Abstract
Gene–environment interactions are important determinants of cancer risk. Traditionally, gene–environment interactions are thought to contribute to tumor-suppressor-gene penetrance by facilitating or inhibiting the acquisition of additional somatic mutations required for tumorigenesis. Here, we demonstrate that a distinctive type of gene–environment interaction can occur during development to enhance the penetrance of a tumor-suppressor-gene defect in the adult. Using rats carrying a germ-line defect in the tuberous sclerosis complex 2 (Tsc-2) tumor-suppressor gene predisposed to uterine leiomyomas, we show that an early-life exposure to diethylstilbestrol during development of the uterus increased tumor-suppressor-gene penetrance from 65% to >90% and tumor multiplicity and size in genetically predisposed animals, but it failed to induce tumors in wild-type rats. This exposure was shown to impart a hormonal imprint on the developing uterine myometrium, causing an increase in expression of estrogen-responsive genes before the onset of tumors. Loss of function of the normal Tsc-2 allele remained the rate-limiting event for tumorigenesis; however, tumors that developed in exposed animals displayed an enhanced proliferative response to steroid hormones relative to tumors that developed in unexposed animals. These data suggest that exposure to environmental factors during development can permanently reprogram normal physiological tissue responses and thus lead to increased tumor-suppressor-gene penetrance in genetically susceptible individuals.
Keywords: developmental programming, gene–environment interaction, Eker rat, uterine leiomyoma, Tsc-2
Inheritance of a defect in a tumor-suppressor gene confers a high risk for developing cancer. However, defects in these genes are rarely 100% penetrant, and, even in families harboring the same genetic mutation, the penetrance of the tumor-suppressor-gene defect can vary significantly (1–4). The importance of gene–environment interactions in contributing to individual differences in tumor-suppressor-gene penetrance was recently highlighted in the New York Breast Cancer Study, which evaluated the breast and ovarian cancer risk in female relatives harboring mutations in the BRCA1 and BRCA2 tumor-suppressor genes. The magnitude of increase in breast cancer risk in the birth cohort born after 1940 was substantially greater than in those born before 1940 (5). These data suggest that additional factors, such as diet, exercise, hormonal milieu, and environmental exposures, can significantly modify cancer risk in the presence of an inherited cancer-susceptibility gene.
Traditionally, gene–environment interactions that influence cancer risk are thought to occur over an individual's lifetime by facilitating or inhibiting acquisition of the multiple somatic mutations required for tumorigenesis (6). However, for some diseases, such as cardiovascular disease and adult-onset diabetes, a developmental programming hypothesis is proposed as an alternative mechanism for modulating adult disease. This hypothesis proposes that, at critical times during development, the exposure of developing tissues to an adverse stimulus or insult can permanently reprogram normal physiological responses and, so, give rise to metabolic and hormonal disorders later in life (7–10). The underlying mechanisms for what has been termed “developmental programming” have not been definitively established, although reprogramming of the hypothalamo–pituitary–adrenal axis as a result of poor maternal nutrition and exposure of developing fetal tissues to excess glucocorticoids is thought to underlie developmental programming for some metabolic diseases of both humans and rodents (11).
The reproductive tract has been shown to be a target for developmental programming as a result of inappropriate hormone exposure. From the 1940s to the 1970s, the xenoestogen diethylstilbestrol (DES) was extensively prescribed to pregnant women at risk for miscarriage. Women who had been exposed to DES in utero during critical periods of reproductive-tract development developed several types of reproductive-tract abnormalities as well as an increased incidence of cervical–vaginal cancer later in life (12). Animal studies that simulate the human DES experience have since shown that exposure of the developing reproductive tracts of CD-1 mice to DES imparts a permanent estrogen imprint that alters reproductive-tract morphology, induces persistent expression of the lactoferrin and c-fos genes, and induces a high incidence of uterine adenocarcinoma (13–15). Because DES is readily metabolized and cleared within days after exposure, the persistent alterations resulting from developmental DES exposure cannot be explained simply by residual body burden of the compound (16, 17). DES-induced developmental programming has been demonstrated to require estrogen receptor (ER) α (18), suggesting that signaling through this receptor is crucial for establishing the imprint. However, the cancer risk for women exposed to DES in utero is only 0.1%, and the uterine adenocarcinoma observed in mice is induced only in the CD-1 strain (C57BL/6 mice are resistant) (18, 19), suggesting a possible link between genetic susceptibility and DES-induced developmental programming.
We propose that developmental programming resulting from an environmental exposure, for example, to a xenoestrogen, can alter risk in genetically susceptible individuals and influence the penetrance of tumor-susceptibility genes. To test this hypothesis, we have used an animal model for hormone-dependent tumorigenesis of the female reproductive tract. In women, tumor-suppressor genes such as BRCA1/2 and PTEN are known to increase the risk for hormone-dependent tumors of the breast and female reproductive tract, but the availability of mouse models in which these or other specific tumor-suppressor-gene defects result in the development of spontaneous hormone-dependent tumors of the female reproductive tract is limited (5, 20). However, Eker rats carrying a germ-line defect in one allele of the Tsc-2 tumor-suppressor gene are a well characterized model for hormone-dependent tumors of the female reproductive tract (21–23). Tsc-2 functions as an important regulator of phosphatidylinositol 3-kinase signaling downstream from AKT, serving to negatively regulate mTOR (24). Loss of function of the Tsc-2 gene product, tuberin, results in the development of tumors in multiple organs, including the kidney, brain, lung, and uterus in humans and rodents (25–28). Tsc-2 participates in tumor formation by the classic “two-hit” mechanism proposed by Knudson (28), with loss of function of tuberin observed in human, rat, and mouse TSC-2-associated lesions. Female rats carrying the Eker mutation (Tsc-2Ek/+) spontaneously develop uterine leiomyomas with a penetrance of 65% by 16 mo of age (23). In women, uterine leiomyoma is an ovarian hormone-dependent tumor that is the most common tumor of the reproductive tract and the major cause of hysterectomy in women of reproductive age. Uterine leiomyoma development in Eker rats is similarly hormone-dependent. Ovariectomy virtually ablates tumor development in this model, and leiomyoma-derived cells display an estrogen-responsive phenotype (23).
Here, using the Eker rat model, we show that exposure of the developing reproductive tract to the xenoestrogen DES can reprogram the normal hormonal responses of the uterus, significantly increasing the penetrance of the Tsc-2 tumor-suppressor-gene defect and increasing the incidence, multiplicity, and size of tumors that result from loss of Tsc-2 function. These results establish developmental programming as a type of gene–environment interaction that could modify risk in genetically susceptible individuals.
Methods
Animals. The care and handling of rats were in accord with National Institutes of Health guidelines and Association for the Accreditation of Laboratory Animal Care-accredited facilities, and all protocols involving the use of these animals were approved by the M. D. Anderson Animal Care and Use Committee. Neonatal female Eker rats obtained from an on-site colony received s.c. injections of either 10 μg of DES (Sigma) per rat per day or 50 μl of sesame seed oil [vehicle (VEH) control] on days 3, 4, and 5 after birth. The rats were genotyped for the Eker mutation (Tsc-2+/+ vs. Tsc-2Ek/+) and maintained on a 12-hr light/dark cycle. Animals were killed at 21 days or at 5, 10, 13, or 16 mo of age, and reproductive-tract tissues and livers were collected and fixed in 10% neutral buffered formalin before paraffin embedding or were frozen in liquid N2. The myometrium was isolated by removing the endometrium with a sterile scalpel. Tumor incidence was determined by gross and microscopic examination of uterine tissue, and tumor size was measured at necropsy. Clonally distinct, multiple tumors within the same uterus were identified either by gross examination or by differences in loss of heterozygosity (LOH) at the Tsc-2 locus by denaturing HPLC (see below). To determine tumor proliferation indices, 16-mo-old females received a 100 mg/kg i.p. injection of sterile BrdUrd (Sigma) in PBS 2 h before being killed. A total of 33 DES-exposed and 34 VEH-exposed Tsc-2Ek/+ female rats were in the 16-mo study. Nine DES-exposed and six VEH-exposed animals became moribund and were killed before 16 mo, but all Tsc-2+/+ rats completed the study. There were no rats killed in the interim because of morbidity in the 21-day or 5-, 10-, or 13-mo groups.
The reproductive stage (proestrus, estrus, metestrus, or diestrus) of each 5-mo-old animal was determined by histological examination of the vagina and ovaries and analysis of blood serum estrogen and progesterone levels. Hormone levels were measured by RIA (Diagnostic Systems Laboratories, Webster, TX) according to the manufacturer's instructions. For 16-mo-old rats, the stage of reproductive senescence [pseudopregnant (PP), persistent estrus (PE), and anestrus (AN)] was determined by vaginal, ovarian, and uterine histology. Each stage is associated with a specific hormonal mileu and reproductive tract histology. PP is associated with large corpora lutea in the ovaries and a mucified vaginal epithelium, whereas in PE, the ovaries contain numerous follicles and the vaginal epithelium is cornified. In AN, the ovaries are atrophied, and the vaginal epithelium is quiescent (29).
LOH at the Tsc-2 Locus. The mean ratio of mutant to wild-type Tsc-2 alleles in heterozygous Eker rats was calculated from ear DNA by multiplex PCR followed by high-performance liquid chromatography as described in ref. 30. Briefly, genomic DNA from frozen tissue was isolated by using the DNEasy kit (Qiagen) by following the manufacturer's protocol. A multiplex PCR designed to discriminate between the wild-type (240-bp product) and mutant (180-bp product) Tsc-2 alleles was conducted, and 10 μl of each PCR mixture was analyzed on the WAVE HPLC system with a DNA separation column (Transgenomic, Santa Clara, CA). DNA was eluted at a flow rate of 0.9 ml/min with a linear acetonitrile gradient consisting of buffer A [0.1 M triethylammonium acetate (TEAA), pH 7.0] and buffer B (0.1 M TEAA/25% acetonitrile, pH 3–4) at 50°C under nondenaturing conditions. The PCR products were separated according to size: the 180-bp fragment eluted at 4.4 min and the 240-bp fragment at 5.5 min. The area ratio between the mutant and wild-type peaks was empirically determined, and the mean (±SD) area ratio for heterozygotes was calculated to be 1.23 ± 0.24 for 24 animals. The same reaction was used to assess LOH in neoVEH and neoDES uterine leiomyomas. Tumors with area ratios >2 SDs greater than the mean area ratio (i.e., area ratio >1.72) were determined to have LOH at the Tsc-2 locus.
Immunohistochemistry. To assess tissue-proliferation rates, paraffin-embedded tissues were sectioned and deparaffinized in xylene and 100% ethanol. Endogenous peroxidase activity was quenched with 1% H2O2 in methanol for 30 min, and the tissues were denatured in 1 M HCl for 20 min. The tissues were treated with 0.05% protease type XXIV (Sigma) in deionized H2O for 20 min. Anti-BrdUrd antibody (Becton Dickinson) or anit-Ki-67 antibody (DAKO) was diluted 1:150 in 0.5% Tween 20 with 0.1% BSA in PBS and hybridized to the tissue overnight at 4°C. Staining was visualized by treatment with biotinylated anti-mouse IgG and the VEC-TASTAIN ABC kit (Vector Laboratories). The apoptotic cells in tumor-tissue sections were visualized with the ApopTag TUNEL kit (Intergen, Purchase, NY) by following the manufacturer's instructions for formalin-fixed, paraffin-embedded tissues. CD-31 staining to assess tumor vascularity was performed by using an anti-CD-31 antibody (Santa Cruz Biotechnology) diluted 1:500 in PBS. CD-31 was visualized with biotinylated anti-goat IgG (Vector Laboratories) as described above, and tumor microvessel density was determined by pathological examination of all tumor sections.
Western Blot Analysis. Western blot analysis of tuberin, ERα, progesterone receptor (PR), and androgen receptor (AR) was conducted on 50 μg of myometrial and liver protein lysate by using a mouse anti-PR antibody (NeoMarkers, Fremont, CA) that recognizes the PR-A and PR-B isoforms and rabbit anti-tuberin, anti-AR, and anti-ERα antibodies (Santa Cruz Biotechnology).
Results
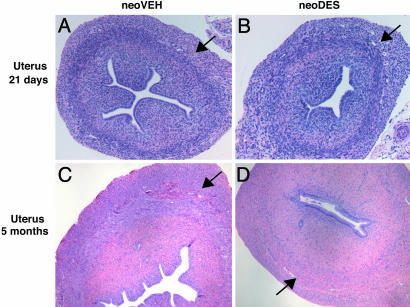
Reproductive Tracts of Eker Rats Exhibit Hormonal Imprinting in Response to Developmental Exposure to Xenoestrogen. Brief exposures during critical periods of development to xenoestrogens, including phytoestrogens and pharmaceuticals such as DES, impart a hormonal imprint on the developing tissue that modulates its appearance and function in the adult. This hormonal imprinting has been demonstrated for several hormone-responsive tissues, including the breast, prostate, and female reproductive tract (14, 31–34). In the Eker rat model, inheritance of a defect in one allele of the Tsc-2 tumor-suppressor gene predisposes to uterine leiomyomas that arise from the myometrial compartment of the uterus. To determine the effect of an environmental exposure during development on the myometrium of Eker rats, animals (Tsc-2+/+ and Tsc-2Ek/+) were exposed to the xenoestrogen DES during neonatal days 3–5 of development, a critical period of myometrial differentiation (35). At postnatal day 21, before the onset of puberty (which in the rat is ≈day 35), no differences were observed in the morphology of the immature uteri of animals exposed to VEH (neoVEH) or DES (neoDES) neonatally (Fig. 1 A and B). However, after the onset of puberty, several morphological and functional differences were observed in the reproductive tracts of neoVEH vs. neoDES females at 5 mo of age. All neoDES females displayed persistent vaginal cornification, condensed and hyalinized endometrial stroma, and a paucity of endometrial glands, hallmarks of early-life estrogen exposure and hormonal imprinting of the female reproductive tract (Fig. 1 C and D). Furthermore, of all neoDES-exposed rats evaluated, 58% displayed endometrial hyperplasia as compared with 0% for neoVEH rats [P < 0.01 (χ2 test); neoDES n = 57; neoVEH n = 60] (see Fig. 6 E and F, which is published as supporting information on the PNAS web site). The architecture of the myometrium was fairly normal in DES-exposed animals, although it was somewhat hypoplastic compared with VEH-exposed myometria (Fig. 1 C and D). The ovaries of the neoDES females lacked corpora lutea and had more interstitial tissue than did neoVEH ovaries (Fig. 6 C and D), resulting in disruption of normal ovarian cycling. There were no differences between VEH-exposed Tsc-2+/+ and Tsc-2Ek/+ females for any of these measures.
Fig. 1.
Effects of neonatal DES exposure on uterine morphology. Histological sections of uteri from 21-day-old (A and B) and 5-mo-old (C and D) neoVEH- and neoDES-exposed rats (×40). All tissue sections were stained with hematoxylin and eosin. Arrows denote the myometrial layer of the uterus.
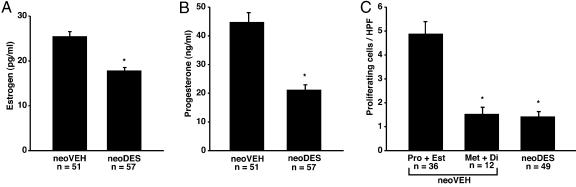
Consistent with the aberrant ovarian histology observed in neoDES females, steroid hormone production also was significantly compromised. Levels of circulating estrogen and progesterone were significantly reduced in neoDES females relative to neoVEH animals, and neoDES females failed to exhibit normal estrus cycles (Fig. 2 A and B). Quantitation of cell proliferation in the myometrium of adult neoDES females revealed that myometrial cells from exposed animals had very low proliferative indices, similar to normal postovulatory neoVEH females in metestrus or diestrus, when hormone-driven cell proliferation is lowest (Fig. 2C). Thus, the appearance of the myometrium of 5-mo-old neoDES females was consistent with the hypoestrogenic milieu found in postovulatory animals. These findings raised the question of what would be the overall impact on tumorigenesis in the target tissue of the combined presence of a tumor-suppressor-gene defect that predisposed to hormone-dependent uterine leiomyoma and a developmental exposure that had hormonally imprinted the reproductive tract and compromised ovarian function.
Fig. 2.
DES-induced developmental programming alters steroid hormone levels and myometrial proliferation. (A and B) Levels of estrogen (A) and progesterone (B) in blood serum of 5-mo-old rats exposed neonatally to either VEH or DES. *, P < 0.01, unpaired t test. (C) Ki-67 was used to determine the proliferative rates of myometrial tissue in neoDES- and neoVEH-treated 5-mo-old Eker rats. For neoVEH animals, the proliferative rates were combined for the reproductive stages of proestrus and estrus (Pro + Est) or metestrus and diestrus (Met + Di). The neoDES animals were not similarly staged because these animals did not cycle. * denotes significant difference from the Pro + Est group. *, P < 0.01, Kruskall–Wallis test.
Tsc-2 Tumor-Suppressor-Gene Penetrance Is Increased in Animals in Response to Hormonal Imprinting. To determine the impact of the hormonal imprint imparted by DES exposure on the myometrium, tumorigenesis was evaluated in neoDES and neoVEH females at 16 mo of age. In wild-type rats (Tsc-2+/+), DES failed to induce uterine leiomyomas; no gross or microscopic uterine leiomyomas were observed in the uteri of (Tsc-2+/+) neoDES or (Tsc-2+/+) neoVEH animals in the absence of a Tsc-2 defect (Table 1). Surprisingly, considering the hypoplastic appearance of the myometrium and the reduction in circulating levels of ovarian steroids, tumor-suppressor-gene penetrance was significantly enhanced in female rats carrying the Eker mutation (Tsc-2Ek/+). neoVEH carriers had a 64% tumor incidence, similar to the 65% incidence of historical controls, whereas neoDES carriers exhibited nearly complete penetrance, with a tumor incidence of 92% (P < 0.02). Tumor multiplicity also increased from 0.82 to 1.33 tumors per rat (P < 0.02), and the tumors in neoDES carriers were 5 times larger than the tumors in neoVEH animals (P < 0.02) (Table 1). Therefore, whereas DES exposure imparted a hormonal imprint on the female reproductive tract in both carriers and noncarriers, it was not sufficient to induce tumors in the absence of the tumor-suppressor-gene defect, indicating that the impact of DES exposure on tumorigenesis was specific for genetically predisposed animals.
Table 1. Developmental DES exposure increases tumor incidence, multiplicity, and size in genetically susceptible animals.
| Age, mo | Genotype | Treatment | No. of rats | % tumor incidence | Multiplicity (mean no. of tumors per rat ± SEM) | Size, cm3 (mean ± SEM) |
|---|---|---|---|---|---|---|
| 16 | Tsc-2Ek/+ | VEH | 28 | 64 | 0.82 ± 0.15 | 2.30 ± 1.10 |
| DES | 24 | 92* | 1.33 ± 0.17* | 10.50 ± 2.70* | ||
| 16 | Tsc-2+/+ | VEH | 34 | 0 | N/A | N/A |
| DES | 34 | 0 | N/A | N/A | ||
| 13 | Tsc-2Ek/+ | VEH | 27 | 48 | 0.63 ± 0.17 | 1.10 ± 0.42 |
| DES | 26 | 69 | 0.73 ± 0.10 | 13.70 ± 7.80 | ||
| 10 | Tsc-2Ek/+ | VEH | 30 | 23 | 0.27 ± 0.10 | 0.01 ± 0.00 |
| DES | 29 | 10 | 0.10 ± 0.06 | 0.16 ± 0.18 |
, P < 0.02 (determined by the χ2 test for tumor incidence and Student's t test for multiplicity and tumor size). N/A, not applicable
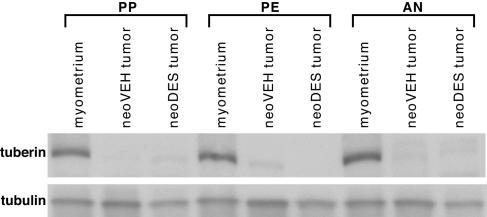
Because DES could impart a hormonal imprint to the uteri of all exposed animals, but tumors arose only in heterozygotes, we asked whether loss of function of the Tsc-2 tumor-suppressor gene was still required for tumorigenesis in DES-exposed animals. Loss of tuberin expression was detected in the vast majority of both neoDES and neoVEH tumors that arose in carrier females, whereas normal myometrium from age-matched Eker rats continued to express high levels of tuberin regardless of the stage of reproductive senescence (Fig. 3). More than 75% of these tumors could be demonstrated by Western analysis to have greatly diminished or absent tuberin expression, and LOH (36) accounted for loss of Tsc-2 function with the same frequency (58% vs. 50%) in neoDES and neoVEH tumors. When tumor incidence was analyzed as a function of time, the difference in tumor incidence observed between neoDES and neoVEH animals at 16 mo of age was less pronounced at 13 mo of age (69% vs. 48%) (Table 1). At the earliest time point examined (10 mo of age), there was no significant difference in tumor incidence between neoDES and neoVEH carriers (10% vs. 23%). However, even at 10 mo, the earliest time at which grossly observable tumors were seen, tumors were larger in the neoDES vs. neoVEH animals (0.16 vs. 0.01 cm3), and this trend continued in the 13-mo-old females (13.70 vs. 1.10 cm3) and became significant by 16 mo (P < 0.05, Table 1). Therefore, consistent with previous studies in this model, loss of function of the remaining wild-type allele appeared to be a rate-limiting event for tumorigenesis. Importantly, the hormonal imprint acquired in response to DES exposure appeared to accelerate the growth of tumors that had lost Tsc-2 gene function, enhancing tumor-suppressor-gene penetrance, even in the presence of a hypoestrogenic milieu present in neoDES females.
Fig. 3.
Tuberin expression in 16-mo-old Eker rats. Shown is Western blot analysis of tuberin expression in normal myometrium and in tumors from neoVEH- and neoDES-exposed animals at each stage of reproductive senescence. Tubulin expression is used as a loading control.
Developmental Programming Enhances the Estrogen-Responsiveness of Target Tissues to Increase Tumor-Suppressor-Gene Penetrance. To determine the mechanism by which developmental DES exposure enhanced tumor-suppressor-gene penetrance, we examined developmental programming of the target tissue as a candidate mechanism for modulation of tumor outcome in genetically susceptible animals. Uterine leiomyomas are hormone-dependent, and tumor development in the Eker rat requires the steroid hormone estrogen (23). To determine whether developmental programming had occurred in the target tissue before tumor development, we examined the myometria of female rats for expression of the PR and AR, two well characterized estrogen-responsive genes.
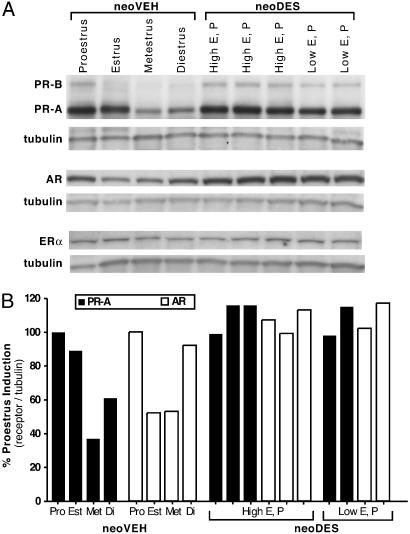
PR and AR expression vary throughout the estrous cycle in the normal myometrium. They are induced during proestrus, when estrogen levels peak, and are reduced to low-to-undetectable levels during later phases of the cycle, when estrogen levels decline (Fig. 4). The myometria of neoVEH females exhibited the typical cyclical estrogen-driven expression pattern of PR and AR. However, in the myometria of neoDES females, PR and AR expression was elevated, even under conditions of low estrogen (Fig. 4). There were no differences in ERα expression in the myometria of neoDES or neoVEH females (Fig. 4). In contrast to the uterine myometrium, the liver, which is fully differentiated in the neonate at the time of DES exposure, exhibited no differences in expression of PR or AR between neoDES and neoVEH animals, which also expressed comparable levels of ERα in their livers (see Fig. 7, which is published as supporting information on the PNAS web site). Deregulation of PR and AR expression in the myometrium demonstrated reprogramming of steroid-hormone responsiveness at the cellular level in neoDES females and also suggested that the hyperresponsiveness to estrogen characteristic of uterine leiomyoma was exacerbated in these animals, permitting tumors to develop subsequent to loss of Tsc-2 tumor-suppressor-gene function, even under the hypoestrogenic hormonal milieu present in neoDES carriers.
Fig. 4.
Developmental DES exposure deregulated PR and AR protein expression in adult myometrium. (A) Western blot analysis of PR, AR, and ERα protein expression in the myometria of 5-mo-old neoVEH-exposed rats at each stage of the reproductive cycle and in neoDES rats. Because neoDES rats did not cycle normally, they were classified by low and high estrogen and progesterone levels, which correspond to proestrus and metestrus hormone levels, respectively. (B) Densitometric quantitation of PR-A and AR protein levels shown in A. Receptor protein levels were normalized to tubulin expression and reported as percent induction of proestrus levels. This quantitation is representative of three independent biological replicates (data not shown).
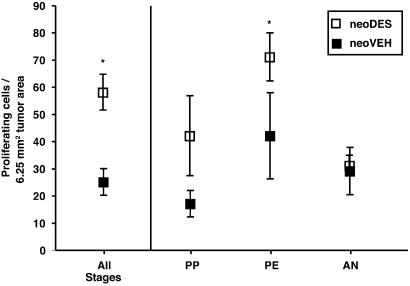
To extend this finding to tumors, we compared the proliferative potential of neoDES vs. neoVEH tumors under similar hormonal milieus. Hormone levels and stage of reproductive senescence were determined for all tumor-bearing animals (neoDES and neoVEH), and the proliferative indices of the tumors were measured by BrdUrd incorporation. neoDES tumors, overall, were twice as proliferative as neoVEH tumors (P < 0.05, Fig. 5). After controlling for hormonal milieu, neoDES tumors were more proliferative than matched tumors from neoVEH carriers at both the PP and PE stages of reproductive senescence (Fig. 5). However, in AN, which is characterized by atrophy of the ovary, absence of ovarian function, and negligible estrogen levels, there was no difference between the proliferative indices of neoVEH and neoDES tumors. Apoptosis, which we have previously shown is not regulated by estrogen in these tumors (37), was not significantly different between neoDES [2.2 ± 0.3 (SEM) apoptotic cells per 6.25 mm2 of tumor area] and neoVEH (2.1 ± 0.3) tumors, as determined by TUNEL staining. Tumor vascularity also did not differ between neoDES and neoVEH tumors as determined by CD31 staining and visualization of vessel density and structure (data not shown). Taken together, these data indicate that developmental programming as a result of inappropriate DES exposure imparted a hormonal imprint on the developing myometrium that enhanced the hormone responsiveness of target cells, leading to increased tumor-suppressor-gene penetrance.
Fig. 5.
Tumor-cell proliferation. Comparison of the tumor-cell-proliferation rates, as assessed by BrdUrd incorporation, for neoDES vs. neoVEH 16-mo-old Eker rats without accounting for stage of reproductive senescence, i.e., All stages group (*, P < 0.01, Mann–Whitney test; neoVEH n = 19, neoDES n = 26). Animals are classified as belonging to one of three stages of reproductive senescence (AN, PE, and PP) according to estrogen and progesterone levels and reproductive tract histology. Statistical analysis was performed with the Mann–Whitney test. For rats in PP, P = 0.07 (neoVEH n = 9, neoDES n = 3); for rats in PE, *, P < 0.05 (neoVEH n = 4, neoDES n = 16); for rats in AN, P = 0.41 (neoVEH n = 4, neoDES n = 5); for PE + PP neoDES vs. neoVEH rats, P < 0.01.
Discussion
Here, we show that an environmental exposure during development of the uterus reprograms the normal hormone-responsiveness of target cells, leading to increased tumor-suppressor-gene penetrance. Eker rats are genetically predisposed to develop hormone-dependent uterine leiomyomas. Treatment of neonatal Eker rats with the xenoestrogen DES during a critical period of reproductive-tract development induced developmental programming of target tissues, resulting in alterations in reproductive-tract morphology and deregulation of normal estrogen-driven patterns of gene expression in the myometrium of adult animals. In conjunction with a genetic susceptibility, DES-induced developmental programming significantly increased tumor incidence, multiplicity, and size in susceptible animals; however, no tumors were induced by DES in wild-type rats, even though programming occurred in the reproductive tracts of these animals.
Developmental programming of estrogen-responsive genes was evidenced in the myometrium before tumor development by the elevated expression of AR and PR in postpubertal, adult neoDES females. This developmental programming occurred as a result of the hormonal imprint imparted on the developing tissue by a brief early-life exposure to DES that permanently altered gene-expression profiles in the adult. However, the exact nature of this imprint or how it is imparted by DES exposure has not been established. One possible mechanism by which a xenoestrogen such as DES could impart a hormonal imprint is by association of the ligand-activated ER with DNA, inducing epigenetic changes in chromatin structure and/or DNA methylation. Induction of reproductive tract tumors by DES appears to require ERα, because ERα-knockout mice exposed to DES do not show reduced expression of these genes or develop tumors (18). These studies and others have led to the suggestion that the hormonal imprint is mediated by inappropriate transactivation of the ER during reproductive-tract development. There is precedent for inappropriate hormone exposure to induce a hormonal imprint by means of epigenetic changes. Abnormal expression of the lactoferrin and c-fos genes in the endometrium of adult CD-1 mice developmentally exposed to DES correlates with an altered pattern of CpG methylation in these genes (13, 15, 38). DNA methylation has also been shown to provide memory of the first hormone induction during development (39). The tyrosine aminotransferase (Tat) gene is induced in rat fetal hepatocytes by exposure to glucocorticoids. Interaction of the glucocorticoid–receptor complex with the glucocorticoid response unit in the Tat enhancer induces chromatin remodeling and recruitment of the basal transcription machinery and leads to selective demethylation of CpG sites within this region. Once demethylated, these sites remain unmethylated, even in the absence of hormone, because of the clonal inheritance of DNA-methylation patterns. These changes in methylation provide a memory of the first hormonal stimulation that contributes to a stronger subsequent hormonal response to glucocorticoids in exposed cells relative to naïve cells.
In our experiments, developmental programming by DES occurred in all exposed animals (Tsc-2+/+ and Tsc-2Ek/+), as evidenced by alterations in reproductive tract morphology, changes in ovarian hormone levels, and deregulated AR and PR expression in the myometrium. However, although all exposed animals exhibited developmental programming, only Tsc-2Ek/+ animals developed tumors. Our data suggest that, whereas early-life DES exposure can induce developmental programming, these changes may not be sufficient to induce tumors in exposed individuals. However, in the presence of a tumor-suppressor-gene defect, the combined effect of a genetic susceptibility and developmental programming can result in enhanced tumor-suppressor-gene penetrance. Supporting our observations, the CD-1 strain of mice exposed to DES develop uterine adenocarcinomas, whereas C57BL/6 mice are resistant to tumor induction by DES, pointing to the importance of genetic background as a determinant of response to xenoestrogen exposure (18, 40). A previous report by Couse et al. (18) proposed that developmental exposure to DES functions as a promoter rather than as an initiator of tumorigenesis. In our study, we show that DES induces developmental programming in all exposed animals; however, only animals heterozygous for the Tsc-2-gene defect were at an increased risk of tumor development. The increased proliferative potential of tumor cells arising in neoDES Tsc-2Ek/+ females would be consistent with this developmental programming having a promotional effect on nascent tumor cells that had lost or inactivated the normal allele of the Tsc-2 gene.
Since the discovery of xenoestrogens, considerable controversy has existed over the actual impact of these exposures on cancer risk in the human population (41). This question is particularly important, given the recent observation that nongenetic factors, such as environmental exposures, have had a significant impact on breast cancer risk over the past 60 years in predisposed women carrying BRCA1/2 defects (5). Gene–environment interaction has been defined as coparticipation in the same causal mechanism leading to disease (42). Our data indicate that developmental programming as a result of early-life xenoestogen exposure can coparticipate with a preexisting genetic susceptibility to enhance disease penetrance. Increased or decreased cancer risk as a result of environmental exposures has traditionally been linked to the ability of gene–environment interactions to induce somatic mutations that participate in multistage carcinogenesis. However, our data indicate that there is an alternative mechanism in which environmental exposures program the response of a tissue to physiological signals that can modulate the penetrance and phenotype of tumors that develop in genetically susceptible individuals. Therefore, developmental programming could contribute to the variability in disease penetrance observed in hereditary cancer predispositions and could represent an important determinant of risk in individuals genetically susceptible to hormone-dependent tumors such as uterine, breast, and prostate cancers and, perhaps, other tumor types as well.
Supplementary Material
Acknowledgments
We thank Deena Walker, Melisa Portis, and Pam Huskey for excellent technical assistance. This work was supported by National Institutes of Health Grants ES08263 and HD46282 (to C.L.W.) and ES07784 and CA16672 (to J.D.C.) and by an American Legion Auxiliary Fellowship in Cancer Research (to J.D.C.).
Author contributions: J.C.B. and C.L.W. designed research; J.D.C., B.J.D., and S.-L.C. performed research; J.D.C., B.J.D., C.L.W., S.-L.C., and C.J.C. analyzed data; and J.D.C. wrote the paper.
Abbreviations: AN, anestrus; AR, androgen receptor; DES, diethylstilbestrol; LOH, loss of heterozygosity; PE, persistent estrus; PP, pseudopregnant; PR, progesterone receptor; VEH, vehicle.
References
- 1.Thorlacius, S., Struewing, J. P., Hartge, P., Olafsdottir, G. H., Sigvaldason, H., Tryggvadottir, L., Wacholder, S., Tulinius, H. & Eyfjord, J. E. (1998) Lancet 352, 1337-1339. [DOI] [PubMed] [Google Scholar]
- 2.Struewing, J. P., Hartge, P., Wacholder, S., Baker, S. M., Berlin, M., McAdams, M., Timmerman, M. M., Brody, L. C. & Tucker, M. A. (1997) N. Engl. J. Med. 336, 1401-1408. [DOI] [PubMed] [Google Scholar]
- 3.Rahman, N., Arbour, L., Houlston, R., Bonaiti-Pellie, C., Abidi, F., Tranchemontagne, J., Ford, D., Narod, S., Pritchard-Jones, K., Foulkes, W. D., et al. (2000) J. Natl. Cancer Inst. 92, 650-652. [DOI] [PubMed] [Google Scholar]
- 4.Knudson, A. G. (2002) Am. J. Med. Genet. 111, 96-102. [DOI] [PubMed] [Google Scholar]
- 5.King, M. C., Marks, J. H. & Mandell, J. B. (2003) Science 302, 643-646. [DOI] [PubMed] [Google Scholar]
- 6.Shields, P. G. & Harris, C. C. (2000) J. Clin. Oncol. 18, 2309-2315. [DOI] [PubMed] [Google Scholar]
- 7.Couzin, J. (2002) Science 296, 2167-2169. [DOI] [PubMed] [Google Scholar]
- 8.Barker, D. J. (2002) Trends Endocrinol. Metab. 13, 364-368. [DOI] [PubMed] [Google Scholar]
- 9.Frankel, S., Elwood, P., Sweetnam, P., Yarnell, J. & Smith, G. D. (1996) Lancet 348, 1478-1480. [DOI] [PubMed] [Google Scholar]
- 10.Hattersley, A. T. & Tooke, J. E. (1999) Lancet 353, 1789-1792. [DOI] [PubMed] [Google Scholar]
- 11.Bertram, C. E. & Hanson, M. A. (2002) Reproduction 124, 459-467. [DOI] [PubMed] [Google Scholar]
- 12.Herbst, A. L., Ulfelder, H. & Poskanzer, D. C. (1971) N. Engl. J. Med. 284, 878-881. [DOI] [PubMed] [Google Scholar]
- 13.Newbold, R. R., Hanson, R. B. & Jefferson, W. N. (1997) Biol. Reprod. 56, 1147-1157. [DOI] [PubMed] [Google Scholar]
- 14.Newbold, R. R., Bullock, B. C. & McLachlan, J. A. (1990) Cancer Res. 50, 7677-7681. [PubMed] [Google Scholar]
- 15.Li, S., Hansman, R., Newbold, R., Davis, B., McLachlan, J. A. & Barrett, J. C. (2003) Mol. Carcinog. 38, 78-84. [DOI] [PubMed] [Google Scholar]
- 16.Marselos, M. & Tomatis, L. (1992) Eur. J. Cancer 29A, 149-155. [DOI] [PubMed] [Google Scholar]
- 17.Marselos, M. & Tomatis, L. (1992) Eur. J. Cancer 28A, 1182-1189. [DOI] [PubMed] [Google Scholar]
- 18.Couse, J. F., Dixon, D., Yates, M., Moore, A. B., Ma, L., Maas, R. & Korach, K. S. (2001) Dev. Biol. 238, 224-238. [DOI] [PubMed] [Google Scholar]
- 19.Melnick, S., Cole, P., Anderson, D. & Herbst, A. (1987) N. Engl. J. Med. 316, 514-516. [DOI] [PubMed] [Google Scholar]
- 20.Ali, I. U. (2000) J. Natl. Cancer Inst. 92, 861-863. [DOI] [PubMed] [Google Scholar]
- 21.Yeung, R. S., Xiao, G. H., Jin, F., Lee, W. C., Testa, J. R. & Knudson, A. G. (1994) Proc. Natl. Acad. Sci. USA 91, 11413-11416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi, T., Hirayama, Y., Kobayashi, E., Kubo, Y. & Hino, O. (1995) Nat. Genet. 9, 70-74. [DOI] [PubMed] [Google Scholar]
- 23.Walker, C. L., Hunter, D. & Everitt, J. I. (2003) Genes Chromosomes Cancer 38, 349-356. [DOI] [PubMed] [Google Scholar]
- 24.Li, Y., Corradetti, M. N., Inoki, K. & Guan, K. L. (2004) Trends Biochem. Sci. 29, 32-38. [DOI] [PubMed] [Google Scholar]
- 25.Walker, C., Goldsworthy, T. L., Wolf, D. C. & Everitt, J. (1992) Science 255, 1693-1695. [DOI] [PubMed] [Google Scholar]
- 26.Everitt, J. I., Goldsworthy, T. L., Wolf, D. C. & Walker, C. L. (1992) J. Urol. 148, 1932-1936. [DOI] [PubMed] [Google Scholar]
- 27.Everitt, J. I., Wolf, D. C., Howe, S. R., Goldsworthy, T. L. & Walker, C. (1995) Am. J. Pathol. 146, 1556-1567. [PMC free article] [PubMed] [Google Scholar]
- 28.Cheadle, J. P., Reeve, M. P., Sampson, J. R. & Kwiatkowski, D. J. (2000) Hum. Genet. 107, 97-114. [DOI] [PubMed] [Google Scholar]
- 29.Mulholland, J. & Jones, C. J. (1993) Microsc. Res. Tech. 25, 148-168. [DOI] [PubMed] [Google Scholar]
- 30.Kleymenova, E. & Walker, C. L. (2001) J. Biochem. Biophys. Methods 47, 83-90. [DOI] [PubMed] [Google Scholar]
- 31.Tomooka, Y. & Bern, H. A. (1982) J. Natl. Cancer Inst. 69, 1347-1352. [PubMed] [Google Scholar]
- 32.Hilakivi-Clarke, L., Cho, E. & Clarke, R. (1998) Oncol. Rep. 5, 609-616. [DOI] [PubMed] [Google Scholar]
- 33.Prins, G. S., Birch, L., Couse, J. F., Choi, I., Katzenellenbogen, B. & Korach, K. S. (2001) Cancer Res. 61, 6089-6097. [PubMed] [Google Scholar]
- 34.Newbold, R. R., Banks, E. P., Bullock, B. & Jefferson, W. N. (2001) Cancer Res. 61, 4325-4328. [PubMed] [Google Scholar]
- 35.Brody, J. R. & Cunha, G. R. (1989) Am. J. Anat. 186, 1-20. [DOI] [PubMed] [Google Scholar]
- 36.Hunter, D. S., Klotzbucher, M., Kugoh, H., Cai, S.-L., Mullen, J. P., Manfioletti, G., Fuhrman, U. & Walker, C. L. (2002) Cancer Res. 62, 3766-3772. [PubMed] [Google Scholar]
- 37.Burroughs, K. D., Kiguchi, K., Howe, S. R., Fuchs-Young, R., Trono, D., Barrett, J. C. & Walker, C. (1997) Endocrinology 138, 3056-3064. [DOI] [PubMed] [Google Scholar]
- 38.Li, S., Washburn, K. A., Moore, R., Uno, T., Teng, C., Newbold, R. R., McLachlan, J. A. & Negishi, M. (1997) Cancer Res. 57, 4356-4359. [PubMed] [Google Scholar]
- 39.Thomassin, H., Flavin, M., Espinas, M. L. & Grange, T. (2001) EMBO J. 20, 1974-1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miller, C., Degenhardt, K. & Sassoon, D. A. (1998) Nat. Genet. 20, 228-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crisp, T. M., Clegg, E. D., Cooper, R. L., Wood, W. P., Anderson, D. G., Baetcke, K. P., Hoffmann, J. L., Morrow, M. S., Rodier, D. J., Schaeffer, J. E., et al. (1998) Environ. Health Perspect. 106, 11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Greenland, S. & Rothman, K. J. (1998) in Modern Epidemiology, eds. Rothman, K. J. & Greenland, S. (Lippincott Williams and Wilkins, Philadelphia), pp. 329-342.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.