Abstract
This study aimed to count Enterobacteriaceae and Escherichia coli in different locations on pig carcasses (shank, loin, abdomen, shoulder, and jowl) from two slaughterhouses (A and B) between September 2019 and July 2021 during different slaughter stages (after bleeding, after passing through the epilator machine, after manual toileting in the dirty area, before and after evisceration, and after the final washing), as well as verify antimicrobial resistance and biofilm formation capacity. The main points of Enterobacteriaceae and E. coli contamination were identified in the two slaughterhouses through three collections. The stages with the highest counts were post-bleeding and evisceration in both slaughterhouses and after manual toileting in slaughterhouse B in the first collection. Most E. coli isolates were resistant to multiple antimicrobials, with higher resistance frequencies to amoxicillin, ampicillin, chloramphenicol, sulfonamides, and streptomycin. The virulence genes eae, stx1, and stx2 were also detected. Three isolates had all three genes and exhibited resistance to at least six antimicrobial classes (β-lactams, macrolides, aminoglycosides, sulfonamides, amphenicols, and quinolones). E. coli isolates also showed a high frequency of strains with moderate and strong in vitro biofilm-forming capacity. This is the first study to characterize microbial contamination by pig slaughter stage in the Federal District region, demonstrating the critical points for hygienic production. E. coli was isolated from the surface of pig carcasses, as well as the virulence genes stx1, stx2, and eae were detected. The multi-antimicrobial resistant isolates also had a moderate-to-strong biofilm formation capacity, thus demonstrating risks to public health.
Keywords: swine, Escherichia coli, biofilms, antimicrobial multiresistance
1. Introduction
Brazilian pork production in 2020 was approximately 4.5 million tons, and more than 1 million tons were exported, with the domestic market using approximately 3 million tons and 16 kg consumption per inhabitant [1]. According to the Brazilian Association of Animal Proteins [1], Brazil is the fourth largest pork exporter worldwide, and the Federal District (DF) is responsible for 0.4% of Brazilian pig breeding, with approximately 200 thousand porcine in 2020 [2].
Production process intensification was attributed to meat market demands, which increased the densities of pig breeding products, causing sanitary pressure in the production system. This increases concern about microorganisms in the production chain and their effects [3,4,5,6].
Microbiological contamination of food is a global problem [7] and responsible for two-thirds of foodborne disease outbreaks. Animals are the main microbial load carriers in products of animal origin due to contamination, both from the gastrointestinal contents and processing environment areas [8,9].
The microorganisms commonly involved in outbreaks of foodborne illnesses (DTAs) include Salmonella, Staphylococcus aureus, Campylobacter, Listeria monocytogenes, and Escherichia coli [9,10,11]. Brazilian legislation requires the microbiological control of cattle and pig carcasses in refrigerated slaughterhouses to evaluate process hygiene and reduce the prevalence of these pathogenic agents [12]. Furthermore, the Enterobacteriaceae family count was included in the evaluation of carcasses after the final wash in slaughterhouses before the cooling chamber to evaluate the hygienic conditions of slaughter, following the international guides of microorganism analyses [12,13].
Enterobacteriaceae contagion was analyzed as an indicator of hygiene conditions at slaughter since they can be found in the environment and slaughtered animal intestines and can also indicate food contamination directly or indirectly by fecal content or environmental factors [14,15]. E. coli is relevant among those of fecal origin [15] as some strains, such as uremic–hemolytic Shiga toxin-producing E. coli (STEC) [16], enteropathogenic E. coli (EPEC), enteroaggregative E. coli (EAgEC), enteroinvasive E. coli (EIEC), and enterohemorrhagic E. coli (EHEC) [9], can cause foodborne diseases.
Few studies have evaluated the microbial counts at each stage of pig slaughter in Brazil [17], with most microorganism studies focusing on just one part of the carcass or industrial processing environment [14,18,19,20,21,22,23]. The Enterobacteriaceae and E. coli count during the technological processing stages of pig slaughter in the Federal District or in various pig carcass points/parts has not been studied. According to Pinto [24] and Costa et al. [25], it has been used to carry out risk analysis and identify critical control points within pig slaughter, aiming to monitor and change whenever necessary, and is a way to ensure food safety [26].
Therefore, this study aimed to evaluate the Enterobacteriaceae and E. coli counts at different carcass points/parts and at different pig slaughter stages in refrigerated slaughterhouses located in the Federal District region to study and identify the stx1, stx2, and eae genes in E. coli isolates, as well as evaluate their biofilm formation capacity and antimicrobial resistance.
2. Materials and Methods
2.1. Sample Origin
This study was conducted in two refrigerated pig slaughterhouses (A and B) located in the Federal District region, under official inspection, between September 2019 and July 2021. The industries agreed to participate spontaneously and voluntarily in this study, and the pigs did not come from integrated farms. Three collections were conducted, and a carcass was randomly selected and monitored at each stage of the slaughter process to swab defined points on the carcass surface. At each visit, a collection was carried out, with one collection/visit at cold storage slaughterhouse A and two visits at cold storage slaughterhouse B.
The samples were obtained from sterile cotton swabs (Absorve® Cotia, São Paulo, Brazil) used with sterilized polyamide and stainless-steel molds, respectively, with an area of 9 cm2 in the collection from the refrigerated slaughterhouse A and 100 cm2 in the collection of the refrigerated slaughterhouse B. The swabs were previously moistened in sterile 0.1% peptone water and, after scrubbing, were placed individually in tubes containing the transport solution as described by Corbellini et al. [14] and Davanzo et al. [27].
Five points/locations were selected for the pig carcass, and six points/stages were selected for the technological processing of pig slaughter. The points/locations chosen to collect swab samples followed the protocols described by Matsubara [28], the MAPA Animal Product Sample Collection Manual [29], and Cê [30].
Swabs were collected from the shank, loin, abdomen, shoulder, and jowl of a randomly selected carcass at selected stages during the technological process of slaughter: after bleeding, after depilation, after manual depilation (toilet), before and after evisceration, after washing the carcass in the clean area, and before moving the carcass into the cooling chamber. Individual sterile swabs (Absorve®) were used at each point for each stage, making 30 swab samples for each slaughterhouse, with 90 samples for both establishments.
2.2. Enterobacteriaceae and E. coli Count
Enterobacteriaceae and E. coli were counted according to the method AOAC method (Association of Official Analytical Chemists) [31] using 3MTM Petrifilm TM Enterobacteriaceae Count Plate (EB) and Petrifilm TM 3MTM E. coli/Coliform Count Plate (EC), respectively. The swabs were homogenized with 1 mL transport solution, inoculated in both EB and EC plates, and incubated at 35 ± 1 °C for 24 h. The counting methodology on Violet Red Bile Glucose agar (Acumedia®) was used, in accordance with the ABNT NBR ISO 21528-2:2020 standard [32], to count Enterobacteriaceae. After inoculating the samples, they were incubated at 35 °C for 24 h. The counts for each analysis were converted into logarithmic values.
2.3. E. coli Isolation
Regarding E. coli isolation by stage of the technological processing of slaughter and location of the carcass in slaughterhouses A on 1st visit and B on 2nd visit, the swabs collected from slaughterhouses A and B were seeded directly on eosin methylene blue (EMB) agar plates (KASVI,Weissópolis, Paraná, Brasil) and incubated at 37 °C for 24 h. Colonies considered typical were subjected to biochemical tests as described by Silva et al. [31] to confirm the presence of microorganisms.
2.4. Isolate Antibiogram
A total of 52 E. coli samples were subjected to an antibiogram using the disk diffusion technique standardized by the Clinical and Laboratory Standards Institute manual [33]. To perform the antibiogram, five disks (SENSIDISC, DME, Araçatuba, São Paulo, Brazil) were placed on the agar surface. The following pharmacological bases were used: Quinolones: nalidixic acid (NAL: 30 μg), ciprofloxacin (CIP: 5 μg); β-Lactams: amoxicillin (AMX: 10 μg), ampicillin (AMP: 10 μg); Cephalosporins: cefazolin (CFZ: 30 μg), ceftazidime (CAZ; 30 μg); Amphenicols: chloramphenicol (CLO: 30 μg); Tetracyclines: tetracycline (TET: 30 μg), doxycycline (DOX: 30 μg); Macrolides: erythromycin (ERI: 15 μg); Aminoglycosides: streptomycin (EST: 10 μg), gentamicin (GEN: 10 μg); Sulfonamide: sulfonamides (SUL: 300 μg). The results followed the CLSI M100-ED31:2021 standard [34].
2.5. E. coli Virulence Gene Detection
Polymerase chain reaction (PCR) was used to investigate the presence of Shiga toxin gene types 1 and 2 (Stx1 and Stx2) and eae genes, as described by China et al. [35]. DNA was extracted through boiling method. Then, 5 μL product containing approximately 5 ng/μL total DNA estimated, as sample, was amplified in a 25 μL reaction mixture containing 1× buffer (200 mM Tris-HCl, pH 8.4, 500 mM KCl—PHT®), 2.0 mM dNTPs, 2 U taq DNA polymerase (PHT®), 2 mM phosphate deoxyribonucleotides (Invitrogen®,Waltham, MA, USA), 3 mM MgCl2 (PHT®), and 1 μM primers on a MyCycler thermocycler (BioRad®,Hercules, CA, USA. The primers, annealing temperatures, and expected fragment size are described in Table 1.
Table 1.
List of primers used to detect virulence genes in isolates of E. coli.
The products were visualized in 1.5% agarose gel (Invitrogen® Waltham, MA, USA) stained with 0.5 μg/mL ethidium bromide on a transilluminator device (Imagequant, Gelifesciences®) and imaged (Major Science UV®, Saratoga, CA, USA). Positive controls for stx1, stx2, and eae were obtained from the bacteriolytic library of the Food Microbiology Laboratory/FAV/UnB.
2.6. In Vitro Biofilm Formation Capacity Evaluation
The in vitro biofilm formation capacity of E. coli isolates was evaluated as described by Djordjevic et al. [36] and adapted by Borges et al. [37]. Each isolate analysis was performed in triplicate at 37, 25, and 10 °C for 24, 48, or 72 h. The analyses at 37 °C and 25 °C were carried out in a bacteriological incubator (Quimis® and Fanem®, respectively), while those at 10 °C were carried out in a refrigerator (Eletrolux®, Stockholm, Sweden)
After the incubation, the inocula were removed using a pipette for subsequent washing with sterile 0.9% saline solution three times. The plates were then dried at room temperature, treated with 200 µL methanol PA (Vetec®—Sigma-Aldrich, St. Louis, MO, USA) for 15 min, and then dried for 15 min at room temperature after removing the methanol by inverting the plates. Thereafter, the plates were treated with 200 µL 1% Hucker’s crystal violet (Newsprov®) to stain the isolate adhesion areas for 10 min, washed with running water, and dried at room temperature. Subsequently, 200 µL 33% acetic acid (J. T. Baker®) was added to each sample, and the absorbance at 490 nm was measured using an ELISA reader (Biotek®Elx800).
The result was considered as the reading average of three samples of each isolate and compared to that of the negative control (Don). The mean absorbance obtained from triplicate readings was used to determine the final optical density of each strain (ODf), which was compared with that of the negative control (ODn). The isolates were categorized into non-biofilm-forming isolates (NF) when ODf ≤ ODn, weakly biofilm-forming when ODn < ODf ≤ 2× ODn, moderate biofilm-forming when 2× ODn < ODf ≤ 4× ODn, or strong biofilm-forming when 4× ODn < ODf [38].
Statistical analyses were performed to verify the biofilm formation ability of each isolate by correspondence analysis, chi-square test, and Fisher’s test when relevant, thus checking the greater or lesser capacity for formation between temperatures, incubation durations, and the collection sites at slaughter and carcass. All analyses were performed using SAS® software (v.9.4, Cary, NC, USA) at a significance level of 5%.
3. Results and Discussion
3.1. Enterobacteriaceae and E. coli Count
The Enterobacteriaceae and E. coli counts obtained from each pig carcass location/point, as well as each pig slaughter stage, are described in Table 2, Table 3, Table 4, Table 5, Table 6 and Table 7.
Table 2.
Enterobacteriaceae and E. coli count results in UFC/cm2 at different parts/points of the carcass, after bleeding stage, during the technological processing of pig slaughter in slaughterhouses A and B located in the Federal District region. The average counts of all parts of the carcass were converted into log UFC/cm2. (US* Unviable/lost sample; NG** No growth).
| Slaughter Technological Processing Stage |
Visits (Slaughterhouse A and B) |
Microorganism | Carcass Part/Point (Count in CFU/cm2) |
Average Carcass Count (log CFU/cm2) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 Shank |
2 Loin |
3 Shoulder |
4 Abdomen |
5 Jowl |
||||
| After bleeding |
1st visit (slaughterhouse A) | Enterobacteriaceae | 2.5 × 102 2.3log |
1.3 × 101 1.1log |
9.2 × 102 2.9log |
2.6 × 102 2.4log |
US* 0 |
2.55 |
| E. coli | 1.0 × 102
2log |
0.2 × 101
0.3log |
7.0 × 101 1.84log |
2.1 × 101
1.3log |
US* 0 |
1.68 | ||
| 1st visit (slaughterhouse B) | Enterobacteriaceae | 8.0 × 101 1.9log |
6.1 × 101 1.7log |
4.0 × 101
1.6log |
6.0 × 101 1.7log |
1.4 × 102 2.1log |
1.88 | |
| E. coli | 0.4 × 101 0.6log |
0.3 × 101 0.4log |
104
4log |
104 4log |
104 4log |
3.77 | ||
| 2nd visit (slaughterhouse B) | Enterobacteriaceae | NG** 0 |
0.8 × 101
0.9log |
1.2 × 103 3.07log |
0.6 × 102 1.7log |
9.8 × 102 2.9log |
2.65 | |
| E. coli | NG** 0 |
NG** 0 |
2.8 × 102 2.4log |
NG** 0 |
2.1 × 102 2.3log |
1.99 | ||
Table 3.
Enterobacteriaceae and E. coli count results in UFC/cm2 at different parts/points of the carcass after the epilation process during the technological processing of pig slaughter in slaughterhouses A and B located in the Federal District region. The average counts of all parts of the carcass were converted into log UFC/cm2. (NG** No growth).
| Slaughter Technological Processing Stage |
Visits (Slaughterhouse A and B) |
Microorganism | Carcass Part/Point (Count in CFU/cm2) |
Average Carcass Count (log CFU/cm2) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 Shank |
2 Loin |
3 Shoulder |
4 Abdomen |
5 Jowl |
||||
| After epilation process |
1st visit (slaughterhouse A) | Enterobacteriaceae | 3.9 × 101 1.5log |
3.2 × 102 2.5log |
3.8 × 101 1.5log |
2.1 × 102 2.3log |
2.8 × 102 2.4log |
2.24 |
| E. coli | 2.1 × 101 1.3log |
1.3 × 101
1.1log |
0.7 × 101 0.8log |
0.2 × 101 0.3log |
0.4 × 101 0.6log |
0.97 | ||
| 1st visit (slaughterhouse B) | Enterobacteriaceae | 0.4 × 101 0.6log |
2.9 × 101 1.4log |
4.9 × 101 1.6log |
2.0 × 101 1.3log |
3.3 × 101 1.5log |
1.43 | |
| E. coli | 1.3 × 101 1.1log |
1.2 × 101 1log |
1.9 × 101 1.2log |
0.9 × 101 0.9log |
2.1 × 101 | 1.17 | ||
| 2nd visit (slaughterhouse B) | Enterobacteriaceae | 1.2 × 102 2log |
6.0 × 101 1.7log |
2.8 × 102 2.4log |
3.4 × 102 2.5log |
4.8 × 102 2.6log |
2.40 | |
| E. coli | NG** | NG** | NG** | NG** | NG** | 0 | ||
Table 4.
Enterobacteriaceae and E. coli count results in UFC/cm2 at different parts/points of the carcass, after manual skin process during the technological processing of pig slaughter, in slaughterhouses A and B located in the Federal District region. The average counts of all parts of the carcass were converted into log UFC/cm2. (US* Unviable/lost sample; NG** No growth).
| Slaughter Technological Processing Stage |
Visits (Slaughterhouse A and B) |
Microorganism | Carcass Part/Point (Count in CFU/cm2) |
Average Carcass Count (log CFU/cm2) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 Shank |
2 Loin |
3 Shoulder |
4 Abdomen |
5 Jowl |
||||
| After manual skin process |
1st visit (slaughterhouse A) | Enterobacteriaceae | 3.5 × 102 2.5log |
US* 0 |
3.2 × 101 1.5log |
4.2 × 102 2.6log |
3.0 × 102 2.4log |
2.4 |
| E. coli | 9.8 × 101 1.9log |
1.0 × 101 1log |
0.9 × 101 0.9log |
1.3 × 101 1.1log |
US* 0 |
1.51 | ||
| 1st visit (slaughterhouse B) | Enterobacteriaceae | 7.7 × 101 1.8log |
4.0 × 101 1.6log |
2.8 × 101 1.4log |
0.4 × 101 0.6log |
2.9 × 102 2.4log |
1.94 | |
| E. coli | 2.9 × 101 1.4log |
3.0 × 101 1.4log |
1.5 × 101 1.1log |
1.1 × 101 1log |
104 4log |
3.30 | ||
| 2nd visit (slaughterhouse B) | Enterobacteriaceae | 2.4 × 102 2.3log |
1.2 × 102 2log |
NG** | 0.5 × 101 0.6log |
NG** | 1.86 | |
| E. coli | NG** | NG** | 2.0 × 101 1.3log |
NG** | NG** | 0.6 | ||
Table 5.
Enterobacteriaceae and E. coli count results in UFC/cm2 at different parts/points of the carcass before the evisceration stage during the technological processing of pig slaughter in slaughterhouses A and B located in the Federal District region. The average counts of all parts of the carcass were converted into log UFC/cm2. (NG** No growth).
| Slaughter Technological Processing Stage |
Visits (Slaughterhouse A and B) |
Microorganism | Carcass Part/Point (Count in CFU/cm2) |
Average Carcass Count (log CFU/cm2) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 Shank |
2 Loin |
3 Shoulder |
4 Abdomen |
5 Jowl |
||||
| Before evisceration |
1st visit (slaughterhouse A) | Enterobacteriaceae | 4.3 × 103 2.6log |
2.3 × 102 2.3log |
2.5 × 102 2.3log |
5.0 × 101 1.6log |
2.7 × 102 2.4log |
2.39 |
| E. coli | 1.7 × 102 2.2log |
2.4 × 101 1.3log |
NG** | 2.7 × 101 1.4log |
0.9 × 101 0.9log |
1.66 | ||
| 1st visit (slaughterhouse B) | Enterobacteriaceae | 2.7 × 101
31.4log |
3.9 × 101 1.5log |
3.6 × 101 1.5log |
0.7 × 101
0.8log |
6.6 × 101
1.8log |
1.54 | |
| E. coli | 1.1 × 101 1log |
1.3 × 101 1.1log |
1.4 × 101
1.1log |
0.7 × 101 0.8log |
0.8 × 101
0.9log |
1.02 | ||
| 2nd visit (slaughterhouse B) | Enterobacteriaceae | 0.6 × 101 1.7log |
2.0 × 101 1.3log |
0.1 × 101 0 |
NG** | NG** | 1.2 | |
| E. coli | 2.0 × 101 1.3log |
NG** | NG** | NG** | NG** | 0.6 | ||
Table 6.
Enterobacteriaceae and E. coli count results in UFC/cm2 at different parts/points of the carcass after the evisceration stage during the technological processing of pig slaughter in slaughterhouses A and B located in the Federal District region. The average counts of all parts of the carcass were converted into log UFC/cm2. (US* Unviable/lost sample; NG** No growth).
| Slaughter Technological Processing Stage |
Visits (Slaughterhouse A and B) |
Microorganism | Carcass Part/Point (Count in CFU/cm2) |
Average Carcass Count (log CFU/cm2) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 Shank |
2 Loin |
3 Shoulder |
4 Abdomen |
5 Jowl |
||||
| After evisceration |
1st visit (slaughterhouse A) | Enterobacteriaceae | 6.2 × 101 1.7log |
2.8 × 101 1.4log |
4.4 × 102 2.6log |
5.4 × 101 1.7log |
2.2 × 102 2.3log |
2.20 |
| E. coli | 3.4 × 102 2.5log |
3.8 × 101 1.5log |
2.9 × 101 1.4log |
US* | 2.9 × 101 1.4log |
2.03 | ||
| 1st visit (slaughterhouse B) | Enterobacteriaceae | 1.0 × 103
3log |
1.7 × 102 2.2log |
3.1 × 102 2.4log |
1.3 × 103
3.1log |
1.3 × 103
3.1log |
2.91 | |
| E. coli | 106 6log |
106 6log |
106
6log |
106 6log |
106
6log |
6.0 | ||
| 2nd visit (slaughterhouse B) | Enterobacteriaceae | 1.2 × 102 2log |
8.0 × 102 2.9log |
3.6 × 101 1.5log |
1.4 × 102 2.1log |
0.4 × 101 0.6log |
2.3 | |
| E. coli | 2.0 × 101 1.3log |
6.0 × 101 1.7log |
NG** | NG** | NG** | 1.20 | ||
Table 7.
Enterobacteriaceae and E. coli count results in UFC/cm2 at different parts/points of the carcass after washing the carcass and before moving them into the cooling chamber of pig slaughterhouses A and B located in the Federal District region. The average counts of all parts of the carcass were converted into log UFC/cm2. (NG** No growth).
| Slaughter Technological Processing Stage | Visits (Slaughterhouse A and B) |
Microorganism | Carcass Part/Point (Count in CFU/cm2) |
Average Carcass Count (log CFU/cm2) | ||||
|---|---|---|---|---|---|---|---|---|
| 1 Shank |
2 Loin |
3 Shoulder |
4 Abdomen |
5 Jowl |
||||
| After wash and before enter the cooling chamber |
1st visit (slaughterhouse A) | Enterobacteriaceae | 1.1 × 102 2log |
5.1 × 101 1.6log |
3.3 × 101 1.5log |
3.2 × 101 1.5log |
1.8 × 101 1.2log |
1.68 |
| E. coli | 3.3 × 101 1.5log |
8.7 × 101 1.9log |
4.4 × 101 1.6log |
0.2 × 101 0.3log |
4.0 × 101 1.6log |
1.61 | ||
| 1st visit (slaughterhouse B) | Enterobacteriaceae | 7.5 × 101 1.8log |
7.2 × 101 1.8log |
1.0 × 102
2log |
1.5 × 102
2.1log |
1.5 × 102
2.1log |
2.03 | |
| E. coli | 104
4log |
104
4log |
104
4log |
104
4log |
104
4log |
4.0 | ||
| 2nd visit (slaughterhouse B) | Enterobacteriaceae | NG** 0 |
NG** 0 |
NG** 0 |
NG** 0 |
NG** 0 |
0 | |
| E. coli | NG** 0 |
NG** 0 |
NG** 0 |
NG** 0 |
NG** 0 |
0 | ||
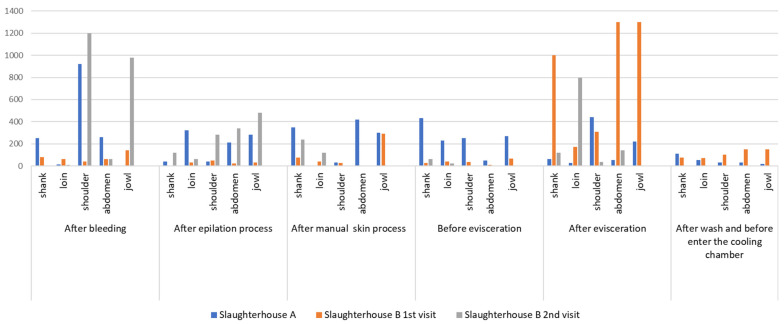
The results obtained for Enterobacteriaceae counts (CFU/cm2) for pig carcass parts from slaughterhouses A and B for each visit and at each slaughter stage are described in Figure 1 and Figure 2.
Figure 1.
Pig carcass count of Enterobacteriaceae (CFU/cm2) in slaughterhouses A and B (1st and 2nd visits) by stage of technological processing of pig slaughter and by location/point on the carcass.
Figure 2.
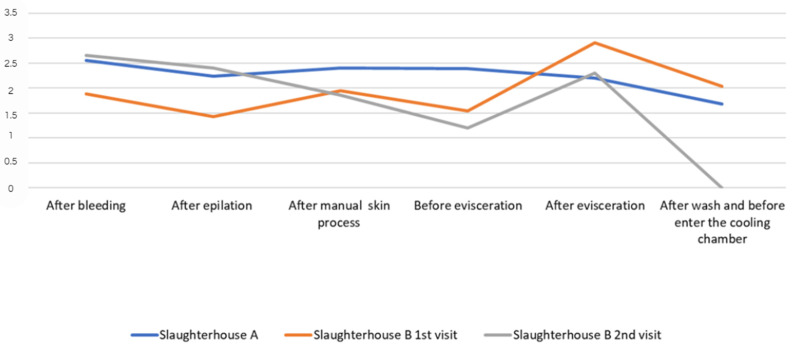
General average of Enterobacteriaceae counts from all parts of the pig carcass, converted into log CFU/cm2, from each stage of the technological processing of slaughter in slaughterhouses A (1st visit) and B (1st and 2nd visits).
In general, few studies in Brazil have focused on Enterobacteriaceae count in pig carcasses, as can be seen in the review conducted by Viltrop et al. [17]. No published studies have reported Enterobacteriaceae count by carcass location/point and at different slaughter stages in the Federal District. Microorganism analysis was established in 2018 through Normative Instruction number 60 from the Ministry of Agriculture, Livestock, and Supply [12], which may explain the absence of related work. Figure 1 and Figure 2 show a similar profile for Enterobacteriaceae counts/cm2 and average counts converted into log CFU/cm2 for all carcass parts at each slaughter stage in slaughterhouses A and B, with higher peaks due to higher counts after bleeding and evisceration, which corroborates the results reported by Wheatley et al. [39], describing greater contamination in the jowl and abdomen areas after evisceration. The high count in the shoulder and jowl regions in the post-bleeding stage in both slaughterhouses corroborates the analysis by Bolton et al. [40], in which a higher bacterial count may be associated with contact of the pig carcass with the slaughterhouse floor or other instruments present in the environment at this stage.
In the analysis of the pig slaughter technology flowchart, a higher count of Enterobacteriaceae count/cm2 was found in slaughterhouse A, in the post-bleeding stage, and in the general carcass average for refrigerated slaughterhouses A and B (second collection), whose log CFU/cm2 values were 2.55 and 2.65, respectively. Subsequently, the count decreased after hair removal and manual skinning, increased after the post-evisceration stage, and decreased after washing and before entering the cooling chamber. These findings demonstrate a reduction in the count of microorganisms after bleeding, probably due to the action of the scalding process, depilation stage, and final washing of the carcasses, which are stages provided by Ordinance 711 of 1995 of the Ministry of Agriculture, Livestock, and Supply [41], which deals with procedural standards for pig slaughter and inspection. The increase in counts observed in this study in the post-evisceration stage corroborates the reports by Bolton et al. [40] regarding the high count of aerobic microorganisms at this stage. According to the authors, the higher count may be related to the spread of microorganisms due to the nature of the evisceration procedure, as well as to equipment/utensils contaminated at this stage and to the ambient air due to the release of fomites.
The average Enterobacteriaceae count, which was obtained from the sum of the counts of all pig carcass points/parts for each slaughter stage and expressed in log CFU/cm2, was 2.5 log CFU/cm2 after bleeding for slaughterhouse A in the first collection, 1.88 log CFU/cm2 for slaughterhouse B in the first collection, and 2.6 log CFU/cm2 in the second collection (Table 2, Table 3, Table 4, Table 5, Table 6 and Table 7 and Figure 2). These values were lower than those obtained by Corbellini et al. [14], who reported 3.5 log CFU/cm2 in pig carcasses in slaughterhouses located in southern Brazil. Although the results of the present study were obtained from a single carcass for each visit to slaughterhouses A and B, the results were close to those verified by Montes et al. [42] in pig carcasses from the western region of Santa Catarina, Brazil (2.37 log CFU/cm2 in the bleeding stage). However, the results of the average carcass in log CFU/cm2 in the stage after bleeding, with values obtained of 2.55 log CFU/cm2 (A first collection), 1.88 log CFU/cm2 (B first collection) and 2.65 log CFU/cm2 (B second collection), were higher than those obtained by Cê (1.83 log CFU/cm2) [30], in pig carcasses from slaughterhouses located in Santa Catarina, Brazil.
Unlike the results of other studies describing recontamination of carcasses during epilation due to the difficulty of sanitizing the equipment and fecal contamination [40,43,44], in this study, there was no large increase in the logarithmic Enterobacteriaceae count after the epilation stage. Comparing the stages before scalding (after bleeding) and after epilation (after scalding), the logarithmic Enterobacteriaceae count decreased to 0.31 log CFU/cm2 in the first collection from slaughterhouse A, 0.45 log CFU/cm2 in the first collection from slaughterhouse B, and 0.25 log CFU/cm2 in the second collection from slaughterhouse B. The reduction in Enterobacteriaceae counts after hair removal (Figure 2) can be explained by scalding followed by epilation and the absence of recontamination in the epilation machine. Thus, these results corroborate those of Spescha et al. [43], Wheatley et al. [39], and Nastasijevic et al. [45], who demonstrated that scalding pig carcasses reduced bacterial contamination.
In both refrigerated Slaughterhouse A and the first collection from Slaughterhouse B, the skin processes were made in the dirty area, according to the classification given by Ordinance No. 711 of 1995 of the Ministry of Agriculture, Livestock, and Supply [41]. The procedure was manually performed on a table, under which the hair remained, and with high humidity, which may have contributed to the increased microbial count at this stage, even though there was a reduction in the previous stage of scalding. However, there was a reduction in 0.54 log CFU/ cm2 for slaughterhouse B in the second collection. The logarithmic results of the overall carcass average (Table 4) from the manual toilet stage, from the first collections from slaughterhouses A and B (2.4 and 1.94 log CFU/cm2 respectively), increased from those from the previous stage (hair removal). Comparing the microbial count at the manual toilet stage in this study with those at the mechanized toilet stage verified by Corbellini et al. [14], in the dirty area, the results are similar to those observed by the authors, including that at the previous steps, such as the post-epilation step. However, these results differed from those observed by Zwirzitz et al. [46], who did not detect Enterobacteriaceae at the skin stage. The results of this study were also different from those obtained by Cê [30] and Rodrigues et al. [22], who did not detect Enterobacteriaceae or only detected a low count at the skin process stage. A possible cause may be related to the knives used by handlers at this stage, which pass through areas close to the oral cavity and perianal region. This, in turn, is related to inadequate ways of carrying out this procedure, thus characterizing cross-contamination, although Buncic and Sofos [47] and Wheatley et al. [39] also verified contamination in the practice of mechanized toileting.
Evaluating the general logarithmic results of the carcasses (Table 2, Table 3, Table 4, Table 5, Table 6 and Table 7 and Figure 1 and Figure 2) before and after evisceration in slaughterhouse B indicated an increase of more than 1 log CFU/cm2 in the two collections (2.91 and 2.3 log CFU/cm2 from the first and second collections, respectively). In relation to the results per point/location on the carcass, the shoulder (4.4 × 102 CFU/cm2) presented the highest Enterobacteriaceae count after evisceration in slaughterhouse A in the first collection. In cold slaughterhouse B, the belly and jowls presented a count of 1.3 × 103 CFU/cm2 in the first collection, and the loin had the highest count (8.0 × 102 CFU/cm2) in the second collection. Wheatley et al. [39] found significant increases in counts in the jowls and abdominal area after evisceration, as seen in this study. According to them, evisceration is frequently the biggest source of contamination in pig slaughter since even healthy animals can be carriers of pathogenic and opportunistic microorganisms due to changes in the permeability of the intestinal wall as a result of stress during slaughter. These microorganisms can penetrate other tissues, as the handler can be the vehicle for transmission to other areas, leading to increased counts [48], thus justifying the results of this study.
MAPA Normative Instruction No. 60, of 20 December 2018 [12] establishes microbiological count standards of Enterobacteriaceae, in log CFU/cm2, for pork carcasses before cooling as acceptable, with the standard being lower than 2.0 log CFUcm2, intermediate standard with counts between 2.0 and 3.0 log CFU/cm2, and unacceptable standard with counts above 3.0 log CFU/cm2. In this study, the carcasses from slaughterhouse A in the first collection and those from slaughterhouse B in the second collection could be classified as acceptable after washing and before cooling. The carcass from the first collection from Slaughterhouse B was classified as intermediate, as it presented a logarithmic count of 2.04 log CFU/cm2.
3.2. E. coli Counts
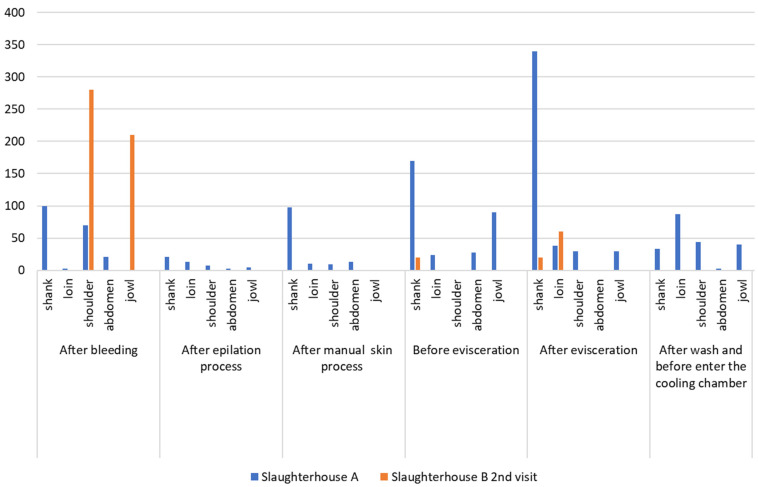
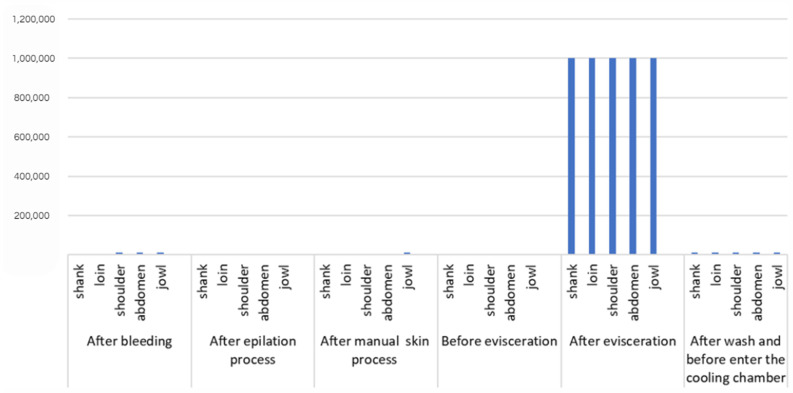
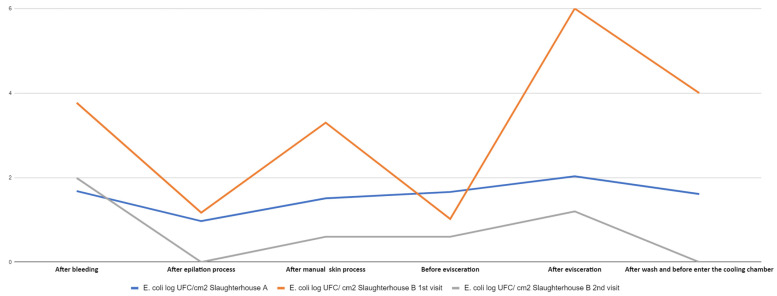
Figure 3 and Figure 4 present the E. coli counts (CFU/cm2) from pig carcass points/parts, which revealed an increased count in the stages after bleeding and evisceration; the E. coli count for all carcass parts in the post-evisceration stage in slaughterhouse B, in the first collection cm2, which decreased to 104 CFU/cm2 after washing the carcass and the jowl. Figure 5 shows the average E. coli counts converted into logarithmic values (log CFU/cm2) from slaughterhouses A and B and for each slaughter stage.
Figure 3.
Count of E. coli/cm2 at different points on the pig carcass and at different stages of the slaughter process in slaughterhouses A and B (2nd collection).
Figure 4.
Count of E. coli/cm2 at different points on the pig carcass and at different stages of the slaughter process in slaughterhouses A and B (1st collection).
Figure 5.
General average of E. coli counts in log CFU/cm2 in different parts of the pig carcass from slaughterhouses A (1st collection) and B (1st and 2nd collection) at different stages of the slaughter process.
According to Ghafir et al. [49], E. coli and Enterobacteriaceae counts are normally correlated and used in slaughterhouses as environmental and fecal contamination indicators; both appear throughout the technological processing of slaughter in this study, indicating contamination. High counts were observed, with a value of 104 CFU/cm2 in the jowl, shoulder, and abdominal regions, at the post-bleeding stage, in the first collection from slaughterhouse B.
In this study, E. coli counts were also higher in the stages after bleeding (overall average carcass count of 1.68 log CFU/cm2 in slaughterhouse A in the first collection, 3.77 log CFU/cm2 in slaughterhouse B in the first collection, and 1.99 log CFU/cm2 in slaughterhouse B in the second collection) and after evisceration (average counts of 2.03 log CFU/cm2 in slaughterhouse A in the first collection, 6.0 log CFU/cm2 in slaughterhouse B in the first collection, and 1.2 log CFU/cm2 in slaughterhouse B in the second collection). The respective peak values are shown in Figure 5, which presents the average E. coli counts converted into log CFU/cm2 from all carcass points/locations by stage and sequence of the technological processing of slaughter. The points/locations on the carcass with the highest E. coli counts were the jowls, shoulder, and abdomen in the stages after bleeding, skinning, evisceration, and before the cooling stage in slaughterhouse B in the first collection. Slaughterhouse A had the highest count, which remained until the post-evisceration stage. The results obtained in this study differed from the E. coli prevalence on pig carcass surfaces in the southern region of Brazil observed by Machado et al. [50], where the jowls had high E. coli counts.
According to Rivas et al. [51], final washing did not reduce the microbial count in the carcass. However, this study verified decreased E. coli counts in carcasses after final washing than those after the previous evisceration stage. Comparing the results of these two stages, the average E. coli count after evisceration (2.03 log CFU/cm2) decreased after washing (1.61 log CFU/cm2) in slaughterhouse A. In this study, the average E. coli count after evisceration also decreased after washing in the first (6.0 and 4.0 log CFU/cm2, respectively) and second (1.20 log CFU/cm2 and undetected, respectively) collections from slaughterhouse B. Even though residual chlorine content was not measured in the washing water in this study, Brazilian legislation determines a 0.2 to 2.0 ppm chlorine limit in drinkable water [13]. Therefore, the presence of chlorine may be a possible justification for the superficial microbial reduction or absence of growth after washing the carcasses observed in this study.
The E. coli count on the pig carcass surface obtained in this study do not provide parameters for Brazilian legislation, which does not consider counting E. coli in pig carcasses for microbiological control. In Brazil, Enterobacteriaceae count and the presence or absence of Salmonella spp. are used, following the model established by the European Union, which also counts aerobic colonies to evaluate the hygiene of the meat production process [52]. However, the E. coli count can be considered as a quality control indicator since the profiles verified for this microorganism were similar to those of Enterobacteriaceae.
Although Enterobacteriaceae and E. coli counts were carried out on a few carcasses, which in turn may not show the overall real profile of the national industry, the results showed the same count profile, in which the peaks with higher values coincided with the same steps between the two analyses, which in turn can contribute to improvements in the pig slaughter flowchart in industries. Furthermore, the counts in different carcass parts showed similarities in places with the highest counts between the two analyses, which can contribute to good manufacturing practices in this type of establishment, making it the first in the region and country.
3.3. E. coli Isolates
Twenty-three E. coli isolates were obtained in the first collection from slaughterhouse A, and 29 isolates in the second collection from slaughterhouse B, for a total of 52 isolates (Table 8). The virulence genes and in vitro biofilm formation capacity of the isolates were studied.
Table 8.
Identification of E. coli isolates by stage of the technological processing of slaughter and location on the carcass, in slaughterhouses A in 1st visit and B in 2nd visit.
| Slaughtering Stage | Slaughterhouse/Visit | Shank | Loin | Shoulder | Abdomen | Jowl |
|---|---|---|---|---|---|---|
| After bleeding | A (1st visit) | 1A | 1B | 1C | 1D | 1E |
| B (2nd visit) | 7A | 7B | 7C | 7D | 7E | |
| After epilation | A (1st visit) | 2A | 2B | 2C | ND | ND |
| B (2nd visit) | 8A | 8B | 8C | 8D | 8E | |
| After manual skin | A (1st visit) | 3A | 3B | ND | 3D | 3E |
| B (2nd visit) | 9A | 9B | 9C | 9D | 9E | |
| Before evisceration | A (1st visit) | 4A | ND | ND | 4D | 4E |
| B (2nd visit) | 10A | 10B | 10C | 10D | 10E | |
| After evisceration | A (1st visit) | 5A | 5B | 5C | 5D | ND |
| B (2nd visit) | 11A | 11B | 11C | 11D | 11E | |
| After final wash | A (1st visit) | 6A | 6B | ND | 6D | 6E |
| B (2nd visit) | 12A | 12B | ND | 12D | 12E |
ND = not detected.
3.4. Antibiogram of E. coli Isolates
The antibiograms and frequency results for the 52 E. coli isolates from slaughterhouses A and B are presented in Table 9 and Table 10. The drugs with the highest percentage of resistance detected in this study were beta-lactams (amoxicillin and ampicillin, 88.5% resistant isolates), followed by sulfonamide (84.61%), chloramphenicol (82.7%), and streptomycin (80%). According to the CLSI [34], the antibiogram results for ampicillin can be used to predict the results for amoxicillin, which explains the same proportion of resistance to both antimicrobials in this study.
Table 9.
Antibiogram of 52 E. coli isolates from slaughterhouses A and B located in the Federal District region, using the disk-diffusion method and interpreted, according to CLSI parameters [34], as resistant I, intermediate (I), or sensitive (S).
| Antimicrobial | Resistant | Intermedite | Sensitive |
|---|---|---|---|
| Nalidixic acid | 1A-1B-1C-1D; 2A-2B-2C; 3A-3D; 4A-4E; 5A-5B-5C-5D; 6A-6B-6D-6E; 7B-7C-7D; 8B-8C-8D; 9A-9B-9D-9E; 10A-10C-10D; 11A-11B-11C-11D-11E; 12E |
3B-3E 7A-7E 8A |
1E; 4D 8E; 9C ‘10B-10E 12A-1-B-12D |
| Amoxicillin | 1A-1B-1C-1D-1E; 2A-2B-2C 3A-3B-3D-3E; 4A-4D-4E; 5A-5B-5C-5D; 6A-6B-6E; 7A-7B-7D-7E; 8A-8B-8C-8D-8E; 9A-9B-9D 10A, 10B, 10D 11A-11B-11C-11D-11E 12A-12B-12D-12E |
- | 6D 7C 9-C-9E 1-C-10E |
| Ampicillin | 1A-1B-1C-1D-1E; 2A-2B-2C 3A-3B-3D-3E; 4A-4D-4E - 5A-5B-5C-5D; 6A-6B-6E 7A-7B-7D-7E 8A-8B-8C-8D-8E; 9A-9B-9D; 10A, 10B, 10D 11A-11B-11C-11D-11E 12A-12B-12D-12E |
- | 6D 7C 9-C-9E 1-C-10E |
| Cefazolin | 1D 8A-8D 10A-10C |
1A-1B-1C-1E; 2A-2B-2C; 3A-3B-3D-3E; 4A-4D-4E; 5A-5B-5C-5D; 6A-6B-6D-6E 8B-8C-8E; 9A; 11E |
7A-7B-7C-7D-7E; 9B-9C-9D-9E; 10B-10D-10E; 11A-11B-11C-11D 12A-12B-12D-12E |
| Ceftazidime | 2A-2B 8A 9C |
1C | 1A-1B-1D-1E; 2C; 3A-3B-3D-3E; 4A-4D-4E; 5A-5B-5C-5D; 6A-6B-6D-6E 7A-7B-7C-7D-7E 8B-8C-8D-8E; 9A-9B-9D-9E; 10A-10B-10C-10D-10E 11A-11B-11C-11D-11E; 12A-12B-12D-12E |
| Ciprofloxacin | 1C-1D; 2C; 3B-3D-3E; 4E 5D; 6D 7C; 9A 10A-10D |
1A; 4A; 5B; 6A 7A-7E; 8A 9D; 10C |
1B-1E; 2A-2B; 3A 4D 5A-5C; 6B-6E 7B-7D; 8B-8C-8D-8E 9B-9C-9E; 10B-10E 11A-11B-11C-11D-11E 12A-12B-12D-12E |
| Chloramphenicol | 1A-1C-1D; 2A-2B-2C 3A-3B-3D-3E; 4A-4E 5A-5B-5D; 6A-6B-6D-6E 7A-7B-7C-7D-7E; 8A-8B-8C-8E; 9A-9B-9D-9E 10A-10B-10C-10D-10E 11A-11B-11C-11D-11E; 12E |
8D | 1B-1E 4D; 5C 9C 12A-12B-12D |
| Doxycycline | 1A-1B; 6D 7A-7B-7C-7D-7E 8A-8B-8C-8D-8E 9A-9B-9D-9E 10A-10B-10C-10D-10E 11A-11B-11C-11D-11E; 12E |
1D-1E | 1C; 2A-2B-2C 3A-3B-3D-3E; 4A-4D-4E 5A-5B-5C-5D 6A-6B-6E 9C 12A-12B-12D |
| Streptomycin | 1A-1C-1D-1E; 2A-2B-2C; 3A-3B-3D-3E; 4A-4D-4E; 5A-5C-5D; 6A-6B-6D-6E 7A-7B-7C-7D-7E 8A-8B-8C-8E; 9A-9B-9D; 10A-10B-10E 11A-11B-11C-11D-11E; 12E |
8D 9E 10C-10D |
1B 5B 9C 12A-12B-12D |
| Erythromycin | 1A-1B-1C-1D-1E; 2A-2B-2C 3A-3B-3D-3E; 4A-4D-4E 5A-5B-5C-5D; 6A-6B-6D-6E 7A; 8A-8B-8C-8D 10C-10D-10E 11A-11B-11C-11D-11E; 12E |
- | 7B-7C-7D-7E; 8E 9A-9B-9C-9D-9E 10A-10B 12A-12B-12D |
| Gentamicin | 1C-1D 2C 4E; 5B-5D 7C |
- | 1A-1B-1E; 2A-2B; 3A-3B-3D-3E; 4A-4D; 5A-5C; 6A-6B-6D-6E 7A-7B-7D-7E; 8A-8B-8C-8D-8E; 9A-9B-9C-9D-9E 10A-10B-10C-10D-10E 11A-11B-11C-11D-11E 12A-12B-12D-12E |
| Sulfonamide | 1A-1C-1D-1E; 2A-2B-2C 3A-3B-3D-3E; 4A-4D-4E 5A-5B-5C-5D; 6A-6B-6D-6E 7A-7B-7C-7D-7E; 8C-8E; 9A-9B-9E; 10A-10B-10C-10D-10E 11A-11B-11C-11D-11E 12D-12E |
- | 1B 8A-8B-8D 9C-9D 12A-12B |
| Tetracycline | 1A-1B; 6D 7A-7B-7C-7D-7E 8A-8B-8C-8D-8E 9A-9B-9D-9E 10A-10B-10C-10D-10E 11A-11B-11C-11D-11E; 12E |
- | 1C-1D-1E; 2A-2B-2C 3A-3B-3D-3E; 4A-4D-4E 5A-5B-5C-5D; 6A-6B-6E 9C 12A-12B-12D |
Table 10.
Frequency results of 52 E. coli isolate antibiograms from slaughterhouses A and B located in the Federal District region, using the disk-diffusion method and interpreted, according to CLSI parameters [34], as resistant I, intermediate (I), or sensitive (S).
| Antimicrobial | Number of Resistant Isolates (%) | Number of Intermediate Resistance Isolates (%) |
Number of Sensitive Isolates (%) | Total of Resistant and Intermediate Isolates (%) |
|---|---|---|---|---|
| Nalidixic acid (NAL) |
73% (38/52) |
9.6% (5/52) |
17.4% (9/52) |
82.6% (43/52) |
| Amoxicillin (AMO) |
88.4% (46/52) |
0% (0/0) |
11.6% (6/52) |
88.4% (46/52) |
| Ampicillin (AMP) |
88.4% (46/52) |
0% (0/0) |
11.6% (6/52) |
88.4% (46/52) |
| Cefazolin (CFZ) |
9.6% (5/52) |
51.9% (27/52) |
38.5% (20/52) |
61.5% (32/52) |
| Ceftazidime (CAZ) |
7.6% (4/52) |
2% (1/52) |
90.4% (47/52) |
9.6% (5/52) |
| Ciprofloxacin (CIP) |
25% (13/52) |
17.3% (9/52) |
57.7% (30/52) |
42.3% (22/52) |
| Chloramphenicol (CLO) |
82.6% (43/52) |
2% (1/52) |
15.4% (8/52) |
84.6% (44/52) |
| Doxycycline (DOX) |
53.7% (28/52) |
4% (2/52) |
42.3% (22/52) |
57.7% (30/52) |
| Streptomycin (EST) |
80.7% (42/52) |
7.7% (4/52) |
11.6% (6/52) |
88.4% (46/52) |
| Erythromycin (ERI) |
71.1% (37/52) |
0% (0/0) |
28.9% (15/52) |
71.1% (37/52) |
| Gentamicin (GEN) |
13.5% (7/52) |
0% (0/0) |
86.5% (45/52) |
13.5% (7/52) |
| Tetracycline (TET) |
53.7% (28/52) |
0% (0/0) |
46.3% (24/52) |
53.7% (28/52) |
| Sulfonamide (SUL) |
84.6% (44/52) |
0% (0/0) |
15.4% (8/52) |
84.6% (44/52) |
According to the definition of multidrug resistance stipulated by Magiorakos et al. [53], 49 E. coli isolates (94.23%) were considered multidrug-resistant, presenting resistance to three or more classes of antimicrobials. Similar results were obtained by Mangal et al. [51], in an evaluation of pork from India and identified 100% resistance to ampicillin in 41 E. coli isolates. Similar to the findings of this study, Pissetti et al. [54] detected a multidrug resistance profile in 71.5% strains from swine carcasses in Santa Catarina, Brazil, with 97.8%, 86.4%, 85.1%, and 81.1% strains being resistant to tetracycline, chloramphenicol, sulfonamide, and ampicillin, respectively.
Sulfonamide was the second most common antimicrobial with resistance among the E. coli isolates in this study. In 2012, in the Federal District region, 74.8% and 70.1% strains in pig feces on breeding farms were resistant to sulfonamides and tetracyclines, respectively [55]. Unlike these reports [56,57,58,59,60], tetracycline resistance frequencies in this study were low, with 28 doxycycline- and tetracycline-resistant isolates each (53.9%).
Forty-four (84.61%) isolates presented resistance to chloramphenicol, which has been banned from use in animals in Brazil since 2003 [61]. The results obtained were corroborated by Mangal et al. [51], who verified resistance to chloramphenicol in 30 isolates (73.2%) in India. Drummond and Perecmanis [52] also identified 34.7% chloramphenicol-resistant strains in pig feces in the Federal District. The persistence of this resistance can be attributed to the maintenance of chloramphenicol resistance genes through co-selection with other resistance and virulence genes [55,56,62,63,64]. However, further studies are necessary to confirm this.
Resistance to the antimicrobials streptomycin and sulphonamide was 80.8% and 84.6%, respectively, which were different from the results of Mangal et al. [51], who obtained 100% sensitivity to streptomycin and sulphonamide in retail pork in India. Streptomycin resistance is a common finding in E. coli isolated from animals and animal products and, along with ampicillin resistance, is among the resistance phenotypes that are frequently co-transferred with sulphonamide resistance [65], which may explain the results of this study since the isolates also showed 81.1% resistance to sulphonamides.
The lowest resistance frequencies were observed for first-generation cephalosporin cefazolin (9.6%) and third-generation ceftazidime (7.7%), followed by gentamicin (13.5%). Moennighoff et al. [66] identified less than 15% resistance to gentamicin in pigs in Germany, as in this study. Third-generation cephalosporins are part of the so-called highest priority antimicrobials, which are critically important in human medicine, together with quinolones, macrolides, ketolides, and glycopeptides [67]. In this study, 7.7% E. coli isolates were ceftazidime-resistant, being the first report of this resistance in pig carcasses in the region (Federal District and Central West).
All 52 isolates were resistant to at least two antimicrobials. The presence of resistance to seven antimicrobials was detected in 15 isolates, which is the highest frequency of multidrug resistance in this study. However, three (5.77%) isolates were resistant to 10 antimicrobials. Only two isolates were sensitive to 12 antimicrobials out of the 13 tested, with the most sensitive antimicrobials being ceftazidime (90.4%) and gentamicin (86.5%).
Antimicrobial resistance is a global problem, posing a growing threat to public health [55]. Resistant bacteria can be transmitted to humans when consuming and handling contaminated food through direct contact with animals and the distribution of these bacteria in the environment [56]. The results obtained in this study demonstrate phenotypic resistance in slaughterhouses A and B, and most E. coli isolates showed multidrug resistance, which may represent an important source of dissemination of antimicrobial resistance in the environment.
3.5. Eae, stx1, and stx2 Virulence Gene Detection in E. coli
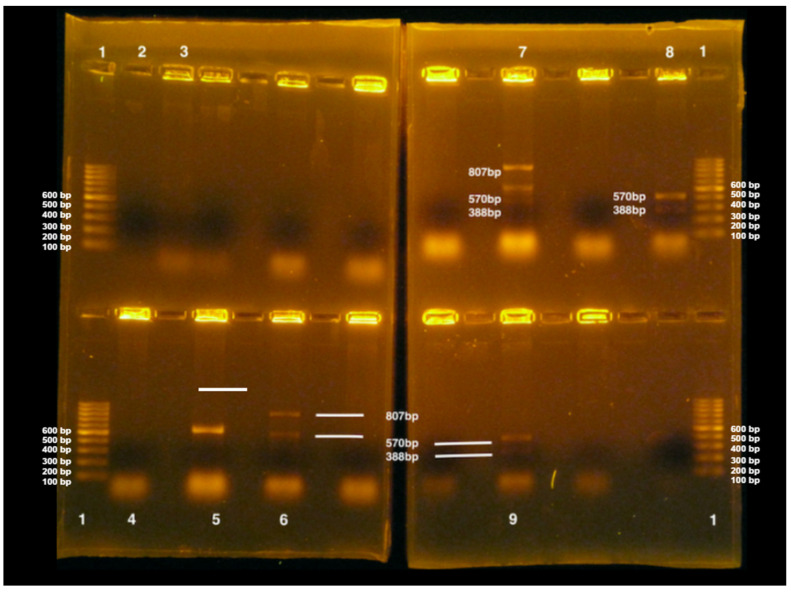
The eae, stx1, and stx2 genes were identified by PCR in 24/52 (46.15%), 16/52 (30.8%), and 9/52 (17.3%) E. coli isolates, respectively. Only three (5.8%) isolates (5D, 8A, and 11D) concomitantly presented all three genes (Figure 6).
Figure 6.
Detection of virulence genes stx1, stx2, and eae by multiple PCR in E. coli isolates in pig carcasses from slaughterhouse B in two gels. (1) 100 bp marker, (2) negative control, (3) positive control for stx1 gene (388 bp) in E. coli, (4) isolate 10D—negative, (5) isolate 10E—positive for eae gene (570 bp), (6) isolate 11A—positive for eae (570 bp) and stx2 (807 bp) genes, (7) isolate 11D—positive for eae (570 bp), stx1 (388 bp) and stx2 (807 bp) genes, (8) isolate 12A—positive for eae genes (570 bp) and stx1 (388 bp), (9) isolate 12D—positive for eae (570 bp) and stx1 (388 bp) genes.
E. coli with stx1 and stx2 genes can be called STEC [57,68]. Among the isolates, 11 isolates had stx1, four isolates had stx2, and five isolates contained both stx1 and stx2 genes. These genes are the main markers for STEC detection using PCR [58]. Corroborating the results regarding the detection of these genes in this study, Bouvet et al. [59] detected 48 STEC isolates in pig carcasses in slaughterhouses in France, 47 of which had the stx2 gene, and one isolate had the stx1 gene. However, this study identified more isolates containing the stx1 gene than stx2. These authors also studied the eae gene but did not identify it, considering the isolates to be of low potential danger to public health, as the simple finding of stx genes in food would not pose a threat to human consumption [60].
In contrast, in this study, 24 isolates containing the eae gene were identified; of these, five also harbored the stx1 gene (1E, 4A, 6B, 12A, and 12D), and three harbored the stx2 gene (3E, 9A, and 11A). Furthermore, three isolates presented all three genes (stx1, stx2, and eae) that could represent a risk to public health, as they contained one of the enhanced factors of STEC infection capacity, the intimin gene [62,63].
In Brazil, STEC outbreaks related to pork consumption have not been described to date. However, three outbreaks between 2014 and 2018 related to STEC O157:H7 have been described in Canada, with the detection of the stx2a and stx1a + stx2a genes and eae in the isolates [64], corroborating the importance of these findings, though the isolates were not serotyped.
In Slaughterhouse A, the stx1 gene was identified in isolates from four points during the slaughter process: after bleeding (1E: jowl), before evisceration (4A: shank, 4D: shoulder), after evisceration (5D: abdomen), and after washing the carcass prior to cooling (6B: loin). The stx2 gene was identified after manual skinning (3E: jowl) and evisceration (5D: shoulder). The eae gene was identified at all slaughter stages: after bleeding (1B: loin, 1C: shoulder, and 1E: jowl), after epilation (2A: shank), after manual skin (3A: shank, 3B: loin, 3E: jowl), before evisceration (4A: shank, 4D: abdomen), after evisceration (5A: shank, 5D: abdomen), and after washing the carcass prior to cooling (6B: loin, 6D: abdomen).
The results of the detection of virulence genes, together with the antibiograms of the isolates in this study, are presented in Table 11.
Table 11.
Antibiogram, PCR detection of the virulence genes stx1, stx2, and eae in E. coli isolates from pig carcasses from slaughterhouses A and B located in the Federal District.
| Isolates | Slaughterhouse | Antimicrobial Resistance | Virulence Gene |
|---|---|---|---|
| 1B | A | AMO AMP ERI DOX TET NAL | eae |
| 1C | A | AMO AMP ERI EST GEN SUL CLO NAL CIP | eae |
| 1E | A | AMO AMP ERI EST SUL | eae, stx1 |
| 2A | A | AMO AMP CAZ ERI EST SUL CLO NAL | eae |
| 3A | A | AMO AMP EST ERI SUL CLO NAL | eae |
| 3B | A | AMO AMP ERI EST SUL CLO CIP | eae |
| 3E | A | AMO AMP ERI EST SUL CLO CIP | eae, stx2 |
| 4A | A | AMO AMP ERI EST SUL CLO NAL | eae, stx1 |
| 4D | A | AMO AMP ERI EST SUL | eae, stx1 |
| 5A | A | AMO AMP ERI EST SUL CLO NAL | eae |
| 5D | A | AMO AMP ERI EST GEN SUL CLO NAL CIP | eae, stx1, stx2 |
| 6B | A | AMO AMP ERI EST SUL CLO NAL | eae, stx1 |
| 6D | A | ERI EST SUL DOX TET CLO NAL CIP | eae |
| 7A | B | AMO AMP ERI EST SUL DOX TET CLO | stx1, stx2 |
| 7B | B | AMO AMP EST SUL DOX TET CLO NAL | stx1 |
| 7C | B | EST GEN SUL DOX TET CLO NAL CIP | stx1 |
| 7D | B | AMO AMP EST SUL DOX TET CLO NAL | stx1 |
| 7E | B | AMO AMP EST SUL DOX TET CLO | stx1 |
| 8A | B | AMO AMP CFZ CAZ ERI EST DOX TET CLO | eae, stx1, stx2 |
| 8B | B | AMO AMP ERI EST DOX TET CLO NAL | stx2 |
| 8E | B | AMO AMP EST SUL DOX TET CLO | eae |
| 9A | B | AMO AMP EST SUL DOX TET CLO NAL CIP | eae, stx2 |
| 9B | B | AMO AMP EST SUL DOX TET CLO NAL | eae |
| 9C | B | CAZ | stx1, stx2 |
| 10B | B | AMO AMP EST SUL DOX TET CLO | eae |
| 10C | B | CFZ ERI SUL DOX TET CLO NAL | stx1 |
| 10E | B | EST SUL DOX TET CLO | eae |
| 11A | B | AMO AMP EST SUL DOX TET CLO NAL | eae, stx2 |
| 11D | B | AMO AMP EST SUL DOX TET CLO NAL | eae, stx1, stx2 |
| 12A | B | AMO AMP ERI | eae, stx1 |
| 12B | B | AMO AMP | eae |
| 12D | B | AMO AMP ERI SUL | eae, stx1 |
AMO = amoxicillin, AMP = ampicillin, CFZ = cefazolin, CAZ = ceftazidime, ERI = erythromycin, EST = streptomycin, GEN = entamicinacin, SUL = Sulfonamide, DOX = doxycycline, TET = tetracycline, CLO = chloramphenicol, NAL = nalidixic acid, CIP = ciprofloxacin.
In slaughterhouse B, the stx1 gene was identified in E. coli isolates from all carcass points after bleeding (7A, 7B, 7C, 7D, 7E), shoulder before evisceration (10C), abdomen after evisceration (11D), and loin and abdomen after washing the carcass prior to cooling (12B, 12D). The stx2 gene was identified in the shank after bleeding (7A), shank and loin after epilation (8A, 8B), jowl and shoulder after manual skin process (9A, 9C), and shank and abdomen after evisceration (11A and 11D). The eae gene was not identified after bleeding but was identified in the shank after depilation (8A), shank and loin after manual skin (9A, 9B), shank and jowl before evisceration (10A, 10E), shank and abdomen after evisceration (11A, 11D), and shank, loin, and abdomen after washing the carcass prior to cooling (12A, 12B, and 12D).
Therefore, the transmission of STEC can potentially occur at all pig slaughter stages in the Federal District region.
3.6. In Vitro Biofilm Formation Capacity Evaluation
The in vitro biofilm-forming capacities of 51 E. coli isolates were tested. Of these, 22 isolates were from Slaughterhouse A, and 29 were from Slaughterhouse B. The ability to form biofilms with respect to the incubation time was not significantly different (p < 0.086).
Regarding temperature variation at 10 °C, the ability to not form biofilm was greater compared to that at other temperature ranges, while the ability to form moderate and strong biofilm was more frequent at 37 and 24 °C.
Regarding the collection stage during the slaughterhouse process, there was a statistically significant difference (p < 0.0001): the toilet had a higher frequency of non-biofilm-forming E. coli, while the post-evisceration stage had a higher frequency of strong biofilm-forming E. coli.
At the isolated points of the carcass, E. coli also showed a significant difference (p < 0.0001), with the isolates from the jowl region showing a higher frequency of non-biofilm-forming E. coli, whereas the isolates from the shank region showed a higher frequency of strong biofilm-forming E. coli.
Twenty E. coli isolates with characteristic virulence factors for STEC (stx1 and stx2 genes) were evaluated for their biofilm-forming capacity. Of these isolates, eight showed weak or no formation capacity at the different temperatures evaluated (1E, 4D, 6B, 7E, 8A, 9A, 10C, and 12D). Four isolates showed a moderate or strong capacity for biofilm formation at the three temperatures evaluated (4A, 7A, 7 B, and 11A). Another eight isolates (3E, 5D, 7C, 7D, 8 B, 9C, 11D, and 12A) showed varying biofilm-formation capabilities according to the temperatures studied.
Of the 11 isolates with the stx1 gene, two (1E and 6B) did not show biofilm formation capacity, two (10C and 12D) showed weak biofilm formation, and only one (4A) showed strong biofilm formation capacity at the three tested temperatures. The other six isolates had biofilm-forming capacities that varied with temperature.
Two E. coli isolates with the stx2 gene presented weak biofilm formation capacity at 37 °C (3E and 9A), and two presented moderate biofilm formation at 24 °C (8B and 9A). Two isolates had moderate capacity at 37 °C (8B and 11A) and 24 °C (3E and 11A) each. No isolate with the stx2 gene was non-forming at the three temperatures and strongly forming at temperatures of 37 and 24 °C.
Evaluation of the joint presence of the stx1 and stx2 virulence genes in an isolate and its biofilm-forming capacity revealed that isolate 8A was a weak former and 7A was a strong former at the three temperatures tested. Isolates 5D and 11D were weak formers at 10 °C and isolate 9C at 37 °C. At 24 °C, isolates 9C and 11D showed moderate biofilm-forming capacities. At 37 °C, isolates 5D and 11D showed a moderate capacity for biofilm formation, and 11D also showed it at 24 °C. Isolates 5D and 9C showed a strong ability to form biofilm at 24 and 10 °C, respectively. Table 12 presents the results.
Table 12.
Antimicrobial resistance and biofilm formation capacity of E. coli containing the stx1 and stx2 virulence genes, at different temperatures, from pig carcasses from slaughterhouses A and B.
| Isolates | Virulence Genes | Biofilm Formation Capacity | Antimicrobial Resistance | ||
|---|---|---|---|---|---|
| 37 °C | 24 °C | 10 °C | |||
| 1E | stx1 | NF | NF | NF | AMO-AMP-ERI-EST-SUL |
| 3E | stx2 | WF | MF | WF | AMO-AMP-ERI-EST-SUL-CLO-CIP |
| 4A | stx1 | SF | SF | SF | AMO-AMP-ERI-EST-SUL-CLO-NAL |
| 4D | stx1 | WF | WF | NF | AMO-AMP-ERI-EST-SUL |
| 5D | stx1, stx2 | MF | FFO | WF | AMO-AMP-ERI-EST-GEN-SUL-CLO-NAL-CIP |
| 6B | stx1 | NF | NF | NF | AMO-AMP-ERI-EST-SUL-CLO-NAL |
| 7A | stx1, stx2 | SF | SF | SF | AMO-AMP-ERI-EST-SUL-DOX-TET-CLO |
| 7B | stx1 | MF | SF | SF | AMO-AMP-EST-SUL-DOX-TET-CLO-NAL |
| 7C | stx1 | MF | WF | WF | EST-GEN-SUL-DOX-TET-CLO-NAL-CIP |
| 7D | stx1 | MF | MF | WF | AMO-AMP-EST-SUL-DOX-TET-CLO-NAL |
| 7E | stx1 | WF | NF | NF | AMO-AMP-EST-SUL-DOX-TET-CLO |
| 8A | stx1, stx2 | WF | WF | WF | AMO-AMP-CFZ-CAZ-ERI-EST-DOX-TET-CLO |
| 8B | stx2 | FM | WF | WF | AMO-AMP-ERI-EST-DOX-TET-CLO-NAL |
| 9A | stx2 | WF | WF | WF | AMO-AMP-EST-SUL-DOX-TET-CLO-NAL-CIP |
| 9C | stx1, stx2 | WF | MF | SF | CAZ |
| 10C | stx1 | WF | WF | WF | CFZ-ERI-SUL-DOX-TET-CLO-NAL |
| 11A | stx2 | MF | MF | SF | AMO-AMP-EST-SUL-DOX-TET-CLO-NAL |
| 11D | stx1, stx2 | MF | MF | WF | AMO-AMP-EST-SUL-DOX-TET-CLO-NAL |
| 12A | stx1 | WF | MF | SF | AMO-AMP-ERI |
| 12D | stx1 | WF | WF | WF | AMO-AMP-ERI-SUL |
NF = non-biofilm forming; WF = weakly biofilm forming; MF= moderate biofilm former; SF= strong biofilm former. AMO = amoxicillin, AMP = ampicillin, CFZ = cefazolin, CAZ = ceftazidime, ERI = erythromycin, EST = streptomycin, GEN = gentamicin, SUL = Sulfonamide, DOX = doxycycline, TET = tetracycline, CLO = chloramphenicol, NAL = nalidixic acid, CIP = ciprofloxacin.
In contrast to the results of this study, Milan and Timm [69] detected STEC isolates of bovine origin in Brazil that were incapable of forming biofilms.
Evaluating the isolates with virulence genes according to their biofilm formation capacity and antimicrobial resistance infer that isolates 4A, 7A, 7B, and 11A have disease-causing factors, can form moderate and strong biofilms at different temperatures, and are resistant to multiple antimicrobials. Isolate 5D also has multidrug resistance, with moderate and strong biofilm formation capacity at 37 and 24 °C, respectively. Therefore, these isolates are relevant to public health. Corroborating these results, Sanchez et al. [70] evaluated isolates from human samples and detected multidrug-resistant E. coli isolates capable of forming biofilms.
Therefore, the results presented in this study on STEC virulence genes in swine carcasses in the Federal District are relevant because of the presence of isolates containing the stx1 and stx2 genes as well as antimicrobial multiresistance phenotypes.
This is the first study in Brazil to evaluate the capacity to form biofilms, the presence of stx1, stx2, and eae virulence genes, and antimicrobial resistance in E. coli isolates from different locations on the pig carcass in the process stages of slaughter. The results demonstrate varied biofilm formation capabilities and the presence of STEC isolates at different slaughter stages in different locations on carcasses, thus making them relevant to public health.
4. Conclusions
In this study, the Enterobacteriaceae and E. coli counts in pig carcasses were measured, which were relevant as quality controls between slaughter processing stages as they revealed the stages with the greatest contamination.
Phenotypic characterization using disk diffusion technique demonstrated high antimicrobial multidrug resistance in E. coli isolates. Furthermore, they were highly sensitive to third-generation cephalosporins and ceftazidime, which are considered critically important for treating serious bacterial infections in humans. However, these isolates are already resistant to quinolones and macrolides, such as nalidixic acid and erythromycin, which are also on the World Health Organization (WHO) list; therefore, there is a warning about antimicrobial use in animals.
In E. coli isolates, virulence genes for STEC have been isolated; these are stx1 and stx2 genes, in large numbers, with or without the eae intimin gene, which can enhance the virulence of the strains. Another factor identified, which may enhance virulence, in addition to keeping such genes persistent and disseminating them along with multidrug resistance in the environment, is the ability to form biofilms, which varies at different temperatures, with few non-biofilm formers.
The presence of these pathogens, together with the indicator microorganism counts, reflect the hygienic conditions of the slaughterhouses and technological processes of slaughter in the establishments evaluated; they can contribute to the industry in general as this is the first study on the counts of these microorganisms on the pig carcass surface and other parts during the different stages of slaughter in Brazil.
This is the first microbial characterization study on pig carcasses in the Federal District, both in different parts of the carcass and at different stages of slaughter, demonstrating the presence of stx1, stx2, and eae genes, multidrug resistance, and various biofilm formation in E. coli. Both pathogen and virulence gene identification as well as antimicrobial resistance can represent a risk to public health, given their presence at different slaughter stages.
Author Contributions
Conceptualization: A.P.S.; Data curation: M.M.S.C.; Software: B.S.L.D.; Methodology: M.M.S.C., E.F.A.D. and A.P.S.; Validation: M.M.S.C. and A.P.S.; Formal analysis: A.P.S., R.L.d.S., E.F.A.D., H.M.B.d.C., V.H.d.L.C. and B.S.L.D.; investigation: M.M.S.C. and E.F.A.D.; Project administration: A.P.S.; Writing—original draft preparation: M.M.S.C.; Writing—review and editing: R.L.d.S. and A.P.S.; Resources: A.P.S. and S.P.; Supervision: A.P.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable. This study did not require permission from an ethics committee as no human or animal experimentation was involved.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Universidade de Brasília. Grant number R$ 8.500.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Associação Brasileira de Proteína Animal Relatório Anual. 2021. [(accessed on 3 February 2020)]. Available online: https://abpa-br.org/abpa-relatorio-anual/
- 2.Instituto Brasileiro de Geografia e Estatística Produção da Pecuária Municipal 2020. Rio de Janeiro. [(accessed on 13 June 2020)];2021 Available online: http://www.cidades.ibge.gov.br/brasil/df/brasilia/pesquisa/18/16459.
- 3.Morés N., Amaral A.L., Lima G.J.M.M., Costa O.A.D., Miele M. Produção de Suínos em Família Sem Uso Coletivo de Antimicrobianos, 1st ed.; 2018. [(accessed on 5 July 2021)]. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1093916/producao-de-suinos-em-familia-sem-uso-coletivo-de-antimicrobianos.
- 4.Wilbert C.A., Mores N., Klein C.H., Lima G.J.M.M., Bassi N.S.S. Sistema de Produção de Suínos em Família Sem o Uso Coletivo de Antimicrobianos—Regulamento. [(accessed on 9 February 2023)];Embrapa Suínos Aves. 2019 557:6. Available online: https://www.embrapa.br/busca-de-publicacoes/-/publicacao/1102053/sistema-de-producao-de-suinos-em-familia-sem-o-uso-coletivo-de-antimicrobianos-regulamento. [Google Scholar]
- 5.Lima G.J.M.M. Estratégias no auxílio da redução do uso de antimicrobianos na produção de suínos. Rev. Científica Produção Anim. 2019;20:31–37. [Google Scholar]
- 6.Neitzke D.C., Roza C.R., Weber F.H. Segurança dos alimentos: Contaminação por Salmonella no abate de suínos. Campinas. 2017;20:e2015062. doi: 10.1590/1981-6723.6315. [DOI] [Google Scholar]
- 7.Food and Agriculture Organization/World/World Health Organization (WHO) Guidelines for Risk Analysis of Foodborne Antimicrobial Resistance. 2011. [(accessed on 3 August 2021)]. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXG%2B77-2011%252FCXG_077e.pdf.
- 8.Samulak R.L., Zanetti G.F., Rodrigues S.A., Bittencourt J.V.M. Condição higiênico–sanitária de abatedouro frigorífico e fábrica de embutidos no estado do Paraná. Rev. Bras. Tecnol. Agroind. 2011;5:408–417. doi: 10.3895/S1981-36862011000100004S1. [DOI] [Google Scholar]
- 9.Abebe E., Gugsa G., Ahmed M. Review on major food-borne zoonotic bacterial pathogens. J. Trop Med. 2020;2020:4674235. doi: 10.1155/2020/4674235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Newell D.G., Koopmans M., Verhoef L., Duizer E., Aidara-Kane A., Sprong H., Opsteegh M., Langelaar M., Threfall J., Scheutz F., et al. Foodborne diseases: The challenges of 20 years ago persist, while new ones continue to emerge. Int. J. Food Microbiol. 2012;139:s3–s15. doi: 10.1016/j.ijfoodmicro.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitham H.K., Sundararaman P., Dewey-Matti D., Manikonda K., Marshall K., Griffin P.M., Gleason B.L., Subramhanya S., Crowe S.J. Novel Outbreak-Associated Food Vehicles, United States. Emerg. Infect. Dis. 2021;27:2554–2559. doi: 10.3201/eid2710.204080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brasil Ministério da Agricultura, Pecuária e Abastecimento. Instrução Normativa n° 60, de 20 de Dezembro de 2018. Estabelece o Controle Microbiológico em Carcaça de Suínos e em Carcaça e Carne de Bovinos em Abatedouros Frigoríficos, Registrados No Departamento de Inspeção de Produtos de Origem Animal (DIPOA), Com Objetivo de Avaliar a Higiene do Processo e Reduzir a Prevalência de Agentes Patogênicos. [(accessed on 17 October 2022)];2018 Available online: https://www.gov.br/agricultura/pt-br/acesso-a-informacao/participacao-social/consultas-publicas/documentos/Portaria7518ConsultapblicaINmicrobiologiacarcaas.pdf.
- 13.Brasil Ministério da Saúde. Portaria GM/MS N° 888, de 4 de Maio de 2021. Altera o Anexo XX da Portaria de Consolidação n° 5/GM/MS, de 28 de Setembro de 2017, Para Dispor Sobre os Procedimentos de Controle e de Vigilância da Qualidade da Água Para Consumo Humano e Seu Padrão de Potabilidade. [(accessed on 30 November 2023)];2021 Available online: https://bvsms.saude.gov.br/bvs/saudelegis/gm/2021/prt0888_24_05_2021_rep.html.
- 14.Corbellini L.G., Júnior A.B., Costa E.F., Duarte A.S., Albuquerque E.R., Kich J.D., Cardoso M., Nauta M. Effect of slaughterhouse and day of sampling on the probability of a pig carcass being Salmonella-positive according to the Enterobacteriaceae count in the largest Brazilian pork production region. Int. J. Food Microbiol. 2016;2:58–66. doi: 10.1016/j.ijfoodmicro.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 15.Barco L., Belluco S., Roccato A., Ricci A. Escherichia coli and Enterobacteriaceae counts on pig and ruminant carcasses along the slaughterline, factors influencing the counts and relationship between visual fecal contamination of carcasses and counts: A review. EFSA Support. Publ. 2014;11:634E. doi: 10.2903/sp.efsa.2014.EN-634. [DOI] [Google Scholar]
- 16.World (WHO) E. coli. Fact Sheets. 2018. [(accessed on 3 February 2020)]. Available online: https://www.who.int/news-room/fact-sheets/detail/e-coli.
- 17.Viltrop A., Niine T., Tobias T., Sassu E.L., Bartolo I.D., Pavoni E., Alborali G.L., Burow E., Smith R.P. Slaughter practices and their effectiveness in controlling microbial species Salmonella Contamination of Pig Carcasses. J. Food Prot. 2023;86:100171. doi: 10.1016/j.jfp.2023.100171. [DOI] [PubMed] [Google Scholar]
- 18.Pissetti C., Werlang G.O., Biesus L.L., Kich J.D., Cardoso M.R.d.I. Detecção de Salmonella entericae Listeria monocytogenes em carcaças suínas na etapa de pré-resfriamento. Acta Sci. Vet. 2002;40:1–8. [Google Scholar]
- 19.Zero R.C., Rodrigues J.O. Salmonella: Riscos, transmissão e controle na cadeia de produção suína–revisão da literatura. Nucl. Anim. 2017;9:129–141. doi: 10.3738/21751463.2692. [DOI] [Google Scholar]
- 20.Paim D.S., Pissetti C., Vieira T.R., Werlang G.O., Costa E.F., Kich J.D., Cardoso M. Enumeration, Antimicrobial Resistance and Typing of Salmonella enterica: Profile of Strains Carried in the Intestinal Contents of Pigs at Slaughter in Southern Brazil. Acta Sci. Vet. 2019;47:1636. doi: 10.22456/1679-9216.89668. [DOI] [Google Scholar]
- 21.Viana C., Grossi J.L., Sereno M.J., Yamatogi R.S., Bersot L.S., Call D.R., Nero L.A. Phenotypic and genotypic characterization of non-typhoidal Salmonella isolated from a Brazilian pork production chain. Food Res. Int. 2020;137:109406. doi: 10.1016/j.foodres.2020.109406. [DOI] [PubMed] [Google Scholar]
- 22.Rodrigues G.R., Carvalho I.R., Gandra E.E.A., Carbonera N., Demari G.H., Demari A.C., Lautenchleger F., Moura N.B., Martins T.S., Scheer E.H., et al. Reflexes and microbiological interrelationships during pork processing and bases. Braz. J. Dev. 2021;7:25395–25430. doi: 10.34117/bjdv7n3-308. [DOI] [Google Scholar]
- 23.Rizzotto D., Montes J.H., Kich J.D., Pepipolli V., Bianchi I., Oliveira Júnior J.M., Duval E.H., Schewegler E., Moreira F. Salmonella enterica and enterobacteria in pig carcasses processed on different days after slaughter Pesq. Agropec. Bras. 2022;57:e02813. doi: 10.1590/s1678-3921.pab2022.v57.02813. [DOI] [Google Scholar]
- 24.Pinto A.P.S. Swine carcass microbiological evaluation, hazard analysis, and critical control points (HACCP) in a slaughterhouse in Minas Gerais, Brazil. Rev. Soc. Ven. Microbiol. 2004;24:83–88. [Google Scholar]
- 25.Costa E.F., Cardoso M., Kich J.D., Corbellini L.G. Qualitative risk assessment approach for foodborne microbial hazards. Microb. Risk Anal. 2020;15:1000105. doi: 10.1016/j.mran.2020.100105. [DOI] [Google Scholar]
- 26.Food and Agriculture Organization/World Health Organization (FAO/WHO) Risk Assessment of Microbiological Hazards in Food. 1999. [(accessed on 6 March 2023)]. Available online: https://apps.who.int/iris/handle/10665/65973.
- 27.Davanzo E.F.A., Santos R.L., Castro V.H.L., Palma J.M., Pribul B.R., Dallago B.S.L., Fuga B., Medeiros M., Almeida S.S.T., Costa H.M.B., et al. Molecular characterization of Salmonella spp. and Listeria monocytogenes strains from biofilms in cattle and poultry slaughterhouses located in the federal District and State of Goiás, Brazil. PLoS ONE. 2021;16:e0259687. doi: 10.1371/journal.pone.0259687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsubara E.N. Master’s Thesis. University of São Paulo; São Paulo, Brazil: 2005. [(accessed on 3 February 2023)]. Condição Higiênico-Sanitária de Meias-Carcaças de Suínos Após o Abate e Depois do Resfriamento e Análise da Utilização de Lista de Verificação Para Avaliar Boas Práticas no Abate de Suínos. Available online: https://www.teses.usp.br/teses/disponiveis/10/10134/tde-30102006-113224/publico/EstherNaomiMatsubara.pdf. [Google Scholar]
- 29.Brasil Ministério da Agricultura, Pecuária e Abastecimento. Manual de Coleta de Amostras de Produtos de Origem Animal. [(accessed on 2 October 2022)];2017 Available online: https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-animal/anuario-dos-programas-de-controle-de-alimentos-de-origem-animal-do-dipoa/manual-de-coleta-de-amostras-de-produtos-de-origem-animal.pdf.
- 30.Cê E.R. Master’s Thesis. Universidade Tecnológica do Paraná; Curitiba, Brazil: 2016. [(accessed on 5 December 2023)]. Influências das Etapas do Processo de Abate de Suínos na Prevalência de Patógenos e Níveis de Microrganismos Indicadores de Qualidade e Higiene. Available online: https://repositorio.utfpr.edu.br/jspui/handle/1/1665. [Google Scholar]
- 31.Silva N., Junqueira V.C.A., Silveira N.F.A., Tanowaki M.H., Gomes R.A.R., Okazaki M.M. Manual de Métodos de Análise Microbiológica de Alimentos e Água. 5th ed. Blucher; São Paulo, Brazil: 2017. [Google Scholar]
- 32.Microbiologia da Cadeia Produtiva de Alimentos—Método Horizontal Para Detecção e Enumeração de Enterobacteriaceae Parte 2: Método de Contagem de Colônias. 1st ed. Associação Brasileira de Normas Técnicas (ABNT); Rio de Janeiro, Brazil: 2020. [Google Scholar]
- 33.Performance Standards for Antimicrobial Disc Susceptibility Tests: CLSI Document M02-A11. 11th ed. Clinical and Laboratory Standards Institute (CLSI); Wayne, PA, USA: 2012. [(accessed on 9 February 2023)]. Available online: https://www.researchgate.net/file.PostFileLoader.html?id=58139aa4615e27240754da03&assetKey=AS%3A422233756704774%401477679780485. [Google Scholar]
- 34.Performance Standards for Antimicrobial Susceptibility Testing. 31st ed. CLSI Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2021. [(accessed on 3 August 2023)]. Available online: http://em100.edaptivedocs.net/GetDoc.aspx?doc=CLSI%20M100%20ED32:2022&sbssok=CLSI%20M100%20ED32:2022%20TABLE%202A&format=HTML#CLSI%20M100%20ED32:2022%20TABLE%202A. [Google Scholar]
- 35.China B., Pirson V., Mainil J. Typing of Bovine Attaching and Effacing Escherichia coli by Multiplex In Vitro Amplification of Virulence-Associated Genes. Appl. Environ. Microbiol. 1996;62:3462–3465. doi: 10.1128/aem.62.9.3462-3465.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Djordjevic D., Wiedmann M., McLandsborough L.A. Microtiter plate assay for Assessment of Listeria monocytogenes Biofilm Formation. Appl. Environ. Microbiol. 2002;68:2950–2958. doi: 10.1128/AEM.68.6.2950-2958.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borges K.A., Furian T.Q., Sousa S.S., Menezes R., Tondo E.C., Salle C.T.P., Moraes H.L.S., Nascimento V.P. Biofilm-forming capacity of Salmonella serotypes at different temperatures. Pesq. Vet. Bras. 2018;38:71–76. doi: 10.1590/1678-5150-pvb-4928. [DOI] [Google Scholar]
- 38.Stepanovic S., Vukovic D., Dakic I., Savic B., Svabic-Vlahovic M. A modified microtiter plate test was used to quantify Staphylococcal biofilm formation. J. Microbio. Methods. 2000;40:175–179. doi: 10.1016/S0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 39.Wheatley P., Giotis E.S., McKevitt A.I. Effects of slaughter on carcass contamination at an Irish pork production plant. Ir. Vet. J. 2014;67:1. doi: 10.1186/2046-0481-67-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bolton D.J., Pearce R.A., Sheridan J.J., Blair I.S., McDowell D.A., Harrigton D. Washing and chilling as critical control points in pork slaughter hazard analysis and critical control point (HACCP) systems. J. Appl. Microbiol. 2002;92:893–902. doi: 10.1046/j.1365-2672.2002.01599.x. [DOI] [PubMed] [Google Scholar]
- 41.Brasil Ministério da Agricultura, Pecuária e Abastecimento. Portaria n° 711, de 1° de Novembro de 1995. Aprova as Normas Técnicas de Instalações e Equipamentos Para Abate e Industrialização de Suínos. [(accessed on 5 January 2023)];1995 Available online: https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-animal/empresario/arquivos/Portaria7111995alteradaportarian13042018.pdf/view.
- 42.Montes J.H., Rozzoto D.W., Bianchi I., Oliveira J.M., Peripolli V., Moreira F. Contaminação por Enterobactérias em Carcaças Suínas ao Longo da Linha de Abate. In Mostra Nacional de Iniciação Científica e Tecnologia Interdisciplinar—XII MICTI—IFC Campus Brusque. 2019. [(accessed on 22 March 2022)]. Available online: https://www.google.com/search?client=safari&rls=en&q=.+Contamina%C3%A7%C3%A3o+por+enterobact%C3%A9rias+em+carca%C3%A7as+su%C3%ADnas+ao+longo+da+linha+de+abate.&ie=UTF-8&oe=UTF-8.
- 43.Spescha C., Stephan R., Zweifel C. Microbiological contamination of pig carcasses at different stages of slaughter in two European Union-proven abattoirs. J. Food Prot. 2006;69:2568–2575. doi: 10.4315/0362-028X-69.11.2568. [DOI] [PubMed] [Google Scholar]
- 44.Warriner K., Aldsworth T.G., Kaur S., Dodd C.E. Cross-contamination of carcasses and equipment during pork processing. J. Appl. Microbiol. 2002;93:169–177. doi: 10.1046/j.1365-2672.2002.01678.x. [DOI] [PubMed] [Google Scholar]
- 45.Nastasijevic I., Lakicevic B., Raseta M., Djordjevic V.Z., Jankovic V., Mrdovic B., Brankovic-Lazic I. Evaluation of pig welfare in a lairage and process hygiene in a single abattoir. Sci. J. Meat Technol. Quot. 2018;59:8–22. doi: 10.18485/meattech.2018.59.1.2. [DOI] [Google Scholar]
- 46.Zwirzitz B., Wetzels S.U., Dixon E.D., Stessl B., Zaiser A., Rabanser I., Thalguter S., Pinior B., Roch F.F., Strachan C., et al. Sources and transmission routes of microbial populations throughout meat processing facilities. J. Biofilm Microbiome. 2020;6:26. doi: 10.1038/s41522-020-0136-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buncic S., Sofos J. Interventions to control Salmonella contamination during poultry, cattle, and pig slaughter. Food Res. Int. 2012;45:641–655. doi: 10.1016/j.foodres.2011.10.018. [DOI] [Google Scholar]
- 48.Velebit B., Lakicevic B., Semenova A.A., Revutskaya N.M., Yushina Y.K., Nasonova V.V. Factors Influencing Microbial Transmission in Meat Processing Plants. Theor. Pract. Meat Process. 2021;6:183–190. doi: 10.21323/2414-438X-2021-6-2-183-190. [DOI] [Google Scholar]
- 49.Ghafir Y., China B., Dierick K., Zutter L., Daube G. Hygiene indicator microorganisms for selected pathogens on beef, pork, and poultry meat in Belgium. J. Food Prot. 2008;71:35–45. doi: 10.4315/0362-028X-71.1.35. [DOI] [PubMed] [Google Scholar]
- 50.Machado L.A.P., Lucca F., Alves J., Pozzobon A., Bustamante-filho I.C. Prevalência e genotipagem de Escherichia coli patogênica em carcaças de suínos abatidos em frigoríficos comerciais na Região Sul do Brasil. Rev. Bras. Hig. Sanidade Anim. 2014;8:128. [Google Scholar]
- 51.Mangal P., Rao R., Joshi R. Isolation, identification, and antibiotic sensitivity pattern of Escherichia coli in raw pork: A cross-sectional study. J. Entomol. Zool. Stud. 2018;6:395–398. [Google Scholar]
- 52.Drummond V.O., Perecmanis S. Genes de enterotoxinas e perfil antimicrobiano de Escherichia coli isoladas de suínos hígidos no Distrito Federal. Arq. Bras. Med. Veter-E Zootec. 2013;54:1005–1009. doi: 10.1590/S0102-09352013000400010. [DOI] [Google Scholar]
- 53.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant, and pandrug-resistant bacteria: An international expert proposal for interim standard definitions of acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 54.Pissetti C., Werlang G.O., Kich J.D., Cardoso M. Genotyping and antimicrobial resistance in Escherichia coli from pig carcasses. Pesqui. Veter-Bras. 2017;37:1253–1260. doi: 10.1590/s0100-736x2017001100010. [DOI] [Google Scholar]
- 55.Kim H., Baek H., Lee S., Jang Y., Jung S., Kim A., Choe N.N. Prevalence and antimicrobial resistance of Salmonella spp. and Escherichia coli isolated from pigs at slaughterhouses in Korea. Afr. J. Microbiol. Res. 2011;15:823–830. doi: 10.5897/AJMR10.850. [DOI] [Google Scholar]
- 56.Bischoff K.M., White D.G., Hume M.E., Poole T.L., Nisbet D.J. The chloramphenicol resistance gene cmlA is disseminated on transferable plasmids that confer multiple drug resistance in swine Escherichia coli. FEMS Microbiol. Lett. 2005;243:285–291. doi: 10.1016/j.femsle.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 57.Moennighoff C., Thomas N., Niehaus F., Hartmann M., Menrath A., Merkel J., Detlefsen H., Kreienbrock L., Hennig-Pauka I. Phenotypic antimicrobial resistance in Escherichia coli strains isolated from swine husbandry in Northwestern Germany: Temporal patterns in samples from laboratory practice from 2006 to 2017. BMC Vet. Res. 2020;16:37. doi: 10.1186/s12917-020-2268-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.World Health Organisation (WHO) Antimicrobial Resistance in the Food Chain. Food Safety. 2017. [(accessed on 9 October 2020)]. Available online: https://www.who.int/news-room/questions-and-answers/item/antimicrobial-resistance-in-the-food-chain.
- 59.Bouvet J., Montet M.P., Rossel R., Le Roux A., Bavai C., Ray-Gueniot S., Mazuy C., Atrache V., Vernozy-Rozand C. Effects of slaughter on pig carcass contamination with verotoxin-producing Escherichia coli and E. coli O157:H7. Int. J. Food Microbiol. 2002;77:99–108. doi: 10.1016/S0168-1605(02)00053-3. [DOI] [PubMed] [Google Scholar]
- 60.Paton A.W., Paton J.C. Detection and characterization of Shiga toxigenic Escherichia coli using multiplex PCR assays for stx1, stx2, eaeA, enterohemorrhagic E. coli hlyA, rfbO111, and rfbO157. J. Clin. Microbiol. 1998;36:598–602. doi: 10.1128/JCM.36.2.598-602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brasil Ministério da Agricultura, Pecuária e Abastecimento. Instrução Normativa n° 09, de 27 de Junho de 2003. Proíbe a Fabricação, a Manipulação, o Fracionamento, a Comercialização, a Importação e o Uso Dos Princípios Ativos Cloranfenicol Nitrofuranos e os Produtos Que Contenham Estes Princípios Ativos, Para Uso Veterinário e Suscetível de Emprego Na Alimentação de Todos os Animais e Insetos. [(accessed on 26 February 2023)];2003 Available online: https://www.gov.br/agricultura/pt-br/assuntos/insumos-agropecuarios/insumos-pecuarios/alimentacao-animal/arquivos-alimentacao-animal/legislacao/instrucao-normativa-no-9-de-27-de-junho-de-2003.pdf.
- 62.Tseng M., Fratamico P.M., Manning S.D. Shiga toxin-producing Escherichia coli in swine: A public health perspective. Anim. Health Res. Rev. 2014;15:63–75. doi: 10.1017/S1466252313000170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chassagne L., Pradel N., Robin F., Livrelli V., Bonnet R., Delmas J. Detection of stx1, stx2, and eae genes of enterohemorrhagic Escherichia coli using SYBR Green in a real-time polymerase chain reaction. Diag. Microb. Infec. Dis. 2009;64:98–101. doi: 10.1016/j.diagmicrobio.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 64.Zhang P., Essendoubi S., Keenliside J., Reuter T., Stanford K., King R., Lu P., Yang X. Genomic analysis of Shiga toxin-producing Escherichia coli O157:H7 from cattle and pork production-related environments. NPJ Sci. Food. 2021;5:15. doi: 10.1038/s41538-021-00097-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rosengren L.B., Waldner C.L., Reid-Smith R.J. Associations between antimicrobial resistance phenotypes, antimicrobial resistance genes, and virulence genes in fecal Escherichia coli isolates from healthy grow-finishing pigs. Appl. Environ. Microbiol. 2009;75:1373–1380. doi: 10.1128/AEM.01253-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jiang H.X., Lu D.H., Chen Z.L., Wang X.M., Chen J.R., Liu Y.H., Liao X.P., Liu J.H., Zeng Z.L. High prevalence and widespread distribution of multi-resistant Escherichia coli isolates in pigs and poultry in China. Vet. J. 2011;187:99–103. doi: 10.1016/j.tvjl.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 67.Gemeda B.A., Assefa A., Jaleta M.B., Amenu K., Wieland B. Antimicrobial resistance in Ethiopia: A systematic review and meta-analysis of prevalence in foods, food handlers, animals, and the environment. One Health. 2021;13:100286. doi: 10.1016/j.onehlt.2021.100286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tadesse D.A., Zhao S., Tong E., Ayers S., Singh A., Bartholomew M.J., McDermott P.F. Antimicrobial Drug Resistance in Escherichia coli from Humans and Food Animals, United States, 1950–2002. Emerg. Inf. Dis. 2012;18:741–749. doi: 10.3201/eid1805.111153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Milan C., Timm C.D. Avaliação da capacidade de formar biofilme de Escherichia coli produtoras de shiga toxina. Arq. Ciências Veterinárias Zool. UNIPAR. 2013;16:31–33. [Google Scholar]
- 70.Sanchez C.J., Mende K., Beckius K.M., Akers K.S., Romano D.R., Wenke J.C., Murray C.K. Biofilm formation by clinical isolates and the implications in chronic infections. BMC Infec. Dis. 2013;3:47. doi: 10.1186/1471-2334-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.