ABSTRACT
Fungal mastoiditis caused by Aspergillus fumigatus predominantly occurs in immunocompromised patients. Invasive temporal bone mycoses are rare. They are usually associated with host immunodeficiency, are difficult to diagnose, and many cases are fatal. Treatment consists of antifungal chemotherapy, surgical debridement, and attempts to control the underlying immunological condition. Published reports describe patients with previous ear pathology and associated facial nerve dysfunction. We report a case in a patient with systemic lupus erythematosus. A good outcome followed surgical debridement and the use of a new triazole antifungal agent, voriconazole. Our patient's facial nerve function was unaffected. The presence of normal facial nerve function, however, does not exclude the possibility of invasive fungal mastoiditis.
Keywords: Ear, temporal bone, mastoid, fungal infection, immunosuppression, mycoses
CASE REPORT
Aspergillus infections have been associated with paranasal sinusitis and invasive external otitis.1,2 Although mycotic infections of the head and neck are reported with increasing frequency, primary invasive Aspergillus mastoiditis appears to be rare.3,4 Most patients with an Aspergillus infection are immunocompromised either as a result of their primary disease or as a result of their treatment (e.g., leukemia, acquired immunodeficiency syndrome, transplant patients, diabetes). We report a patient who was immunocompromised from treatment with prednisolone and azathioprine for the control of systemic lupus erythematosus (SLE). Few case reports have identified Aspergillus fungal infections in immunocompetent adults.5,6
A 19-year-old female with SLE and no prior otological problems sought treatment after experiencing left otalgia and hearing loss for 2 weeks. She stated that an upper respiratory tract infection had preceded these symptoms. She complained of a flare-up of her lupus symptoms (joint pains) but denied the presence of dizziness, an aural discharge, or tinnitus. She had been taking prednisolone tablets (20 mg) for a month and had recently begun azathioprine (100 mg) to control her SLE.
On clinical examination the patient was afebrile. Her facial nerve function was normal. The skin of the left external auditory canal showed evidence of a mild inflammation that extended to the pars flaccida. Tuning fork tests were normal. The rest of her otolaryngological examination was normal. A diagnosis suggestive of acute otitis media plus mild otitis externa was made, and she began a course of antibiotics (CO-amoxiclav) in addition to topical antibiotic eardrops (Gentisone HC).
A week later she sought treatment for worsening pain radiating behind her ear associated with left hemicranial headaches. On examination she was tender over the mastoid antrum, but there was no clinical evidence of a postauricular abscess. Her left tympanic membrane was hyperemic and thickened, with evidence of an effusion. Tuning fork tests were consistent with a left conductive hearing loss, which was confirmed by a 40- to 50-dB hearing loss on pure tone audiometry. Tympanometry revealed a type B flat trace (with a normal canal volume).
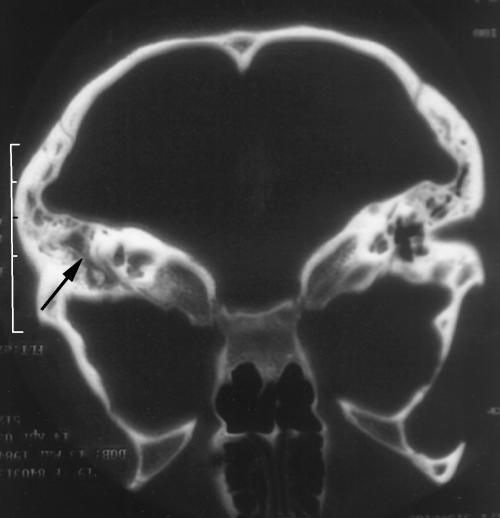
The patient was admitted and treated with intravenous antibiotics (Co-amoxyclav), topical nasal decongestants (ephedrine 1%) and topical steroid nose drops (Betnesol®). Blood investigations revealed a normal white cell count (6.7 × 109/L) and mildly elevated level of C-reactive protein (11.1 mg/L; normal range 0 to 8 mg/L). A compute tomography (CT) scan showed opacification of her left mastoid air cell system and middle ear with no evidence of an intracranial collection (Fig. 1).
Figure 1.
Computed tomography scan demonstrates opacification with coalescence of left mastoid air cells (black arrow).
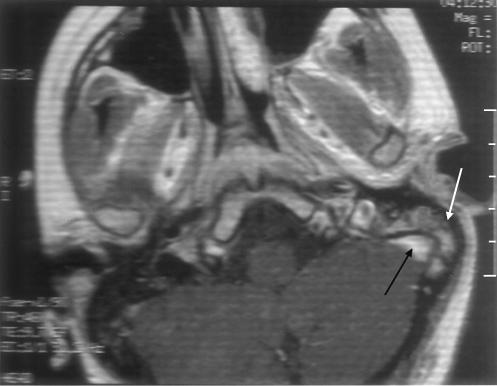
A grommet was inserted in her left ear under local anesthetic. At surgery, a small amount of thin serous fluid was aspirated. The aspirate was cultured, but no growth was reported. She continued to have an aural discharge, which on repeat cultures grew Aspergillus fumigatus. Magnetic resonance imaging of her petrous temporal bones obtained because of her worsening pain and the increased aural discharge showed fluid and an enhancing soft tissue in the left mastoid air cells, consistent with mastoiditis (Fig. 2).
Figure 2.
Magnetic resonance imaging shows mastoiditis (white arrow. The adjacent cavernous sinus (black arrow) is normal.
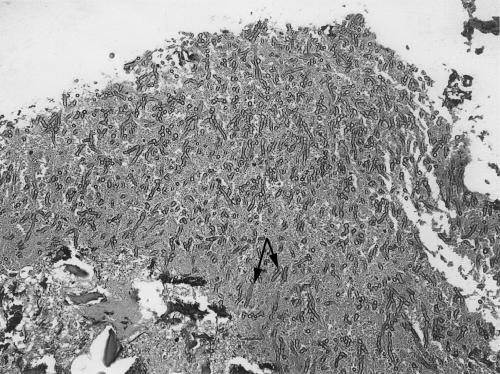
A wide cortical mastoidectomy with opening and clearing of the mastoid antrum was performed urgently and proceeded uneventfully. Large amounts of granulation tissue were identified. Specimens sent for histological examination and bacteriological and cell culture confirmed the presence of an invasive Aspergillus infection (Fig. 3). The patient was treated with intravenous voriconazole (400 mg) twice daily for 1 week followed by 200 mg administered orally twice daily for 6 weeks. At her follow-up examination, 6 weeks later, she was pain free with no evidence of infection.
Figure 3.
Biopsy specimen of granulation tissue shows branching septate fungal hyphae (black arrows) invading bone and vessels (hematoxylen and eosin stain, magnification × 400).
DISCUSSION
Aspergillus is a common saprophyte that occurs worldwide. In Europe the most common isolate is Aspergillus fumigatus, but many other species have been identified as pathogenic to humans. The fungus is often found in soil dust, water, and decaying organisms. Inhalation of the spores (Conidia) causes fungal sinus infections and diffuse lung infections, which subsequently spread to other sites. The dissemination may occur directly or via the bloodstream to organs such as the liver, kidney, and brain.
Initially, the classification of Aspergillus infection was restricted to paranasal sinus disease.7,8 Hall and Farrior3 then included temporal bone infections. Their classification recognizes three forms. Noninvasive aspergillosis is localized and does not invade tissue. It responds to simple conservative removal. Invasive aspergillosis, which is characterized by bony invasion, granulomatous response, and fibrosis, occurs in immunocompetent patients. Finally, fulminant aspergillosis, which occurs in immunocompromised patients, is characterized by tissue and angioinvasion, no granulomatous response, and little tissue reaction.
Sources of the spread of mycotic infection within the head and neck include direct extension from the nasopharynx, hematogenic, tympanogenic, and meningogenic routes.9 The present case highlights a rare presentation of invasive/fulminant aspergillosis via tympanogenic spread. In most cases of Aspergillus infection, temporal bone involvement can be attributed to spread from the external auditory canal, via paranasal sinuses, or hematogenic spread. Previous reports have documented facial nerve involvement.3,6,10 Aspergillus infection should be considered part of the differential diagnosis of patients with mastoiditis, who are relatively immunocompromised and who do not respond to antibiotic treatment regardless of facial nerve involvement.
This case highlights the difficulty of diagnosing invasive/fulminant aspergillosis. Deep tissue biopsy or isolation from blood cultures is required for histopathological confirmation. Colonization with saprophytic fungi in chronic otitis media and superficial fungal otitis externa is common.9 Consequently, no diagnostic conclusion can be drawn from single positive cultures from the external auditory canal or from middle ears with chronic perforations.
Once the diagnosis is confirmed, treatment options for Aspergillus mastoiditis consist of antifungal therapy, aggressive surgical debridement, and attempts to control the patient's underlying immune status. Previous reports have described amphotericin B and itraconazole as effective against invasive Aspergillus.11 Voriconazole is a new broad spectrum triazole antifungal agent. Structurally it is related to fluconazole, but as a second-generation drug its potency and spectrum of activity are improved. Voriconazole is primarily used to treat acute invasive aspergillosis.
Like other azole antifungal agents, voriconazole principally works by inhibiting the enzyme cytochrome P450 14a-demethylase. This inhibition is involved in the sterole biosynthesis pathway. It thereby inhibits ergosterol in the cell membrane, disrupting fungal growth.12 Voriconazole inhibits this enzyme to a greater extent and is dose dependent. Consequently, the potency and spectrum of activity are improved compared with other azoles. In vitro tests have shown good inhibitory activity against Aspergillus fumigatus and other species of mold.13 Large-phase II and III clinical studies and case reports have demonstrated the clinical efficacy of voriconazole against Aspergillus and other mycoses. Voriconazole is more effective than amphotericin B for the treatment of invasive aspergillosis.14 Voriconazole has three important side effects:15,16,17 liver abnormalities and hepatitis; skin abnormalities, including photosensitivity and rashes; and transient visual disturbances that do not threaten sight and are reported in as many as 30% of patients.
The drug interaction profile of voriconizole needs careful evaluation. The inhibition of the metabolism of the cytochrome P450 enzyme indicates a high potential for drug interactions. This potential requires attention to dosage regimes, monitoring serum levels, and monitoring the effects of other interacting drugs.17 Voriconazole is available in both oral and intravenous preparations, and the bioavailability of both is excellent. With its introduction as the first-line treatment of serious infections, great progress in the therapy of invasive fungal infections is likely.
PAPER PRESENTED
Presented as a poster presentation at the 24th Politzer Society Meeting (International Otological Society Meeting) held on August 31, 2003, in Amsterdam, The Netherlands. Presented at the 126th Semon Club meeting, held on November 14, 2003, Guys Hospital in London, United Kingdom.
REFERENCES
- Adams N F., Jr Infections involving the ethmoid, maxillary and sphenoid sinus and the orbit due to Aspergillus fumigatus. Arch Surg. 1923;26:999–1009. [Google Scholar]
- Petrak R M, Pottage J C, Levin S. Invasive external otitis caused by Aspergillus fumigatus in an immunocompromised patient. J Infect Dis. 1985;151:196. [Google Scholar]
- Hall P J, Farrior J B. Aspergillus mastoiditis. Otolaryngol Head Neck Surg. 1993;108:167–170. doi: 10.1177/019459989310800210. [DOI] [PubMed] [Google Scholar]
- Teh W, Matti B S, Marisiddaiah H, Minamoto G Y. Aspergillus sinusitis in patients with AIDS: report of three cases and review. Clin Infect Dis. 1995;21:529–535. doi: 10.1093/clinids/21.3.529. [DOI] [PubMed] [Google Scholar]
- Cunningham M, Yu V L, Turner J, Curtin H. Necrotizing otitis externa due to Aspergillus in an immunocompetent patient. Arch Otolaryngol Head Neck Surg. 1988;114:554–556. doi: 10.1001/archotol.1988.01860170084024. [DOI] [PubMed] [Google Scholar]
- Bryce G E, Phillips P, Lepawsky M, Gribble M J. Invasive Aspergillus tympanomastoiditis in an immunocompetent patient. J Otolaryngol. 1997;26:266–269. [PubMed] [Google Scholar]
- Hora J F. Primary aspergillosis of the paranasal sinuses and associated areas. Laryngoscope. 1965;75:768–773. doi: 10.1288/00005537-196505000-00004. [DOI] [PubMed] [Google Scholar]
- McGill T J. Mycotic infection of the temporal bone. Arch Otolaryngol. 1978;104:140–144. doi: 10.1001/archotol.1978.00790030026006. [DOI] [PubMed] [Google Scholar]
- Stanley R J, McCaffrey T V, Weiland L H. Fungal mastoiditis in the immunocompromised host. Arch Otolaryngol Head Neck Surg. 1988;114:198–199. doi: 10.1001/archotol.1988.01860140096030. [DOI] [PubMed] [Google Scholar]
- Slack C, Watson D W, Abzug M J, Shaw C, Chan K H. Fungal mastoiditis in immunocompromised children. Arch Otolaryngol Head Neck Surg. 1999;125:73–75. doi: 10.1001/archotol.125.1.73. [DOI] [PubMed] [Google Scholar]
- Denning D W, Tucker R M, Hanson L H, Stevens D A. Treatment of invasive Aspergillus with itraconazole. Am J Med. 1989;86:791–800. doi: 10.1016/0002-9343(89)90475-0. [DOI] [PubMed] [Google Scholar]
- Patterson T F. Role of newer azoles in surgical patients. J Chemother. 1999;11:504–512. doi: 10.1179/joc.1999.11.6.504. [DOI] [PubMed] [Google Scholar]
- Sheehan D J, Hitchcock C A, Sibley C M. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999;12:40–79. doi: 10.1128/cmr.12.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhnke M. Voriconazole—application and perspectives. Mycoses. 2002;45:42–47. doi: 10.1111/j.1439-0507.2002.tb04769.x. [DOI] [PubMed] [Google Scholar]
- Johnson L B, Kaufmann C A. Voriconazole: a new triazole antifungal agent. Clin Infect Dis. 2003;36:630–637. doi: 10.1086/367933. [DOI] [PubMed] [Google Scholar]
- Ullmann A J. Review of the safety, tolerability and drug interactions of the new antifungal agents caspofungin and voriconazole. Curr Med Res Opin. 2003;19:263–271. doi: 10.1185/030079903125001884. [DOI] [PubMed] [Google Scholar]
- Hoffman H L, Rathbun R C. Review of the safety and efficacy of voriconazole. Expert Opin Investig Drug. 2002;11:409–429. doi: 10.1517/13543784.11.3.409. [DOI] [PubMed] [Google Scholar]