Abstract
Asian tarsiid and sivaladapid primates maintained relictual distributions in southern Asia long after the extirpation of their close Holarctic relatives near the Eocene–Oligocene boundary. We report here the discovery of amphipithecid and eosimiid primates from Oligocene coastal deposits in Pakistan that demonstrate that stem anthropoids also survived in southern Asia beyond the climatic deterioration that characterized the Eocene–Oligocene transition. These fossils provide data on temporal and paleobiogeographic aspects of early anthropoid evolution and significantly expand the record of stem anthropoid evolution in the Paleogene of South Asia.
Keywords: phylogeny, Paleogene, South Asia
For decades, the abundant primate fossils from the Paleogene of Afro–Arabia provided the primary record of early anthropoid primate evolution. However, over the last decade, it has become increasingly clear that Asia (China, Thailand, and Myanmar) also played an important role in the origins and early diversification of that group (1–10). Eocene amphipithecid and eosimiid primates now figure prominently in models of the early higher-primate radiation. Regardless of the ongoing controversy over their affinities (11–17), notably their anthropoid status, Eosimiidae and Amphipithecidae document a long history of primate evolution during the Eocene of Asia. Such a successful adaptive radiation naturally raises questions regarding the subsequent evolutionary history of Eosimiidae and Amphipithecidae in Asia. In the absence of an adequate Oligocene fossil record from South Asia, it was generally hypothesized that both families may have left no descendant in Asia (18, 19), as was the case for the flourishing Eocene primates from northern continents (adapiforms and omomyiforms), which are virtually unknown during the paleontologically well documented Oligocene (20). Recent collaborative field expeditions (February and March 2000–2004) to the South Gandoï syncline of the Bugti Hills (Balochistan, Pakistan) (Fig. 1) by the Mission Paléontologique Française au Balouchistan and the Earth Sciences Division of the Pakistan Museum of Natural History have enabled us to excavate the primate-bearing locality of Paali Nala (DBC2). This site is situated in the lowermost levels of the Lower Chitarwata Formation, which is early Oligocene in age (21). During the course of this field work, intensive screen-washing operations have allowed the recovery of several dozen primate fossils (primarily isolated teeth). Here, we describe two previously undescribed genera that we refer to the families Amphipithecidae and Eosimiidae. These fossils extend the stratigraphic range of both families into the Oligocene, thereby underscoring the taxonomic diversity that was achieved by anthropoid primates in South Asia during the Paleogene.
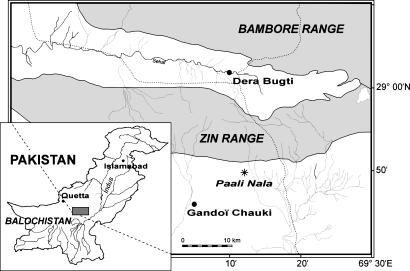
Fig. 1.
Map of the Bugti Hills (central Pakistan, eastern Balochistan) showing the geographic location of the primate-bearing locality of Paali Nala (DBC2, denoted by an asterisk) in the lowermost part of the Chitarwata Formation (Oligocene, Bugti Member) (21). The locality is situated in the South Gandoï syncline (southern side of the Zin anticline), ≈30 km from the village of Dera Bugti.
Systematic Paleontology
Order Primates Linnaeus, 1758; Suborder Anthropoidea Mirvart, 1864; Family Amphipithecidae Godinot, 1994.
Bugtipithecus, Gen. Nov. Type species. Bugtipithecus inexpectans sp. nov.
Etymology. The genus name refers to the Bugti tribe (Greek pithekos, apes).
Diagnosis. As for the type species.
Bugtipithecus inexpectans Sp. Nov. Etymology. Epithet in reference to the unexpected occurrence of higher primates in the Oligocene of South Asia.
Holotype. UMC-DBC 2174, right M1 (Fig. 2J), temporarily housed in the Palaeontology Department, University of Montpellier.
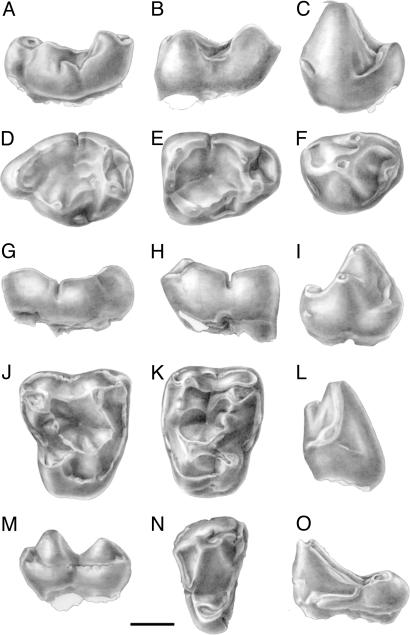
Fig. 2.
Amphipithecidae from Paali Nala DBC2. Bugtipithecus inexpectans gen. sp. nov.: UMC-DBC 2175, right M3: buccal (A), occlusal (D), and lingual (G) views; UMC-DBC 2177, right M1: buccal (B), occlusal (E), and lingual (H) views; UMC-DBC 2178, left P4: buccal (C), occlusal (F), lingual (I), and mesial (L) views; UMC-DBC 2174, right M1 [holotype]: occlusal (J), and buccal (M) views; UMC-DBC 2173, left M2: occlusal view (K); UMC-DBC 2191, left P4: occlusal (N), and mesial (O) views. (Scale bar, 1 mm.) Drawings are from L. Meslin (Centre National de la Recherche Scientifique).
Horizon and locality. Bugti Member, Lower Chitarwata Formation (early Oligocene), Paali Nala DBC2, Bugti Hills (Balochistan, Pakistan; Fig. 1).
Diagnosis. Small-bodied amphipithecid, similar in size to the modern mouse lemur Mirza coquereli. Differs from Myanmarpithecus, Siamopithecus, and Pondaungia (including “Amphipithecus”) (7) in having cusps less inflated and more marginally positioned, upper molars with a relatively high degree of “waisting” lingual to the metacone, a stronger hypoparacrista, a complete lingual cingulum, lower molars showing a massive but deeply notched lingual talonid wall (strong postmetacristid and preentocristid), a smaller and more lingually positioned M3 heel, and in lacking enamel wrinkles on the occlusal surfaces of both upper and lower teeth. Body-mass estimate of 350 g is based on M1 area (from all primate least-squares regression equation) (22). For hypodigm, description, and metrics, see supporting information, which is published on the PNAS web site.
Comparisons. The Amphipithecidae are generally known to be large-bodied [6–9 kg (Pondaungia and Siamopithecus)] or medium-sized [1–2 kg (Myanmarpithecus)] primates, having upper and lower cheek teeth strongly bunodont with moderately to highly crenulated enamel surfaces. Bugtipithecus gen. nov. contrasts with the classic amphipithecid condition in being much smaller and in having teeth with more acute cusps and smooth enamel surfaces. Although differing in these respects, Bugtipithecus also exhibits a set of features otherwise found only in the Amphipithecidae. For instance, as in Pondaungia, Siamopithecus, and Myanmarpithecus, M1–2 in Bugtipithecus exhibit a relatively well developed hypocone on the distolingual cingulum that is united with the protocone (via the postprotocrista) by a strong prehypocrista. In Bugtipithecus, the postprotocrista is not as buccally restricted as it is in Siamopithecus. Rather, it extends distobuccally, as it does in a number of other Paleogene taxa customarily regarded as Anthropoidea (Eosimiidae, Proteopithecidae, Oligopithecidae, and Propliopithecidae) and also, but to a lesser degree, in some omomyiform (e.g., Omomys) and adapiform (e.g., sivaladapids) lineages. In contrast to Siamopithecus, Myanmarpithecus and Pondaungia have small conules on their upper molars. The M1–2 of Bugtipithecus have a distinct metaconule but differ from those of Myanmarpithecus and Pondaungia in lacking the paraconule. The presence of well developed conules on the upper molars is a fairly widespread condition in omomyiforms and also in some adapiforms. In contrast, most of the early anthropoid lineages have reduced [Eosimiidae (Eosimias and Phenacopithecus), and Proteopithecidae] or absent [Eosimiidae (Bahinia), Oligopithecidae, and Propliopithecidae] conules, although exceptions to this generalization are found among parapithecids (e.g., Parapithecus and Apidium) and Algeripithecus (23), which resemble amphipithecids (Pondaungia and Myanmarpithecus) in bearing well developed conules. In Bugtipithecus, the metaconule is connected lingually to the postprotocrista (and not to the “hypometaconule crista” as in omomyiforms and adapiforms) and buccally to a very short crest, which is probably homologous with the hypometacrista found in Siamopithecus and in many stem and crown anthropoids (e.g., Bahinia, Eosimias, Phenacopithecus, Catopithecus, Oligopithecus, Moeripithecus, Aegyptopithecus, pliopithecoids, and platyrrhines). Mesial and parallel to this short hypometacrista, upper molars of Bugtipithecus display an oblique hypoparacrista (distinct from the postparaconule crista occurring in most omomyiforms and some adapiforms), which is a character observable in many anthropoids (except Proteopithecus) but with different degrees of development (i.e., lower, thinner, or shorter). In Bugtipithecus, the hypoparacrista is long, strong, and elevated, as in Siamopithecus and Bahinia. In most species of early primates, the preprotocrista connects to the paraconule or terminates midway between the paracone and protocone if the paraconule is absent. The preparaconule crista generally represents a buccal extension of the preprotocrista and usually connects to a small parastyle. Upper molars of Bugtipithecus, as well as those of Pondaungia, exhibit a condition unusual among early primates in lacking the preparaconule crista. The anterior cingulum is therefore not interrupted, as it is in most primate species, extending continuously from the protocone to the parastyle. Upper molars in Bugtipithecus bear a strong and continuous lingual cingulum, as in early anthropoids (except Parapithecus and Siamopithecus, which show considerable lingual inflation of the protocone). This aspect of the lingual cingulum differs from the condition found in many omomyiforms and adapiforms, in which this cingulum is not continuous but broken lingual to the protocone. As in propliopithecids (Propliopithecus, Moeripithecus, and Aegyptopithecus) and all other amphipithecids, the M1–2 in Bugtipithecus have a cuspate hypocone. This character is not as well developed in oligopithecids (especially Oligopithecus) and proteopithecids, in which a small enamel swelling occurs on a distolingual expansion of the lingual cingulum (Catopithecus and Proteopithecus). Eosimiids have a similar distolingual expansion of the M1–2 talon region but have no hypocone.
The P4 of Bugtipithecus closely resembles that of Siamopithecus in being moderately exodaenodont and simply constructed with a low and mesiodistally short talonid (lingually closed by the strong development of the hypocristid) and in having a small and low hypoconid. As in Siamopithecus, the trigonid of P4 in Bugtipithecus does not have a distinct paraconid, and the metaconid is present and situated inferiorly and distally with respect to the strong protoconid. The P4 of Bugtipithecus differs from that of Siamopithecus in lacking the cristid obliqua and shows a stronger development of the lingual talonid crest. These aspects of P4 morphology match conditions in eosimiids and differ from conditions in oligopithecids, proteopithecids, propliopithecids, and, to some extent, Pondaungia, in all of which P4 has a stronger metaconid and a more important mesiodistal development of the talonid (broad basin, except for Serapia), with the occasional presence of a distinct entoconid (especially in oligopithecids). The P4 in Bugtipithecus further differs from that of oligopithecids in lacking the paraconid. The M1 and M3 of Bugtipithecus are remarkably similar to those of Myanmarpithecus in showing a very strong development of both the postmetacristid and preentocristid, M1 without any development of the paraconid and hypoconulid and M3 with a narrow talonid heel that is lingual in position. These aspects of lower molar morphology in Bugtipithecus differ from the condition observable in eosimiids, oligopithecids, and proteopithecids, in which the lower molars (notably M1) possess a paraconid and have a well developed and cuspidate hypoconulid. Lower molars of propliopithecids lack development of a paraconid as well but, in contrast, possess a strong hypoconulid, as in all other early and more recent Old-World anthropoid primates. The absence of a hypoconulid on the lower molars of Bugtipithecus and Myanmarpithecus is a shared–derived character of both genera but does not characterize all amphipithecids, inasmuch as Siamopithecus and Pondaungia show weak development of that cuspid.
Family Eosimiidae. Beard et al., 1994.
Phileosimias Gen. Nov. Type species. Phileosimias kamali sp. nov.
Included species. Phileosimias brahuiorum sp. nov.
Etymology. The name means “the ally of Eosimias” (Greek philios, ally).
Diagnosis. Eosimiid of the size of Phenacopithecus. Upper molars differ from those of Eosimias, Phenacopithecus, and Bahinia in having cuspate conules, weaker development of lingual and buccal cingula, less waisting distolingual to the metacone (particularly evident in Eosimias and Phenacopithecus), and in lacking both hypoparacrista and hypometacrista. Lower molars have the hypoconulid located slightly more lingual to the midline than in other eosimiids, and the P4 has no mesiolingual cingulid.
Phileosimias kamali Sp. Nov. Etymology. The species name is in honor of our intrepid friend Kamal Madjidulah (Director of The Star, Karachi, Pakistan), in recognition of his high efficiency in organizing the French paleontological missions in the Bugti Hills and his efforts toward promoting cultural knowledge in Pakistan. Holotype. UMC-DBC 2199, right M1 (Fig. 3F), temporarily housed in the Palaeontology Department, University of Montpellier.
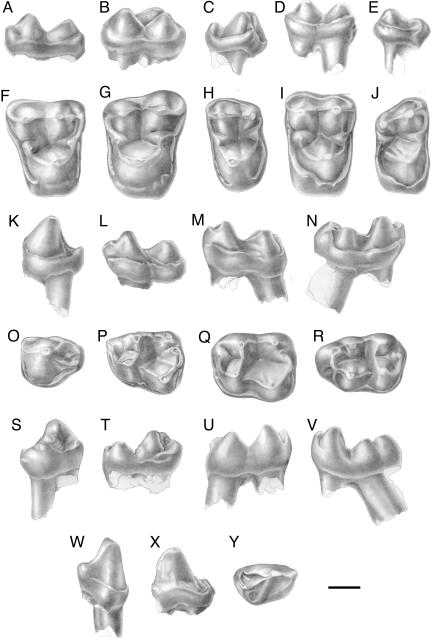
Fig. 3.
Eosimiidae from Paali Nala DBC2. Phileosimias kamali gen. sp. nov.: UMC-DBC 2199, right M1 [holotype]: buccal (A), and occlusal (F) views; UMC-DBC 2197, left M2: buccal (B), and occlusal (G) views; UMC-DBC 2198, left M3: buccal (C), and occlusal (H) views; UMC-DBC 2204, left P4: buccal (K), occlusal (O), lingual (S), and mesial (W) views; UMC-DBC 2206, left M1: buccal (L), occlusal (P), and lingual (T) views; UMC-DBC 2207, left M2: buccal (M), occlusal (Q), and lingual (U) views; UMC-DBC 2208, right M3: buccal (N), occlusal (R), and lingual (V) views; UMC-DBC 2203, right P3: lingual (X), and occlusal (Y) views. Phileosimias brahuiorum gen. sp. nov.: UMC-DBC 2221, right M2: buccal (D), and occlusal (I) views; UMC-DBC 2220, right M3: buccal (E), and occlusal (J) views. (Scale bar, 1 mm.) Drawings are from L. Meslin (Centre National de la Recherche Scientifique).
Horizon and locality. Bugti Member, Lower Chitarwata Formation (early Oligocene), Paali Nala DBC2, Bugti Hills (Balochistan, Pakistan; Fig. 1).
Diagnosis. Differs from Phileosimias brahuiorum sp. nov. in being slightly larger, in showing more triangular and distally waisted upper molars, a buccal expansion of the metacone, no hypocone, a massive and lingually inflated protocone, the metacone of M3 distal to the paracone, and in having less extensive lingual and buccal cingula. Body-mass estimate of 250 g, based on M1 area (22). For hypodigm, description, and metrics, see supporting information.
Phileosimias brahuiorum Sp. Nov. Etymology. The name derives from “Brahui,” the second Baloch language, in homage to all speakers of this language.
Holotype. UMC-DBC 2221, right M2 (Fig. 3I), temporarily housed in the Palaeontology Department, University of Montpellier.
Horizon and locality. As for Phileosimias kamali sp. nov.
Diagnosis. Differs from Phileosimias kamali sp. nov. in having upper molars with more rectangular and transverse outline (not distally waisted), better development of buccal and lingual cingula, the presence of a minute but distinct hypocone, a slender protocone, no buccal expansion of the metacone, and in showing the metacone of M3 distally more lingual with respect to the paracone. For hypodigm, description, and metrics, see supporting information.
Comparisons. At first glance, when considering upper molars only, Phileosimias may appear morphologically divergent with respect to other eosimiids (Eosimias, Bahinia, and Phenacopithecus). Indeed, the presence of cuspate conules, weaker development of the buccal and lingual cingula (especially in Phileosimias kamali), and the absence of both hypoparacrista and hypometacrista differ from the typical eosimiid dental pattern and could even better match that of omomyiforms (notably Omomys). Eosimiids generally exhibit an important distolingual expansion of the lingual cingulum (talon region), have a moderately (Eosimias and Phenacopithecus) to strongly (Bahinia) developed buccal cingulum, and show minute (Eosimias and Phenacopithecus) to indistinct (Bahinia) conules. However, the weak development of buccal and lingual cingula on the upper molars of Phileosimias is not uniform within the genus because a couple of specimens attributed to Phileosimias brahuiorum show well developed cingula. As in all eosimiids and more generally in early anthropoids (oligopithecids, proteopithecids, parapithecids, propliopithecids, and amphipithecids), both species of Phileosimias have upper molars without metaconule cristae (pre-, post-, and hypo-) and postparaconule crista, and lack even rudimentary development of a postprotocingulum (Nannopithex fold), common characters in adapiforms and omomyiforms (except Omomys). Upper molars of Phileosimias show, in contrast, well developed and buccally oriented pre- and postprotocristae (U-shaped protocone), which connect the paraconule and the metaconule, respectively. These teeth also exhibit a buccal expansion of their stylar regions (parastyle and metastyle), a feature that is particularly well developed in eosimiids, but which also occurs in some omomyiforms, such as Macrotarsius, Shoshonius, and Altiatlasius.
The morphology of the lower molars of Phileosimias does not depart significantly from that of other eosimiid primates. The main anatomical difference is the position of the hypoconulid, which is lingual to midline in Phileosimias and not centrally located, as in Phenacopithecus, or slightly buccal to midline, as in Eosimias and those omomyiforms that have a hypoconulid. The location of this distal cuspid in Phileosimias recalls the condition that occurs in early anthropoids from North Africa (Catopithecus, Oligopithecus, Proteopithecus, Serapia, and Arsinoea) and sivaladapid adapiforms from Asia (e.g., Hoanghonius and Guangxilemur), in which the hypoconulid is more lingual and frequently twinned with the entoconid. In Phileosimias, as in all anthropoids, the cristid obliqua on M1 is invariably lateral and reaches the base of the trigonid wall at a point distal to the protoconid rather than distolingual to the protoconid or to the metaconid, as in omomyiforms and adapiforms. Lower molars in Phileosimias, as in all other eosimiids, have trigonids that are open lingually and possess a strongly cuspidate paraconid. Except for M1, on which the paraconid is commonly reduced in early anthropoids (notably Proteopithecus, Serapia, Arsinoea, Catopithecus, and Oligopithecus), the paraconid is generally absent on M1–3 and the trigonid is closed lingually in anthropoids (propliopithecids, parapithecids, amphipithecids, pliopithecoids, and platyrrhines). The presence of a paraconid on all lower molars is observed in omomyiforms and tarsiids. In eosimiids, the paraconid on M2–3 is, however, widely spaced from the metaconid and sometimes mesiolingually positioned between the protoconid and the metaconid (in Phenacopithecus and Phileosimias), whereas it is generally mesial and twinned to the metaconid in omomyiforms.
The P4 of Phileosimias is remarkably similar to those of other eosimiids in showing a slight degree of exodaenodonty, a low and short talonid bearing only a small hypoconid distal to the strong protoconid, a minute to crestiform metaconid situated distally and inferiorly with respect to the protoconid, and having a simple swelling of enamel in place of the paraconid. The P4 of Phileosimias shows, however, a talonid that is more pinched buccolingually and with stronger development of the postmetacristid. These aspects of the P4 metaconid morphology and location differ from conditions in other early anthropoids (e.g., proteopithecids, oligopithecids, and propliopithecids), in which this cuspid is relatively much larger and located farther mesially with respect to the protoconid.
Discussion and Conclusions
Until recently, the Oligocene in southern Asia remained undocumented paleontologically. The discovery of a diverse primate fauna in early Oligocene coastal deposits from Pakistan (21), including representatives of sivaladapids (24), lemur-like strepsirrhines (25), eosimiids, and amphipithecids, has revealed an extensive radiation of primates in South Asia, whereas primate communities otherwise disappeared across the Holarctic continents at that time. The late Eocene–early Oligocene interval was, indeed, one of the most significant episodes of climatic deterioration during the Tertiary (26), involving environmental changes that coincide with drastic changes in faunal structure (27, 28). The temporal persistence of primate communities in southern Asia (29–31) was probably mediated by the paleogeography of this Province, which extended into lower latitudes, thus allowing virtually continuous access to tropical refugia during the middle Cenozoic climatic event (32–34). The paleolatitude of the Bugti Hills ≈31 million years ago was, indeed, ≈14° farther south than in recent times (29°N) because of the northward drift of the Indian Plate (35).
The results of our various phylogenetic analyses (Fig. 4), primarily based on morphological characters (see supporting information), consistently point toward the monophyly of a large clade, including Asian Eosimiidae, Amphipithecidae, Arabo–African Oligopithecidae, Propliopithecidae, African Proteopithecidae, Parapithecidae, and South American platyrrhine primates. Assuming this clade to be the Anthropoidea clade (10), from the present evidence, eosimiids and amphipithecids (and by extension Phileosimias and Bugtipithecus, respectively) are stem anthropoids (17) and, as such, support the hypothesis that Asia was the ancestral homeland of the Anthropoidea clade (1–6, 10). The discovery of Phileosimias and Bugtipithecus from the Oligocene of Pakistan demonstrates that eosimiids remained highly evolutionary conservative through time and that amphipithecids were very autapomorphic with respect to their coeval African relatives, which had evolved into advanced species with more or less modern anatomy (19, 36–38). This apparent evolutionary disparity between Eocene–Oligocene anthropoids of Asia and Africa suggests that anthropoids must have dispersed rapidly between the two continents (39) just after their common Asian ancestry and evolved in relative isolation on both continents during the Paleogene. The cooccurrence of eosimiids and amphipithecids in Pakistan extends considerably the paleogeographic distribution of both families, which were apparently restricted to Southeast Asia during the Eocene. Their fossil record is still scarce but is now sufficient for demonstrating that anthropoids were a diverse and successful group in South Asia during the Paleogene. A simple examination of body weights of the well known Eocene forms (predicted from the M1 area) (22) reveals a large spectrum of body sizes (17), ranging from very tiny species [100–400 g (Eosimias, Phenacopithecus, and Bahinia)] to much larger-bodied forms [1–2 kg (Myanmarpithecus) and 6–9 kg (Pondaungia and Siamopithecus)]. Bugtipithecus and Phileosimias document an unsuspected and more recent phase of the evolutionary history of small-bodied anthropoids in Asia. This discovery presumably provides only a limited perspective on the total anthropoid diversity in this region during the early Oligocene because it represents only one locus, and, furthermore, there is an apparent taphonomic bias because of size sorting regarding large-bodied mammals: only small [Bugtilemur, ≈100 g (25); Phileosimias, ≈250 g; and Bugtipithecus, ≈350 g] to medium-sized [Guangxilemur, ≈2 kg (24)] primate taxa have been unearthed thus far.
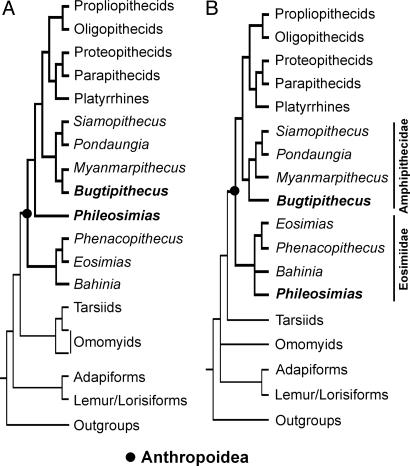
Fig. 4.
Results of phylogenetic analyses. Only simplified high-level trees of strict consensus are presented here, showing the main dichotomies among higher taxonomic primate groups (see supporting information). (A) Strict consensus of two equally most-parsimonious trees of 2,810 steps each [consistency index (CI) = 0.273; retention index (RI) = 0.53] obtained from heuristic searches performed on the dataset including some ordered multistate characters (Option 1). (B) Strict consensus of 38 equally most-parsimonious trees of 2,646 steps each (CI = 0.289; RI = 0.51) obtained from heuristic searches made on the same dataset but considering all characters unordered (Option 2).
The demonstration that anthropoids persisted in southern Asia raises the critical issue of their subsequent evolution. Early Neogene anthropoid communities from South Asia consist of members of the Dionysopithecidae, a group of primates generally considered as stem pliopithecoids among catarrhine anthropoids (40), which seemingly entered Asia from Africa (19, 40) by the early Miocene (as part of the well known faunal interchanges that occurred as a result of the collision between the Afro–Arabian and Eurasian plates) or even earlier, during the Oligocene (41, 42). The eventual extinction of eosimiids and amphipithecids may have resulted from a phenomenon of competitive exclusion when pliopithecoids arrived in South Asia. However, the possibility of continuity and Asian ancestry for some of the Miocene Asian anthropoids from Paleogene Asian forms (43), although widely contested (17, 19, 40), cannot be totally precluded in the light of these discoveries from the Oligocene of Pakistan. In many ways, the dental morphology of the genus Bugtipithecus and, in particular, the morphology of its upper molars, is strikingly reminiscent of that of dionysopithecids, notably Dionysopithecus shuangouensis Li, 1978 (44). For instance, upper molars of both taxa exhibit a strong development of the prehypocrista linking the strong hypocone to the postprotocrista, have a strong and continuous hypoparacrista connected to the preprotocrista, which is limited lingually (lack of a labial extension, i.e., preparaconule crista), and show a labial extension of the anterocingulum (mesial fovea). These upper molar similarities between Bugtipithecus and Dionysopithecus are not observed in the Propliopithecidae (Aegyptopithecus, Propliopithecus, and Moeripithecus), the group of primates from the Oligocene of Arabo–Africa customarily regarded as stem catarrhines, in which pliopithecoids are usually nested (40). Upper molars of Bugtipithecus differ, however, from those of Dionysopithecus in showing an important degree of waisting lingual to the metacone, a minute metaconule, and having a stronger parastyle. Lower molars of Bugtipithecus also differ substantially in lacking the pliopithecine triangle but also in lacking a strong hypoconulid and the distal fovea. Additional paleontological data are therefore necessary for evaluating whether these striking dental resemblances between amphipithecids and dionysopithecids are the result of functional convergences related to dietary specializations or are phylogenetically significant apomorphies.
Supplementary Material
Acknowledgments
We thank Nawab M. A. K. Bugti, Lord of the Bugti Tribes, for his invitation to visit the Bugti territory; K. Madjidulah for his courtesy and high efficiency in organizing the French paleontological missions in Balochistan; M. de Grossouvre, former Attaché de Coopération Scientifique et Universitaire (French Embassy, Islamabad, Pakistan) for his great interest in our French–Pakistani collaboration program; K. C. Beard (Carnegie Museum of Natural History, Pittsburgh); E. Seiffert (University of Oxford, Oxford) and M. Godinot (Muséum National d'Histoire Naturelle, Paris) for valuable comments and advice on fossils; R. F. Kay and B. A. Williams (Duke University, Durham, NC) for providing the data matrix of their cladistic analysis; K. C. Beard, E. Seiffert, R. Kay, and M. Richard-Marivaux for improving the English version of the manuscript; and L. Meslin [Institut des Sciences de l'Evolution de Montpellier (ISEM)] for artwork. Fieldwork was funded by the program Centre National de la Recherche Scientifique-ÉCLIPSE and the Ministry of Foreign Affairs (French Embassy, Islamabad). P.-O.A., M.B., G.M., J.-Y.C., and D.D.F. are members of the Mission Paléontologique Française au Balouchistan (MPFB). J.-L.W. and S.R.H.B. are principal investigators of the Bugti paleontological program. L.M. gratefully acknowledges the Fondation Fyssen and the Fondation Singer Polignac for funding his postdoctoral work. This is ISEM publication 2005-045 and MPFB publication 027.
Author contributions: L.M. designed research; L.M. performed research; L.M. and J.-J.J. analyzed data; L.M. wrote the paper; G.R. was manager of the field expedition and coresponsible for the Pakistani–French paleontolgical program; P.-O.A., M.B., G.M., and J.-Y.C. were responsible for researching on associated fossil mammals; D.D.F. was responsible for researching on paleobotanic fossil remains; N.I. directed many aspects of the excavations; and G.M., Y.C., J.-J.J., J.-Y.C., and D.D.F. directed many aspects of the excavations, including the recovery of primate teeth.
Abbreviations: DBC2, Dera Bugti locus C2; UMC, Université Montpellier II Collections.
References
- 1.Beard, K. C., Qi, T., Dawson, M. R., Wang, B. & Li, C. (1994) Nature 368, 604-609. [DOI] [PubMed] [Google Scholar]
- 2.Beard, K. C., Tong, Y., Dawson, M. R., Wang, J. & Huang, X. (1996) Science 272, 82-85. [Google Scholar]
- 3.Beard, K. C. & Wang, J. (2004) J. Hum. Evol. 46, 401-432. [DOI] [PubMed] [Google Scholar]
- 4.Chaimanee, Y., Suteethorn, V., Jaeger, J.-J. & Ducrocq, S. (1997) Nature 385, 429-431. [DOI] [PubMed] [Google Scholar]
- 5.Chaimanee, Y., Thein, T., Ducrocq, S., Naing Soe, A., Benammi, M., Tun, T., Lwin, T., Wai, S. & Jaeger, J.-J. (2000) Proc. Natl. Acad. Sci. USA 97, 4102-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jaeger, J.-J., Thein, T., Benammi, M., Chaimanee, Y., Soe, A. N., Lwin, T., Wai, S. & Ducrocq, S. (1999) Science 286, 528-530. [DOI] [PubMed] [Google Scholar]
- 7.Jaeger, J.-J., Chaimanee, Y., Tafforeau, P., Ducrocq, S., Soe, A. N., Marivaux, L., Sudre, J., Tun, S. T., Htoon, W. & Marandat, B. (2004) C. R. Palevol 3, 241-253. [Google Scholar]
- 8.Takai, M., Shigehara, N., Aung, A. K., Tun, S. T., Soe, A. N., Tsubamoto, T. & Thein, T. (2001) J. Hum. Evol. 40, 393-409. [DOI] [PubMed] [Google Scholar]
- 9.Marivaux, L., Chaimanee, Y., Ducrocq, S., Marandat, B., Sudre, J., Soe, A. N., Tun, S. T., Htoon, W. & Jaeger, J.-J. (2003) Proc. Natl. Acad. Sci. USA 100, 13173-13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kay, R. F., Williams, B. A., Ross, C. F., Takai, M. & Shigehara, N. (2004) in Anthropoid Origins: New Visions, eds. Ross, C. F. & Kay, R. F. (Plenum, New York), pp. 91-135.
- 11.Ciochon, R. L., Gingerich, P. D., Gunnell, G. F. & Simons, E. L. (2001) Proc. Natl. Acad. Sci. USA 98, 7672-7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ciochon, R. L. & Gunnell, G. F. (2002) Evol. Anthropol. 11, 156-168. [Google Scholar]
- 13.Schwartz, J. H. (2003) in Tarsiers: Past, Present, and Future, eds. Wright, P. C., Simons, E. L. & Gursky, S. (Rutgers Univ. Press, New Brunswick, NJ), pp. 50-96.
- 14.Simons, E. L. (2003) in Tarsiers: Past, Present, and Future, eds. Wright, P. C., Simons, E. L. & Gursky, S. (Rutgers Univ. Press, New Brunswick, NJ), pp. 9-34.
- 15.Ciochon, R. L. & Gunnell, G. F. (2004) in Anthropoid Origins: New Visions, eds. Ross, C. F. & Kay, R. F. (Plenum, New York), pp. 249-282.
- 16.Shigehara, N. & Takai, M. (2004) in Anthropoid Origins: New Visions, eds. Ross, C. F. & Kay, R. F. (Plenum, New York), pp. 323-340.
- 17.Kay, R. F., Schmitt, D., Vinyard, C. J., Perry, J. M. G., Shigehara, N., Takai, M. & Egi, N. (2004) J. Hum. Evol. 46, 3-25. [DOI] [PubMed] [Google Scholar]
- 18.Beard, K. C. (2002) in The Primate Fossil Record, ed. Hartwig, W. C. (Cambridge Univ. Press, Cambridge, U.K.), pp. 133-149.
- 19.Seiffert, E. R., Simons, E. L. & Simons, C. V. M. (2004) in Anthropoid Origins: New Visions, eds. Ross, C. F. & Kay, R. F. (Plenum, New York), pp. 157-181.
- 20.Köhler, M. & Moyà-Solà, S. (1999) Proc. Natl. Acad. Sci. USA 96, 14664-14667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Welcomme, J.-L., Benammi, M., Crochet, J.-Y., Marivaux, L., Métais, G., Antoine, P.-O. & Baloch, I. (2001) Geol. Mag. 138, 397-405. [Google Scholar]
- 22.Conroy, G. C. (1987) Int. J. Primatol. 8, 115-137. [Google Scholar]
- 23.Godinot, M. & Mahboubi, M. (1992) Nature 357, 324-326. [DOI] [PubMed] [Google Scholar]
- 24.Marivaux, L., Welcomme, J.-L., Ducrocq, S. & Jaeger, J.-J. (2002) J. Hum. Evol. 42, 379-388. [DOI] [PubMed] [Google Scholar]
- 25.Marivaux, L., Welcomme, J.-L., Antoine, P.-O., Métais, G., Baloch, I. M., Benammi, M., Chaimanee, Y., Ducrocq, S. & Jaeger, J.-J. (2001) Science 294, 587-591. [DOI] [PubMed] [Google Scholar]
- 26.Berggren, W. A. & Prothero, D. R. (1992) Eocene-Oligocene Climatic and Biotic Evolution: An Overview (Princeton Univ. Press, Princeton).
- 27.Janis, C. M. (1993) Annu. Rev. Ecol. Syst. 24, 467-500. [Google Scholar]
- 28.Meng, J. & McKenna, M. C. (1998) Nature 394, 364-367. [Google Scholar]
- 29.Thomas, H. & Verma, S. N. (1979) C. R. Acad. Sci. 289, 833-836. [Google Scholar]
- 30.Gingerich, P. D. & Sahni, A. (1984) Int. J. Primatol. 5, 63-79. [Google Scholar]
- 31.Ginsburg, L. & Mein, P. (1987) C. R. Acad. Sci. 304, 1213-1215. [Google Scholar]
- 32.Beard, K. C. (1998) in Dawn of the Age of Mammals in Asia, eds. Beard, K. C. & Dawson, M. R. (Bull. Carnegie Mus. Nat. Hist., Pittsburgh), pp. 260-277.
- 33.Qi, T. & Beard, K. C. (1998) J. Hum. Evol. 35, 211-220. [DOI] [PubMed] [Google Scholar]
- 34.Jablonski, N. G. (2003) in Tarsiers: Past, Present, and Future, eds. Wright, P. C., Simons, E. L. & Gursky, S. (Rutgers Univ. Press, New Brunswick, NJ), pp. 35-49.
- 35.Mattauer, M., Matte, P. & Olivet, J.-L. (1999) C. R. Acad. Sci. 328, 499-508. [Google Scholar]
- 36.Kay, R. F., Fleagle, J. G. & Simons, E. L. (1981) Am. J. Phys. Anthropol. 55, 293-322. [Google Scholar]
- 37.Simons, E. L. (1995) Yearb. Phys. Anthropol. 38, 199-238. [Google Scholar]
- 38.Seiffert, E. R. & Simons, E. L. (2001) J. Hum. Evol. 41, 577-606. [DOI] [PubMed] [Google Scholar]
- 39.Tabuce, R. & Marivaux, L. (2005) Anthropol. Sci. 113, 27-32. [Google Scholar]
- 40.Harrison, T. & Yumin, G. (1999) J. Hum. Evol. 37, 225-277. [DOI] [PubMed] [Google Scholar]
- 41.Bohlin, B. (1946) Paleontol. Sinica, 28, 1-259. [Google Scholar]
- 42.Antoine, P.-O., Welcomme, J.-L., Marivaux, L., Baloch, I., Benammi, M. & Tassy, P. (2003) J. Vertebr. Paleontol. 23, 977-980. [Google Scholar]
- 43.Jaeger, J.-J., Chaimanee, Y. & Ducrocq, S. (1998) C. R. Acad. Sci. 321, 73-78. [DOI] [PubMed] [Google Scholar]
- 44.Li, C. (1978) Vertebr. PalAsiatica 16, 187-192. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.