Abstract
In alders, where fertilization occurs ≈8 weeks after pollination, the pollen tube (male gametophyte) grows intermittently in four steps in close association with the development of the ovary and its ovules. Pollen tubes stop growing in the style, at the ovarian locule, and at the chalaza (ovule), before reaching an embryo sac for fertilization. At the stage when the ovary develops an ovule primordium in each of the two locules, many pollen tubes germinate on the stigma, and a few of them reach the style, where they remain for ≈7 weeks. Thereafter, a single tube resumes growing; with a short stop in the upper space of the ovarian locule, it reaches the older of the two ovules when it has developed a two-nucleate embryo sac. Except in the last step, where the tube grows from the chalaza to an embryo sac (female gametophyte), an eight-nucleate mature embryo sac is not necessary for pollen-tube guidance in the pistil. Although the intermittent pollen-tube growth appears to play an important role in the selection of a single pollen tube from many and one ovule from two, its detection provides insight into the study of the mechanism of pollen-tube guidance.
Keywords: Betulaceae, chalazogamy, Fagales, fertilization, ovule
In flowering plants, fertilization occurs when the pollen tube (male gametophyte) reaches the embryo sac (female gametophyte) through the tissue of the pistil (sporophyte), unifying a sperm nucleus with an egg nucleus. This process suggests interaction not only between the pollen tube and the embryo sac, but also between the pollen tube (and embryo sac) and an intervening tissue, i.e., the tissue of the pistil. Recent studies using mutants of Arabidopsis thaliana that lacked normally developed ovules or embryo sacs showed that the pollen tubes lost their way to the target within the pistil (1-3). However, these studies mainly paid attention to interaction between pollen tubes and the embryo sac, and analysis of the correlation between the pollen-tube growth and the developmental stage of the pistils is still scarce.
In a majority of cases, the length of time between pollination and fertilization ranges from 24 to 48 h, and in some plants it is even shorter (4-8). In fagalean species, however, the pollen tube spends >1 month in the pistil before fertilization occurs, because the ovules are not yet mature when the pistil receives pollen grains on the stigma (9-11). We recently reported the pollen-tube growth pattern in Casuarinaceae (Fagales), where a unique mode of fertilization (i.e., chalazogamy) in place of the ordinary mode (i.e., porogamy) was reported (12, 13) (for details regarding chalazogamy and porogamy, see Fig. 5A, which is published as supporting information on the PNAS web site). In Casuarina equisetifolia (12), fertilization occurs in five discontinuous steps of pollen tube growth from the stigma to the embryo sac. We suggested that the pollen tube exhibited repeated steps of growth in accordance with the development of the ovary and ovule(s). However, C. equisetifolia develops four to eight embryo sacs in one ovule, and these embryo sacs differ in developmental stage and are surrounded by a thick nucellar tissue. Because of such conditions, the exact developmental stages of embryo sacs were not determined, and it remains unclear when or in what developmental stages the pollen tube reaches the ovule or the embryo sac.
This paper presents the pollen-tube growth pattern in Alnus firma Siebold et Zucc. and Alnus sieboldiana Matsum., two closely related species within the Betulaceae. Like C. equisetifolia, both Alnus species have a period of >1 month (≈8 weeks in Alnus) before fertilization after pollination, but, unlike C. equisetifolia, they develop only one embryo sac in each ovule. Therefore, these Alnus species allow us to compare pollen-tube growth in pistils with the developmental stages of their ovules or embryo sacs.
Materials and Methods
Plant Materials. Both A. firma and A. sieboldiana have male flowers and female flowers on separate inflorescences. Several hundred female flowers were collected from a single tree of A. firma and from a total of three trees of A. sieboldiana at wild sites in Kyoto Prefecture, Japan, weekly from the end of March to the end of May in 2001-2003. The flowers were fixed in FAA (5 parts stock formalin, 5 parts glacial acetic acid; and 90 parts 50% ethanol).
Observation of Pollen-Tube Growth. To observe pollen-tube growth in pistils or ovules, we removed some pistils or ovules by dissecting female flowers in a solution of 50% ethanol. Thereafter, they were cleared in a diluted solution (1.0%) of sodium hypochlorite (NaClO) at room temperature overnight. After being rinsed two or three times in water for several minutes each, the pistils or ovules were macerated in 1 M NaOH at 60°C for 2 h, then stained with 0.5% decolorized aniline blue in 0.1 M K3PO4 for 3-12 h and observed under a fluorescence microscope. Observations were made with a Zeiss LSM410 using UV filter set (model no. 01) with excitation filter (365 nm; band pass, 12 nm), dichroic mirror (FT395), and barrier filter (LP397), so that the callose of pollen tubes was detected.
Observations of Ovary and Ovule Development. To observe the ovary or ovule development, some pistils were dehydrated through a t-butyl alcohol series and then embedded in Paraplast for microtoming. Sections serially cut 6 or 7 μm thick were stained with Heidenhain's hematoxylin, safranin, and fast green FCF, and mounted in Entellan (Merck). To observe the fine detail of stylar cells, a few pistils were dehydrated by using an acetone series, then embedded in Spurr's resin and sectioned by using an ultra-microtome. Sections serially cut 1 μm thick were stained with Toluidine blue O and observed with an Olympus BX-51 microscope.
Observation of Pollen-Tube Growth and Ovule Development Using the Same Ovary. To compare the relative positions of the pollen tube at the different developmental stages of the ovule or its embryo sac in the same ovary, we removed one side of the ovary wall, detached the two ovules from the inside, and observed their respective embryo sacs. The pollen tube in the ovary was observed by fluorescence microscopy after the ovary, minus its ovules, was stained with aniline blue. The two detached ovules were macerated and cleared in Hoyer's solution (1.5 g of gum arabic, 20 g of chloral hydrate, and 1 ml of glycerol in 6 ml of water), and their respective nucellus was observed by differential interference microscopy (Olympus BX-51). The ovule itself was later stained with aniline blue and observed by fluorescence microscopy.
Results
Pollination in both A. firma and A. sieboldiana occurs early in March. Like other Betulaceae (14), the species are dichogamous. The anthers (male flowers) mature before the pistils (female flowers) on the same tree, so that pollen grains are dispersed before the pistils are receptive in these species. Therefore, the pistils receive pollen grains from other trees, avoiding self-pollination. Table 1 presents the number of pistils that had the pollen-tube tip at particular positions in the pistil or ovule after pollination in A. sieboldiana, showing where and when the pollen tubes are present in growing pistils. The following descriptions are based on A. sieboldiana, but no differences existed in the pattern of pollen-tube growth and the development of ovary and ovule between the two species.
Table 1. Position of the pollen-tube tip in developing pistils of A. sieboldiana.
| Developmental stages of ovules
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Position of pollen-tube tip | Primordial | Megaspore mother cell | Megaspore tetrad | One-nucleate embryo sac | Two-nucleate embryo sac | Four-nucleate embryo sac | Eight-nucleate embryo sac | Mature embryo sac |
| Style | 34 | 10 | 4 | |||||
| Upper space of the ovarian locule | 3 | 7 | 5 | |||||
| Chalaza | 11 | 10 | 2 | 4 | ||||
| Embryo sac | 24* | |||||||
Numbers indicate the number of postils observed.
Including embryo sacs just after fertilization.
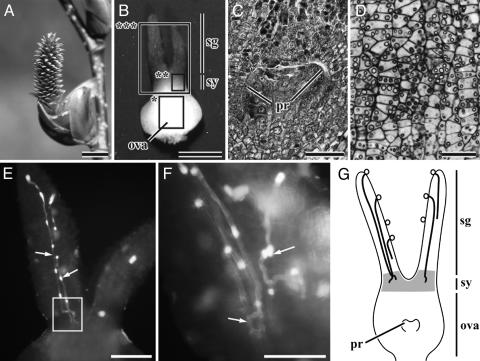
At the time of pollination, the female inflorescences are erect, 13-16 mm long, and terete in shape (Fig. 1A), whereas male inflorescences (catkins) are pendulous. The female flower lacks perianth segments and has a single bicarpellate pistil with two elongate stigmas (Fig. 1B). When the stigmas receive pollen grains, the pistils are 1.3-1.5 mm long including the stigmas. Compared to the large stigmas, the ovary is small and 0.5-0.65 mm long. The ovary has two locules, with an ovule primordium as a small emergence from the central placenta in each locule (Fig. 1 C and G). Within the style, all cells, excluding those of the epidermal and subdermal layers, contain rich starchy grains (Fig. 1 D and G). In 34 such pistils examined, one to seven pollen tubes (3.4 on average) grew downward in the stigmatic tissue (Fig. 1 E and G and Table 1). At the style, the pollen tubes lay in a zigzag line, and their growth is arrested there for about 7 weeks (Fig. 1 F and G). Each pollen tube forms a callose plug(s), and leaves it behind as it grows, but it is not formed if the pollen-tube growth is arrested. A similar pattern of pollen-tube growth is known from other fagalean species such as Betula pendula (9), Quercus (10, 11), C. equisetifolia (12), and Gymnostoma poissonianum (13).
Fig. 1.
Pollen-tube growth and development of the ovary and ovule at the time of pollination (the end of March) in Alnus.(A) Female inflorescence consisting of ≈300 female flowers. (B) A pistil at the time of pollination. (C) Longitudinal section (LS) of the part of the ovary indicated with a rectangle (asterisk) in B.(D) LS of the part of the style indicated with a rectangle (double asterisks) in B, showing that cells of the style contain many starchy grains. (E) Two stigmas and a style, indicated by a rectangle (triple asterisk) in B, showing pollen tubes (arrows). (F) Part of E magnified to show the pollen tubes (arrows) lying zigzag in the style. (G) A diagram illustrating pollen-tube growth in the pistil soon after pollination. Bold curved lines show pollen tubes, and gray shading shows cells with many starch grains. ova, ovary; pr, ovule primodium; sg, stigma; sy, style. (Scale bar, 5 mm in A, 0.5 mm in B, 200 μmin E, 100 μmin C and F, and 50 μmin D.)
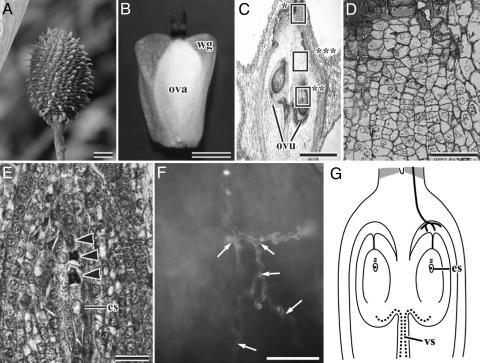
During a period of 7 weeks, female inflorescences elongate 21-25 mm and become oval in shape (Fig. 2A). The two stigmas in each pistil wither, but the ovary develops to become 2.5-3.0 mm long with a large bilateral wing (Fig. 2B). The size of the ovary hardly changes until the fertilization stage. One ovule develops within each ovarian locule (Fig. 2C, see Fig. 5B). Each ovule undergoes meiosis in the nucellus to form a tetrad of megaspores, in which the megaspore lying closest to the chalaza develops into an embryo sac (Fig. 2 E and G, see Fig. 5C).
Fig. 2.
Pollen-tube growth and development of ovule and embryo sac in older pistils of Alnus.(A) Female inflorescence ≈7 weeks after pollination. (B) A single pistil detached from A.(C) Longitudinal section (LS) of ovary. (D) Magnified view the top box in C, showing the disappearing starchy grains in the cells of the style. (E) Magnified view of the bottom box in C, showing a tetrad of megaspores in the nucellus. Arrowheads indicate three degenerating megaspores. (F) Magnified view of the middle box in C, showing that the pollen tube lies in a zigzag line and is divided into small branches (arrows) on the surface of the upper region of the ovarian locule. (G) A diagram illustrating pollen-tube growth in an older pistil. Bold curved lines show pollen tubes and the gray shading shows cells which contained many starch grains in the younger pistil. es, one-nucleate embryo sac; ova, ovary; ovu, ovule; vs., vascular tissue; wg, wing. (Scale bar, 10 mm in A, 1 mm in B, 500 μmin C, 100 μmin D and F, and 50 μmin E.)
Next, a pollen tube starts growing again from the style to the ovarian locule through the stylar transmitting tissue. Before the continuation of pollen-tube growth, the starchy grains mostly vanish in cells of the style (Fig. 2 D and G). Those starchy grains seem to have been digested as nutrition for the pollen tubes as in the case of Actinidia (15) and Prunus (16, 17).
In three of the seven pistils with a tetrad of megaspores in the ovule that we examined, the pollen tube reached the ovarian locule; however, the pollen tube did not reach the ovarian locule in the remaining four (Table 1). This fact indicates that the pollen-tube growth begins again shortly after meiotic division has completed in the ovule. Similarly, in Betula pendula (9), Quercus rubra, Quercus velutina, Quercus alba (10), and C. equisetifolia (12), the pollen tube is known to resume its growth from the style to the locule after meiotic divisions in the ovule. Only one, and rarely two (in 8 of the 115 pistils examined for this purpose), pollen tube reached the upper space of the ovarian locule. The pollen tube does not grow straight downward, but is branched and/or lies in a zigzag line in the upper space of the ovarian locule (Fig. 2 F and G). At that point, pollen-tube growth appeared to be arrested again for several days. Pollen-tube tip might grow during this period, but the directional growth ceases. Indeed the pollen-tube tips were observed in the upper space of the ovarian locule in three of seven pistils with a tetrad of megaspores in the ovule, all seven pistils with a one-nucleate embryo sac in the ovule, and 5 of 16 pistils with a two-nucleate embryo sac in the ovule (Table 1). This finding shows that the pollen tubes remain there until the ovule develops from the megaspore tetrad stage to the two-nucleate embryo sac stage.
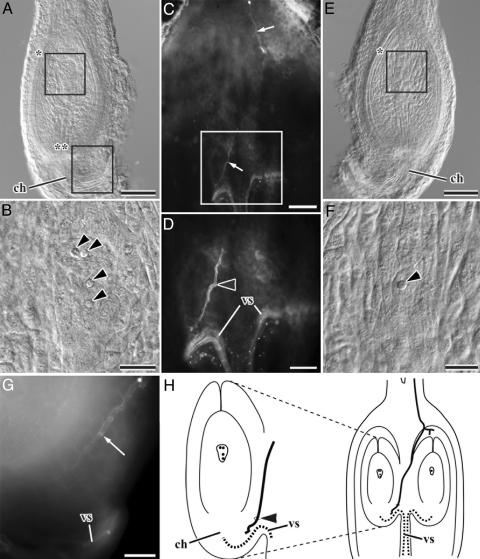
Thereafter, a single pollen tube resumes its growth from the upper space of the ovarian locule toward the ovule after the ovule reaches the two-nucleate embryo sac stage (Table 1). In all of the 21 ovaries examined, the pollen tube reached the chalaza of one of the two ovules with a two- or four-nucleate embryo sac (Fig. 3). The pollen tube penetrates the surface of the funiculus into the ovule (Fig. 3D), as in C. equisetifolia (12). In most cases, the two ovules of the same ovary are at somewhat different stages of development, and the older ovule receives a pollen tube. Indeed, in 15 of the 21 ovaries, one ovule was at the two- to four-nucleate embryo sac stage (Fig. 3 A-D, G, and H), receiving the pollen tube, and the other was at the one- or two-nucleate embryo sac or even the megaspore tetrad stage (Fig. 3 D-F). In four of the six remaining ovaries, both ovules were at the same developmental stage (two- or four-nucleate embryo sac); the ovule with a two-nucleate embryo sac received the pollen tube instead of the one with a four-nucleate embryo sac in only two ovaries. Thus, the pollen tube usually reaches the older of the two ovules in a single ovary. The pollen tube that reaches the chalaza of the ovule stops growing again and stays there for several days until the ovule matures (Table 1).
Fig. 3.
Pollen-tube growth and development of ovule and embryo sac in older pistils of Alnus.(A) One of two ovules, removed from the left side of an ovarian locule (C). (B) Magnified view of the upper box in A, showing an embryo sac with four nuclei (arrowheads). (C) An ovary with two ovules removed, showing that the pollen tube (arrow) grew downward. (D) Magnified view of the rectangle in C, showing that the pollen tube grows to the left ovule. An arrowhead shows the point where the pollen tube penetrated the funiculus. (E) Another ovule, removed from the right side of an ovarian locule (C). (F) Magnified view of the rectangle in E, showing an embryo sac with one nucleus (arrowhead). (G) Magnified view of the lower rectangle in A, showing that the pollen tube (arrow) grew down to the chalaza of the ovule on the left side. (H) A diagram illustrating pollen-tube growth in an older ovary. Bold curved lines show pollen tubes. An arrowhead shows the point where the pollen tube penetrated the funiculus. ch, chalaza; vs., vascular tissue. (Scale bar, 200 μmin A, C, and E, 100 μmin D, and 50 μmin B, F, and G.)
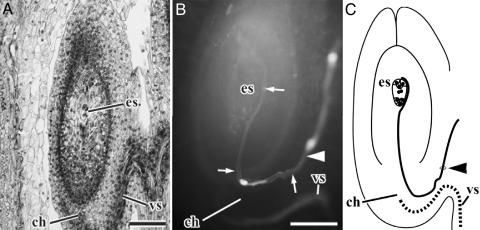
The pollen tube enters the nucellus from the chalaza soon after the embryo sac reaches maturity (Fig. 4 and Table 1). Thus, it takes ≈1 week for pollen-tube guidance from the style up to the embryo sac. The mode of fertilization was chalazogamy in all of the 24 ovules with the mature embryo sac. The pollen tube grows from the chalaza upward along the periphery of the mature embryo sac and enters the embryo sac from the micropylar side (Fig. 4 B and C), as in other species that utilize chalazogamy, such as Pistacia (18-21), Carya (22), Juglans (22-26), Betula (27), Alnus (27), and Corylus (28). No case was observed in which the pollen tube entered the embryo sac from the antipodal side.
Fig. 4.
Pollen-tube growth and development of an embryo sac at the time of fertilization in Alnus.(A) Longitudinal section of mature ovule. (B) Pollen tube (arrow) in the mature ovule. (C) A diagram illustrating pollen tube growth in the mature ovary. The bold curved line shows the pollen tube. Arrowheads in B and C show the point where the pollen tube penetrated the funiculus. ch, chalaza; es, embryo sac; vs, vascular tissue. (Scale bar, 200 μmin A and 100 μmin B.)
Discussion
Relationship Between Pollen-Tube Growth and Ovule Development. Because pollen-tube growth is often arrested in the pistil of Alnus, its progress toward the embryo sac occurs intermittently, not continuously. Arrested growth is summarized in four steps, as illustrated in Fig. 6, which is published as supporting information on the PNAS web site: (i) from the stigma to the style, (ii) from the style to the upper space of the ovarian locule, (iii) from the upper space of the ovarian locule to the chalaza of the ovule, and (iv) from the chalaza to the embryo sac. In Casuarina equisetifolia, the third step was divided into two phases because the pollen tube stops growing at the surface of the funiculus as well before reaching the chalaza (12).
The present study showed that, except for the first step, pollen-tube growth proceeds in close association with the development of ovules and/or embryo sacs. The first step proceeds irrespective of ovules and embryo sacs. The pollen tubes stay in the style for the longest period (7 weeks), probably digesting starchy grains. That period contrasts with the short period (≈1 week) it takes the pollen tube to grow from the style onward until fertilization. The second step occurs after the ovule undergoes meiosis in the nucellus. The third step occurs when one of the two ovules reaches a two-nucleate embryo sac stage, and the fourth step occurs when the embryo sac matures. When the pollen tube reaches the chalaza in the third step, the embryo sac is not yet ready for fertilization; i.e., it has to wait there until the embryo sac matures. In other words, although immature embryo sacs may also participate therein, it seems more likely that the whole developing ovule itself and the chalaza directly guide the pollen tube in the second and third steps, respectively. A mature embryo sac is not necessary to guide the pollen tube up to the chalaza (ovule). Only the pollen-tube growth from the chalaza to the embryo sac (fourth step) is guided by the matured embryo sac (female gametophyte). More exactly, as known in Torenia (29), synergids in the mature embryo sac attract the pollen tube.
In vitro Torenia ovules, the maximum distance between the synergid and synergid-attracted pollen tube was estimated to be a few hundred micrometers (30). In Alnus, the distance between the synergid and chalaza is ≈500-600 μm, indicating that the synergid can attract a pollen tube that is 500-600 μm away. On the other hand, the ovule that does not receive the pollen tube appears to stop growing. The fourth step seems to occur in response to the pollen tube that reaches the chalaza.
Evolutionary Implications of the Intermittent Pollen-Tube Growth. There is no doubt that a delayed development of the ovary compared to the stigma has required the development of the ovary and the ovule after pollination, and consequently prolonged the time between pollination and fertilization. Such a prolonged period between pollination and fertilization is known in some other fagalean species (9-13, 22, 27). In the case of Betula pendula, which also has an immature ovary at the time of pollination, it has been suggested that the retarded ovary provides all pollen tubes with a fair start to the ovules regardless of the different arrival times at the stigma surface (9); this seems likely to be the case for the other species, including alders. However, some time (≈1 week in alders) is also wasted for the pollen tube to grow from the style to the embryo sac, and the growth is not continuous, but intermittent. Throughout the intermittent pollen-tube growth, a single pollen tube (male gametophyte) is selected from many in the first step, and one ovule (with female gametophyte) is selected from two in the third step. In the latter, the pollen tube selected the older ovule rather than the retarded one in alders. These findings suggest that the prolonged period between pollination and fertilization provides the pistil with time for mutual selection between the male and the female gametophyte. Fagales, including Betulaceae, are anemophilous, producing large quantities of pollen grains (14, 31) that lead to random mating. Such anemophily is considered a derived state in angiosperm evolution (31). In those anemophilous plants, however, the intermittent growth of the pollen tube plays an important role in selecting the male and female gamete to be fertilized.
The Study of the Mechanism of Pollen-Tube Guidance in Angiosperms. The results of the present study provide new insight into the mechanism of pollen-tube guidance in angiosperms. In mutants of Arabidopsis thaliana which lacked normally developed ovules or embryo sacs, the pollen tubes lost their way to the target in the ovarian locule (1, 2) or the funiculus (3). Because the pollen tube in those mutants did not grow correctly after passage the funiculus, the matured embryo sac was considered essential for the pollen-tube guidance beyond the funiculus (1-3). However, this study showed that the pollen tube could reach the ovule (chalaza) beyond the funiculus without the matured embryo sac, but only when the embryo sac became two-nucleate. One might consider that the intermittent growth of the pollen tube is restricted to Fagales, but an arrested growth of pollen tubes in the pistil is known in unrelated plant groups such as Rosaceae (17) and Orchidaceae (32, 33). Attention should be paid to detecting mutants strictly destitute of any of the steps of the pollen-tube growth we reported because no mutants are known yet in which the pollen tube stops growing in the style or ovule.
It has been suggested that the Arabidopsis POP2 gene controls the concentration of GABA and regulates pollen-tube growth toward the micropyle (34) (corresponding to the third step we presented in alders). It seems worth investigating whether the Arabidopsis POP2 gene or GABA works in alders as well. Combined analyses of molecular and morphological features known from model and wild plants respectively are expected to provide a clue for the elucidation of the pollen-tube guidance mechanism.
Supplementary Material
Acknowledgments
We are grateful to Hiroaki Setoguchi, Junko Noguchi, and Jung Sung Kim for assistance in collecting materials. The study was supported by Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research no. 14405012 and Biodiversity Research of the 21st Century COE Grant A14.
Author contributions: A.S. and H.T. designed research; A.S. and H.T. performed research; A.S. and H.T. analyzed data; and A.S. and H.T. wrote the paper.
References
- 1.Hülskamp, M., Schneitz, K. & Pruitt, R. E. (1995) Plant Cell 7, 57-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ray, S., Park, S. S. & Ray, A. (1997) Development (Cambridge, U.K.) 124, 2489-2498. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu, K. K. & Okada, K. (2000) Development (Cambridge, U.K.) 127, 4511-4518. [DOI] [PubMed] [Google Scholar]
- 4.Maheshwari, P. (1950) in An Introduction to the Embryology of Angiosperms (McGraw-Hill, New York).
- 5.Gao, X., Francis, D., Ormrod, J. C. & Bennett, M. D. (1992) Ann. Bot. 70, 561-568. [Google Scholar]
- 6.Tian, H.-Q. & Russell, S. D. (1997) Planta 202, 93-105. [Google Scholar]
- 7.Higashiyama, T., Kuroiwa, H., Kawano, S. & Kuroiwa, T. (1997) Planta 203, 101-110. [Google Scholar]
- 8.Faure, J.-E., Rotman, N., Fortuné, P. & Dumas, C. (2002) Plant J. 30, 481-488. [DOI] [PubMed] [Google Scholar]
- 9.Dahl, Å. E. & Fredrikson, M. (1996) Am. J. Bot. 83, 895-902. [Google Scholar]
- 10.Cecich, R. A. (1997) Forest Sci. 43, 140-146. [Google Scholar]
- 11.Boavida, L. C., Varela, M. C. & Feijo, J. A. (1999) Sex Plant Reprod. 11, 347-353. [Google Scholar]
- 12.Sogo, A., Noguchi, J., Jaffré, T. & Tobe, H. (2004) J. Plant Res. 117, 37-46. [DOI] [PubMed] [Google Scholar]
- 13.Sogo, A., Jaffré, T. & Tobe, H. (2004) J. Plant Res. 117, 249-251. [DOI] [PubMed] [Google Scholar]
- 14.Kubizki, K. (1993) in The Families and Genera of Vascular Plants, eds. Kubizki, K., Rohwer, J. G. & Bittrich, V. (Springer, New York), pp. 152-157.
- 15.González, M. V., Coque, M. & Herrero, M. (1996) Am. J. Bot. 83, 148-154. [Google Scholar]
- 16.Arbeloa, A. & Herrero, M. (1987) Ann. Bot. 60, 681-685. [Google Scholar]
- 17.Herrero, M. & Arbeloa, A. (1989) Am. J. Bot. 76, 1441-1447. [Google Scholar]
- 18.Copeland, H. C. (1955) Phytomorphology 6, 440-449. [Google Scholar]
- 19.Grundwag, M. (1976) Bot. J. Linn. Soc. 73, 353-370. [Google Scholar]
- 20.Shuraki, Y. D. & Sedgley, M. (1997) Ann. Bot. 79, 361-369. [Google Scholar]
- 21.Martínez-Pallé, E. & Herrero, M. (1998) Int. J. Plant. Sci. 159, 566-574. [Google Scholar]
- 22.Langdon, L. M. (1934) Bot. Gaz. 96, 93-117. [Google Scholar]
- 23.Karsten, G. (1902) Flora 90, 316-333. [Google Scholar]
- 24.Nast, C. G. (1935) Hilgardia 9, 345-381. [Google Scholar]
- 25.Nast, C. G. (1941) Lilloa 6, 163-205. [Google Scholar]
- 26.Luza, J. G. & Polito, V. S. (1991) Bot. Gaz. 152, 100-106. [Google Scholar]
- 27.Benson, M. (1894) Trans. Linn. Soc. London Bot. 3, 409-424. [Google Scholar]
- 28.Nawaschin, S. (1895) Bot. Centralbl. 63, 104-106. [Google Scholar]
- 29.Higashiyama, T., Yabe, S., Sasaki, N., Nishimura, Y., Miyagishima, S., Kuroiwa, H. & Kuroiwa, T. (2001) Science 293, 1480-1483. [DOI] [PubMed] [Google Scholar]
- 30.Higashiyama, T. (2002) J. Plant Res. 115, 149-160. [DOI] [PubMed] [Google Scholar]
- 31.Culley, T. M., Weller, S. G. & Sakai, A. K. (2002) Trends Ecol. Evol. 17, 361-369. [Google Scholar]
- 32.Carlson, M. C. (1940) Bot. Gaz. 102, 295-301. [Google Scholar]
- 33.Duncan, R. E. & Curtis, J. T. (1942) Bul. Torrey Bot. Club 69, 167-183. [Google Scholar]
- 34.Palanivelu, R., Brass, L., Edlund, A. F. & Preuss, D. (2003) Cell 114, 47-59. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.