Abstract
Objectives: Ankylosing spondylitis (AS) is an autoinflammatory, chronic disease. Patients with AS are at increased risk of cardiovascular disease (CVD). The link between AS and subclinical atherosclerosis is multifactorial and still not completely understood. The aim of this study was to examine the potential associations between carotid intima–media thickness (cIMT) and different cardiometabolic biomarkers in individuals with AS. Methods: A total of 96 patients with AS were prospectively included. cIMT was measured via ultrasonography. Multiple linear regression analysis was used to find the best predictors of cIMT values. Principal component analysis (PCA) was implemented to extract factors that were further tested via binary logistic regression analysis in relation to cIMT. Results: Waist circumference (WC), low-density lipoprotein cholesterol (LDL-c), and the BASDAI score were independently correlated with cIMT in AS patients (p = 0.037, p = 0.060, and p = 0.048, respectively; adjusted R2 = 0.113). PCA extracted four panels of biomarkers, i.e., “haematology–lipid-related factor” (i.e., ferritin, haemoglobin, HDL-c, and triglycerides), “proinflammatory–prothrombotic-related factor” (i.e., platelets, neutrophils, and C-reactive protein), “LDL-c–vitamin-related factor” (i.e., vitamins D and B12, and LDL-c), and “age–glucometabolic-related factor” (i.e., age and HbA1c), in relation to higher cIMT in patients with AS. Among these four clusters, “age–glucometabolic-related factor” was an independent predictor of increased cIMT (p < 0.001). Conclusions: In addition to traditional cardiometabolic risk factors, WC and LDL-c, the disease activity score (BASDAI) is independently related to subclinical atherosclerosis in AS patients. The joint involvement of heterogeneous cardiometabolic risk factors may reflect different pathophysiological processes of subclinical atherosclerosis in patients with AS.
Keywords: ankylosing spondylitis, BASDAI score, cardiometabolic risk factors, cIMT, principal component analysis
1. Introduction
Ankylosing spondylitis (AS) is an autoinflammatory, chronic disease that affects not only the sacroiliac joints and spine but also the cardiovascular system, skin, kidneys, gastrointestinal tract, lungs, and eyes [1,2]. AS has a long asymptomatic stage, and it is assumed that genetic factors and the activated inflammatory cascade are the pathophysiological mechanisms of the disease [2]. Given the underlying chronic inflammation in AS, these individuals are at increased risk of CVD [3]. Compared with the general population, subjects with AS are at a 30–50% risk of incident cardiovascular events [4].
Endothelial dysfunction and subclinical atherosclerosis can be observed in patients with AS even without manifesting CV risk factors [2]. Endothelial dysfunction is the first and reversible stage of atherosclerosis onset [5]. Atherosclerosis, the principal cause of CVD, involves an enhanced immune-inflammatory response, a disbalance between antioxidants and pro-oxidants, lipid peroxidation, endothelial injury and dysfunction, compromised vasodilatation, perturbed haemostasis, and an enhanced prothrombotic state. All such disturbances promote the development of atherosclerotic plaques that, if ruptured, precede CVD [5]. However, the link between AS and subclinical atherosclerosis is multifactorial and still not completely understood [1].
The identification of individuals with high CVD risk is of paramount importance, and such patients would benefit from an immediate therapeutic strategy before experiencing CVD onset and its complications. Although much progress has been made in identifying novel endothelial dysfunction biomarkers that could be reliable parameters of subclinical atherosclerosis [1,5,6,7], traditional biomarkers should not be neglected, especially concerning their joint involvement in different pathophysiological processes of subclinical atherosclerosis.
Carotid intima-media thickness (cIMT) reflects the degree of endothelial dysfunction and is regarded as a simple, cost-effective, non-invasive surrogate parameter of subclinical atherosclerosis [8]. Indeed, previous studies, although with relatively small sample sizes, reported higher cIMT in patients with AS than in healthy individuals [2,3,9,10]. To gain deeper insight into the pathophysiological traits of AS and subclinical atherosclerosis, the aim of the current study was to examine the potential associations between cIMT and different cardiometabolic biomarkers, such as proinflammatory, prothrombotic, and proatherogenic biomarkers, in individuals with AS via a thorough statistical approach, such as principal component analysis.
2. Materials and Methods
2.1. Study Population
A total of 96 patients diagnosed with AS according to the Assessment in Spondylarthritis International Society (ASAS) criteria [11] were prospectively included in this study involving a consultation-based cohort. The participants’ age, sex, smoking status, alcohol consumption status, medication use, family disease history, and disease duration were recorded. Those who smoked, consumed alcohol, had active and chronic infections, dyslipidaemia, diabetes mellitus, hypertension, coronary artery disease, or malignant diseases, had any spine surgeries, used steroids, lipid-lowering agents, or antihyperglycemic agents, were pregnant, or were lactating were excluded from this study. This study was conducted in accordance with the Declaration of Helsinki (ethical approval no: E-40465587-050.01.04-1041, 2024/92). Informed consent was obtained from all participants.
2.2. Measurement of Demographic, Clinical, Disease Activity, and Laboratory Parameters
The participants’ physical examination was performed, and height and weight measurements were taken. Body mass index (BMI) was calculated by dividing weight (kilograms) by height (meters) squared. The BATH Ankylosing Spondylitis Disease Activity Index was measured in the patients [12].
Blood samples were taken after at least eight hours of fasting. The complete blood count (including white blood cell (WBC) count, neutrophils, haemoglobin, and platelets), C-reactive protein levels (CRP), and lipid levels, including triglycerides, total cholesterol (TC), high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), folate, ferritin, vitamin B12, and vitamin D, were analysed.
2.3. Carotid Artery Intima–Media Thickness Measurement
Carotid IMT was measured by two experienced radiologists using the LA2-14A probe on the Samsung RS85 Prestige device. In images obtained in the sagittal plane during greyscale ultrasonography, the intima-media layer was measured at the 1 cm proximal level of the carotid bifurcation distal to the common carotid artery, with appropriate magnification. If there was an atherosclerotic plaque on the walls of the common internal carotid artery at all levels within the examination area, it was noted.
2.4. Statistical Analysis
The results are presented as the median and 25th to 75th percentile values because of a non-normal distribution. Differences between groups were examined by the Kruskal–Wallis and Mann–Whitney U tests. Bivariate correlations were analysed via Spearman’s correlation analysis. Multiple linear regression (MLR) analysis with backwards selection was used to find the best predictors of cIMT values. Factorial analysis (principal component analysis, PCA) with varimax-normalized rotation was implemented to extract factors that were further tested via binary logistic regression analysis. In particular, an eigenvalue >1 was used to extract the factors, whereas variables with factor loadings >0.5 were included in further PCA. Analysis adequacy was tested via the Kaplan–Meier–Olkin (KMO) test and Bartlett’s sphericity test. Binary logistic regression analysis was used to test a possible independent association of PCA-produced factors (scores) with high cIMT values. Statistical analysis was performed via PASW® Statistic v.18 (Chicago, IL, USA) software, and p values <0.05 were considered statistically significant.
3. Results
Table 1 shows the demographic, general clinical, and biochemical characteristics of the AS patients included in this study.
Table 1.
General clinical and biochemical data of ankylosing spondylitis patients.
| Parameter | AS Patients (N = 96) |
|---|---|
| Age, years | 36.5 (31.0–47.0) |
| Gender | |
| Female | 38 (39.6) |
| Male | 58 (60.4) |
| Alcohol use | |
| NO | 90 (93.8) |
| YES | 6 (6.3) |
| Smoking | |
| NO | 59 (61.5) |
| YES | 37 (38.5) |
| Educational background | |
| Primary school | 15 (15.6) |
| Secondary school | 14 (14.6) |
| High school | 29 (30.2) |
| University | 38 (39.6) |
| Marital status | |
| Single | 23 (24.0) |
| Married | 69 (71.9) |
| Divorced | 4 (4.2) |
| Delay in diagnosis, months | 2 (1–3) |
| Family history of AS | |
| NO | 47 (49.0) |
| YES | 49 (51.0) |
| Comorbidities | |
| Diabetes mellitus | |
| NO | 96 (100) |
| CAD | |
| NO | 96 (100) |
| Hypertension | |
| NO | 96 (100) |
| Hyperlipidaemia | |
| NO | 96 (100) |
| Inflammatory back pain | |
| NO | 6 (6.3) |
| YES | 90 (93.7) |
| Peripheral arthritis | |
| NO | 81 (84.4) |
| YES | 15 (15.6) |
| Uveitis | |
| NO | 81 (84.4) |
| YES | 15 (15.6) |
| Dactylitis | |
| NO | 96 (100) |
| Enthesitis | |
| NO | 71 (74.0) |
| YES | 25 (26.0) |
| Sacroiliac MRI enhancement status | |
| None | 6 (6.3) |
| Right unilateral | 7 (7.3) |
| Left unilateral | 7 (7.3) |
| Bilateral | 76 (79.2) |
| Drugs used to treat AS | |
| NSAID | 33 (34.4) |
| Anti TNF | |
| etanercept | 12 (12.5) |
| adalimumab | 28 (29.2) |
| certolizumab | 7 (7.3) |
| infliximab | 6 (6.3) |
| golimumab | 10 (10.4) |
| Drug use duration, years | 3 (2–5) |
| Duration of disease, years | 5.0 (4.0–10.0) |
| BMI, kg/m2 | 25.9 (22.6–29.4) |
| WC, cm | 91.0 (86.0–104.0) |
| WtHR | 0.546 (0.505–0.596) |
| WBC | 7.52 (6.49–8.71) |
| Neutrophil count, ×109 /L | 4.33 (3.34–5.32) |
| Lymphocyte count, ×109/L | 2.39 (1.98–2.92) |
| Haemoglobin, g/L | 140 (120–151) |
| Platelet count, ×109/L | 285 (252–337) |
| Total cholesterol, mg/dL | 209.5 (180.0–237.0) |
| Triglycerides, mg/dL | 109.5 (74.0–173.0) |
| HDL-c, mg/dL | 49.5 (43.0–60.0) |
| LDL-c, mg/dL | 133.5 (108.0–152.0) |
| Vitamin B12, pg/mL | 326.0 (264.0–411.0) |
| Folate, ng/mL | 8.0 (6.9–11.0) |
| Vitamin D, ng/mL | 10.00 (6.00–16.00) |
| Ferritin, ng/mL | 62.5 (22.0–95.0) |
| Hba1c, % | 5.55 (5.20–5.80) |
| BASDAI score | 4.40 (3.40–5.90) |
Abbreviations: CAD, coronary artery disease; MRI, magnetic resonance imaging; AS, ankylosing spondylitis; NSAID, nonsteroidal anti-inflammatory drug; TNF, tumour necrosis factor; BMI, body mass index; WC, waist circumference; WtHR, waist-to-height ratio; WBC, white blood cell; HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; HbA1c, glycated haemoglobin A1c; BASDAI, bath ankylosing spondylitis disease activity index.
This group of patients was considered younger (the median age was less than 40 years), with more men than women (60% vs. 40%). The patients were not generally alcohol users (approximately 6%), and 38.5% of them were smokers. The dominant group of patients (almost 40%) had a university degree, and more than 70% were married. The delay in diagnosis for approximately 75% of the patients was less than 3 months. Approximately 50% of the patients had a positive family history of AS. The patients did not have any comorbidities other than inflammatory back pain (more than 93%), whereas 16% of the patients had peripheral arthritis or uveitis. Enthesitis occurred in 26% of the patients. The majority of patients (79%) had bilateral enhancement of the disease documented by MRI. With respect to AS therapy, 34% of the patients did not use any drugs, whereas 29% of the patients used two different drugs. The average disease duration was 5 years.
Table 2 shows the biochemical and clinical parameter comparisons in the cIMT tertile subgroups of ankylosing spondylitis patients. Older patients and patients with higher BMIs, WCs, WtHRs, and total cholesterol concentrations, such as LDL-c and HbA1c, had significantly higher cIMT values in the current study. The folate concentration was the highest in the patients with the highest cIMT values. Additionally, the BASDAI score was significantly greater in the third cIMT tertile group.
Table 2.
Comparison of biochemical and clinical parameters among cIMT tertile subgroups of ankylosing spondylitis patients.
| cIMT (mm) | p | |||
|---|---|---|---|---|
| ≤0.480 | 0.481–0.565 | ≥0.566 | ||
| Age, years | 30 (26–34) | 37 (33–42) ** | 49 (41–52) ***,### | <0.001 |
| BMI, kg/m2 | 23.5 (21.4–26.7) | 26.8 (23.2–28.7) * | 28.5 (24.3–30.9) ** | 0.007 |
| WC, cm | 89.0 (83.5–92.5) | 92.0 (86.0–108.0) * | 101.0 (88.0–108.0) ** | 0.011 |
| WtHR | 0.511 (0.494–0.534) | 0.547 (0.509–0.606) ** | 0.582 (0.548–0.632) *** | <0.001 |
| Total cholesterol, mg/dL | 193.0 (164.5–221.0) | 206.0 (177.0–231.0) | 224.0 (191.5–241.5) * | 0.038 |
| LDL-c, mg/dL | 122 (98–150) | 127 (110–143) | 145 (124–156) * | 0.109 |
| HbA1c, % | 5.30 (5.10–5.55) | 5.50 (5.00–5.80) | 5.80 (5.55–6.00) ***,## | <0.001 |
| Folate, ng/mL | 8.0 (6.0–12.0) | 7.9 (5.0–9.0) | 9.0 (8.0–11.0) ## | 0.037 |
| BASDAI score | 3.90 (3.25–5.1) | 4.00 (3.30–4.90) | 5.50 (4.40–6.60) *,# | 0.043 |
| Right carotid thickness, mm | 0.440 (0.400–0.455) | 0.520 (0.500–0.560) *** | 0.650 (0.620–0.710) ***,### | <0.001 |
| Left carotid thickness, mm | 0.440 (0.400–0.450) | 0.520 (0.500–0.540) *** | 0.650 (0.620–0.710) ***,### | <0.001 |
For the P-Kruskal–Wallis test, subgroup comparisons were performed via the Mann–Whitney U test. *, **, and *** p < 0.05, 0.01, and 0.001 vs. first cIMT tertile subgroup, respectively. #, ##, and ### p < 0.05, 0.01, and 0.001 vs. second cIMT tertile subgroup, respectively. Abbreviations: BMI, body mass index; WC, waist circumference; WtHR, waist-to-height ratio; LDL-c, low-density lipoprotein cholesterol; HbA1c, glycosylated haemoglobin A1c; BASDAI, bath ankylosing spondylitis disease activity index.
cIMT was significantly positively correlated with age, BMI, WC, WtHR, total and LDL-c concentrations, and HbA1c level (Table 3). These correlations are expected because all of them are well-established risk factors for atherosclerosis, and cIMT is a physical measure of plaque magnitude.
Table 3.
Spearman’s nonparametric correlation between the cIMT average value and other parameters.
| cIMT Average | |
|---|---|
| Age, years | 0.414 *** |
| BMI, kg/m2 | 0.213 * |
| WC, cm | 0.253 * |
| Total cholesterol, mg/dL | 0.231 * |
| LDL-c, mg/dL | 0.232 * |
| HbA1c, % | 0.254 * |
| WtHR | 0.328 *** |
Abbreviations: BMI, body mass index; WC, waist circumference; LDL-c, low-density lipoprotein cholesterol; HbA1c, glycated haemoglobin A1c; WtHR, waist-to-height ratio, * p < 0.05; *** p < 0.01.
Considering the existing relationship between cIMT levels and other patient-related parameters which are characteristic of the disease, we performed MLR to find the best model of cIMT value predictors. This analysis is presented in Table 4. Parameters were selected among those that, in Spearman’s nonparametric correlations, showed a significant correlation with cIMT. The starting model consisted of the following parameters: BASDAI score, LDL-c, triglycerides, BMI, folate, and WC. This analysis enabled the best model selection, which comprises the parameters WC, LDL-C, and BASDAI score, with an adjusted R2 = 0.113.
Table 4.
Multiple linear regression analysis (backwards selection) of the best predictor model.
| Parameters (Model’s Adjusted R2 = 0.113) | Unstandardized Coefficients | p | |
|---|---|---|---|
| B (95th CI) | Std. Error | ||
| WC, cm | 0.002 (0.000–0.005) | 0.001 | 0.037 |
| LDL-c, mg/dL | 0.001 (0.000–0.001) | 0.000 | 0.060 |
| Bath ankylosing spondylitis disease activity index (BASDAI) score | 0.013 (0.000–0.026) | 0.006 | 0.048 |
CI—confidence interval. Abbreviations: WC, waist circumference; LDL-c, low-density lipoprotein cholesterol.
Factorial analysis (principal component analysis, PCA) was implemented to reduce the number of variables to a small number of factors grouped by the same variability. Additionally, this analysis enabled the factors’ transformation into numerically characterized variables, i.e., scores, which could be further used for subsequent analysis to test their predictive ability. The total model explained 41% of the variability in the set of parameters. Analysis adequacy was confirmed by the KMO measure of sample adequacy (0.551) and Bartlett’s test of sphericity (p < 0.001). The detailed results are presented in Table 5.
Table 5.
PCA-derived factors in AS patients.
| Factors | Variables Included in the Factor | Loadings of the Variables | Factor Variability, % (Total Variance: 41%) |
|---|---|---|---|
| Haematology–lipid-related factor | Ferritin | 0.719 | 12 |
| Haemoglobin | 0.704 | ||
| HDL-c | −0.536 | ||
| Triglycerides | 0.530 | ||
| Proinflammatory–prothrombotic-related factor | Platelet count | 0.730 | 11 |
| Neutrophil count | 0.696 | ||
| C-reactive protein | 0.661 | ||
| LDL-c–vitamin-related factor | Vitamin D level | 0.731 | 9 |
| Vitamin B12 level | 0.621 | ||
| LDL-c | −0.506 | ||
| Age–glucometabolic-related factor | Age | 0.739 | 9 |
| HbA1c | 0.707 |
Abbreviations: HDL-c, high-density lipoprotein cholesterol; LDL-c, low-density lipoprotein cholesterol; HbA1c, glycosylated haemoglobin A1c.
The first factor, the haematology- and lipid-related factor, included ferritin, haemoglobin, HDL-c, and triglycerides, among which only HDL-c had negative loading, and this factor explained 12% of the data variability. The second factor (proinflammatory–prothrombotic-related factor) consisted of three parameters, platelet count, neutrophil count, and CRP, all of which had positive loadings, explaining 11% of the variability. The third factor was the LDL-c–vitamin-related factor, which included vitamins D and B12, as well as the LDL-c concentration, and explained 9% of the total model variation. The fourth factor (age–glucometabolic-related factor) consisted of age and HbA1c, both of which had positive loadings and 9% variability. Afterwards, we performed binary logistic regression analysis with the scores produced in the PCA to test its ability to predict high cIMT (third tertile vs. first tertile of the average cIMT value). The results of this analysis are presented in Table 6.
Table 6.
Univariate binary logistic regression analysis for high cIMT and high BASDAI value prediction.
| Factors (Univariate Analysis) | B (SE) |
Wald Coeff. | OR (95% CI) |
p |
|---|---|---|---|---|
| cIMT | ||||
| Age–glucometabolic-related factor | 2.084 (0.500) | 17.359 | 8.035 (3.015–21.414) | <0.001 |
SE, standard error; OR, odds ratio (95th CI, confidence interval); p, binary logistic regression analysis. Abbreviations: cIMT, carotid intima–media thickness.
High cIMT (third tertile and above) was significantly predictive of age-related factors (age and HbA1c).
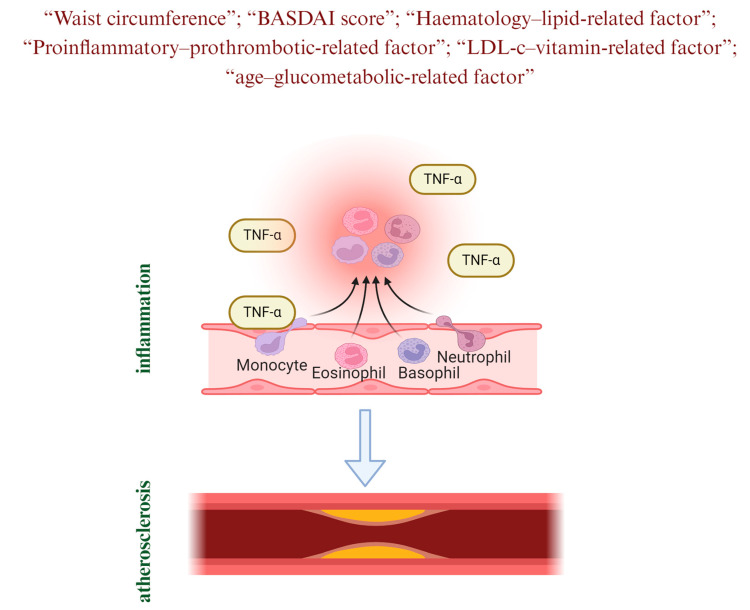
A schematic representation created on the basis of the results of the current study is presented in Figure 1.
Figure 1.
Pathophysiology of cardiovascular disease in ankylosing spondylitis.
4. Discussion
The current study examined the relationship between AS and subclinical atherosclerosis via heterogeneous parameters, such as biochemical markers and cIMT, as simple, cost-effective, and non-invasive diagnostic tools. To the best of our knowledge, this is the first study that has examined a variety of cardiometabolic factors in relation to cIMT in patients with AS via a comprehensive statistical approach by clustering different cardiometabolic risk factors in relation to subclinical atherosclerosis. Although some of the studies reported no difference in cIMT between AS patients and controls [13,14,15], others reported higher cIMT in AS patients than in healthy individuals [2,3,10,15].
The main findings of the current study indicate that WC, LDL-c, and the disease activity score, i.e., the BASDAI score, are independently correlated with cIMT in AS patients. This is the first study that has reported an independent association between WC and cIMT in patients with AS; the findings were similar between non-AS patients and nondiabetic patients in studies with a cross-sectional [16,17] and longitudinal design [18]. Central obesity was also shown to be significantly associated with cIMT in clinically healthy individuals [18].
Visceral compartments of adipose tissue are a significant source of proinflammatory cytokines that favour an increase in neutrophil infiltration and changes in the functional characteristics of macrophages. The potential of neutrophils to secrete proinflammatory cytokines, reactive oxygen species, and a variety of proteolytic enzymes, along with their ability to invade the vascular wall, can provoke damage to the endothelium, which precedes the development of atherosclerosis [5,19].
The novel finding is also the independent positive correlation between LDL-c and cIMT in AS patients. This finding contrasts with previous findings [8,20,21] that included a smaller sample size than our current study did. In the general population, the results are also contradictory [18,22].
LDL is involved in the early stage of atherosclerosis. The recruitment of inflammatory cells to the subendothelial layer and the promotion of prothrombotic alterations on the cell surface of the endothelium are important properties of LDL. In the presence of ROS (macrophages and smooth muscle cells are the major sources of ROS), LDL becomes oxidized and is thus transformed into oxidized (ox)-LDL, which exerts prothrombotic effects through the activation of platelets and thrombus formation [23].
Our findings of the association between the BASDAI score and cIMT support the hypothesis that disease activity may influence endothelial dysfunction [21]. The obtained results are in line with those of some studies that reported a relationship between the BASDAI score and cIMT [3,21] but oppose those of other studies that reported no relationship between the disease activity score and subclinical atherosclerosis [8,20]. However, Yilmaz et al. [3] reported only a weak but not independent correlation between cIMT and the BASDAI score. Similarly, Verma et al. [21] did not confirm the independent correlation between the BASDAI score and cIMT in AS patients.
There are no previous studies on factorial analysis (PCA) in relation to cIMT in AS patients. In the present study, a factorial analysis extracted four panels of biomarkers, i.e., “haematology- and lipid-related factors” (i.e., ferritin, haemoglobin, HDL-c, and triglycerides), “proinflammatory–prothrombotic-related factors” (i.e., platelet count, neutrophil count, and CRP), “LDL-c–vitamin-related factors” (i.e., vitamins D and B12, and LDL-c), and “age–glucometabolic-related factors” (i.e., age and HbA1c).
Among these four clusters, the latter (i.e., age and HbA1c) showed the ability to independently predict high cIMT (third tertile vs. first tertile of the average cIMT value). The independent relationship between age and cIMT in AS patients has recently been confirmed [3,9]. Herein, we have shown the joint influence of age and HbA1c on increased cIMT in AS patients. Zhou et al. [24] reported that in a study of a Chinese nondiabetic population that included nearly 3500 participants, HbA1c was associated with an increased risk of cIMT. Age exhibited an interaction influence on this relationship, which was more evident in individuals aged <60 years in particular.
Atherosclerosis is a multifactorial process. Increased inflammation and oxidative stress, dyslipidaemia, and a prothrombotic state are related to atherosclerosis [5].
There is an assumption that inflammation per se can worsen the lipid profile in terms of increasing serum LDL-c and triglyceride levels and lowering serum HDL-c and can thus aggravate atherosclerosis, which might at least partially explain the increased cardiovascular risk in patients with AS (1).
To the best of our knowledge, the relationship between ferritin and cIMT has not been previously shown in individuals with AS, whereas such a correlation was previously confirmed in a healthy population [25]. As an acute-phase reactant, serum ferritin levels increase in parallel with the increase in inflammation levels [26], which might in part explain the higher levels of ferritin and dyslipidaemia in the highest tertile of the cIMT group.
Inflammation, oxidative stress, and thrombosis are mutually involved in atherosclerosis progression [7]. In parallel with the enhanced proinflammatory milieu, the process of thrombosis is accelerated. Cytokines may influence the physiological functions, size, and morphology of haematological parameters and aggravate the prothrombotic milieu [27].
In the present study, we found that clusters of higher vitamin D and vitamin B12 and lower LDL-c were related to higher cIMT in AS patients (i.e., “LDL-c–vitamin-related factor”). These unexpected results might be explained by the assumption that some of the patients might have used vitamin D and B12 supplementation, which might reduce cIMT and inflammation [28,29].
One of the strengths of the current study is the relatively larger sample size included compared to that of previous studies [2,3,8,9,10] that examined the association between cIMT and AS. Moreover, we have applied PCA to investigate the relationships between different clusters of cardiometabolic biomarkers and cIMT in patients with AS in greater detail since different panels of biomarkers can reflect different signalling pathways of the disease. One of the limitations of this study is its cross-sectional design, which does not allow us to examine the causality between the examined variables. The inclusion of a control group with propensity score-based matching would be highly important for future investigations to gain deeper knowledge regarding AS and cardiometabolic risk. Additionally, we were limited in terms of data on nutritional habits and vitamin D and B12 supplementation, which might influence the related results. Since this was a single-centre study, multiethnic and multicentre studies are needed to further examine such issues. We were not able to measure lipoprotein subclasses or ox-LDL, which might significantly contribute to a deeper understanding of atherogenic dyslipidaemia in AS.
5. Conclusions
In addition to traditional cardiometabolic risk factors such as WC and LDL-c, the disease activity score (BASDAI) is independently related to subclinical atherosclerosis in AS patients. The joint involvement of heterogeneous cardiometabolic risk factors, such as a panel of biomarkers, i.e., “haematology–lipid-related factors” (i.e., ferritin, haemoglobin, HDL-c, and triglycerides), “proinflammatory–prothrombotic-related factors” (i.e., platelet count, neutrophil count, and CRP), “LDL-c–vitamin-related factors” (i.e., vitamins D and B12, and LDL-c), and “age–glucometabolic-related factors” (i.e., age and HbA1c), may reflect different pathophysiological processes of subclinical atherosclerosis in patients with AS. New longitudinal studies are needed to confirm our results.
Acknowledgments
This work was financially supported in part by a grant from the Ministry of Education, Science and Innovation, Montenegro, and the Ministry of Education, Science and Technological Development, Republic of Serbia (project number: 451-03-65/2024-03/200161).
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and laboratory analyses were performed by O.C., B.K., F.B.C., H.E. and F.M. Statistical analysis was performed by J.K.-S. The first draft of the manuscript was written by A.K., and all authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Recep Tayyip Erdogan University Non-invasive Clinical Research Ethics Committee (protocol code E-40465587-050.01.04-1041, 2024/92 and date of approval 7 May 2024).
Informed Consent Statement
Informed consent was obtained from all participants.
Data Availability Statement
All of the data generated or analysed during this study are included in this article. The data will be available upon reasonable request (contact persons: filiz.mercantepe@saglik.gov.tr and aleksandranklisic@gmail.com).
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
The authors thank the Recep Tayyip Erdogan University Development Foundation who provided funding for the open access publishing of this study (grant number: 02024004018071).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Łosińska K., Korkosz M., Kwaśny-Krochin B. Endothelial Dysfunction in Patients with Ankylosing Spondylitis. Reumatologia. 2019;57:100–105. doi: 10.5114/reum.2019.84815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sertdemir A.L., Şahin A.T., Duran M., Çelik M., Tatar S., Oktay İ., Alsancak Y. Association between Syndecan-4 and Subclinical Atherosclerosis in Ankylosing Spondylitis. Medicine. 2024;103:E37019. doi: 10.1097/MD.0000000000037019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yilmaz P.D., Kadiyoran C., Goktepe M.H., Akkubak Y., Icli A., Kucuk A. Syndecan 1 May Slow the Progression of Subclinical Atherosclerosis in Patients with Ankylosing Spondylitis. Clin. Exp. Hypertens. 2023;45:2156529. doi: 10.1080/10641963.2022.2156529. [DOI] [PubMed] [Google Scholar]
- 4.Eriksson J.K., Jacobsson L., Bengtsson K., Askling J. Is Ankylosing Spondylitis a Risk Factor for Cardiovascular Disease, and How Do These Risks Compare with Those in Rheumatoid Arthritis? Ann. Rheum. Dis. 2017;76:364–370. doi: 10.1136/annrheumdis-2016-209315. [DOI] [PubMed] [Google Scholar]
- 5.Macvanin M., Gluvic Z., Klisic A., Manojlovic M., Suri J., Rizzo M., Isenovic E. The Link between miRNAs and PCKS9 in Atherosclerosis. Curr. Med. Chem. 2023:6926–6956. doi: 10.2174/0109298673262124231102042914. [DOI] [PubMed] [Google Scholar]
- 6.Klisic A., Patoulias D. The Role of Endocan in Cardiometabolic Disorders. Metabolites. 2023;13:640. doi: 10.3390/metabo13050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vukicevic P., Klisic A., Neskovic V., Babic L., Mikic A., Bogavac-Stanojevic N., Matkovic M., Putnik S., Aleksic N., Kotur-Stevuljevic J. New Markers of Platelet Activation and Reactivity and Oxidative Stress Parameters in Patients Undergoing Coronary Artery Bypass Grafting. Oxid. Med. Cell. Longev. 2021;2021:8915253. doi: 10.1155/2021/8915253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cure E., Icli A., Uslu A.U., Sakiz D., Cure M.C., Baykara R.A., Yavuz F., Arslan S., Kucuk A. Atherogenic Index of Plasma: A Useful Marker for Subclinical Atherosclerosis in Ankylosing Spondylitis: AIP Associate with CIMT in AS. Clin. Rheumatol. 2018;37:1273–1280. doi: 10.1007/s10067-018-4027-0. [DOI] [PubMed] [Google Scholar]
- 9.Aydoğan Baykara R., Küçük A., Tuzcu A., Tuzcu G., Cüre E., Uslu A.U., Omma A. The Relationship of Serum Visfatin Levels with Clinical Parameters, Flow-Mediated Dilation, and Carotid Intima-Media Thickness in Patients with Ankylosing Spondylitis. Turk. J. Med. Sci. 2021;51:1865–1874. doi: 10.3906/sag-2012-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tocoglu A.G., Dogan B., Tuzun S., Akcay E.U., Altas A.E., Ayhan L.T., Yilmaz S., Genc A.C., Gonullu E., Tamer A. The Relationship between Salusin-α, Salusin-β, and Klotho Levels with Subclinical Atherosclerosis in Ankylosing Spondylitis. Eur. Rev. Med. Pharmacol. Sci. 2023;27:9838–9845. doi: 10.26355/eurrev_202310_34160. [DOI] [PubMed] [Google Scholar]
- 11.Rudwaleit M., Van Der Heijde D., Landewé R., Akkoc N., Brandt J., Chou C.T., Dougados M., Huang F., Gu J., Kirazli Y., et al. The Assessment of SpondyloArthritis International Society Classification Criteria for Peripheral Spondyloarthritis and for Spondyloarthritis in General. Ann. Rheum. Dis. 2011;70:25–31. doi: 10.1136/ard.2010.133645. [DOI] [PubMed] [Google Scholar]
- 12.Bönisch A., Ehlebracht-König I. The BASDAI-D—An Instrument to Defining Disease Status in Ankylosing Spondylitis and Related Diseases. Z. Rheumatol. 2003;62:251–263. doi: 10.1007/s00393-003-0519-6. [DOI] [PubMed] [Google Scholar]
- 13.Sari I., Okan T., Akar S., Cece H., Altay C., Secil M., Birlik M., Onen F., Akkoc N. Impaired Endothelial Function in Patients with Ankylosing Spondylitis. Rheumatology. 2006;45:283–286. doi: 10.1093/rheumatology/kei145. [DOI] [PubMed] [Google Scholar]
- 14.Erre G.L., Sanna P., Zinellu A., Ponchietti A., Fenu P., Sotgia S., Carru C., Ganau A., Passiu G. Plasma Asymmetric Dimethylarginine (ADMA) Levels and Atherosclerotic Disease in Ankylosing Spondylitis: A Cross-Sectional Study. Clin. Rheumatol. 2011;30:21–27. doi: 10.1007/s10067-010-1589-x. [DOI] [PubMed] [Google Scholar]
- 15.Hatipsoylu E., Şengül I., Kaya T., Karatepe A.G., Akçay S., Isayeva L., Bozkaya G., Tatar E. Assessment of Subclinical Atherosclerotic Cardiovascular Disease in Patients with Ankylosing Spondylitis. Anatol. J. Cardiol. 2019;22:185–191. doi: 10.14744/AnatolJCardiol.2019.13367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alizargar J., Bai C.H. Factors Associated with Carotid Intima Media Thickness and Carotid Plaque Score in Community-Dwelling and Non-Diabetic Individuals. BMC Cardiovasc. Disord. 2018;18:21. doi: 10.1186/s12872-018-0752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alizargar J., Bai C.H. Value of the Arterial Stiffness Index and Ankle Brachial Index in Subclinical Atherosclerosis Screening in Healthy Community-Dwelling Individuals. BMC Public. Health. 2019;19:65. doi: 10.1186/s12889-019-6398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szabóová E., Lisovszki A., Kolarčik P., Fatĺová E., Molnár T., Bujdoš M., Szabó P. Impact of Classical Risk Factors on Subclinical Carotid Atherosclerosis Progression: Insights from a Non-Diabetic Cohort. Rev. Cardiovasc. Med. 2024;25:103. doi: 10.31083/j.rcm2503103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klisic A., Radoman Vujačić I., Vučković L.J., Ninic A. Total Leukocyte Count, Leukocyte Subsets and Their Indexes in Relation to Cardiovascular Risk in Adolescent Population. Eur. Rev. Med. Pharmacol. Sci. 2021;25:3038–3044. doi: 10.26355/eurrev_202104_25557. [DOI] [PubMed] [Google Scholar]
- 20.Gupta N., Saigal R., Goyal L., Agrawal A., Bhargava R., Agrawal A. Carotid Intima Media Thickness as a Marker of Atherosclerosis in Ankylosing Spondylitis. Int. J. Rheumatol. 2014;2014:839135. doi: 10.1155/2014/839135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verma I., Krishan P., Syngle A. Predictors of Atherosclerosis in Ankylosing Spondylitis. Rheumatol. Ther. 2015;2:173–182. doi: 10.1007/s40744-015-0017-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Song P., Fang Z., Wang H., Cai Y., Rahimi K., Zhu Y., Fowkes F.G.R., Fowkes F.J.I., Rudan I. Global and Regional Prevalence, Burden, and Risk Factors for Carotid Atherosclerosis: A Systematic Review, Meta-Analysis, and Modelling Study. Lancet Glob. Heal. 2020;8:e721–e729. doi: 10.1016/S2214-109X(20)30117-0. [DOI] [PubMed] [Google Scholar]
- 23.Gąsecka A., Rogula S., Szarpak Ł., Filipiak K.J. LDL-Cholesterol and Platelets: Insights into Their Interactions in Atherosclerosis. Life. 2021;11:39. doi: 10.3390/life11010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou M., Zhang J., Jia J., Liu Y., Guo M., Lv X., Zhao X., Chen S. Association between Hemoglobin A1c and Asymptomatic Carotid Intima-Media Thickness in Middle-Aged and Elderly Populations without Diabetes. Nutr. Metab. Cardiovasc. Dis. 2022;32:1463–1469. doi: 10.1016/j.numecd.2022.02.003. [DOI] [PubMed] [Google Scholar]
- 25.Ma H., Lin H., Hu Y., Li X., He W., Jin X., Gao J., Zhao N., Song B., Pan B., et al. Serum Ferritin Levels Are Associated with Carotid Atherosclerosis in Chinese Postmenopausal Women: The Shanghai Changfeng Study. Br. J. Nutr. 2015;114:1064–1071. doi: 10.1017/S0007114515001944. [DOI] [PubMed] [Google Scholar]
- 26.Sciacqua A., Ventura E., Tripepi G., Cassano V., D’Arrigo G., Roumeliotis S., Maio R., Miceli S., Perticone M., Andreozzi F., et al. Ferritin Modifies the Relationship between Inflammation and Arterial Stiffness in Hypertensive Patients with Different Glucose Tolerance. Cardiovasc. Diabetol. 2020;19:123. doi: 10.1186/s12933-020-01102-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Welsh C., Welsh P., Mark P.B., Celis-Morales C.A., Lewsey J., Gray S.R., Lyall D.M., Iliodromiti S., Gill J.M.R., Pell J., et al. Association of Total and Differential Leukocyte Counts with Cardiovascular Disease and Mortality in the UK Biobank. Arterioscler. Thromb. Vasc. Biol. 2018;38:1415–1423. doi: 10.1161/ATVBAHA.118.310945. [DOI] [PubMed] [Google Scholar]
- 28.Säidifard N., Tangestani H., Djafarian K., Shab-Bidar S. Serum Vitamin D Level and Carotid Intima-Media Thickness: A Systematic Review and Meta-Analysis of Observational Studies and Randomized Control Trials. Horm. Metab. Res. 2020;52:305–315. doi: 10.1055/a-1153-0657. [DOI] [PubMed] [Google Scholar]
- 29.Shirode P.S., Parekh A.D., Patel V.V., Vala J., Jailmalani A.M., Vora N.M., Gummala V., Patel J.S., Shriram N. Early Detection of Subclinical Atherosclerosis: Hyperhomocysteinaemia as a Promising Marker in Adolescents With Vitamin B Deficiency. Cureus. 2023;15:6–13. doi: 10.7759/cureus.41571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All of the data generated or analysed during this study are included in this article. The data will be available upon reasonable request (contact persons: filiz.mercantepe@saglik.gov.tr and aleksandranklisic@gmail.com).