. [DOI] [PubMed] [Google Scholar]
- 33.Ogiwara, K., Shinohara, M. & Takahashi, T. (2004) Gene 337, 79-89. [DOI] [PubMed] [Google Scholar]
- 34.Baranczuk, R. J. & Fainstat, T. (1976) Am. J. Obstet. Gynecol. 124, 517-522. [DOI] [PubMed] [Google Scholar]
- 35.Talbot, P. (1983) J. Exp. Zool. 225, 141-148. [DOI] [PubMed] [Google Scholar]
- 36.Osman, P. & Lieuwma-Noordanus, C. (1980) J. Reprod. Fert. 59, 431-436. [DOI] [PubMed] [Google Scholar]
- 37.Rose, U. M., Hanssen, R. G. J. M. & Kloosterboer, H. J. (1999) Biol. Reprod. 61, 503-511. [DOI] [PubMed] [Google Scholar]