Abstract
Pulmonary hypertension (PH) is a severe and chronic disease characterized by increased pulmonary vascular resistance and remodeling, often precipitating right-sided heart dysfunction and death. Although the condition is progressive and incurable, current therapies for the disease focus on multiple different drugs and general supportive therapies to manage symptoms and prolong survival, ranging from medications more specific to pulmonary arterial hypertension (PAH) to exercise training. Moreover, there are multiple studies exploring novel experimental drugs and therapies including unique neurostimulation, to help better manage the disease. Here, we provide a narrative review focusing on current PH treatments that target multiple underlying biochemical mechanisms, including imbalances in vasoconstrictor–vasodilator and autonomic nervous system function, inflammation, and bone morphogenic protein (BMP) signaling. We also focus on the potential of novel therapies for managing PH, focusing on multiple types of neurostimulation including acupuncture. Lastly, we also touch upon the disease’s different subgroups, clinical presentations and prognosis, diagnostics, demographics, and cost.
Keywords: pulmonary hypertension (PH), pulmonary arterial hypertension (PAH), chronic thromboembolic pulmonary hypertension (CTEPH), cardiac dysfunction, neurostimulation and electroacupuncture, vascular remodeling, alveolar hypoxia
1. Introduction
Pulmonary hypertension (PH) is a progressive, incurable clinical condition defined hemodynamically by a mean pulmonary arterial pressure (mPAP) of ≥20 mmHg at rest, as measured by right heart catheterization (RHC) [1,2]. In accordance with the guidelines of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), PH is generally classified into five etiological groups: pulmonary arterial hypertension (PAH; Group 1), PH associated with left heart disease (PH-LHD; Group 2), PH associated with lung disease or hypoxia (Group 3), PH associated with chronic thromboembolic disease (CTEPH) or other pulmonary artery obstructions (Group 4), and PH with unclear or multifactorial mechanisms (Group 5) [1]. There are multiple subtypes within each group, as listed below in Table 1. In this paper, we focus particularly on the mechanisms and treatments for these groups.
Table 1.
Etiological and clinical classifications of pulmonary hypertension (PH) [1].
Group 1: Pulmonary Arterial Hypertension (PAH)
|
Reproduced with permission of the © European Society of Cardiology and the European Respiratory Society 2024: European Respiratory Journal 61 (1) 2200879; DOI: 10.1183/13993003.00879-2022. Published 6 January 2023 [1]. HIV = human immunodeficiency virus; PCH = pulmonary capillary hemangiomatosis; PVOD = pulmonary veno-occlusive disease.
PH patients may also be categorized into different groups based on their functional class (FC); the World Health Organization (WHO), for instance, separates PH patients into groups, from FC I (least severe) to IV (most severe), based on the limitations and symptoms that arise during physical activity (dyspnea, fatigue, chest pain, syncope), as well as comfort at rest [1]. Utilizing the functional classes is important in determining initial treatment plans and defining goals in stages, for both the patient and physician. For instance, physicians can set the goal as containing symptoms at FC I or II during the management of PH [1,3]. As such, the survival rate is better in patients who improve from a more severe to a less severe FC, compared to those remaining at the same FC [4,5].
This review article will explore how care teams treat PH, including exploring FDA (Food and Drug Administration)-approved and potential emerging therapies for the disease as well as the mechanisms they target. The paper will also mention the diagnosis, cost, and demographics of the disease.
2. Demographics and Cost
The prevalence of PH varies significantly across different populations. Older patients over the age of sixty-five have an estimated PH prevalence of 10%, and those with comorbidities such as aortic stenosis or heart failure with preserved ejection fraction (HFpEF) have a higher prevalence of PH, ranging from around 30 to 90% [6,7,8,9]. Despite scanty evidence, studies also suggest that those with residence in economically developing nations, poor healthcare access, low annual income, higher risk of diseases such as tuberculosis and human immunodeficiency virus (HIV) infection, and low health literacy have a higher prevalence of PH and worse FC and are less likely to receive adequate diagnosis and treatment [10,11,12,13,14,15]. Furthermore, multiple studies find that PH-LHD is the most common type of PH, followed by PH due to lung disease and/or hypoxia [1,6].
Through mechanisms such as inflammation and vascular injury, COVID-19 (Coronavirus Disease 2019) cohorts are also associated with a higher prevalence of PH. This could be in part due to the higher prevalence of pre-existing lung and heart comorbidities in patients affected by COVID-19; consequently, such patients could have already developed PH previously [16,17,18]. On the other hand, some studies suggest that COVID-19 could precipitate elevated pulmonary arterial pressure and PH in patients, from those with venous thromboembolic disease to those without any previous cardiovascular complications [19,20,21,22]. Indeed, multiple studies have reported that COVID-19 could contribute to acute or chronic cardiovascular and pulmonary events in patients that did not previously have them, including increased pulmonary arterial pressure, diastolic dysfunction, pericardial thickening, and impaired left ventricular function [23,24,25]. Regardless, given the lack of abundant data, particularly those which establish patients as having no prior signs of PH, more studies are necessary to establish PH as a COVID-19 sequela.
Reports also indicate that the prevalence of PH is rising, in part due to the increasing use of diagnostic tools such as echocardiography, an aging population, and the increasing burden of diseases such as heart failure [26,27]. Consequently, it is necessary to understand and tackle certain risk factors associated with PH, such as disease prevention and healthcare equity programs, along with finding novel therapies [1,10].
PH can also impose a large cost on the patient, their caregivers, and the broader economy. For instance, in the United States (US), drugs for PAH can cost anywhere between USD 5000 and USD 250,000 annually according to different studies, which is evidently compounded for those on combination therapies or in worse FCs; similar costs for PAH-related drugs have also been reported in other countries [28,29,30,31,32]. Including costs such as hospitalizations, medical devices, diagnostics, supportive therapies, and transplants, PAH can cost patients upwards of tens of thousands of dollars and countries hundreds of millions of dollars [28,29,30,33]. For other types of PH, such as PH associated with systemic sclerosis and PH associated with chronic obstructive pulmonary disease (COPD), total costs per patient may also amount to thousands of dollars annually [34,35,36]. Moreover, studies on patients with PAH and CTEPH report that they have their work and consequently their income significantly affected by the disease, due to factors including being unable to work at all, taking extended sick and disability leave, or considering early retirement. Similarly, caregivers of these patients often have their own work and income affected and experience exhaustion, leading to an indirect annual cost of thousands of dollars and tens of millions of dollars nationally [28,33,37,38,39,40].
Those with PH also often present with comorbidities that increase the cost. For instance, renal disease is associated with PH: studies estimate that PH affects 17 to 56 percent of patients receiving hemodialysis, with a varying incidence between 9 and 39 percent in those with end-stage renal disease (ESRD) [41,42,43,44]. Thyroid disease has also been associated with PH, with one study reporting the prevalence of PH to be 35–47% in patients with hyperthyroidism and 10–24% in patients with hypothyroidism; interestingly, reports suggest a mechanistic link between thyroid hormones and the pulmonary vasculature [45,46]. Furthermore, as mentioned previously, both PH-LHD and PH associated with chronic lung disease and/or hypoxia are the most common groups of PH. The cost of managing these diseases in combination with PH could place additional burden on both the patient and their caregivers.
3. Diagnosis and Biomarkers
Diagnosis and classification of PH relies on mPAP; indeed, medications such as riociguat, epoprostenol, and sildenafil decrease mPAP in patients and provide symptomatic benefit [47,48]. However, additional measures, such as pulmonary arterial wedge pressure (PAWP; mmHg units) to measure pulmonary vein and left-sided heart pressure and pulmonary vascular resistance (PVR; Wood units) to measure pulmonary arterial resistance and narrowing, are important in the classification of PH [1].
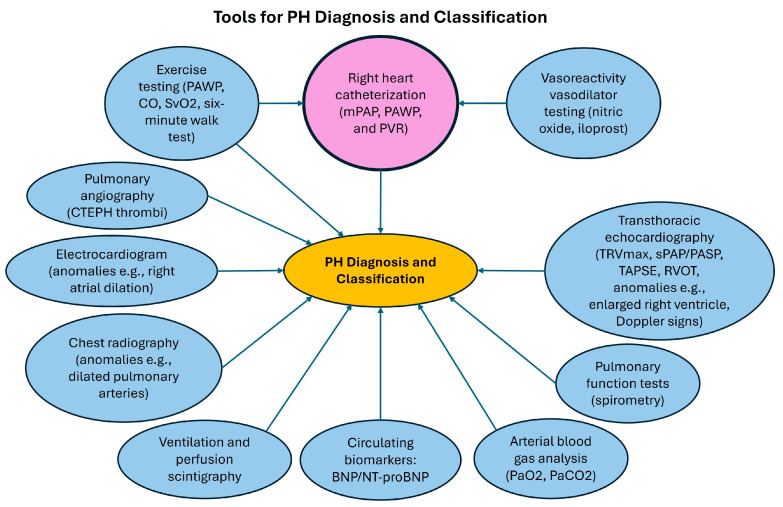
Multiple diagnostic techniques can be utilized when diagnosing and classifying PH through various parameters (Figure 1); however, official ESC/ERS guidelines require RHC for diagnosing and classifying PH. Care teams typically provide RHC as an outpatient procedure in cardiac catheterization labs at experienced centers, whereby a catheter is inserted into a patient’s arm, neck, groin, or other areas to measure pressures inside the heart and lungs [1]. The test measures parameters including mPAP, PAWP, and PVR for subsequent classification into pre-capillary PH (often due to pathological pulmonary vascular remodeling and resistance), post-capillary PH (often due to conditions such as left-sided heart failure), or combined pre- and post-capillary PH; a PAWP of ≤15 mmHg is generally necessary for a diagnosis of pre-capillary PH, including PAH [1,2]. Differentiating between these groups is crucial to avoid misclassification and mistreatment in PH patients [1].
Figure 1.
Tools used for pulmonary hypertension (PH) diagnosis and classification. Right heart catheterization is uniquely colored because it is required for the diagnosis of PH. Tools such as exercise testing or vasoreactivity testing can be utilized when performing right heart catheterization. This figure is only a summary: it does not include all the parameters that can be measured through diagnostic tools (e.g., chest radiography can also measure factors such as right atrial dilation) nor novel diagnostics currently being explored but not yet established (e.g., serum uric acid). mPAP = mean pulmonary arterial pressure, PAWP = pulmonary arterial wedge pressure, PVR = pulmonary vascular resistance, CO = cardiac output, SvO2 = mixed venous oxygen saturation, CTEPH = chronic thrombo-embolic PH, BNP = brain natriuretic peptide, NT-proBNP = N-terminal prohormone of BNP, TRVmax = maximum peak tricuspid regurgitation velocity, sPAP/PASP = pulmonary arterial systolic pressure, TAPSE = tricuspid annular plane systolic excursion, RVOT = right ventricular outflow tract, PaO2/PaCO2 = partial pressure of oxygen/carbon dioxide.
Care teams often perform additional tests, such as vasoreactivity and exercise testing, and measure further parameters, such as mixed venous oxygen saturation (SvO2) and cardiac output (CO), while conducting RHC [1]. For instance, in vasoreactivity challenges, patients with PAH inhale compounds such as nitric oxide (NO) or iloprost to evaluate their pulmonary arteries’ response to vasodilators and their candidacy for calcium channel blocker therapies; in this test, CO increasing or remaining unchanged helps indicate a positive response [1]. Furthermore, care teams can use RHC with exercise testing to assess patients with symptoms such as unexplained dyspnea during physical activity; in such tests, teams measure parameters such as PAWP and CO help diagnose exercise PH, while guidelines also recommend taking SvO2 and arterial saturation [1].
In addition to RHC, clinicians often use echocardiography, particularly transthoracic echocardiography (TTE), as a first-line tool when there is suspicion of PH, because it is non-invasive and provides valuable information. Official ESC/ERS guidelines recommend measuring the maximum peak tricuspid regurgitation velocity (TRVmax), which confers a high probability for PH if it is >2.8 m/s [1]. Another important parameter is pulmonary arterial systolic pressure (sPAP, PASP) [1,49]. Although there are potential inaccuracies in measuring variables that contribute to sPAP, such as right atrial pressure, an sPAP above 60 mmHg can also confer a high probability of PH [49]. Other important echocardiographic parameters include tricuspid annular plane systolic excursion (TAPSE) and right ventricular outflow tract (RVOT). Such values are not only important in suggesting disease—indeed, lower TAPSE/sPAP ratios may support a diagnosis of PH—but also can help differentiate between subgroups of PH—for instance, different RVOT patterns may indicate pre-capillary PH or PH with HFpEF [1,49,50,51].
TTE can also analyze other signs which point to PH, such as enlarged right ventricles, a flattened interventricular septum, or left ventricular hypertrophy [1,49]. Such signs can also be important in the classification of PH; for instance, in PH associated with HFpEF, left ventricular hypertrophy and a deviated interatrial septum tend to be present [49]. Similarly, left atrial enlargement can indicate PH-LHD [49]. Other signs that clinicians can measure include Doppler echocardiographic signs, such as E/A (early diastolic mitral inflow velocity to late atrial contraction mitral inflow velocity) or E/E′ (early diastolic mitral inflow velocity to early diastolic mitral annulus velocity) ratios. Lastly, it is important to note that while TTE can provide a probability estimate for PH, RHC is still necessary for a definitive diagnosis.
Multiple other tests may also aid in diagnosis. For instance, electrocardiograms can provide evidence for right atrial dilation, right ventricular hypertrophy and strain, right axis deviation, P pulmonale, right bundle branch block, and a prolonged QTc interval (corrected interval between Q and T waves) [1,48,52,53]. Because patients with PH can face complications such as arrythmias, electrocardiograms can be crucial in establishing treatment, such as rhythm-control therapy [1,53,54]. Chest radiography can also check for comparable results to echocardiography, including right atrial enlargement, dilated pulmonary arteries, calcifications, and aneurysms [1,48,55]. Pulmonary function tests and arterial blood gas analysis can help distinguish between groups such as idiopathic PAH and PH associated with lung disease by measuring parameters such as total lung capacity and forced expiratory volume, while ventilation/perfusion scintigraphy and pulmonary angiography can help diagnose CTEPH [1]. Other tests include exercise tests and abdominal ultrasound for portal hypertension [1]. Typically, PAH diagnosis occurs only after excluding other groups of PH as defined in Table 1 [1,56].
In addition to tests, different biomarkers can be crucial in assessing PH early. One such biomarker is brain natriuretic peptide (BNP) and its inactive, more stable counterpart, N-terminal prohormone of BNP (NT-proBNP). Both peptides derive from proBNP (prohormone of BNP) in a process which occurs in ventricular cardiac myocytes in response to stretch, such as stretch due to increased pressure or volume overload. Consequently, a blood test can also measure both peptides to aid in the diagnosis of PH [1,57]. Indeed, elevated BNP is present in idiopathic PAH, PH associated with COPD, CTEPH, and multiple other classes of PH; similarly, one study reports elevated NT-proBNP in a heterogeneous group of patients with pre-capillary PH [57]. Moreover, along with other markers, such as functional class, as mentioned above, and the six-minute walk test, both BNP and NT-proBNP are strong predictors of mortality in PAH patients and, in part, constitute PAH risk stratification tools [1,57]. Currently, BNP and NT-proBNP are the primary biomarkers measured at PH centers to evaluate for the disease [1]. However, it is important to note that BNP and NT-proBNP are not specific for PH and can increase in other cardiac and renal diseases [1,57].
In addition to BNP/NT-proBNP, multiple markers could aid as potential diagnostic or predictive markers in PH, although care teams have yet to apply them clinically as such. For instance, increased serum endothelin-1 (ET-1), a potent vasoconstrictor, and reduced serum NO, an important vasodilator, are both implicated in PH, and these compounds are mentioned in more detail below [57]. Hyperuricemia is also present in patients with PAH and PH-LHD, and studies report that serum uric acid levels correlate with mortality in PAH patients; however, hyperuricemia is also present in other diseases and other states, such as renal dysfunction or in cases of diuretic use [57]. Additional studies are investigating factors such as bone morphogenic proteins (BMPs) and chemokines as biomarkers for PH [57].
Those with PH typically present with symptoms not entirely specific to the disease but important for physicians to suspect and initiate prompt action for therapy and referral. For instance, a cardinal feature of PH is progressive exercise dyspnea, while other symptoms are fatigue, tachycardia, and syncope upon exertion. On physical examination, patients may also display lower extremity edema, dilated jugular veins, and ascites. Additional symptoms observed in PH patients are augmented heart sounds, such as a pronounced pulmonary component in the second heart sound, cyanosis, and exertional and nocturnal hypoxia. Moreover, mental stress due to sleep problems, anxiety, and depression can arise in patients with PH, in which case treatment plans could include adequate psychosocial support and the appropriate medications [1,58,59].
4. Mechanisms and Established Treatments
PH is associated with multiple changes at a tissue and cellular level. The vasoconstriction of the pulmonary arteries with the potential involvement of the pulmonary vein and capillaries contributes to an elevated mPAP [1]. Histologically, patients with PAH and CTEPH show various forms of pathological vascular remodeling, vascular lesions, and associated inflammation [48,60,61]. Multiple studies also report pulmonary vascular pathology, such as arterial thickening and hypertrophy, in those with PAH, CTEPH, and PH-LHD [62,63,64,65]. Vascular fibroblast migration, increased production of matrix proteins, endothelial cell proliferation, and neovascularization could also contribute to PH pathology [61,62,66,67]. Lesions also increase the chance of surgical risk in patients with CTEPH [48,68,69].
Hypoxia, infection and inflammation, shear stress, genetics, vasodilator–vasoconstrictor imbalances, and dysautonomia contribute to or are results of such vascular pathology in PH. The sections below will discuss these mechanisms and related treatments.
4.1. Vasoconstrictor–Vasodilator Imbalances
PH has long been associated with the increased production of vasoconstrictors, such as endothelin-1 (ET-1), and the decreased production of vasodilators, such as NO and prostacyclin.
For instance, ET-1 is overexpressed in both the plasma and lungs of PH patients in multiple different PH groups, such as CTEPH, PH associated with hypoxia, and PAH; one study also reports that ET-1 concentration positively correlates with the severity of symptoms in CTEPH patients [70,71,72,73,74]. Mechanistically, ET-1 activates two G-coupled protein receptor subtypes, endothelin receptor A (ETA) and endothelin receptor B (ETB). ETA receptors typically dominate over ETB receptors in the pulmonary vasculature; the activation of the former leads to vasoconstriction while the latter induces vasodilator release [73,75,76]. ET-1 promotes intracellular calcium release through the phospholipase C (PLC)/inositol triphosphate pathway, causing smooth muscle contraction. Diacylglycerols produced by PLC activation further potentiate smooth muscle contraction [76,77]. Furthermore, ET-1 can induce the transcription of genes to promote smooth muscle cell growth and proliferation by the extracellular signal-regulated kinase (ERK) or sodium/hydrogen pump [75,78,79,80].
In accordance with these mechanisms, multiple current drugs aim to block ETA activation to help manage symptoms; however, these drugs are more specific to PAH and have minimal effect on improving symptoms such as exercise capacity in other groups of PH patients [1,81,82,83,84]. Endothelin antagonists include drugs such as bosentan, ambrisentan, and macitentan. Bosentan and macitentan are both oral medications that competitively inhibit ETA and ETB receptors, promoting pulmonary vasodilation through the pathways mentioned above [1]. Ambrisentan is also an oral medication but selectively inhibits ETA receptors. Although there are side effects associated with these medications, including liver dysfunction and thrombocytopenia, these drugs are effective in improving mortality and morbidity rates, WHO-defined FC, and exercise capacity as measured by the six-minute walk distance test, as well as hemodynamic parameters such as PVR and mPAP in patients with PAH [85,86,87,88].
Vasodilator mechanisms also play a vital role in the pathogenesis of PH. For instance, ETB receptor activation on endothelial cells can trigger nitric oxide synthase (NOS) to produce NO; NO subsequently diffuses to vascular smooth muscle cells and activates soluble guanylate cyclase (sGC), which generates cyclic guanosine monophosphate (cGMP) that leads to smooth muscle relaxation and vasodilation [78]. Moreover, NO synthesis depends on multiple additional factors: for instance, NOS often uses both oxygen and L-arginine to produce NO [89]. Additionally, studies not only report reduced lung expression of NOS in those with PAH and secondary PH, but also increased phosphodiesterase 5 (PDE5) expression, an enzyme which degrades cGMP, in PAH lung tissue [90,91].
There are multiple drugs which target these pathways; however, these drugs are more specific for PAH rather than PH-LHD or PH associated with chronic lung disease or hypoxia. For instance, PDE5 inhibitors (PDE5i) include tadalafil and sildenafil, which target the NO pathway by acting as reversible competitive inhibitors of PDE5; they both improve hemodynamic values and functional status in patients with PAH [92,93]. There are also sGC stimulators including riociguat, which help to treat those affected by both PAH and CTEPH. For instance, riociguat targets the NO pathway by increasing the sensitivity of sGC to NO and directly stimulating the sGC enzyme independently of NO. Accordingly, riociguat also helps increase exercise, improve FC, and improve hemodynamic parameters in these patients [47,94].
Prostacyclin is a small lipid molecule that acts on prostacyclin (IP) receptors on the smooth muscle to induce potent cyclic adenosine monophosphate (cAMP)-mediated vasodilation and anti-proliferative pathways [1,95]. However, research finds that PAH patients can have abnormalities in the prostacyclin pathway, such as a reduction in lung and pulmonary arterial prostacyclin synthase expression [1,95]. Similar to drugs targeting the NO pathway, prostacyclin analogs aim to correct vasoconstrictor–vasodilator imbalances by binding to prostacyclin receptors present on endothelial cells, smooth muscle cells, and platelets. In binding to these receptors, they promote cAMP production in both smooth muscle cells and platelets, promoting vasodilation and inhibiting platelet aggregation [1].
Prostacyclin analogs for PAH treatment include epoprostenol, iloprost, and treprostinil. Clinicians can administer epoprostenol intravenously, and the drug is more effective for those in more severe FC compared to other therapies [96,97]. Although there are more stable versions, epoprostenol typically has a short half-life and consequently requires continuous infusion [1]. Regardless, intravenous (IV) epoprostenol improves symptoms such as exercise capacity as well as hemodynamic parameters [96].
Treprostinil is similar to epoprostenol but allows administration via subcutaneous injection or inhalation; through these means, it has shown tremendous benefit in improving exercise capacity, hemodynamic parameters, symptoms, quality of life measures, and even certain biomarkers such as NT-proBNP [98,99]. Oral treprostinil shows more mixed results in benefits, as measured by the 6 min walk test [100,101]. Interestingly, inhaled treprostinil has shown marked benefits in PH patients with interstitial lung disease; in the INCREASE clinical trial, patients undergoing the treatment for 16 weeks showed better exercise capacity, an increase in time until certain clinical worsening events (such as hospitalization, transplantation, or death), and a reduction in NT-proBNP. Despite limitations such as in measuring what constituted an acute exacerbation, these studies report treprostinil effectively helps a subset of patients with Group 3 PH [102,103].
Iloprost is another inhaled prostacyclin analog that has shown benefit symptomatically and hemodynamically in PAH patients [104]. Selexipag is yet another prostacyclin receptor agonist and can be orally available; clinical trials have shown that it helps to ameliorate hemodynamic parameters and reduce the relative risk of morbidity and mortality events [105,106]. Lastly, beraprost improves exercise capacity, at least in the short term, for PAH patients, but not hemodynamic parameters [107,108].
For PAH patients, a combination of a PDE5i and endothelin receptor antagonist is often the standard of care for both mild and more severe cases [3]. Typically, common side effects of prostacyclin analogs vary, but include headache, flushing, jaw pain, diarrhea, and errors related to administration, such as IV injection site infection, infusion site pain, and sepsis [1].
4.2. Hypoxia and Oxygen Therapy
Hypoxia is known to be a contributing factor to PH in both patients and animal models; the mechanisms underlying this process, however, are multifactorial and not well-established in PH. Generally, pre-clinical studies have discovered that low oxygen can drive hypoxic pulmonary vasoconstriction (HPV) in smooth muscle cells; research has suggested that these cells are responsible for sensing changes in oxygen, but the nature of this oxygen sensor is less clear [109,110]. Moreover, the nature of HPV in PH is unclear, given that studies on patients who lived, in part, in high altitudes still maintain a relatively elevated pulmonary arterial hypertension even when entering normoxic conditions [109].
Studies on animal pulmonary arterial tissue and vascular smooth muscle cells report that hypoxia can trigger alterations in mitochondria-mediated reactive oxygen species (ROS) production, ROS-dependent reduction in NO bioavailability and NO-related enzyme activity, and redox-dependent modification of potassium and calcium channels in smooth muscle cells, promoting vasoconstriction [111,112,113,114,115,116,117,118]. Indeed, a blood-based study on those living in high altitudes showed that they have an increased pulmonary output of free radicals, inflammatory cytokines, and a loss of NO bioavailability, suggesting PH and HPV could be related via ROS and a loss of available pulmonary NO [119]. Moreover, patients with PAH display elevated levels of arginase, an enzyme that competes with NOS for the substrate arginine; similarly, pre-clinical research also shows that hypoxia can reduce arginine transport into endothelial cells for NO synthesis [120,121].
There is also evidence that pulmonary neuroendocrine cells (PNECs), oxygen-sensitive chemoreceptors in the alveolar and bronchiole endothelium, may play a role in the pathogenesis of PH. For instance, studies on lung tissue show an increased amount of PNECs in patients with PAH, as well as related diseases such as COPD [122,123]. Moreover, PNECs store important vasoconstrictors such as serotonin and vasodilators such as calcitonin gene-related peptide (CGRP) [123,124]. Given that animal studies have shown that hypoxia can trigger serotonin release from and CGRP depletion in PNECs [123,125], it is possible that PNECs can play a crucial role in hypoxia-associated PH and HPV; however, researchers must conduct more studies on PNECs to establish their link to PH.
Hypoxia also activates multiple hypoxia-inducible factors (HIFs) [126,127]. In animal and human cellular models, both low oxygen and ROS, triggered by hypoxia, play a role in promoting hypoxia-inducible factor 1 (HIF-1) stabilization and signaling [128,129,130,131]. HIF-1 is also associated with vascular remodeling and smooth muscle hypertrophy in hypoxic animal models [132] as well as multiple molecular changes, including the downregulation of potassium channel expression and the upregulation of sodium–hydrogen exchangers in animal smooth muscle cells, both of which are associated with smooth muscle hypertrophy and hyperplasia [133,134]. Hypoxia also increases ET-1 expression through HIF-1 complex-mediated binding to the transcription site for ET-1 in human cell models [135]. Moreover, in endothelial cells from patients with PAH and CTEPH, HIF-1 subunits are overexpressed [136]; endothelial cell research from CTEPH patients also suggests that HIF-1 can play a role in controlling platelet adhesion to endothelial cell walls through activating the neural precursor cell expressed developmentally down-regulated protein 9 (NEDD9) protein [136,137]. Lastly, human and animal cell studies show that hypoxia triggers fibroblast migration and proliferation through complex signaling pathways involving protein kinase B (Akt), as well as vascular remodeling through HIF-mediated increases in activin, a ligand associated with PH [138,139,140].
With regard to treatment, care teams can supplement oxygen therapy to maintain pulmonary and systemic oxygen levels when pO2 is low in each group of PH [1]. Clinical studies on PAH have reported that oxygen therapy does not have a significant clinical or survival effect on the prognosis [1,141]. However, other clinical studies have also shown oxygen helps patients with PAH, including by lowering hemodynamic values and providing symptomatic benefit such as exercise function [110]. In this respect, it is possible that oxygen therapy helps those with PH via the mechanisms listed above, specifically by promoting vasodilation and reducing HPV. Regardless, ESC/ERS guidelines recommend reserving oxygen for those with low O2 saturation during exercise or sleep, although care teams can employ ambulatory oxygen if there is symptomatic benefit [1].
4.3. Genetics and Signaling Pathways
Imbalances in proliferative and anti-proliferative signaling via the transforming growth factor-β (TGF-β) receptor have been associated with PAH. For instance, mutations in the BMP receptor type II (BMPR2) gene, a subtype of TGF-β receptor, are the most common heritable form of PAH and are associated with increased intima, smooth muscle, and adventitia thickening in pulmonary arteries through complex signaling pathways [61]. However, it is likely that mutations in other genes constitute the pathology of PAH, since not all of those with BMPR2 gene mutations develop the PAH phenotype.
BMPR2 signaling is complex and controls multiple signaling pathways. First, BMPR2 downstream targets include BMP4 (bone morphogenic protein 2) and BMP7 (bone morphogenic protein 7) in human vascular smooth muscle cells, which help repress the secretion of ET-1; moreover, ET-1 can downregulate BMPR2 expression and signaling in these cells [142,143]. This could underlie, in part, the reason those with BMPR2 gene mutations are at risk for developing PAH.
Immunologically, BMPR2 deficiency is also associated with increased cytokine production from human vascular smooth muscle cells, which is associated with reductions in extracellular superoxide dismutase 3 (SOD3) activity and the increased production of ROS [144]. Additionally, pre-clinical studies on both animal and human cell and tissue models, including those modeling PAH, associate BMPR2 deficiency with increased lymphocyte, neutrophil, monocyte, and macrophage activity and migration [145,146,147]. This is understandable, given that BMPR2 activation on macrophages can inhibit macrophage activation and the upregulation of adhesive membrane proteins [145,146,147]. Interestingly, from a viral standpoint, HIV infection can lead to the miRNA-mediated downregulation of BMPR2 expression [148].
Metabolically, BMPR2 loss is associated with mitochondrial dysfunction in endothelial cells and cardiomyocytes by shifting away from glucose and fatty acid oxidation, respectively; studies suggest that these changes can predispose PAH clinical symptoms such as endothelial inflammation and right ventricular failure [149,150].
More pertinently, impaired BMPR2 activity may result in the impaired regulation of activin, a pro-proliferative ligand that reduces bone morphogenic protein (BMP) activity and promotes myogenic proliferation and remodeling; indeed, activin A is overexpressed in pulmonary vessels of both human and animals affected by PAH [151,152,153]. Accordingly, sotatercept, a fusion protein which acts as a ligand trap to inhibit activin signaling, improves clinical outcomes for PAH patients, including exercise capacity, as measured by the six-minute walk test [154,155]. By inhibiting activin’s activity, sotatercept also interferes in activin’s ability to compete with surface receptors with anti-proliferative BMP signals.
4.4. Inflammation
Inflammation is a crucial factor in the pathology of PH, especially in those with PH associated with infection or connective tissue diseases. For instance, vascular lesions in human lung samples harbor lymphocytes, dendritic cells, mast cells, macrophages, and proliferating endothelial cells [156,157,158,159]. Patients with PH also show higher serum cytokines including multiple interleukins (IL), tumor necrosis factor alpha (TNFα), and chemokines [160,161,162].
Multiple cytokines and pathways contribute to PH pathogenesis. For instance, one study on a mice model showed that hypoxia increases lung production of IL-1β, which activates receptors on smooth muscle cell and triggers proliferation [163]. Moreover, IL-6 triggers the signal transducer and activator of transcription 3 (STAT3) in human cell cultures, causing the increase in miRNAs that degrade BMPR2 mRNA; as mentioned previously, a loss of BMPR2 signaling can contribute to PH pathology [164]. Indeed, studies on animal models and human cell cultures have shown that Il-6 can increase endothelial growth factor receptors and matrix metalloproteinases that promote smooth muscle cell proliferation and the endothelial–mesenchymal transition seen in PH [165,166]. Additionally, research suggests that IL-6 and other cytokines, such as IL-8 and IL-10, can be important predictors for survival in PAH patients, similarly to traditional markers such as the 6 min walk test or hemodynamic parameters, signifying their importance in PH pathogenesis [160,167]. Other interleukins, such as IL-8 and IL-13, act through pathways that promote endothelial cell proliferation and arginase upregulation, respectively [168,169].
The mechanisms related to increased lymphocyte activity and consequent cytokine production are less clear in PH. Studies have reported that patients may have dysregulated regulatory T-cell function which could predispose high immune system reactivity [170,171]. A considerable proportion of PH patients have circulating autoantibodies [172,173], which could precipitate endothelial cell damage and further PH pathogenesis. Infections such as HIV or COVID-19 are also associated with damage to the vascular endothelia, cytokine storms, increased inflammation, and PH pathogenesis. Research studies report that COVID-19 potentiates intense platelet activation, which might contribute to thrombosis seen in multiple groups of PH patients [174,175].
With regard to therapies, PAH-specific drugs have limited anti-inflammatory effects, and prostacyclins are even stronger mediators of immune activity [176]. For instance, sotatercept not only acts as an activin ligand trap and restores BMP signaling, but also contributes to immunosuppression: in a rat model of PAH, sotatercept reduced pulmonary inflammation and inflammatory gene expression profiles [177]. Another example is riociguat, which in part mediates inflammation by blocking TGF-β signaling and inhibiting fibrosis, as shown in a pre-clinical study [178]. In animal models, bosentan suppresses the cytokine TNFα; in patients with PAH due to systemic sclerosis, bosentan leads to a reduction in proinflammatory cytokines [179,180].
4.5. General Therapies
ESC/ERC guidelines do not recommend most PAH-specific drugs for those with PH-LHD or PH associated with chronic lung disease or hypoxia, as the evidence suggests a lack of clinical improvement and potentially adverse outcomes [1]. However, the FDA has approved riociguat for helping those with CTEPH, and reports suggest that macitentan can also benefit CTEPH patients [1,181]. Moreover, diuretics are a general supportive therapy for PH patients to relieve pulmonary congestion and right-sided heart failure [1].
For patients with CTEPH, pulmonary thromboendarterectomy (PEA) is typically the treatment of choice and is potentially curative; physicians can also employ percutaneous balloon pulmonary angioplasty (BPA) if PEA is ineffective or unfeasible due to surgery risk [1]. In addition, guidelines recommend lifelong anticoagulation in patients with CTEPH, such as Vitamin K antagonists and Factor Xa inhibitors like rivaroxaban. Reports advise Vitamin K antagonists for those with antiphospholipid syndrome [1,182]. Lastly, while there is benefit for those with CTEPH, anticoagulation treatment use for PAH is unclear. Research suggests that anticoagulation therapies such as warfarin may help improve survival and quality of life in patients with idiopathic PAH, but other studies report no significant advantage in the same group of patients [1,183,184].
Other general therapies include managed exercise training, which improves hemodynamics, oxygen uptake, and quality of life in patients affected by PH [185,186]. ESC/ERC guidelines recommend limiting salt and water consumption to reduce volume overload and hypertension as well as correcting iron deficiency, which often occurs in PH [1]. They also strongly advise women against pregnancy, as studies link pregnancy with higher maternal mortality rates in those affected by PH [1]. ESC/ERC protocol also recommends routine vaccinations to prevent infections such as influenza and pneumococcal pneumonia as a general safety measure [1].
The management of PH patients in Group 2 and Group 3 often focus on addressing the underlying issue with therapies that focus on problems such as heart failure, valvulopathy, or obstructive or restrictive lung diseases. Currently, lung transplants are the only potentially curative treatment for PH associated with chronic lung disease, and clinicians can also consider such transplants for patients in other PH groups who are unresponsive to medications and in a high FC [1].
5. Potential Therapies
In addition to FDA-approved medications and general therapies, current investigations are studying potential therapies in pre-clinical or clinical studies. We highlight specific therapies below.
5.1. Prostacyclin Analogs
Ralinepag, similar to other FDA-approved prostacyclin analogs listed above, is a potential therapy for PAH. Ralinepag also exhibits similarities mechanistically to other prostacyclin drugs, promoting vasodilation and discouraging smooth muscle cell proliferation through binding to the IP receptor, leading to an intracellular increase in cAMP [187]. In terms of administration, patients can take ralinepag orally [187]. Most pertinently, ralinepag has shown benefits in PAH patients, including improvement in six-minute walk testing and reductions in PVR [187,188]. Currently, clinical studies and data analysis are ongoing via Phase 3 ADVANCE trials, which aim to elucidate the efficacy of ralinepag in endpoints such as time to clinical worsening events and exercise capacity, as well as the drug’s long-term safety and efficacy [189,190,191].
5.2. sGC Stimulators
MK-5475 is a sGC stimulator that is in an inhalable dry powder form. Multiple research efforts are currently investigating the drug: for instance, a Phase I study demonstrated that MK-5475 is not only safe but reduces PVR in patients with PH-COPD [192]. MK-5475 has also shown the same benefits in a phase I study which involved patients with PAH [193]. The INSIGNIA-PAH trial, a Phase II and III trial assessing the efficacy and safety of MK-5475 in patients with PAH, was recently finished [194]. Currently, no Phase II trials testing MK-5475 in PH-COPD patients exist.
5.3. CGRP
Research finds that reduced CGRP, a potent vasodilator found in cells such as pulmonary neuroendocrine cells, in the plasma of rats affected by hypoxia-induced PH [195,196]. The administration of CGRP can stimulate pulmonary vasodilation in hypoxic-induced PH animal models and attenuate pathological vascular remodeling in PAH rat models in part by suppression of ET-1 release and attenuating the cGMP-AMP synthase (cGAS)-simulator of inferior genes (STING)-nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) pathway, respectively [197,198,199,200]. CGRP ligands may also activate the calcitonin receptor-like receptor (CLR)/receptor activity-modifying protein 1 (RAMP1) receptor, leading to the triggering of multiple signaling pathways that, in turn, regulate not only intracellular energy metabolic patterns but also reduce the mitochondrial damage involved in PAH rat models [199]. A study also reports that the replacement of the CGRP gene with an adenoviral vector ameliorates hypobaric chamber-induced pulmonary hypertension in mice models [201]. However, researchers would need to perform more studies to investigate CGRP’s efficacy in subjects with PH.
5.4. HIF-Related Therapies
Currently, no approved medications exist targeting HIF pathways in PH patients. However, research suggests that inhibitors of the hypoxia-inducible factor 2 (HIF-2) pathway can help improve symptoms such as right heart function and pulmonary remodeling in rat models of PAH and hypoxia-induced PH [202,203]. Moreover, numerous pre-clinical studies have reported similar results with HIF-1 inhibitors [204,205,206,207,208].
5.5. BMPR2-Related Therapies
Tacrolimus is an immunosuppressive medication typically used for those with organ transplants or autoimmune diseases. However, tacrolimus also ameliorates right ventricular systolic pressure, right ventricular hypertrophy, and reduced pathological vascular changes in PAH rat models with dysfunctional endothelial BMPR2 signaling [209]. Moreover, tacrolimus potentially rescues BMP signaling in pulmonary artery endothelial cells by blocking an inhibitor protein of BMPR1 and activating SMAD1/5 and mitogen-activated protein kinase (MAPK) signaling [209]. Furthermore, a phase IIa randomized controlled trial showed that PAH patients tolerated tacrolimus well [210]. Still, additional studies need to confirm the efficacy of tacrolimus.
Etanercept targets the BMPR2 pathway in part by inhibiting TNFα, a cytokine that is overexpressed in PAH patients and leads to the downregulation of BMPR2 mRNA in rat PAH models [160,211]. Although research shows that etanercept reduces pulmonary hypertension and lung tissue cytokine levels in rat models of PAH, there are no clinical trials on the drug to date [212].
Studies have also investigated other routes of therapy for in PAH models. For instance, the targeted delivery of exosomes to replace defective BMPR2 has the ability to reverse monocrotaline-induced PH [213]. Studies on rats indicate that SRT2104, an activator of Sirtuin 1 (SIRT1), can mediate the restoration of tuberous sclerosis complex 2 (TSC2), a growth suppressor protein on smooth muscle cells reduced in PAH, to ameliorate disease [214].
5.6. Additional Immunological Therapies
Immunosuppressive drugs such as dexamethasone, mycophenolate mofetil, cyclosporine, tacrolimus, and etanercept reduce neutrophil migration, decrease lymphocyte proliferation, and attenuate endothelial cell dysfunction and hemodynamic parameters in animal models of PAH. However, these preclinical findings warrant further translational and clinical investigation [215].
Rituximab, a monoclonal antibody against CD20 (a B-cell protein), has shown modest but not statistically significant symptomatic benefits, as measured by the six-minute walk test in PAH patients [216]. Anakinra, which acts as an IL-1 receptor antagonist, has shown symptomatic benefits in PAH patients, as measured by different heart failure and disease severity scores. However, both will need more robust clinical trials to establish their safety and efficacy [216,217].
5.7. Potential Neurostimulation and Autonomic-Related Therapies
There is also increasing evidence for a role for the autonomic nervous system in PH. Studies have suggested both increased sympathetic activity and decreased parasympathetic activity in PAH patients [218,219]. Additional pre-clinical tests suggest a role for imbalanced autonomic activity in other PH subtypes; for instance, both sympathetic denervation and parasympathetic stimulation attenuated pulmonary vascular remodeling in CTEPH rat models [220]. Furthermore, chronic intermittent hypoxia led to rostral ventrolateral medulla (rVLM)-mediated heightened sympathetic tone, although research has not yet directly linked such a finding to PH [221].
5.7.1. Multiple Pharmacological Therapies Have Been Tested to Assess the Role of the Autonomic System in the Pathogenesis of PH
ESC/ERC guidelines, together with other studies, have stated that clinical evidence for beta (β)-blocker use in PH management is currently lacking [1,222]. However, research shows that the beta-blocker carvedilol improves heart rate and right ventricular (RV) function in hypoxia-induced PH rats, inhibits smooth muscle cell proliferation in vitro, and helps control heart rate in patients with PAH [223,224,225]. Moreover, nebivolol, a β1 antagonist and β2 agonist, reduces the proliferation of vasoactive and proinflammatory factors from pulmonary artery endothelial cells from patients with PAH [226]. Other similar drugs, such as bisoprolol and arotinolol, improve RV function by preventing RV hypertrophy and improving RV contractility in rats with monocrotaline-induced PH [227,228]. However, more extensive clinical trials with beta-blockers have not yet been conducted, let alone shown efficacy in patients with PH, although these pre-clinical studies detail some promising prospects [1,222,229,230,231,232,233].
With regard to the parasympathetic nervous system, the acetylcholinesterase inhibitor pyridostigmine attenuates right-sided heart dysfunction and pulmonary remodeling in rat models of hypoxia-induced PH and CTEPH, respectively [219,220].
5.7.2. Non-Pharmacological Therapies: Sympathetic Activity Modulation and Vagal Nerve Stimulation (VNS)
Sympathetic modulation and neurostimulation of parasympathetic nerves could also become promising therapies for PH. For instance, researchers have conducted sympathetic modulation through methods such as sympathetic ganglion block, renal sympathetic denervation, and pulmonary artery denervation (PADN), which itself involves techniques such as radiofrequency ablation or high-energy ultrasound [220,234,235,236]. Studies with animal models of PAH and CTEPH have reported that reducing sympathetic activity attenuates pulmonary vascular remodeling, reduces hemodynamic parameters including right ventricular pressure and mPAP, and ameliorates pulmonary wall thickness [220,237,238,239,240,241,242,243,244]. Mechanistically, sympathetic modulation leads to increased NO signaling, an altered expression of genes that are related to inflammation and vasoconstriction, and the downregulation of the activity of the renin–angiotensin–aldosterone system [220,237,238,239].
Clinically, studies have reported that PADN leads to a decrease in hemodynamic parameters, such as mPAP and PVR, as well as an improvement in exercise capacity and cardiac function in patients with PAH [235,236,245,246]. Other studies suggest that PADN could have similar benefits in those with residual CTEPH [247,248], but more extensive, randomized studies are warranted to assess PADN’s effectiveness in patients with PH [1].
Vagal nerve stimulation (VNS) may also help to ameliorate PH, as suggested by multiple pre-clinical studies. Chronic VNS helps prolong survival, reduce dysautonomia and inflammation, and improve right heart function and hemodynamic parameters in various rat models of PAH [249]. One study also reported that neurostimulation helps to preserve right ventricular function in rats with significant right ventricular overload and PH induced by pulmonary arterial banding [250]. Currently, no clinical studies have investigated the efficacy of VNS in patients with PH.
5.7.3. Potential for Additional Integrative Therapies
Research has not yet investigated manual acupuncture (MA) and electroacupuncture (EA), both of which stimulate somatosensory nerves, for patients with PH. However, EA treatment via specific acupoints ameliorates elevated mPAP, vascular remodeling, and right ventricular hypertrophy in rat models of hypoxia-induced PH, in part by normalizing ET-1 and endothelial nitric oxide synthase (eNOS) imbalances [251]. EA also attenuates pulmonary vascular remodeling, via pathways involving vascular endothelial growth factor (VEGF)/phosphoinositide 3-kinases (PI3K)/Akt and ET-1 reduction, in rats affected by COPD [252]. These findings corroborate those from pre-clinical and clinical studies in different diseases, such as systemic hypertension, asthma, and systemic sclerosis, which report acupuncture’s amelioration of ET-1, NO, and CGRP imbalances [253,254,255,256,257,258,259].
MA and EA have also shown additional benefit in both animal models and patients with comorbidities commonly found in PH, such as heart failure, lung disease, and systemic sclerosis. For instance, studies in patients with COPD have demonstrated acupuncture’s role in improving lung and pulmonary function, exercise capacity and endurance, efficiency of oxygen uptake, oxygen saturation, and quality of life [260,261,262,263,264,265,266]. Additionally, acupuncture improves the ejection fraction and the regulation of pathological ventricular enlargement in subjects with heart failure, both in preclinical and clinical studies [267,268,269]. These findings are listed in Table 2, together with other studies that will be discussed below. Regardless, although the studies do not make a link to PH, it could be possible that acupuncture provides symptomatic benefit to PH patients with these comorbidities. However, more research needs to be conducted, via both pre-clinical and clinical studies, to establish acupuncture as a therapy for comorbidities in those with PH. Moreover, an analysis of seven randomized controlled trials on heart failure patients concluded that acupuncture studies displayed methodological heterogeneity and an inconclusive efficacy [270].
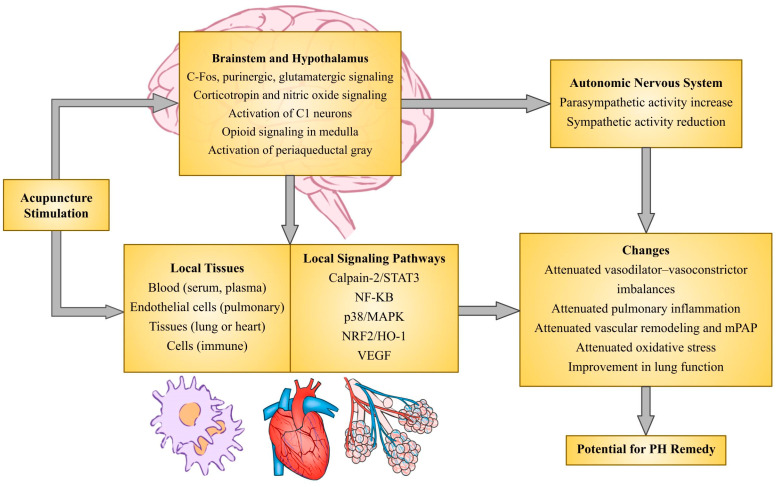
Acupuncture’s mechanisms with regard to these reported benefits have not been fully elucidated. Similar to the PADN studies mentioned above, it is possible that acupuncture mediates its benefit through modulating sympathetic activity: studies have reported that EA can dampen elevated sympathetic responses, including in rat models of cardiac dysfunction such as heart failure and myocardial infarction [269,271,272,273]. In these rats and other sympathetically stressed animals, EA-mediated reduction in sympathetic activity in such heart failure studies depend on C-fibers and thinly myelinated group A delta (Aδ)-fibers within the median nerve [271,272,274,275]. Indeed, such somatosensory input through the median nerve has been shown to activate processes within the hypothalamus, midbrain, and medulla to modulate elevated sympathetic activity, including increasing opioid expression, consequently altering signaling in the rVLM [271,274,276,277] and activating glutamatergic, reciprocal, and excitatory pathways between the arcuate nucleus (ARC) and the midbrain ventrolateral periaqueductal gray (vlPAG) [273,278]. Pre-clinical studies with median nerve stimulation have also suggested EA reduces gamma-aminobutyric acid (GABA) release in the vlPAG, disinhibiting vlPAG neurons, and in turn suppressing sympathetic neuronal activity in the rVLM through a serotonergic-mediated pathway [279,280,281]. EA also activates opioid neurons in the ARC which project to the rVLM, reducing elevated activity of the pre-sympathetic neurons [281]. Lastly, EA can reduce sympathetic activity through other central pathways. For instance, research suggests that the hyperactivity of sympathetic neurons within the hypothalamic paraventricular nucleus (PVN) and their ensuing connections with the rVLM play a role in multiple diseases, including in models of hypoxia-induced PH [282,283,284,285]. Elevated levels of corticotropin-releasing hormone (CRH) synthesis and neuronal activity contribute to this sympathetic overactivity, including in disease models of PH [282,283]. Moreover, studies observe activated CRH neurons in the PVN and nucleus tractus solitarius (NTS) during acute hypoxic conditions leading to increased sympathetic outflow [286,287,288,289,290]. Although not in PH models, studies report that EA reduces CRH signaling in the rVLM and PVN in animal models of stress, cardiovascular disease, and multiple other sympathetic excitatory-related conditions [291,292,293,294]. Reductions in other nitric oxide synthases, including neuronal nitric oxide synthases (nNOS), in the PVN and other areas such as the lung exposed to hypoxia, promote hypoxia-induced PH and are associated with increased sympathetic activity [295,296]. However, these mechanisms with relation to EA effects in PH are not clear, although one study has shown that EA decreases nNOS levels in the hypothalamus in a rat model of systemic hypertension [297].
It is important to note that more studies are necessary not only to establish EA’s efficacy in pre-clinical models of PH (for which there is one study listed, [251]), but also if the EA modulation of sympathetic activity would apply to attenuating symptoms in PH models, and whether the mechanisms above would underlie such a change. There is evidence that heightened sympathetic activity plays a role in PH pathogenesis as mentioned above, but these studies do not yet detail a firm link between acupuncture and PH.
Table 2.
Studies supporting the role of acupuncture in the pathologies of PH.
| References | Model | Technique | Findings Potentially Relevant to Pulmonary Hypertension (PH) |
|---|---|---|---|
| [251] | Pre-clinical Hypoxic-induced PH |
Electroacupuncture (EA) | Mean pulmonary arterial pressure (mPAP) ↓, right ventricular (RV) size ↓ Pathological pulmonary remodeling ↓ Serum/lung endothelial nitric oxide synthase (eNOS) ↑, serum/lung endothelin-1 (ET-1) ↓ |
| [253,297] | Pre-clinical Hypertension |
EA Non-EA |
Sympathetic activity (e.g., via nitric oxide synthase (NOS) pathways) ↓ Serum norepinephrine ↓ Serum interleukins/C-reactive protein ↓ Serum ET-1 ↓, myocardial eNOS ↑ |
| [256,257] | Clinical Hypertension |
Non-EA + enhanced external counterpulsation (EECP) | Serum nitric oxide (NO) ↑, serum ET-1 ↓ |
| [260,261,262,263,264,265,266] | Clinical Chronic obstructive pulmonary disease (COPD) |
EA Non-EA |
Oxygen utilization/efficiency ↑, dyspnea ↓, exercise capacity ↑ |
| [298,299,300,301,302,303,304] | Pre-clinical Systemic inflammation |
EA Non-EA |
Serum/lung tumor necrosis factor alpha (TNF-α), interleukins ↓ Parasympathetic (vagus) outflow ↑ Ejection fraction ↑ |
| [267,268,269,272] | Pre-clinical Heart failure/cardiac insult |
EA | Sympathetic outflow ↓ Heart function ↑ (i.e., left ventricle ejection fraction ↑, left ventricle size ↓ |
| [271,273,274,275,276,277,278,279,280,281,291,292,294] | Pre-clinical Sympathetically stressed |
EA | Sympathetic outflow ↓ (i.e., via central opioid, corticotropin-releasing hormone (CRH) pathways) Serum CRH, cortisol, norepinephrine, adrenaline ↓ |
| [305] | Clinical Post-surgery secondary to lung cancer |
EA | Arterial oxygen partial pressure (PaO2)/fraction of inspired oxygen (FiO2) ↑ Superoxide dismutase (SOD) activity ↑ Length of hospital stay ↓ |
| [258] | Clinical Systemic sclerosis |
EA | Plasma ET-1 ↓ |
| [306,307,308] | Pre-clinical Lung injury |
EA | Lung SOD activity ↑ Serum/lung cytokines ↓ PaO2 ↑ Lung injury score ↓ |
| [252,309] | Pre-clinical COPD |
EA | Pathological pulmonary remodeling ↓ Lung cytokines ↓ Lung function (i.e., expiratory volume) ↑ |
PH = pulmonary hypertension; EA = electroacupuncture; ET-1 = endothelin-1, NO = nitric oxide, eNOS = endothelial nitric oxide synthase; EECP = enhanced external counterpulsation; COPD = chronic obstructive pulmonary disease; CRH = corticotropin-releasing hormone; PaO2/FiO2 = arterial oxygen pressure/fraction of inspired oxygen; SOD = superoxide dismutase. ↓ indicates a decrease in the stated parameter, while ↑ indicates an increase.
In addition to autonomic dysfunction, acupuncture may ameliorate PH through actions within the lungs. For instance, EA reduces serum levels of the vasoconstrictor ET-1 in patients with systemic sclerosis and systemic hypertension, the former of which is associated with PAH [253,257,258]. As mentioned above, a preclinical study showed EA-mediated reversal of hypoxia-induced PH via the attenuation of elevated mean pulmonary arterial pressure, right ventricular hypertrophy, and pulmonary vascular remodeling and reductions in ET-1 levels [251]. Mounting evidence also shows that neurostimulation techniques such as acupuncture may also reduce inflammation, which is found in various disease pathologies including PH. For instance, multiple studies report that both MA and EA at varying acupoints help reduce serum cytokines—including TNFα, IL-1β, and IL-6—in rat models of endotoxin-mediated inflammation in part through vagal efferents [268,298,299,300,301,302]. Moreover, studies suggest that acupuncture influences the activity of the vagal–adrenal axis through the cholinergic–anti-inflammatory pathway, namely via the activation of the vagus nerve, dopamine release from the adrenal gland, and the suppression of systemic inflammation [268,300,301,310,311]. Lastly, there is evidence suggesting acupuncture’s role in improving pulmonary function and regulating oxidative stress and inflammation. For instance, EA increased lung tissue SOD activity to improve pulmonary lung function in rat models; moreover, EA similarly increased serum SOD activity and improved lung function and recovery in patients recovering from operations due to lung cancer [305,306,312]. Other studies have demonstrated that EA affects multiple signaling pathways to reduce inflammation and oxidative stress in animal models through various other signaling pathways, such as the inhibition of calpain-2 and STAT3 pathways in cardiomyocytes, the activation of local cannabinoid receptors and the inhibition of Toll-like receptor 4 (TLR4)-NF-κB signaling in peripheral immune cells or lung tissue, and the modulation of the nuclear factor erythroid 2-related factor (Nrf2)/heme oxygenase-1 (HO-1) pathway [268,302,303,304,306,307,308]. There is also evidence that acupuncture reduces the expression of genes related to oxidative stress and inflammation as well, limiting ROS and cytokine production, albeit in animal models with varying conditions like inflammation and ischemia-induced hypoxia [303,313,314]. Other studies indicate that acupuncture could also mediate immune cell migration in lung tissue [309,315]. Figure 2 below diagrams the mechanisms this section details.
Figure 2.
Potential mechanisms of acupuncture-mediated neurostimulation for pulmonary hypertension (PH).
Acupuncture helps manage multiple other diseases. Clinically, care teams use acupuncture to help manage various types of pain, especially chronic pain [316]; however, there are also studies suggesting that acupuncture could be beneficial in managing symptoms in diabetes [317,318], depression and sleep disorders [319,320], hypertension, cardiovascular problems, and other conditions listed above. A considerable number of the studies listed are pre-clinical; hence, more investigations are needed to establish acupuncture’s efficacy in patients affected by PH, given acupuncture’s potential role in mediating pulmonary vascular remodeling, autonomic dysfunction, and inflammation, as mentioned in studies above.
6. Conclusions
PH is a complex disease that affects millions of people globally. While studies have revealed multiple pharmacological treatments alleviating suffering and mechanisms associated with the disease, furthser investigations would be beneficial in exploring both emerging therapies and complementary non-pharmacological treatments. Moreover, given that PH is on the rise in part due to the greater utilization of diagnostics [26,27], more studies are necessary for care teams to offer preventive and potentially curative treatments for PH.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The authors S. Tjen-A-Looi and S. Malik are National Center for Complementary and Integrative Health (NCCIH) Research Project Grant (RO1) AT011306 award recipients.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Humbert M., Kovacs G., Hoeper M.M., Badagliacca R., Berger R.M., Brida M., Carlsen J., Coats A.J., Escribano-Subias P., Ferrari P., et al. 2022 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Respir. J. 2022;61:2200879. doi: 10.1183/13993003.00879-2022. [DOI] [PubMed] [Google Scholar]
- 2.Simonneau G., Montani D., Celermajer D.S., Denton C.P., Gatzoulis M.A., Krowka M., Williams P.G., Souza R. Haemo-dynamic definitions and updated clinical classification of pulmonary hypertension. Eur. Respir. J. 2019;53:1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoeper M.M., Ghofrani H.A., Grünig E., Klose H., Olschewski H., Rosenkranz S. Pulmonary Hypertension. Dtsch. Arztebl. Int. 2017;114:73–84. doi: 10.3238/arztebl.2016.0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barst R.J., Chung L., Zamanian R.T., Turner M., McGoon M.D. Functional Class Improvement and 3-Year Survival Outcomes in Patients with Pulmonary Arterial Hypertension in the REVEAL Registry. Chest. 2013;144:160–168. doi: 10.1378/chest.12-2417. [DOI] [PubMed] [Google Scholar]
- 5.Sitbon O., Humbert M., Nunes H., Parent F., Garcia G., Hervé P., Rainisio M., Simonneau G. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: Prognostic factors and survival. J. Am. Coll. Cardiol. 2002;40:780–788. doi: 10.1016/S0735-1097(02)02012-0. [DOI] [PubMed] [Google Scholar]
- 6.Hoeper M.M., Humbert M., Souza R., Idrees M., Kawut S.M., Sliwa-Hahnle K., Jing Z.-C., Gibbs J.S.R. A global view of pulmonary hypertension. Lancet Respir. Med. 2016;4:306–322. doi: 10.1016/S2213-2600(15)00543-3. [DOI] [PubMed] [Google Scholar]
- 7.Zlotnick D.M., Ouellette M.L., Malenka D.J., DeSimone J.P., Leavitt B.J., Helm R.E., Olmstead E.M., Costa S.P., DiScipio A.W., Likosky D.S., et al. Effect of Preoperative Pulmonary Hypertension on Outcomes in Patients With Severe Aortic Stenosis Following Surgical Aortic Valve Replacement. Am. J. Cardiol. 2013;112:1635–1640. doi: 10.1016/j.amjcard.2013.07.025. [DOI] [PubMed] [Google Scholar]
- 8.Tichelbäcker T., Dumitrescu D., Gerhardt F., Stern D., Wissmüller M., Adam M., Schmidt T., Frerker C., Pfister R., Halbach M., et al. Pulmonary hypertension and valvular heart disease. Herz. 2019;44:491–501. doi: 10.1007/s00059-019-4823-6. [DOI] [PubMed] [Google Scholar]
- 9.Ovchinnikov A., Potekhina A., Belyavskiy E., Ageev F. Heart Failure with Preserved Ejection Fraction and Pulmonary Hypertension: Focus on Phosphodiesterase Inhibitors. Pharmaceuticals. 2022;15:1024. doi: 10.3390/ph15081024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mocumbi A., Humbert M., Saxena A., Jing Z.-C., Sliwa K., Thienemann F., Archer S.L., Stewart S. Pulmonary hypertension. Nat. Rev. Dis. Prim. 2024;10:1. doi: 10.1038/s41572-023-00486-7. [DOI] [PubMed] [Google Scholar]
- 11.Thienemann F., Dzudie A., Mocumbi A.O., Blauwet L., Sani M.U., Karaye K.M., Ogah O.S., Mbanze I., Mbakwem A., Udo P., et al. The causes, treatment, and outcome of pulmonary hypertension in Africa: Insights from the Pan African Pulmonary Hypertension Cohort (PAPUCO) Registry. Int. J. Cardiol. 2016;221:205–211. doi: 10.1016/j.ijcard.2016.06.242. [DOI] [PubMed] [Google Scholar]
- 12.Katoto P.D.M.C., Mukasa S.L., Sani M.U., Karaye K.M., Mbanze I., Damasceno A., Mocumbi A.O., Dzudie A., Sliwa K., Thienemann F. HIV status and survival of patients with pulmonary hypertension due to left heart disease: The Pan African Pulmonary Hypertension Cohort. Sci. Rep. 2023;13:9790. doi: 10.1038/s41598-023-36375-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schikowski E.M., Swabe G., Chan S.Y., Magnani J.W. Association between income and likelihood of right heart catheterization in individuals with pulmonary hypertension: A US claims database analysis. Pulm. Circ. 2022;12:e12132. doi: 10.1002/pul2.12132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harikrishnan S., Mani A., Sanjau G., Ashishkumar M., Menon J., Rajesh G., Kumar R.K., Koshy A.G., Attacheril T.V., George R., et al. Pulmonary Hypertension Registry of Kerala, India (PRO-KERALA): One-year outcomes. Indian Heart J. 2021;74:34–39. doi: 10.1016/j.ihj.2021.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talwar A., Sahni S., Talwar A., Kohn N., Klinger J.R. Socioeconomic Status Affects Pulmonary Hypertension Disease Severity at Time of First Evaluation. Pulm. Circ. 2016;6:191–195. doi: 10.1086/686489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Caravita S., Baratto C., Di Marco F., Calabrese A., Balestrieri G., Russo F., Faini A., Soranna D., Perego G.B., Badano L.P., et al. Haemodynamic characteristics of COVID-19 patients with acute respiratory distress syndrome requiring mechanical ventilation. An invasive assessment using right heart catheterization. Eur. J. Hear Fail. 2020;22:2228–2237. doi: 10.1002/ejhf.2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pagnesi M., Baldetti L., Beneduce A., Calvo F., Gramegna M., Pazzanese V., Ingallina G., Napolano A., Finazzi R., Ruggeri A., et al. Pulmonary hypertension and right ventricular involvement in hospitalised patients with COVID-19. Heart. 2020;106:1324–1331. doi: 10.1136/heartjnl-2020-317355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Charif F., Dakroub F., Akl I.B., Kularatne M., Montani D. Pulmonary arterial hypertension and COVID-19: Piecing the puzzle. Respir. Med. Res. 2023;84:101053. doi: 10.1016/j.resmer.2023.101053. [DOI] [PubMed] [Google Scholar]
- 19.Tudoran C., Tudoran M., Lazureanu V.E., Marinescu A.R., Pop G.N., Pescariu A.S., Enache A., Cut T.G. Evidence of Pulmonary Hypertension after SARS-CoV-2 Infection in Subjects without Previous Significant Cardiovascular Pathology. J. Clin. Med. 2021;10:199. doi: 10.3390/jcm10020199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan A.W., Ullah I., Khan K.S., Tahir M.J., Masyeni S., Harapan H. Pulmonary arterial hypertension post COVID-19: A sequala of SARS-CoV-2 infection? Respir. Med. Case Rep. 2021;33:101429. doi: 10.1016/j.rmcr.2021.101429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scudiero F., Silverio A., Di Maio M., Russo V., Citro R., Personeni D., Cafro A., D′Andrea A., Attena E., Pezzullo S., et al. Pulmonary embolism in COVID-19 patients: Prevalence, predictors and clinical outcome. Thromb. Res. 2020;198:34–39. doi: 10.1016/j.thromres.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vlachou M., Drebes A., Candilio L., Weeraman D., Mir N., Murch N., Davies N., Coghlan J.G. Pulmonary thrombosis in Covid-19: Before, during and after hospital admission. J. Thromb. Thrombolysis. 2021;51:978–984. doi: 10.1007/s11239-020-02370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tudoran C., Tudoran M., Cut T.G., Lazureanu V.E., Oancea C., Marinescu A.R., Pescariu S.A., Pop G.N., Bende F. Evolution of Echocardiographic Abnormalities Identified in Previously Healthy Individuals Recovering from COVID-19. J. Pers. Med. 2022;12:46. doi: 10.3390/jpm12010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: A review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 25.Satterfield B.A., Bhatt D.L., Gersh B.J. Cardiac involvement in the long-term implications of COVID-19. Nat. Rev. Cardiol. 2021;19:332–341. doi: 10.1038/s41569-021-00631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wijeratne D.T., Lajkosz K., Brogly S.B., Lougheed M.D., Jiang L., Housin A., Barber D., Johnson A., Doliszny K.M., Archer S.L. Increasing Incidence and Prevalence of World Health Organization Groups 1 to 4 Pulmonary Hypertension: A Population-Based Cohort Study in Ontario, Canada. Circ. Cardiovasc. Qual. Outcomes. 2018;11:e003973. doi: 10.1161/CIRCOUTCOMES.117.003973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NHS Digital National Audit of Pulmonary Hypertension 10th Annual Report, Great Britain, 2018–2019. [(accessed on 15 August 2024)]. Available online: https://digital.nhs.uk/data-and-information/publications/statistical/national-pulmonary-hypertension-audit/2019#.
- 28.Zozaya N., Abdalla F., Moreno I.C., Crespo-Diz C., Gallardo A.M.R., Soriano J.R., Galán M.A., Hidalgo-Vega A. The economic burden of pulmonary arterial hypertension in Spain. BMC Pulm. Med. 2022;22:105. doi: 10.1186/s12890-022-01906-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Burger C.D., Ghandour M., Menon D.P., Helmi H., Benza R.L. Early intervention in the management of pulmonary arterial hypertension: Clinical and economic outcomes. Clin. Outcomes Res. 2017;9:731–739. doi: 10.2147/CEOR.S119117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bergot E., De Leotoing L., Bendjenana H., Tournier C., Vainchtock A., Nachbaur G., Humbert M. Hospital burden of pulmonary arterial hypertension in France. PLoS ONE. 2019;14:e0221211. doi: 10.1371/journal.pone.0221211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selexipag (Uptravi) for pulmonary arterial hypertension. Med. Lett. Drugs Ther. 2016;58:21–23. [PubMed] [Google Scholar]
- 32.Sikirica M., Iorga S.R., Bancroft T., Potash J. The economic burden of pulmonary arterial hypertension (PAH) in the US on payers and patients. BMC Heal Serv. Res. 2014;14:676. doi: 10.1186/s12913-014-0676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogbomo A., Tsang Y., Mallampati R., Panjabi S. The direct and indirect health care costs associated with pulmonary arterial hypertension among commercially insured patients in the United States. J. Manag. Care Spéc. Pharm. 2022;28:608–616. doi: 10.18553/jmcp.2022.28.6.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherif A.A., Gilvaz V.J., Abraham S., Saji A.M., Mathew D., Isath A., Rajendran A., Contreras J., Lanier G.M., Reginato A.M. Systemic sclerosis is associated with increased in-patient mortality in patients hospitalized for heart failure. ESC Hear Fail. 2024;11:1900–1910. doi: 10.1002/ehf2.14457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Said Q., Martin B.C., Joish V.N., Kreilick C., Mathai S.C. The cost to managed care of managing pulmonary hypertension. J. Med. Econ. 2012;15:500–508. doi: 10.3111/13696998.2012.665109. [DOI] [PubMed] [Google Scholar]
- 36.Weiss T., Near A.M., Zhao X., Ramey D.R., Banerji T., Xie H., Nathan S.D. Healthcare resource utilization in patients with pulmonary hypertension associated with chronic obstructive pulmonary disease (PH-COPD): A real-world data analysis. BMC Pulm. Med. 2023;23:455. doi: 10.1186/s12890-023-02698-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delcroix M., Howard L. Pulmonary arterial hypertension: The burden of disease and impact on quality of life. Eur. Respir. Rev. 2015;24:621–629. doi: 10.1183/16000617.0063-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fuge J., Park D.-H., von Lengerke T., Richter M.J., Gall H., Ghofrani H.A., Kamp J.C., Hoeper M.M., Olsson K.M. Impact of Pulmonary Arterial Hypertension on Employment, Work Productivity, and Quality of Life—Results of a Cross-Sectional Multi-Center Study. Front. Psychiatry. 2022;12:781532. doi: 10.3389/fpsyt.2021.781532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Runheim H., Kjellström B., Beaudet A., Ivarsson B., Husberg M., Pillai N., Levin L., Bernfort L. Societal costs associated with pulmonary arterial hypertension: A study utilizing linked national registries. Pulm. Circ. 2023;13:e12190. doi: 10.1002/pul2.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kjellström B., Runheim H., Beaudet A., Husberg M., Ivarsson B., Pillai N., Levin L., Bernfort L. Societal costs associated to chronic thromboembolic pulmonary hypertension: A study utilizing linked national registries. Pulm. Circ. 2023;13:e12254. doi: 10.1002/pul2.12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eskandarian R., Sakhdari M.S., Yarmohamadi M., Mirmohammadkhani M. Pulmonary Artery Pressure Status in Hemodialysis Patients and Its Association with Nutritional and Biochemical Markers. Middle East J. Rehabil. Health Stud. 2023;11:e129698. doi: 10.5812/mejrh-129698. [DOI] [Google Scholar]
- 42.Mukhtar K.N., Mohkumuddin S., Mahmood S.N. Frequency of Pulmonary Hypertension in hemodialysis patients. Pak. J. Med. Sci. 2014;30:1319–1322. doi: 10.12669/pjms.306.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tudoran C., Tudoran M., Ciocarlie T., Mates A., Pescariu S., AbuAwwad A. Pulmonary hypertension in patients with end stage renal disease undergoing hemodialysis. Niger. J. Clin. Pr. 2020;23:198–204. doi: 10.4103/njcp.njcp_278_19. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y., Ding X.-H., Rao R., Wang Y., Pang F., Tang S., Nie L., Bian S.-Z. The Prevalence of Pulmonary Hypertension Among Maintenance Dialysis Patients with ESRD and Its Associated Factors: A Retrospective Study. Front. Med. 2020;7:570874. doi: 10.3389/fmed.2020.570874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lang I.M., Palazzini M. The burden of comorbidities in pulmonary arterial hypertension. Eur. Heart J. Suppl. 2019;21:K21–K28. doi: 10.1093/eurheartj/suz205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marvisi M., Balzarini L., Mancini C., Mouzakiti P. Thyroid gland and pulmonary hypertension. What’s the link? Panminerva Med. 2013;55:93–97. [PubMed] [Google Scholar]
- 47.Ghofrani H.-A., Galiè N., Grimminger F., Grünig E., Humbert M., Jing Z.-C., Keogh A.M., Langleben D., Kilama M.O., Fritsch A., et al. Riociguat for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2013;369:330–340. doi: 10.1056/NEJMoa1209655. [DOI] [PubMed] [Google Scholar]
- 48.Montani D., Günther S., Dorfmüller P., Perros F., Girerd B., Garcia G., Jaïs X., Savale L., Artaud-Macari E., Price L.C., et al. Pulmonary arterial hypertension. Orphanet J. Rare Dis. 2013;8:97. doi: 10.1186/1750-1172-8-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanwar M.K., Tedford R.J., Thenappan T., De Marco T., Park M., McLaughlin V. Elevated Pulmonary Pressure Noted on Echocardiogram: A Simplified Approach to Next Steps. J. Am. Hear Assoc. 2021;10:e017684. doi: 10.1161/JAHA.120.017684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guazzi M., Dixon D., Labate V., Beussink-Nelson L., Bandera F., Cuttica M.J., Shah S.J. RV Contractile Function and its Coupling to Pulmonary Circulation in Heart Failure with Preserved Ejection Fraction: Stratification of Clinical Phenotypes and Outcomes. JACC Cardiovasc. Imaging. 2017;10:1211–1221. doi: 10.1016/j.jcmg.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 51.Tello K., Axmann J., Ghofrani H.A., Naeije R., Narcin N., Rieth A., Seeger W., Gall H., Richter M.J. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int. J. Cardiol. 2018;266:229–235. doi: 10.1016/j.ijcard.2018.01.053. [DOI] [PubMed] [Google Scholar]
- 52.Rich S., Dantzker D.R., Ayres S.M., Bergofsky E.H., Brundage B.H., Detre K.M., Fishman A.P., Goldring R.M., Groves B.M., Koerner S.K., et al. Primary Pulmonary Hypertension. A national prospective study. Ann. Intern. Med. 1987;107:216–223. doi: 10.7326/0003-4819-107-2-216. [DOI] [PubMed] [Google Scholar]
- 53.Henkens I.R., Mouchaers K.T.B., Vonk-Noordegraaf A., Boonstra A., Swenne C.A., Maan A.C., Man S.-C., Twisk J.W.R., van der Wall E.E., Schalij M.J., et al. Improved ECG detection of presence and severity of right ventricular pressure load validated with cardiac magnetic resonance imaging. Am. J. Physiol. Circ. Physiol. 2008;294:H2150–H2157. doi: 10.1152/ajpheart.01312.2007. [DOI] [PubMed] [Google Scholar]
- 54.Wanamaker B., Cascino T., McLaughlin V., Oral H., Latchamsetty R., Siontis K.C. Atrial Arrhythmias in Pulmonary Hypertension: Pathogenesis, Prognosis and Management. Arrhythmia Electrophysiol. Rev. 2018;7:43–48. doi: 10.15420/aer.2018.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Diller G.-P., Gatzoulis M.A. Pulmonary Vascular Disease in Adults with Congenital Heart Disease. Circulation. 2007;115:1039–1050. doi: 10.1161/CIRCULATIONAHA.105.592386. [DOI] [PubMed] [Google Scholar]
- 56.McLaughlin V.V., Archer S.L., Badesch D.B., Barst R.J., Farber H.W., Lindner J.R., Mathier M.A., McGoon M.D., Park M.H., Rosenson R.S., et al. American College of Cardiology Foundation Task Force on Expert Consensus Documents, American Heart Association, American College of Chest Physicians, American Thoracic Society, Inc, & Pulmonary Hypertension Association (2009). ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association developed in collaboration with the American College of Chest Physicians; American Thoracic Society, Inc.; and the Pulmonary Hypertension Association. J. Am. Coll. Cardiol. 2009;53:1573–1619. doi: 10.1016/j.jacc.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 57.Warwick G., Thomas P.S., Yates D.H. Biomarkers in pulmonary hypertension. Eur. Respir. J. 2008;32:503–512. doi: 10.1183/09031936.00160307. [DOI] [PubMed] [Google Scholar]
- 58.Pfeuffer E., Krannich H., Halank M., Wilkens H., Kolb P., Jany B., Held M. Anxiety, Depression, and Health-Related QOL in Patients Diagnosed with PAH or CTEPH. Lung. 2017;195:759–768. doi: 10.1007/s00408-017-0052-z. [DOI] [PubMed] [Google Scholar]
- 59.Harzheim D., Klose H., Pinado F.P., Ehlken N., Nagel C., Fischer C., Ghofrani A., Rosenkranz S., Seyfarth H.-J., Halank M., et al. Anxiety and depression disorders in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Respir. Res. 2013;14:104. doi: 10.1186/1465-9921-14-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tuder R.M., Abman S.H., Braun T., Capron F., Stevens T., Thistlethwaite P.A., Haworth S.G. Development and Pathology of Pulmonary Hypertension. J. Am. Coll. Cardiol. 2009;54:S3–S9. doi: 10.1016/j.jacc.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 61.Stacher E., Graham B.B., Hunt J.M., Gandjeva A., Groshong S.D., McLaughlin V.V., Jessup M., Grizzle W.E., Aldred M.A., Cool C.D., et al. Modern Age Pathology of Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2012;186:261–272. doi: 10.1164/rccm.201201-0164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chazova I.E., Loyd J., Zhdanov V.S., Newman J.H., Belenkov Y., Meyrick B. Pulmonary artery adventitial changes and venous involvement in primary pulmonary hypertension. Am. J. Pathol. 1995;146:389–397. [PMC free article] [PubMed] [Google Scholar]
- 63.Cool C.D., Stewart J.S., Werahera P., Miller G.J., Williams R.L., Voelkel N.F., Tuder R.M. Three-Dimensional Reconstruction of Pulmonary Arteries in Plexiform Pulmonary Hypertension Using Cell-Specific Markers. Evidence for a dynamic and heterogeneous process of pulmonary endothelial cell growth. Am. J. Pathol. 1999;155:411–419. doi: 10.1016/S0002-9440(10)65137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Al-Omary M.S., Sugito S., Boyle A.J., Sverdlov A.L., Collins N.J. Pulmonary Hypertension Due to Left Heart Disease. Hypertension. 2020;75:1397–1408. doi: 10.1161/HYPERTENSIONAHA.119.14330. [DOI] [PubMed] [Google Scholar]
- 65.Valentini A., Franchi P., Cicchetti G., Messana G., Chiffi G., Strappa C., Calandriello L., del Ciello A., Farchione A., Preda L., et al. Pulmonary Hypertension in Chronic Lung Diseases: What Role Do Radiologists Play? Diagnostics. 2023;13:1607. doi: 10.3390/diagnostics13091607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stenmark K.R., Gerasimovskaya E., Nemenoff R.A., Das M. Hypoxic activation of adventitial fibroblasts: Role in vascular remodeling. Chest. 2002;122:326S–334S. doi: 10.1378/chest.122.6_suppl.326S. [DOI] [PubMed] [Google Scholar]
- 67.Davie N.J., Crossno J.T., Frid M.G., Hofmeister S.E., Reeves J.T., Hyde D.M., Carpenter T.C., Brunetti J.A., McNiece I.K., Stenmark K.R., et al. Hypoxia-induced pulmonary artery adventitial remodeling and neovascularization: Contribution of progenitor cells. Am. J. Physiol. Cell Mol. Physiol. 2004;286:L668–L678. doi: 10.1152/ajplung.00108.2003. [DOI] [PubMed] [Google Scholar]
- 68.Tuder R.M. Pulmonary vascular remodeling in pulmonary hypertension. Cell Tissue Res. 2016;367:643–649. doi: 10.1007/s00441-016-2539-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Delcroix M., Torbicki A., Gopalan D., Sitbon O., Klok F.A., Lang I., Jenkins D., Kim N.H., Humbert M., Jais X., et al. ERS statement on chronic thromboembolic pulmonary hypertension. Eur. Respir. J. 2020;57:2002828. doi: 10.1183/13993003.02828-2020. [DOI] [PubMed] [Google Scholar]
- 70.Giaid A., Yanagisawa M., Langleben D., Michel R.P., Levy R., Shennib H., Kimura S., Masaki T., Duguid W.P., Stewart D.J. Expression of Endothelin-1 in the Lungs of Patients with Pulmonary Hypertension. N. Engl. J. Med. 1993;328:1732–1739. doi: 10.1056/NEJM199306173282402. [DOI] [PubMed] [Google Scholar]
- 71.Reesink H.J., Meijer R.C., Lutter R., Boomsma F., Jansen H.M., Kloek J.J., Bresser P. Hemodynamic and Clinical Correlates of Endothelin-1 in Chronic Thromboembolic Pulmonary Hypertension. Circ. J. 2006;70:1058–1063. doi: 10.1253/circj.70.1058. [DOI] [PubMed] [Google Scholar]
- 72.Goerre S., Wenk M., Bärtsch P., Lüscher T.F., Niroomand F., Hohenhaus E., Oelz O., Reinhart W.H. Endothelin-1 in Pulmonary Hypertension Associated with High-Altitude Exposure. Circulation. 1995;91:359–364. doi: 10.1161/01.CIR.91.2.359. [DOI] [PubMed] [Google Scholar]
- 73.Cacoub P., Dorent R., Nataf P., Carayon A., Riquet M., Noe E., Piette J.C., Godeau P., Gandjbakhch I. Endothelin-1 in the lungs of patients with pulmonary hypertension. Cardiovasc. Res. 1997;33:196–200. doi: 10.1016/S0008-6363(96)00189-7. [DOI] [PubMed] [Google Scholar]
- 74.Galié N., Manes A., Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovasc. Res. 2004;61:227–237. doi: 10.1016/j.cardiores.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 75.Liu R., Yuan T., Wang R., Gong D., Wang S., Du G., Fang L. Insights into Endothelin Receptors in Pulmonary Hypertension. Int. J. Mol. Sci. 2023;24:10206. doi: 10.3390/ijms241210206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brewster L.M.M., Garcia V.P., Levy M.V., Stockelman K.A., Goulding A., DeSouza N.M., Greiner J.J., Hijmans J.G., DeSouza C.A. Endothelin-1-induced endothelial microvesicles impair endothelial cell function. J. Appl. Physiol. 2020;128:1497–1505. doi: 10.1152/japplphysiol.00816.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Marsden P.A., Danthuluri N.R., Brenner B.M., Ballermann B.J., Brock T.A. Endothelin action on vascular smooth muscle involves inositol trisphosphate and calcium mobilization. Biochem. Biophys. Res. Commun. 1989;158:86–93. doi: 10.1016/S0006-291X(89)80180-9. [DOI] [PubMed] [Google Scholar]
- 78.Zhang Y.-M., Wang K.-Q., Zhou G.-M., Zuo J., Ge J.-B. Endothelin-1 promoted proliferation of vascular smooth muscle cell through pathway of extracellular signal-regulated kinase and cyclin D1. Acta Pharmacol. Sin. 2003;24:563–568. [PubMed] [Google Scholar]
- 79.Douglas S.A., Ohlstein E.H. Signal Transduction Mechanisms Mediating the Vascular Actions of Endothelin. J. Vasc. Res. 1997;34:152–164. doi: 10.1159/000159219. [DOI] [PubMed] [Google Scholar]
- 80.Simonson M.S., Wann S., Mené P., Dubyak G.R., Kester M., Nakazato Y., Sedor J.R., Dunn M.J. Endothelin stimulates phospholipase C, Na+/H+ exchange, c-fos expression, and mitogenesis in rat mesangial cells. J. Clin. Investig. 1989;83:708–712. doi: 10.1172/JCI113935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koller B., Steringer-Mascherbauer R., Ebner C., Weber T., Ammer M., Eichinger J., Pretsch I., Herold M., Schwaiger J., Ulmer H., et al. Pilot Study of Endothelin Receptor Blockade in Heart Failure with Diastolic Dysfunction and Pulmonary Hypertension (BADDHY-Trial) Hear Lung Circ. 2017;26:433–441. doi: 10.1016/j.hlc.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 82.Vachiéry J.-L., Delcroix M., Al-Hiti H., Efficace M., Hutyra M., Lack G., Papadakis K., Rubin L.J. Macitentan in pulmonary hypertension due to left ventricular dysfunction. Eur. Respir. J. 2018;51:1701886. doi: 10.1183/13993003.01886-2017. [DOI] [PubMed] [Google Scholar]
- 83.Packer M., McMurray J.J., Krum H., Kiowski W., Massie B.M., Caspi A., Pratt C.M., Petrie M.C., DeMets D., Kobrin I., et al. Long-Term Effect of Endothelin Receptor Antagonism with Bosentan on the Morbidity and Mortality of Patients with Severe Chronic Heart Failure. Primary Results of the ENABLE Trials. JACC Hear Fail. 2017;5:317–326. doi: 10.1016/j.jchf.2017.02.021. [DOI] [PubMed] [Google Scholar]
- 84.Park J., Song J.H., Park D.-A., Lee J.S., Lee S.-D., Oh Y.-M. Systematic Review and Meta-Analysis of Pulmonary Hypertension Specific Therapy for Exercise Capacity in Chronic Obstructive Pulmonary Disease. J. Korean Med. Sci. 2013;28:1200–1206. doi: 10.3346/jkms.2013.28.8.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rubin L.J., Badesch D.B., Barst R.J., Galiè N., Black C.M., Keogh A., Pulido T., Frost A., Roux S., Leconte I., et al. Bosentan Therapy for Pulmonary Arterial Hypertension. N. Engl. J. Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 86.Galiè N., Rubin L., Hoeper M., Jansa P., Al-Hiti H., Meyer G., Chiossi E., Kusic-Pajic A., Simonneau G. Treatment of patients with mildly symptomatic pulmonary arterial hypertension with bosentan (EARLY study): A double-blind, randomised controlled trial. Lancet. 2008;371:2093–2100. doi: 10.1016/S0140-6736(08)60919-8. [DOI] [PubMed] [Google Scholar]
- 87.Pulido T., Adzerikho I., Channick R.N., Delcroix M., Galiè N., Ghofrani A., Jansa P., Jing Z.-C., Le Brun F.-O., Mehta S., et al. Macitentan and Morbidity and Mortality in Pulmonary Arterial Hypertension. N. Engl. J. Med. 2013;369:809–818. doi: 10.1056/NEJMoa1213917. [DOI] [PubMed] [Google Scholar]
- 88.Galiè N., Olschewski H., Oudiz R.J., Torres F., Frost A., Ghofrani H.A., Badesch D.B., McGoon M.D., McLaughlin V.V., Roecker E.B., et al. Ambrisentan for the treatment of pulmonary arterial hypertension: Results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation. 2008;117:3010–3019. doi: 10.1161/CIRCULATIONAHA.107.742510. [DOI] [PubMed] [Google Scholar]
- 89.Lumb A.B., Slinger P. Hypoxic Pulmonary Vasoconstriction. Anesthesiology. 2015;122:932–946. doi: 10.1097/ALN.0000000000000569. [DOI] [PubMed] [Google Scholar]
- 90.Giaid A., Saleh D. Reduced Expression of Endothelial Nitric Oxide Synthase in the Lungs of Patients with Pulmonary Hypertension. N. Engl. J. Med. 1995;333:214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 91.Wharton J., Strange J.W., Møller G.M.O., Growcott E.J., Ren X., Franklyn A.P., Phillips S.C., Wilkins M.R. Antiproliferative Effects of Phosphodiesterase Type 5 Inhibition in Human Pulmonary Artery Cells. Am. J. Respir. Crit. Care Med. 2005;172:105–113. doi: 10.1164/rccm.200411-1587OC. [DOI] [PubMed] [Google Scholar]
- 92.Galiè N., Ghofrani H.A., Torbicki A., Barst R.J., Rubin L.J., Badesch D., Fleming T., Parpia T., Burgess G., Branzi A., et al. Sildenafil Citrate Therapy for Pulmonary Arterial Hypertension. N. Engl. J. Med. 2005;353:2148–2157. doi: 10.1056/NEJMoa050010. [DOI] [PubMed] [Google Scholar]
- 93.Galiè N., Brundage B.H., Ghofrani H.A., Oudiz R.J., Simonneau G., Safdar Z., Shapiro S., White R.J., Chan M., Beardsworth A., et al. Tadalafil Therapy for Pulmonary Arterial Hypertension. Circulation. 2009;119:2894–2903. doi: 10.1161/CIRCULATIONAHA.108.839274. [DOI] [PubMed] [Google Scholar]
- 94.Ghofrani H.-A., D’Armini A.M., Grimminger F., Hoeper M.M., Jansa P., Kim N.H., Mayer E., Simonneau G., Wilkins M.R., Fritsch A., et al. Riociguat for the Treatment of Chronic Thromboembolic Pulmonary Hypertension. N. Engl. J. Med. 2013;369:319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 95.Tuder R.M., Cool C.D., Geraci M.W., Wang J., Abman S.H., Wright L., Badesch D., Voelkel N.F. Prostacyclin Synthase Expression Is Decreased in Lungs from Patients with Severe Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 1999;159:1925–1932. doi: 10.1164/ajrccm.159.6.9804054. [DOI] [PubMed] [Google Scholar]
- 96.Barst R.J., Rubin L.J., Long W.A., McGoon M.D., Rich S., Badesch D.B., Groves B.M., Tapson V.F., Bourge R.C., Brundage B.H., et al. A Comparison of Continuous Intravenous Epoprostenol (Prostacyclin) with Conventional Therapy for Primary Pulmonary Hypertension. N. Engl. J. Med. 1996;334:296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 97.Badesch D.B., Tapson V.F., McGoon M.D., Brundage B.H., Rubin L.J., Wigley F.M., Rich S., Barst R.J., Barrett P.S., Kral K.M., et al. Continuous Intravenous Epoprostenol for Pulmonary Hypertension Due to the Scleroderma Spectrum of Disease. Ann. Intern. Med. 2000;132:425–434. doi: 10.7326/0003-4819-132-6-200003210-00002. [DOI] [PubMed] [Google Scholar]
- 98.Simonneau G., Barst R.J., Galie N., Naeije R., Rich S., Bourge R.C., Keogh A., Oudiz R., Frost A., Blackburn S.D., et al. Continuous subcutaneous infusion of treprostinil, a prostacyclin analogue, in patients with pulmonary arterial hypertension: A double-blind, randomized, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 2002;165:800–804. doi: 10.1164/ajrccm.165.6.2106079. [DOI] [PubMed] [Google Scholar]
- 99.McLaughlin V.V., Benza R.L., Rubin L.J., Channick R.N., Voswinckel R., Tapson V.F., Robbins I.M., Olschewski H., Rubenfire M., Seeger W. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: A randomized controlled clinical trial. J. Am. Coll. Cardiol. 2010;55:1915–1922. doi: 10.1016/j.jacc.2010.01.027. [DOI] [PubMed] [Google Scholar]
- 100.Tapson V.F., Jing Z.-C., Xu K.-F., Pan L., Feldman J., Kiely D.G., Kotlyar E., McSwain C.S., Laliberte K., Arneson C., et al. Oral Treprostinil for the Treatment of Pulmonary Arterial Hypertension in Patients Receiving Background Endothelin Receptor Antagonist and Phosphodiesterase Type 5 Inhibitor Therapy (The FREEDOM-C2 Study) Chest. 2013;144:952–958. doi: 10.1378/chest.12-2875. [DOI] [PubMed] [Google Scholar]
- 101.Tapson V.F., Torres F., Kermeen F., Keogh A.M., Allen R.P., Frantz R.P., Badesch D.B., Frost A.E., Shapiro S.M., Laliberte K., et al. Oral Treprostinil for the Treatment of Pulmonary Arterial Hypertension in Patients on Background Endothelin Receptor Antagonist and/or Phosphodiesterase Type 5 Inhibitor Therapy (The FREEDOM-C Study): A randomized controlled trial. Chest. 2012;142:1383–1390. doi: 10.1378/chest.11-2212. [DOI] [PubMed] [Google Scholar]
- 102.Nathan S.D., Tapson V.F., Elwing J., Rischard F., Mehta J., Shapiro S., Shen E., Deng C., Smith P., Waxman A. Efficacy of Inhaled Treprostinil on Multiple Disease Progression Events in Patients with Pulmonary Hypertension due to Parenchymal Lung Disease in the INCREASE Trial. Am. J. Respir. Crit. Care Med. 2021;205:198–207. doi: 10.1164/rccm.202107-1766OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Waxman A., Restrepo-Jaramillo R., Thenappan T., Ravichandran A., Engel P., Bajwa A., Allen R., Feldman J., Argula R., Smith P., et al. Inhaled Treprostinil in Pulmonary Hypertension Due to Interstitial Lung Disease. N. Engl. J. Med. 2021;384:325–334. doi: 10.1056/NEJMoa2008470. [DOI] [PubMed] [Google Scholar]
- 104.Olschewski H., Simonneau G., Galiè N., Higenbottam T., Naeije R., Rubin L.J., Nikkho S., Speich R., Hoeper M.M., Behr J., et al. Inhaled Iloprost for Severe Pulmonary Hypertension. N. Engl. J. Med. 2002;347:322–329. doi: 10.1056/NEJMoa020204. [DOI] [PubMed] [Google Scholar]
- 105.Simonneau G., Torbicki A., Hoeper M.M., Delcroix M., Karlócai K., Galiè N., Degano B., Bonderman D., Kurzyna M., Efficace M., et al. Selexipag: An oral, selective prostacyclin receptor agonist for the treatment of pulmonary arterial hypertension. Eur. Respir. J. 2012;40:874–880. doi: 10.1183/09031936.00137511. [DOI] [PubMed] [Google Scholar]
- 106.Sitbon O., Channick R., Chin K.M., Frey A., Gaine S., Galiè N., Ghofrani H.-A., Hoeper M.M., Lang I.M., Preiss R., et al. Selexipag for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2015;373:2522–2533. doi: 10.1056/NEJMoa1503184. [DOI] [PubMed] [Google Scholar]
- 107.Galiè N., Humbert M., Vachiéry J.-L., Vizza C., Kneussl M., Manes A., Sitbon O., Torbicki A., Delcroix M., Naeije R., et al. Effects of beraprost sodium, an oral prostacyclin analogue, in patients with pulmonary arterial hypertension: A randomized, double-blind, placebo-controlled trial. J. Am. Coll. Cardiol. 2002;39:1496–1502. doi: 10.1016/S0735-1097(02)01786-2. [DOI] [PubMed] [Google Scholar]
- 108.Barst R.J., McGoon M., McLaughlin V., Tapson V., Oudiz R., Shapiro S., Robbins I.M., Channick R., Badesch D., Rayburn B.K., et al. Beraprost therapy for pulmonary arterial hypertension. J. Am. Coll. Cardiol. 2003;41:2119–2125. doi: 10.1016/S0735-1097(03)00463-7. [DOI] [PubMed] [Google Scholar]
- 109.Sylvester J.T., Shimoda L.A., Aaronson P.I., Ward J.P.T. Hypoxic Pulmonary Vasoconstriction. Physiol. Rev. 2012;92:367–520. doi: 10.1152/physrev.00041.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Green S., Stuart D. Oxygen and pulmonary arterial hypertension: Effects, mechanisms, and therapeutic benefits. Eur. J. Prev. Cardiol. 2020;28:127–136. doi: 10.1093/eurjpc/zwaa001. [DOI] [PubMed] [Google Scholar]
- 111.Rawat M., Lakshminrusimha S., Vento M., Rawat M., Lakshminrusimha S., Vento M. Pulmonary hypertension and oxidative stress: Where is the link? Semin. Fetal Neonatal Med. 2022;27:101347. doi: 10.1016/j.siny.2022.101347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Dunham-Snary K.J., Wu D., Sykes E.A., Thakrar A., Parlow L.R., Mewburn J.D., Parlow J.L., Archer S.L. Hypoxic pulmonary vasoconstriction: From molecular mechanisms to medicine. Chest. 2017;151:181–192. doi: 10.1016/j.chest.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Post J.M., Hume J.R., Archer S.L., Weir E.K. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am. J. Physiol. Physiol. 1992;262:C882–C890. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- 114.McMurtry I.F., Davidson A.B., Reeves J.T., Grover R.F. Inhibition of hypoxic pulmonary vasoconstriction by calcium antagonists in isolated rat lungs. Circ. Res. 1976;38:99–104. doi: 10.1161/01.RES.38.2.99. [DOI] [PubMed] [Google Scholar]
- 115.Rhoades R.A., Packer C.S., Roepke D.A., Jin N., Meiss R.A. Reactive oxygen species alter contractile properties of pulmonary arterial smooth muscle. Can. J. Physiol. Pharmacol. 1990;68:1581–1589. doi: 10.1139/y90-241. [DOI] [PubMed] [Google Scholar]
- 116.Black S.M., Johengen M.J., Soifer S.J. Coordinated Regulation of Genes of the Nitric Oxide and Endothelin Pathways during the Development of Pulmonary Hypertension in Fetal Lambs. Pediatr. Res. 1998;44:821–830. doi: 10.1203/00006450-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 117.Steinhorn R.H., Russell J.A., Morin F.C. Disruption of cGMP production in pulmonary arteries isolated from fetal lambs with pulmonary hypertension. Am. J. Physiol. Circ. Physiol. 1995;268:H1483–H1489. doi: 10.1152/ajpheart.1995.268.4.H1483. [DOI] [PubMed] [Google Scholar]
- 118.Ivy D.D., Ziegler J.W., Dubus M.F., Fox J.J., Kinsella J.P., Abman S.H. Chronic Intrauterine Pulmonary Hypertension Alters Endothelin Receptor Activity in the Ovine Fetal Lung. Pediatr. Res. 1996;39:435–442. doi: 10.1203/00006450-199603000-00010. [DOI] [PubMed] [Google Scholar]
- 119.Bailey D.M., Dehnert C., Luks A.M., Menold E., Castell C., Schendler G., Faoro V., Gutowski M., Evans K.A., Taudorf S., et al. High-altitude pulmonary hypertension is associated with a free radical-mediated reduction in pulmonary nitric oxide bioavailability. J. Physiol. 2010;588:4837–4847. doi: 10.1113/jphysiol.2010.194704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu W., Kaneko F.T., Zheng S., Comhair S.A.A., Janocha A.J., Goggans T., Thunnissen F.B.J.M., Farver C., Hazen S.L., Jennings C., et al. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J. 2004;18:1746–1748. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]
- 121.Block E.R., Herrera H., Couch M. Hypoxia inhibits L-arginine uptake by pulmonary artery endothelial cells. Am. J. Physiol. Cell Mol. Physiol. 1995;269:L574–L580. doi: 10.1152/ajplung.1995.269.5.L574. [DOI] [PubMed] [Google Scholar]
- 122.Heath D., Yacoub M., Gosney J., Madden B., Caslin A., Smith P. Pulmonary endocrine cells in hypertensive pulmonary vascular disease. Histopathology. 1990;16:21–28. doi: 10.1111/j.1365-2559.1990.tb01055.x. [DOI] [PubMed] [Google Scholar]
- 123.Noguchi M., Furukawa K.T., Morimoto M. Pulmonary neuroendocrine cells: Physiology, tissue homeostasis and disease. Dis. Model. Mech. 2020;13:dmm046920. doi: 10.1242/dmm.046920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Tjen-A-Looi S.C., Fu L.W., Nguyen A.T., Gong Y., Malik S. Autonomic Function and Electroacupuncture. In: Xia Y., editor. Advanced Acupuncture Research: From Bench to Bedside. Springer; Cham, Switzerland: 2022. pp. 345–360. [DOI] [Google Scholar]
- 125.Fu X.W., Nurse C.A., Wong V., Cutz E. Hypoxia-induced secretion of serotonin from intact pulmonary neuroepithelial bodies in neonatal rabbit. J. Physiol. 2002;539:503–510. doi: 10.1113/jphysiol.2001.013071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pullamsetti S.S., Mamazhakypov A., Weissmann N., Seeger W., Savai R. Hypoxia-inducible factor signaling in pulmonary hypertension. J. Clin. Investig. 2020;130:5638–5651. doi: 10.1172/JCI137558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hewes J.L., Lee J.Y., Fagan K.A., Bauer N.N. The changing face of pulmonary hypertension diagnosis: A historical perspective on the influence of diagnostics and biomarkers. Pulm. Circ. 2020;10:2045894019892801. doi: 10.1177/2045894019892801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jaitovich A., Jourd’heuil D. A Brief Overview of Nitric Oxide and Reactive Oxygen Species Signaling in Hypoxia-Induced Pulmonary Hypertension. Adv. Exp. Med. Biol. 2017;967:71–81. doi: 10.1007/978-3-319-63245-2_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Brunelle J.K., Bell E.L., Quesada N.M., Vercauteren K., Tiranti V., Zeviani M., Scarpulla R.C., Chandel N.S. Oxygen sensing requires mitochondrial ROS but not oxidative phosphorylation. Cell Metab. 2005;1:409–414. doi: 10.1016/j.cmet.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 130.Guzy R.D., Hoyos B., Robin E., Chen H., Liu L., Mansfield K.D., Simon M.C., Hammerling U., Schumacker P.T. Mitochondrial complex III is required for hypoxia-induced ROS production and cellular oxygen sensing. Cell Metab. 2005;1:401–408. doi: 10.1016/j.cmet.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 131.Mansfield K.D., Guzy R.D., Pan Y., Young R.M., Cash T.P., Schumacker P.T., Simon M.C. Mitochondrial dysfunction resulting from loss of cytochrome c impairs cellular oxygen sensing and hypoxic HIF-α activation. Cell Metab. 2005;1:393–399. doi: 10.1016/j.cmet.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shimoda L.A., Manalo D.J., Sham J.S.K., Semenza G.L., Sylvester J.T. Partial HIF-1α deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am. J. Physiol. Cell Mol. Physiol. 2001;281:L202–L208. doi: 10.1152/ajplung.2001.281.1.L202. [DOI] [PubMed] [Google Scholar]
- 133.Bonnet S., Michelakis E.D., Porter C.J., Andrade-Navarro M.A., Thébaud B., Bonnet S., Haromy A., Harry G., Moudgil R., McMurtry M.S., et al. An abnormal mitochondrial–hypoxia inducible factor-1α–kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: Similarities to human pulmonary arterial hypertension. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 134.Shimoda L.A., Fallon M., Pisarcik S., Wang J., Semenza G.L. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am. J. Physiol. Cell Mol. Physiol. 2006;291:L941–L949. doi: 10.1152/ajplung.00528.2005. [DOI] [PubMed] [Google Scholar]
- 135.Yamashita K., Discher D.J., Hu J., Bishopric N.H., Webster K.A. Molecular Regulation of the endothelin-1 gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, AND p300/CBP. J. Biol. Chem. 2001;276:12645–12653. doi: 10.1074/jbc.M011344200. [DOI] [PubMed] [Google Scholar]
- 136.Tuder R.M., Chacon M., Alger L., Wang J., Taraseviciene-Stewart L., Kasahara Y., Cool C.D., Bishop A.E., Geraci M., Semenza G.L., et al. Expression of angiogenesis-related molecules in plexiform lesions in severe pulmonary hypertension: Evidence for a process of disordered angiogenesis. J. Pathol. 2001;195:367–374. doi: 10.1002/path.953. [DOI] [PubMed] [Google Scholar]
- 137.Alba G.A., Samokhin A.O., Wang R.-S., Zhang Y.-Y., Wertheim B.M., Arons E., Greenfield E.A., Slingsby M.H.L., Ceglowski J.R., Haley K.J., et al. NEDD9 Is a Novel and Modifiable Mediator of Platelet–Endothelial Adhesion in the Pulmonary Circulation. Am. J. Respir. Crit. Care Med. 2021;203:1533–1545. doi: 10.1164/rccm.202003-0719OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chai X., Sun D., Han Q., Yi L., Wu Y., Liu X. Hypoxia induces pulmonary arterial fibroblast proliferation, migration, differentiation and vascular remodeling via the PI3K/Akt/p70S6K signaling pathway. Int. J. Mol. Med. 2018;41:2461–2472. doi: 10.3892/ijmm.2018.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ryanto G.R.T., Ikeda K., Miyagawa K., Tu L., Guignabert C., Humbert M., Fujiyama T., Yanagisawa M., Hirata K.-I., Emoto N. An endothelial activin A-bone morphogenetic protein receptor type 2 link is overdriven in pulmonary hypertension. Nat. Commun. 2021;12:1720. doi: 10.1038/s41467-021-21961-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Merfeld-Clauss S., Lu H., Wu X., March K.L., Traktuev D.O. Hypoxia-induced activin A diminishes endothelial cell vasculogenic activity. J. Cell. Mol. Med. 2017;22:173–184. doi: 10.1111/jcmm.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sandoval J., Aguirre J.S., Pulido T., Martinez-Guerra M.L., Santos E., Alvarado P., Rosas M., Bautista E. Nocturnal Oxygen Therapy in Patients with the Eisenmenger Syndrome. Am. J. Respir. Crit. Care Med. 2001;164:1682–1687. doi: 10.1164/ajrccm.164.9.2106076. [DOI] [PubMed] [Google Scholar]
- 142.Din S., Sarathchandra P., Yacoub M.H., Chester A.H. Interaction between bone morphogenetic proteins and endothelin-1 in human pulmonary artery smooth muscle. Vasc. Pharmacol. 2009;51:344–349. doi: 10.1016/j.vph.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 143.Maruyama H., Dewachter C., Belhaj A., Rondelet B., Sakai S., Remmelink M., Vachiery J.-L., Naeije R., Dewachter L. Endothelin-Bone morphogenetic protein type 2 receptor interaction induces pulmonary artery smooth muscle cell hyperplasia in pulmonary arterial hypertension. J. Hear Lung Transplant. 2015;34:468–478. doi: 10.1016/j.healun.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 144.Soon E., Crosby A., Southwood M., Yang P., Tajsic T., Toshner M., Appleby S., Shanahan C.M., Bloch K.D., Pepke-Zaba J., et al. Bone Morphogenetic Protein Receptor Type II Deficiency and Increased Inflammatory Cytokine Production. A Gateway to Pulmonary Arterial Hypertension. Am. J. Respir. Crit. Care Med. 2015;192:859–872. doi: 10.1164/rccm.201408-1509OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Talati M., West J., Zaynagetdinov R., Hong C.C., Han W., Blackwell T., Robinson L., Blackwell T.S., Lane K. BMP Pathway Regulation of and by Macrophages. PLoS ONE. 2014;9:e94119. doi: 10.1371/journal.pone.0094119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim C.W., Song H., Kumar S., Nam D., Kwon H.S., Chang K.H., Son D.J., Kang D.-W., Brodie S.A., Weiss D., et al. Anti-Inflammatory and Antiatherogenic Role of BMP Receptor II in Endothelial Cells. Arter. Thromb. Vasc. Biol. 2013;33:1350–1359. doi: 10.1161/ATVBAHA.112.300287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sawada H., Saito T., Nickel N.P., Alastalo T.-P., Glotzbach J.P., Chan R., Haghighat L., Fuchs G., Januszyk M., Cao A., et al. Reduced BMPR2 expression induces GM-CSF translation and macrophage recruitment in humans and mice to exacerbate pulmonary hypertension. J. Exp. Med. 2014;211:263–280. doi: 10.1084/jem.20111741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Palakeel J.J., Ali M., Chaduvula P., Chhabra S., Lamichhane S.L., Ramesh V., Opara O.C., Khan F.Y., Kabiraj G., Kauser H., et al. An Outlook on the Etiopathogenesis of Pulmonary Hypertension in HIV. Cureus. 2022;14:e27390. doi: 10.7759/cureus.27390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Diebold I., Hennigs J.K., Miyagawa K., Li C.G., Nickel N.P., Kaschwich M., Cao A., Wang L., Reddy S., Chen P.-I., et al. BMPR2 Preserves Mitochondrial Function and DNA during Reoxygenation to Promote Endothelial Cell Survival and Reverse Pulmonary Hypertension. Cell Metab. 2015;21:596–608. doi: 10.1016/j.cmet.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sutendra G., Michelakis E.D. The Metabolic Basis of Pulmonary Arterial Hypertension. Cell Metab. 2014;19:558–573. doi: 10.1016/j.cmet.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 151.Ryanto G.R.T., Musthafa A., Hara T., Emoto N. Inactivating the Uninhibited: The Tale of Activins and Inhibins in Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2023;24:3332. doi: 10.3390/ijms24043332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Olsen O.E., Sankar M., Elsaadi S., Hella H., Buene G., Darvekar S.R., Misund K., Katagiri T., Knaus P., Holien T. BMPR2 inhibits activin and BMP signaling via wild-type ALK2. J. Cell Sci. 2018;131:jcs.213512. doi: 10.1242/jcs.213512. [DOI] [PubMed] [Google Scholar]
- 153.Yung L.-M., Yang P., Joshi S., Augur Z.M., Kim S.S.J., Bocobo G.A., Dinter T., Troncone L., Chen P.-S., McNeil M.E., et al. ACTRIIA-Fc rebalances activin/GDF versus BMP signaling in pulmonary hypertension. Sci. Transl. Med. 2020;12:eaaz5660. doi: 10.1126/scitranslmed.aaz5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Humbert M., McLaughlin V., Gibbs J.S.R., Gomberg-Maitland M., Hoeper M.M., Preston I.R., Souza R., Waxman A., Subias P.E., Feldman J., et al. Sotatercept for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2021;384:1204–1215. doi: 10.1056/NEJMoa2024277. [DOI] [PubMed] [Google Scholar]
- 155.Hoeper M.M., Badesch D.B., Ghofrani H.A., Gibbs J.S.R., Gomberg-Maitland M., McLaughlin V.V., Preston I.R., Souza R., Waxman A.B., Grünig E., et al. Phase 3 Trial of Sotatercept for Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2023;388:1478–1490. doi: 10.1056/NEJMoa2213558. [DOI] [PubMed] [Google Scholar]
- 156.Tuder R.M., Marecki J.C., Richter A., Fijalkowska I., Flores S. Pathology of Pulmonary Hypertension. Clin. Chest Med. 2007;28:23–42. doi: 10.1016/j.ccm.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Heath D., Yacoub M. Lung mast cells in plexogenic pulmonary arteriopathy. J. Clin. Pathol. 1991;44:1003–1006. doi: 10.1136/jcp.44.12.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Perros F., Dorfmuller P., Souza R., Durand-Gasselin I., Mussot S., Mazmanian M., Herve P., Emilie D., Simonneau G., Humbert M. Dendritic cell recruitment in lesions of human and experimental pulmonary hypertension. Eur. Respir. J. 2007;29:462–468. doi: 10.1183/09031936.00094706. [DOI] [PubMed] [Google Scholar]
- 159.Tuder R.M., Groves B., Badesch D.B., Voelkel N.F. Exuberant endothelial cell growth and elements of inflam-mation are present in plexiform lesions of pulmonary hypertension. Am. J. Pathol. 1994;144:275–285. [PMC free article] [PubMed] [Google Scholar]
- 160.Soon E., Holmes A.M., Treacy C.M., Doughty N.J., Southgate L., Machado R.D., Trembath R.C., Jennings S., Barker L., Nicklin P., et al. Elevated Levels of Inflammatory Cytokines Predict Survival in Idiopathic and Familial Pulmonary Arterial Hypertension. Circulation. 2010;122:920–927. doi: 10.1161/CIRCULATIONAHA.109.933762. [DOI] [PubMed] [Google Scholar]
- 161.Humbert M., Monti G., Brenot F., Sitbon O., Portier A., Grangeot-Keros L., Duroux P., Galanaud P., Simonneau G., Emilie D. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am. J. Respir. Crit. Care Med. 1995;151:1628–1631. doi: 10.1164/ajrccm.151.5.7735624. [DOI] [PubMed] [Google Scholar]
- 162.Smolders V.F.E.D., Lodder K., Rodríguez C., Tura-Ceide O., Barberà J.A., Jukema J.W., Quax P.H.A., Goumans M.J., Kurakula K. The Inflammatory Profile of CTEPH-Derived Endothelial Cells Is a Possible Driver of Disease Progression. Cells. 2021;10:737. doi: 10.3390/cells10040737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Parpaleix A., Amsellem V., Houssaini A., Abid S., Breau M., Marcos E., Sawaki D., Delcroix M., Quarck R., Maillard A., et al. Role of interleukin-1 receptor 1/MyD88 signalling in the development and progression of pulmonary hypertension. Eur. Respir. J. 2016;48:470–483. doi: 10.1183/13993003.01448-2015. [DOI] [PubMed] [Google Scholar]
- 164.Brock M., Trenkmann M., Gay R.E., Michel B.A., Gay S., Fischler M., Ulrich S., Speich R., Huber L.C. Interleukin-6 Modulates the Expression of the Bone Morphogenic Protein Receptor Type II Through a Novel STAT3–microRNA Cluster 17/92 Pathway. Circ. Res. 2009;104:1184–1191. doi: 10.1161/CIRCRESAHA.109.197491. [DOI] [PubMed] [Google Scholar]
- 165.Steiner M.K., Syrkina O.L., Kolliputi N., Mark E.J., Hales C.A., Waxman A.B. Interleukin-6 Overexpression Induces Pulmonary Hypertension. Circ. Res. 2009;104:236–244. doi: 10.1161/CIRCRESAHA.108.182014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Coll-Bonfill N., Musri M.M., Ivo V., Barberà J.A., Tura-Ceide O. Transdifferentiation of endothelial cells to smooth muscle cells play an important role in vascular remodelling. Am. J. Stem. Cells. 2015;4:13–21. [PMC free article] [PubMed] [Google Scholar]
- 167.Selimovic N., Bergh C.-H., Andersson B., Sakiniene E., Carlsten H., Rundqvist B. Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Eur. Respir. J. 2009;34:662–668. doi: 10.1183/09031936.00174908. [DOI] [PubMed] [Google Scholar]
- 168.Li A., Varney M.L., Valasek J., Godfrey M., Dave B.J., Singh R.K. Autocrine Role of Interleukin-8 in Induction of Endothelial Cell Proliferation, Survival, Migration and MMP-2 Production and Angiogenesis. Angiogenesis. 2005;8:63–71. doi: 10.1007/s10456-005-5208-4. [DOI] [PubMed] [Google Scholar]
- 169.Zimmermann N., King N.E., Laporte J., Yang M., Mishra A., Pope S.M., Muntel E.E., Witte D.P., Pegg A.A., Foster P.S., et al. Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J. Clin. Investig. 2003;111:1863–1874. doi: 10.1172/JCI17912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tamosiuniene R., Tian W., Dhillon G., Wang L., Sung Y.K., Gera L., Patterson A.J., Agrawal R., Rabinovitch M., Ambler K., et al. Regulatory T Cells Limit Vascular Endothelial Injury and Prevent Pulmonary Hypertension. Circ. Res. 2011;109:867–879. doi: 10.1161/CIRCRESAHA.110.236927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Huertas A., Tu L., Gambaryan N., Girerd B., Perros F., Montani D., Fabre D., Fadel E., Eddahibi S., Cohen-Kaminsky S., et al. Leptin and regulatory T-lymphocytes in idiopathic pulmonary arterial hypertension. Eur. Respir. J. 2012;40:895–904. doi: 10.1183/09031936.00159911. [DOI] [PubMed] [Google Scholar]
- 172.Becker M.O., Kill A., Kutsche M., Guenther J., Rose A., Tabeling C., Witzenrath M., Kühl A.A., Heidecke H., Ghofrani H.A., et al. Vascular Receptor Autoantibodies in Pulmonary Arterial Hypertension Associated with Systemic Sclerosis. Am. J. Respir. Crit. Care Med. 2014;190:808–817. doi: 10.1164/rccm.201403-0442OC. [DOI] [PubMed] [Google Scholar]
- 173.Isern R.A., Yaneva M., Weiner E., Parke A., Rothfield N., Dantzker D., Rich S., Arnett F.C. Autoantibodies in patients with primary pulmonary hypertension: Association with anti-Ku. Am. J. Med. 1992;93:307–312. doi: 10.1016/0002-9343(92)90238-7. [DOI] [PubMed] [Google Scholar]
- 174.Zaid Y., Puhm F., Allaeys I., Naya A., Oudghiri M., Khalki L., Limami Y., Zaid N., Sadki K., Ben El Haj R., et al. Platelets Can Associate With SARS-CoV-2 RNA and Are Hyperactivated in COVID-19. Circ. Res. 2020;127:1404–1418. doi: 10.1161/CIRCRESAHA.120.317703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Connors J.M., Levy J.H. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Voelkel N.F., Tamosiuniene R., Nicolls M.R. Challenges and opportunities in treating inflammation associated with pulmonary hypertension. Expert Rev. Cardiovasc. Ther. 2016;14:939–951. doi: 10.1080/14779072.2016.1180976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Joshi S.R., Liu J., Bloom T., Atabay E.K., Kuo T.-H., Lee M., Belcheva E., Spaits M., Grenha R., Maguire M.C., et al. Sotatercept analog suppresses inflammation to reverse experimental pulmonary arterial hypertension. Sci. Rep. 2022;12:7803. doi: 10.1038/s41598-022-11435-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Beyer C., Zenzmaier C., Palumbo-Zerr K., Mancuso R., Distler A., Dees C., Zerr P., Huang J., Maier C., Pachowsky M.L., et al. Stimulation of the soluble guanylate cyclase (sGC) inhibits fibrosis by blocking non-canonical TGFβ signalling. Ann. Rheum. Dis. 2014;74:1408–1416. doi: 10.1136/annrheumdis-2013-204508. [DOI] [PubMed] [Google Scholar]
- 179.Uzun N., Durmus S., Gercel G., Aksu B., Misirlioglu N.F., Uzun H. Effects of Bosentan on Hypoxia, Inflammation and Oxidative Stress in Experimental Blunt Thoracic Trauma Model. Medicina. 2024;60:1148. doi: 10.3390/medicina60071148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Bellisai F., Morozzi G., Scaccia F., Chellini F., Simpatico A., Pecetti G., Galeazzi M. Evaluation of the Effect of Bosentan Treatment on Proinflammatory Cytokine Serum Levels in Patients Affected by Systemic Sclerosis. Int. J. Immunopathol. Pharmacol. 2011;24:261–264. doi: 10.1177/039463201102400134. [DOI] [PubMed] [Google Scholar]
- 181.Ghofrani H.-A., Simonneau G., D’Armini A.M., Fedullo P., Howard L.S., Jaïs X., Jenkins D.P., Jing Z.-C., Madani M.M., Martin N., et al. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): Results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir. Med. 2024;12:e21–e30. doi: 10.1016/S2213-2600(24)00027-4. [DOI] [PubMed] [Google Scholar]
- 182.Ordi-Ros J., Sáez-Comet L., Pérez-Conesa M., Vidal X., Riera-Mestre A., Castro-Salomó A., Cuquet-Pedragosa J., Ortiz-Santamaria V., Mauri-Plana M., Solé C., et al. Rivaroxaban Versus Vitamin K Antagonist in Antiphospholipid Syndrome: A randomized noninferiority trial. Ann. Intern. Med. 2019;171:685–694. doi: 10.7326/M19-0291. [DOI] [PubMed] [Google Scholar]
- 183.Frank H., Ruber K., Mlczoch J., Schuster E., Gurtner H.P., Kneussl M., Huber K. The Effect of Anticoagulant Therapy in Primary and Anorectic Drug-Induced Pulmonary Hypertension. Chest. 1997;112:714–721. doi: 10.1378/chest.112.3.714. [DOI] [PubMed] [Google Scholar]
- 184.Preston I.R., Roberts K.E., Miller D.P., Sen G.P., Selej M., Benton W.W., Hill N.S., Farber H.W. Effect of Warfarin Treatment on Survival of Patients with Pulmonary Arterial Hypertension (PAH) in the Registry to Evaluate Early and Long-Term PAH Disease Management (REVEAL) Circulation. 2015;132:2403–2411. doi: 10.1161/CIRCULATIONAHA.115.018435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ehlken N., Lichtblau M., Klose H., Weidenhammer J., Fischer C., Nechwatal R., Uiker S., Halank M., Olsson K., Seeger W., et al. Exercise training improves peak oxygen consumption and haemodynamics in patients with severe pulmonary arterial hypertension and inoperable chronic thrombo-embolic pulmonary hypertension: A prospective, randomized, controlled trial. Eur. Heart J. 2015;37:35–44. doi: 10.1093/eurheartj/ehv337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Pandey A., Garg S., Khunger M., Garg S., Kumbhani D.J., Chin K.M., Berry J.D. Efficacy and Safety of Exercise Training in Chronic Pulmonary Hypertension. Circ. Hear Fail. 2015;8:1032–1043. doi: 10.1161/CIRCHEARTFAILURE.115.002130. [DOI] [PubMed] [Google Scholar]
- 187.Barberà J., Jansa P., Klings E., Ristić A., Keogh A., Solum D., Rao Y., Grover R., Saib I., Sood N. Ralinepag Phase II Open-Label Extension Study in Patients with Pulmonary Arterial Hypertension. Adv. Ther. 2024;41:1062–1074. doi: 10.1007/s12325-023-02769-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Torres F., Farber H., Ristic A., McLaughlin V., Adams J., Zhang J., Klassen P., Shanahan W., Grundy J., Hoffmann I., et al. Efficacy and safety of ralinepag, a novel oral IP agonist, in PAH patients on mono or dual background therapy: Results from a phase 2 randomised, parallel group, placebo-controlled trial. Eur. Respir. J. 2019;54:1901030. doi: 10.1183/13993003.01030-2019. [DOI] [PubMed] [Google Scholar]
- 189.A Study Evaluating the Efcacy and Safety of Ralinepag to Improve Treatment Outcomes in Pah Patients, NCT03626688. [(accessed on 19 April 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03626688.
- 190.A Study of Ralinepag to Evaluate Efects on Exercise Capacity by CPET in Subjects with WHO Group 1 PH (CAPACITY), NCT04084678. [(accessed on 19 April 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04084678.
- 191.A Study Evaluating the Long-Term Efcacy and Safety of Ralinepag in Subjects with PAH via an Open-Label Extension. [(accessed on 19 April 2023)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03683186.
- 192.Bajwa E.K., Cislak D., Kumar A., Li D., Messina E.J., Reynders T., Denef J.-F., Corcea V., Buch K.P., Lai E., et al. Phase 1 Study of MK-5475, an Inhaled Soluble Guanylate Cyclase Stimulator, in Participants with Pulmonary Hypertension Associated with Chronic Obstructive Pulmonary Disease. Int. J. Chronic Obstr. Pulm. Dis. 2024;19:1105–1121. doi: 10.2147/COPD.S454905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Bajwa E.K., Cislak D., Palcza J., Feng H.-P., Messina E.J., Reynders T., Denef J.-F., Corcea V., Lai E., Stoch S.A. Effects of an inhaled soluble guanylate cyclase (sGC) stimulator MK-5475 in pulmonary arterial hypertension (PAH) Respir. Med. 2022;206:107065. doi: 10.1016/j.rmed.2022.107065. [DOI] [PubMed] [Google Scholar]
- 194.A Study of the Efcacy and Safety of Mk-5475 in Participants with Pulmonary Arterial Hypertension (Insignia-Pah: Phase 2/3 Study of an Inhaled Sgc Stimulator In Pah) (Mk-5475-007) [(accessed on 21 August 2024)]; Available online: https://Clinicaltrials.Gov/Ct2/Show/Nct04732221.
- 195.Keith I.M., Ekman R. Dynamic Aspects of Regulatory Lung Peptides in Chronic Hypoxic Pulmonary Hypertension. Exp. Lung Res. 1992;18:205–224. doi: 10.3109/01902149209031681. [DOI] [PubMed] [Google Scholar]
- 196.Keith I.M., Tjen-A-Looi S., Kraiczi H., Ekman R., Norton C.E., Segal S.S., Masood A., Yi M., Belcastro R., Li J., et al. Three-week neonatal hypoxia reduces blood CGRP and causes persistent pulmonary hypertension in rats. Am. J. Physiol. Circ. Physiol. 2000;279:H1571–H1578. doi: 10.1152/ajpheart.2000.279.4.H1571. [DOI] [PubMed] [Google Scholar]
- 197.Tjen-A-Looi S., Ekman R., Lippton H., Cary J., Keith I. CGRP and somatostatin modulate chronic hypoxic pulmonary hypertension. Am. J. Physiol. Circ. Physiol. 1992;263:H681–H690. doi: 10.1152/ajpheart.1992.263.3.H681. [DOI] [PubMed] [Google Scholar]
- 198.Lo C.C.W., Moosavi S.M., Bubb K.J. The Regulation of Pulmonary Vascular Tone by Neuropeptides and the Implications for Pulmonary Hypertension. Front. Physiol. 2018;9:1167. doi: 10.3389/fphys.2018.01167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yan X., Huang J., Zeng Y., Zhong X., Fu Y., Xiao H., Wang X., Lian H., Luo H., Li D., et al. CGRP attenuates pulmonary vascular remodeling by inhibiting the cGAS-STING-NFκB pathway in pulmonary arterial hypertension. Biochem. Pharmacol. 2024;222:116093. doi: 10.1016/j.bcp.2024.116093. [DOI] [PubMed] [Google Scholar]
- 200.Tjen-A-Looi S., Kraiczi H., Ekman R., Keith I. Sensory CGRP depletion by capsaicin exacerbates hypoxia-induced pulmonary hypertension in rats. Regul. Pept. 1998;74:1–10. doi: 10.1016/S0167-0115(98)00007-X. [DOI] [PubMed] [Google Scholar]
- 201.Champion H.C., Bivalacqua T.J., Toyoda K., Heistad D.D., Hyman A.L., Kadowitz P.J. In Vivo Gene Transfer of Prepro-Calcitonin Gene–Related Peptide to the Lung Attenuates Chronic Hypoxia-Induced Pulmonary Hypertension in the Mouse. Circulation. 2000;101:923–930. doi: 10.1161/01.CIR.101.8.923. [DOI] [PubMed] [Google Scholar]
- 202.Macias D., Moore S., Crosby A., Southwood M., Du X., Tan H., Xie S., Vassallo A., Wood A.J., Wallace E.M., et al. Targeting HIF2α-ARNT hetero-dimerisation as a novel therapeutic strategy for pulmonary arterial hypertension. Eur. Respir. J. 2020;57:1902061. doi: 10.1183/13993003.02061-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Dai Z., Zhu M.M., Peng Y., Machireddy N., Evans C.E., Machado R., Zhang X., Zhao Y.-Y. Therapeutic Targeting of Vascular Remodeling and Right Heart Failure in Pulmonary Arterial Hypertension with a HIF-2α Inhibitor. Am. J. Respir. Crit. Care Med. 2018;198:1423–1434. doi: 10.1164/rccm.201710-2079OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Chen T., Zhou Q., Tang H., Bozkanat M., Yuan J.X., Raj J.U., Zhou G. miR-17/20 Controls Prolyl Hydroxylase 2 (PHD2)/Hypoxia-Inducible Factor 1 (HIF1) to Regulate Pulmonary Artery Smooth Muscle Cell Proliferation. J. Am. Hear. Assoc. 2016;5:e004510. doi: 10.1161/JAHA.116.004510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Docherty C.K., Nilsen M., MacLean M.R. Influence of 2-Methoxyestradiol and Sex on Hypoxia-Induced Pulmonary Hypertension and Hypoxia-Inducible Factor-1-α. J. Am. Hear Assoc. 2019;8:e011628. doi: 10.1161/JAHA.118.011628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Jiang Y., Zhou Y., Peng G., Liu N., Tian H., Pan D., Liu L., Yang X., Li C., Li W., et al. Topotecan prevents hypoxia-induced pulmonary arterial hypertension and inhibits hypoxia-inducible factor-1α and TRPC channels. Int. J. Biochem. Cell Biol. 2018;104:161–170. doi: 10.1016/j.biocel.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 207.Cheng C.-C., Chi P.-L., Shen M.-C., Shu C.-W., Wann S.-R., Liu C.-P., Tseng C.-J., Huang W.-C. Caffeic Acid Phenethyl Ester Rescues Pulmonary Arterial Hypertension through the Inhibition of AKT/ERK-Dependent PDGF/HIF-1α In Vitro and In Vivo. Int. J. Mol. Sci. 2019;20:1468. doi: 10.3390/ijms20061468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Luo Y., Teng X., Zhang L., Chen J., Liu Z., Chen X., Zhao S., Yang S., Feng J., Yan X. CD146-HIF-1α hypoxic reprogramming drives vascular remodeling and pulmonary arterial hypertension. Nat. Commun. 2019;10:3551. doi: 10.1038/s41467-019-11500-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Spiekerkoetter E., Tian X., Cai J., Hopper R.K., Sudheendra D., Li C.G., El-Bizri N., Sawada H., Haghighat R., Chan R., et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J. Clin. Investig. 2013;123:3600–3613. doi: 10.1172/JCI65592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Spiekerkoetter E., Sung Y.K., Sudheendra D., Scott V., Del Rosario P., Bill M., Haddad F., Long-Boyle J., Hedlin H., Zamanian R.T. Randomised placebo-controlled safety and tolerability trial of FK506 (tacrolimus) for pulmonary arterial hypertension. Eur. Respir. J. 2017;50:1602449. doi: 10.1183/13993003.02449-2016. [DOI] [PubMed] [Google Scholar]
- 211.Hurst L.A., Dunmore B.J., Long L., Crosby A., Al-Lamki R., Deighton J., Southwood M., Yang X., Nikolic M.Z., Herrera B., et al. TNFα drives pulmonary arterial hypertension by suppressing the BMP type-II receptor and altering NOTCH signalling. Nat. Commun. 2017;8:14079. doi: 10.1038/ncomms14079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Zhang L.-L., Lu J., Li M.-T., Wang Q., Zeng X.-F. Preventive and remedial application of etanercept attenuate monocrotaline-induced pulmonary arterial hypertension. Int. J. Rheum. Dis. 2014;19:192–198. doi: 10.1111/1756-185X.12304. [DOI] [PubMed] [Google Scholar]
- 213.Aliotta J.M., Pereira M., Wen S., Dooner M.S., Del Tatto M., Papa E., Goldberg L.R., Baird G.L., Ventetuolo C.E., Quesenberry P.J., et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc. Res. 2016;110:319–330. doi: 10.1093/cvr/cvw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Shen Y., Goncharov D.A., Pena A., Baust J., Barragan A.C., Ray A., Rode A., Bachman T.N., Chang B., Jiang L., et al. Cross-talk between TSC2 and the extracellular matrix controls pulmonary vascular proliferation and pulmonary hypertension. Sci. Signal. 2022;15:eabn2743. doi: 10.1126/scisignal.abn2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Meloche J., Renard S., Provencher S., Bonnet S. Anti-inflammatory and immunosuppressive agents in PAH. Hand Book Exp. Pharmacol. 2013;218:437–476. doi: 10.1007/978-3-642-38664-0_18. [DOI] [PubMed] [Google Scholar]
- 216.Zamanian R.T., Badesch D., Chung L., Domsic R.T., Medsger T., Pinckney A., Keyes-Elstein L., D’Aveta C., Spychala M., White R.J., et al. Safety and Efficacy of B-Cell Depletion with Rituximab for the Treatment of Systemic Sclerosis–associated Pulmonary Arterial Hypertension: A Multicenter, Double-Blind, Randomized, Placebo-controlled Trial. Am. J. Respir. Crit. Care Med. 2021;204:209–221. doi: 10.1164/rccm.202009-3481OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Trankle C.R., Canada J.M., Kadariya D., Markley R., De Chazal H.M., Pinson J., Fox A., Van Tassell B.W., Abbate A., Grinnan D. IL-1 Blockade Reduces Inflammation in Pulmonary Arterial Hypertension and Right Ventricular Failure: A Single-Arm, Open-Label, Phase IB/II Pilot Study. Am. J. Respir. Crit. Care Med. 2019;199:381–384. doi: 10.1164/rccm.201809-1631LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Velez-Roa S., Ciarka A., Najem B., Vachiery J.-L., Naeije R., van de Borne P. Increased Sympathetic Nerve Activity in Pulmonary Artery Hypertension. Circulation. 2004;110:1308–1312. doi: 10.1161/01.CIR.0000140724.90898.D3. [DOI] [PubMed] [Google Scholar]
- 219.Bós D.D.S.G., Van Der Bruggen C.E.E., Kurakula K., Sun X.-Q., Casali K.R., Casali A.G., Rol N., Szulcek R., Dos Remedios C., Guignabert C., et al. Contribution of Impaired Parasympathetic Activity to Right Ventricular Dysfunction and Pulmonary Vascular Remodeling in Pulmonary Arterial Hypertension. Circulation. 2018;137:910–924. doi: 10.1161/circulationaha.117.027451. [DOI] [PubMed] [Google Scholar]
- 220.Karpov A.A., Vachrushev N.S., Shilenko L.A., Smirnov S.S., Bunenkov N.S., Butskih M.G., Chervaev A.-K.A., Vaulina D.D., Ivkin D.Y., Moiseeva O.M., et al. Sympathetic Denervation and Pharmacological Stimulation of Parasympathetic Nervous System Prevent Pulmonary Vascular Bed Remodeling in Rat Model of Chronic Thromboembolic Pulmonary Hypertension. J. Cardiovasc. Dev. Dis. 2023;10:40. doi: 10.3390/jcdd10020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Silva A.Q., Schreihofer A.M. Altered sympathetic reflexes and vascular reactivity in rats after exposure to chronic intermittent hypoxia. J. Physiol. 2011;589:1463–1476. doi: 10.1113/jphysiol.2010.200691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Perros F., de Man F.S., Bogaard H.J., Antigny F., Simonneau G., Bonnet S., Provencher S., Galiè N., Humbert M. Use of β-Blockers in Pulmonary Hypertension. Circ. Hear Fail. 2017;10:e003703. doi: 10.1161/CIRCHEARTFAILURE.116.003703. [DOI] [PubMed] [Google Scholar]
- 223.Patel M.K., Chan P., Betteridge L.J., Schachter M., Sever P.S. Inhibition of Human Vascular Smooth Muscle Cell Proliferation by the Novel Multiple-Action Antihypertensive Agent Carvedilol. J. Cardiovasc. Pharmacol. 1995;25:652–657. doi: 10.1097/00005344-199504000-00020. [DOI] [PubMed] [Google Scholar]
- 224.Farha S., Saygin D., Park M.M., Cheong H.I., Asosingh K., Comhair S.A., Stephens O.R., Roach E.C., Sharp J., Highland K.B., et al. Pulmonary arterial hypertension treatment with carvedilol for heart failure: A randomized controlled trial. J. Clin. Investig. 2017;2:e95240. doi: 10.1172/jci.insight.95240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bogaard H.J., Natarajan R., Mizuno S., Abbate A., Chang P.J., Chau V.Q., Hoke N.N., Kraskauskas D., Kasper M., Salloum F.N., et al. Adrenergic Receptor Blockade Reverses Right Heart Remodeling and Dysfunction in Pulmonary Hypertensive Rats. Am. J. Respir. Crit. Care Med. 2010;182:652–660. doi: 10.1164/rccm.201003-0335OC. [DOI] [PubMed] [Google Scholar]
- 226.Perros F., Ranchoux B., Izikki M., Bentebbal S., Happé C., Antigny F., Jourdon P., Dorfmüller P., Lecerf F., Fadel E., et al. Nebivolol for Improving Endothelial Dysfunction, Pulmonary Vascular Remodeling, and Right Heart Function in Pulmonary Hypertension. J. Am. Coll. Cardiol. 2015;65:668–680. doi: 10.1016/j.jacc.2014.11.050. [DOI] [PubMed] [Google Scholar]
- 227.Ishikawa M., Sato N., Asai K., Takano T., Mizuno K. Effects of a Pure. ALPHA/BETA.-Adrenergic Receptor Blocker on Monocrotaline-Induced Pulmonary Arterial Hypertension with Right Ventricular Hypertrophy in Rats. Circ. J. 2009;73:2337–2341. doi: 10.1253/circj.CJ-09-0213. [DOI] [PubMed] [Google Scholar]
- 228.de Man F.S., Handoko M.L., van Ballegoij J.J., Schalij I., Bogaards S.J., Postmus P.E., van der Velden J., Westerhof N., Paulus W.J., Vonk-Noordegraaf A. Bisoprolol Delays Progression Towards Right Heart Failure in Experimental Pulmonary Hypertension. Circ. Hear Fail. 2012;5:97–105. doi: 10.1161/CIRCHEARTFAILURE.111.964494. [DOI] [PubMed] [Google Scholar]
- 229.van Campen J.S., de Boer K., van de Veerdonk M.C., van der Bruggen C.E., Allaart C.P., Raijmakers P.G., Heymans M.W., Marcus J.T., Harms H.J., Handoko M.L., et al. Bisoprolol in idiopathic pulmonary arterial hypertension: An explorative study. Eur. Respir. J. 2016;48:787–796. doi: 10.1183/13993003.00090-2016. [DOI] [PubMed] [Google Scholar]
- 230.Moretti C., Marra W.G., D’Ascenzo F., Omedè P., Cannillo M., Libertucci D., Fusaro E., Meynet I., Giordana F., Salera D., et al. Beta blocker for patients with pulmonary arterial hypertension: A single center experience. Int. J. Cardiol. 2015;184:528–532. doi: 10.1016/j.ijcard.2015.02.033. [DOI] [PubMed] [Google Scholar]
- 231.Provencher S., Herve P., Jais X., Lebrec D., Humbert M., Simonneau G., Sitbon O. Deleterious Effects of β-Blockers on Exercise Capacity and Hemodynamics in Patients with Portopulmonary Hypertension. Gastroenterology. 2006;130:120–126. doi: 10.1053/j.gastro.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 232.So P.P.-S., Davies R.A., Chandy G., Stewart D., Beanlands R.S., Haddad H., Pugliese C., Mielniczuk L.M. Usefulness of Beta-Blocker Therapy and Outcomes in Patients with Pulmonary Arterial Hypertension. Am. J. Cardiol. 2012;109:1504–1509. doi: 10.1016/j.amjcard.2012.01.368. [DOI] [PubMed] [Google Scholar]
- 233.Martyniuk T.V., Konosova I.D., Chazova I.E. Use of nebivolol in patients with idiopathic pulmonary hypertension: Results of the pilot study. Ter. Arkhiv. 2012;84:49–53. [PubMed] [Google Scholar]
- 234.Xu W., Wang D.-Y., Chen Z.-Y., Gao Q., Zou Y.-L., Sun D.-H., Zhang S., Zhao X.-B., Gong Y.-T., Zhang Y., et al. Noninvasive Stereotactic Radiotherapy for PADN in an Acute Canine Model of Pulmonary Arterial Hypertension. JACC Basic Transl. Sci. 2024;9:244–256. doi: 10.1016/j.jacbts.2023.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Chen S.-L., Zhang F.-F., Xu J., Xie D.-J., Zhou L., Nguyen T., Stone G.W. Pulmonary artery denervation to treat pulmonary arterial hypertension: The single-center, prospective, first-in-man PADN-1 study (first-in-man pulmonary artery denervation for treatment of pulmonary artery hypertension) J. Am. Coll. Cardiol. 2013;62:1092–1100. doi: 10.1016/j.jacc.2013.05.075. [DOI] [PubMed] [Google Scholar]
- 236.Rothman A.M.K., Vachiery J.-L., Howard L.S., Mikhail G.W., Lang I.M., Jonas M., Kiely D.G., Shav D., Shabtay O., Avriel A., et al. Intravascular Ultrasound Pulmonary Artery Denervation to Treat Pulmonary Arterial Hypertension (TROPHY1): Multicenter, Early Feasibility Study. JACC Cardiovasc. Interv. 2020;13:989–999. doi: 10.1016/j.jcin.2019.12.027. [DOI] [PubMed] [Google Scholar]
- 237.Liu C., Jiang X.-M., Zhang J., Li B., Li J., Xie D.-J., Hu Z.-Y. Pulmonary artery denervation improves pulmonary arterial hypertension induced right ventricular dysfunction by modulating the local renin-angiotensin-aldosterone system. BMC Cardiovasc. Disord. 2016;16:192. doi: 10.1186/s12872-016-0366-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Na S., Kim O.S., Ryoo S., Kweon T.D., Choi Y.S., Shim H.S., Oh Y.J. Cervical Ganglion Block Attenuates the Progression of Pulmonary Hypertension via Nitric Oxide and Arginase Pathways. Hypertension. 2014;63:309–315. doi: 10.1161/HYPERTENSIONAHA.113.01979. [DOI] [PubMed] [Google Scholar]
- 239.Zhou L., Zhang J., Jiang X.-M., Xie D.-J., Wang J.-S., Li L., Li B., Wang Z.-M., Rothman A.M., Lawrie A., et al. Pulmonary Artery Denervation Attenuates Pulmonary Arterial Remodeling in Dogs with Pulmonary Arterial Hypertension Induced by Dehydrogenized Monocrotaline. JACC: Cardiovasc. Interv. 2015;8:2013–2023. doi: 10.1016/j.jcin.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 240.Chen S.-L., Zhang Y.-J., Zhou L., Xie D.-J., Zhang F.-F., Jia H.-B., Wong S.S., Kwan T.W. Percutaneous pulmonary artery denervation completely abolishes experimental pulmonary arterial hypertension in vivo. Eurointervention J. EuroPCR Collab. Work. Group Interv. Cardiol. Eur. Soc. Cardiol. 2013;9:269–276. doi: 10.4244/EIJV9I2A43. [DOI] [PubMed] [Google Scholar]
- 241.Rothman A.M., Arnold N.D., Chang W., Watson O., Swift A.J., Condliffe R., Elliot C.A., Kiely D.G., Suvarna S.K., Gunn J., et al. Pulmonary Artery Denervation Reduces Pulmonary Artery Pressure and Induces Histological Changes in an Acute Porcine Model of Pulmonary Hypertension. Circ. Cardiovasc. Interv. 2015;8:e002569. doi: 10.1161/CIRCINTERVENTIONS.115.002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bos D.d.S.G., Happé C., Schalij I., Pijacka W., Paton J.F., Guignabert C., Tu L., Thuillet R., Bogaard H.-J., van Rossum A.C., et al. Renal Denervation Reduces Pulmonary Vascular Remodeling and Right Ventricular Diastolic Stiffness in Experimental Pulmonary Hypertension. JACC Basic Transl. Sci. 2017;2:22–35. doi: 10.1016/j.jacbts.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Qingyan Z., Xuejun J., Yanhong T., Zixuan D., Xiaozhan W., Xule W., Zongwen G., Wei H., Shengbo Y., Congxin H. Beneficial Effects of Renal Denervation on Pulmonary Vascular Remodeling in Experimental Pulmonary Artery Hypertension. Rev. Espanola Cardiol. 2015;68:562–570. doi: 10.1016/j.recesp.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 244.Huang Y., Liu Y.-W., Pan H.-Z., Zhang X.-L., Li J., Xiang L., Meng J., Wang P.-H., Yang J., Jing Z.-C., et al. Transthoracic Pulmonary Artery Denervation for Pulmonary Arterial Hypertension. Arter. Thromb. Vasc. Biol. 2019;39:704–718. doi: 10.1161/ATVBAHA.118.311992. [DOI] [PubMed] [Google Scholar]
- 245.Chen S.-L., Zhang H., Xie D.-J., Zhang J., Zhou L., Rothman A.M., Stone G.W. Hemodynamic, functional, and clinical responses to pulmonary artery denervation in patients with pulmonary arterial hypertension of different causes. Circ. Cardiovasc. Interv. 2015;8:e002837. doi: 10.1161/CIRCINTERVENTIONS.115.002837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Zhang H., Wei Y., Zhang C., Yang Z., Kan J., Gu H., Fan F., Gu H., Wang Q., Xie D., et al. Pulmonary Artery Denervation for Pulmonary Arterial Hypertension. JACC Cardiovasc. Interv. 2022;15:2412–2423. doi: 10.1016/j.jcin.2022.09.013. [DOI] [PubMed] [Google Scholar]
- 247.Zhang H., Zhang J., Chen M., Xie D.-J., Kan J., Yu W., Li X.-B., Xu T., Gu Y., Dong J., et al. Pulmonary Artery Denervation Significantly Increases 6-Min Walk Distance for Patients with Combined Pre- and Post-Capillary Pulmonary Hypertension Associated With Left Heart Failure. JACC Cardiovasc. Interv. 2018;12:274–284. doi: 10.1016/j.jcin.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 248.Romanov A., Cherniavskiy A., Novikova N., Edemskiy A., Ponomarev D., Shabanov V., Losik D., Elesin D., Stenin I., Mikheenko I., et al. Pulmonary Artery Denervation for Patients with Residual Pulmonary Hypertension After Pulmonary Endarterectomy. J. Am. Coll. Cardiol. 2020;76:916–926. doi: 10.1016/j.jacc.2020.06.064. [DOI] [PubMed] [Google Scholar]
- 249.Yoshida K., Saku K., Kamada K., Abe K., Tanaka-Ishikawa M., Tohyama T., Nishikawa T., Kishi T., Sunagawa K., Tsutsui H. Electrical Vagal Nerve Stimulation Ameliorates Pulmonary Vascular Remodeling and Improves Survival in Rats with Severe Pulmonary Arterial Hypertension. JACC Basic Transl. Sci. 2018;3:657–671. doi: 10.1016/j.jacbts.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Yoshida K., Saku K., Bogaard H.J., Abe K., Sunagawa K., Tsutsui H. Vagal nerve stimulation preserves right ventricular function in a rat model of right ventricular pressure overload. Pulm. Circ. 2022;12:e12154. doi: 10.1002/pul2.12154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Pan P., Zhang X., Qian H., Shi W., Wang J., Bo Y., Li W. Effects of electro-acupuncture on endothelium-derived endothelin-1 and endothelial nitric oxide synthase of rats with hypoxia-induced pulmonary hypertension. Exp. Biol. Med. 2010;235:642–648. doi: 10.1258/ebm.2010.009353. [DOI] [PubMed] [Google Scholar]
- 252.Zhang L., Tian Y., Zhao P., Jin F., Miao Y., Liu Y., Li J. Electroacupuncture attenuates pulmonary vascular remodeling in a rat model of chronic obstructive pulmonary disease via the VEGF/PI3K/Akt pathway. Acupunct. Med. 2022;40:389–400. doi: 10.1177/09645284221078873. [DOI] [PubMed] [Google Scholar]
- 253.Xin J.-J., Gao J.-H., Liu Q., Zhao Y.-X., Zhou C., Yu X.-C. Involvement of ET-1/eNOS in the ameliorating effect of electroacupuncture on cardiac dysfunction in rats with spontaneously hypertensive. Search Life Sci. Lit. 2022;42:647–653. doi: 10.13703/j.0255-2930.20211228-k0006. [DOI] [PubMed] [Google Scholar]
- 254.Shi Y.-R., Yi W., Qiao Y., Zhuo S.-Y., Zhang Q., Yang X.-J., Liang T., Ling X. Effects of acupuncture on pulmonary function and airway smooth muscle spasm in asthma rats. Zhongguo Zhen Jiu. 2023;43:937–943. doi: 10.13703/j.0255-2930.20220618-k0002. [DOI] [PubMed] [Google Scholar]
- 255.Qiao Y., Liang Y., Zhuo S., Yang X., Liang T., Ling X., Zhang Q., Shi Y., Yi W. Effect and mechanism of acupuncture on airway smooth muscle relaxation during acute asthma attack in rats. Zhongguo Zhen Jiu. 2024;44:295–302. doi: 10.13703/j.0255-2930.20230426-k0004. [DOI] [PubMed] [Google Scholar]
- 256.Lin M., Wang X., Ye B., Zhang J., Lin S., Xu Y., Zhou J., Liu S., Zhou S., Guan X., et al. External counterpulsation stimulation combined with acupuncture for vascular endothelial function in patients with hypertension: A randomized pilot trial. Clin. Exp. Hypertens. 2023;45:2181355. doi: 10.1080/10641963.2023.2181355. [DOI] [PubMed] [Google Scholar]
- 257.Jiang X. Effects of magnetic needle acupuncture on blood pressure and plasma ET-1 level in the patient of hypertension. J. Tradit. Chin. Med. = Chung I Tsa Chih Ying Wen Pan. 2003;23:290–291. [PubMed] [Google Scholar]
- 258.Maeda M., Kachi H., Ichihashi N., Oyama Z., Kitajima Y. The effect of electrical acupuncture-stimulation therapy using thermography and plasma endothelin (ET-1) levels in patients with progressive systemic sclerosis (PSS) J. Dermatol. Sci. 1998;17:151–155. doi: 10.1016/S0923-1811(97)00078-9. [DOI] [PubMed] [Google Scholar]
- 259.Li W.-J., Li S.-M., Ding Y., He B., Keegan J., Dong H., Ruan J.-W., Zeng Y.-S. Electro-acupuncture upregulates CGRP expression after rat spinal cord transection. Neurochem. Int. 2012;61:1397–1403. doi: 10.1016/j.neuint.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 260.Maekura T., Miki K., Miki M., Kitada S., Maekura R. Clinical Effects of Acupuncture On The Pathophysiological Mechanism Of Chronic Obstructive Pulmonary Disease During Exercise. Int. J. Chronic Obstr. Pulm. Dis. 2019;14:2787–2798. doi: 10.2147/COPD.S225694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Gao J., Ouyang B.S., Sun G., Fan C., Wu Y.J., Ji L.L. Comparative research on effect of warm needling therapy on pulmonary function and life quality of patients with COPD in the stable phase. Zhongguo Zhen Jiu = Chin. Acupunct. Moxibustion. 2011;31:893–897. [PubMed] [Google Scholar]
- 262.Ge Y., Yao H., Tong J., He Y., Li G., Kong X. Effects of acupuncture on peripheral skeletal muscle exercise ability in patients with chronic obstructive pulmonary disease at stable phase. Zhongguo Zhen Jiu = Chin. Acupunct. Moxibustion. 2017;37:366–371. doi: 10.13703/j.0255-2930.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 263.Feng J., Wang X., Li X., Zhao D., Xu J. Acupuncture for chronic obstructive pulmonary disease (COPD) Medicine. 2016;95:e4879. doi: 10.1097/MD.0000000000004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Tong J., Guo Y.M., He Y., Li G.Y., Chen F., Yao H. Regulatory effects of acupuncture on exercise tolerance in patients with chronic obstructive pulmonary disease at stable phase: A randomized controlled trial. Zhongguo Zhen Jiu = Chin. Acupunct. Moxibustion. 2014;34:846–850. [PubMed] [Google Scholar]
- 265.Suzuki M., Muro S., Ando Y., Omori T., Shiota T., Endo K., Sato S., Aihara K., Matsumoto M., Suzuki S., et al. A Randomized, Placebo-Controlled Trial of Acupuncture in Patients with Chronic Obstructive Pulmonary Disease (COPD): The COPD-acupuncture trial (CAT). Archives of internal medicine. Arch. Intern. Med. 2012;172:878–886. doi: 10.1001/archinternmed.2012.1233. [DOI] [PubMed] [Google Scholar]
- 266.Suzuki M., Namura K., Ohno Y., Tanaka H., Egawa M., Yokoyama Y., Akao S., Fujiwara H., Yano T. The Effect of Acupuncture in the Treatment of Chronic Obstructive Pulmonary Disease. J. Altern. Complement. Med. 2008;14:1097–1105. doi: 10.1089/acm.2007.0786. [DOI] [PubMed] [Google Scholar]
- 267.Zhou C., Zhou C., Wu M.-Z., Wu M.-Z., Liu Q., Liu Q., Xin J.-J., Xin J.-J., Wu S., Wu S., et al. Synergistic and Attenuating Effect of Electroacupuncture on Aconitine in Improving Heart Failure and Its Calcium Regulation Mechanism. Evid.-Based Complement. Altern. Med. 2022;2022:4940745. doi: 10.1155/2022/4940745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Wu Z., Xia Y., Wang C., Lu W., Zuo H., Wu D., Li Y., Guo R., Lu J., Zhang L. Electroacupuncture at Neiguan (PC6) attenuates cardiac dysfunction caused by cecal ligation and puncture via the vagus nerve. Biomed. Pharmacother. 2023;162:114600. doi: 10.1016/j.biopha.2023.114600. [DOI] [PubMed] [Google Scholar]
- 269.Ma L., Cui B., Shao Y., Ni B., Zhang W., Luo Y., Zhang S. Electroacupuncture improves cardiac function and remodeling by inhibition of sympathoexcitation in chronic heart failure rats. Am. J. Physiol. Circ. Physiol. 2014;306:H1464–H1471. doi: 10.1152/ajpheart.00889.2013. [DOI] [PubMed] [Google Scholar]
- 270.Lee H., Kim T.-H., Leem J. Acupuncture for heart failure: A systematic review of clinical studies. Int. J. Cardiol. 2016;222:321–331. doi: 10.1016/j.ijcard.2016.07.195. [DOI] [PubMed] [Google Scholar]
- 271.Chao D.M., Shen L.L., Tjen-A-Looi S.C., Pitsillides K.F., Li P., Longhurst J.C., Guo Z.-L., Ma L., Cui B., Shao Y., et al. Naloxone reverses inhibitory effect of electroacupuncture on sympathetic cardiovascular reflex responses. Am. J. Physiol. Circ. Physiol. 1999;276:H2127–H2134. doi: 10.1152/ajpheart.1999.276.6.H2127. [DOI] [PubMed] [Google Scholar]
- 272.Li P., Pitsillides K.F., Rendig S.V., Pan H.-L., Longhurst J.C. Reversal of Reflex-induced myocardial ischemia by median nerve stimulation: A feline model of electroacupuncture. Circulation. 1998;97:1186–1194. doi: 10.1161/01.CIR.97.12.1186. [DOI] [PubMed] [Google Scholar]
- 273.Li P., Tjen-A-Looi S.C., Longhurst J.C. Excitatory projections from arcuate nucleus to ventrolateral periaqueductal gray in electroacupuncture inhibition of cardiovascular reflexes. Am. J. Physiol. Circ. Physiol. 2006;290:H2535–H2542. doi: 10.1152/ajpheart.00972.2005. [DOI] [PubMed] [Google Scholar]
- 274.Li P., Tjen-A-Looi S., Longhurst J.C. Rostral ventrolateral medullary opioid receptor subtypes in the inhibitory effect of electroacupuncture on reflex autonomic response in cats. Auton. Neurosci. 2001;89:38–47. doi: 10.1016/S1566-0702(01)00247-8. [DOI] [PubMed] [Google Scholar]
- 275.Tjen-A-Looi S.C., Fu L.-W., Wei Z., Syuu Z., Longhurst J.C. Role of unmyelinated fibers in electroacupuncture cardiovascular responses. Auton. Neurosci. 2005;118:43–50. doi: 10.1016/j.autneu.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 276.Tjen-A-Looi S.C., Li P., Longhurst J.C. Prolonged inhibition of rostral ventral lateral medullary premotor sympathetic neurons by electroacupuncture in cats. Auton. Neurosci. 2003;106:119–131. doi: 10.1016/S1566-0702(03)00076-6. [DOI] [PubMed] [Google Scholar]
- 277.Zhou W., Fu L.-W., Guo Z.-L., Longhurst J.C. Role of glutamate in the rostral ventrolateral medulla in acupuncture-related modulation of visceral reflex sympathoexcitation. Am. J. Physiol. Circ. Physiol. 2007;292:H1868–H1875. doi: 10.1152/ajpheart.00875.2006. [DOI] [PubMed] [Google Scholar]
- 278.Guo Z.-L., Longhurst J.C. Activation of reciprocal pathways between arcuate nucleus and ventrolateral periaqueductal gray during electroacupuncture: Involvement of VGLUT3. Brain Res. 2010;1360:77–88. doi: 10.1016/j.brainres.2010.08.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Tjen-A-Looi S.C., Li P., Longhurst J.C. Processing cardiovascular information in the vlPAG during electroacupuncture in rats: Roles of endocannabinoids and GABA. J. Appl. Physiol. 2009;106:1793–1799. doi: 10.1152/japplphysiol.00142.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Fu L.-W., Longhurst J.C. Electroacupuncture modulates vlPAG release of GABA through presynaptic cannabinoid CB1 receptors. J. Appl. Physiol. 2009;106:1800–1809. doi: 10.1152/japplphysiol.91648.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Li P., Tjen-A-Looi S.C., Guo Z.-L., Fu L.-W., Longhurst J.C., Li M., Chitravanshi V.C., Kawabe K., Sapru H.N., Arakawa H., et al. Long-loop pathways in cardiovascular electroacupuncture responses. J. Appl. Physiol. 2009;106:620–630. doi: 10.1152/japplphysiol.91277.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Oliveira A.C., Karas M.M., Alves M., He J., de Kloet A.D., Krause E.G., Richards E.M., Bryant A.J., Raizada M.K. ACE2 overexpression in corticotropin-releasing-hormone cells offers protection against pulmonary hypertension. Front. Neurosci. 2023;17:1223733. doi: 10.3389/fnins.2023.1223733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Goncharuk V.D., Van Heerikhuize J., Swaab D.F., Buijs R.M. Paraventricular nucleus of the human hypothalamus in primary hypertension: Activation of corticotropin-releasing hormone neurons. J. Comp. Neurol. 2002;443:321–331. doi: 10.1002/cne.10124. [DOI] [PubMed] [Google Scholar]
- 284.Dampney R.A., Michelini L.C., Li D.-P., Pan H.-L. Regulation of sympathetic vasomotor activity by the hypothalamic paraventricular nucleus in normotensive and hypertensive states. Am. J. Physiol. Circ. Physiol. 2018;315:H1200–H1214. doi: 10.1152/ajpheart.00216.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Li D.-P., Zhou J.-J., Zhang J., Pan H.-L. CaMKII Regulates Synaptic NMDA Receptor Activity of Hypothalamic Presympathetic Neurons and Sympathetic Outflow in Hypertension. J. Neurosci. 2017;37:10690–10699. doi: 10.1523/JNEUROSCI.2141-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Ruyle B.C., Martinez D., Heesch C.M., Kline D.D., Hasser E.M. The PVN enhances cardiorespiratory responses to acute hypoxia via input to the nTS. Am. J. Physiol. Integr. Comp. Physiol. 2019;317:R818–R833. doi: 10.1152/ajpregu.00135.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.King T.L., Heesch C.M., Clark C.G., Kline D.D., Hasser E.M. Hypoxia activates nucleus tractus solitarii neurons projecting to the paraventricular nucleus of the hypothalamus. Am. J. Physiol. Integr. Comp. Physiol. 2012;302:R1219–R1232. doi: 10.1152/ajpregu.00028.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Silva T.M., Takakura A.C., Moreira T.S. Acute hypoxia activates hypothalamic paraventricular nucleus-projecting catecholaminergic neurons in the C1 region. Exp. Neurol. 2016;285:1–11. doi: 10.1016/j.expneurol.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 289.Wang L.A., Nguyen D.H., Mifflin S.W. Corticotropin-releasing hormone projections from the paraventricular nucleus of the hypothalamus to the nucleus of the solitary tract increase blood pressure. J. Neurophysiol. 2019;121:602–608. doi: 10.1152/jn.00623.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Wang L.A., Nguyen D.H., Mifflin S.W. CRHR2 (Corticotropin-Releasing Hormone Receptor 2) in the Nucleus of the Solitary Tract Contributes to Intermittent Hypoxia-Induced Hypertension. Hypertension. 2018;72:994–1001. doi: 10.1161/HYPERTENSIONAHA.118.11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Zhu L., Ye Z., Zhang M., Xu W., Wang R., Wu S., Gao H. Electroacupuncture intervention on stress-induced cardiac autonomic imbalance in rats involves corticotropin-releasing hormone system activity. NeuroReport. 2023;34:401–410. doi: 10.1097/WNR.0000000000001905. [DOI] [PubMed] [Google Scholar]
- 292.Ye Z., Zhu L., Li X.-J., Gao H.-Y., Wang J., Wu S.-B., Wu Z.-J., Gao H.-R. PC6 electroacupuncture reduces stress-induced autonomic and neuroendocrine responses in rats. Heliyon. 2023;9:e15291. doi: 10.1016/j.heliyon.2023.e15291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Zhang M., Sun J., Wang Y., Tian Z. Secretagogin Mediates the Regulatory Effect of Electroacupuncture on Hypothalamic-Pituitary-Adrenal Axis Dysfunction in Surgical Trauma. Neural Plast. 2021;2021:1–11. doi: 10.1155/2021/8881136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Zhou Q.Z., Peng X.H., Wu Q.F., Yu S.G., Wei J.L., Xu H.Y., Wang W., Yin H.Y. Effect of electroacupuncture on the expression of corticotropin releasing hormone (CRH) and CRHR 1 mRNA in paraventricular nucleus of hypothalamus in stress-induced anxiety rats. Zhen Ci Yan Jiu = Acupunct. Res. 2008;33:372–376. [PubMed] [Google Scholar]
- 295.Huang J., Tamisier R., Ji E., Tong J., Weiss W.J. Chronic intermittent hypoxia modulates nNOS mRNA and protein expression in the rat hypothalamus. Respir. Physiol. Neurobiol. 2007;158:30–38. doi: 10.1016/j.resp.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 296.Ogoshi T., Tsutsui M., Kido T., Sakanashi M., Naito K., Oda K., Ishimoto H., Yamada S., Wang K.-Y., Toyohira Y., et al. Protective Role of Myelocytic Nitric Oxide Synthases against Hypoxic Pulmonary Hypertension in Mice. Am. J. Respir. Crit. Care Med. 2018;198:232–244. doi: 10.1164/rccm.201709-1783OC. [DOI] [PubMed] [Google Scholar]
- 297.Kim J.-I., Kim Y.-S., Kang S.-K., Kim C., Park C., Lee M.S., Huh Y. Electroacupuncture decreases nitric oxide synthesis in the hypothalamus of spontaneously hypertensive rats. Neurosci. Lett. 2008;446:78–82. doi: 10.1016/j.neulet.2008.09.049. [DOI] [PubMed] [Google Scholar]
- 298.Lim H.-D., Kim M.-H., Lee C.-Y., Namgung U. Anti-Inflammatory Effects of Acupuncture Stimulation via the Vagus Nerve. PLoS ONE. 2016;11:e0151882. doi: 10.1371/journal.pone.0151882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Liu S., Wang Z., Su Y., Qi L., Yang W., Fu M., Jing X., Wang Y., Ma Q. A neuroanatomical basis for electroacupuncture to drive the vagal–adrenal axis. Nature. 2021;598:641–645. doi: 10.1038/s41586-021-04001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Liu S., Wang Z.-F., Su Y.-S., Ray R.S., Jing X.-H., Wang Y.-Q., Ma Q. Somatotopic Organization and Intensity Dependence in Driving Distinct NPY-Expressing Sympathetic Pathways by Electroacupuncture. Neuron. 2020;108:436–450.e7. doi: 10.1016/j.neuron.2020.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Torres-Rosas R., Yehia G., Peña G., Mishra P., Thompson-Bonilla M.d.R., Moreno-Eutimio M.A., Arriaga-Pizano L.A., Isibasi A., Ulloa L. Dopamine mediates vagal modulation of the immune system by electroacupuncture. Nat. Med. 2014;20:291–295. doi: 10.1038/nm.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Zhao Y.X., He W., Jing X.H., Liu J.L., Rong P.J., Ben H., Liu K., Zhu B. Transcutaneous Auricular Vagus Nerve Stimulation Protects Endotoxemic Rat from Lipopolysaccharide-Induced Inflammation. Evid.-Based Complement. Altern. Med. 2012;2012:627023. doi: 10.1155/2012/627023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Li X., Wang L., Ying X., Zheng Y., Tan Q., Yu X., Gong J., Li M., Deng X., Yang G., et al. Electroacupuncture pre-treatment alleviates sepsis-induced cardiac inflammation and dysfunction by inhibiting the calpain-2/STAT3 pathway. Front. Physiol. 2022;13:961909. doi: 10.3389/fphys.2022.961909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Chen T., Xiong Y., Long M., Zheng D., Ke H., Xie J., Yin N., Chen Z. Electro-acupuncture Pretreatment at Zusanli (ST36) Acupoint Attenuates Lipopolysaccharide-Induced Inflammation in Rats by Inhibiting Ca2+ Influx Associated with Cannabinoid CB2 Receptors. Inflammation. 2018;42:211–220. doi: 10.1007/s10753-018-0885-5. [DOI] [PubMed] [Google Scholar]
- 305.Ju S., Liu M., Wang B., Yu D., Zhang H., Zhang M., Li J. Transcutaneous electrical acupoint stimulation improves pulmonary function by regulating oxidative stress during one-lung ventilation in patients with lung cancer undergoing thoracoscopic surgery: A randomized controlled trial. BMC Complement. Med. Ther. 2023;23:463. doi: 10.1186/s12906-023-04304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Ma W., Li Z., Lu Z., Tan W., Zhang Z., Li Y., Yang Z., Zhou J., Tang H., Cui H. Protective Effects of Acupuncture in Cardiopulmonary Bypass-Induced Lung Injury in Rats. Inflammation. 2017;40:1275–1284. doi: 10.1007/s10753-017-0570-0. [DOI] [PubMed] [Google Scholar]
- 307.Gong L.-R., Kan Y.-X., Lian Y., Dong S.-A., Zhao D.-H., Shi J., Yu J.-B. Electroacupuncture Attenuates Limb Ischemia-Reperfusion-Induced Lung Injury Via p38 Mitogen-Activated Protein Kinase-Nuclear Factor Erythroid-2-Related Factor-2/Heme Oxygenase Pathway. J. Surg. Res. 2020;246:170–181. doi: 10.1016/j.jss.2019.08.021. [DOI] [PubMed] [Google Scholar]
- 308.Luo J., Yan R., Ding L., Ning J., Chen M., Guo Y., Liu J., Chen Z., Zhou R. Electroacupuncture Attenuates Ventilator-Induced Lung Injury by Modulating the Nrf2/HO-1 Pathway. J. Surg. Res. 2024;295:811–819. doi: 10.1016/j.jss.2023.11.055. [DOI] [PubMed] [Google Scholar]
- 309.Lu J., Xie J.J., Xiang S.Y., Li Y., Cong W.J., Lin X.G., Liu Z.B. Electroacupuncture Improves Pulmonary Function of Rats with Chronic Obstructive Pulmonary Disease by Down-regulating Inflammatory Reaction and Expression of Macrophage Migration Inhibitory Factor/CD 74-CD 44/p 38 MAPK Signaling in Lung Tissues. Zhen Ci Yan Jiu = Acupunct. Res. 2018;43:759–766. doi: 10.13702/j.1000-0607.180494. [DOI] [PubMed] [Google Scholar]
- 310.Zhang L., Wu Z., Zhou J., Lu S., Wang C., Xia Y., Ren H., Tong Z., Ke L., Li W. Electroacupuncture Ameliorates Acute Pancreatitis: A Role for the Vagus Nerve–Mediated Cholinergic Anti-Inflammatory Pathway. Front. Mol. Biosci. 2021;8:647647. doi: 10.3389/fmolb.2021.647647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Abe C., Inoue T., Inglis A.M., Viar E.K., Huang L., Ye H., Rosin D.L., Stornetta R.L., Okusa M.D., Guyenet P.G. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat. Neurosci. 2017;20:700–707. doi: 10.1038/nn.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Tang W., Dong M., Teng F., Cui J., Zhu X., Wang W., Wuniqiemu T., Qin J., Yi L., Wang S., et al. TMT-based quantitative proteomics reveals suppression of SLC3A2 and ATP1A3 expression contributes to the inhibitory role of acupuncture on airway inflammation in an OVA-induced mouse asthma model. Biomed. Pharmacother. 2020;134:111001. doi: 10.1016/j.biopha.2020.111001. [DOI] [PubMed] [Google Scholar]
- 313.Li Y., Zhang H., Yang J., Zhan M., Hu X., Liu Y., Yu L., Yan X., Liang S., Zhang R., et al. P2Y12 receptor as a new target for electroacupuncture relieving comorbidity of visceral pain and depression of inflammatory bowel disease. Chin. Med. 2021;16:139. doi: 10.1186/s13020-021-00553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Jung Y.S., Lee S.-W., Park J.H., Seo H.B., Choi B.T., Shin H.K. Electroacupuncture preconditioning reduces ROS generation with NOX4 down-regulation and ameliorates blood-brain barrier disruption after ischemic stroke. J. Biomed. Sci. 2016;23:32. doi: 10.1186/s12929-016-0249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Zhang X.-F., Zhu J., Geng W.-Y., Zhao S.-J., Jiang C.-W., Cai S.-R., Cheng M., Zhou C.-Y., Liu Z.-B. Electroacupuncture at Feishu (BL13) and Zusanli (ST36) down-regulates the expression of orexins and their receptors in rats with chronic obstructive pulmonary disease. J. Integr. Med. 2014;12:417–424. doi: 10.1016/S2095-4964(14)60040-6. [DOI] [PubMed] [Google Scholar]
- 316.Vickers A.J., Vertosick E.A., Lewith G., MacPherson H., Foster N.E., Sherman K.J., Irnich D., Witt C.M., Linde K. Acupuncture for Chronic Pain: Update of an Individual Patient Data Meta-Analysis. J. Pain. 2017;19:455–474. doi: 10.1016/j.jpain.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Yuan C.-X., Wang X., Yu Z., Lian X.-Y., Xu B. Electroacupuncture improves peripheral neuropathy by up-regulating Sirt1/PGC-1α/TFAM pathway in type 2 diabetes rats with peripheral neuropathy. Zhen Ci Yan Jiu. 2024;49:349–357. doi: 10.13702/j.1000-0607.20221318. [DOI] [PubMed] [Google Scholar]
- 318.Hoerder S., Habermann I.V., Hahn K., Meyer-Hamme G., Ortiz M., Grabowska W., Roll S., Willich S.N., Schroeder S., Brinkhaus B., et al. Acupuncture in diabetic peripheral neuropathy-neurological outcomes of the randomized acupuncture in diabetic peripheral neuropathy trial. World J. Diabetes. 2023;14:1813–1823. doi: 10.4239/wjd.v14.i12.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Yang N.-N., Lin L.-L., Li Y.-J., Li H.-P., Cao Y., Tan C.-X., Hao X.-W., Ma S.-M., Wang L., Liu C.-Z. Potential Mechanisms and Clinical Effectiveness of Acupuncture in Depression. Curr. Neuropharmacol. 2022;20:738–750. doi: 10.2174/1570159X19666210609162809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Chen S., Li J., Yan L., Zhang X., Huang J., Zhou P. Electroacupuncture alleviates the symptom of depression in mice by regulating the cGAS-STING-NLRP3 signaling. Aging. 2024;16:6731–6744. doi: 10.18632/aging.205596. [DOI] [PMC free article] [PubMed] [Google Scholar]