Abstract
The expectation that cell-mediated immunity is important in the control of feline leukemia virus (FeLV) infection led us to test a DNA vaccine administered alone or with cytokines that favored the development of a Th1 immune response. The vaccine consisted of two plasmids, one expressing the gag/pol genes and the other expressing the env gene of FeLV-A/Glasgow-1. The genetic adjuvants were plasmids encoding the feline cytokines interleukin-12 (IL-12), IL-18, or gamma interferon (IFN-γ). Kittens were immunized by three intramuscular inoculations of the FeLV DNA vaccine alone or in combination with plasmids expressing IFN-γ, IL-12, or both IL-12 and IL-18. Control kittens were inoculated with empty plasmid. Following immunization, anti-FeLV antibodies were not detected in any kitten. Three weeks after the final immunization, the kittens were challenged by the intraperitoneal inoculation of FeLV-A/Glasgow-1 and were then monitored for a further 15 weeks for the presence of virus in plasma and, at the end of the trial, for latent virus in bone marrow. The vaccine consisting of FeLV DNA with the IL-12 and IL-18 genes conferred significant immunity, protecting completely against transient and persistent viremia, and in five of six kittens protecting against latent infection. None of the other vaccines provided significant protection.
The retrovirus, feline leukemia virus (FeLV), is a significant pathogen of domestic cats throughout the world, such that there is a demand for methods to protect against the infection. Cats exposed to FeLV may either become persistently viremic or recover from infection. Viremic cats are anergic to FeLV proteins, showing little evidence of an immune response to the virus, and are at very high risk of developing a fatal disease within 2 to 4 years. In contrast, recovered cats produce virus neutralizing antibodies (20) and FeLV-specific cytotoxic T cells (CTLs) (14), are resistant to reinfection, and do not develop FeLV-related diseases (20). A third possible outcome is the establishment of a “latent” infection, in which cats are not viremic but have a covert infection of bone marrow cells and, like fully recovered cats, have virus-neutralizing antibodies (43). In most cats with latent infections the virus is eventually eliminated, but occasionally the infection persists for several years (53) and may be reactivated at a later date so that cats become viremic.
The observation that cats can recover naturally from exposure to FeLV led to the development of vaccines designed to protect against FeLV infection and disease. Several FeLV vaccines are commercially available. These contain inactivated virions (22, 61, 77), subunits of virions (41), or a recombinant Env protein (8, 33, 45). The ideal vaccine should protect against the establishment of both viremia and latent infection. In practice, however, although the efficacy of these FeLV vaccines has been demonstrated (24, 41, 45, 55–57, 77), the level of protection has not always been complete (31, 38, 39, 58). Attempts have been made to produce more effective vaccines, using novel adjuvants such as immune-stimulating complexes (51, 52) or live virus vectors, including vaccinia virus, canarypox virus, and feline herpesvirus, that contain one or more FeLV genes. The immune stimulating complex vaccine conferred excellent protection from persistent viremia but has not been developed for commercial use. Of the live recombinant vaccines, the vaccinia virus was ineffective (16), while the canarypoxvirus (67) and herpesvirus vaccines (73, 76) provided a level of protection from viremia that was no better than that produced by existing vaccines. At present, therefore, no experimental or commercially available FeLV vaccine consistently provides total protection against the development of transient or persistent viremia or latent bone marrow infection, following exposure to virus (65).
Naked DNA vaccination provides a new approach to the development of stable and affordable vaccines. Experimental DNA vaccines against viral, bacterial, and parasitic diseases have been described (12), and their utility in the prophylaxis of retroviral infections, such as with human immunodeficiency virus, simian immunodeficiency virus, and feline immunodeficiency virus (FIV), has been explored, with promising results (3, 15, 25, 70, 72). In several studies, the coinoculation of plasmids encoding cytokine genes as adjuvants significantly enhanced the immune response raised to DNA vaccines (9), modulating both the amplitude and the nature of the response (7, 34, 35, 69). Although the details of the immune response that leads to recovery from FeLV infection are not yet known, it seemed reasonable to suppose that killing of virus-infected cells by CTL activity is a key element in immunity. Therefore, this approach seemed an attractive proposition for FeLV since DNA vaccination leads to antigen presentation through the endogenous pathway, and genetic adjuvants can be used to promote cell-mediated immunity. In this study we investigated the ability of a DNA vaccine capable of expressing defective FeLV particles to induce protection in cats against challenge with live virus. The vaccine was evaluated either alone or in combination with the genes of cytokines that might be expected to promote a Th1 immune response.
MATERIALS AND METHODS
Cell lines and virus strains.
Cell culture media and supplements were obtained from Gibco/Life Technologies, Inc., Paisley, United Kingdom. The QN10S feline cell line was used for FeLV detection and assays of FeLV infectivity and virus-neutralizing antibodies (59). The 293 cell line, human embryonic kidney cells transformed by sheared human adenovirus type 5 DNA (18), the derivative 293T cell line expressing the simian virus 40 (SV40) T antigen (44), and the FEA cell line of feline embryonic fibroblasts (28) were maintained as described previously. Primary feline alveolar macrophage cultures were established from freshly isolated lung tissue. The macrophages were cultured in Dulbecco modified Eagle medium (Gibco-BRL) containing 20 mM HEPES, 2 mM glutamine, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 10% fetal calf serum (Gibco-BRL) at 37°C with 5% CO2.
The FeLV challenge virus was derived from a molecular clone of FeLV-A/Glasgow-1 (66) and was grown in FEA cells. The batch used had a titer of 2.2 × 106 focus-forming units (FFU)/ml.
Preparation of FeLV antigen DNA constructs.
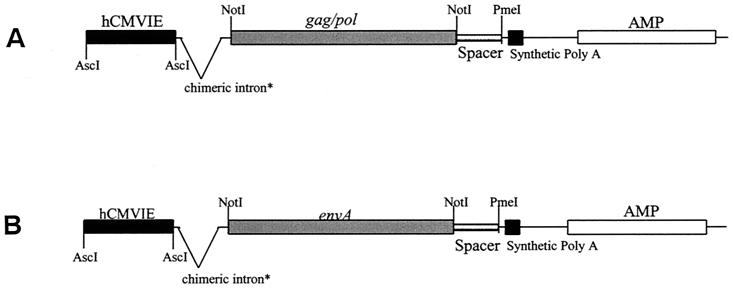
The FeLV DNA vaccine consisted of two separate pUSE1− series constructs, one expressing FeLV gag/pol genes and the other expressing the FeLV subgroup A env gene. The pUSE1− plasmid is a mammalian expression vector derived from the vector pCI-neo (Promega). The cytomegalovirus (CMV) promoter was cloned into the AscI site in pUSE1− to create pUSE1−CMVT. The gag/pol genes from the FeLV A/Glasgow-1 molecular clone (66) were then subcloned from the vector pCDNA3 (Invitrogen B.V., De Schelp, The Netherlands) into pUSE1−CMVT as a NotI fragment to create pUSE1−CMVT(gagpol), as illustrated in Fig. 1A. To create the pUSE1−CMVT(envA) construct, the env gene from the FeLV A/Glasgow-1 molecular clone was subcloned from pCDNA3 into pUSE1−CMVT. The env gene was first excised from pCDNA3 by PstI/BamHI digestion and then blunt ended. pUSE1−CMVT(gagpol) was digested with NotI, gel purified, and blunt ended to create pUSE1−CMVT. Finally, env was blunt end ligated into pUSE1−CMVT to create pUSE1−CMVT(envA), as illustrated in Fig. 1B. The plasmid pCI-neo (Promega) was used as control DNA.
FIG. 1.
(A) pUSE1−CMVT(gagpol) DNA construct. (B) pUSE1−CMVT(envA) DNA construct. ∗, 5′ donor site from the first intron of the human beta-globin gene and the branch and 3′-acceptor of an immunoglobulin gene heavy-chain variable region. AMP, ampicillin resistance gene. The pUSE1− plasmid is a mammalian expression vector derived from the vector pCI-neo (Promega).
In vitro expression of FeLV DNA constructs.
Confirmation that the FeLV DNA constructs pUSE1−CMVT(gagpol) and pUSE1−CMVT(envA) were expressed was demonstrated by fixed and live cell immunofluorescence. Duplicate cultures of 293T cells were transfected with pUSE1−CMVT(gagpol) or pUSE1−CMVT(envA) by using calcium phosphate transfection (75). After 48 h transfected cells and nontransfected negative control cells were harvested, and 4 × 105 cells per well were coated onto multispot slides. After an overnight incubation at 37°C, cells transfected with pUSE1−CMVT(gagpol) were fixed with ice-cold methanol, following two washes with phosphate-buffered saline (PBS). Cells were then incubated for 90 min at 37°C with a gp70-specific monoclonal antibody (MAb 6-15; a gift of Kees Weijer, Netherlands Cancer Institute) [controls and cells transfected with the pUSE1−CMVT(envA) construct] or a p27-specific MAb (PF12J-10A; a gift of Chris Grant, Custom Monoclonals, Sacramento, Calif.) [controls and fixed cells transfected with the pUSE1−CMVT(gagpol) construct]. After three PBS washes the cells were incubated with 25 μl of anti-mouse immunoglobulin G antibody conjugated to fluorescein isothiocyanate, diluted in RPMI medium (Gibco), for 1 h at 37°C. After three PBS washes, coverslips were placed on the slides, and the cells were examined on a microscope with UV light. Fixed or live FeLV-infected FEA cells, labeled with the anti-p27 and anti-gp70 MAbs, respectively, served as positive controls.
In addition, the production of empty virions by 293 cells cotransfected with the FeLV constructs and a packageable lacZ reporter gene was demonstrated. Three plasmids were used for this purpose: pUSE1−CMVT(gagpol) and pUSE1−CMVT(envA), as described above, and pHIT111. pHIT111 comprised a recombinant Moloney murine leukemia virus vector genome, containing the lacZ gene driven by the CMV promoter (64). 293 cells were seeded in 25-cm2 flasks and were incubated overnight to provide cultures that were approximately 30% confluent for transfection. Then, 5 μg of each of the three plasmids was cotransfected into the 293 cells by using calcium phosphate transfection (75), according to the manufacturer's protocol (ProFection Mammalian Transfection System; Promega). After 16 h, the culture fluid was replaced with 5 ml of fresh medium, and 2 days after transfection the culture supernatants were harvested and passed through a 0.45-μm (pore-size) syringe filter. Polybrene was added to the filtrate to a final concentration of 8 μg/ml, and 1 ml was added to duplicate 25-cm2 flasks of FEA cells. After 2 h the inoculum was replaced with fresh medium. Three days after infection the cells were labeled for lacZ expression by using the β-galactosidase assay method. The number of blue cells in each flask was counted to determine if recombinant retrovirus, containing the reporter gene lacZ (expressing the enzyme β-galactosidase), had been produced as a result of in vitro expression of the FeLV DNA constructs.
Preparation of cytokine adjuvant DNA constructs.
The cloning and characterization of the feline gamma interferon (IFN-γ) gene have been described previously (1, 2). For use as an adjuvant gene, the IFN-γ cDNA was subcloned into the pCI-neo vector (Promega) as an EcoRI-NotI fragment. Feline interleukin-12 (IL-12) and IL-18 genes were isolated utilizing RT-PCR of mRNA from feline alveolar macrophages stimulated with LPS (19). The isolation and characterization of the full-length feline IL-18 gene have been described (19), and the DNA sequence has been submitted to EMBL under accession number Y13923. For use as an adjuvant gene, the IL-18 cDNA, in the mature form lacking the inactive precursor sequence, was subcloned into the PsecI vector as an EcoRV-NotI fragment. The PsecI vector, derived from the pCI-neo (Promega) and pSecTag plasmids (Invitrogen), contained a synthetic immunoglobulin secretory component to facilitate the secretion of the active form of IL-18.
The cloning and sequencing of the p35 and p40 subunits of feline IL-12 have been described (19). To create the adjuvant constructs employed in this study, the IL-12 p40 and p35 cDNAs were subcloned into the pCI-neo vector (Promega) as EcoRI and XhoI-NotI fragments, respectively.
In vitro expression of cytokine DNA constructs.
Expression of feline IL-18 and the p35 and p40 subunits of IL-12 in vitro was assessed using Northern blot analysis of mRNA isolated from transfected 293T cells. To detect the expression of the full-length IL-18 protein, lysates of cells transfected with IL-18 DNA were also analyzed by Western blotting (68) by using a cross-reacting rabbit polyclonal antibody against equine IL-18. Feline IL-12 protein expression was similarly confirmed by Western blot using a rabbit polyclonal antibody raised against a synthetic peptide homologous to feline IL-12 p40; both denaturing and nondenaturing conditions were used. There is currently no antibody available specific for either feline IL-12 p35 or p70 (IL-12 heterodimer).
DNA immunization, virus challenge, and blood sampling protocol.
The plasmid DNAs were purified by using Endofree Plasmid Giga kits (Qiagen) according to the manufacturer's protocol. Endotoxin levels were quantified by Q1 Biotech, Glasgow, United Kingdom, using the Limulus amebocyte lysate technique and were determined to be less than 0.5 endotoxin units/ml.
Twenty-nine specific-pathogen-free kittens, aged between 13 and 15 weeks old, were obtained from a commercial source. They were arranged randomly into five groups, each with six kittens, except for group B, which contained five kittens. Each group was split between two rooms, each with 9.36 m2 of floor space and a height of 2.36 m. The kittens were fed proprietary wet and dry cat food and also water. The experiment was conducted in accordance with the guidelines of the UK Home Office Inspectorate.
The kittens were immunized by the inoculation of 100 μg of each DNA construct, in a volume of 200 μl of endotoxin-free PBS (BioWhittaker), into one site in the quadriceps femoris muscle. Cats were immunized with the FeLV DNA constructs alone (group A) or with these constructs and plasmids expressing IFN-γ (group B), IL-12 (group C), or both IL-12 and IL-18 (group D). Control cats (group E) were immunized with empty plasmid (pCI-neo; Promega). Inoculations were performed on three occasions, at 7, 5, and 3 weeks before challenge with virus. As a challenge, a dose of 2 × 105 FFU of FeLV-A/Glasgow-1 was administered in 1 ml of endotoxin-free PBS (BioWhittaker) by intraperitoneal inoculation.
Blood samples were collected by jugular venipuncture before the start of the trial, 48 h after each immunization, on the day of challenge, and at intervals of approximately 3 weeks after challenge. The samples were collected in heparin tubes, and the plasma was separated and stored in aliquots at −70°C. The trial was terminated 15 weeks after challenge, when blood and bone marrow samples were collected and tested for the presence of active and, in the case of the marrow, latent FeLV infection.
Detection of infectious FeLV.
Plasma samples were tested for the presence of FeLV by infection of QN10S cells, as described elsewhere (31). Bone marrow samples were collected from all cats at the end of the trial, and the cells were cultured for 14 to 17 days, as described previously (43). When the primary cultures became confluent after 14 days, the cells were subcultured 1:2. At 7, 14, and 17 days of culture, the culture fluids were examined for infectious virus using QN10S cells to identify latent FeLV infection that was reactivated in vitro.
Detection of anti-FeLV antibodies.
Plasma samples were tested for virus-neutralizing antibodies to FeLV-A/Glasgow-1 using a focus reduction assay (31). Plasma samples taken on the day of challenge were also analyzed by two other methods for the presence of anti-FeLV antibodies induced by vaccination. An enzyme-linked immunosorbent assay (ELISA) (74) was used to detect anti-FeLV gp70 antibodies, and Western blot analysis (13, 26) against a lysate of purified FeLV-A virions was performed to detect antibodies to any FeLV protein.
Statistical analyses.
Statistical analysis was performed on the virus isolation results. Measurements were taken from each of the five treatment groups (A to E) at the four time points after challenge. Fisher's exact test was performed for each time point on the 2 × 5 contingency tables, constructed to illustrate the number of positive and negative virus isolation results in each group. When a significant overall test was detected for the data at a particular time point, pairwise Fisher's exact tests were performed between all pairs of treatments.
Feline IL-18 gene accession number.
The feline IL-18 DNA sequence has been submitted to the Nucleotide Sequence Database, EMBL, Outstation EBI, Cambridge, England, and has been referenced under accession number Y13923.
RESULTS
FeLV DNA constructs express viral proteins that are assembled to produce virions in vitro.
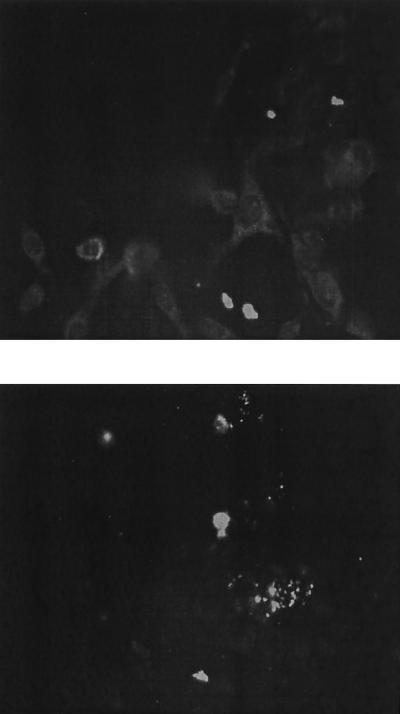
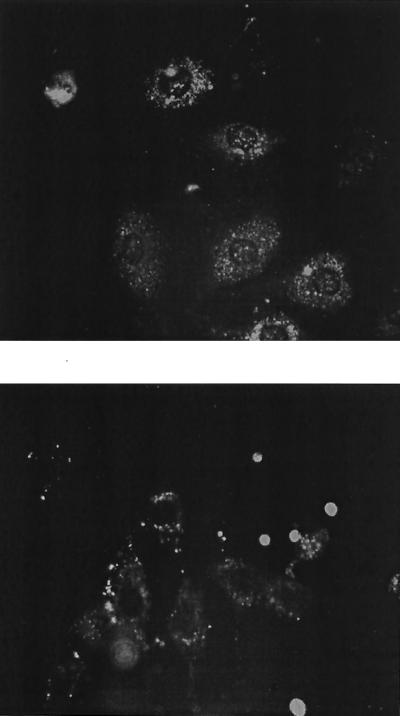
The expression of pUSE1−CMVT(gagpol) and pUSE1−CMVT(envA) constructs in transfected 293T cells was demonstrated by using fixed- and live-cell immunofluorescence. Fixed-cell immunofluorescence, utilizing an anti-p27 MAb, demonstrated a diffuse expression pattern of FeLV p27 protein in the cytoplasm of 293T cells transfected with pUSE1−CMVT(gagpol) (Fig. 2). Live-cell immunofluorescence, utilizing an anti-gp70 MAb, demonstrated the expression of FeLV gp70 protein on the surface of 293T cells transfected with pUSE1−CMVT(envA) (Fig. 2). Expression of these proteins was not detected in untransfected control cells (Fig. 3). Fixed and live FeLV-infected FEA cells labeled with anti-p27 and anti-gp70 MAbs, respectively, acted as positive controls (Fig. 4).
FIG. 2.
Photographs of fixed 293T cells transfected with pUSE1−CMVT (gagpol) DNA construct and labeled with anti-p27 MAb PF12J-10A (top) and live 293T cells transfected with the pUSE1−CMVT(envA) DNA construct and labeled with anti-gp70 MAb 6–15 (bottom). Magnification, ×200.
FIG. 3.
Photographs of untransfected fixed 293T cells labeled with an anti-p27 MAb (PF12J-10A) (top), and untransfected live 293T cells labeled with an anti-gp70 MAb (6–15) (bottom). Magnification, ×200.
FIG. 4.
Photographs of fixed FeLV-A-infected FEA cells labeled with anti-p27 MAb PF12J-10A (top) and live FeLV-A-infected FEA cells labeled with anti-gp70 MAb 6–15 (bottom). Magnification, ×200.
The capacity of the FeLV DNA constructs to drive the production and release of virus-like particles was also determined. The constructs were transfected together with a lacZ reporter gene construct into 293 cells, and the culture fluid from the transfected cells was subsequently inoculated onto FEA cells, which are highly susceptible to FeLV. After 3 days of incubation, the FEA cells were labeled for lacZ expression. Approximately 0.1% of the cells in each of two flasks were found by the β-galactosidase assay method to have stained blue, compared to none in the control flasks. These data confirmed that the transfected FeLV DNA constructs were able to produce particles in vitro that could infect cat cells.
Cytokine DNA constructs are expressed in vitro in mammalian cells.
It was not possible to ensure that all the cytokine genes used in the study were expressed in a biologically active form in cat cells. This has been achieved for the IFN-γ that was used (1), but assays of biological activity are not yet available for feline IL-12 or IL-18. However, it was possible to demonstrate expression of the products of the IL-12 and IL-18 genes after transfection of the constructs into 293T cells. Northern blot analysis to assess in vitro mRNA expression of the feline IL-18, p35, and p40 IL-12 constructs revealed the presence of bands at approximately 1.2, 1.5, and 1.9 kb, respectively, that corresponded to the expected sizes of the pCI-neo derived IL-18, p35, and p40 IL-12 mRNA transcripts (L. Hanlon, C. McGillivray, L. Nicolson, D. E. Onions, D. J. Argyle, and S. Dunham, unpublished data). In addition, Western blot analysis to detect in vitro IL-18 protein expression, using a cross-reactive anti-equine IL-18 polyclonal antibody, demonstrated the presence of a single 24-kDa species, the predicted size of the feline pro-IL-18 protein (Hanlon et al., unpublished) Similarly, in using a rabbit anti-peptide antibody specific for feline p40, a single 40-kDa protein was demonstrated by Western blotting in both IL-12 p40 transfected cell supernatants and lysates. After nondenaturing polyacrylamide gel electrophoresis, it was possible to detect a band corresponding to the predicted size of the IL-12 p70 heterodimer in supernatants from cells transfected with both p40 and p35 DNA but not in supernatants from cells transfected with either subunit alone (S. Dunham, L. Hanlon, J. Bruce, and J. Neil, unpublished data). Taken together, these data confirmed that IL-12 and IL-18 DNA constructs were expressed in mammalian cells in vitro.
Infectious virus is not produced in cats as a result of homologous recombination between FeLV vaccine DNA and endogenous retroviral sequences.
In the vaccine trial, kittens were inoculated with FeLV DNA and cytokine DNA on three occasions, with an interval of 2 weeks between the inoculations. Three weeks following the administration of the final dose of vaccine the kittens were challenged by the intraperitoneal inoculation of FeLV. The safety of DNA vaccines is a concern, both in terms of immediate adverse reactions and of the longer-term consequences arising from the expression of transfected DNA in vivo. Although the FeLV vaccine constructs were expressed and assembled into FeLV virions, these empty particles lacked a functional viral genome and, consequently, should not be capable of replication, proviral integration, or establishing productive infection. However, there is a theoretical risk that the FeLV DNA constructs might be able to recombine in vivo with endogenous feline retroviral sequences (49), resulting in the production of replication-competent virions capable of establishing productive FeLV infection in cats.
To investigate the appearance of such recombinant viruses, the pretrial, postimmunization, and day-of-challenge plasma samples were tested for infectious virus. Virus was not isolated from any blood sample collected before challenge (Table 1). This result indicated that a recombination event between vaccine DNA and endogenous retroviral sequences, generating replication-competent virus, was unlikely to have occurred in these cats. Moreover, no local or systemic reactions were observed in any kitten after immunization. Therefore, it was concluded that the FeLV DNA vaccine and cytokine constructs were safe to administer to cats.
TABLE 1.
Results of virus isolation and virus-neutralizing antibody assay on day of challenge and at intervals following challenge
| DNA inoculum groupa | Catalog no. | Outcome of FeLV challenge of vaccinated cats atb:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wk 0
|
Wk 3
|
Wk 6
|
Wk 9
|
Wk 13
|
Wk 15
|
||||||||
| VI | VN | VI | VN | VI | VN | VI | VN | VI | VN | VI | VIBM | ||
| Group A | L1 | − | 0 | + | 0 | + | 0 | + | 0 | + | 0 | + | + |
| L2 | − | 0 | − | 0 | − | 0 | + | 0 | + | 0 | + | + | |
| L3 | − | 0 | + | 0 | − | 0 | − | 32 | − | 128 | − | + | |
| L4 | − | 0 | − | 0 | − | 0 | − | 16 | − | 128 | − | + | |
| L5 | − | 0 | − | 0 | − | 0 | − | 16 | − | 32 | − | − | |
| L6 | − | 0 | − | 8 | − | 64 | − | 64 | − | 1,024 | − | − | |
| Group B | L8 | − | 0 | + | 0 | + | 0 | + | 0 | + | 0 | + | + |
| L9 | − | 0 | + | 0 | + | 0 | + | 0 | + | 0 | + | + | |
| L10 | − | 0 | − | 0 | − | 0 | − | 0 | − | 0 | − | − | |
| L11 | − | 0 | − | 0 | − | 0 | − | 0 | − | 0 | − | − | |
| L12 | − | 0 | − | 0 | − | 0 | − | 0 | − | 16 | − | − | |
| Group C | L13 | − | 0 | + | 0 | + | 0 | + | 0 | + | 0 | + | + |
| L14 | − | 0 | + | 0 | + | 0 | + | 0 | + | 0 | + | + | |
| L15 | − | 0 | + | 0 | + | 0 | + | 0 | + | 0 | + | + | |
| L16 | − | 0 | + | 0 | + | 0 | − | 0 | − | 0 | + | + | |
| L17 | − | 0 | − | 0 | − | 16 | − | 64 | − | 512 | − | − | |
| L18 | − | 0 | − | 0 | − | 8 | − | 64 | − | 1,024 | − | − | |
| Group D | L19 | − | 0 | − | 0 | − | 8 | − | 32 | − | 64 | − | − |
| L20 | − | 0 | − | 0 | − | 0 | − | 8 | − | 32 | − | − | |
| L21 | − | 0 | − | 0 | − | 0 | − | 16 | − | 64 | − | + | |
| L22 | − | 0 | − | 0 | − | 0 | − | 16 | − | 64 | − | − | |
| L23 | − | 0 | − | 0 | − | 0 | − | 32 | − | 128 | − | − | |
| L24 | − | 0 | − | 16 | − | 16 | − | 64 | − | 1,024 | − | − | |
| Group E | L25 | − | 0 | + | 0 | + | 0 | + | 0 | + | 0 | + | + |
| L26 | − | 0 | + | 0 | + | 0 | + | 0 | + | 0 | + | + | |
| L27 | − | 0 | + | 0 | + | 0 | + | 0 | + | 0 | + | + | |
| L28 | − | 0 | + | 0 | − | 0 | − | 8 | − | 128 | − | + | |
| L29 | − | 0 | + | 0 | − | 16 | − | 64 | − | 512 | − | + | |
| L30 | − | 0 | − | 0 | − | 0 | − | 32 | − | 128 | − | − | |
Group A, FeLV env + gag/pol; group B, FeLV env +gag/pol + IFN-γ; group C, FeLV env + gag/pol + IL-12; group D, FeLV env + gag/pol + IL-12 + IL-18; group E, pCI-neo plasmid.
VI, virus isolation on plasma; VN, virus-neutralizing antibody assay; VIBM, virus isolation performed on cultured bone marrow supernatants. Values are expressed as titers for the virus-neutralizing antibody assay.
DNA immunization does not elicit antiviral antibodies.
The capacity of the vaccines to induce FeLV-specific antibodies was determined. Antibodies were not detected by virus neutralization (Table 1), anti-gp70 ELISA or Western blotting in the plasma samples taken from the kittens on the day of challenge. These results illustrated that immunization in this way did not initiate an FeLV-specific antibody response. Further, the lack of an antibody response is consistent with the conclusion that no replication-competent FeLV was generated during immunization.
The FeLV DNA vaccine alone does not provide significant protection against challenge with infectious virus.
The efficacy of the vaccines was assessed by their capacity to prevent the development of transient, persistent, or latent infection after challenge in comparison with the response of unvaccinated control cats. Cats were monitored at 3, 6, 9, 13, and 15 weeks following challenge for the presence of infectious FeLV in plasma. Sampling 3 weeks after challenge can be particularly revealing, since it is at this time that a transient viremia is most likely to be found in cats that eventually recover from infection (32). Protection against a transient infection would indicate that vaccination had led to a reduction in viral growth very early after infection. Persistent viremia was defined as the presence of virus in the plasma at the end of the trial, i.e., 15 weeks after challenge. Virus-neutralizing antibody titers were determined at each time point, since a significant postchallenge antibody titer usually correlates with protection (23). Virus isolation was also performed on cultured bone marrow samples, collected 15 weeks postchallenge, when the trial ended. The latter procedure was carried out to find if ostensibly immune, nonviremic cats harbored latent FeLV infection in bone marrow.
The results are shown in Table 1 and are summarized in Table 2. Virus was isolated from five of the six unvaccinated, control cats in group E at 3 weeks after challenge. For the remainder of the trial, only three of these cats remained viremic. However, virus was isolated from bone marrow cell cultures from all five cats at 15 weeks after challenge. In contrast, only two of the six cats in group A, inoculated with the FeLV DNA vaccine alone, were viremic 3 weeks after challenge. One of these two cats became persistently viremic, while the second appeared to recover. A third cat (L2) became persistently infected from 9 weeks after infection. At 15 weeks, virus was isolated from the bone marrow of these three cats and from a fourth cat. These results indicate that the vaccine might have provided a degree of protection from transient viremia at 3 weeks after challenge, although the difference in the proportion of viremic cats was not statistically significant between the groups. Later, at 15 weeks after challenge, the proportion of viremic cats in both groups A and E was almost identical. These results, however, did not necessarily reflect a reduction in the protective effect of the vaccine as the trial progressed but rather that some of the infected cats in the control group (E) cleared the virus and became nonviremic. From the results at the end of the trial, it was concluded that the FeLV DNA vaccine alone did not provide significant protection against persistent viremia or latent FeLV infection, although it may have reduced the number of transiently infected cats.
TABLE 2.
Summary of the number of cats with transient viremia, persistent viremia, and latent infection following challenge
| Group | DNA inoculum | Gene adjuvant | No. of animals with:
|
No. of animals with persistent and latent infections/total no. | ||
|---|---|---|---|---|---|---|
| Transient viremiaa/total no. | Persistent viremia/total no. | Additional latent infection/total no. | ||||
| A | FeLV env + gag/pol | None | 1/6 | 2/6 | 2/6 | 4/6 |
| B | FeLV env + gag/pol | IFN-γ | 0/5 | 2/5 | 0/5 | 2/5 |
| C | FeLV env + gag/pol | IL-12 | 0/6 | 4/6 | 0/6 | 4/6 |
| D | FeLV env + gag/pol | IL-12 + IL-18 | 0/6 | 0/6 | 1/6 | 1/6 |
| E | pCI-neo plasmid | None | 2/6 | 3/6 | 2/6 | 5/6 |
Transient viremia defined as viremia at 3 weeks postchallenge that is cleared by 6 weeks postchallenge.
A combination of IL-12 and IL-18 cytokine DNA constructs act as vaccine adjuvants enhancing protection against persistent and latent FeLV infection.
To assess the ability of cytokine DNA constructs to enhance any immunity induced by the FeLV DNA vaccine alone, cats in the three other groups were coinoculated with plasmids encoding IFN-γ (group B), IL-12 (group C), or a combination of IL-12 and IL-18 (group D) and were challenged with FeLV.
Three of the five cats of group B, inoculated with the vaccine and IFN-γ DNA constructs, were consistently free from viremia throughout the trial. However, statistical analysis revealed no significant difference between the proportions of viremic cats in this group (B), the FeLV DNA-alone group (A), or the control group (E). Therefore, it was concluded that the FeLV DNA vaccine and IFN-γ constructs did not protect cats from the development of transient or persistent viremia and that IFN-γ was not an effective vaccine adjuvant in this system.
Three of the six cats inoculated with the vaccine and IL-12 DNA constructs (group C) were consistently viremic from 3 weeks after challenge. A fourth cat that was viremic at 3 and 6 weeks and then appeared to recover became viremic again on week 15 and was considered to be persistently viremic. Throughout the trial, the proportion of viremic cats in group C was similar to that of group E (the control group) and, remarkably, was consistently higher than that of group A (the FeLV DNA vaccine alone group), although the differences were not statistically significant. These results indicated that the FeLV DNA vaccine and IL-12 constructs did not protect cats from the development of transient and persistent viremia; that IL-12 was not an effective vaccine adjuvant in this system, and that the inclusion of IL-12 DNA constructs with the FeLV DNA vaccine might actually have reduced vaccine efficacy.
The best protection was achieved by immunization with FeLV DNA and the combination of IL-12 and IL-18 genes DNA (group D). None of the vaccinated cats was viremic at any point during the trial. Compared with control group E, the proportion of viremic cats was significantly lower in group D, at 3 weeks post-challenge (P < 0.5). However, the difference was not significant at 15 weeks because three of the control cats had recovered. At that time, however, two additional control cats had a latent bone marrow infection compared to only one of the six vaccinated cats. In addition, the virus load in the bone marrow of the vaccinated cat must have been extremely low, since virus was found only at the last sampling, 17 days after establishment of the cultures. (In comparison, virus was detected within 14 days in all cultures from the control cats.) This low level of infection was confirmed when cultures from a second bone marrow biopsy, collected from the latently infected cat in group D 7 weeks later, failed to yield virus.
Therefore, as shown in Table 2, the combined total of cats persistently and latently infected in group D was much lower than in either group E (controls) or group A (vaccine alone). Hence, the combination of IL-12 and IL-18 DNA constructs acted as a potent vaccine adjuvant, producing significant protection against FeLV challenge.
DISCUSSION
We describe here the construction of a FeLV DNA vaccine that, when administered to cats together with a combination of feline IL-12 and IL-18 genes, provided a high level of protection from challenge with virus. This protection was achieved without the induction of a detectable anti-FeLV antibody response. Based on the proportion of cats that was viremic at the end of the trial, the differences between the group given this vaccine and the control group were not statistically significant, due mainly to the fact that half of the control cats recovered naturally from the challenge. It had been expected that all of the control cats would become persistently infected following the intraperitoneal challenge. The recovery from viremia may have been due to the innate resistance of 20-week-old cats (23). Indeed it was in an attempt to overcome this potential resistance that the intraperitoneal method of administration of the challenge was used (45). Another possible explanation is that the inoculation of “empty” plasmid DNA induced a nonspecific immune enhancement in the control cats. However, overall, when the lack of transient and persistent viremia in all cats and of latent infection in all but one cat is considered, the level of protection conferred by the FeLV DNA together with IL-12 and IL-18 DNA was impressive.
Since the crucial immunogens for establishing protective immunity against FeLV are not yet known, we elected to develop a vaccine that should express all of the viral genes. As anticipated, cotransfection of cells with the plasmids containing either gag/pol or env led to the expression of viral proteins that underwent processing and were assembled into virus-like particles capable of infecting feline cells. Such authentic processing might be expected to promote efficient presentation of viral antigens to the immune system.
It is generally considered that env is an important protective antigen (30) certainly in terms of priming for the production of virus-neutralizing antibodies. This belief is to some extent confirmed by the success of the commercial vaccine that contains only p45env as antigen (45). However, other viral antigens may contribute to the generation of cell-mediated immunity. While virus-neutralizing antibodies are important in blocking the further spread of virus to uninfected cells and in establishing resistance to FeLV infection (23), cell-mediated immunity is likely to be important in the elimination of cells already infected with virus during transient infection (5) and in protecting against the development of latent infection. Indeed, in studies of vaccination against murine leukemia virus, protection against challenge with tumor cells has been achieved by immunization with gag DNA alone (6). Accordingly, we attempted to potentiate a Th1-type cell-mediated immune response by using the genes of the cytokines IFN-γ, IL-12, and IL-18 as adjuvants. Studies in vitro have demonstrated that IL-12 and IL-18 act synergistically on T cells to produce an increase in IFN-γ production (37, 46), an extremely pleiotropic cytokine that is integral to the development of a functional cellular immune response (11). In vivo, the efficacy of DNA vaccines can be augmented by the coinoculation of plasmids expressing either IFN-γ, IL-12, or IL-18 that enhance the development of Th1 cell populations, the production of Th1 cytokines, and the generation of antigen-specific CTL responses, which are essential in the development of functional cellular immunity (7, 25, 27, 34, 62, 63, 69). Therefore, in this study, it is likely that the combination of IL-12 and IL-18, which potently enhanced the protection conferred by the FeLV DNA vaccine, mediated its adjuvant effect in vivo through a synergistic increase in IFN-γ production.
Paradoxically, the IFN-γ DNA construct was not an effective adjuvant in this trial, although plasmids expressing IFN-γ have been found to enhance other DNA vaccines (25, 54). Our IFN-γ DNA construct may have been ineffective because the levels of IFN-γ protein expressed were too low or too localized to stimulate the immune system effectively. In contrast, as a result of their synergistic action, the IL-12 and IL-18 DNA constructs may have stimulated the production of enhanced levels of IFN-γ, which overcame the deficiency. An alternative explanation is that, because IFN-γ may directly inhibit the expression of genes driven by the CMV or SV40 promoters (21), the simultaneous administration of the plasmid expressing IFN-γ with the vaccine DNA (which is under the control of a CMV promoter) decreased the expression of the FeLV antigens to such an extent that an effective immune response was not elicited.
Due to logistical constraints we were unable to include in this study a group of vaccinates given FeLV DNA and IL-18 DNA alone, which might have answered the question of whether a single component or the combination of IL-12 and IL-18 was responsible for enhancing the efficacy of the successful vaccine. It was surprising that inoculation of vaccine and IL-12 alone as an adjuvant appeared to reduce vaccine efficacy. Thus, the proportion of cats that became viremic following challenge was similar in groups C (vaccine and IL-12) and E (control group) but was consistently greater than in group A (vaccine alone), although the differences were not statistically significant. The reason for the failure of the IL-12 adjuvant alone to enhance vaccine efficacy is not known. The IL-12 construct consisted of two separate plasmids, expressing either the p35 or the p40 subunit. Studies of human and murine IL-12 have shown that overexpression of p40 relative to p35 may result in the generation of p40 homodimers (which behave as receptor antagonists in vitro), inhibiting the biological activity of endogenous IL-12 (17, 42). Similarly, inoculation with the separate feline p35 and p40 IL-12 constructs may have resulted in the overexpression of the p40 subunit due to differences in transfection levels of target cells. The consequent formation of p40 homodimers and the inhibition of the immunostimulatory effects of endogenous IL-12 (4) may have downregulated the cellular immune response and thus reduced the efficacy of the vaccine. Due to the lack of feline IL-12- and IL-18-specific bioassays, the biological activity of our expressed IL-12 and IL-18 proteins could not be evaluated. Therefore, it is difficult to draw definite conclusions regarding the in vivo effects of these cytokines. Studies are in progress to develop feline-specific bioassays that will allow the bioactivity of a particular cytokine construct to be thoroughly assessed in vitro, before the inoculation of animals. Future vaccine trials should include a group with vaccine and IL-18 alone so as to determine whether IL-18, rather than the combination of IL-12 and IL-18, mediated the potent adjuvant effect that was observed here.
The absence of a detectable antibody response in any cat prior to viral challenge was very striking. Although most other FeLV vaccines do not induce significant levels of virus-neutralizing antibodies (29, 31), antibodies to gp70 or other FeLV antigens are commonly elicited (8, 55). While DNA vaccines have been shown to induce potent cellular and humoral immune responses in a variety of animal disease models (12, 47), relatively few DNA vaccination trials have been conducted in cats. Studies investigating the feline humoral immune response induced by immunization with naked plasmid DNA have provided conflicting results (10, 25, 50, 60). Intramuscular DNA vaccination with a defective FIV provirus conferred significant protection against FIV infection in the absence of a detectable prechallenge antiviral antibody titer (25). Similarly, inoculation with a minimalistic immunogenic defined gene expression vector (MIDGE) vaccine containing the genes coding for FIV gp140 and feline IL-12 conferred significant protection in the absence of a detectable virus-specific humoral immune response (40). In contrast, intramuscular immunization with DNA plasmids encoding the FIV gp120 protein was able to elicit an FIV-specific humoral immune response (10).
The inability of the FeLV DNA vaccines to induce FeLV-specific antibodies before challenge may reflect the pathway by which the viral proteins were processed by antigen-presenting cells. Alternatively, anti-DNA or anti-cytokine antibodies, produced as a result of immunization with plasmid DNA or in vivo expression of cytokine adjuvant DNA constructs (48, 71), may have interfered with the humoral immune response against the antigenic components of the DNA vaccine (36). If such inhibition did occur, it was not a permanent effect, since after challenge high levels of virus-neutralizing antibodies appeared in all but two of the cats that did not develop a persistent infection. As expected, neutralizing antibodies were not found in viremic cats, which appear to be anergic or immunotolerant to FeLV antigens. There is no evidence from our results that any vaccine even primed the cats for an antiviral antibody response since the antibodies that were produced after challenge neither appeared any earlier in the vaccinates than in the control cats that recovered naturally from infection nor achieved higher titers. This result also indicates that the effective vaccine did not induce “sterilizing” immunity and that there was a transient, undetected growth of the challenge virus that induced antibody production. It is most likely that protection was achieved through the induction of cell-mediated immunity. The induction of FeLV-specific cytotoxic T cells following vaccination and before challenge could not be investigated in this study. It may be significant, however, that very much higher levels of effector CTL were demonstrated in a selection of the vaccinated, protected cats than in viremic or naturally recovered cats that were tested subsequently (14).
Certainly, the FeLV DNA and IL-12 and IL-18 adjuvant combination was an effective vaccine, being safe to administer and conferring a degree of protection from transient, persistent, and latent FeLV infection that was at least equal to, and in most cases superior to, that produced by other commercial and experimental FeLV vaccines (65). Future studies should elucidate fully the protective mechanism of the vaccine and the cytokine adjuvants. The potential for manipulating the nature and quantity of an immune response by cytokine gene adjuvants might also be investigated in the use of DNA vaccines for immunotherapy aimed at curing cats of FeLV viremia.
ACKNOWLEDGMENTS
The Wellcome Trust and Q1 Biotech supported this work.
We are grateful to Margaret Hosie for helpful discussions regarding trial design and to Alan Kingsman for providing the pHIT111 plasmid. We also thank Graham Law, Davina Graham, Paul McGowan, Angela Pacitti, and Richard Irvine for their assistance.
REFERENCES
- 1.Argyle D J, Harris M, Lawrence C, McBride K, Barron R, McGillivray C, Onions D E. Expression of feline recombinant interferon-gamma in baculovirus and demonstration of biological activity. Vet Immunol Immunopathol. 1998;64:97–105. doi: 10.1016/s0165-2427(98)00127-5. [DOI] [PubMed] [Google Scholar]
- 2.Argyle D J, Smith K, McBride K, Fulton R, Onions D E. Nucleotide and predicted peptide sequence of feline interferon-gamma (IFN-gamma) DNA Seq. 1995;5:169–171. doi: 10.3109/10425179509029357. [DOI] [PubMed] [Google Scholar]
- 3.Boyer J D, Ugen K E, Wang B, Agadjanyan M, Gilbert L, Bagarazzi M L, Chattergoon M, Frost P, Javadian A, Williams W V, Refaeli Y, Ciccarelli R B, McCallus D, Coney L, Weiner D B. Protection of chimpanzees from high-dose heterologous HIV-1 challenge by DNA vaccination. Nat Med. 1997;3:526–532. doi: 10.1038/nm0597-526. [DOI] [PubMed] [Google Scholar]
- 4.Brunda M J. Interleukin-12. J Leukoc Biol. 1994;55:280–288. doi: 10.1002/jlb.55.2.280. [DOI] [PubMed] [Google Scholar]
- 5.Charreyre C, Pedersen N C. Study of feline leukemia virus immunity. J Am Vet Med Assoc. 1991;199:1316–1324. [PubMed] [Google Scholar]
- 6.Chen W, Qin H, Chesebro B, Cheever M A. Identification of a gag-encoded cytotoxic T-lymphocyte epitope from FBL-3 leukemia shared by Friend, Moloney, and Rauscher murine leukemia virus-induced tumors. J Virol. 1996;70:7773–7782. doi: 10.1128/jvi.70.11.7773-7782.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chow Y H, Chiang B L, Lee Y L, Chi W K, Lin W C, Chen Y T, Tao M H. Development of Th1 and Th2 populations and the nature of immune responses to hepatitis B virus DNA vaccines can be modulated by codelivery of various cytokine genes. J Immunol. 1998;160:1320–1329. [PubMed] [Google Scholar]
- 8.Clark N, Kushner N N, Barrett C B, Kensil C R, Salsbury D, Cotter S. Efficacy and safety field trials of a recombinant DNA vaccine against feline leukemia virus infection. J Am Vet Med Assoc. 1991;199:1433–1443. [PubMed] [Google Scholar]
- 9.Cohen A D, Boyer J D, Weiner D B. Modulating the immune response to genetic immunization. FASEB J. 1998;12:1611–1626. [PubMed] [Google Scholar]
- 10.Cuisinier A M, Mallet V, Meyer A, Caldora C, Aubert A. DNA vaccination using expression vectors carrying FIV structural genes induces immune response against feline immunodeficiency virus. Vaccine. 1997;15:1085–1094. doi: 10.1016/s0264-410x(97)00004-2. [DOI] [PubMed] [Google Scholar]
- 11.De Maeyer E, De Maeyer-Guignard J. Interferon-gamma. Curr Opin Immunol. 1992;4:321–36. doi: 10.1016/0952-7915(92)90083-q. [DOI] [PubMed] [Google Scholar]
- 12.Donnelly J J, Ulmer J B, Liu M A. DNA vaccines. Life Sci. 1997;60:163–172. doi: 10.1016/s0024-3205(96)00502-4. [DOI] [PubMed] [Google Scholar]
- 13.Flynn J N, Cannon C A, Neil J C, Jarrett O. Vaccination with a feline immunodeficiency virus multiepitopic peptide induces cell-mediated and humoral immune responses in cats, but does not confer protection. J Virol. 1997;71:7586–7592. doi: 10.1128/jvi.71.10.7586-7592.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flynn J N, Hanlon L, Jarrett O. Feline leukaemia virus: protective immunity is mediated by virus-specific cytotoxic T lymphocytes. Immunology. 2000;101:120–125. doi: 10.1046/j.1365-2567.2000.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuller D H, Corb M M, Barnett S, Steimer K, Haynes J R. Enhancement of immunodeficiency virus-specific immune responses in DNA-immunized rhesus macaques. Vaccine. 1997;15:924–926. doi: 10.1016/s0264-410x(96)00271-x. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert J H, Pedersen N C, Nunberg J H. Feline leukemia virus envelope protein expression encoded by a recombinant vaccinia virus: apparent lack of immunogenicity in vaccinated animals. Virus Res. 1987;7:49–67. doi: 10.1016/0168-1702(87)90057-8. [DOI] [PubMed] [Google Scholar]
- 17.Gillessen S, Carvajal D, Ling P, Podlaski F J, Stremlo D L, Familletti P C, Gubler U, Presky D H, Stern A S, Gately M K. Mouse interleukin-12 (IL-12) p40 homodimer: a potent IL-12 antagonist. Eur J Immunol. 1995;25:200–206. doi: 10.1002/eji.1830250133. [DOI] [PubMed] [Google Scholar]
- 18.Graham F L, Smiley J, Russell W C, Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977;36:59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- 19.Hanlon L. Feline IL-12 and IL-18: adjuvants in FeLV DNA vaccination studies. Ph.D. thesis. Glasgow, United Kingdom: University of Glasgow; 2000. [Google Scholar]
- 20.Hardy W D J. Feline leukaemia virus diseases. In: Hardy W D J, Essex M, McClelland A J, editors. Feline leukaemia virus. Amsterdam, The Netherlands: Elsevier Science Publishers; 1980. pp. 3–31. [Google Scholar]
- 21.Harms J S, Splitter G A. Interferon-gamma inhibits transgene expression driven by SV40 or CMV promoters but augments expression driven by the mammalian MHC I promoter. Hum Gene Ther. 1995;6:1291–1297. doi: 10.1089/hum.1995.6.10-1291. [DOI] [PubMed] [Google Scholar]
- 22.Hines D L, Cutting J A, Dietrich D L, Walsh J A. Evaluation of efficacy and safety of an inactivated virus vaccine against feline leukemia virus infection. J Am Vet Med Assoc. 1991;199:1428–1430. [PubMed] [Google Scholar]
- 23.Hoover E A, Olsen R G, Hardy W D J, Schaller J P, Mathes L E. Feline leukemia virus infection: age-related variation in response of cats to experimental infection. J Natl Cancer Inst. 1976;57:365–369. doi: 10.1093/jnci/57.2.365. [DOI] [PubMed] [Google Scholar]
- 24.Hoover E A, Perigo N A, Quackenbush S L, Mathiason-DuBard C K, Overbaugh J M, Kloetzer W S, Elder J H, Mullins J I. Protection against feline leukemia virus infection by use of an inactivated virus vaccine. J Am Vet Med Assoc. 1991;199:1392–1401. [PubMed] [Google Scholar]
- 25.Hosie M J, Flynn J N, Rigby M A, Cannon C, Dunsford T, Mackay N A, Argyle D, Willett B J, Miyazawa T, Onions D E, Jarrett O, Neil J C. DNA vaccination affords significant protection against feline immunodeficiency virus infection without inducing detectable antiviral antibodies. J Virol. 1998;72:7310–7319. doi: 10.1128/jvi.72.9.7310-7319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hosie M J, Jarrett O. Serological responses of cats to feline immunodeficiency virus. AIDS. 1990;4:215–220. doi: 10.1097/00002030-199003000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Iwasaki A, Stiernholm B J, Chan A K, Berinstein N L, Barber B H. Enhanced CTL responses mediated by plasmid DNA immunogens encoding costimulatory molecules and cytokines. J Immunol. 1997;158:4591–4601. [PubMed] [Google Scholar]
- 28.Jarrett O. Determinants of the host range of feline leukaemia virus. J Gen Virol. 1973;20:169–175. doi: 10.1099/0022-1317-20-2-169. [DOI] [PubMed] [Google Scholar]
- 29.Jarrett O. Efficacy of recombinant feline leukemia virus vaccines. AIDS Res Hum Retrovir. 1996;12:435–436. doi: 10.1089/aid.1996.12.435. [DOI] [PubMed] [Google Scholar]
- 30.Jarrett O. The relevance of feline retroviruses to the development of vaccines against HIV. AIDS Res Hum Retrovir. 1996;12:385–387. doi: 10.1089/aid.1996.12.385. [DOI] [PubMed] [Google Scholar]
- 31.Jarrett O, Ganiere J P. Comparative studies of the efficacy of a recombinant feline leukaemia virus vaccine. Vet Rec. 1996;138:7–11. doi: 10.1136/vr.138.1.7. [DOI] [PubMed] [Google Scholar]
- 32.Jarrett O, Golder M C, Stewart M F. Detection of transient and persistent feline leukaemia virus infections. Vet Rec. 1982;110:225–228. doi: 10.1136/vr.110.10.225. [DOI] [PubMed] [Google Scholar]
- 33.Kensil C R, Barrett C, Kushner N, Beltz G, Storey J, Patel U, Recchia J, Aubert A, Marciani D. Development of a genetically engineered vaccine against feline leukemia virus infection. J Am Vet Med Assoc. 1991;199:1423–1427. [PubMed] [Google Scholar]
- 34.Kim J J, Ayyavoo V, Bagarazzi M L, Chattergoon M A, Dang K, Wang B, Boyer J D, Weiner D B. In vivo engineering of a cellular immune response by coadministration of IL-12 expression vector with a DNA immunogen. J Immunol. 1997;158:816–826. [PubMed] [Google Scholar]
- 35.Kim J J, Trivedi N N, Nottingham L K, Morrison L, Tsai A, Hu Y, Mahalingam S, Dang K, Ahn L, Doyle N K, Wilson D M, Chattergoon M A, Chalian A A, Boyer J D, Agadjanyan M G, Weiner D B. Modulation of amplitude and direction of in vivo immune responses by co-administration of cytokine gene expression cassettes with DNA immunogens. Eur J Immunol. 1998;28:1089–1103. doi: 10.1002/(SICI)1521-4141(199803)28:03<1089::AID-IMMU1089>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 36.Klinman D M, Takeno M, Ichino M, Gu M, Yamshchikov G, Mor G, Conover J. DNA vaccines: safety and efficacy issues. Springer Semin Immunopathol. 1997;19:245–256. doi: 10.1007/BF00870272. [DOI] [PubMed] [Google Scholar]
- 37.Kohno K, Kataoka J, Ohtsuki T, Suemoto Y, Okamoto I, Usui M, Ikeda M, Kurimoto M. IFN-gamma-inducing factor (IGIF) is a costimulatory factor on the activation of Th1 but not Th2 cells and exerts its effect independently of IL-12. J Immunol. 1997;158:1541–1550. [PubMed] [Google Scholar]
- 38.Legendre A M, Hawks D M, Sebring R, Rohrbach B, Chavez L, Chu H J, Acree W M. Comparison of the efficacy of three commercial feline leukemia virus vaccines in a natural challenge exposure. J Am Vet Med Assoc. 1991;199:1456–1462. [PubMed] [Google Scholar]
- 39.Legendre A M, Mitchener K L, Potgieter L N. Efficacy of a feline leukemia virus vaccine in a natural exposure challenge. J Vet Intern Med. 1990;4:92–98. doi: 10.1111/j.1939-1676.1990.tb00882.x. [DOI] [PubMed] [Google Scholar]
- 40.Leutenegger C M, Boretti F S, Mislin C N, Flynn J N, Schroff M, Habel A, Junghans C, Koenig-Merediz S A, Sigrist B, Aubert A, Pedersen N C, Wittig B, Lutz H. Immunization of cats against feline immunodeficiency virus (FIV) infection by using minimalistic immunogenic defined gene expression vector vaccines expressing FIV gp140 alone or with feline interleukin-12 (IL-12), IL-16, or a CpG Motif. J Virol. 2000;74:10447–10457. doi: 10.1128/jvi.74.22.10447-10457.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lewis M G, Mathes L E, Olsen R G. Protection against feline leukemia by vaccination with a subunit vaccine. Infect Immun. 1981;34:888–894. doi: 10.1128/iai.34.3.888-894.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ling P, Gately M K, Gubler U, Stern A S, Lin P, Hollfelder K, Su C, Pan Y C, Hakimi J. Human IL-12 p40 homodimer binds to the IL-12 receptor but does not mediate biologic activity. J Immunol. 1995;154:116–127. [PubMed] [Google Scholar]
- 43.Madewell B R, Jarrett O. Recovery of feline leukaemia virus from non-viraemic cats. Vet Rec. 1983;112:339–342. doi: 10.1136/vr.112.15.339. [DOI] [PubMed] [Google Scholar]
- 44.Marazzi S, Blum S, Hartmann R, Gundersen D, Schreyer M, Argraves S, von, V F, Pytela R, Ruegg C. Characterization of human fibroleukin, a fibrinogen-like protein secreted by T lymphocytes. J Immunol. 1998;161:138–147. [PubMed] [Google Scholar]
- 45.Marciani D J, Kensil C R, Beltz G A, Hung C H, Cronier J, Aubert A. Genetically-engineered subunit vaccine against feline leukaemia virus: protective immune response in cats. Vaccine. 1991;9:89–96. doi: 10.1016/0264-410x(91)90262-5. [DOI] [PubMed] [Google Scholar]
- 46.Micallef M J, Ohtsuki T, Kohno K, Tanabe F, Ushio S, Namba M, Tanimoto T, Torigoe K, Fujii M, Ikeda M, Fukuda S, Kurimoto M. Interferon-gamma-inducing factor enhances T helper 1 cytokine production by stimulated human T cells: synergism with interleukin-12 for interferon-gamma production. Eur J Immunol. 1996;26:1647–1651. doi: 10.1002/eji.1830260736. [DOI] [PubMed] [Google Scholar]
- 47.Montgomery D L, Ulmer J B, Donnelly J J, Liu M A. DNA vaccines. Pharmacol Ther. 1997;74:195–205. doi: 10.1016/s0163-7258(97)82003-7. [DOI] [PubMed] [Google Scholar]
- 48.Mor G, Singla M, Steinberg A D, Hoffman S L, Okuda K, Klinman D M. Do DNA vaccines induce autoimmune disease? Hum Gene Ther. 1997;8:293–300. doi: 10.1089/hum.1997.8.3-293. [DOI] [PubMed] [Google Scholar]
- 49.Neil J C, Fulton R, Rigby M, Stewart M. Feline leukaemia virus: generation of pathogenic and oncogenic variants. Curr Top Microbiol Immunol. 1991;171:67–93. doi: 10.1007/978-3-642-76524-7_4. [DOI] [PubMed] [Google Scholar]
- 50.Osorio J E, Tomlinson C C, Frank R S, Haanes E J, Rushlow K, Haynes J R, Stinchcomb D T. Immunization of dogs and cats with a DNA vaccine against rabies virus. Vaccine. 1999;17:1109–1116. doi: 10.1016/s0264-410x(98)00328-4. [DOI] [PubMed] [Google Scholar]
- 51.Osterhaus A, Weijer K, UytdeHaag F, Jarrett O, Sundquist B, Morein B. Induction of protective immune response in cats by vaccination with feline leukemia virus iscom. J Immunol. 1985;135:591–596. [PubMed] [Google Scholar]
- 52.Osterhaus A, Weijer K, UytdeHaag F, Knell P, Jarrett O, Akerblom L, Morein B. Serological responses in cats vaccinated with FeLV ISCOM and an inactivated FeLV vaccine. Vaccine. 1989;7:137–141. doi: 10.1016/0264-410x(89)90053-4. [DOI] [PubMed] [Google Scholar]
- 53.Pacitti A M, Jarrett O. Duration of the latent state of feline leukaemia virus infections. Vet Rec. 1985;117:472–474. doi: 10.1136/vr.117.18.472-a. [DOI] [PubMed] [Google Scholar]
- 54.Pasquini S, Xiang Z, Wang Y, He Z, Deng H, Blaszczyk-Thurin M, Ertl H C. Cytokines and costimulatory molecules as genetic adjuvants. Immunol Cell Biol. 1997;75:397–401. doi: 10.1038/icb.1997.62. [DOI] [PubMed] [Google Scholar]
- 55.Pedersen N C. Immunogenicity and efficacy of a commercial feline leukemia virus vaccine. J Vet Intern Med. 1993;7:34–39. doi: 10.1111/j.1939-1676.1993.tb03166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pedersen N C, Johnson L, Birch D, Theilen G H. Possible immunoenhancement of persistent viremia by feline leukemia virus envelope glycoprotein vaccines in challenge-exposure situations where whole inactivated virus vaccines were protective. Vet Immunol Immunopathol. 1986;11:123–148. doi: 10.1016/0165-2427(86)90093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pedersen N C, Theilen G H, Werner L L. Safety and efficacy studies of live- and killed-feline leukemia virus vaccines. Am J Vet Res. 1979;40:1120–1126. [PubMed] [Google Scholar]
- 58.Pollock R V, Scarlett J M. Randomized blind trial of a commercial FeLV vaccine. J Am Vet Med Assoc. 1990;196:611–616. [PubMed] [Google Scholar]
- 59.Ramsey I K. Recombinant surface glycoproteins of feline leukaemia virus. Ph.D. thesis. Glasgow, United Kingdom: University of Glasgow; 1993. [Google Scholar]
- 60.Richardson J, Moraillon A, Baud S, Cuisinier A M, Sonigo P, Pancino G. Enhancement of feline immunodeficiency virus (FIV) infection after DNA vaccination with the FIV envelope. J Virol. 1997;71:9640–9649. doi: 10.1128/jvi.71.12.9640-9649.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sebring R W, Chu H J, Chavez L G, Sandblom D S, Hustead D R, Dale B, Wolf D, Acree W M. Feline leukemia virus vaccine development. J Am Vet Med Assoc. 1991;199:1413–1419. [PubMed] [Google Scholar]
- 62.Sin J I, Kim J J, Arnold R L, Shroff K E, McCallus D, Pachuk C, McElhiney S P, Wolf M W, Pompa-de Bruin S J, Higgins T J, Ciccarelli R B, Weiner D B. IL-12 gene as a DNA vaccine adjuvant in a herpes mouse model: IL-12 enhances Th1-type CD4+ T cell-mediated protective immunity against herpes simplex virus-2 challenge. J Immunol. 1999;162:2912–2921. [PubMed] [Google Scholar]
- 63.Sin J I, Kim J J, Boyer J D, Ciccarelli R B, Higgins T J, Weiner D B. In vivo modulation of vaccine-induced immune responses toward a Th1 phenotype increases potency and vaccine effectiveness in a herpes simplex virus type 2 mouse model. J Virol. 1999;73:501–509. doi: 10.1128/jvi.73.1.501-509.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soneoka Y, Cannon P M, Ramsdale E E, Griffiths J C, Romano G, Kingsman S M, Kingsman A J. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sparkes A H. Feline leukaemia virus: a review of immunity and vaccination. J Small Anim Pract. 1997;38:187–194. doi: 10.1111/j.1748-5827.1997.tb03339.x. [DOI] [PubMed] [Google Scholar]
- 66.Stewart M A, Warnock M, Wheeler A, Wilkie N, Mullins J I, Onions D E, Neil J C. Nucleotide sequences of a feline leukemia virus subgroup A envelope gene and long terminal repeat and evidence for the recombinational origin of subgroup B viruses. J Virol. 1986;58:825–834. doi: 10.1128/jvi.58.3.825-834.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tartaglia J, Jarrett O, Neil J C, Desmettre P, Paoletti E. Protection of cats against feline leukemia virus by vaccination with a canarypox virus recombinant, ALVAC-FL. J Virol. 1993;67:2370–2375. doi: 10.1128/jvi.67.4.2370-2375.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsuji T, Hamajima K, Fukushima J, Xin K Q, Ishii N, Aoki I, Ishigatsubo Y, Tani K, Kawamoto S, Nitta Y, Miyazaki J, Koff W C, Okubo T, Okuda K. Enhancement of cell-mediated immunity against HIV-1 induced by coinoculation of plasmid-encoded HIV-1 antigen with plasmid expressing IL-12. J Immunol. 1997;158:4008–4013. [PubMed] [Google Scholar]
- 70.Ugen K E, Boyer J D, Wang B, Bagarazzi M, Javadian A, Frost P, Merva M M, Agadjanyan M G, Nyland S, Williams W V, Coney L, Ciccarelli R, Weiner D B. Nucleic acid immunization of chimpanzees as a prophylactic/immunotherapeutic vaccination model for HIV-1: prelude to a clinical trial. Vaccine. 1997;15:927–930. doi: 10.1016/s0264-410x(96)00254-x. [DOI] [PubMed] [Google Scholar]
- 71.van der Meide P H, Schellekens H. Anti-cytokine autoantibodies: epiphenomenon or critical modulators of cytokine action. Biotherapy. 1997;10:39–48. doi: 10.1007/BF02678216. [DOI] [PubMed] [Google Scholar]
- 72.Wang B, Ugen K E, Srikantan V, Agadjanyan M G, Dang K, Refaeli Y, Sato A I, Boyer J, Williams W V, Weiner D B. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci USA. 1993;90:4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wardley R C, Berlinski P J, Thomsen D R, Meyer A L, Post L E. The use of feline herpesvirus and baculovirus as vaccine vectors for the gag and env genes of feline leukaemia virus. J Gen Virol. 1992;73:1811–1818. doi: 10.1099/0022-1317-73-7-1811. [DOI] [PubMed] [Google Scholar]
- 74.Weijer K, Uytdehaag F G, Jarrett O, Lutz H, Osterhaus A D. Post-exposure treatment with monoclonal antibodies in a retrovirus system: failure to protect cats against feline leukemia virus infection with virus neutralizing monoclonal antibodies. Int J Cancer. 1986;38:81–87. doi: 10.1002/ijc.2910380114. [DOI] [PubMed] [Google Scholar]
- 75.Wigler M, Silverstein S, Lee L S, Pellicer A, Cheng Y, Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977;11:223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- 76.Willemse M J, van Schooneveld S H, Chalmers W S, Sondermeijer P J. Vaccination against feline leukaemia using a new feline herpesvirus type 1 vector. Vaccine. 1996;14:1511–1516. doi: 10.1016/s0264-410x(96)00108-9. [DOI] [PubMed] [Google Scholar]
- 77.York S M, York C J. Development of a whole killed feline leukemia virus vaccine. J Am Vet Med Assoc. 1991;199:1419–1422. [PubMed] [Google Scholar]