Abstract
Background: Distal bile duct cancer is an aggressive malignancy. Tumor-infiltrating immune cells (TIICs) in the tumor microenvironment are crucial for predicting prognosis in various cancers. In this study, we analyzed TIICs based on CD11b, CD163, and CD8 expression, and evaluated their association with clinicopathologic factors and prognosis in distal bile duct cancer. Methods: A total of 90 patients who underwent curative resection for distal bile duct cancer were enrolled. We analyzed CD11b+ tumor-infiltrating myeloid cells (TIMs), CD163+ tumor-infiltrating macrophages (TAMs), and CD8+ tumor-infiltrating lymphocytes (TILs) using immunohistochemistry and tissue microarrays. The correlation between TIICs and clinicopathologic characteristics was assessed. Results: Low levels of CD11b+ TIMs (p < 0.001) and high levels of CD8+ TILs (p = 0.003) were significantly associated with improved overall survival (OS). A combined low level of CD11b+ TIMs and high level of CD8+ TILs was identified as an independent favorable prognostic factor (hazard ratio, 0.159; confidence interval, 0.061–0.410; p < 0.001). Conclusions: CD11b+ TIMs play a crucial role in the tumor microenvironment and the prognosis of distal bile duct cancer. The combined analysis of CD11b+ TIMs and CD8+ TILs can predict survival in patients with distal bile duct cancer.
Keywords: distal bile duct cancer, CD11b, CD8, CD163, tumor microenvironment, prognosis, immunotherapy
1. Introduction
Distal bile duct cancer is a rare and aggressive malignant tumor, typically classified as an extrahepatic cholangiocarcinoma. It primarily originates from the biliary epithelium between the ampulla of Vater and the junction of the cystic duct [1,2]. The 5-year survival rate for patients who undergo surgical resection for distal bile duct cancers is only 35.4–44.4% [3,4]. While new targeted therapies based on genetic mutation, such as FGFR2 and IDH-1/2, have shown promising results, they are predominantly applicable to intrahepatic cholangiocarcinoma rather than distal bile duct cancer [2,5]. Recently, HER2, KRAS, and EGFR mutations have been identified in some patients with distal bile duct cancer, making them potential candidates for targeted therapy. However, the overall benefits remain limited due to the small number of patients with these mutations [6]. As a result, there is an urgent need for new treatment options for distal bile duct cancer, with immunotherapy emerging as a promising approach.
Immunotherapy has achieved significant success as a treatment modality and has become a standard treatment for melanoma, lung cancer, genitourinary tract cancer, and head and neck cancer [7,8,9,10]. For immunotherapy to be effective, it is essential to understand the tumor microenvironment and the immune status of each tumor [11,12,13].
The tumor microenvironment consists of tumor cells themselves, along with various subsets of innate and adaptive immune cells, the vascular network, and stromal cells. These components interact through complex networks, involving growth factors, cytokines, chemokines, adhesion molecules, and extracellular matrices [14]. Among these, immune cells play dynamic roles in modulating either the promotion or suppression of tumor growth by interacting with other immune cells, and thus, they are now extensively utilized by immune therapy [15,16].
Immune dysregulation plays a crucial role in modulating the development and prognosis of distal bile duct cancer [17]; considerable research has been applied to elucidate the underlying mechanisms of immune dysregulation. However, only some studies can be used to study the tumor immune cells in distal bile duct cancer [18,19].
CD11b, the ligand that binds the subunits of the dimeric integrin CD11b/CD18b, is a receptor for both fibrinogen and endothelial intracellular adhesion molecule-1 and is expressed on most myeloid cells, including macrophages, monocytes, neutrophils, and some dendritic cell subsets. Recently, CD11b+ tumor-infiltrating myeloid cells (TIMs) were shown to interact with other immune cells, especially CD163+ tumor-associated macrophages (TAMs) and CD8+ tumor-infiltrating lymphocytes (TILs), in the tumor microenvironment [20,21]. Further, CD11b expression in myeloid cells can affect tumor characteristics and prognosis in various solid cancers, including gastric cancer, liver metastases from lung cancer, breast cancer, and intestinal tumors [22,23,24,25,26].
In this study, we aimed to analyze CD11b+ TIMs, CD8+ TILs, and CD163+ TAMs in relation to the clinicopathologic characteristics of distal bile duct cancer, and to assess the prognostic significance of the combined analysis of CD11b+ TIMs and CD8+ TILs.
2. Materials and Methods
2.1. Patients
This study was conducted as a non-randomized and retrospective analysis at a single institution. A total of 90 patients with distal bile duct cancer were enrolled in this study. All the participants were diagnosed with distal bile duct cancer and underwent curative resection between 2015 and 2020 at Chung-Ang University Hospital, Seoul, Republic of Korea. We subsequently collected cases with sufficient tissue samples for immunohistochemistry, along with the available clinicopathologic data. Clinical data, including patient age, sex, and overall survival (OS), were extracted from electronic medical records. The pathologic characteristics were meticulously re-evaluated, encompassing the depth of invasion, nodal status, lymphovascular invasion, perineural invasion, and resection margin status. The pathologic primary tumor (pT) and pathologic regional lymph node (pN) stages were reclassified according to the TNM Classification of Malignant Tumours, eighth edition.
2.2. Immunohistochemistry
Tissue microarrays (TMAs) were constructed by punch tissue cores (2 mm) from two representative areas and placed into recipient blocks. TMAs were processed with antigen retrieval and incubated primary antibodies against CD11b (1:200; Abcam, Cambridge, UK), CD8 (1:1000, C8/144B; Dako, Cambridge, UK), and CD163 (1:400; Abcam, Cambridge, UK). All the immunostains were carried out in the Venta Biotech automated system (Venta Medical System, Tucson, AZ, USA) according to the manufacturer’s protocol.
2.3. Analysis of Tumor-Infiltrating Immune Cells According to CD11b, CD8, and CD163 Expression
Immunostained slides were evaluated by two pancreaticobiliary pathologists (S.A.H and J.Y.K.) who were blinded to the clinicopathological information of the patients. Any discrepancies between the two pathologists were resolved by reviewing the cases together using a multi-head microscope until a consensus was reached.
The numbers of CD11b-, CD8-, and CD163-positive tumor-infiltrating immune cells (TIICs) were counted in five foci, with the highest density of immunostained cells found in the intratumoral area at a magnification of 400× (BX53; Olympus, Tokyo, Japan). High TIICs were defined as values above the medians for CD11b-, CD8-, and CD163-positive TIICs.
2.4. Statistical Analyses
Categorical variables were analyzed using the Chi-squared or Fisher’s exact test, as appropriate, while continuous variables were evaluated using Student’s T-test. The cut-off values for high or low levels of CD11b+ TIMs, CD8+ TILs, and CD163+ TAMs were determined using the receiver operating characteristic (ROC) curve and Youden’s index, with overall survival as the endpoint criterion. The correlation between the mean number of CD8+ TILs and CD163+ TAMs with CD11b+ TIMs was evaluated using a Pearson correlation test. OS was defined as the period from diagnosis to death from any cause, or the period from diagnosis to the last follow-up. The Kaplan–Meier method and log-rank test were used to plot survival times. Cox proportional hazards regression models determined the prognostic significance. The proportional hazards assumption was tested using the Schoenfeld residuals test. A two-sided p-value < 0.05 indicated statistical significance in all the tests and models. Data analyses were performed with the R statistical software version 4.3.2 (http://www.r-project.org, 25 July 2024).
3. Results
3.1. Clinicopathologic Characteristics
All the patients underwent curative resections, with R0 resections performed in 80 patients (88.9%) and R1 resections in 10 patients (11.1%). The median age was 68 years, and 53 patients (58.9%) were men. The median tumor size was 2.75 cm. According to TNM staging, 24 patients (26.7%) were pT1, 30 (33.3%) were pT2, and 36 (40.0%) were pT3. Regarding nodal status, 61 patients (67.8%) were N0, and 29 (32.2%) were N1/N2. Lymphovascular invasion and perineural invasion were observed in 45 patients (50.0%) and 71 patients (78.9%), respectively.
3.2. Correlation of CD11b+, CD8+, and CD163+ TIICs with Clinicopathologic Characteristics
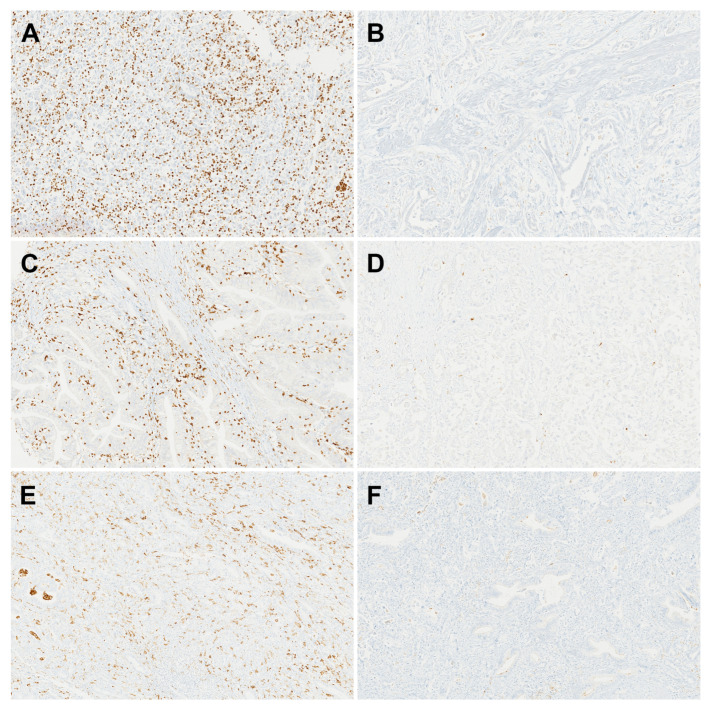
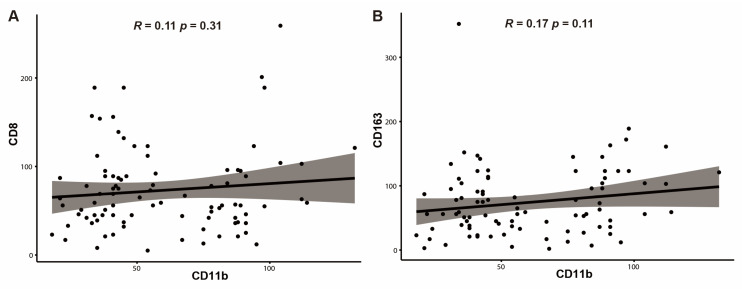
The median numbers of CD11b+ TIMs, CD8+ TILs, and CD163+ TAMs were 46, 58, and 67, respectively. Based on the results from the ROC curve and Youden’s index, the cut-off values were determined to be 58 for CD11b+ TIMs, 68 for CD8+ TILs, and 31 for CD163+ TAMs. Tumors were then classified as high or low based on these values. High-level CD11b+ TIMs were found in 38 patients (42.2%), high-level CD8+ TILs in 40 patients (44.4%), and high-level CD163+ TAMs in 70 patients (77.8%) (Table 1), (Figure 1A–F). The cases with low levels of CD8+ TILs were significantly associated with a high T stage (p = 0.002). The number of CD11b+ TIMs was not significantly correlated with the number of CD8+ TILs (R = 0.11; p = 0.311) (Figure 2A), or with the number of CD163+ TAMs (R = 0.17; p = 0.113) (Figure 2B).
Table 1.
Association between CD11b, CD8, CD163, and clinicopathologic findings of distal bile duct cancer.
| CD11b | CD8 | CD163 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | High (n = 38) (%) |
Low (n = 52) (%) |
p-Value | High (n = 40) (%) |
Low (n = 50) (%) |
p-Value | High (n = 70) (%) |
Low (n = 20) (%) |
p-Value |
| Age (mean ± SD) | 66.7 ± 10.4 | 68.0 ± 9.6 | 0.557 * | 66.1 ± 9.4 | 68.5 ± 10.3 | 0.247 * | 66.9± 10.0 | 69.4 ± 9.5 | 0.317 * |
| Gender | 0.626 | 0.981 | 0.510 | ||||||
| Male | 24 (63.2) | 29 (55.8) | 23 (57.5) | 30 (60.0) | 43 (61.4) | 10 (50.0) | |||
| Female | 14 (36.8) | 23 (44.2) | 17 (42.5) | 20 (40.0) | 27 (38.6) | 10 (50.0) | |||
| Tumor size (mean ± SD) | 3.2± 1.5 | 3.0 ± 1.3 | 0.726 * | 3.0 ± 1.3 | 3.2 ± 1.5 | 0.549 * | 3.1 ± 1.3 | 3.1 ± 1.5 | 0.936 * |
| Histologic grade | 0.358 | 0.707 | 0.206 | ||||||
| Well | 8 (21.1) | 16 (30.8) | 9 (22.5) | 15(30.0) | 16 (22.9) | 8 (40.0) | |||
| Moderate Poorly |
23 (60.5) 7 (18.4) |
31 (59.6) 5 (9.6) |
25 (62.5) 6 (15.0) |
29(58.0) 6 (12.0) |
43 (61.4) 11 (15.7) |
11 (55.0) 1 (5.0) |
|||
| T stage | 0.318 | 0.002 | 0.372 | ||||||
| T1 | 7 (18.4) | 17 (32.7) | 15 (37.5) | 9 (18.0) | 21 (30.0) | 3 (15.0) | |||
| T2 | 14 (36.8) | 16 (30.8) | 17(42.5) | 13(26.0) | 23 (32.9) | 7 (35.0) | |||
| T3 | 17 (44.7) | 19 (36.5) | 18(20.0) | 28(56.0) | 26 (37.1) | 10 (50.0) | |||
| N stage | 0.723 | 0.338 | 0.678 | ||||||
| N0 | 24 (63.2) | 37 (71.2) | 30(75.0) | 31(62.0) | 46 (65.7) | 15 (75.0) | |||
| N1 N2 |
12 (31.6) 2 (5.3) |
13 (25.0) 2 (3.8) |
8 (20.0) 2 (5.0) |
17(34.0) 2(4.0%) |
21 (30.0) 3 (4.3) |
4 (20.0) 1 (5.0) |
|||
| Lymphovascular invasion | 1 | 0.832 | 1 | ||||||
| Yes | 19 (50.0) | 26 (50.0) | 19(47.5) | 26(52.0) | 35 (50.0) | 10 (50.0) | |||
| No | 19 (50.0) | 26 (50.0) | 21(52.5) | 24 (48.0) | 35 (50.0) | 10 (50.0) | |||
| Perineural invasion | 0.803 | 0.583 | 0.654 | ||||||
| Yes | 29 (76.3) | 42 (80.8) | 30(75.0) | 41 (82.0) | 54 (77.1) | 17 (85.0) | |||
| No | 9 (23.7) | 10 (19.2) | 10(25.0) | 9 (18.0) | 16 (22.9) | 3 (15.0) | |||
| Margin status of the bile duct | 0.386 | 0.189 | 0.560 | ||||||
| R0 | 32 (84.2) | 48 (92.3) | 38(95.0) | 42(84.0) | 61 (87.1) | 19 (95.0) | |||
| R1 | 6 (15.8) | 4 (7.7) | 2 (5.0) | 8 (16.0) | 9 (12.9) | 1 (5.0) | |||
SD, standard deviation; *, t-test; R0, clear resection margin; R1, positive resection margin on microscopic examination.
Figure 1.
Representative immunohistochemistry of CD11b+ tumor-infiltrating myeloid cells (TIMs), CD8+ tumor-infiltrating lymphocytes (TILs), and CD163+ tumor-associated macrophages (TAMs). (A) High CD11b+ TIM level. (B) Low CD11b+ TIM level. (C) High CD8+ TIL level. (D) Low CD8+ TIL level. (E) High CD163+ TAM level. (F) Low CD163+ TAM level.
Figure 2.
The correlation of the mean number of CD8+ tumor-infiltrating lymphocytes (TILs) and CD163+ tumor-associated macrophages (TAMs) according to those of CD11b+ tumor-infiltrating myeloid cells (TIMs) in distal bile duct cancer. The correlation is not identified significantly in CD8+ TILs (R = 0.11, p = 0.31) (A) and CD163+ TAMs (R = 0.17, p = 0.11) (B) with those of CD11b+ TIMs, respectively.
3.3. Survival Effects with Respect to CD11b+, CD8+, and CD163+ TIICs
The median OS was 22.25 months. The patients with either low levels of CD11b+ TIMs or high levels of CD8+ TILs had longer OS compared to those with high levels of CD11b+ TIMs or low levels of CD8+ TILs (low CD11b+, p < 0.001; high CD8+, p = 0.003). Conversely, CD163+ TAM status was not significantly related to OS (p = 0.18) (Figure 3A–C).
Figure 3.
Prognostic analysis of CD11b+ tumor-infiltrating myeloid cells (TIMs), CD8+ tumor-infiltrating lymphocytes (TILs), and CD163+ tumor-associated macrophages (TAMs) in distal bile duct cancer. The Kaplan–Meier curves demonstrate a significant increase in overall survival (OS) in patients with low levels of CD11b+ TIMs (A) and high levels of CD8+ TILs (B); no significant difference in OS is observed according to CD163+ TMA levels (C).
3.4. Clinicopathologic Features and Survival Effects with Respect to Concomitant Low CD11b+ and High CD8+ TIICs
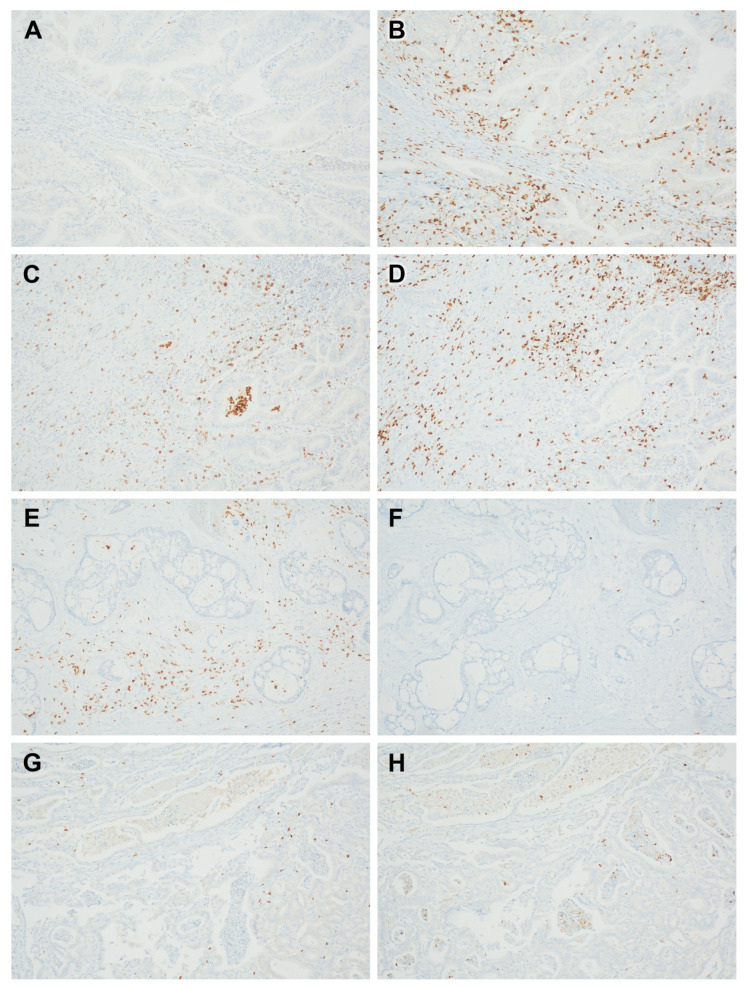
Concomitant low levels of CD11b+ TIMs and high levels of CD+8 TILs were found in 27 patients (30.0%) (Figure 4A–H). The tumors with low levels of CD11b+ TIMs and high levels of CD8+ TILs were significantly associated with a low T stage (p < 0.001) (Table 2).
Figure 4.
Representative immunohistochemistry for paired cases of CD11b+ tumor-infiltrating myeloid cells (TIMs) and CD8+ tumor-infiltrating lymphocytes (TILs): low CD11b+ TIMs (A) and high CD8+ TILs (B); high CD11b+ TIMs (C) and high CD8+ TILs (D); high CD11b+ TIMs (E) and low CD8+ TILs (F); and low CD11b+ TIMs (G) and low CD8+ TILs (H).
Table 2.
Association between high CD11b/high CD8 and clinicopathologic findings of distal bile duct cancer.
| Low CD11b/ High CD8 | |||
|---|---|---|---|
| Variable | Present (n = 27) (%) |
Absent (n = 63) (%) |
p-Value |
| Age (mean ± SD) | 66.6 ± 9.6 | 67.8 ± 10.1 | 0.585 |
| Gender | 0.782 | ||
| Male | 15 (55.6) | 38 (60.3) | |
| Female | 12 (44.4) | 25 (39.7) | |
| Tumor size (mean ± SD) | 3.0 ± 1.2 | 3.1 ± 1.5 | 0.633 |
| Histologic grade | 0.902 | ||
| Well | 7 (25.9) | 17 (27.0) | |
| Moderate Poorly |
17 (63.0) 3 (11.1) |
37 (58.7) 9 (14.3) |
|
| T stage | <0.001 | ||
| T1 | 13 (48.1) | 11 (17.5) | |
| T2 | 11 (40.7) | 19 (30.2) | |
| T3 | 3 (11.1) | 33 (52.4) | |
| N stage | 0.178 | ||
| N0 | 22 (81.5) | 39 (61.9) | |
| N1 N2 |
4 (14.8) 1 (3.7) |
21 (33.3) 3 (4.8) |
|
| Lymphovascular invasion | 0.358 | ||
| Yes | 11 (40.7) | 34 (54.0) | |
| No | 16 (59.3) | 29 (46.0) | |
| Perineural invasion | 0.310 | ||
| Yes | 19 (70.4) | 52 (82.5) | |
| No | 8 (29.6) | 11 (17.5) | |
| Margin status of the bile duct | 0.067 | ||
| R0 | 27 (100) | 53 (84.1) | |
| R1 | 0 (0) | 10 (15.9) | |
SD, standard deviation; R0, clear resection margin; R1, positive resection margin on microscopic examination.
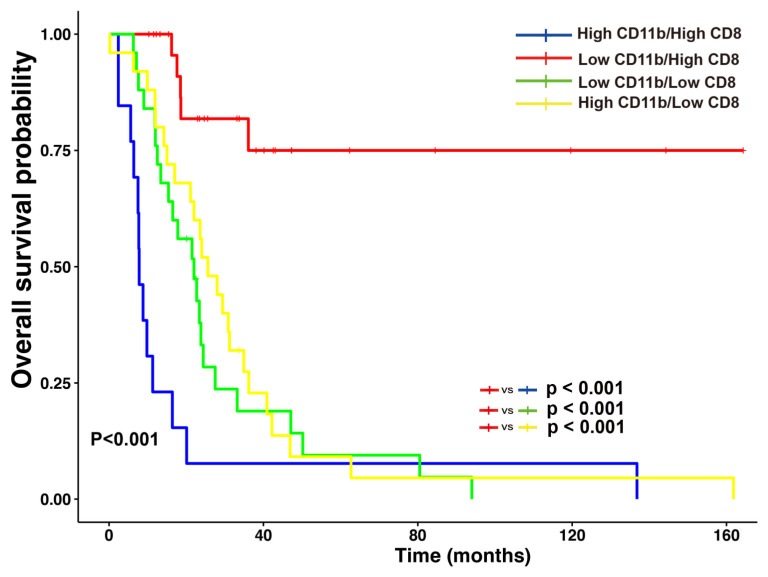
According to the status of combined CD11b+ TIMs and CD8+ TILs, patients had significantly increased OS (p < 0.001). In detail, the patients with combined low CD11b+ TIMs and high CD8+ TILs had longer OS than the patients with the following combinations: combined high CD11b+ TIMs and high CD8+ TILs (p < 0.001); combined low CD11b+ TIMs and low CD8+ TILs (p < 0.001); or combined high CD11b+ TIMs and low CD8+ TILs (p < 0.001) (Figure 5).
Figure 5.
Combined analysis of CD11b+ tumor-infiltrating myeloid cells (TIMs) and CD8+ tumor-infiltrating lymphocytes (TILs) in distal bile duct cancer. Patients with a combined low level of CD11b+ TIMs and high level of CD8+ TILs have a favorable prognosis relative to other groups.
In a univariate Cox proportional hazards analysis, shorter OS was significantly and inversely correlated with high T stage, nodal metastasis, and R1 margin status, whereas the patients with combined low CD11b+ TIMs and high CD8+ TILs had significantly increased OS. A multivariate analysis revealed that combined low CD11b+ TIM with high CD8+ TIL status was an independent and significantly favorable prognostic factor (hazard ratio = 0.159; 95% confidence interval: 0.061–0.410, p < 0.001) (Table 3).
Table 3.
Univariate and multivariate analyses of factors associated with overall survival of distal bile duct cancer.
| Clinicopathologic Factors | Univariate Analysis |
Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p-Value | HR | 95% CI | p-Value | |
| Age (years) | 1.006 | 0.980–1.033 | 0.649 | |||
| Gender | ||||||
| Male | ||||||
| Female | 0.971 | 0.590–1.599 | 0.909 | |||
| Tumor size | 0.993 | 0.828–1.190 | 0.937 | |||
| Histologic grade | ||||||
| Well | ||||||
| Moderate | 1.410 | 0.800–2.484 | 0.235 | |||
| Poorly | 1.372 | 0.615–3.062 | 0.440 | |||
| pT stage | ||||||
| pT1 | ||||||
| PT2 | 4.967 | 2.061–11.974 | <0.001 | 5.221 | 1.989–13.704 | <0.001 |
| PT3 | 9.584 | 4.008–22.920 | <0.001 | 6.785 | 2.646–17.397 | <0.001 |
| pN stage | ||||||
| pN0 | ||||||
| pN1 | 1.760 | 1.019–3.040 | 0.043 | 1.267 | 0.721–2.226 | 0.411 |
| pN2 | 4.565 | 1.334–15.615 | 0.016 | 2.711 | 0.782–9.390 | 0.116 |
| Lymphovascular invasion | ||||||
| No | ||||||
| Yes | 1.595 | 0.970–2.624 | 0.066 | |||
| Margin status of the bile duct | ||||||
| R0 | ||||||
| R1 | 2.046 | 1.030–4.066 | 0.041 | 0.969 | 0.479–1.962 | 0.931 |
| Perineural invasion | ||||||
| No | ||||||
| Yes | 0.764 | 0.406–1.436 | 0.403 | |||
| Low CD11b/ high CD8 | ||||||
| No | ||||||
| Yes | 0.124 | 0.050–0.311 | <0.001 | 0.159 | 0.061–0.410 | <0.001 |
R0, clear resection margin; R1, positive resection margin on microscopic examination.
4. Discussion
In the present study, low levels of CD11b+ TIMs were associated with a favorable prognosis. The number of CD11b+ TIMs was not significantly associated with the numbers of CD8+ TILs and CD163+ TAMs. Further, a low level of CD11b+ TIMs combined with a high level of CD8+ TILs was an independent prognostic factor in distal bile duct cancer.
Tumor immunotherapy, including immune checkpoint inhibitors, vaccinations, immune cell therapies, and cytokine therapies, has become a practical strategy for tumor treatment [27,28,29]. For the feasibility of successful tumor immunotherapy, the characteristics of tumor immune status in each tumor must be evaluated.
CD11b expression has been identified in hematopoietic tumor cells and TIICs in various solid cancers [30,31,32]. Among TIICs, CD11b is predominantly expressed in myeloid cells, including dendritic cells, mononuclear cells, and myeloid-derived suppressor cells [33]. CD11b+ TIICs significantly contribute to the tumor microenvironment and play crucial roles in carcinogenesis. Targeting CD11b reduces myeloid cell infiltration, particularly in cells expressing S100A8 and matrix metalloproteinase-9, which are key factors in tumor regrowth [34]. In prostate cancer, tumor-derived cytokines drive CD11b+ cells to differentiate into osteoclasts, promoting bone metastasis [35]. Additionally, the BG34-200 ligand binds to a novel CD11b epitope, inducing monocyte-to-dendritic cell differentiation and enhancing T-cell activation, offering the potential for novel immunotherapies [36]. These mechanisms highlight the pivotal role of CD11b in both tumor progression and immune modulation.
In our study, the number of CD11b+ TIMs was similar to the numbers of CD8+ TILs and CD163+ TAMs. In previous studies, CD11b+ TIMs in gastric cancer and pancreatic cancer were abundant in tumors [24,37]. We confirmed that CD11b+ TIMs are one of the main components of TIICs in distal bile duct cancer.
Most studies suggest that CD163+ TAMs are associated with a poor prognosis in various solid cancers [38,39]. In our study, the level of CD163+ TAMs was not significantly associated with OS in distal bile duct cancer. Similarly, Miura et al. reported that CD163+ macrophages were not associated with OS [40], indicating that the prognostic role of CD163+ TMAs per se is limited in distal bile duct cancer.
CD11b can modulate some TIICs within the tumor microenvironment. Dendritic cells expressing major histocompatibility complex II+/CD11b+/CD11chigh can suppress CD8+ T-cell function in tumors [41]. Schmid et al. reported that the inhibition of CD11b in myeloid cells can lead to immune suppressive macrophage polarization by regulating M2-related cytokines [21]. Despite comparing the quantitative numbers of TIICs characterized by CD11b+, CD8+, and CD163+ markers, we did not observe significant changes in CD8+ TILs and CD163+ TAMs in line with the level of CD11b+ TIMs. Our results suggest that CD11b+ TIMs do not affect the quantitative levels of CD8+ TILs and CD163+ TAMs in distal bile duct cancer.
Some studies suggest that CD11b+ TIMs contribute to a poor prognosis in solid cancers, including gastric cancer, and are associated with recurrence after chemoradiotherapy in head and neck cancer [20,24]. We also found that the patients with high levels of CD11b+ TIMs had reduced OS (p = 0.042).
High levels of CD8+ TILs are a good prognostic marker in various solid cancers [42,43]. We also identified that the patients with high levels of CD8+ TILs had a favorable prognosis (p = 0.003). OS in the patients with low CD11b+ TIMs and high CD8+TILs was significantly different from that in the patients with high CD11b+ TIMs and high CD8+TILs (p < 0.001). Moreover, the patients with combined low CD11b+ TIMs and high CD8+ TILs showed the best OS compared to the patients in the other combined groups. Based on our data, we concluded that the CD8+ T-cell antitumor effect could be hindered by CD11b+ TIMs in distal bile duct cancer.
According to the possible mechanism by which CD11b+ TIM suppresses the tumoricidal effect of CD8+ TILs, CD11b+ myeloid-derived suppressor cells regulate the entry of activated CD8+ TILs into the tumor site. However, our results in distal bile duct cancer indicate that the levels of CD11b+ TIMs do not significantly affect the number of CD8+ TILs.
In line with our results, Yu et al. demonstrated that activated antigen-specific Fas+CD8+ T-cells undergo apoptosis following interaction with FasL+CD11b+F4/80+ monocyte-derived macrophages that contributed to immune deserts in a murine model of liver metastasis. Thus, CD11b+ TIMs may hinder antitumor immunity by suppressing CD8+ T-cells [44]. Furthermore, another potential mechanism involves CD11b+IL-4Rα+ monocytes. These cells, activated by IFN-γ from T cells, produce IL-13 and IFN-γ, suppressing antigen-activated CD8+ T cells [45]. This immunosuppressive action reduces immune surveillance and the effectiveness of immunotherapy, highlighting the need to target CD11b+ myeloid cells to restore T-cell function and improve treatment outcomes.
Unlike our results, Duong et al. reported that CD11b+ conventional dendritic cells activated by type 1 interferon promote antitumor CD8+ T-cell immunity [46]. Moreover, CD11b activation facilitates pro-inflammatory macrophage polarization by inducing the expression of microRNA Let-7a, demonstrated in mouse models of melanoma, breast, and lung cancer. In contrast, inhibiting CD11b reduces Let-7a levels and increases cMyc expression, driving immune suppressive macrophage polarization, enhancing vascular maturation, and promoting faster tumor growth [21]. These conflicting results could stem from the heterogeneity of CD11b+ myeloid cells because CD11b expression is observed in various inflammatory cells, including tumor-infiltrating monocytes, TAMs, granulocytes, and dendritic cells, and rarely, T-cells, B-cells, and NK and NK-T cells [37]. TIIC proportions in each tumor can vary according to the type of tumor [47]. We assume that the conflicting role of CD11b+ myeloid cells might originate from the differences in dominancy between tumor immune suppression or the antitumor immunity of CD11b+ myeloid cells.
The first limitation of our study is that we could not evaluate the sub-lineages of CD11b+ TIMs. As previously mentioned, various myeloid cells, including macrophages, monocytes, neutrophils, and some dendritic cells, even lymphoid cells, can express CD11b [37]. Further studies are needed to clarify the subpopulations of CD11b+ TIMs that play a significant prognostic role and to determine how to modulate T-cell immunity in distal bile duct cancer. A second limitation is that the study design is non-randomized and retrospective. This resulted in a relatively small sample size, which hindered the ability to perform power calculations for the appropriate number of patients. Consequently, the cut-off values for CD11b+ TIMs and CD8+ TILs in this study may not be definitive. Further large-scale, prospective validation studies are needed to establish optimized cut-off values. Despite our study limitation, we believe that evaluating levels of CD11b+ TIMs and CD8+ TILs is helpful in predicting prognosis in patients with distal bile duct cancer.
5. Conclusions
In this study, TIICs, including CD11b+ TIMs and CD8+ TILs, showed a significant prognostic role in distal bile duct cancer. Of note, we found that the combination of a low level of CD11b+ TIMs with a high level of CD8+ TILs was an independent prognostic factor in distal bile duct cancer; moreover, CD11b+ TIMs may hinder the antitumor activity of CD8+ T-cells. Overall, our data suggest that the control of CD11b+ TIMs could be one of the keys to the antitumor effect in distal bile duct cancer.
Abbreviations
The following abbreviations are used in this manuscript:
| TICC | Tumor-infiltrating immune cells |
| TIM | Tumor-infiltrating myeloid cell |
| TAM | Tumor-infiltrating macrophages |
| TIL | Tumor-infiltrating lymphocytes |
| OS | Overall survival |
| TMA | Tissue microarray |
| pT | The pathologic primary tumor stage |
| pN | The pathologic regional lymph node stage |
Author Contributions
Conceptualization, S.A.H. and M.Y.L.; methodology, J.H.C., J.Y.K., G.Y.L. and M.H.; software, H.W.H. and G.Y.L.; validation, S.A.H. and K.R.L.; formal analysis, J.H.C. and J.Y.K.; investigation, J.H.C. and J.Y.K.; resources, M.H. and H.W.H.; data curation, K.R.L. and M.K.K.; writing—original draft preparation, J.H.C. and J.Y.K.; writing—review and editing, S.A.H. and M.Y.L.; visualization, S.A.H.; supervision, M.Y.L.; project administration, S.A.H. and M.Y.L.; funding acquisition, S.A.H. and M.Y.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Chung-Ang University Hospital (IRB No. 2406-014-63).
Informed Consent Statement
The requirement for informed consent was waived due to the retrospective design of this study.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was supported by the Chung-Ang University Research Scholarship Grant in 2021 and a grant from the MD-PhD/Medicine Scientist Training Program through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Razumilava N., Gores G.J. Cholangiocarcinoma. Lancet. 2014;383:2168–2179. doi: 10.1016/S0140-6736(13)61903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brindley P.J., Bachini M., Ilyas S.I., Khan S.A., Loukas A., Sirica A.E., Teh B.T., Wongkham S., Gores G.J. Cholangiocarcinoma. Nat. Rev. Dis. Primers. 2021;7:65. doi: 10.1038/s41572-021-00300-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y., Liu S., Wu L., Wan T. Survival after surgical resection of distal cholangiocarcinoma: A systematic review and meta-analysis of prognostic factors. Asian J. Surg. 2017;40:129–138. doi: 10.1016/j.asjsur.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Jang J.-Y., Kim S.-W., Park D.J., Ahn Y.J., Yoon Y.-S., Choi M.G., Suh K.-S., Lee K.U., Park Y.-H. Actual Long-term Outcome of Extrahepatic Bile Duct Cancer After Surgical Resection. Ann. Surg. 2005;241:77–84. doi: 10.1097/01.sla.0000150166.94732.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zugman M., Botrus G., Pestana R.C., Uson Junior P.L.S. Precision Medicine Targeting FGFR2 Genomic Alterations in Advanced Cholangiocarcinoma: Current State and Future Perspectives. Front. Oncol. 2022;12:860453. doi: 10.3389/fonc.2022.860453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wheless M., Agarwal R., Goff L., Lockney N., Padmanabhan C., Heumann T. Current Standards, Multidisciplinary Approaches, and Future Directions in the Management of Extrahepatic Cholangiocarcinoma. Curr. Treat. Options Oncol. 2024;25:127–160. doi: 10.1007/s11864-023-01153-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen E.E.W., Bell R.B., Bifulco C.B., Burtness B., Gillison M.L., Harrington K.J., Le Q.T., Lee N.Y., Leidner R., Lewis R.L., et al. The Society for Immunotherapy of Cancer consensus statement on immunotherapy for the treatment of squamous cell carcinoma of the head and neck (HNSCC) J. ImmunoTherapy Cancer. 2019;7:184. doi: 10.1186/s40425-019-0662-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ralli M., Botticelli A., Visconti I.C., Angeletti D., Fiore M., Marchetti P., Lambiase A., de Vincentiis M., Greco A. Immunotherapy in the Treatment of Metastatic Melanoma: Current Knowledge and Future Directions. J. Immunol. Res. 2020;2020:9235638. doi: 10.1155/2020/9235638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U Gandhy S., Madan R.A., Aragon-Ching J.B. The immunotherapy revolution in genitourinary malignancies. Immunotherapy. 2020;12:819–831. doi: 10.2217/imt-2020-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lahiri A., Maji A., Potdar P.D., Singh N., Parikh P., Bisht B., Mukherjee A., Paul M.K. Lung cancer immunotherapy: Progress, pitfalls, and promises. Mol. Cancer. 2023;22:40. doi: 10.1186/s12943-023-01740-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lv B., Wang Y., Ma D., Cheng W., Liu J., Yong T., Chen H., Wang C. Immunotherapy: Reshape the Tumor Immune Microenvironment. Front. Immunol. 2022;13:844142. doi: 10.3389/fimmu.2022.844142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sadeghi Rad H., Monkman J., Warkiani M.E., Ladwa R., O’Byrne K., Rezaei N., Kulasinghe A. Understanding the tumor microenvironment for effective immunotherapy. Med. Res. Rev. 2021;41:1474–1498. doi: 10.1002/med.21765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Binnewies M., Roberts E.W., Kersten K., Chan V., Fearon D.F., Merad M., Coussens L.M., Gabrilovich D.I., Ostrand-Rosenberg S., Hedrick C.C. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 2018;24:541–550. doi: 10.1038/s41591-018-0014-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson N.M., Simon M.C. The tumor microenvironment. Curr. Biol. 2020;30:R921–R925. doi: 10.1016/j.cub.2020.06.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katsuta E., Rashid O.M., Takabe K. Clinical relevance of tumor microenvironment: Immune cells, vessels, and mouse models. Hum. Cell. 2020;33:930–937. doi: 10.1007/s13577-020-00380-4. [DOI] [PubMed] [Google Scholar]
- 16.Tiwari A., Trivedi R., Lin S.-Y. Tumor microenvironment: Barrier or opportunity towards effective cancer therapy. J. Biomed. Sci. 2022;29:83. doi: 10.1186/s12929-022-00866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ni L., Xu J., Li Q., Ge X., Wang F., Deng X., Miao L. Focusing on the Immune Cells: Recent Advances in Immunotherapy for Biliary Tract Cancer. Cancer Manag. Res. 2024;16:941–963. doi: 10.2147/CMAR.S474348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chellappa S., Hugenschmidt H., Hagness M., Subramani S., Melum E., Line P.D., Labori K.-J., Wiedswang G., Taskén K., Aandahl E.M. CD8+ T Cells That Coexpress RORγt and T-bet Are Functionally Impaired and Expand in Patients with Distal Bile Duct Cancer. J. Immunol. 2017;198:1729–1739. doi: 10.4049/jimmunol.1600061. [DOI] [PubMed] [Google Scholar]
- 19.Miyahara Y., Takashi S., Shimizu Y., Ohtsuka M. The prognostic impact of neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR) in patients with distal bile duct cancer. World J. Surg. Oncol. 2020;18:78. doi: 10.1186/s12957-020-01847-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balermpas P., Rödel F., Liberz R., Oppermann J., Wagenblast J., Ghanaati S., Harter P.N., Mittelbronn M., Weiss C., Rödel C., et al. Head and neck cancer relapse after chemoradiotherapy correlates with CD163+ macrophages in primary tumour and CD11b+ myeloid cells in recurrences. Br. J. Cancer. 2014;111:1509–1518. doi: 10.1038/bjc.2014.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmid M.C., Khan S.Q., Kaneda M.M., Pathria P., Shepard R., Louis T.L., Anand S., Woo G., Leem C., Faridi M.H., et al. Integrin CD11b activation drives anti-tumor innate immunity. Nat. Commun. 2018;9:5379. doi: 10.1038/s41467-018-07387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Q.-Q., Hu X.-W., Liu Y.-L., Ye Z.-J., Gui Y.-H., Zhou D.-L., Qi C.-L., He X.-D., Wang H., Wang L.-J. CD11b deficiency suppresses intestinal tumor growth by reducing myeloid cell recruitment. Sci. Rep. 2015;5:15948. doi: 10.1038/srep15948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kudo-Saito C., Ogiwara Y., Imazeki H., Boku N., Uemura Y., Zhang R., Kawano-Nagatsuma A., Kojima M., Ochiai A. CD11b+ DIP2A+ LAG3+ cells facilitate immune dysfunction in colorectal cancer. Am. J. Cancer Res. 2021;11:5428. [PMC free article] [PubMed] [Google Scholar]
- 24.Okita Y., Tanaka H., Ohira M., Muguruma K., Kubo N., Watanabe M., Fukushima W., Hirakawa K. Role of tumor-infiltrating CD11b+ antigen-presenting cells in the progression of gastric cancer. J. Surg. Res. 2014;186:192–200. doi: 10.1016/j.jss.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 25.Jiang Y., Wang C., Wang Y., Zhang W., Liu L., Cheng J. Prognostic role of CD11b+ myeloid-derived suppressor cells in oral squamous cell carcinoma. Arch. Med. Sci. 2021;19:171–179. doi: 10.5114/aoms/116683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang C., Li L., Ye Z., Zhang A., Bao Y., Wu X., Ren G., Jiang C., Wang O., Wang Z. Mechanisms underlying neutrophils adhesion to triple-negative breast cancer cells via CD11b-ICAM1 in promoting breast cancer progression. Cell Commun. Signal. 2024;22:340. doi: 10.1186/s12964-024-01716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahin U., Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359:1355–1360. doi: 10.1126/science.aar7112. [DOI] [PubMed] [Google Scholar]
- 28.Jenkins R.W., Barbie D.A., Flaherty K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br. J. Cancer. 2018;118:9–16. doi: 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xue D., Hsu E., Fu Y.-X., Peng H. Next-generation cytokines for cancer immunotherapy. Antib. Ther. 2021;4:123–133. doi: 10.1093/abt/tbab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen M.-H., Atenafu E., Craddock K.J., Brandwein J., Chang H. CD11b expression correlates with monosomal karyotype and predicts an extremely poor prognosis in cytogenetically unfavorable acute myeloid leukemia. Leuk. Res. 2013;37:122–128. doi: 10.1016/j.leukres.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 31.Saed G.M., Fletcher N.M., Diamond M.P., Morris R.T., Gomez-Lopez N., Memaj I. Novel expression of CD11b in epithelial ovarian cancer: Potential therapeutic target. Gynecol. Oncol. 2018;148:567–575. doi: 10.1016/j.ygyno.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 32.Liu C.-Y., Wang Y.-M., Wang C.-L., Feng P.-H., Ko H.-W., Liu Y.-H., Wu Y.-C., Chu Y., Chung F.-T., Kuo C.-H., et al. Population alterations of l-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14−/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2009;136:35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PubMed] [Google Scholar]
- 33.Daurkin I., Eruslanov E., Vieweg J., Kusmartsev S. Generation of antigen-presenting cells from tumor-infiltrated CD11b myeloid cells with DNA demethylating agent 5-aza-2′-deoxycytidine. Cancer Immunol. Immunother. 2009;59:697–706. doi: 10.1007/s00262-009-0786-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ahn G.O., Tseng D., Liao C.-H., Dorie M.J., Czechowicz A., Brown J.M. Inhibition of Mac-1 (CD11b/CD18) enhances tumor response to radiation by reducing myeloid cell recruitment. Proc. Natl. Acad. Sci. USA. 2010;107:8363–8368. doi: 10.1073/pnas.0911378107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mizutani K., Sud S., Pienta K.J. Prostate cancer promotes CD11b positive cells to differentiate into osteoclasts. J. Cell. Biochem. 2009;106:563–569. doi: 10.1002/jcb.22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roche V., Sandoval V., Wolford C., Senders Z., Kim J.A., Ribeiro S.P., Huang A.Y., Sekaly R.-P., Lyons J., Zhang M. Carbohydrate ligand engagement with CD11b enhances differentiation of tumor-associated myeloid cells for immunotherapy of solid cancers. J. ImmunoTherapy Cancer. 2023;11:e006205. doi: 10.1136/jitc-2022-006205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panni R.Z., Herndon J.M., Zuo C., Hegde S., Hogg G.D., Knolhoff B.L., Breden M.A., Li X., Krisnawan V.E., Khan S.Q., et al. Agonism of CD11b reprograms innate immunity to sensitize pancreatic cancer to immunotherapies. Sci. Transl. Med. 2019;11:eaau9240. doi: 10.1126/scitranslmed.aau9240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kinoshita J., Fushida S., Yamaguchi T., Moriyama H., Saito H., Shimada M., Terai S., Okamoto K., Nakamura K., Ninomiya I., et al. Prognostic value of tumor-infiltrating CD163+macrophage in patients with metastatic gastric cancer undergoing multidisciplinary treatment. BMC Cancer. 2022;22:608. doi: 10.1186/s12885-022-09713-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ni C., Yang L., Xu Q., Yuan H., Wang W., Xia W., Gong D., Zhang W., Yu K. CD68- and CD163-positive tumor infiltrating macrophages in non-metastatic breast cancer: A retrospective study and meta-analysis. J. Cancer. 2019;10:4463–4472. doi: 10.7150/jca.33914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miura T., Yoshizawa T., Hirai H., Seino H., Morohashi S., Wu Y., Wakiya T., Kimura N., Kudo D., Ishido K., et al. Prognostic Impact of CD163+ Macrophages in Tumor Stroma and CD8+ T-Cells in Cancer Cell Nests in Invasive Extrahepatic Bile Duct Cancer. Anticancer Res. 2017;37:183–190. doi: 10.21873/anticanres.11304. [DOI] [PubMed] [Google Scholar]
- 41.Norian L.A., Rodriguez P.C., O’Mara L.A., Zabaleta J., Ochoa A.C., Cella M., Allen P.M. Tumor-Infiltrating Regulatory Dendritic Cells Inhibit CD8+ T Cell Function via l-Arginine Metabolism. Cancer Res. 2009;69:3086–3094. doi: 10.1158/0008-5472.CAN-08-2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hong S.A., Hwang H.W., Kim M.K., Lee T.J., Yim K., Won H.S., Sun D.S., Kim E.Y., Ko Y.H. High endothelial venule with concomitant high CD8+ tumor-infiltrating lymphocytes is associated with a favorable prognosis in resected gastric cancer. J. Clin. Med. 2020;9:2628. doi: 10.3390/jcm9082628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raskov H., Orhan A., Christensen J.P., Gögenur I. Cytotoxic CD8+ T cells in cancer and cancer immunotherapy. Br. J. Cancer. 2020;124:359–367. doi: 10.1038/s41416-020-01048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu J., Green M.D., Li S., Sun Y., Journey S.N., Choi J.E., Rizvi S.M., Qin A., Waninger J.J., Lang X., et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat. Med. 2021;27:152–164. doi: 10.1038/s41591-020-1131-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gallina G., Dolcetti L., Serafini P., De Santo C., Marigo I., Colombo M.P., Basso G., Brombacher F., Borrello I., Zanovello P., et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J. Clin. Investig. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duong E., Fessenden T.B., Lutz E., Dinter T., Yim L., Blatt S., Bhutkar A., Wittrup K.D., Spranger S. Type I interferon activates MHC class I-dressed CD11b+ conventional dendritic cells to promote protective anti-tumor CD8+ T cell immunity. Immunity. 2022;55:308–323.e309. doi: 10.1016/j.immuni.2021.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thorsson V., Gibbs D.L., Brown S.D., Wolf D., Bortone D.S., Ou Yang T.H., Porta-Pardo E., Gao G.F., Plaisier C.L., Eddy J.A., et al. The Immune Landscape of Cancer. Immunity. 2018;48:812–830.e814. doi: 10.1016/j.immuni.2018.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.