Abstract
The geographic situation of the Iberian Peninsula makes it a natural link between Europe and North Africa. However, it is a matter of debate to what extent African influences via the Straits Gibraltar have affected Iberia's prehistoric development. Because early African pastoralist communities were dedicated to cattle breeding, a possible means to detect prehistoric African–Iberian contacts might be to analyze the origin of cattle breeds on the Iberian Peninsula. Some contemporary Iberian cattle breeds show a mtDNA haplotype, T1, that is characteristic to African breeds, generally explained as being the result of the Muslim expansion of the 8th century A.D., and of modern imports. To test a possible earlier African influence, we analyzed mtDNA of Bronze Age cattle from the Portalón cave at the Atapuerca site in northern Spain. Although the majority of samples showed the haplotype T3 that dominates among European breeds of today, the T1 haplotype was found in one specimen radiocarbon dated 1800 calibrated years B.C. Accepting T1 as being of African origin, this result indicates prehistoric African–Iberian contacts and lends support to archaeological finds linking early African and Iberian cultures. We also found a wild ox haplotype in the Iberian Bronze Age sample, reflecting local hybridization or backcrossing or that aurochs were hunted by these farming cultures.
Keywords: ancient DNA, aurochs, Iberian cattle, mithochondrial DNA, Africa
The geographical proximity of the Iberian Peninsula to Africa makes the Straits of Gibraltar a likely contact zone between the two continents. Early human communities are known to have existed simultaneously on both sides of the Straits, and it seems possible that interaction between these communities took place with an interchange of populations, ideas, goods, and livestock (1, 2). The hypothesis that such contacts took place, resulting in an African influence on Iberia's prehistoric development, is thus not a recent one (3) but was overshadowed in the early 1960s by new ideas claiming a Near Eastern origin for the Iberian Neolithic (4).
Evidence of human occupation in central Spain before the beginning of the Neolithic, as defined by the introduction of agriculture, is scarce (5, 6). However, by 6000 B.C., it is evident that Neolithic cultures were present along the eastern Spanish Mediterranean coast as well as in Andalusia, represented by the cave culture. Only a few centuries later, the signs of Neolithization are also clear in central Spain. This rapid spread of pastoral communities across the peninsula is proposed to have been due either to colonization by the Andalusian cave culture (7–9) or to the spread of new technology and ideas from the Mediterranean cultures to indigenous Mesolithic hunter-gatherers (10, 11). These early Neolithic populations of Andalusia appear to have consisted of a number of distinct groups (12), one of which is suggested to have African origin due to finds of characteristic red ochre ceramics (13, 14). Similarities have also been noted between the predynastic Badarian Egyptian culture dated to the 5th millennium B.C. and the Late Atlantic Neolithic culture in western Andalusia (14). Previously, the appearance of the Late Atlantic Neolithic culture had been placed at a significantly later date than the Egyptian culture, and this chronology and the cultural similarity were interpreted as implying that Egypt was the original source (14). However, more accurate radiocarbon dates obtained from Late Atlantic Neolithic culture sites subsequently redated the origin of this culture to being approximately the same as that of the predynastic Badarian Egyptian culture (15), leading to the hypothesis that these two cultures might derive from a common area, perhaps through pastoral groups living in the Sahara. The culture linked to the Late Atlantic Neolithic period is known to have been dedicated almost exclusively to cattle breeding, secondarily complemented by sheep and goat breeding (14), suggesting that an investigation of the origin of Iberian cattle may offer further insight into early Iberian–African cultural contacts.
The origin and diversification of domestic cattle (Bos taurus) have been investigated with mtDNA analyses on modern and ancient cattle specimens. Five mtDNA haplogroups have been described in cattle from Europe and Africa, denoted T, T1, T2, T3, and the primigenius type. The most frequently observed haplogroup in Europe is T3, which, along with T and T2, is common in the Near East (16, 17). Haplogroup T1 is common in Africa, where it is also believed to have originated (17, 18), and it has also been observed in extant Iberian and Latin American cattle breeds (19–22) but not elsewhere in Europe. The primigenius haplogroup is only known from six British aurochs (Bos primigenius) remains (16, 17).
It is presumed that most cattle breeds on the Iberian Peninsula, like Central European cattle, originate from the Near East (17, 19), either from introduction via the mainland route or via the Mediterranean littoral route (20). Moreover, North African Berbers may have introduced some Iberian breeds from Africa, in conjunction with the Muslim expansion of A.D. 710 (20). However, the finding based on nuclear markers that contemporary Iberian breeds are more closely related to African cattle than to Central European cattle (19, 23) is explained with more recent gene flow from Africa derived in the 1960s and 1970s. It has also been speculated that local aurochs could have contributed to the gene pool (24, 25).
In this study, we test the possibility of a much earlier influence of African cattle on the Iberian Peninsula through the analysis of ancient DNA from excavated domestic cattle remains in Iberia dated to the Bronze Age. The detection of the T1 haplogroup in prehistoric Iberian cattle specimens would suggest an introduction of African cattle into Iberia much earlier than has previously been thought, an event that would imply early contacts over the Straits of Gibraltar.
Materials and Methods
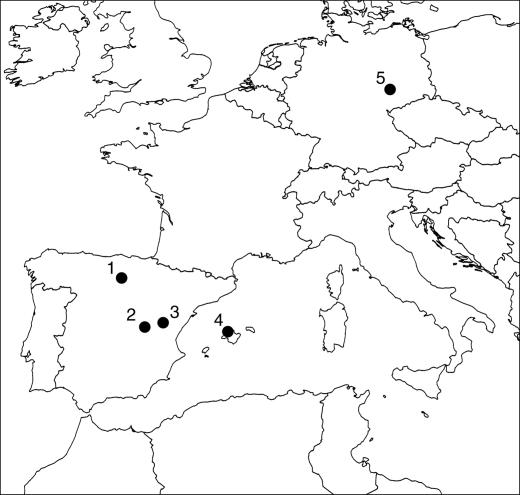
Archaeological Sites, Material, and Sampling. Forty-seven domestic cattle teeth and bones were sampled from the Iberian Peninsula and Central Europe (see Fig. 1). Fourteen of the Iberian samples are Bronze Age material from the Portalón cave at the Atapuerca site, 15 km east of Burgos. Several sites from the Sierra de Atapuerca have provided a valuable record of northern Spain in the Lower and Middle Pleistocence (26, 27), as well as providing a considerable amount of Holocene material (28). The faunal analysis from the Bronze Age period indicates a predominance of domestic species, such as cattle and ovicaprids, over wild species (mostly red deer). The bone and teeth specimens selected for this study derive from an area excavated in 2000. This area of cave stratigraphy is partially disturbed, but the finds could be assigned to two distinct levels (level 3 and 4) belonging to the Bronze Age period, confirmed by radiocarbon dates of 1760–1440 calibrated years (cal) B.C. (beta-153360) and 2200–1940 cal B.C. (beta-153361) for level 3 and 4, respectively (29).
Fig. 1.
Map shows sites sampled in this study. 1, Portalón cave of the Atapuerca site and the medieval site of San Pablo Burgos, both in Spain; 2, Valparasio de Abajo, Cuenca, Spain; 3, Cueva de Joaquin, Teruel, Spain; 4, Cueva Mosset, Mallorca, Escorca, Spain; 5, the Central European sites of Zauschwitz and Werben, both in Germany.
To gain a wider context through a larger sample set from prehistoric Spain, nine additional specimens from Spain kept in the Museo Nacional de Ciencias Naturales were collected. This material had been identified as Neolithic from the archaeological context. Radiocarbon dating was performed on one sample from each of the two main sites represented, Cuenca and Teruel. The sample from the Cuenca site was found to be Bronze Age dated to 2670 cal B.C. (Ua-23541), and the Teruel sample was found to be medieval dated to A.D. 1120 (Ua-23540). Additionally, seven samples dated to the late Middle Ages (14th to 16th century) from a medieval Dominican monastery of San Pablo, in Burgos in northern Spain, were included.
Finally, 17 Early to Late Neolithic Central European samples of morphologically well preserved domestic cattle bones from culturally dated closed contexts were selected. The samples originated from a region between the cities of Dresden (51° N, 13° 45′ E) and Leipzig (51° 20′ N, 12° 20′ E) in Germany. This area (Fig. 1) belongs to the heartland of the first Early Neolithic farmers, the Linearbandkeramik culture dated to 5500–5000 cal B.C. (30), and the region can be regarded as topographically and culturally coherent for this period.
DNA Extraction, Amplification, and Sequencing. DNA was extracted from pulverized cattle bone and teeth by using hybridization and magnetic bead separation after releasing the DNA from the bone apatite complex by using a phosphate buffer. This method (described in the supporting information, which is published on the PNAS web site) is intended to retrieve as many targeted sequences as possible while keeping the extract clean from PCR inhibitors. The control region was amplified in three overlapping fragments by using published primer pairs An2F-An1R and An1F-An3R (16, 31) and an additional primer pair, Ko1-Ko2. A second PCR was performed to label the product with biotin for the pyrosequencing stage (Biotage AB, Uppsala), the method used for sequence determination (32, 33). Sequencing primers were designed along the three overlapping fragments and added following the supplied protocol (see the supporting information).
Authenticity of DNA Sequences. We applied criteria for the authenticity of the ancient DNA sequences as described in ref. 34. Every sequence considered as being authentic was based on three independent amplifications from a minimum of two independent extractions resulting in identical sequence results through sequencing of overlapping fragments by using pyrosequencing or standard chain termination DNA sequencing. Pyrosequencing was used to detect miscoding lesions present in ancient DNA molecules (see the supporting information).
Phylogenetic Analysis. Sequences were edited and aligned by using the PSQ 96 MA snp software system (Biotage AB) and sequencher 4.1.4 (Gene Codes, Ann Arbor, MI). Reduced median networks were constructed according to ref. 35. The 216-bp sequences included in the network analysis cover positions 16042–16158 and 16179–16277 in the B. taurus reference sequence (36).
Results and Discussion
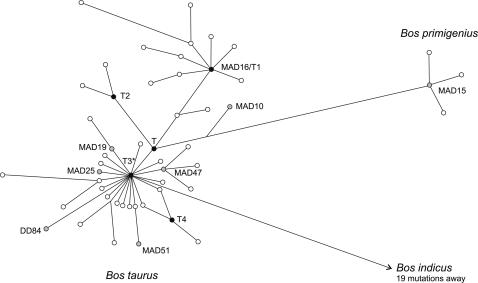
Of the 47 ancient cattle specimens analyzed, 30 gave sufficient reproducible sequence information for the three overlapping fragments (Table 1). The phylogenetic analysis (Fig. 2) assigned 27 samples (all 8 German samples and 19 Iberian samples) to haplogroup T3. One Iberian sample, MAD10, belonged to haplogroup T. This finding shows that haplogroup T3, which dominates among modern domestic European cattle, was already common in Central Europe during Neolithic times and in Iberia during the Bronze Age. Similarly, haplogroup T, present with low frequency in modern European cattle, also existed as an apparently rare variant in northern Iberia during the Bronze Age. This pattern would be compatible with domestic cattle of Near Eastern origin spreading into the Iberian Peninsula from the European mainland via France, or perhaps by a more rapid route from the Near East along the littoral Mediterranean.
Table 1. Samples analyzed in this study and their location, age, and assigned haplogroup.
| Sample | Location | Age | Haplogroup |
|---|---|---|---|
| MAD2* | Portalón, Atapuerca | 1780 cal B.C.† | T3 |
| MAD3 | Portalón, Atapuerca | 1780 cal B.C.† | T3 |
| MAD5 | Portalón, Atapuerca | 1780 cal B.C.† | T3 |
| MAD6* | Portalón, Atapuerca | 1635 cal B.C.‡ | T3 |
| MAD8 | Portalón, Atapuerca | 1780 cal B.C.† | T3 |
| MAD9* | Portalón, Atapuerca | 1780 cal B.C.† | T/T3§ |
| MAD10 | Portalón, Atapuerca | 1780 cal B.C.† | T |
| MAD11 | Portalón, Atapuerca | 1780 cal B.C.† | T3 |
| MAD14 | Portalón, Atapuerca | 1780 cal B.C.† | T3 |
| MAD15* | Portalón, Atapuerca | 1740 cal B.C.‡ | primigenius |
| MAD16* | Portalón, Atapuerca | 1800 cal B.C.‡ | T1 |
| MAD17 | Portalón, Atapuerca | 1780 cal B.C.† | T3 |
| MAD18 | Portalón, Atapuerca | 1780 cal B.C.† | T3 |
| MAD19 | San Pablo, Burgos | A.D. 1300-1500¶ | T3 |
| MAD20* | San Pablo, Burgos | A.D. 1300-1500¶ | T3 |
| MAD22 | San Pablo, Burgos | A.D. 1300-1500¶ | T/T3§ |
| MAD23 | San Pablo, Burgos | A.D. 1300-1500¶ | T3 |
| MAD25* | San Pablo, Burgos | A.D. 1300-1500¶ | T3 |
| MAD45 | Cueva Mosset, Mallorca, Escorca | Ancient∥ | T3 |
| MAD46 | Valparaiso de Abajo, Cuenca | Ancient** | T3 |
| MAD47 | Cueva de Joaquin, Teruel | A.D. 1120‡ | T3 |
| MAD49 | Valparasio de Abajo, Cuenca | Ancient** | T3 |
| MAD50 | Cueva de Joaquin, Teruel | Ancient** | T3 |
| MAD51 | Valparaiso de Abajo, Cuenca | 2670 B.C.‡ | T3 |
| DD24 | Zauschwitz | 4900-4400 B.C.¶ | T3 |
| DD25 | Zauschwitz | 4900-4400 B.C.¶ | T3 |
| DD27 | Zauschwitz | 4900-4400 B.C.¶ | T3 |
| DD29 | Zauschwitz | 3100-2500 B.C.¶ | T3 |
| DD36 | Zauschwitz | 4900-4400 B.C.¶ | T3 |
| DD83 | Werben | 3800-3300 B.C.¶ | T3 |
| DD84 | Werben | 3800-3300 B.C.¶ | T3 |
| DD85 | Werben | 3800-3300 B.C.¶ | T3 |
Sequence data from all three amplified fragments [16042-16158 and 16163-16313, according to the reference sequence (31)] were retrieved for all samples except MAD9 and MAD22, which both provided only reproduced data from two of the fragments and therefore were excluded from the network construction.
Independently reproduced
Date estimates for specimens that were not directly radiocarbon dated were approximated by using the average of the five radiocarbon dates from sediment and bone samples found in the same layer
Direct radiocarbon dating
Not included in the network analysis
Contextual dating
No reliable date available
Single radiocarbon date from the site, but not from the specimen
Fig. 2.
Reduced median network [Network 4.109 (30); threshold set for 2] of 313 sequences downloaded from GenBank and 30 ancient Bos sequences presented in this study. Six haplogroups, T, T1, T2, T3, the Asian haplogroup T4 (38), and the primigenius haplogroup, are labeled. The position for the ancient sequences presented here that do not fall within the center of the T3 cluster are also labeled. The ancient sequences in the T3 cluster, marked with an asterisk, are MAD2, MAD3, MAD5, MAD6, MAD8, MAD11, MAD14, MAD17, MAD18, MAD20, MAD23, MAD45, MAD46, MAD49, MAD50, DD24, DD25, DD27, DD29, DD36, DD83, and DD85.
Two Bronze Age specimens from the cave complex of Atapuerca in northern Spain showed deviating haplotypes. Sample MAD15, a permanent upper left premolar radiocarbon dated to 1740 cal B.C. (Ua-22027), was of the primigenius haplogroup, whereas MAD16, a permanent lower right premolar radiocarbon dated to 1800 cal B.C. (Ua-22028), belonged to the African haplogroup T1. The finding of the primigenius haplogroup was unexpected, because the MAD15 specimen was morphologically classified as representing a domesticated cow. This classification was supported by the archaeological context and the faunal analysis of some 1,000 bovid remains (28). The presence of the primigenius haplotype could be the result of independent domestication of local aurochs or backcrossing between domesticates and the wild ancestor. If correct, this process must have involved wild females, because mtDNA is maternally inherited. Aurochs were once widespread across Asia, Europe, and North Africa and were present on the Iberian Peninsula during the Late Neolithic (37–40); thus, there is a theoretical possibility for backcrossing.
It should be recognized that several of the diagnostic morphological features that distinguish wild bovids from domestic bovids concern horn and cranial elements, none of which are present in the excavated area dated to the Bronze Age. Although a premolar does contain some morphological information, it does not provide conclusive evidence for distinguishing between domestic cattle and aurochs. Even if the archeological context supports a domestic origin, it cannot be ignored that this sample may represent a hunted wild animal from the local area. The finds of red deer and wild boar at the Portalón site are evidence for hunting being practiced, and aurochs could have been one of the hunted game animals.
The presence of T1 in MAD16 is, to our knowledge, the earliest identification of this haplotype on the Iberian Peninsula. Accepting this haplogroup as being of African origin (17) would indicate an influence of African cattle on the Iberian Peninsula during the Bronze Age or earlier. Early signs of Neolithization are present in the Iberian southern coastal regions at ≈6000 B.C. (7, 10). Some of these early communities, whether being locals adapting to new ideas or recently immigrated pastoralists bearing these new ideas, have distinctive cultural traits linking them to Africa (13, 14). These contacts, as well as the spread of agriculture, must be seen as a continuing process in which ideas and practices were being interchanged. Given that only 13 km separate Africa from Iberia over the Straits of Gibraltar, an African influence does not seem implausible. Cattle from Africa are known to carry traits such as heat resistance (41), which could have been desirable to southern Iberian pastoral communities, or the Iberian pastoralists might simply have wished to increase the size of their herds.
It is also necessary to consider the possibility that the ancient distribution of aurochs haplotypes did not reflect the modern distribution in domestic cattle. Specifically, although it is generally thought that T1 originated in Africa (17, 20, 42), this haplogroup may have been present among Iberian aurochs in prehistoric times. If this presence were the case, then the finding of T1 in the MAD16 sample could be taken to indicate local domestication, or at least local aurochs being incorporated into early cattle stock. The best way to test whether the presence of T1 in modern Iberian cattle is due to local aurochs introgression would be with genetic data from ancient Iberian aurochs. Unfortunately, there is no such data available to either support or dismiss this possibility. However, the fact that those European aurochs so far analyzed show only the distinct primegenius haplogroup (16, 17) gives no indication of T1 having been present in Europe.
Supplementary Material
Acknowledgments
We thank Judith Oexle and Uwe Reuter (both of Landesamt für Archäologie mit Landesmuseum für Vorgeschichte, Dresden, Germany) for providing samples from Germany; Begoña Sanchez (Museo Nacional de Ciencias Naturales) for providing samples from central Spain; and Bo Gräslund, Laura Juez, Elena Santos, Rolf Quam, and Jan Storå for providing useful comments on the manuscript. This work was supported by the Swedish Research Council and Dirección General de Investigación Científica y Técnicia of Spain Project BOS 2003-08938-C03-01; Svenska Arkeologiska Samfundet (Rosa and Viktor Tengborgs travel grant), the German Academic Exchange Service, and a Göransson-Sandviken travel grant (all to C.A.); and a research grant from the Atapuerca Foundation of Burgos (to A.I.O.). Funding for fieldwork in Spain was provided by the Consejería de Cultura y Turismo of Junta Castilla y León.
Author contributions: A.G. designed research; C.A., A.B., C.S., and A.G. performed research; J.M.C., A.I.O., R.E., J.L.A., H.E., and A.G. contributed new reagents/analytic tools; C.A., P.P., C.S., J.L.A., H.E., and A.G. analyzed data; and C.A. wrote the paper.
Abbreviation: cal, calibrated years.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database (accession nos. AY847188–AY847219).
References
- 1.Harrison, R. & Gilman, A. (1977) in Ancient Europe and the Mediterranean, ed. Markotic, V. (Aris & Phillips, Warminster, U.K.), pp. 89-104.
- 2.Ramos, J., Castañeda, V., Bernal, D., Herrero, N. & Pérez, M. (2003) in El Abrigo y Cueva de Benzú en la Prehistoria de Ceuta: Aproximación al Estudio de las Sociedades Cazadoras-Recolectoras y Tribales Comunitarias en el Ámbito Norteafricano del Estrecho de Gibraltar, eds. Ramos, J., Bernal, D. & Castañeda, V. (Universidad de Cádiz, Cádiz, Spain), pp. 405-413.
- 3.Martínez Santa-Olalla, J. (1946) Esquema Paleontológico de la Península Hispánica (Seminario de la Historia Primitiva del Hombre, Madrid).
- 4.Tarradell, M. (1960) in Primer Symposium de Prehistoria de la Península Ibérica (Institución Príncipe de Viana, Pamplona, Spain), pp. 45-67.
- 5.Zilhão, J. (1993) J. Med. Archaeol. 61, 5-63. [Google Scholar]
- 6.Zilhão, J. (2000) in Europe's First Farmers, ed. Price, D. T. (Cambridge Univ. Press, Cambridge, U.K.), pp. 144-182.
- 7.Municio, L. (1988) in El Neolítico en España, ed. López, P. (Cátedra, Madrid), pp. 299-327.
- 8.Estremera, M. S. (2003) in Primeros Agricultores y Ganaderos en la Meseta Norte: El Neolítico de la Cueva de Vaquera, Torreiglesias, Segovia (Junta de Castilla y León, Zamora, Spain), pp. 19-26, 47-131, 209-214.
- 9.Delibes, G. & Fernández, J. (2000) in Pré-Historia Recente da Peninsula Ibérica, ed. Oliveira Jorge, V. (ADECAP, Porto, Portugal), Vol. 4, pp. 95-122. [Google Scholar]
- 10.Hernando, A. (1999) Los Primeros Agricultores de la Península Ibérica (Sintesis, Madrid).
- 11.Jiménez, J. (1999) Sagvntvm-Plav Extra 2, 493-501. [Google Scholar]
- 12.Caro, A. (2002) Ensayo Sobre Cerámica en Arqueología (Agrija, Seville, Spain).
- 13.Camps, G. (1984) in Scripta Praehistorica: Francisco Jordá Oblata, ed. Fortea, J. (University of Salamanca, Salamanca, Spain), pp. 187-208.
- 14.Escacena, J. L. (2000) in Prehistoric Iberia: Genetics, Anthropology, and Linguistics, ed. Arnaiz-Villena, A. (Kluwer–Plenum, New York), pp. 125-162.
- 15.Mederos, A. (1996) Spal 5, 45-86. [Google Scholar]
- 16.Bailey, J. F., Richards, M. B., Macaulay, V. A., Colson, I. B., James I. T., Bradley, D. G., Hedges, R. E. & Sykes, B. C. (1996) Proc. R. Soc. London Ser. B 263, 1467-1473. [DOI] [PubMed] [Google Scholar]
- 17.Troy, C. S., MacHugh, D. E., Bailey, J. F., Magee, D. A., Loftus, R. T., Cunningham, P., Chamberlain, A. T., Sykes, B. C. & Bradley, D. G. (2001) Nature 410, 1088-1091. [DOI] [PubMed] [Google Scholar]
- 18.Bradley, D. G., MacHugh, D. E., Cunningham, P. & Loftus, R. T. (1996) Proc. Natl. Acad. Sci. USA 93, 5131-5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beja-Pereira, A., Alexandrino, P., Bessa, I., Carretero, Y., Dunner, S., Ferrand, N., Jordana, J., Laloe, D., Moazami-Goudarzi, K., Sanchez, A., et al. (2003) J. Hered. 94, 243-250. [DOI] [PubMed] [Google Scholar]
- 20.Cymbron, T., Loftus, R. T., Malheiro, M. I. & Bradley, D. G. (1999) Proc. R. Soc. London Ser. B 266, 597-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Magee, D. A., Meghen, C., Harrison, S., Troy, C. S., Cymbron, T., Gaillard, C., Morrow, A., Maillard, J. C. & Bradley, D. G. (2002) J. Hered. 93, 429-432. [DOI] [PubMed] [Google Scholar]
- 22.Miretti, M. M., Dunner, S., Naves, E. P., Contel, E. P. & Ferro, J. A. (2004) J. Hered. 95, 450-453. [DOI] [PubMed] [Google Scholar]
- 23.Beja-Pereira, A., Alexandrino, P., Bessa, I., Gama, L. & Ferrand, N. (2002) Anim. Genet. 33, 295-300. [DOI] [PubMed] [Google Scholar]
- 24.Davidson, I. (1989) in The Walking Larder: Patterns of Domestication, Pastoralism, and Predation, ed. Clutton-Brock, J. (Unwin Hyman, London), pp. 59-71.
- 25.Martín-Burriel, I., García-Muro, E. & Zaragoza, P. (1999) Anim. Genet. 30, 177-182. [DOI] [PubMed] [Google Scholar]
- 26.Arsuaga, J. L., Bermúdez de Castro, J. M. & Carbonell, E., eds. (1997) J. Hum. Evol. 33 (2/3), 105-421. [Google Scholar]
- 27.Bermúdez de Castro, J. M., Carbonell, E. & Arsuaga, J. L., eds. (1999) J. Hum. Evol. 37 (3/4), 309-700. [DOI] [PubMed] [Google Scholar]
- 28.Clark, G. A. (1979) in The North Burgos Archaeological Survey: Bronze Age and Iron Age Archaeology on the Meseta del Norte (Province of Burgos, Northern Spain), ed. Clark, G. A. (Arizona State Univ., Tempe), p. 307.
- 29.Ruiz-Zapata, M. B., Ortega, A. I., Dorado, M., Valdeolmillos, A., Gil, M. J., Arsuaga, J. L., Carretero, J. M., Martínez, I. & Pérez-González, A. (2003) in Quaternary Climatic Changes and Environmental Crisis in the Mediterranean Region, eds. Ruíz-Zapata, M. B., Dorado, M., Valdeolmillos, A., Gil, M. J., Bardají, T., Bustamante, I. & Martínez, I. (Iversidad de Alcalá de Henares, Alcalá de Henares, Madrid), pp. 99-106.
- 30.Benecke, N. (1994) Archäozoologische Studien zur Entwicklung der Haustierhaltung in Mitteleuropa und Südskandinavien von den Anfängen bis zum Ausgehenden Mittelalter (Deutsches Archäologisches Institut, Berlin).
- 31.Loftus, R. T., MacHugh, D. E., Bradley, D. G., Sharp, P. M. & Cunningham P. (1994) Proc. Natl. Acad. Sci. USA 91, 2757-2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ronaghi, M. (2003) Methods Mol. Biol. 212, 189-195. [DOI] [PubMed] [Google Scholar]
- 33.Ronaghi, M., Uhlén, M. & Nyrén, P. (1998) Science 281, 363-365. [DOI] [PubMed] [Google Scholar]
- 34.Cooper, A. & Poinar, H. N. (2000) Science 289, 1139. [DOI] [PubMed] [Google Scholar]
- 35.Bandelt, H.-J., Forster, P., Sykes, B. C. & Richards, M. B. (1995) Genetics 141, 743-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anderson, S., de Bruijn, M. H., Coulson, A. R., Eperon, I. C., Sanger, F. & Young, I. G. (1982) J. Mol. Biol. 156, 683-717. [DOI] [PubMed] [Google Scholar]
- 37.Morales, A. (2003) in Primeros Agricultores y Ganaderos en la Meseta Norte: El Neolítico de la Cueva de Vaquera, Torreiglesias, Segovia (Junta de Castilla y León, Zamora, Spain), pp. 305-313.
- 38.Delibes, G., Romero, F., Escudero, Z., Sanz, C., Mariscal, B., Cubero, C., Uzaquiano, P., Morales, A., Liesau, C. & Calonge, G. (1995) in Arqueología y Medio Ambiente: El Primer Milenio A.C. en el Duero Medio, eds. Delibes, G., Romero, F. & Morales, A. (Junta de Castilla y León, Valladolid, Spain), pp. 543-582.
- 39.Riquelme, J. A. (2003) Numantia 8, 56-61. [Google Scholar]
- 40.Conlin-Hayes, E. (2003) Carel 1, 83-143. [Google Scholar]
- 41.Paula-Lopes, F. F., Chase, C. C., Jr., Al-Katanani, Y. M., Krininger, C. E., III, Rivera, R. M., Tekin, S., Majewski, A. C., Ocon, O. M., Olson, T. A. & Hansen, P. J. (2003) Reproduction 125, 285-294. [DOI] [PubMed] [Google Scholar]
- 42.Hanotte, O., Bradley, D. G., Ochieng, J. W., Verjee, Y., Hill, E. W. & Rege, J. E. (2002) Science 296, 336-339. [DOI] [PubMed] [Google Scholar]
- 43.Mannen, H., Tsuji, S., Loftus, R. T. & Bradley, D. G. (1998) Genetics 150, 1169-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.