Abstract
This article describes the clinical application of gene therapy to a nonlethal disease, rheumatoid arthritis (RA). Intraarticular transfer of IL-1 receptor antagonist (IL-1Ra) cDNA reduces disease in animal models of RA. Whether this procedure is safe and feasible in humans was addressed in a phase I clinical study involving nine postmenopausal women with advanced RA who required unilateral sialastic implant arthroplasty of the 2nd-5th metacarpophalangeal (MCP) joints. Cultures of autologous synovial fibroblasts were established and divided into two. One was transduced with a retrovirus carrying IL-1Ra cDNA; the other provided untransduced, control cells. In a dose escalation, double-blinded fashion, two MCP joints were injected with transduced cells, and two MCP joints received control cells. One week later, injected joints were resected and examined for evidence of successful gene transfer and expression by using RT-PCR, ex vivo production of IL-1Ra, in situ hybridization, and immunohistochemistry. All subjects tolerated the protocol well, without adverse events. Unlike control joints, those receiving transduced cells gave positive RT-PCR signals. Synovia that were recovered from the MCP joints of intermediate and high dose subjects produced elevated amounts of IL-1Ra (P = 0.01). Clusters of cells expressing high levels of IL-1Ra were present on synovia of transduced joints. No adverse events occurred. Thus, it is possible to transfer a potentially therapeutic gene safely to human rheumatoid joints and to obtain intraarticular, transgene expression. This conclusion justifies additional efficacy studies and encourages further development of genetic approaches to the treatment of arthritis and related disorders.
Keywords: clinical trial, interleukin-1 receptor antagonist, retrovirus, synovium
Despite recent advances in therapy, rheumatoid arthritis (RA) remains incurable and often difficult to treat. Biological agents, particularly immunomodulatory and antiinflammatory proteins, are being developed as novel, improved antirheumatic drugs (1). Although potent, these proteins are very expensive. Furthermore, they require repeated systemic introduction by s.c. injection or i.v. infusion, which heightens concerns over adverse side-effects. Delivery of genes encoding therapeutic proteins, rather than administration of the proteins themselves, promises to obviate these problems (2). Gene transfer enables patients to synthesize the cognate gene products endogenously, potentially in a prolonged and regulated manner. Moreover, local gene delivery restricts transgene expression to defined anatomical locations, such as joints, thereby reducing the prospects of systemic adverse effects. This article describes an application of these principles in humans.
A number of genes have shown impressive efficacy in animal models of RA (2), but, for the purposes of initial human studies, we used a cDNA encoding the IL-1 receptor antagonist (IL-1Ra) (3). The recombinant protein has an excellent safety profile (4) and shows efficacy in animal models of RA (5). Efficacy in human RA, however, is modest (6) because IL-1Ra has a remarkably short biological half-life (5). This circumstance makes it a particularly good candidate for gene delivery.
There are several strategies for gene therapy of arthritis (2). Most progress has been made with the approach of Bandara et al. (7, 8), in which antiarthritic genes are transferred to the synovial linings of diseased joints. Although direct, in vivo gene delivery to joints is simple and effective in animal models of RA (9, 10), various technical and safety constraints predicated an ex vivo, retroviral gene transfer strategy for the trial described here. The feasibility and efficacy of ex vivo, retrovirally mediated IL-1Ra gene transfer to joints has been demonstrated in rabbits (11), rats (12), and mice (13). The likelihood of success in humans was reinforced by the results of experiments in which human cartilage and genetically modified synovium were coimplanted into severe combined immunodeficient (SCID) mice (14). Extensive testing in rabbits (15) and mice (16) failed to detect serious adverse events.
Based upon these promising preclinical findings, we designed a phase I human protocol (15) to evaluate the safety and feasibility of IL-1Ra gene transfer to human rheumatoid joints.
Methods
Research Subjects. Eligible participants were postmenopausal or ovariectomized women under age 75 fulfilling the American College of Rheumatology criteria for RA (17). They had elected to undergo replacement of the 2nd-5th metacarpophalangeal (MCP) joints on one hand and additional surgical procedures on at least one other joint. To avoid coercion, eligible subjects were consented into the study by an independent, yet informed, orthopedic surgeon. Where applicable, corticosteroids were discontinued one month before beginning the study, but other medication was allowed.
Study Design. Safety was the overriding concern, particularly because this study proposed to apply gene therapy to a nonlethal disease. Ex vivo gene transfer helps in this regard, because no infectious agents are introduced into the participants, and the genetically modified cells can be extensively screened before implantation. Safety was maximized by delivering genes to joints that were scheduled to undergo total joint replacement.
Human IL-1Ra cDNA was transferred to the 2nd-5th MCPs on one hand of nine individuals with RA (Table 1) by an ex vivo protocol involving the retroviral transduction of autologous synovial fibroblasts (Fig. 1). Synovial tissue was retrieved, at the time of elective surgery involving joints other than the target MCP joints, and used as a source of autologous synoviocytes. Cells were expanded in culture; half were transduced with a retrovirus carrying the human IL-1Ra cDNA, the remaining half serving as untransduced controls. IL-1Ra concentrations of conditioned media were measured by ELISA (R & D Systems) to ensure that the transduced cells met the investigational new drug (IND) release criterion of producing at least 30 ng of IL-1Ra per 106 cells per 48 h. The bulk of each culture was cryopreserved, whereas aliquots were sent to BioWhittaker to be tested for replication-competent retrovirus (RCR), endotoxin, mycoplasma, and other adventitious agents.
Table 1. Demographic characteristics and disease history of participants.
| Subject no. | Age, yr | Disease duration, yr | Rheumatoid factor | Current antirheumatic therapy | Previous arthroplasty |
|---|---|---|---|---|---|
| Group I, low dose | |||||
| 1 | 68 | 20 | + | DMARD and NSAID | + |
| 2 | 73 | 26 | + | DMARD and Corticosteroid | + |
| 3 | 63 | 10 | + | DMARD and NSAID | + |
| Group II, intermediate dose | |||||
| 4 | 56 | 17 | + | DMARD, corticosteroid, and NSAID | − |
| 5 | 61 | 15 | + | DMARD | + |
| 6 | 61 | 10 | − | Corticosteroid and NSAID | + |
| Group III, high dose | |||||
| 7 | 49 | 23 | + | DMARD, corticosteroid, and NSAID | + |
| 8 | 55 | 25 | + | DMARD, corticosteroid, and NSAID | + |
| 9 | 51 | 24 | − | DMARD | − |
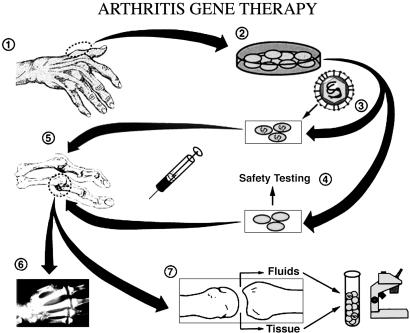
Fig. 1.
Protocol for gene transfer to joints. Surgery of the joints of the hand or foot (step 1) provided autologous synovium, which was used to establish cultures of synovial fibroblasts (step 2). Half the cells were transduced with the retroviral vector (step 3), and all cells were tested for RCR and adventitious agents (step 4) before injection into MCP joints numbers 2-5 on one hand (step 5). In a double-blinded fashion, two joints received transduced cells, and two received control cells. One week later, the injected joints were surgically removed during total joint replacement surgery (step 6), and the retrieved tissues were analyzed for evidence of successful gene transfer and gene expression (step 7). [Reproduced with permission from ref. 2 (Copyright 1999, University of Pittsburgh Medical Center).]
After receipt of negative test data, the cells were prepared for intraarticular injection. In a double-blind procedure, two MCP joints received transduced cells, and two received untransduced cells. Subjects received 106 (group I, low dose), 1.5-5 × 106 (group II, intermediate dose), or 6.5-10 × 106 (group III, high dose) cells per joint (Table 2). Each subject was then contacted daily by telephone to monitor adverse events. On the 7th day, the injected MCP joints underwent sialastic implant arthroplasty, and the retrieved synovial tissues were examined for IL-1Ra expression by RT-PCR and ELISA measurement. Specimens were further examined by in situ hybridization and immunohistochemistry to determine the anatomical location of the transduced cells. Complete medical history and physical examinations, and blood tests to monitor a variety of parameters, including the presence of RCR, were conducted 1, 3, 6, and 12 months after surgery, and then annually. This protocol was approved by the Recombinant DNA Committee of the National Institutes of Health (NIH) (protocol 9406-074), the NIH, and the FDA (IND BB-IND-6235). Details of the protocol are documented in ref. 15.
Table 2. Synovial harvest, cell transduction, and injection.
| IL-1Ra (ng per 106 cells per 48 h) |
||||||
|---|---|---|---|---|---|---|
| Pre-cryopreserve |
Post-cryopreserve |
|||||
| Subject no. | First procedure | Control | Trans. | Control | Trans. | Cells injected/MCP |
| Group I, low dose | ||||||
| 1 | 1st MCP fusion | U.D. | 78 | 0.5 | 208 | 106 |
| 2 | Wrist fusion | 0.1 | 35 | U.D. | 8 | 106 |
| 3 | 1st MCP fusion | 0.2 | 44 | U.D. | 188 | 106 |
| Group II, intermediate dose | ||||||
| 4 | 1st MCP fusion | 0.1 | 50 | U.D. | 103 | 1.5 × 106 |
| 5 | Wrist fusion | 0.2 | 100 | U.D. | 489 | 1.5 × 106 |
| 6 | Wrist fusion | U.D. | 71 | U.D. | 81 | 5 × 106 |
| Group III, high dose | ||||||
| 7 | 1st MCP fusion | 0.1 | 92 | 0.1 | 152 | 6.5 × 106 |
| 8 | 1st MTP fusion | 0.9 | 74 | U.D. | 66 | 107 |
| 9 | 1st MCP fusion | 0.1 | 67 | U.D. | 64 | 107 |
MTP, metatarsophalangeal joint; Trans., transduced cells; U.D., undetectable.
Laboratory Methods. Preparation of retrovirus. MFG-IRAP is a replication-defective, amphotropic, recombinant retrovirus derived from the Moloney murine leukemia virus, carrying the complete coding sequence of human IL-1Ra. It was produced by the ψ-CRIP packaging cell line by using standard techniques. The sequences of the IL-1Ra insert and the upstream and downstream flanking regions were confirmed independently (LARK Sequencing Technologies, Houston). The packaging cell line was certified free from RCR endotoxin, mycoplasma, and other adventitious agents (BioWhittaker Inc.) and transferred to the Human Gene Therapy Applications Laboratory of the University of Pittsburgh School of Medicine, where clinical grade virus was prepared.
Transduction of synovial fibroblasts. Synovial cell cultures were established by standard methods. At confluence, cells were trypsinized and subcultured. When 60% confluent, half of the secondary cultures were incubated with 4 ml of retroviral supernatant containing 8 μg/ml polybrene. Cells were centrifuged at 2,000 × g for 2 h during retroviral transduction (18) and incubated for 18 h before adding fresh growth medium.
At confluence, media were changed, and the amount of IL-1Ra secreted by transduced and untransduced cells was measured by ELISA (R & D Systems). Once production of >30 ng of IL-1Ra per 106 transduced cells per 48 h was confirmed, 1% of the cells and 5% of the conditioned culture medium were sent to BioWhittaker to be tested for RCR and other adventitious agents. The remaining cells were cryopreserved. During this period, conditioned media were further assayed for prostaglandin E2 and IL-6 by ELISA (R & D Systems).
Uncontaminated cultures were thawed, recultured, trypsinized, washed, resuspended in saline, coded, and subjected to a “stat” Gram stain as well as analysis for mycoplasma and endotoxin. The cells were then injected into the appropriate MCP joints. The conditioned media were retained and assayed retrospectively for IL-1Ra.
RT-PCR. Minced tissue was homogenized in TRIzol solution (Invitrogen) and extracted with chloroform. RNA was precipitated with isopropanol, resuspended in 10 mM Tris·HCl (pH 8.0) and 1 mM EDTA, and quantitated spectrophotometrically.
For reverse transcription, 3 μg of RNA were mixed with 50 ng of an oligonucleotide primer complementary to sequences within the human IL-1Ra coding region in 250 mM Tris·HCl (pH 8.3), 0.4 M KCl, 15 mM MgCl2 and 0.4 mM each of dATP, dGTP, dCTP, and dTTP. After heating to 80°C for 5 min, the mixture was placed on ice, and DTT was added to 5 mM followed by 200 units of SuperScript II Rnase- reverse transcriptase (Life Technologies, Rockville, MD). The mixture was incubated at 42°C for 1 h.
For PCR amplification, the upstream primer was complementary to unique sequences in the MFG vector, and the downstream primer was complementary to sequences specific for IL-1Ra. One microgram of each oligonucleotide primer was mixed with 20 μl of cDNA from the reverse transcription reaction in 20 mM Tris·HCl (pH 8.8), 10 mM KCl, 10 mM (NH4)2SO4, 4 mM Mg SO4, 0.1% Triton X-100, and 400 mM each of dATP, dGTP, dCTP, and dTTP, and 2 units of Vent DNA polymerase (New England Biolabs). After 35 rounds of amplification (1 min at 95°C, 1 min at 59°C, and 1 min at 72°C), 20 μl of reaction products were electrophoresed in a 0.8% agarose gel and denatured, and the DNA was transferred to Hybond n plus nylon membrane (DuPont). DNA was UV crosslinked to the membrane, then placed in a hybridization oven in 1% BSA, 1 mM EDTA, 7% SDS, and 0.5 M Na2HPO4 (pH 7.2) at 65°C. Radiolabeled probe was generated by random primer extension by using a portion of the IL-1Ra coding region internal to the oligonucleotide primer pair as template. After incubation overnight at 65°C, the blot was washed, and DNA bands hybridized to the radiolabeled probe were visualized by autoradiography.
In situ hybridization. Frozen sections were thawed, postfixed with 2% paraformaldehyde in PBS, washed three times in PBS, and digested with proteinase K for 10 min. Sections were washed in PBS containing 2 mg/ml glycine, acetylated with acetic anhydride, washed, dehydrated through graded alcohols, and prehybridized in 1 part formamide, 1 part hybridization buffer, and 20 mg/ml dextran sulfate. After 30 min prehybridization, sections were washed in water, dehydrated, and incubated for 4 h at 42°C with 35S-labeled, single-stranded RNA probe complementary to IL-1Ra transcripts. The slides were thoroughly washed and then dehydrated through graded ethanols, air dried, dipped in NTB-2 emulsion, dried, incubated at 4°C for 1-5 days, and developed.
Immunofluorescence. Sections were washed three times in PBS containing 0.5% BSA and 0.15% glycine, pH 7.4 (Buffer A) followed by 30 min incubation with purified goat IgG (50 μg/ml) at 25°C and three additional washes with Buffer A. Sections were incubated for 60 min with a rabbit anti-human IL-1Ra primary antibody followed by three washes in Buffer A and 60 min incubation in fluorescently labeled secondary anti-rabbit IgG (2 μg/ml). Sections were washed thoroughly in Buffer A, stained with Hoechst stain, mounted in Gelvatol and coverslipped. For both the in situ hybridization and fluorescence microscopy studies an Olympus Provis microscope equipped with an Optronic cooled CCD camera was used for image collection. Images were assembled in Photoshop (Adobe) with no postprocessing.
Statistical Analysis. Cells were randomized to individual MCP joints by a Monte Carlo program. Production of prostaglandin E2 and IL-6 by transduced and untransduced cell cultures was compared by a two-tailed Student's t test.
Ex vivo IL-1Ra production by cells recovered surgically from MCP joints was analyzed by repeated measures analysis of variance by using the natural logarithm of the IL-1Ra concentrations.
Results
Participant Characteristics. All participants were Caucasians with long-standing, erosive, progressive RA. Seven had required previous surgical intervention. At the time of entering the study, all subjects were taking one or more disease-modifying antirheumatic drugs (DMARDs), nonsteroidal antiinflammatory drugs (NSAIDs), or oral corticosteroids (Table 1).
Synovial Harvest, Culture, and Transduction. Synthesis of IL-1Ra by cultures of untransduced cells was very low or undetectable. Transduction elevated IL-1Ra production beyond the 30 ng per 106 cells per 48 h investigational new drug requirement in all cases (Table 2). High synthesis of IL-1Ra persisted and, in most instances, increased after cryopreservation and reculture.
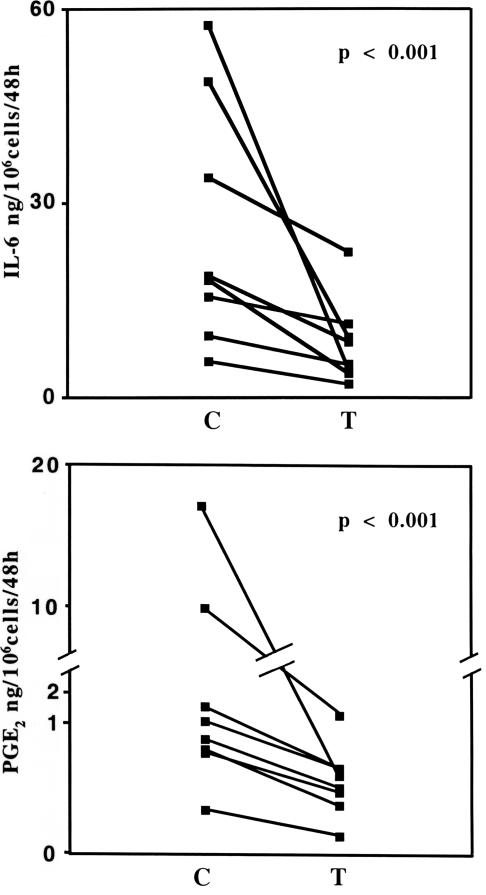
Transduced cells produced less IL-6 and prostaglandin E2 than unmodified controls (Fig. 2), demonstrating that synthesis of these mediators was partially driven by the autocrine production of IL-1 and confirming that the transgene product was biologically active.
Fig. 2.
Effect of IL-1Ra gene transfer on in vitro mediator production. Media were recovered from paired cultures of synovial fibroblasts before (C) and after (T) transduction with the IL-1Ra gene. Concentrations of IL-6 (Upper) and prostaglandin E2 (Lower) were determined by ELISA.
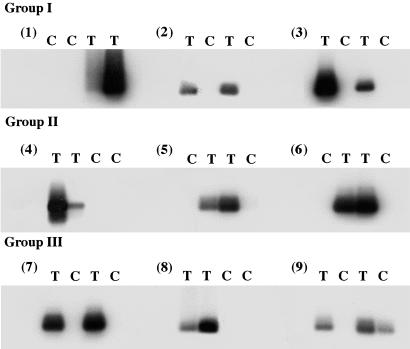
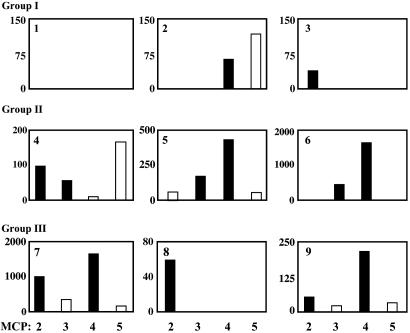
Intraarticular Expression of the IL-1Ra Transgene. RT-PCR analysis confirmed expression of mRNA derived from the transgene in all joints injected with the genetically modified cells (Fig. 3). Only one of the control joints, the 5th MCP joint of subject 9, gave a positive RT-PCR signal. Cultures established from the joints of individuals receiving the lowest dose of cells (106 cells per joint) synthesized little IL-1Ra, regardless of the presence or absence of the transgene (Fig. 4; subjects 1-3). However, in 11 of the 12 joints receiving the higher doses of cells (groups II and III), IL-1Ra production was substantially elevated (Fig. 4; subjects 4-9; P = 0.01).
Fig. 3.
Expression of IL-1Ra transgene in retrieved synovial tissues. RNA was extracted and subjected to the RT-PCR procedure described in Methods. The reaction products were separated by agarose gel electrophoresis, subjected to Southern blotting, and visualized by autoradiography. Each of the four MCP joints of each subject (subjects 1-9) from the low (group I, subjects 1-3), medium (group II, subjects 4-6), and high (group III, subjects 7-9) dose groups are indicated as receiving transduced (T) or control (C) cells.
Fig. 4.
Synthesis of IL-1Ra by retrieved synovial tissues. Cells were recovered from synovium that was surgically harvested 1 week after gene transfer and placed in monolayer culture. Conditioned media were assayed for IL-1Ra by ELISA. Data for MCP joints 2-5 of each of the samples from the low (group I, subjects 1-3), medium (group II, subjects 4-6), and high (group III, subjects 7-9) dose groups are shown. Values (IL-1Ra pg/ml) for cells recovered from joints receiving transduced cells are shown with filled bars, and those from joints receiving untransduced cells are shown with open bars. Note that scales on the y axes of the individual panels differ.
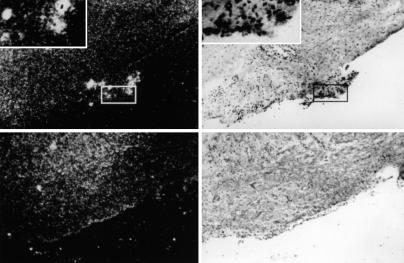
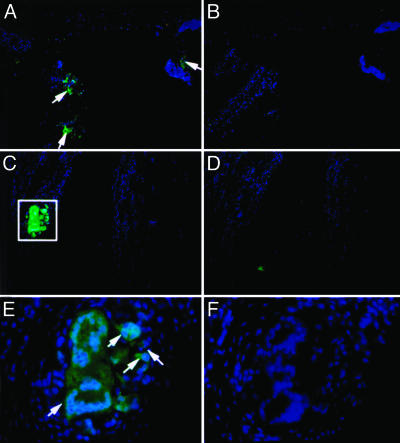
In situ hybridization (Fig. 5) and immunohistochemistry (Fig. 6) identified clusters of cells expressing large amounts of IL-1Ra attached to the surface of synovia recovered from joints receiving the transduced cells. Such cells were absent from sections of control joints.
Fig. 5.
Localization of transduced cells in synovium determined by in situ hybridization. Autoradiograms of processed synovial tissue retrieved from joints receiving transduced (Upper) or untransduced (Lower) cells, shown by dark field (Left) and bright field (Right) illumination. (Magnification: ×50.) Boxes identify clusters of cells, present only on synovia of joints receiving transduced cells, expressing high levels of IL-1Ra mRNA. (Insets) Shown are such cell clusters at ×200 magnification.
Fig. 6.
Localization of transduced cells in synovium determined by immunohistochemistry. (A, C, and E) Shown (at magnifications of ×50, ×200, and ×400, respectively) is the presence of cells attached to the synovial surface, expressing high levels of IL-1Ra (green fluorescence). These cells are absent from control joints (B, D, and F). Nuclei are stained blue with DAPI.
Safety. Some subjects experienced transient discomfort during the intraarticular injections, with subsequent ecchymosis. No adverse events were reported during daily telephone contact. Participants are now >5 yr post-gene transfer, and they remain free from RCR and adverse events related to the study.
Discussion
These data demonstrate that it is possible to transfer genes to human joints and to achieve intraarticular transgene expression in a safe and acceptable manner. Further studies to evaluate efficacy seem justified, especially because clinical findings suggest that recombinant IL-1Ra (Kineret, Amgen Biologicals) has antiinflammatory and antierosive properties in patients with RA (6). Kineret is delivered by daily s.c. injection. Intraarticular delivery by gene transfer promises to confer a therapeutic advantage because of its ability to generate a high, sustained, local concentration of IL-1Ra that, unlike its recombinant counterpart, has undergone authentic posttranslational processing. Furthermore, daily s.c. delivery of recombinant protein fails to maintain therapeutic serum levels of IL-1Ra between injections (5).
Preclinical data (15) suggest that cells are most likely to escape from inflamed joints during the first week after injection. Thus, although the injected joints were surgically removed after one week, genetically modified cells that had migrated to extraarticular locations would remain in the body as a potential source of delayed adverse events. This concern was amplified by reports of leukemia in children with X-linked severe combined immunodeficiency (SCID) who had received ex vivo gene therapy with the same viral vector backbone, MFG, as used in our study (19). Because of the critical importance of safety in the application of gene therapy to nonlethal diseases, we delayed publication of these data until a 5-yr period had passed without clinical or molecular evidence of side-effects.
The ex vivo protocol used here was effective, but it was tedious, time-consuming, and expensive. Local gene therapy for RA would be expedited by the development of safe and effective in vivo vectors (20, 21) that facilitate long-term transgene expression. Candidates include high titer retrovirus (22), adenovirus (23, 24), adeno-associated virus (AAV) (25, 26), and lentivirus (9, 27). Of these, AAV is perceived to be the safest and has several empirical advantages (25); recent improvements in the technology of recombinant AAV production facilitate its use in human clinical trials.
The genetic approaches to therapy illustrated in this study are not restricted to RA. Promising preclinical data have been obtained for gene therapy of other inflammatory (28) and autoimmune (29) diseases, as well as osteoarthritis (30) and a variety of orthopaedic conditions (31).
Acknowledgments
We thank Drs. John Barranger, Joseph Glorioso, and Michael Lotze for their advice and encouragement; Drs. John Barranger and Albert Bahnson for preparing the clinical grade virus; Dr. Timothy Wright and Dr. Terrence Starz for their support of this project; Dr. Steven Conti for referring patient number 9; Lisa Rossi for recruiting patient number 8; Helga Georgescu, Robbie Maillard, Catherine Trumpower, and Lori Miller for expert and proficient technical assistance; Helen Clark, Lou Duerring, Dr. Shirley Fitzgerald, Anne Cunningham, and Lauren Ward for logistical and organizational support; Dr. David Nickerson for statistical analysis; Maria Del Vecchio for considerable assistance in the preparation of this manuscript; and Drs. Michael Weinblatt, Malcolm Brenner, Richard Gelberman, and Thomas Medsger for critical review of an earlier version of this manuscript. This work was supported by National Institutes of Health Grant AR 43623 and Orthogen, A.G.
Author contributions: C.H.E. and P.D.R. designed research; C.H.E. and M.V. analyzed data; P.D.R. contributed new reagents/analytic tools; S.C.G., E.M.E., T.L.W., and S.C.W. performed research; M.C.W. supervised patient care/evaluation (rheumatology); M.M.T. obtained consent of subjects for study and follow-up; R.K. and T.A.M. supervised patient recruitment/care/evaluation; J.H.H. performed intraarticular injections and joint replacement surgery; and C.H.E. wrote the paper.
Abbreviations: RA, rheumatoid arthritis; IL-1Ra, IL-1 receptor antagonist; MCP, metacarpophalangeal; RCR, replication-competent retrovirus; DMARD, disease-modifying antirheumatic drug; NSAID, nonsteroidal antiinflammatory drug.
References
- 1.Furst, D. E., Breedveld, F. C., Kalden, J. R., Smolen, J. S., Burmester, G. R., Dougados, M., Emery, P., Gibofsky, A., Kavanaugh, A. F., Keystone, E. C., et al. (2003) Ann. Rheum. Dis. 62 Suppl. 2, ii2-ii9, and [DOI] [PMC free article] [PubMed] [Google Scholar]; erratum (2004) 63 114.
- 2.Evans, C. H., Ghivizzani, S. C., Kang, R., Muzzonigro, T., Wasko, M. C., Herndon, J. H. & Robbins, P. D. (1999) Arthritis Rheum. 42 1-16. [DOI] [PubMed] [Google Scholar]
- 3.Arend, W. P. & Evans, C. H. (2003) in The Cytokine Handbook, eds. Thompson, A. W. & Lotze, M. (Academic, London), Vol. 2, pp. 687-732. [Google Scholar]
- 4.Fleishmann, R. M. (2002) Clin. Exp. Rheumatol. 20 S35-S41. [PubMed] [Google Scholar]
- 5.Bendele, A., McAbee, T., Sennello, G., Frazier, J., Chlipala, E. & McCabe, D. (1999) Arthritis Rheum. 42 498-506. [DOI] [PubMed] [Google Scholar]
- 6.Bresnihan, B. (2002) Clin. Exp. Rheumatol 20 S32-S34. [PubMed] [Google Scholar]
- 7.Bandara, G., Robbins, P. D., Georgescu, H. I., Mueller, G. M., Glorioso, J. C. & Evans, C. H. (1992) DNA Cell Biol. 11 227-231. [DOI] [PubMed] [Google Scholar]
- 8.Bandara, G., Mueller, G. M., Galea-Lauri, J., Tindal, M. H., Georgescu, H. I., Suchanek, M. K., Hung, G. L., Glorioso, J. C., Robbins, P. D. & Evans, C. H. (1993) Proc. Natl. Acad. Sci. USA 90 10764-10768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gouze, E., Pawliuk, R., Gouze, J. N., Pilapil, C., Fleet, C., Palmer, G. D., Evans, C. H., Leboulch, P. & Ghivizzani, S. C. (2003) Mol. Ther. 7 460-466. [DOI] [PubMed] [Google Scholar]
- 10.Lechman, E. R., Jaffurs, D., Ghivizzani, S. C., Gambotto, A., Kovesdi, I., Mi, Z., Evans, C. H. & Robbins, P. D. (1999) J. Immunol. 163 2202-2208. [PubMed] [Google Scholar]
- 11.Otani, K., Nita, I., Macaulay, W., Georgescu, H. I., Robbins, P. D. & Evans, C. H. (1996) J. Immunol. 156 3558-3562. [PubMed] [Google Scholar]
- 12.Makarov, S. S., Olsen, J. C., Johnston, W. N., Anderle, S. K., Brown, R. R., Baldwin, A. S., Jr., Haskill, J. S. & Schwab, J. H. (1996) Proc. Natl. Acad. Sci. USA 93 402-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakker, A. C., Joosten, L. A., Arntz, O. J., Helsen, M. M., Bendele, A. M., van de Loo, F. A. & van den Berg, W. B. (1997) Arthritis Rheum. 40 893-900. [DOI] [PubMed] [Google Scholar]
- 14.Muller-Ladner, U., Roberts, C. R., Franklin, B. N., Gay, R. E., Robbins, P. D., Evans, C. H. & Gay, S. (1997) J. Immunol. 158 3492-3498. [PubMed] [Google Scholar]
- 15.Evans, C. H., Robbins, P. D., Ghivizzani, S. C., Herndon, J. H., Kang, R., Bahnson, A. B., Barranger, J. A., Elders, E. M., Gay, S., Tomaino, M. M., et al. (1996) Hum. Gene Ther. 7 1261-1280. [DOI] [PubMed] [Google Scholar]
- 16.Boggs, S. S., Patrene, K. D., Mueller, G. M., Evans, C. H., Doughty, L. A. & Robbins, P. D. (1995) Gene Ther. 2 632-638. [PubMed] [Google Scholar]
- 17.Arnett, F. C., Edworthy, S. M., Bloch, D. A., McShane, D. J., Fries, J. F., Cooper, N. S., Healey, L. A., Kaplan, S. R., Liang, M. H., Luthra, H. S., et al. (1988) Arthritis Rheum. 31 315-324. [DOI] [PubMed] [Google Scholar]
- 18.Del Vecchio, M. A., Georgescu, H. I., McCormack, J. E., Robbins, P. D. & Evans, C. H. (2001) Arthritis Res. 3 259-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Check, E. (2003) Nature 421 305. [DOI] [PubMed] [Google Scholar]
- 20.Nita, I., Ghivizzani, S. C., Galea-Lauri, J., Bandara, G., Georgescu, H. I., Robbins, P. D. & Evans, C. H. (1996) Arthritis Rheum. 39 820-828. [DOI] [PubMed] [Google Scholar]
- 21.Ghivizzani, S. C., Oligino, T. J., Glorioso, J. C., Robbins, P. D. & Evans, C. H. (2001) Drug Discov. Today 6 259-267. [DOI] [PubMed] [Google Scholar]
- 22.Ghivizzani, S. C., Lechman, E. R., Tio, C., Mule, K. M., Chada, S., McCormack, J. E., Evans, C. H. & Robbins, P. D. (1997) Gene Ther. 4 977-982. [DOI] [PubMed] [Google Scholar]
- 23.Ghivizzani, S. C., Lechman, E. R., Kang, R., Tio, C., Kolls, J., Evans, C. H. & Robbins, P. D. (1998) Proc. Natl. Acad. Sci. USA 95 4613-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roessler, B. J., Allen, E. D., Wilson, J. M., Hartman, J. W. & Davidson, B. L. (1993) J. Clin. Invest. 92 1085-1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goater, J., Muller, R., Kollias, G., Firestein, G. S., Sanz, I., O'Keefe, R. J. & Schwarz, E. M. (2000) J. Rheumatol. 27 983-989. [PubMed] [Google Scholar]
- 26.Watanabe, S., Imagawa, T., Boivin, G. P., Gao, G., Wilson, J. M. & Hirsch, R. (2000) Mol. Ther. 2 147-152. [DOI] [PubMed] [Google Scholar]
- 27.Gouze, E., Pawliuk, R., Pilapil, C., Gouze, J. N., Fleet, C., Palmer, G. D., Evans, C. H., Leboulch, P. & Ghivizzani, S. C. (2002) Mol. Ther. 5 397-404. [DOI] [PubMed] [Google Scholar]
- 28.Evans, C. H. & Robbins, P. D., eds. (2000) Gene Therapy in Inflammatory Diseases (Birkhauser, Basle), p. 260.
- 29.Evans, C. H., Ghivizzani, S. C., Oligino, T. J. & Robbins, P. D. (2000) J. Clin. Immunol. 20 334-346. [DOI] [PubMed] [Google Scholar]
- 30.Evans, C. H., Gouze, J. N., Gouze, E., Robbins, P. D. & Ghivizzani, S. C. (2004) Gene Ther. 11 379-389. [DOI] [PubMed] [Google Scholar]
- 31.Evans, C. H., Ghivizzani, S. C. & Robbins, P. D. (2004) Clin. Orthop. 429 316-329. [DOI] [PubMed] [Google Scholar]