Selective lysis of siblings appears to be a highly evolved and complex process.
Streptococcus pneumoniae, a leading cause of serious infections, including pneumonia, infections of the middle ear, and meningitis (recently reviewed in ref. 1), is an organism of great public health concern. Antibiotic resistance among S. pneumoniae strains is rapidly increasing, complicating treatment (2). S. pneumoniae, previously known as Diplococcus pneumoniae or pneumococcus, played a central role in discovery of DNA as the “transforming principle,” the molecule that contained the encoded information necessary to transform a bacterial cell from an unencapsulated avirulent type to a virulent encapsulated type (3, 4). Since these seminal observations, the mechanism of DNA uptake by S. pneumoniae and its ecological and medical significance have been subjects of great interest. In this issue of PNAS, Jean-Pierre Claverys and coworkers (5) describe a novel mechanism that ties together the development of competence (the ability to take up DNA from the environment) with the fratricide of siblings, leading to the release of a key S. pneumoniae virulence factor, pneumolysin [Ply (6)], in the process. The release of Ply and other cytoplasmic contents, which occurs at the expense of a large portion of the bacterial population, suggests that the survival benefit of being virulent or alerting the host to its presence outweighs the disadvantage of losing many members of the S. pneumoniae population.
Proficiency in uptake of DNA from the environment by S. pneumoniae has contributed to the development of multidrug-resistant strains, providing direct evidence for its importance in the continuing evolution of this species. For example, the mosaic penicillin-binding proteins that render S. pneumoniae resistant to β-lactam antibiotics appear to have originated in oral streptococci, such as Streptococcus mitis (7). The ability of S. pneumoniae to take up DNA is transient and regulated by a cell density-dependent, or quorum-sensing, pathway. During logarithmic growth, an S. pneumoniae population passes through a phase where a subpopulation of cells becomes competent for DNA internalization (8). Before achieving this state, low levels of a competence-stimulating peptide (CSP) are secreted by S. pneumoniae cells into the environment. The accumulation of this peptide in the environment is detected by a two-component signaling system, termed ComDE. Detection of CSP by ComDE leads to induction of higher levels of expression of CSP, resulting in its exponential accumulation in the environment. Detection of CSP in the environment also leads to expression of an alternate σ factor, encoded by comX, which in turn leads to the induction of >100 genes (9, 10). Only 23 of these genes have direct roles in DNA uptake (9), providing prima facie evidence that this quorum-sensing system plays a greater role in the biology of S. pneumoniae than merely regulating the ability of the organism to internalize DNA from the environment.
Ply is a member of the cholesterol-dependent, pore-forming toxin family produced by many Gram-positive species of bacteria. Ply has been shown to compromise the function of ciliated bronchial epithelial cells, leading to reduced clearance of both mucous and the organism from the lower respiratory tract (reviewed in ref. 1). Ply is unusual among members of this toxin class, in that it is translated without a secretion signal and remains cytoplasmic until released by lysis of the S. pneumoniae cell.
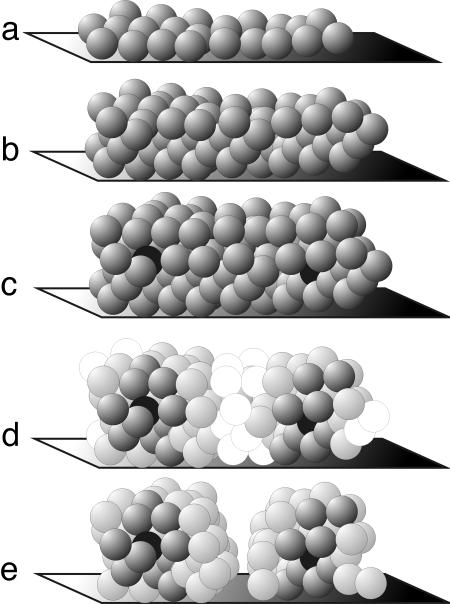
In the present study, Claverys and his research team (5) noticed that a mutant of S. pneumoniae spontaneously gave rise to clear hemolytic zones surrounding some of the colonies on a blood agar plate, as opposed to the more characteristic green discoloration that usually occurs around S. pneumoniae colonies. They found that this hemolysis could be induced with the addition of exogenous CSP, and that hemolysis depended on the production of Ply. These investigators screened a variety of mutants of this hemolytic variant to identify the pathway that resulted in CSP-induced release of Ply. It was found that three cell-wall hydrolases played overlapping roles in lysing the cells, leading to Ply release. It was further found that an inferred two-component bacteriocin, termed CibAB (competence-induced bacteriocin), was involved in inducing autolysis by the cell-wall hydrolases. This bacteriocin-induced cell lysis leads to release of cytoplasmic Ply. CibAB-expressing cells appear to be protected from autoinduction of cell-wall hydrolases by expression of a proposed immunity factor, CibC. It was further shown that competent CibAB-expressing cells could induce the lysis of noncompetent cells, resulting in release of Ply from the latter population. The predatory release of Ply from cells within the population that had yet to achieve the competent state by competent cells induced to express CibAB and protected by CibC from autolysis was termed allolysis (5) (see the model proposed in Fig. 1).
Fig. 1.
Proposed model for fratricidal killing of siblings. S. pneumoniae or other bacterial species accumulate in number (a and b) and secrete low levels of a quorum-sensing molecule such as CSP. Some cells (c, cells shaded in black) are first to respond to accumulating CSP through the ComDE two-component sensing system. Responding cells in turn express high levels of the CSP quorum signal, which would appear to increase the proportion of competent cells in the immediate microenvironment, within a radius that would depend in part on the quantity of CSP expressed and its diffusion rate. Clusters of competent cells then express the CibAB bacteriocin, resulting in allolysis of siblings that had yet to achieve competence and express the protective cibC gene product (d). This lysis may result in the release of Ply and inflammatory cell-wall debris in the case of S. pneumoniae; it may result in the release of nutrients that delay the completion of sporulation by survivors in the case of B. subtilis; or in the enhancement of surface-to-volume relationships, the deposition of matrix DNA, and the outgrowth of biofilm architectures (or the release of pieces of biofilm into the circulation) in the case of P. aeruginosa or other biofilm-forming organisms.
S. pneumoniae has long been known to possess a pathway leading to the lytic death of a large proportion of cells within a population, and this lytic death and release of DNA were recently shown to be induced by the competence pathway (11, 12). The demonstration that lysis is induced exogenously by a subpopulation of protected organisms provides interesting insights into S. pneumoniae colonization and infection and also natural selection as it applies to bacterial populations. With respect to S. pneumoniae colonization and infection, it was recently shown that Ply functions, at least in part, by signaling through the toll-like receptor TLR4 (13). It was suggested that, in concert with stimulation of TLR2 by cell walls of lysed S. pneumoniae, these inflammatory stimuli serve to alert the host to the presence of the organism, activating innate defenses and ultimately protecting the host from infection. From this perspective, S. pneumoniae may benefit from actively alerting the host to its presence, thereby extending the period of colonization of the nasopharynx in asymptomatic carriers. The exacerbation of disease in a minority of colonized hosts that results from cell lysis and release of Ply may be an inadvertent consequence of this effort toward symbiosis.
Predation or killing of a subpopulation of organisms as a means for perpetuating the genetic lineage has been described recently in Bacillus subtilis (14). In that system, an antibiotic and a killing factor are produced by a subpopulation of organisms immediately prior to commitment to sporulation, which results in killing of nonsporulating sister cells within the population. It was suggested that nutrients released by lysed cells are cannibalized by the sporulating siblings, delaying their commitment to completing the process of spore formation. Independently, it has been observed that DNA forms an integral part of Pseudomonas aeruginosa biofilms (16). In the development of biofilm by some strains of P. aeruginosa, a phage is induced that results in lysis of a fraction of the P. aeruginosa cells within the population (16), resulting in the release of DNA and other cellular constituents. The released DNA then appears to be bound by type IV pili on the surface of P. aeruginosa (17), contributing to accretion of the biofilm.
In summary, the selective lysis of siblings by a subpopulation of bacterial cells appears to be a highly evolved and complex process. This process is used by widely divergent organisms toward varied but related ends, presumably leading to enhanced survival of species. In the case of S. pneumoniae, lysis of a subpopulation and release of Ply may either stabilize the relationship with its host by activating host innate defenses or exacerbate an infection by releasing a virulence trait and inflammatory mediators. In B. subtilis, it may serve to buy additional time for the lineage by scavenging the nutrients released from lysis of siblings, permitting completion of the sporulation process and thereby ensuring survival. In P. aeruginosa, lysis may contribute to the formation of higher-order architectures by both vacating occupied spaces to optimize surface-to-volume relationships and providing important matrix materials for the further growth of the biofilm community. Evidently, within a population, being at the leading edge is also a key to survival in the microbial world.
See companion article on page 8710.
References
- 1.Jedrzejas, M. J. (2001) Microbiol. Mol. Biol. Rev. 65, 187-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knobler, S. L., Lemon, S. M., Najafi, M. & Burroughs, T., eds. (2003) The Resistance Phenomenon in Microbes and Infectious Disease Vectors (Natl. Acad. Press, Washington, DC), pp. 3-12. [PubMed]
- 3.Griffith, F. (1928) J. Hyg. 27, 113-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avery, O. T., MacLeod, C. M. & McCarty, M. (1944) J. Exp. Med. 79, 137-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guiral, S., Mitchell, T. J., Martin, B. & Claverys, J.-P. (2005) Proc. Natl. Acad. Sci. USA 102, 8710-8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry, A. M., Yother, J., Briles, D. E., Hansman, D. & Paton, J. C. (1989) Infect. Immun. 57, 2037-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hakenbeck, R., Konig, A., Kern, I., van der Linden, M., Keck, W., Billot-Klein, D., Legrand, R., Schoot, B. & Gutmann, L. (1998) J. Bacteriol. 180, 1831-1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Claverys, J. P. & Håvarstein, L. S. (2002) Front. Biosci. 7, 1798-1814. [DOI] [PubMed] [Google Scholar]
- 9.Peterson, S. N., Sung, C. K., Cline, R., Desai, B. V., Snesrud, E. C., Luo, P., Walling, J., Li, H., Mintz, M., Tsegaye, G., et al. (2004) Mol. Microbiol. 51, 1051-1070. [DOI] [PubMed] [Google Scholar]
- 10.Dagkessamanskaia, A., Moscoso, M., Henard, V., Guiral, S., Overweg, K., Reuter, M., Martin, B., Wells, J. & Claverys, J. P. (2004) Mol. Microbiol. 51, 1071-1086. [DOI] [PubMed] [Google Scholar]
- 11.Steinmoen, H., Knutsen, E. & Håvarstein, L. S. (2002) Proc. Natl. Acad. Sci. USA 99, 7681-7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moscoso, M. & Claverys, J. P. (2004) Mol. Microbiol. 54, 783-794. [DOI] [PubMed] [Google Scholar]
- 13.Malley, R., Henneke, P., Morse, S. C., Cieslewicz, M. J., Lipsitch, M., Thompson, C. M., Kurt-Jones, E., Paton, J. C., Wessels, M. R. & Golenbock, D. T. (2003) Proc. Natl. Acad. Sci. USA 100, 1966-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez-Pastor, J. E., Hobbs, E. C. & Losick, R. (2003) Science 301, 510-513. [DOI] [PubMed] [Google Scholar]
- 15.Whitchurch, C. B., Tolker-Nielsen, T., Ragas, P. C. & Mattick, J. S. (2002) Science 295, 1487. [DOI] [PubMed] [Google Scholar]
- 16.Webb, J. S., Thompson, L. S., James, S., Charlton, T., Tolker-Nielsen, T., Koch, B., Givskov, M. & Kjelleberg, S. (2003) J. Bacteriol. 185, 4585-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Schaik, E. J., Giltner, C. L., Audette, G. F., Keizer, D. W., Bautista, D. L., Slupsky, C. M., Sykes, B. D. & Irvin, R. T. (2005) J. Bacteriol. 187, 1455-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]