Abstract
Onychomycosis is a common fungal infection that affects the nails and accounts for approximately 50% of all nail diseases. The main pathogens involved include dermatophytes, such as Trichophyton rubrum, members of the T. mentagrophytes complex, and emerging pathogens in this infection, T. schoenleinii and T. tonsurans. Tea tree (Melaleuca alternifolia Cheel) essential oil (EO) has been proposed as a promising natural alternative to traditional treatments due to its antimicrobial properties. Among its more than 100 compounds, terpinen-4-ol is one of the main contributors to the antifungal action of this EO. To determine the antifungal activity of tea tree EO against dermatophytes, we designed an in vitro study using EUCAST-AFST protocols to obtain the values of MIC (minimum inhibitory concentration) and MFC (minimum fungicidal concentration) of several commercial M. alternifolia Cheel EOs against three species of dermatophytes isolated from clinical samples with suspected toenail onychomycosis. The results showed that the microorganism most sensitive to the action of the EO was T. rubrum, which had an MIC value more than 13 times lower than the value obtained for T. schoenleinii (0.4% v/v), the most resistant isolate. No differences in antifungal activity were observed by the analysed EOs or between the MIC and MFC values. These in vitro results suggest that tea tree EO is a viable option for the alternative treatment of onychomycosis, although clinical studies are needed to confirm the long-term antifungal activity, safety and efficacy of the oils studied in a clinical context.
Keywords: antifungal activity, clinical isolates, dermatophytes, essential oil, fungal infection, onychomycosis
1. Introduction
Onychomycosis is a fungal infection affecting the nails, and one of the most common nail disorders. It is thought to account for approximately 50% of all nail diseases and has an estimated global prevalence of around 5.5% [1,2,3]. The microorganisms most frequently associated with onychomycosis include dermatophytes, such as Trichophyton rubrum, and members of the T. mentagrophytes complex, and yeasts of the genus Candida and non-dermatophyte moulds, such as Scopulariopsis brevicaulis and Aspergillus spp. [4,5,6,7,8]. In the genus Trichophyton, the species T. schoenleinii and T. tonsurans [9,10,11,12] are frequently identified. Both of these are responsible for tinea capitis (scalp ringworm), as they are able to colonise and degrade keratinised tissues of the human body [13].
Nail infections can cause nail deformities, pain, and discomfort [14,15]. Onychomycosis has a considerable psychological impact on self-image and can affect self-esteem [16]. It is also hard to treat because of the keratinic nature of the infected tissue, which is difficult for antifungal agents to penetrate [17].
In the search for effective and natural novel treatments against onychomycosis, tea tree (Melaleuca alternifolia Cheel) essential oil (EO) has proven to be a promising solution. It has proven antimicrobial properties, and its use is supported by growing scientific evidence [18,19,20,21]. Although several species of the genus Melaleuca are suitable for this type of treatment, M. alternifolia Cheel is the most frequently used because of the high concentration of terpinen-4-ol in its EO [22,23], one of the main reasons for its antifungal activity [24]. Another reason for the frequent use of this EO is its efficacy against a wide range of microorganisms, including those that cause onychomycosis [25,26]. This compound, together with alpha-terpineol and eucalyptol (also known as 1.8-cineole), are known to increase the cell membrane permeability of fungi and alter mycelial morphology and cell ultrastructure [27].
The marketing and composition of tea tree EO are regulated by international standards. ISO 4730:2017/Amd 1:2018 [28] states the requirements for Melaleuca spp. EO and determines the quality criteria for its sale, including the chemical composition, impurity levels and physical parameters.
Several studies have analysed the antifungal activity of M. alternifolia Cheel EO and established the values of MIC (minimum inhibitory concentration) and MFC (minimum fungicidal concentration) against various dermatophytes [18,24,29,30], using reference strains of the main infectious agents [31,32]. However, little information is available about clinical isolates obtained during examination of the feet. The aim of this study is to determine the MIC and MFC of three commercial M. alternifolia Cheel EO against clinical isolates of the main causative agents of onychomycosis of the genus Trichophyton. This will help to define the initial procedures before clinical trials are conducted to assess the potential of EO therapies as alternative or complementary treatments to traditional antifungal approaches.
2. Materials and Methods
2.1. Origin and Composition of Essential Oils
Three commercial M. alternifolia Cheel EO (Esencias Lozano®, Naissance® and Marnys®) were used. The three companies are based in Europe and have online stores: Esencias Lozano® in Caravaca de la Cruz, Spain; Naissance® in Neath, United Kingdom; and Marnys® in Cartagena, Spain.
Essential oil composition was analysed using the information provided by the commercial brands. All EO had a terpinene-4-ol percentage higher than 40%, and the compounds alpha-terpineol and eucalyptus did not exceed 4.41% (Table 1). All concentrations were within the ranges defined by ISO 4730:2017/Amd 1:2018 [28] and were indicated in the product information sheets.
Table 1.
Concentration of the EO compounds with antifungal activity obtained by gas chromatography coupled with mass spectrometry according to the product information sheets.
| Component | Naissence® | Marnys® | Esencias Lozano® |
|---|---|---|---|
| Terpinen-4-ol | 41.90% | 44.84% | 40.76% |
| Alpha terpineol | n.d. | 2.78% | 4.41% |
| Eucalyptol | 2.30% | 4.23% | 1.74% |
| Para Mentha-1,4-Diene | 22.20% | n.d. | n.d. |
| Alpha Terpinene | 10.90% | 10.92% | 10.10% |
| Gammaterpinene | n.d. | 22.31% | 20.23% |
| Alpha Terpinolene | 3.0% | n.d. | n.d. |
| Terpinolene | n.d. | 2.77% | 3.17% |
| Alpha Pinene | 3.90% | 3.94% | 2.17% |
| Beta Pinene | n.d. | 0.38% | n.d. |
| Para Cymene | 3.60% | 2.72% | 2.44% |
| Limonene | 2.80% | 2.67% | n.d. |
| Sabinene | 0.20% | 0.24% | n.d. |
| Aromadendrene | 0.70% | 2.13% | 0.45% |
| Aromadendrene Isomer | n.d. | 0.09% | n.d. |
| Allo Aromandendrene | n.d. | 0.31% | n.d. |
| Cadinene | n.d. | 0.01% | n.d. |
| Delta Cadineno | n.d. | n.d. | 1.62% |
| Globulol | n.d. | 0.24% | n.d. |
| Viridiflorol | n.d. | 0.06% | n.d. |
| Para Ment-3-Ene | n.d. | 0.08% | n.d. |
| Beta Myrcene | n.d. | 0.07% | n.d. |
| Alpha Phellandrene | n.d. | 0.15% | n.d. |
| Linalool | n.d. | 0.03% | n.d. |
| Beta Caryophyllene | n.d. | 0.17% | 0.27% |
| Cis-P-Menth-2-En-1-Ol | n.d. | 0.07% | n.d. |
| Cadina-3,5-Diene | n.d. | 0.23% | n.d. |
| Zonarene | n.d. | 0.04% | n.d. |
| Alpha Humulene + Cis-Piperitol | n.d. | 0.05% | n.d. |
| Neral | n.d. | 0.05% | n.d. |
| Ledene | n.d. | 0.78% | n.d. |
| Alpha Muurolene + B-Selinene | n.d. | 0.09% | n.d. |
| Geranial + A-Selinene | n.d. | 0.14% | n.d. |
| Spathulenol | n.d. | 0.00% | n.d. |
®: trademark; %: percentage; n.d.: no data available.
2.2. Microbial Strains and Inoculum Preparation
The clinical isolates used were obtained during examination of the feet of three patients with suspected onychomycosis and handled at the University Centre of Plasencia. The study comprised samples taken from three women with average age 55.7 years (±7.4 years) and mean body mass index 25.5 kg/m2 (±2.4 kg/m2). Participants had no history of toenail fungal infection and no concomitant illnesses.
Toenail tissue samples were taken following Pérez Pico et al. [33]. Samples were observed under conventional light microscopy with 30% (p/v) KOH solution (Labbox, Barcelona, Spain) to determine the presence of dermatophytes. The species of Trichophyton were identified using lactophenol blue solution (Labbox, Barcelona, Spain), following [34,35]. Microbiological cultures of each sample were grown in triplicate in Sabouraud dextrose agar selective medium with chloramphenicol and cycloheximide (Condalab, Torrejón de Ardoz, Spain), incubated at a constant temperature of 25–28 °C for 2–6 weeks.
The T. schoenleinii, T. tonsurans, and T. rubrum isolates were subcultured in Sabouraud dextrose agar (Condalab, Madrid, Spain) supplemented with cycloheximide 300 mg/L and chloramphenicol 50 mg/L and incubated 7–10 days at 30 °C to adequate sporulation.
2.3. Antifungal Susceptibility Testing
The MIC values were determined following the protocols of the European Committee on Antimicrobial Susceptibility Testing Subcommittee on Antifungal Susceptibility Testing (EUCAST-AFST) for dermatophytes [36]. The EO concentrations analysed were from 0.01% (v/v) to 1.25% (v/v), dissolved in dimethyl sulfoxide (DMSO; PanReac AppliChem, Barcelona, Spain) at a final concentration of 2% (v/v). The RPMI 1640 culture medium with L-glutamine (Sigma-Aldrich, Taufkirchen, Germany) supplemented with 2% (w/v) glucose (PanReac AppliChem, Barcelona, Spain) and buffered with 3-(N-morpholino) propanesulfonic acid (MOPS; Sigma-Aldrich, Taufkirchen, Germany) was inoculated with a suspension adjusted to 2–5 × 105 CFU/mL, supplemented with cycloheximide 300 mg/L and chloramphenicol 50 mg/L (PanReac AppliChem, Barcelona, Spain). Microdilutions were incubated for 7 days at 30 °C without agitation until they were read. MIC was established as the lowest EO concentration in mg/L that inhibited fungal growth [36].
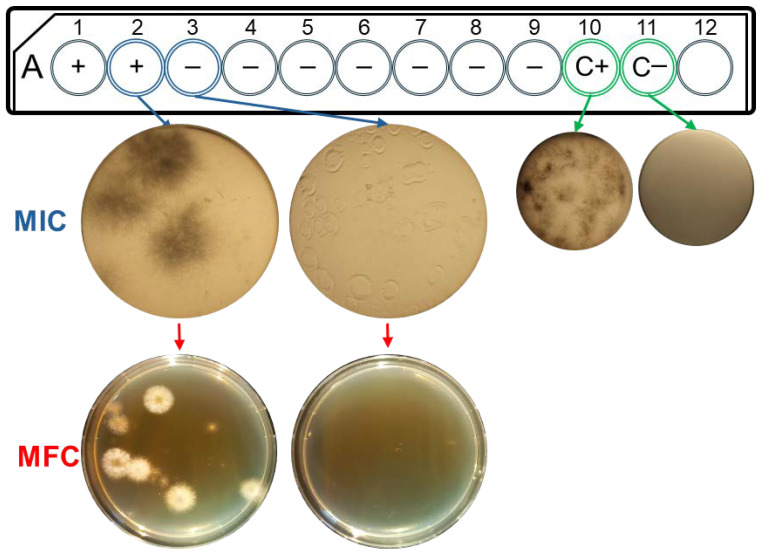
To determine MFC, 10 µL from wells without growth was inoculated onto Sabouraud dextrose agar medium (Condalab, Madrid, Spain), supplemented with cycloheximide 300 mg/L and chloramphenicol 50 mg/L, and incubated for 2 days at 30 °C [37,38]. Minimum inhibitory concentration was established as the lowest EO concentration without fungal growth (Figure 1). All experiments were performed independently in triplicate.
Figure 1.
Antifungal susceptibility testing of T. tonsurans against Esencias Lozano® M. alternifolia Cheel tea tree EO. A: diagram of microdilution plates with well numbers (1-12); +: with fungal growth; −: without fungal growth; C+: positive growth control; C−: negative growth control. MIC: minimum inhibitory concentration in well microdilution plate. Images taken at 2.5× for greater detail. MFC: minimum fungicidal concentration in Petri dishes.
3. Results
Antifungal Susceptibility Results
The antifungal activity of the three EO was analysed by determining the MIC and MFC values of the clinical isolates (Figure 1). All isolates were sensitive to EO concentrations lower than 0.5% (v/v). T. schoenleinii was the most resistant (0.4% v/v), and T. rubrum (0.03% v/v) was the most sensitive. Therefore, the microorganisms showed different responses to M. alternifolia Cheel tea tree EO. However, no differences were detected in MIC or MFC between the three commercial EOs tested, as they all showed the same result (Table 2).
Table 2.
Antifungal activity of commercial M. alternifolia Cheel essential oils.
| Strain | Units | Esencias Lozano® | Naissance® | Marnys® | |||
|---|---|---|---|---|---|---|---|
| MIC | MFC | MIC | MFC | MIC | MFC | ||
| T. schoenleinii | % (v/v) | 0.4 3.582 |
0.4 3.582 |
0.4 3.582 |
0.4 3.582 |
0.4 3.582 |
0.4 3.582 |
| mg/L | |||||||
| T. tonsurans | % (v/v) | 0.09 805.95 |
0.09 805.95 |
0.09 805.95 |
0.09 805.95 |
0.09 805.95 |
0.09 805.95 |
| mg/L | |||||||
| T. rubrum | % (v/v) | 0.03 268.65 |
0.03 268.65 |
0.03 268.65 |
0.03 268.65 |
0.03 268.65 |
0.03 268.65 |
| mg/L | |||||||
®: Registered trademark; MIC: minimum inhibitory concentration; MFC: minimum fungicidal concentration.
4. Discussion
The results of this research demonstrate the in vitro efficacy of tea tree EO in inhibiting the growth of microorganisms that cause toenail onychomycosis, assessed by determining the MIC and MFC of three samples from clinical isolates. The antifungal activity observed can be attributed to certain compounds of the EO, especially terpinen-4-ol, alpha-terpineol, and eucalyptol, as described elsewhere [23,27,28]. All three commercial EOs analysed complied with ISO 4730:2017/Amd 1:2018 [28], which defines the amounts of the terpinen-4-ol component (not less than 35%) and eucalyptol (15% or more) in the EO. This standard also establishes a maximum and minimum range for other tea tree EO compounds to ensure that the product characteristics are homogeneous, safe, and good-quality.
Studies by Roana et al. [21] and Hammer et al. [39] on the efficacy of M. alternifolia Cheel EO against T. rubrum in onychomycosis clinical samples reported 0.06–0.3% MIC and 0.06–0.25% MFC. In contrast, our study found 0.03% MIC and MFC, indicating a greater antifungal efficacy of the three EOs analysed. These discrepancies could reflect differences in the testing methodology, as the methods to determine antifungal activity differed between the three studies. They may also be explained by the chemotype of the EO used, especially the terpinen-4-ol concentration. Roana et al. [21] used a different concentration from ours, and Hammer et al. [39] did not indicate the concentration. These and other variables highlight the difficulty of obtaining comparable and reproducible results using different standards. The proven ability of the analysed EO to act at low concentrations would reduce the risk of side effects. These EOs could be an attractive choice for patients seeking treatments with fewer adverse effects, polymedicated patients, or those who are resistant or intolerant to synthetic antifungal treatments [40,41]. Because the literature does not mention the MIC and MFC of tea tree EO against clinical isolates of T. schoenleinii and T. tonsurans, the results reported in this study are a novel contribution.
The lack of differences detected in MIC and MFC among the commercial EO tested suggests homogeneity in the composition and quality of the products available on the market, which are subject to standardisation regulations [28]. This is crucial to ensure therapeutic efficacy in the use of tea tree EO to treat onychomycosis, provided that quality standards are maintained [28,42].
The higher resistance of T. schoenleinii and T. tonsurans compared to T. rubrum could be associated with differences in the cell wall and the structure of the membrane. The first two microorganisms are larger under the microscope and have a thicker, more partitioned cell wall than T. rubrum. These factors influence susceptibility to antifungal agents [43,44]. Efflux pumps identified in the plasmatic membrane of members of the Trichophyton genus are responsible for resistance to multiple antifungal therapies. In T. rubrum, differences in expression detected in function of the presence of antifungal agents could explain the differences in antifungal activity among species of Trichophyton [45]. Although T. rubrum is the dermatophyte most frequently associated with onychomycosis (8), T. schoenleinii and T. tonsurans are becoming more prevalent in toenail infections [46]. This suggests the adaptation of these species for infection, at least in toenails. Differences in geographical distribution between species of this genus must also be taken into account [47].
Commercial tea tree EO shows considerable potential as an alternative antifungal treatment for dermatophyte onychomycosis, with demonstrated efficacy in vitro. The results provide the information needed to commence the next stage of clinical trials to assess the activity of these commercial EOs in vivo. Clinical studies will determine the long-term safety and efficacy of the commercial EOs studied, both individually and in combination with other antifungal treatments. They will also help to define the formula for topical administration of the EO to provide a comprehensive, alternative solution for managing onychomycosis.
Acknowledgments
We thank Jane McGrath for assistance with the translation and final language review.
Author Contributions
Conceptualization, E.M.Á., J.V.R. and R.M.; methodology, E.M.Á. and J.V.R.; software, E.M.Á., J.V.R. and O.L.R.; validation, E.M.Á., J.V.R., O.L.R. and R.M.; formal analysis, E.M.Á.; investigation, E.M.Á., J.V.R., O.L.R. and R.M.; resources, R.M.; data curation, E.M.Á., J.V.R., O.L.R. and R.M.; writing—original draft preparation, E.M.Á. and J.V.R.; writing—review and editing, E.M.Á., J.V.R., O.L.R. and R.M.; supervision, R.M.; project administration, R.M.; funding acquisition, R.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Universidad de Extremadura (Ref. 195/2022).
Informed Consent Statement
Written informed consent was obtained from all participants to publish this paper.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This study was funded by the Extremadura Regional Government and the European Regional Development Fund (ERDF) through a grant to the research group (code CTS020, reference GR21077).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Lipner S.R., Scher R.K. Onychomycosis: Clinical overview and diagnosis. J. Am. Acad. Dermatol. 2019;80:835–851. doi: 10.1016/j.jaad.2018.03.062. [DOI] [PubMed] [Google Scholar]
- 2.Lee D.K., Lipner S.R. Optimal diagnosis and management of common nail disorders. Ann. Med. 2022;54:694–712. doi: 10.1080/07853890.2022.2044511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker B.A., Childress M.A. Common Foot Problems: Over-the-Counter Treatments and Home Care. Am. Fam. Physician. 2018;98:298–303. [PubMed] [Google Scholar]
- 4.Findley K., Oh J., Yang J., Conlan S., Deming C., Meyer J.A., Schoenfeld A., Nomicos E., Park M., NIH Intramural Sequencing Center Comparative Sequencing Program et al. H. Topographic diversity of fungal and bacterial communities in human skin. Nature. 2013;498:367–370. doi: 10.1038/nature12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhatia V.K., Sharma P.C. Epidemiological studies on dermatophytosis in human patients in Himachal Pradesh. Springerplus. 2014;3:134. doi: 10.1186/2193-1801-3-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teixeira de Aguilar Péres N., Albuquerque Maranhão F.C., Rossi A., Martinez-Rossi N.M. Dermatófitos: Interação patógeno-hospedeiro e resistência a antifúngicos. An. Bras. Dermatol. 2010;85:657–667. doi: 10.1590/S0365-05962010000500009. [DOI] [PubMed] [Google Scholar]
- 7.Hube B., Hay R., Brasch J., Veraldi S., Schaller M. Dermatomycoses and inflammation: The adaptive balance between growth, damage, and survival. J. Mycol. Med. 2015;25:44–58. doi: 10.1016/j.mycmed.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Joyce A., Gupta A.K., Koenig L., Wolcott R., Carviel J. Fungal diversity and onychomycosis. J. Am. Podiatr. Med. Assoc. 2019;109:57–63. doi: 10.7547/17-070. [DOI] [PubMed] [Google Scholar]
- 9.Macura A.B., Krzyściak P., Skóra M., Gniadek A. Case report: Onychomycosis due to Trichophyton schoenleinii. Mycoses. 2012;55:e18–e19. doi: 10.1111/j.1439-0507.2011.02072.x. [DOI] [PubMed] [Google Scholar]
- 10.Brillowska-Dąbrowska A., Saunte D.M., Arendrup M.C. Five-Hour Diagnosis of Dermatophyte Nail Infections with Specific Detection of Trichophyton rubrum. J. Clin. Microbiol. 2007;45:1200–1204. doi: 10.1128/JCM.02072-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bunyaratavej S., Bunyaratavej S., Muanprasart C., Matthapan L., Varothai S., Tangjaturonrusamee C., Pattanaprichakul P. Endonyx onychomycosis caused by Trichophyton tonsurans. Indian J. Dermatol. Venereol. Leprol. 2015;81:390–392. doi: 10.4103/0378-6323.157460. [DOI] [PubMed] [Google Scholar]
- 12.Sato T., Kitahara H., Honda H., Katsukawa F., Hiruma M., Yaguchi T. Onychomycosis of the Middle Finger of a Japanese Judo Athlete due to Trichophyton tonsurans. Med. Mycol. J. 2019;60:1–4. doi: 10.3314/mmj.18-00012. [DOI] [PubMed] [Google Scholar]
- 13.Gao Y., Zhan P., Hagen F., Menken S.B.J., Sun J., Rezaei-Matehkolaei A., de Hoog S. Molecular epidemiology and in vitro antifungal susceptibility of Trichophyton schoenleinii, agent of tinea capitis favosa. Mycoses. 2019;62:466–474. doi: 10.1111/myc.12889. [DOI] [PubMed] [Google Scholar]
- 14.Hoy N.Y., Leung A.K.C., Metelitsa A.I., Adams S. New concepts in median nail dystrophy, onychomycosis, and hand, foot, and mouth disease nail pathology. ISRN Dermatol. 2012;64:680163. doi: 10.5402/2012/680163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gupta A.K., Gupta G., Jain H.C., Lynde C.W., Foley K.A., Daigle D., Cooper E.A., Summerbell R.C. The prevalence of unsuspected onychomycosis and its causative organisms in a multicentre Canadian sample of 30 000 patients visiting physicians’ offices. J. Eur. Acad. Dermatol. Venereol. 2016;30:1567–1572. doi: 10.1111/jdv.13677. [DOI] [PubMed] [Google Scholar]
- 16.Chacon A., Franca K., Fernandez A., Nouri K. Psychosocial impact of onychomycosis: A review. Int. J. Dermatol. 2013;52:1300–1307. doi: 10.1111/ijd.12122. [DOI] [PubMed] [Google Scholar]
- 17.Leung A.K.C., Lam J.M., Leong K.F., Hon K.L., Barankin B., Leung A.A.M., Wong A.H.C. Onychomycosis: An updated review. Recent. Pat. Inflamm. Allergy Drug Discov. 2020;14:32–45. doi: 10.2174/1872213X13666191026090713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Villar Rodríguez J., Pérez Pico A.M., Mingorance Álvarez E., Mayordomo Acevedo R. Meta-analysis of the antifungal activities of three essential oils as alternative therapies in dermatophytosis infections. J. Appl. Microbiol. 2022;133:241–253. doi: 10.1111/jam.15539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammer K.A., Carson C.F., Riley T.V. Antifungal activity of the components of Melaleuca alternifolia (tea tree) oil. J. Appl. Microbiol. 2003;95:853–860. doi: 10.1046/j.1365-2672.2003.02059.x. [DOI] [PubMed] [Google Scholar]
- 20.Carson C.F., Hammer K.A., Riley T.V. Melaleuca alternifolia (tea tree) oil: A review of antimicrobial and other medicinal properties. Clin. Microbiol. Rev. 2006;19:50–62. doi: 10.1128/CMR.19.1.50-62.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roana J., Mandras N., Scalas D., Campagna P. Antifungal Activity of Melaleuca alternifolia Essential Oil (TTO) and Its Synergy with Itraconazole or Ketoconazole against Trichophyton rubrum. Molecules. 2021;26:461. doi: 10.3390/molecules26020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Essential Oil of Melaleuca, Terpinen-4-ol Type (Tea Tree Oil) AMENDMENT 1: Enantiomeric Distribution. ISO-International Organization for Standardization; Geneva, Switzerland: 2018. [(accessed on 17 September 2024)]. Available online: https://www.iso.org/standard/74547.html. [Google Scholar]
- 23.Vázquez A., Tabanca N., Kendra P.E. HPTLC Analysis and Chemical Composition of Selected Melaleuca Essential Oils. Molecules. 2023;28:3925. doi: 10.3390/molecules28093925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D’agostino M., Tesse N., Frippiat J.P., Machouart M., Debourgogne A. Essential Oils and Their Natural Active Compounds Presenting Antifungal Properties. Molecules. 2019;24:3713. doi: 10.3390/molecules24203713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang S.-A., Jeon S.-K., Lee E.-J., Im N.-K., Jung J.-Y., Lee I.-S. Bioactivity and Chemical Composition of the Essential oil of Tea Tree (Melaleuca alternifolia) J. Life Sci. 2008;18:1644–1650. doi: 10.5352/JLS.2008.18.12.1644. [DOI] [Google Scholar]
- 26.Winkelman W.J. Aromatherapy, botanicals, and essential oils in acne. Clin. Dermatol. 2018;36:299–305. doi: 10.1016/j.clindermatol.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Yu D., Wang J., Shao X., Xu F., Wang H. Antifungal modes of action of tea tree oil and its two characteristic components against Botrytis cinerea. J. Appl. Microbiol. 2015;119:1253–1262. doi: 10.1111/jam.12939. [DOI] [PubMed] [Google Scholar]
- 28.Essential Oil of Melaleuca, Terpinen-4-Ol Type (Tea Tree Oil) International Organization for Standardization; Geneva, Switzerland: 2017. [(accessed on 17 September 2024)]. Available online: https://www.iso.org/standard/69082.html. [Google Scholar]
- 29.Abd Rashed A., Rathi D.-N.G., Ahmad Nasir N.A.H., Abd Rahman A.Z. Antifungal Properties of Essential Oils and Their Compounds for Application in Skin Fungal Infections: Conventional and Nonconventional Approaches. Molecules. 2021;26:1093. doi: 10.3390/molecules26041093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Da Cruz Cabral L., Pinto Fernadez V., Patriarca A. Application of plant derived compounds to control fungal spoilage and mycotoxin production in foods. Int. J. Food Microbiol. 2013;166:1–14. doi: 10.1016/j.ijfoodmicro.2013.05.026. [DOI] [PubMed] [Google Scholar]
- 31.Mahmoudvand H., Sepahvand A., Jahanbakhsh S., Ezatpour B., Ayatollahi Mousavi S.A. Evaluation of antifungal activities of the essential oil and various extracts of Nigella sativa and its main component, thymoquinone against pathogenic dermatophyte strains. J. Mycol. Med. 2014;24:e155–e161. doi: 10.1016/j.mycmed.2014.06.048. [DOI] [PubMed] [Google Scholar]
- 32.Mertas A., Garbusińska A., Szliszka E., Jureczko A., Kowalska M., Król W. The Influence of Tea Tree Oil (Melaleuca alternifolia) on Fluconazole Activity against Fluconazole-Resistant Candida albicans Strains. Biomed. Res. Int. 2015;2015:590470. doi: 10.1155/2015/590470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pérez Pico A.M., Mingorance Álvarez E., Pérez Luque C., Mayordomo Acevedo R. Adquisición de competencias para el diagnóstico de onicomicosis mediante entrenamiento práctico podológico preclínico y clínico. Educ. Med. Super. 2019;33:e1962. [Google Scholar]
- 34.Baran R., Haneke E., Hay R.J., Piraccini B.M., Tosti A. Onychomycosis. In: Baran R., Haneke E., Hay R.J., Piraccini B.M., Tosti A., editors. The Current Approach to Diagnosis and Therapy. 2nd ed. CRC Press; London, UK: 1999. [Google Scholar]
- 35.Koneman E.W., Roberts G.D. Micología Práctica de Laboratorio. 3rd ed. Editorial Panamericana; Buenos Aires, Argentina: 1987. [Google Scholar]
- 36.Arendrup M.C., Kahlmeter G., Guinea J., Meletiadis J. How to: Perform antifungal susceptibility testing of microconidia-forming dermatophytes following the new reference EUCAST method E.Def 11.0, exemplified by Trichophyton. Clin. Microbiol. Infect. 2021;27:55–60. doi: 10.1016/j.cmi.2020.08.042. [DOI] [PubMed] [Google Scholar]
- 37.Trifan A., Bostănaru A.-C., Luca S.V., Temml V., Akram M., Herdlinger S., Kulinowski Ł., Skalicka-Woźniak K., Granica S., Czerwińska M.E., et al. Honokiol and magnolol: Insights into their antidermatophytic effects. Plants. 2021;10:2522. doi: 10.3390/plants10112522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Trifan A., Luca S.V., Bostănaru A.-C., Brebu M., Jităreanu A., Romeo-Teodor C., Skalicka-Woźniak K., Granica S., Czerwińska M.E., Kruk A., et al. Apiaceae essential oils: Boosters of terbinafine activity against dermatophytes and potent anti-inflammatory effectors. Plants. 2021;10:2378. doi: 10.3390/plants10112378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammer K.A., Carson C.F., Riley T.V. In vitro activity of Melaleuca alternifolia (tea tree) oil against dermatophytes and other filamentous fungi. J. Antimicrob. Chemoth. 2002;50:195–199. doi: 10.1093/jac/dkf112. [DOI] [PubMed] [Google Scholar]
- 40.Gupta A.K., Mays R.R., Versteeg S.G., Shear N.H., Piguet V. Update on current approaches to diagnosis and treatment of onychomycosis. Expert. Rev. Anti Infect. Ther. 2018;16:929–938. doi: 10.1080/14787210.2018.1544891. [DOI] [PubMed] [Google Scholar]
- 41.Gupta A.K., Sibbald R.G., Andriessen A., Belley R., Boroditsky A., Botros M., Chelin R., Gulliver W., Keast D., Raman M. Toenail onychomycosis—A Canadian approach with a new transungual treatment: Development of a clinical pathway. J. Cutan. Med. Surg. 2015;19:440–449. doi: 10.1177/1203475415581310. [DOI] [PubMed] [Google Scholar]
- 42.Oil T. The European Cosmetics Association Colipa. Recommendations on Tea-Tree Oil. 2002. [(accessed on 17 September 2024)]. Available online: https://colipa.eu/european-cosmetics-association/
- 43.Flores F.C., Beck R.C.R., da Silva C.B. Essential oils for treatment for onychomycosis: A mini-review. Mycopathology. 2015;181:9–15. doi: 10.1007/s11046-015-9957-3. [DOI] [PubMed] [Google Scholar]
- 44.Pontón J. La pared celular de los hongos y el mecanismo de acción de la anidulafungina. Rev. Iberoam. Micol. 2008;25:78–82. doi: 10.1016/S1130-1406(08)70024-X. [DOI] [PubMed] [Google Scholar]
- 45.Martinez-Rossi N.M., Peres N.T.A., Rossi A. Antifungal resistance mechanisms in dermatophytes. Mycopathology. 2008;166:369–383. doi: 10.1007/s11046-008-9110-7. [DOI] [PubMed] [Google Scholar]
- 46.Iglesias Sánchez M., Pérez Pico A.M., Muñoz del Rey J., Ledesma Alcázar M., Mayordomo Acevedo R. Métodos moleculares: Reacción en cadena de la polimerasa (PCR), frente a medios de cultivo convencionales. Análisis comparativo en la detección de hongos dermatofitos. Rev. Esp. Pod. 2011;22:146–149. [Google Scholar]
- 47.Chatterjee M., Datta D. Trichophyton: Changing Nomenclature and Practical Implications. Indian J. Dermatol. 2023;68:503–507. doi: 10.4103/ijd.ijd_827_23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.