Abstract
Aim: To explore the predictive value of miR-486-5p in early cervical cancer and the associations of miR-486-5p with different clinical symptoms.
Materials & methods: A total of 185 women were recruited. The relative expression levels of serum miR-486-5p were analyzed by real-time polymerase chain reaction. The receiver operator characteristic curves were utilized to reflect the predictive performance of miR-486-5p and squamous cell carcinoma antigen for early cervical cancer. Univariate logistic regression and ranked logistic regression were used to explore the associations of miR-486-5p with different clinical symptoms of early cervical cancer, with odd ratios (ORs) and 95% confidence intervals.
Results: Eighty-one women (44.26%) had early cervical cancer. The relative expression of serum miR-486-5p was 1.99-fold higher in early cervical cancer patients than that in controls (p < 0.0001). The predictive performance of miR-486-5p for early cervical cancer was significantly superior to that of squamous cell carcinoma antigen, with an area under the curve of 0.865 (p < 0.001), sensitivity of 1.000 and specificity of 0.804. In addition, overexpressed miR-486-5p was associated with high odds of maximum tumor diameter increase (OR = 1.30, 95% CI: [1.01–1.66]).
Conclusion: MiR-486-5p may be a potential biomarker for the early cervical cancer diagnosis, and was linked to the risk of maximum tumor diameter.
Keywords: : cervical cancer, early prediction, maximum tumor diameter, miR-486-5p, real-time polymerase chain reaction
Plain language summary
Article highlights.
Background
Early-stage cervical cancers are asymptomatic and nonspecific, early detection is extremely hard.
The potential target for miR-486-5p are linked to cervical cancer differentiation.
Materials & methods
This study contains real cases from a medical center in China.
Relative expression of miR-486-5p between control and patients and the predictive performance of miR-486-5p on early-stage cervical cancer are assessed.
Results
Relative expression of serum miR-486-5p was significantly higher in early cervical cancer patients.
The predictive value of miR-486-5p in early cervical cancer was superior to that of SCC-Ag.
Overexpressed miR-486-5p was related to an increased risk of maximum tumor diameter increase.
Discussion
Serum miR-486-5p play an important role in early-stage cervical cancer and could be involved in multiple mechanisms.
MiR-486-5p had the potential to be a competitive biomarker for early-stage cervical cancer due to it is a convenient, efficient and nonintrusive method.
The link between miR-486-5p and maximum tumor diameter may help improve cervical cancer prognosis.
Conclusion
MiR-486-5p could be a potential biomarker for early-stage cervical cancer.
MiR-486-5p should be included in the strategies for screening and prognosis improvement in cervical cancer.
1. Introduction
Cervical cancer has been the fourth cancer-killer and represents a substantial portion of global cancer burden in women [1]. The 3-year disease-free survival rate of cervical cancer patients in stages I A1 to II A1 was 87% [2], and the total survival rate fell to 15% in patients with stage IV A [3]. Due to early-stage cervical cancers are asymptomatic and nonspecific, early detection is extremely hard, resulting in the high mortality rate [4]. Hence, early diagnosis plays a crucial role in improving cervical cancer prognosis and reducing the disease burdens.
At present, thinprep cytologic test (TCT) combined with human papillomavirus (HPV)-DNA detection is one of the most common cervical cancer screening methods [5]. This combined detection can detect cervical cells and conduct cytological classification diagnosis and has a higher detection rate, with pooled sensitivity of 0.86 and combined specificity of 0.79 [6]. However, the application for TCT is limited because diagnosis for high-risk cervical cancer patients usually based on pathological examination, and TCT is often a better choice only for individuals with higher economic ability [6]. Cervical biopsy is still the gold standard for cervical cancer diagnosis, but they are invasive and time-consuming, potentially delaying the treatment and incurring additional costs and risks [7]. Additionally, several serum tumor biomarkers have also been used for the diagnosis and monitoring of cervical cancer [8,9]. It is valuably to search for a noninvasive, economic and convenient serum biomarker with high sensitivity and specificity for early cervical cancer diagnosis.
The deregulation of short, non-coding single strands of RNAs, namely microRNAs (miRNAs), has been widely observed to play a critical role in cervical cancer and many other tumors, such as cellular proliferation, invasion, apoptosis and metastasis [10,11], and thus, miRNAs have high potential to be used as serum biomarkers [12]. A number of miRNAs, including miR-20a, miR-205, miR-218, miR-29a, miR-200a, miR-486-5p, have been studied and considered as potential ideal biomarkers for noninvasive cervical cancer detection [13,14]. Among them, miR-486-5p has high sensitivity and specificity [15]. Human olfactomedin 4 (OLFM4) [16] and hypoxia-induced transcription factor 1α (HIF1α) [17] are linked to cervical intraepithelial neoplasia progression and cervical cancer differentiation, and are potential targets for miR-486-5p. A series of in vitro tests also showed that miR-486-5p can regulate the tumor suppressor gene phosphatase and tensin homolog (PTEN) and induce PI3K/AKT tumor signaling pathway, which can promote cervical cancer cells’ proliferation, invasion and migration, and tumor tissue growth in nude mice [18,19]. Nevertheless, association between miR-486-5p and cervical cancer have not been fully elucidated, and limited study has explored the diagnosis value of miR-486-5p for early cervical cancer diagnosis.
Herein, this study aims to assess the diagnosis value of miR-486-5p in early cervical cancer, and explore the associations of miR-486-5p with different clinical symptoms of cervical cancer, in order to provide some reference for looking for reliable biomarkers of early screening and diagnosis of cervical cancer and reduce the mortality risk and disease burdens.
2. Materials & methods
2.1. Study samples & characteristics
A total of 83 cervical cancer patients, together with 102 controls (including healthy individuals and patients with cervical lesions and regular gynecopathy) were recruited from 1 November 2021 to 31 December 2022 in the First Affiliated Hospital of Bengbu Medical College. Patients with cervical cancer diagnosed via pathology, not receive surgery, chemotherapy or radiotherapy, and without history of cervical surgery, hysterectomy or cervical epithelial disease were included. Those who were pregnant or during puerperal period, or diagnosed with other malignancies were excluded. All methods were carried out in accordance with relevant guidelines and regulations (Declaration of Helsinki). The current study has obtained the ethical approval from the Institutional Review Board of the First Affiliated Hospital of Bengbu Medical College (approval no. 2022127). Written informed consent was obtained from the participants.
2.2. MiRNA extraction, cDNA synthesis & quantification
Tubes without anticoagulant were used for peripheral blood samples collection, and then leave the samples for half an hour for separating sera, and storage at -20°C for further analytics. After excluding haemolytic blood samples, the miRNA was extracted using TRIzon Reagent (CW0580S, Cowin Biotech Co., Ltd, China) according to manufacturer’s instructions. Triploid TRIzon solution was added to the blood and blended to lyse the cells. Trichloromethane solution (0.2 ml for per 1 ml TRIzon) was added after the mixed solution quiesced at room temperature for 5 min. An aliquot of 600 μl of supernatant was transferred into a fresh RNase-Free tube after centrifugation at 12,000 rpm for 15 min at 4°C. Equal volume isopropyl alcohol solution was added into the supernatant and quiesced at room temperature for 10 min after upside down mixing, and then centrifuged at 12,000 rpm for 10 min at 4°C. Sediment obtained from separation was washed by 75% ethanol solution (1:1 TRIzon) and centrifuged at 12,000 rpm for 3 min at 4°C to obtain the miRNA (i.e., sediment). After drying the miRNA, 30–100 μl RNase-Free water was added to dissolve and for subsequent analysis.
Then, miRNA was reverse-transcribed into cDNA using the HiFiScript cDNA Synthesis Kit (CW2569M, Cowin Biotech Co., Ltd, China) with the 20 μl reaction system according to the manufacturer's instructions. SYBR Green-based quantitative polymerase chain reaction was utilized to quantify MiR-486-5p using the FastStart Universal SYBR Green Master mix (Roche Diagnostics Ltd, Switzerland). The primer of miR-486-5p and U6 small nuclear RNA (U6 snRNA) were synthesized by Sangon Biotech Corp (Shanghai, China). Polymerase chain reaction was performed in 10 μl reaction volume on the Applied Biosystems QuantStudioTM 3 (Thermo Fisher Scientific, MA, US) according to the following cycle parameters: 95°C for 600 s, followed by 40 repeats of 95°C for 15 s and 60°C for 30 s. In addition, the relative expression levels were calculated though the 2-ΔΔCt method and normalized to levels of U6 snRNA.
2.3. Definition of early cervical cancer
The International Federation of Gynecology and Obstetrics (FIGO) staging system was used for the staging of cervical cancer [20]. In this study, FIGO I stage and FIGO II stage were classified into early cervical cancer. Individuals in control group were considered as non-cervical cancer persons.
2.4. Data collection
We also collected information on other characteristics of participants, including age, body mass index, weight, height, marital status (unmarried/married), menopause (yes/no), gravidity, parity, diabetes mellitus (DM) (yes/no), hypertension (yes/no), irregular vaginal bleeding (yes/no), abnormal vaginal discharge (yes/no), cervical erosion (yes/no), HPV infection (yes/no), medical history (yes/no), other complications (yes/no), the status of CA 125, CA 199, CEA, and squamous cell carcinoma antigen (SCC-Ag), maximum tumor diameter (<2, 2–4 and >4 cm), parametrial invasiveness (yes/no), invasive range (no/cervix/extrauterine and in a third of the vagina or pelvic wall), parametrial infiltration (yes/no), lymphatic metastasis (yes/no), lymph-vascular space infiltration (yes/no), tumor differentiation stage (low, medium and high) and histological type [nonkeratinizing squamous cell carcinoma, keratosis squamous cell carcinoma, cervical duct adenocarcinoma, endometrioid adenocarcinoma, cervical intraepithelial neoplasia (CIN) II and CIN III].
2.5. Statistical analyses
Normally distributed data were expressed by mean ± standard deviation (Mean ± SD). T-test was used to compare the differences between cervical cancer group and control group. Non-normally distributed data were expressed using median and quartiles [M (Q1, Q3)], and Mann–Whitney U test was used for comparison. Enumeration data were expressed as frequency and constituent ratio [N (%)], and Chi-square test (χ2) or Fisher’s exact test was used for comparison. Predictive performance of miR-486-5p and SCC-Ag for early cervical cancer were reflected by the receiver operator characteristic (ROC) curves (excluding 21 patients with advanced cervical cancer). The Delong test was used for the comparation of the area under the curves (AUCs) of ROC, sensitivity and specificity between miR-486-5p and SCC-Ag. Univariate logistic regression and ranked logistic regression were utilized to explore the associations of miR-486-5p and different clinical symptoms of cervical cancer, with odds ratios (ORs) and 95% confidence intervals (CIs). Two-sided p < 0.05 was considered as statistically significant. Statistical analyses were performed using GraphPad Prism 8.0.1 (GraphPad Software, La Jolla, CA, USA), SAS 9.4 (SAS Institute, Cary, NC, USA) and R v. 4.0.3 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Relative expression of serum miR-486-5p
The relative expression levels of miR-486-5p in controls and early cervical cancer patients were shown in the Figure 1. It was clearly that the relative expression level of serum miR-486-5p in early cervical cancer patients was 1.99-fold higher than that in noncervical cancer individuals (p < 0.0001), indicating that the overexpressed miR-486-5p may be involved in the occurrence and progression of cervical cancer [21].
Figure 1.
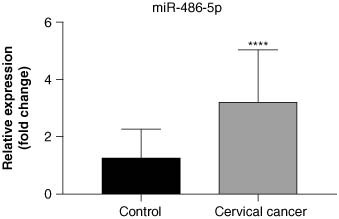
The relative expression of miR-486-5p between control group and cervical cancer group. Data represent the mean ± SD. ****p < 0.0001.
SD: Standard deviation.
3.2. Characteristics of participants
Initially, 83 cervical cancer patients, together with 102 controls were recruited. After excluding two cervical cancer patients with other malignancies, 183 women were finally eligible. The characteristics of women in early cervical cancer group and control group were shown in the Supplementary Table S1. Among eligible participants, 81 (44.26%) had early cervical cancer. The average age of patients was 55.07 years old and that of controls were 44.75 years old. More than half of early cervical cancer patients were menopause (48 [59.26%]), while the majority of controls were not menopause (76 [74.51%]). The median concentrations of serum CEA (2.29 vs 1.70) μg/l and SCC-Ag (2.95 vs 1.60) μg/l in early cervical cancer group were both significantly higher than that in control group. In most early cervical cancer patients, the maximum tumor diameter was 2–4 cm, without parametrial invasiveness, parametrial infiltration, lymphatic metastasis or lymph-vascular space infiltration, the tumor differentiation stage was medium and histological type was nonkeratosis squamous cell carcinoma.
3.3. The predictive performance of miR-486-5p & SCC-Ag for early cervical cancer
Figure 2 is the ROC curves reflecting the predictive performance of miR-486-5p and SCC-Ag for the prevalence of early cervical cancer. Only patients with early cervical cancer (i.e., FIGO stage I and II) were included in this exploration (n = 162). We observed that the predictive performance of miR-486-5p on early cervical cancer diagnosis was relatively superior to that of SCC-Ag, with an AUC of 0.865 (p < 0.001), sensitivity of 1.000 and specificity of 0.804. More details on comparation of predictive performance on early cervical cancer diagnosis between miR-486-5p and SCC-Ag were shown in the Table 1.
Figure 2.
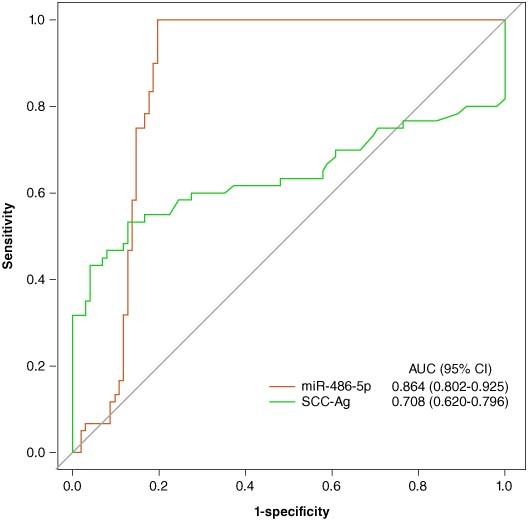
The receiver operator characteristic curves of the predictive performance of miR-486-5p and squamous cell carcinoma antigen for early cervical cancer.
Table 1.
Predictive performance of miRNA-486-5p on diagnosis for early cervical cancer.
| Variable | AUC (95% CI) | Sensitivity (95% CI) | Specificity (95% CI) | PPV (95% CI) | NPV (95% CI) | Accuracy (95% CI) |
|---|---|---|---|---|---|---|
| miRNA | 0.864 (0.802–0.925) | 1.000 (0.955–1.000) | 0.804 (0.714–0.876) | 1.000 (0.956–1.000) | 0.802 (0.711–0.875) | 0.891 (0.836–0.932) |
| SCC-Ag | 0.708 (0.620–0.796) | 0.543 (0.435–0.652) | 0.961 (0.923–0.998) | 0.726 (0.651–0.801) | 0.917 (0.838–0.995) | 0.776 (0.716–0.836) |
AUC: Area under the curve; CC: Cervical cancer; CI: Confidence interval; NPV: Negative predictive value; PPV: Positive predictive value; SCC-Ag: Squamous cell carcinoma antigen.
3.4. Associations of miR-486-5p with different clinical symptoms of cervical cancer
Table 2 shows the associations between relative expression of serum miR-486-5p and different clinical symptoms of cervical cancer, including maximum tumor diameter, parametrial invasiveness, lymphatic metastasis, lymph-vascular space infiltration and tumor differentiation stage of cervical cancer. The results showed that overexpressed miR-486-5p was associated with an increased risk of maximum tumor diameter increase (OR = 1.30, 95% CI: [1.01–1.66]).
Table 2.
Association between miRNA-486-5p and different clinic characteristics of early cervical cancer.
| Variables | OR (95% CI) | p-value |
|---|---|---|
| maximum tumor diameter | 1.30 (1.01–1.66) | 0.039 |
| Parametrial invasiveness | 0.98 (0.76–1.26) | 0.875 |
| Lymphatic metastasis | 0.76 (0.50–1.13) | 0.175 |
| Lymph-vascular space infiltration | 0.91 (0.67–1.24) | 0.542 |
| Tumor differentiation stage | 1.09 (0.85–1.40) | 0.475 |
CC: Cervical cancer; CI: Confidence interval; OR: Odd ratio.
4. Discussion
The current study analyzed the relative expression levels of serum miR-486-5p in early cervical cancer patients, assessed the predictive value of miR-486-5p for early cervical cancer diagnosis, and explored the associations of miR-486-5p with different clinical symptoms of cervical cancer. We found that relative expression of serum miR-486-5p in early cervical cancer patients is 1.99-fold higher than that in controls. The predictive performance of miR-486-5p on early cervical cancer diagnosis was relatively superior to that of SCC-Ag. Also, overexpressed miR-486-5p was linked to a high risk of maximum tumor diameter increase.
The roles of miRNAs as potential biomarkers for cervical cancer diagnosis have been discussed [22,23]. Li et al. [21] suggested that miR-486-5p can stimulate cell proliferation, migration and invasion via inhibition of PTEN expression and activation of oncogenic PI3K/Akt pathway in cervical cancer. Du et al. [24] established a unique biomarker panel for detection of early-stage cervical cancer including SCC-Ag degree and serum miR-29a, miR-25, miR-486-5p levels, and found that the combining analyses of multiple serum biomarkers achieved both high sensitivity (80.0%) and high specificity (96.7%). Similarly, our study found the relative expression of serum miR-486-5p was significantly higher in early cervical cancer patients than that in controls. Also, comparing to SCC-Ag, miR-486-5p had a superior predictive performance on early cervical cancer diagnosis (the AUC of ROC was 0.865). Our findings supplemented that single miR-486-5p index have the potential to serve as a biomarker for early cervical cancer diagnosis, which may be simpler and less costly. Nevertheless, the causal association between miR-486-5p and cervical cancer occurrence need further clarification.
Mechanisms that miR-486-5p taking part in the occurrence and progression of cervical cancer have not been revealed up to now. MiR-486-5p functions as an oncogenic miRNA in cervical cancer, and its biological functions are mediated through its overexpression to block the directly targeted PTEN and protein expression, thus leading to Akt phosphorylation, and resulting in tumor growth in vivo [25,26]. In addition to the PI3K/Akt pathway, impairment of estrogen receptor alpha-mediated OLFM4 expression has been associated with progression of CIN and differentiation of cervical cancer [27,28]. MiR-486-5p has been recognized as a potential regulator in upstream of OLFM4 [29]. Although previous conclusions provide possible speculations, the specific mechanisms that miR-486-5p influencing occurrence and development of cervical cancer need further discussion.
SCC-Ag is a common biomarker for cervical cancer, but it has unsatisfactory performance for early-stages diagnosis [30]. In study conducted by Zhang et al. [31], SCC-Ag showed moderate diagnostic performances for cervical squamous cell carcinoma, and as many as 63 (57.3%) early-stage patients would be falsely considered as healthy. Zhu et al. [32] measured α-Actinin 4 and SCC-Ag levels in 211 patients from negative for intra epithelial lesion to cervical cancer, to analyze single or combined effect on the early cervical lesion diagnosis through a CIN III cut-off, and found that AUC of combination was superior to AUC of SCC-Ag (81.4 and 75.4, respectively). In the current study, we found a superior diagnostic value of miR-486-5p for early cervical cancer to SCC-Ag, indicating that miR-486-5p may be an idea serum biomarker for early cervical cancer diagnosis. Studies have suggested that aberrant expression of miRNAs may be used as a potential in the prediction of prognosis and diagnosis of cancer patients [33,34]. Similarly, we hold opinion that miR-486-5p detection could be a convenient and efficient approach for early cervical cancer diagnosis due to the cancer biomarker’s identification by noninvasive methods is very useful for early diagnosis, treatment response monitoring, risk assessment and cancer control [35]. Besides, as mentioned before, comparing to the cervical biopsy, which is a gold standard for cervical cancer diagnosis, miR-486-5p may overcome cervical biopsy’s shortcoming that invasive, time-consuming, high-cost [6,7]. Herein, since early cervical cancer patients often have no significant clinical features before diagnosis, nonintrusive miR-486-5p detection may be more recommended.
We also found a positive association between miR-486-5p and maximum tumor diameter increase. In fact, the maximum tumor diameter is one of the primary descriptive prognostic factors that can indicate the tumor progression and lymph node metastasis in cancer patients [36,37]. Li et al. [38] showed that tumor diameter larger than 5 cm is closely related to the prognosis after surgery in adult patients with low-grade glioma. Also, Nakamura et al. [39] suggested that increased tumor anteroposterior diameter is an independent negative prognostic factor for overall survival in patients with endometrial carcinoma. Among our study population, 54.32% patients had a maximum tumor diameter of 2–4 cm, 34.57% had that of >4 cm, and 11.11% had that of <2 cm. Herein, since miR-486-5p may play an important role in the progression of cervical cancer, regular monitoring should be taken into consideration in the strategies for improving cervical cancer prognosis, in order to convenient and timely modifying the treatments.
This study based on true cases from a medical center in China to assess the predictive value of miR-486-5p in early cervical cancer diagnosis, which provided some theoretical basis for further exploration of early biomarkers with high sensitivity and specificity for cervical cancer diagnosis. However, there are still some limitations. The study subjects were all Chinese that limited the extrapolation of the findings. As a cross-sectional study, we could not conclude the causal association of miR-486-5p and early cervical cancer occurrence due to the absence of follow-up information. Additionally, we could not investigate the specific mechanism that miR-486-5p taking part in the development of cervical cancer.
5. Conclusion
In this cross-sectional study, true cervical cancer patients and controls were recruited from our hospital to assess the diagnosis value of miR-486-5p in early cervical cancer. We observed that the relative expression level of serum miR-486-5p in early cervical cancer patients is 1.99-fold higher than that in controls, and it has a relatively superior predictive performance in early cervical cancer to that of SCC-Ag, with high AUC (0.865), sensitivity (1.000) and specificity (0.804). These findings suggested that comparing to previous common biomarker for cervical cancer diagnosis, such as SCC-Ag, TCT and cervical biopsy, miR-486-5p had the potential to be a noninvasive and more convenient and cheaper tool for early cervical cancer diagnosis. Also, miR-486-5p expression was positively linked to the risk of maximum tumor diameter increase among patients with cervical cancer. Since the maximum tumor diameter is a prognostic factor indicating the tumor progression and lymph node metastasis in cancer patients, our results indicated that miR-486-5p expression may be also applicated in dynamically monitoring progress of cervical cancer. Overall, this study provided some reference for looking for reliable biomarkers of early screening and diagnosis of cervical cancer, which may further reduce the mortality risk and disease burdens. However, the causal association of miR-486-5p with occurrence of cervical cancer could not be concluded, and the specific mechanism that relative expression of miR-486-5p involving in cervical cancer was still unclear. More prospective studies including large multicentric samples should be conducted to verify the predictive value of miR-486-5p in cervical cancer, and extend its application possibilities to people of different races. The focus of future research is still to find non-invasive biomarkers that can indicate the diagnosis of asymptomatic early cervical cancer, reduce the side effects of screening and diagnosis, improve the detection rate, save medical costs and reduce the risk of death.
Supplementary Material
Funding Statement
This study was supported by the Natural Science Research Project of Anhui Educational Committee (Project No. KJ2021A0754); Bengbu Science and Technology Innovation Guidance Project in 2021 (Project No. 20210337); the Natural Science Research Project of Anhui Educational Committee (Project 2022AH051428).
Supplemental material
Supplemental data for this article can be accessed at https://doi.org/10.1080/17520363.2024.2400963
Author contributions
L Wang, G Ye, conceiving and designing the study; L Wang, Y Wang, K Yang, X Hu, collecting the data; L Wang, Y Wang, K Yang, X Hu, analyzing and interpreting the data; L Wang, writing the manuscript; G Ye, providing critical revisions that are important for the intellectual content; L Wang, Y Wang, K Yang, X Hu, G Ye, approving the final version of the manuscript.
Financial disclosure
This study was supported by the Natural Science Research Project of Anhui Educational Committee (Project No. KJ2021A0754); Bengbu Science and Technology Innovation Guidance Project in 2021 (Project No. 20210337); the Natural Science Research Project of Anhui Educational Committee (Project 2022AH051428). The authors have no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Competing interests disclosure
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, stock ownership or options and expert testimony.
Writing disclosure
No writing assistance was utilized in the production of this manuscript.
Ethical conduct of research
The authors state that they have obtained appropriate Institutional Review Board approval (the First Affiliated Hospital of Bengbu Medical College (approval no. 2022127)) and/or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.Tabatabaei FS, Saeedian A, Azimi A, et al. Evaluation of survival rate and associated factors in patients with cervical cancer: a retrospective cohort study. J Res Health Sci. 2022;22(2):e00552. doi: 10.34172/jrhs.2022.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pimple SA, Mishra GA. Global strategies for cervical cancer prevention and screening. Minerva Ginecol. 2019;71(4):313–320. doi: 10.23736/S0026-4784.19.04397-1 [DOI] [PubMed] [Google Scholar]
- 4.Du S, Zhao Y, Lv C, et al. Applying serum proteins and microRNA as novel biomarkers for early-stage cervical cancer detection. Sci Rep. 2020;10(1):10. doi: 10.1038/s41598-020-65850-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bedell SL, Goldstein LS, Goldstein AR, et al. Cervical cancer screening: past, present, and future. Sex Med Rev. 2020;8(1):28–37. doi: 10.1016/j.sxmr.2019.09.005 [DOI] [PubMed] [Google Scholar]
- 6.Li T, Lai Y, Yuan J. The diagnostic accuracy of TCT + HPV-DNA for cervical cancer: systematic review and meta-analysis. Ann Transl Med. 2022;10(14):761. doi: 10.21037/atm-22-1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pretorius RG, Zhang WH, Belinson JL, et al. Colposcopically directed biopsy, random cervical biopsy, and endocervical curettage in the diagnosis of cervical intraepithelial neoplasia II or worse. Am J Obstet Gynecol. 2004;191(2):430–434. doi: 10.1016/j.ajog.2004.02.065 [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Zhang H, Huang Y, et al. Expression of immune cell markers and tumor markers in patients with cervical cancer. Int J Gynecol Cancer. 2020;30(7):969–974. doi: 10.1136/ijgc-2020-001254 [DOI] [PubMed] [Google Scholar]
- 9.Meng H, Zhang Y, Chen Y. Diagnosis value of colposcope combined with serum squamous cell carcinoma antigen, carbohydrate antigen 125, and carcinoembryonic antigen for moderate to advanced cervical cancer patients treated with modified Fuzheng Peiyuan Decoction. Evid Based Complement Alternat Med. 2021;2021:4355805. doi: 10.1155/2021/4355805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sammarco ML, Tamburro M, Pulliero A, et al. Human papillomavirus infections, cervical cancer and microRNAs: an overview and implications for public health. MicroRNA. 2020;9(3):174–186. doi: 10.2174/2211536608666191026115045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fattahi M, Shahrabi S, Saadatpour F, et al. microRNA-382 as a tumor suppressor? Roles in tumorigenesis and clinical significance. Int J Biol Macromol. 2023;250:125863. doi: 10.1016/j.ijbiomac.2023.125863 [DOI] [PubMed] [Google Scholar]
- 12.Rezaee D, Saadatpour F, Akbari N, et al. The role of microRNAs in the pathophysiology of human central nervous system: a focus on neurodegenerative diseases. Ageing Res Rev. 2023;92:102090. doi: 10.1016/j.arr.2023.102090 [DOI] [PubMed] [Google Scholar]
- 13.Nahand JS, Vandchali NR, Darabi H, et al. Exosomal microRNAs: novel players in cervical cancer. Epigenomics. 2020;12(18):1651–1660. doi: 10.2217/epi-2020-0026 [DOI] [PubMed] [Google Scholar]
- 14.Lv A, Tu Z, Huang Y, et al. Circulating exosomal miR-125a-5p as a novel biomarker for cervical cancer. Oncol Lett. 2021;21(1):54. doi: 10.3892/ol.2020.12316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li G, Hu X, Wang G, et al. Methylation detection of circulating tumor cell miR-486-5p/miR-34c-5p in the progression of colorectal cancer. Clin Transl Oncol. 2023;25(3):673–684. doi: 10.1007/s12094-022-02973-x [DOI] [PubMed] [Google Scholar]
- 16.Li J, Liu C, Li D, et al. OLFM4 inhibits epithelial-mesenchymal transition and metastatic potential of cervical cancer cells. Oncol Res. 2019;27(7):763–771. doi: 10.3727/096504018X15399955297355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tian T, Dong Y, Zhu Y, et al. Hypoxia-induced CNPY2 upregulation promotes glycolysis in cervical cancer through activation of AKT pathway. Biochem Biophys Res Commun. 2021;551:63–70. doi: 10.1016/j.bbrc.2021.02.116 [DOI] [PubMed] [Google Scholar]; •• References associated with mechanisms that miR-486-5p plays a role in cervical cancer.
- 18.Douvris A, Vinas J, Burns KD. miRNA-486-5p: signaling targets and role in non-malignant disease. Cell Mol Life Sci. 2022;79(7):376. doi: 10.1007/s00018-022-04406-y [DOI] [PMC free article] [PubMed] [Google Scholar]; •• References associated with mechanisms that miR-486-5p plays a role in cervical cancer.
- 19.Gao ZJ, Yuan WD, Yuan JQ, et al. miR-486-5p functions as an oncogene by targeting PTEN in non-small cell lung cancer. Pathol Res Pract. 2018;214(5):700–705. doi: 10.1016/j.prp.2018.03.013 [DOI] [PubMed] [Google Scholar]; •• References associated with mechanisms that miR-486-5p plays a role in cervical cancer.
- 20.Bhatla N, Singhal S, Dhamija E, et al. Implications of the revised cervical cancer FIGO staging system. Indian J Med Res. 2021;154(2):273–283. doi: 10.4103/ijmr.IJMR_4225_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li C, Zheng X, Li W, et al. Serum miR-486-5p as a diagnostic marker in cervical cancer: with investigation of potential mechanisms. BMC Cancer. 2018;18(1):61. doi: 10.1186/s12885-017-3753-z [DOI] [PMC free article] [PubMed] [Google Scholar]; •• References associated with mechanisms that miR-486-5p plays a role in cervical cancer.
- 22.Shen S, Zhang S, Liu P, et al. Potential role of microRNAs in the treatment and diagnosis of cervical cancer. Cancer Genet. 2020;248-249:25–30. doi: 10.1016/j.cancergen.2020.09.003 [DOI] [PubMed] [Google Scholar]
- 23.Miao J, Regenstein JM, Xu D, et al. The roles of microRNA in human cervical cancer. Arch Biochem Biophys. 2020;690:108480. doi: 10.1016/j.abb.2020.108480 [DOI] [PubMed] [Google Scholar]
- 24.Du S, Zhao Y, Lv C, et al. Applying serum proteins and microRNA as novel biomarkers for early-stage cervical cancer detection. Sci Rep. 2020;10(1):9033. doi: 10.1038/s41598-020-65850-z [DOI] [PMC free article] [PubMed] [Google Scholar]; • References associated with potential predictive value of miR-486-5p in cervical cancer.
- 25.Alexander MS, Casar JC, Motohashi N, et al. MicroRNA-486-dependent modulation of DOCK3/PTEN/AKT signaling pathways improves muscular dystrophy-associated symptoms. J Clin Invest. 2014;124(6):2651–2667. doi: 10.1172/JCI73579 [DOI] [PMC free article] [PubMed] [Google Scholar]; •• References associated with mechanisms that miR-486-5p plays a role in cervical cancer.
- 26.Xu J, Li R, Workeneh B, et al. Transcription factor FoxO1, the dominant mediator of muscle wasting in chronic kidney disease, is inhibited by microRNA-486. Kidney Int. 2012;82(4):401–411. doi: 10.1038/ki.2012.84 [DOI] [PMC free article] [PubMed] [Google Scholar]; •• References associated with mechanisms that miR-486-5p plays a role in cervical cancer.
- 27.Yu L, He M, Yang Z, et al. Olfactomedin 4 is a marker for progression of cervical neoplasia. Int J Gynecol Cancer. 2011;21(2):367–372. doi: 10.1097/IGC.0b013e31820866fe [DOI] [PubMed] [Google Scholar]; •• References associated with mechanisms that miR-486-5p plays a role in cervical cancer.
- 28.Duan C, Liu X, Liang S, et al. Oestrogen receptor-mediated expression of olfactomedin 4 regulates the progression of endometrial adenocarcinoma. J Cell Mol Med. 2014;18(5):863–874. doi: 10.1111/jcmm.12232 [DOI] [PMC free article] [PubMed] [Google Scholar]; •• References associated with mechanisms that miR-486-5p plays a role in cervical cancer.
- 29.Ma H, Tian T, Liang S, et al. Estrogen receptor-mediated miR-486-5p regulation of OLFM4 expression in ovarian cancer. Oncotarget. 2016;7(9):10594–10605. doi: 10.18632/oncotarget.7236 [DOI] [PMC free article] [PubMed] [Google Scholar]; •• References associated with mechanisms that miR-486-5p plays a role in cervical cancer.
- 30.Henry NL, Hayes DF. Cancer biomarkers. Mol Oncol. 2012;6(2):140–146. doi: 10.1016/j.molonc.2012.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J, Dong D, Wei Q, et al. CXCL10 serves as a potential serum biomarker complementing SCC-Ag for diagnosing cervical squamous cell carcinoma. BMC Cancer. 2022;22(1):1052. doi: 10.1186/s12885-022-10142-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu B, Dong B, Hong S, et al. Combined detection of ACTN4 and SCC-Ag is a promising serological biomarker for cervical intraepithelial neoplasia 3 or worse: a case-control study. Risk Manag Healthc Policy. 2020;13:2677–2687. doi: 10.2147/RMHP.S278809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sayad A, Najafi S, Hussen BM, et al. The role of circular RNAs in pancreatic cancer: new players in tumorigenesis and potential biomarkers. Pathol Res Pract. 2022;232:153833. doi: 10.1016/j.prp.2022.153833 [DOI] [PubMed] [Google Scholar]; • References associated with potential predictive value of miR-486-5p in cervical cancer.
- 34.Fattahi M, Rezaee D, Fakhari F, et al. microRNA-184 in the landscape of human malignancies: a review to roles and clinical significance. Cell Death Discov. 2023;9(1):423. doi: 10.1038/s41420-023-01718-1 [DOI] [PMC free article] [PubMed] [Google Scholar]; • References associated with potential predictive value of miR-486-5p in cervical cancer.
- 35.Aftab M, Poojary SS, Seshan V, et al. Urine miRNA signature as a potential non-invasive diagnostic and prognostic biomarker in cervical cancer. Sci Rep. 2021;11(1):10323. doi: 10.1038/s41598-021-89388-w [DOI] [PMC free article] [PubMed] [Google Scholar]; • References associated with potential predictive value of miR-486-5p in cervical cancer.
- 36.Detterbeck FC, Boffa DJ, Kim AW, et al. The eighth edition lung cancer stage classification. Chest. 2017;151(1):193–203. doi: 10.1016/j.chest.2016.10.010 [DOI] [PubMed] [Google Scholar]
- 37.Jia B, Chen B, Long H, et al. Tumor volume is more reliable to predict nodal metastasis in non-small cell lung cancer of 3.0 cm or less in the greatest tumor diameter. World J Surg Oncol. 2020;18(1):168. doi: 10.1186/s12957-020-01946-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li X, Li R, Ren H, et al. Predicting factors of tumor progression in adult patients with low-grade glioma within five years after surgery. Transl Cancer Res. 2021;10(4):1907–1915. doi: 10.21037/tcr-21-589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakamura K, Nakayama K, Ishikawa N, et al. Preoperative tumor size is associated with deep myometrial invasion and lymph node metastases and is a negative prognostic indicator for patients with endometrial carcinoma. Oncotarget. 2018;9(33):23164–23172. doi: 10.18632/oncotarget.25248 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


