Abstract
This study analyzed the effects of Trichoderma harzianum on the growth of continuous cropping Lagenaria siceraria and the physical and chemical properties of rhizosphere soil and microbial community structure, using Illumina Miseq (PE300) high-throughput sequencing technology along with physiological and biochemical detection. The results indicated that after applying T. harzianum, the growth of L. siceraria was significantly promoted, with increases in plant height, fresh weight, and dry weight of 21.42%, 24.5%, and 4.5%, respectively. The pH of the rhizosphere soil decreased from 7.78 to 7.51, while the electrical conductivity, the available phosphorus, the available potassium, and the total nitrogen were markedly higher compared to the control group and increased by 13.95%, 22.54%, 21.37%, and 16.41%, respectively. The activities of catalase and sucrase in the rhizosphere increased by 18.33% and 61.47%, and the content of soil organic carbon (SOC) increased by 27.39%, which indicated that T. harzianum could enhance soil enzyme activity and promotes the transformation of organic matter. The relative abundance of beneficial bacteria such as Pseudomonas increased, while the relative abundance of harmful fungi such as Fusarium and Podosphaera decreased significantly.
Keywords: chemical properties, rhizosphere soil, soil enzyme activity
1. Introduction
Lagenaria siceraria (Molina) Standl, commonly known as L. siceraria, gourd, or lupa, is an annual climbing vine herb [1]. L. siceraria possesses substantial nutritional and economic value due to its chemical composition, which includes glucose, pentosan, lignans, and other compounds [2]. These components contribute to its diuretic and anti-inflammatory effects, thereby enhancing its medicinal value [3]. The annual sales of L. siceraria crafts in Liaocheng, Shandong Province, China reached more than a few million dollars. In recent years, the intensive planting and improper field management measures of L. siceraria have caused the emergence of continuous cropping barriers. This induced the quality and yield of L. siceraria fruit to continue to decline, which has emerged as the primary obstacle to the advancement of the L. siceraria industry.
Continuous cropping refers to a planting model in which a single crop is cultivated on the same land for an extended period. This planting model is prone to continuous cropping obstacles, which are manifested by growth impediments, exacerbation of pests and diseases, yield reduction, and quality decline [4,5,6]. The resistance and stability of soil microorganisms within the soil ecosystem, as well as their roles in the circulation of soil materials, are critical. These microorganisms facilitate water transport, organic matter decomposition, nutrient absorption, and soil transformation, significantly impacting plant disease and pest management [7]. After long-term continuous cropping of watermelon, the spectrum of rhizosphere bacteria decreased, whereas the spectrum of fungi increased, with a corresponding increase in the relative abundance of pathogenic fungi like Fusarium, which became the leading dominant group [8]. Similarly, continuous cropping of cucumber significantly increased the amount of fungi in rhizosphere soil, with a notable rise in harmful Fusarium species and a decline in the prevalence of beneficial bacteria such as Pseudomonas [9]. Long-term continuous cropping of Cucurbitaceae plants maybe lead to alterations in the number, biodiversity, and species richness of soil rhizosphere microorganisms, and structural imbalance is a significant factor related to the development of continuous cropping challenges [10]. Therefore, addressing these obstacles by modifying the soil rhizosphere microbial population structure is viewed as an effective strategy.
Trichoderma harzianum is a biological control agent that effectively alleviates the sustained cropping obstacles in the production of cucumber, melon and pepper production [11,12]. It not only inhibits the survival and activity of pathogens, preventing soil pathogens multiplication, but also enhances plant growth, increases resistance to stress and disease, and comprehensively improves the quality of crops [13,14]. T. ghanense and T. citrinoviride have been shown to restrict the growth of cucumber pathogens such as Pythium aphanidermatum while facilitating cucumber growth [12]. Following the application of T. saturnisporum, the damage caused by Pythium ultimum to melon seedlings was reduced, with disease severity decreasing by 63% [15]. In greenhouse experiments, the execution of T. harzianum effectively curtailed the incidence of peanut brown root rot and promoted plant growth [16]. The concurrent application of biochar and Trichoderma increased the variability of bacterial communities within the rhizosphere soil of continuous cropped cucumber, optimized the physical and chemical attributes of the soil, and stimulated root activity [17]. Under sterile laboratory conditions, the application of Trichoderma harzianum T-22 biofertilizer to the carrot rhizosphere soil significantly increased the numbers of Bacillus and Pseudomonas, as well as beneficial fungi such as Trichoderma [18]. Additionally, inoculation of T. harzianum ST02 in the rhizosphere soil of sweet sorghum significantly increased the relative abundance of beneficial nitrogen-fixing bacteria, such as Arthrobacter [19].
Therefore, the effects of T. harzianum were evaluated on the performance of L. siceraria, as well as on the physico-chemical properties and microbial community structure of the rhizosphere soil under continuous planting conditions. This study aimed to evaluate the remediation potential of T. harzianum for continuous L. siceraria cultivation and to provide a scientific basis for effectively alleviating the adverse effects of continuous cropping.
2. Materials and Methods
2.1. Experimental Materials
T. harzianum (T-22) was produced by Shandong Cangyuan Biotechnology Co., Ltd. (Weifang, China) (effective viable count: ≥1 billion/g).
The experimental material L. siceraria variety is Yayao gourd, which is the largest gourd variety planted in Liaocheng, Shandong Province. It is preserved by the Muhongmei Research Group of the College of Agriculture and Biology of Liaocheng University.
2.2. L. siceraria Cultivation Methods
The soil used for the pot experiment was sourced from the plantation of the College of Agronomy and Biology at Liaocheng University, located in Liaocheng City, Shandong Province, China, where L. siceraria has been continuously cultivated for two years. This region falls within the temperate monsoon climate zone, with an annual average temperature of approximately 13.1 °C, annual precipitation of about 600 mm, and annual frost-free period of about 200 days. Before planting, the soil had a pH of 7.23, organic carbon content of 8.20 g/kg, total nitrogen of 710.45 mg/kg, available phosphorus of 45.63 mg/kg, available potassium of 121.52 mg/kg, and electrical conductivity of 0.56 mS/cm.
The L. siceraria was planted in a black plastic basin (bottom length 6.8 cm, height 8.5 cm, top length 10 cm), which was filled with L. siceraria rhizosphere soil (500 g) that had been continuously planted in the same field for two years. The crop continues (CC) group was irrigated with sterile water, and the crop treatment (CT) group was irrigated with 200 times diluted fertilizer per kilogram of liquid. Each treatment had 5 replicates and was irrigated every 5 days. The conditions of the greenhouse were as follows: room temperature 25 °C, relative air humidity 50%. Other growth conditions remained unchanged when growing in the controlled environment chamber of the College of Agronomy and Biology of Liaocheng University for 25 days, growing to the climbing stage.
2.3. Soil Sample Collection
After extracting the soil from the pot of L. siceraria, the soil was softly tapped away from the root system, and the soil sample adhered to the root exterior was coated utilizing a sterile brush. Then, the plant debris and root residues were removed through a 40-mesh sieve. The rhizosphere soil samples from the same pot were thoroughly combined to achieve a completely uniform sample. Frozen at −80 °C for storage, the soil microbial sequencing was entrusted to Shanghai Meiji Biological Company (Shanghai, China) (https://www.majorbio.com/, accessed on 15 July 2024) to analyze the chemical composition and enzymatic functions of the remaining soil.
2.4. Detection of Soil Chemical Properties
The soil pH and electrical conductivity were measured by potentiometric method and conductivity method (with a water-to-soil ratio of 2.5:1). The contents of soil organic matter, total nitrogen, available phosphorus, and available potassium were quantified by the potassium dichromate volumetric method, Kjeldahl method, combined extraction–colorimetric method, and flame photometer method, respectively [20,21,22].
2.5. Examination of Soil Enzyme Activity
Soil urease activity was measured using the indigo colorimetric method [23], soil sucrase activity was assessed by the 3,5-dinitrosalicylic acid colorimetric method [24], neutral phosphatase activity in the soil was measured using the disodium phenyl phosphate colorimetric method [23], and soil catalase activity was evaluated through potassium permanganate titration [25].
2.6. Soil Microbial Sequencing
Microbial genomic DNA was extracted from 0.25 g soil samples according to the E.Z.N.A.® Soil DNA Kit (Omega Bio-tek, Norcross, GA, USA). DNA quality and concentration were assessed via 1.0% agarose gel electrophoresis and the NanoDrop2000 spectrophotometer (Thermo Scientific, Waltham, MA, USA). The hypervariable regions V3–V4 of the bacterial 16S rRNA gene were amplified with primer pairs 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′), and the ITS1 rDNA regions of fungi were amplified with a forward primer ITS1F (CTTGGTCATTTAGAGGAAGTAA) and reverse primer ITS2R (GCTGCGTTCTTCATCGATGC) pair, using a T100 Thermal Cycler PCR thermocycler (BIO-RAD, Hercules, CA, USA) [26]. PCR products were purified from a 2% agarose gel using the PCR Clean-Up Kit (YuHua, Shanghai, China), and quantified with a Qubit 4.0 fluorometer (Thermo Fisher Scientific, Waltham, MA, USA).
2.7. Sequencing Data Processing
The soil microbial diversity was sequenced using the Illumina Miseq PE300 platform (Illumina, San Diego, CA, USA). The purified amplicons were initially assembled according to the standard of equimolar amounts, and then subjected to paired-end sequencing.
The raw FASTQ files were de-multiplexed and then quality filtered using fastp version 0.19.6 [27] and merged to a reference standard using FLASH version 1.2.7 [28]. The representative sequences of bacteria and fungi were classified [29,30] and analyzed separately using an RDP Classifier version 2.2 [31] with a confidence level of 70%.
2.8. Data Statistics and Analysis
Using Mothur version 1.30.2, the dilution curve and α-diversity index were calculated. The Bray–Curtis distance matrix served as the basis for Principal Coordinate Analysis (PCoA) (Vegan v2.5-3 package). Species differences were assessed using Linear Discriminant Analysis Effect Size (LEfSe) (http://galaxy.biobakery.org/, accessed on 15 July 2024) software, with a significance level set at p < 0.05 and an LDA effect size threshold of >3.5. FUNGuild software (http://www.funguild.org/, accessed on 15 July 2024) and the Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt, version 1.1.0) were employed to obtain functional predictions of bacterial and fungal communities. The Duncan’s Multiple Range Test was used to examine differences across treatments, with significance levels set at p < 0.05 and p < 0.01. All results are presented as mean ± standard deviation (SD).
3. Results
3.1. Effects of T. harzianum on Growth Indexes of L. siceraria
The application of T. harzianum was found to enhance the growth of L. siceraria. Specifically, this treatment resulted in a significant increase in plant height, as well as both fresh and dry biomass (Table 1). The height of the plants, along with their fresh and dry weight, exhibited increases of 21.42%, 24.5%, and 4.5%, respectively, compared with the CC group.
Table 1.
Effects of T. harzianum on growth indexes of L. siceraria.
| Sample | Plant Height (cm) | Fresh Weight (g) | Dry Weight (g) |
|---|---|---|---|
| CC | 18.06 ± 0.94 b | 9.02 ± 0.06 b | 3.29 ± 0.05 b |
| CT | 21.93 ± 1.70 a | 11.23 ± 0.15 a | 3.44 ± 0.03 a |
Note: Data are presented as mean ± SD. Different lowercase superscript letters indicate notable differences at p < 0.05.
3.2. Effects of T. harzianum on Soil Physical and Chemical Properties of L. siceraria
The application of T. harzianum notably altered the chemical characteristics of the rhizosphere soil (Table 2). In the CT group, the electrical conductivity, the organic matter, the available phosphorus, the available potassium, and the total nitrogen were markedly higher compared to the CC group and increased by 13.95%, 27.39%, 22.54%, 21.37%, and 16.41%, respectively. The pH of rhizosphere soil decreased from 7.78 to 7.51. This suggests that T. harzianum enhances soil nutrient levels while also affecting soil pH.
Table 2.
Effects of T. harzianum on soil chemical properties of L. siceraria.
| Sample | SOC/(g/kg) | AP/(mg/kg) | AK/(mg/kg) | TN/(mg/kg) | EC/(mS/cm) | pH |
|---|---|---|---|---|---|---|
| CC | 7.3 ± 0.34 b | 41.29 ± 1.52 b | 110.4 ± 1.89 b | 640.49 ± 12.27 b | 0.43 ± 0.01 b | 7.78 ± 0.11 a |
| CT | 9.3 ± 0.20 a | 50.60 ± 0.89 a | 134.0 ± 0.81 a | 745.63 ± 11.34 a | 0.49 ± 0.01 a | 7.51 ± 0.01 b |
Note: Data are presented as mean ± SD. Different lowercase superscript letters indicate notable differences at p < 0.05. SOC: soil organic carbon, AP: available phosphorus, AK: available potassium, TN: total nitrogen, and EC: electrical conductivity.
3.3. Effect of T. harzianum on Enzyme Activity in Rhizosphere Soil of L. siceraria
The application of T. harzianum preparations enhanced the activities of urease, phosphatase, catalase, and sucrase to different extents (Table 3). The soil treated with T. harzianum exhibited substantial increases in catalase and sucrase activities, with enhancements of 18.33% and 61.47%, respectively, compared to the CC group.
Table 3.
Effects of T. harzianum on soil enzyme activities.
| Sample | Urease (mg/g/d) | NAP (mg/g/d) | CAT (mg/g/d) | Sucrase (mg/g/d) |
|---|---|---|---|---|
| CC | 0.48 ± 0.01 a | 0.97 ± 0.01 a | 1.20 ± 0.04 b | 7.89 ± 0.05 b |
| CT | 0.49 ± 0.02 a | 1.04 ± 0.06 a | 1.42 ± 0.06 a | 12.74 ± 0.44 a |
Note: Data are presented as mean ± SD. Different lowercase superscript letters indicate notable differences at p < 0.05. NAP: neutral phosphatase. CAT: catalase.
3.4. Effects of T. harzianum on Soil Microbial Diversity and Species Richness
3.4.1. Microbial Cluster Analysis
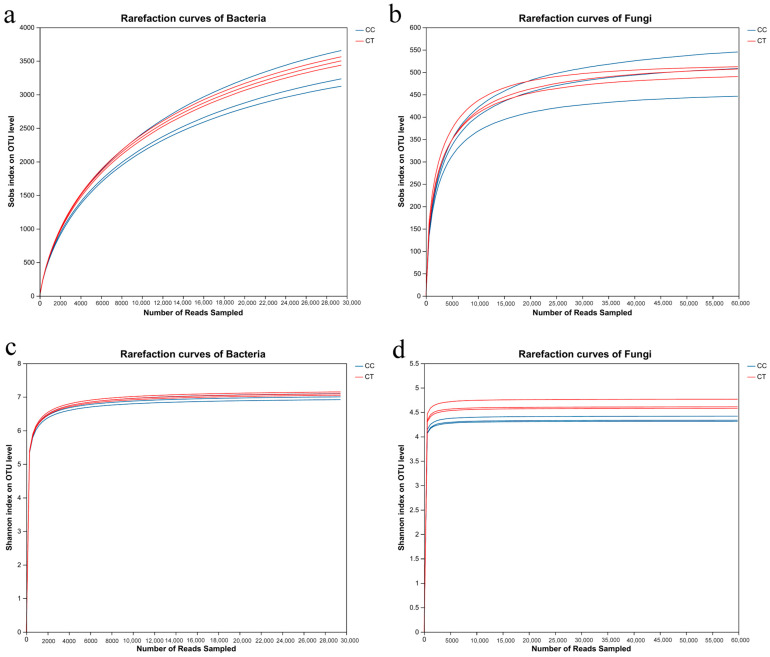
Following the quality control and filtering of sequencing data, a total of 56,149 and 53,216 bacterial 16S rRNA sequences, and 77,010 and 66,073 fungal ITS sequences were obtained from the CC and CT groups, respectively. Sequences exhibiting over 97% similarity to OTU representative sequences were chosen for annotation and further analysis. The Sobs diversity curves for both bacteria and fungi did not reach a plateau at a distance of 0.03 (Figure 1a,b), suggesting that the sequencing data may not fully capture all the communities present in the samples. In contrast, the Shannon diversity curves approached a plateau as the number of reads increased (Figure 1c,d), indicating that the sequencing data adequately represent the majority of the microbial diversity within the samples.
Figure 1.
Dilution curves under CC and CT treatments. (a,b) represent the Sobs curves of bacteria and fungi, respectively, and (c,d) represent the Shannon curves of bacteria and fungi, respectively. CC refers to the continuous cropping treatment group; CT refers to the T harzianum treatment group.
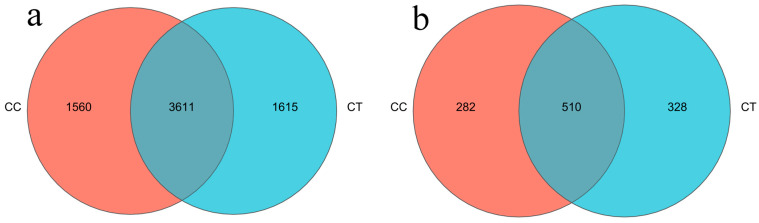
A similarity threshold of 97% was applied and a Venn diagram was utilized to depict the number of unique and shared OTUs across various treatments (Figure 2). The analysis revealed 3611 common bacterial OTUs between the treatments. The CC group exhibited 1560 unique OTUs, while the CT group had 1615. Additionally, 519 fungal OTUs were common, with 283 unique to the CC group and 322 unique to the CT group.
Figure 2.
Unique and shared genera of the CC and CT groups in Venn diagram form. (a,b) represent bacteria and fungi, respectively.
3.4.2. Microbial Alpha and Beta Diversity Analysis
Notable differences were detected in the Shannon and Simpson indices in the fungal communities between the CC and CT groups (Table 4). Specifically, the Shannon index was higher in the CT group compared to the CC group, whereas the Simpson index was lower in the CT group. It is important to highlight that a higher Shannon index indicates greater community richness, while a lower Simpson index reflects increased community evenness. The indices for bacterial community diversity and richness did not differ significantly between the CC and CT groups. Similar results were observed for the Chao1 and ACE indices in the fungal communities.
Table 4.
Diversity along with richness indices of the bacterial and fungal communities under the CC and CT soil treatments.
| Sample | OTUs | ACE | Chao | Shannon | Simpson | Coverage | |
|---|---|---|---|---|---|---|---|
| Bacterial | CC | 3337.00 ± 280.83 a | 4076.75 ± 413.76 a | 3919.7 ± 361.43 a | 7.01 ± 0.09 a | 0.0022 ± 0.0001 a | 0.968223 |
| CT | 3499.67 ± 63.01 a | 4278.05 ± 88.28 a | 4122.7 ± 106.67 a | 7.10 ± 0.05 a | 0.0021 ± 0.0002 a | 0.969683 | |
| Fungal | CC | 499.67 ± 50.02 a | 512.96 ± 57.98 a | 516.86 ± 65.16a | 4.36 ± 0.06 b | 0.0309 ± 0.0022 a | 0.999498 |
| CT | 503.00 ± 11.53 a | 509.37 ± 10.85 a | 510.12 ± 11.04 a | 4.65 ± 0.10 a | 0.0215 ± 0.0022 b | 0.999693 |
Note: Data are presented as mean ± SD. Different lowercase superscript letters indicate notable differences at p < 0.05, Cutoff < 0.03.
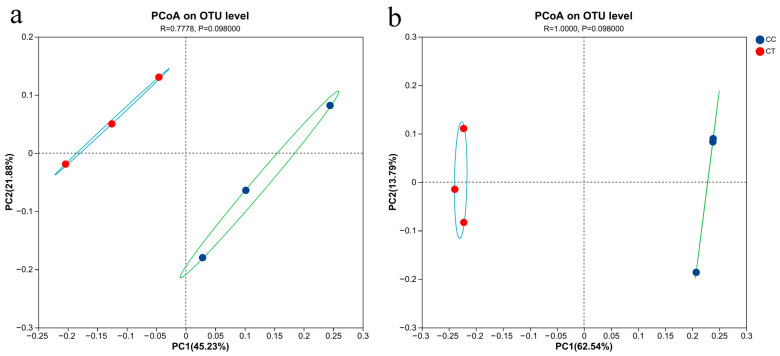
PCoA ordination was conducted on various groups based on the OTU data (Figure 3). The analysis revealed that the application of T. harzianum notably altered the microbial community structure in the rhizosphere soil of L. siceraria. For bacteria, the PCoA (Figure 3a) indicated that the first and second principal components accounted for 45.23% and 21.88% of the total variance, respectively. In the case of fungi, the first and second principal components explained 62.54% and 13.79% of the variance, respectively.
Figure 3.
PCoA analysis under CC and CT treatments. (a,b) represent the β-diversity of bacteria and fungi, respectively. The blue dots represent the fertilizer treatment group of T. harzianum, and the red dots represent the continuous cropping treatment group.
3.5. The Community Composition and Structure of CC and CT Groups
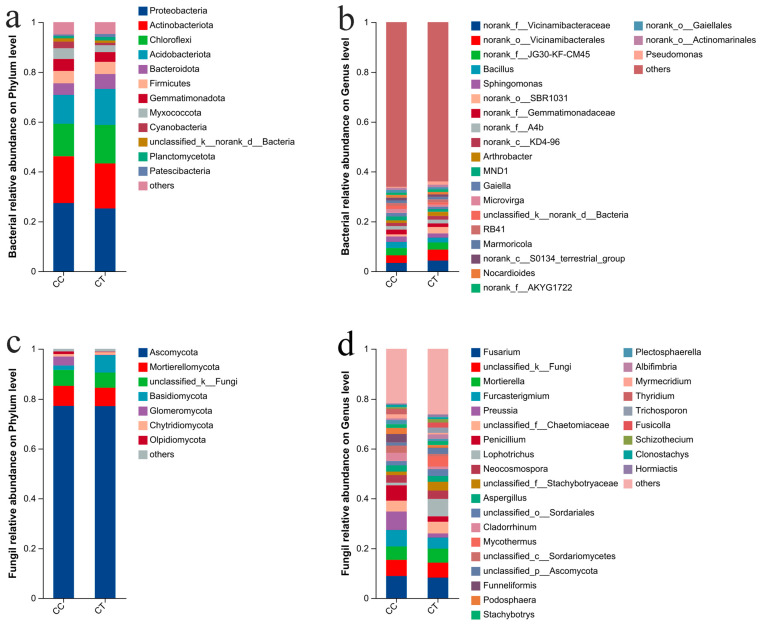
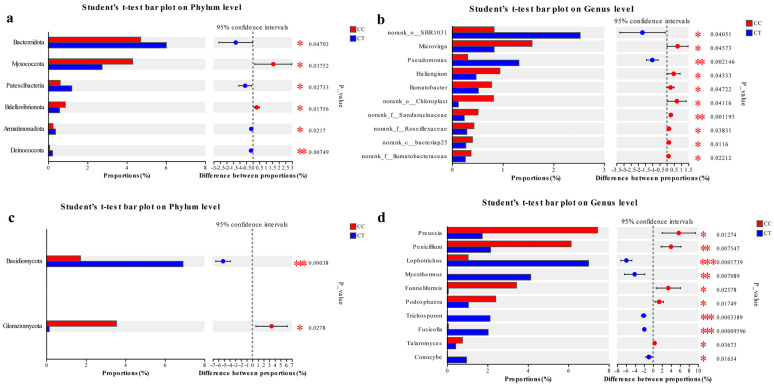
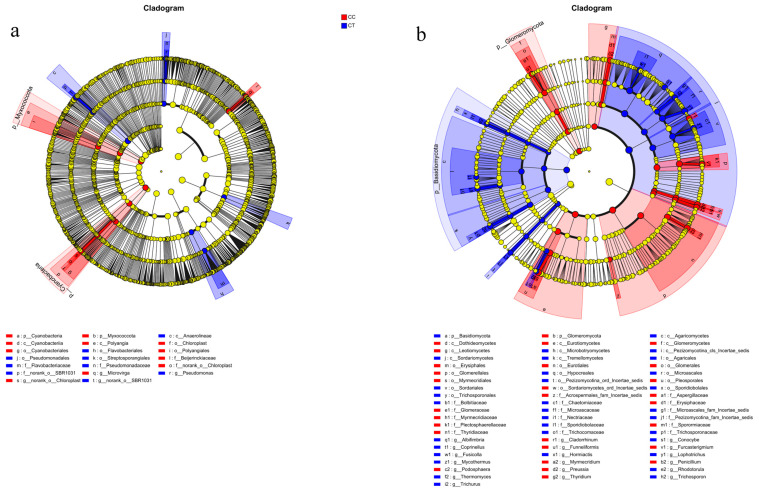
Following sequence classification with the Mothur program, the sample data were annotated and summarized. At the phylum level (Figure 4a and Figure 5a), the predominant phyla in the soil bacterial community were Proteobacteria, Actinobacteriota, Chloroflexi, Acidobacteriota, and Bacteroidota. These phyla constituted 75.49% of the bacterial community in the CC group and 79.22% in the CT group. Among them, Proteobacteria had the highest proportion in the two groups, with 27.39% and 25.19% for the CC and CT groups, respectively. Among the top 12 most abundant bacterial phyla, Bacteroidota, Patescibacteria, and Armatimonadota were found at significantly higher levels in the CT group compared to the CC group (p < 0.05). Additionally, Deinococcota showed a significantly greater presence in the CT group (p < 0.01). Conversely, Myxococcota and Bdellovibrionota were significantly less abundant in the CT group relative to the CC group. Overall, the distribution of soil bacteria in the two groups was similar at the phylum level. At the phylum level, the soil fungi in the samples were analyzed and annotated (Figure 4c and Figure 5c). The predominant fungal phyla were Ascomycota, Mortierellomycota, and Basidiomycota, which together comprised 86.9% and 91.39% of the fungal community in the CC and CT groups, respectively. Notably, the relative abundance of Ascomycota far exceeded the proportion of other phylums, which was 77.18% and 77.12% in the CC and CT groups, respectively, and was the most important component of the entire fungal community. The relative abundance of Basidiomycota was significantly higher in the CT group compared to the CC group (p < 0.001), while Glomeromycota was found at significantly lower levels in the CT group.
Figure 4.
Composition analysis under CC and CT treatments. (a) Relative abundance of bacteria at the phylum level; (b) relative abundance of bacteria at the genus level; (c) relative abundance of fungi at the phylum level; and (d) relative abundance of fungi at the genus level.
Figure 5.
Difference analysis under CC and CT treatments. (a,b) are differences in bacterial communities at the phylum and genus levels. (c,d) are the differences in fungal communities at the phylum and genus levels. On the right side is the p value. * indicates that there is a significant difference between the two sets of data (p < 0.05), ** indicates that there is a very significant difference between the two sets of data (p < 0.01), *** indicates that there is a highly significant difference between the two sets of data (p < 0.001), using the Student’s t-test.
At the genus level, the bacterial differences in the data of different treated samples were analyzed (Figure 4b and Figure 5b). Significant differences in genus distribution were observed. After the application of T. harzianum, Pseudomonas displayed a markedly higher relative abundance compared to the continuous cropping (CC) group. In contrast, the levels of Microvirga, Haliangium, and Ilumatobacter were notably reduced in the T. harzianum-treated samples relative to the CC group. Similarly, analysis of the fungal community at the genus level (Figure 4d and Figure 5d) revealed substantial differences in fungal genus distribution across treatments. Penicillium, Podosphaera, and Talaromyces were significantly more abundant in the CC group compared to the CT group (p < 0.05). The abundance of Preussia was significantly higher in the CC group (p < 0.01). Conversely, Conocybe was more prevalent in the CT group (p < 0.05), and Mycothermus was also significantly more abundant in the CT group (p < 0.01). Additionally, Lophotrichus, Trichosporon, and Fusicolla were present at significantly higher levels in the CT group compared to the CC group (p < 0.001). Furthermore, Fusarium showed a higher proportion in the CC group, with relative abundances of 8.86% and 8.21% in the CC and CT groups, respectively. Previous studies have shown that Fusarium and Podosphaera can cause a variety of diseases in plants.
3.6. Microbial LEfSe Analysis
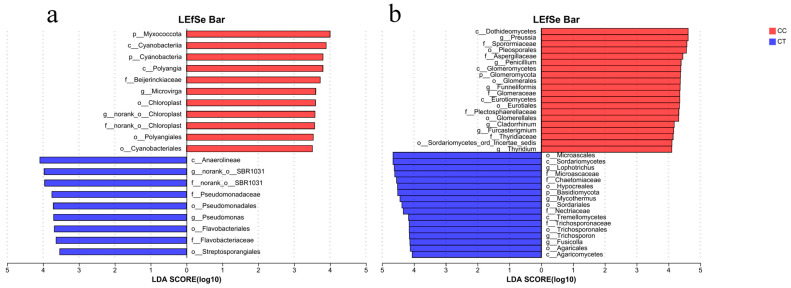
Linear discriminant analysis (LEfSe) was employed to assess differences in bacterial and fungal communities across four treatment groups, aiming to identify microbial species with significant variations in relative abundance. Species differentiation was determined using an LDA score greater than 3.5. The results of bacterial community analysis (Figure 6a and Figure 7a) showed that the biomarkers in the CC group included the following: Microvirga, Cyanobacteriia, Polyangia, Chloroplast, Polyangiales, and Cyanobacteriales. The biomarkers in the CT group, such as Pseudomonas, Flavobacteriaceae, and Streptosporangiales, were significantly enriched (p < 0.05). Additionally, these biomarkers demonstrated substantial enrichment (p < 0.05) within this group. The results of fungal community analysis (Figure 6b and Figure 7b) showed that the biomarkers in the CC group included the following: Preussia, Penicillium, Funneliformis, Cladorrhinum, Furcasterigmium, Thyridium, Podosphaera, and Myrmecridium, and they were significantly enriched (p < 0.05). The biomarkers in the CT group mainly include the following: Lophotrichus, Mycothermus, Trichosporon, Fusicolla, Albifimbria, Conocybe, Coprinellus, Trichurus, Rhodotorula, Hormiactis, and Thermomyces, and were significantly enriched (p < 0.05).
Figure 6.
Difference analysis of community LEfSe under CC and CT treatments. (a) Bacteria. (b) Fungi.
Figure 7.
LDA discrimination results under CC and CT processing. (a) Bacteria. (b) Fungi.
3.7. Functions of Bacterial and Fungal Communities
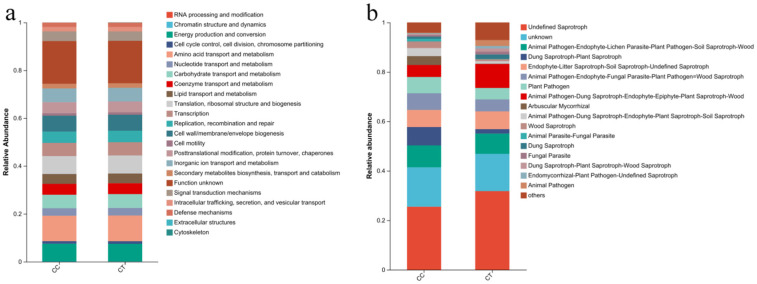
Functional prediction analyses provide an initial interpretation of the microbial community functions within the samples (Figure 8). The application of T. harzianum significantly affects the relative abundance of soil fungal communities (Figure 8b). Among the dominant fungi with higher abundance, the relative abundance of Animal Pathogen-Endophyte-Fungal Parasite-Plant Pathogen-Wood Saprotroph, Animal Pathogen-Endophyte-Lichen Parasite-Plant Pathogen-Soil Saprotroph-Wood Saprotroph, Dung Saprotroph-Plant Saprotroph, and Plant Pathogen were significantly higher in the CC group compared to the CT group. Conversely, in the CT group, the relative abundance of Endophyte-Litter Saprotroph-Soil Saprotroph-Undefined Saprotroph fungi was notably higher than in the CC group. The primary functions of the dominant bacterial communities include (Figure 8a) the following: amino acid transport and metabolism, translation, ribosomal structure and biogenesis, energy production and conversion, and biosynthesis of cell wall/membrane/envelope components. No significant differences were observed between the CT and CC groups in these functions (Figure 8a).
Figure 8.
Function prediction of soil microorganisms under CC and CT treatments. (a) Bacteria. (b) Fungi.
4. Discussion
4.1. The Promoting Effect of T. harzianum on the Growth of L. siceraria
Dry weight and fresh weight are important bases for evaluating plant adaptability and stress resistance [32]. Trichoderma, when used as a biofertilizer, can promote plant growth and induce plant disease resistance. Research has indicated that under greenhouse and field conditions, the application of irrigation methods using the Trichoderma strain TH1 can enhance the dry weight of lentil plants by as much as 120% [33]. T. harzianum has a beneficial effect on the fresh weight and moisture content of Cannabis sativa inflorescences [34]. In addition, T. harzianum significantly promoted the stem length and whole plant weight of Bupleurum chinense [35]. In pot experiments, mixing T. harzianum with soil from continuously cropped apple significantly promoted the growth of apple seedlings and increased enzyme activity in the rhizosphere soil [36]. Under greenhouse conditions, spraying T. harzianum TW21990 liquid on strawberry leaves also significantly enhanced strawberry growth and development. These results indicate that T. harzianum can promote plant growth through various application methods [37]. In this study, irrigation was used to treat continuously cropped L. siceraria seedlings, resulting in significant increases in plant height, dry weight, and fresh weight, similar to previously reported effects of other types of fertilizers on crop growth.
4.2. Effects of T. harzianum on Soil Chemical Properties and Enzyme Activities
Soil pH is a crucial indicator of soil health, and too acidic and alkaline soil environments are not conducive to plant growth [38]. In this study, the pH value was reduced from 7.78 to 7.51, which was more conducive to the survival of soil microorganisms suitable for the growth of gourd. Previous studies have demonstrated that functional microbial fertilizers can substantially enhance the availability of soil nutrients and alter the composition of soil microbial communities. This, in turn, regulates the accessibility and supply of rhizosphere nutrients, thereby improving soil fertility [39]. For instance, inoculating the rhizosphere of sweet sorghum with Trichoderma under salt stress led to significant increases in TN, AP, AK, and organic matter [19]. In this study, the changes in soil after applying T. harzianum are consistent with previous research. Soil enzymes, originating from microorganisms, plants, and animals are essential for the metabolic processes within soil ecosystems and play a vital role in nutrient cycling and energy flow [39]. The combined application of biochar and Trichoderma not only enhanced soil electrical conductivity, organic matter, AP, AK, and TN but also significantly boosted the activities of soil catalase and invertase [9]. Similarly, treatment with T. harzianum MHT114 in continuously cropped pepper fields resulted in increased levels of TN, organic matter, AP, and AK, alongside heightened soil enzyme activity [11]. In this study, the activities of urease, phosphatase, catalase, and sucrase in the soil were significantly increased after the application of T. harzianum, which was consistent with the reported studies. T. harzianum increased the activity of soil enzymes, promoting the transformation of nutrients, and improved soil conditions.
4.3. Effects of T. harzianum on Microbial Community Structure in the Rhizosphere Soil of L. siceraria
Proteobacteria dominated in different treatments. Proteobacteria, being the most prevalent in soil, plays a critical role in the carbon cycle. Following the application of T. harzianum, the relative abundance of Bacteroidota, Patescibacteria, and Armatimonadota in the CT group notably exceeded that observed in the CC group. These phyla are commonly found in soils and are integral to nutrient transformation processes. Research has demonstrated that Bacteroidota can promote the absorption of N, P, K, and other nutrients in plants, and can improve the defense stress ability of plants [40]. Proteobacteria can increase the content of soluble phosphorus in soil and the utilization rate of phosphorus fertilizer by plants to promote plant growth [41]. Armatimonadota can degrade complex carbohydrates [42]. This is consistent with previous studies. After the application of rhizosphere probiotic Bacillus, plant growth was promoted, and Proteobacteria was also induced to a significant enrichment of Proteobacteria in the plant rhizosphere [43]. In addition, many strains of Pseudomonas are plant growth-promoting rhizobacteria [41]. It was confirmed by Lefse analysis that it was a significantly enriched biomarker in the CT group. Similarly, inoculation of soil with Trichoderma M45a increased the relative abundance of Pseudomonas in the rhizosphere and promoted plant growth, corroborating the results of this study [44]. Thus, the application of T. harzianum markedly enhances the relative abundance of beneficial bacteria, such as Pseudomonas, within the bacterial community.
Ascomycota represents the predominant phylum within fungal communities and constitutes a major element of the overall fungal population. As the largest and most diverse fungal phylum in soil, it is a key organic decomposer. Fusarium can survive in normal plants and can cause plant disease when conditions are suitable. Previous studies have shown that Fusarium causes replant disease of apple trees [45]. After continuous cropping of watermelon, the concentration of Fusarium spores in the soil increased, which enhanced its reproductive capacity [46]. In this study, the application of T. harzianum resulted in a reduced proportion of Fusarium and Podosphaera in the CT group compared to CC group. At the same time, LEFse analysis confirmed that Podosphaera was a significantly enriched biomarker in the fungal community. This observation aligns with previous findings. The addition of Bacillus subtilis significantly reduced the abundance of Fusarium in the rhizosphere soil of Panax notoginseng. The application of Trichoderma asperellum also significantly reduced the proportion of Fusarium [47]. Thus, the incorporation of T. harzianum enhances plant growth by modifying the rhizosphere microbial community structure, increasing the relative abundance of beneficial bacteria like Pseudomonas and decreasing the prevalence of Fusarium and Podosphaera.
4.4. Effects of T. harzianum on Microbial Community Function in the Rhizosphere Soil of L. siceraria
Functional prediction of fungi revealed significant differences between the CC and CT groups. Previous studies have indicated that an increase in Animal Pathogen-Endophyte-Lichen Parasite-Plant Pathogen-Soil Saprotroph-Wood Saprotroph and a decrease in Endophyte-Litter Saprotroph-Soil Saprotroph-Undefined Saprotroph are associated with the onset of saffron rot compared to healthy saffron [48]. Similarly, research on Perilla frutescens showed that an increase in Plant Pathogen within the microbial community led to a decline in soil nutrients and inhibited plant growth [49]. These findings align with our results, where the relative abundance of harmful fungi was higher and beneficial fungi lower in the CC group compared to the CT group. In terms of bacterial functional prediction, no significant differences were observed between the CC and CT groups. This lack of difference might be due to the large diversity and abundance of soil microorganisms, where the functional changes in a few specific bacteria may not be reflected in the overall data. The application of T. harzianum was effective in reducing the abundance of harmful fungi and increasing the relative abundance of beneficial fungi, thereby improving the structure of the soil microbial community.
5. Conclusions
Using root irrigation methods with T. harzianum on soil from continuously cropped L. siceraria significantly promoted L. siceraria growth, improved the physico-chemical properties of the rhizosphere soil, and enhanced soil enzyme activity, thereby increasing the soil’s nutrient transformation capacity. High-throughput sequencing revealed that T. harzianum altered the microbial community structure of the soil, increasing the relative abundance of beneficial bacteria such as Proteobacteria while reducing the abundance of harmful fungi like Fusarium and Podosphaera. Therefore, T. harzianum is an effective microbial agent for alleviating the adverse effects of continuous L. siceraria cropping.
Author Contributions
Methodology, J.W.; Software, S.L.; Validation, J.W. and S.L.; Formal analysis, S.Q.; Writing—original draft, J.W. and S.M.; Writing—review & editing, H.M.; Visualization, J.W. and S.Q.; Supervision, H.M.; Project administration, H.M.; Funding acquisition, H.M. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by [The Natural Science Fund of Shandong Province] grant number [ZR2020MC031], [The Open Project of Liaocheng University Landscape Architecture Discipline] grant number [No. 319462212] and [Liaocheng University Student Innovation Project] grant number [No. 2022404360].
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Nkosi L.S., Ntuli N.R., Mavengahama S. Morpho-Agronomic Evaluation of Lagenaria siceraria Landraces and Their Populations. Plants. 2022;11:1558. doi: 10.3390/plants11121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maja D., Mavengahama S., Mashilo J. Cucurbitacin biosynthesis in cucurbit crops, their pharmaceutical value and agricultural application for management of biotic and abiotic stress: A review. S. Afr. J. Bot. 2022;145:3–12. doi: 10.1016/j.sajb.2021.08.044. [DOI] [Google Scholar]
- 3.Yao J., Wu C.Y., Fan L.J., Kang M.H., Liu Z.R., Huang Y.H., Xu X.L., Yao Y.J. Effects of the Long-Term Continuous Cropping of Yongfeng Yam on the Bacterial Community and Function in the Rhizospheric Soil. Microorganisms. 2023;11:274. doi: 10.3390/microorganisms11020274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sui J.K., Li C.Y., Wang Y.P., Li X.Y., Liu R., Hua X.W., Liu X.L., Qi H., Seo T. Microecological Shifts in the Rhizosphere of Perennial Large Trees and Seedlings in Continuous Cropping of Poplar. Microorganisms. 2024;12:58. doi: 10.3390/microorganisms12010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ul Haq M.Z., Yu J., Yao G.L., Yang H.G., Iqbal H.A., Tahir H., Cui H.G., Liu Y., Wu Y.G. A Systematic Review on the Continuous Cropping Obstacles and Control Strategies in Medicinal Plants. Int. J. Mol. Sci. 2023;24:12470. doi: 10.3390/ijms241512470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharyya S.S., Ros G.H., Furtak K., Iqbal H.M.N., Parra-Saldívar R. Soil carbon sequestration-An interplay between soil microbial community and soil organic matter dynamics. Sci. Total Environ. 2022;815:152928. doi: 10.1016/j.scitotenv.2022.152928. [DOI] [PubMed] [Google Scholar]
- 7.Liu F.Y., Zhu Q., Yang H.R., Zhou J., Dai C.C., Wang X.X. An integrated prevention strategy to address problems associated with continuous cropping of watermelon caused by Fusarium oxysporum. Eur. J. Plant Pathol. 2019;155:293–305. doi: 10.1007/s10658-019-01771-6. [DOI] [Google Scholar]
- 8.Gu X., Yang N., Zhao Y., Liu W.H., Li T.F. Long-term watermelon continuous cropping leads to drastic shifts in soil bacterial and fungal community composition across gravel mulch fields. BMC Microbiol. 2022;22:189. doi: 10.1186/s12866-022-02601-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou X.G., Gao D.M., Liu J., Qiao P.L., Zhou X.L., Lu H.B., Wu X., Liu D., Jin X., Wu F.Z. Changes in rhizosphere soil microbial communities in a continuously monocropped cucumber (Cucumis sativus L.) system. Eur. J. Soil. Biol. 2014;60:1–8. doi: 10.1016/j.ejsobi.2013.10.005. [DOI] [Google Scholar]
- 10.Liu J., Zhang M., Xu J., Yao X., Lou L., Hou Q., Zhu L., Yang X., Liu G., Xu J. A Transcriptomic Analysis of Bottle Gourd-Type Rootstock Roots Identifies Novel Transcription Factors Responsive to Low Root Zone Temperature Stress. Int. J. Mol. Sci. 2024;25:8288. doi: 10.3390/ijms25158288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao T.T., Jiang X.L. Changes in microbial community and enzyme activity in soil under continuous pepper cropping in response to Trichoderma hamatum MHT1134 application. Sci. Rep. 2021;11:21585. doi: 10.1038/s41598-021-00951-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al-Shuaibi B.K., Kazerooni E.A., Al-Maqbali D., Al-Kharousi M., Al-Yahya’ei M.N., Hussain S., Velazhahan R., Al-Sadi A.M. Biocontrol Potential of Trichoderma ghanense and Trichoderma citrinoviride toward Pythium aphanidermatum. J. Fungi. 2024;10:284. doi: 10.3390/jof10040284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tyskiewicz R., Nowak A., Ozimek E., Jaroszuk-Scisel J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022;23:2329. doi: 10.3390/ijms23042329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woo S.L., Hermosa R., Lorito M., Monte E. Trichoderma: A multipurpose, plant-beneficial microorganism for eco-sustainable agriculture. Nat. Rev. Microbiol. 2023;21:312–326. doi: 10.1038/s41579-022-00819-5. [DOI] [PubMed] [Google Scholar]
- 15.Sánchez-Montesinos B., Diánez F., Moreno-Gavira A., Gea F.J., Santos M. Plant Growth Promotion and Biocontrol of Pythium ultimum by Saline Tolerant Trichoderma Isolates under Salinity Stress. Int. J. Environ. Res. Public Health. 2019;16:2053. doi: 10.3390/ijerph16112053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erazo J.G., Palacios S.A., Pastor N., Giordano F.D., Rovera M., Reynoso M.M., Venisse J.S., Torres A.M. Biocontrol mechanisms of Trichoderma harzianum ITEM 3636 against peanut brown root rot caused by Fusarium solani RC 386. Biol. Control. 2021;164:104774. doi: 10.1016/j.biocontrol.2021.104774. [DOI] [Google Scholar]
- 17.Cao X., Zhang N., Zeng X.Y., Lan Q.L., Ma N., Wu C.C. Effects of biochar and Trichoderma on bacterial community diversity in continuous cropping soil. Hortic. Environ. Biotechnol. 2022;63:1–12. doi: 10.1007/s13580-021-00373-8. [DOI] [Google Scholar]
- 18.Patkowska E., Mielniczuk E., Jamiołkowska A., Skwaryło-Bednarz B., Błażewicz-Woźniak M. The Influence of Trichoderma harzianum Rifai T-22 and Other Biostimulants on Rhizosphere Beneficial Microorganisms of Carrot. Agronomy. 2020;10:1637. doi: 10.3390/agronomy10111637. [DOI] [Google Scholar]
- 19.Wei Y., Yang H., Hu J., Li H., Zhao Z., Wu Y., Li J., Zhou Y., Yang K., Yang H. Trichoderma harzianum inoculation promotes sweet sorghum growth in the saline soil by modulating rhizosphere available nutrients and bacterial community. Front. Plant Sci. 2023;14:1258131. doi: 10.3389/fpls.2023.1258131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu X., Jiang Q., Ma T., Wu R. Release risk of soil phosphorus under different farming systems: Indoor experiments and in-situ measurement. Soil. Tillage Res. 2024;240:106106. doi: 10.1016/j.still.2024.106106. [DOI] [Google Scholar]
- 21.Walkley A. An Examination of Methods for Determining Organic Carbon and Nitrogen in Soils. (with One Text-figure.) J. Agric. Sci. 1935;25:598–609. doi: 10.1017/S0021859600019687. [DOI] [Google Scholar]
- 22.Bremner J.M. Determination of nitrogen in soil by the Kjeldahl method. J. Agric. Sci. 1960;55:11–33. doi: 10.1017/S0021859600021572. [DOI] [Google Scholar]
- 23.Meena S.N., Sharma S.K., Singh P., Meena B.P., Ram A., Meena R.L., Singh D., Meena R.B., Nogiya M., Jain D., et al. Comparative analysis of soil quality and enzymatic activities under different tillage based nutrient management practices in soybean–wheat cropping sequence in Vertisols. Sci. Rep. 2024;14:6840. doi: 10.1038/s41598-024-54512-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhaoying L. Soil Sucrase: Detection Conditions Based on DNS Colorimetric. Chin. Agric. Sci. 2016;27:171–176. [Google Scholar]
- 25.Shah T., Khan Z., Asad M., Imran A., Niazi M.B.K., Dewil R., Ahmad A., Ahmad P. Straw incorporation into microplastic-contaminated soil can reduce greenhouse gas emissions by enhancing soil enzyme activities and microbial community structure. J. Environ. Manag. 2024;351:119616. doi: 10.1016/j.jenvman.2023.119616. [DOI] [PubMed] [Google Scholar]
- 26.Liu C., Zhao D., Ma W., Guo Y., Wang A., Wang Q., Lee D.-J. Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. Appl. Microbiol. Biotechnol. 2016;100:1421–1426. doi: 10.1007/s00253-015-7039-6. [DOI] [PubMed] [Google Scholar]
- 27.Chen S., Zhou Y., Chen Y., Gu J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i890. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Magoč T., Salzberg S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Edgar R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 30.Stackebrandt E., Goebel B.M. Taxonomic Note: A Place for DNA-DNA Reassociation and 16S rRNA Sequence Analysis in the Present Species Definition in Bacteriology. Int. J. Syst. Evol. Microbiol. 1994;44:846–849. doi: 10.1099/00207713-44-4-846. [DOI] [Google Scholar]
- 31.Wang Q., Garrity G.M., Tiedje J.M., Cole J.R. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussein H.-A.A., Alshammari S.O., Abd El-Sadek M.E., Kenawy S.K.M., Badawy A.A. The Promotive Effect of Putrescine on Growth, Biochemical Constituents, and Yield of Wheat (Triticum aestivum L.) Plants under Water Stress. Agriculture. 2023;13:587. doi: 10.3390/agriculture13030587. [DOI] [Google Scholar]
- 33.Marra R., Lombardi N., Piccolo A., Bazghaleh N., Prashar P., Vandenberg A., Woo S. Mineral Biofortification and Growth Stimulation of Lentil Plants Inoculated with Trichoderma Strains and Metabolites. Microorganisms. 2022;10:87. doi: 10.3390/microorganisms10010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang L.L., Fan C., Yuan H.M., Wu G.W., Sun J., Zhang S.Q. The Effect of Rotational Cropping of Industrial Hemp (Cannabis sativa L.) on Rhizosphere Soil Microbial Communities. Agronomy. 2022;12:2293. doi: 10.3390/agronomy12102293. [DOI] [Google Scholar]
- 35.Liu L., Xu Y.S., Cao H.L., Fan Y., Du K., Bu X., Gao D.M. Effects of Trichoderma harzianum biofertilizer on growth, yield, and quality of Bupleurum chinense. Plant Direct. 2022;6:e461. doi: 10.1002/pld3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang R., Yan Z., Wang Y., Chen X., Yin C., Mao Z. Effects of Trichoderma harzianum Fertilizer on the Soil Environment of Malus hupehensis Rehd. Seedlings under Replant Conditions. HortScience. 2021;56:1073–1079. doi: 10.21273/HORTSCI15970-21. [DOI] [Google Scholar]
- 37.Li W., Fu Y., Jiang Y., Hu J., Wei Y., Li H., Li J., Yang H., Wu Y. Synergistic Biocontrol and Growth Promotion in Strawberries by Co-Cultured Trichoderma harzianum TW21990 and Burkholderia vietnamiensis B418. J. Fungi. 2024;10:551. doi: 10.3390/jof10080551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng X.F., Wang Z.R., Zhu Y.J., Wang J.P., Liu B. Effects of a microbial restoration substrate on plant growth and rhizosphere bacterial community in a continuous tomato cropping greenhouse. Sci. Rep. 2020;10:13729. doi: 10.1038/s41598-020-70737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z.R., Tang S.S., Liu X.D., Ren X.L., Wang S.N., Gao Z.G. The Effects of Trichoderma viride T23 on Rhizosphere Soil Microbial Communities and the Metabolomics of Muskmelon under Continuous Cropping. Agronomy. 2023;13:1092. doi: 10.3390/agronomy13041092. [DOI] [Google Scholar]
- 40.Pan X., Raaijmakers J.M., Carrión V.J. Importance of Bacteroidetes in host–microbe interactions and ecosystem functioning. Trends Microbiol. 2023;31:959–971. doi: 10.1016/j.tim.2023.03.018. [DOI] [PubMed] [Google Scholar]
- 41.Emenike C.U., Agamuthu P., Fauziah S.H., Omo-Okoro P.N., Jayanthi B. Enhanced Bioremediation of Metal-Contaminated Soil by Consortia of Proteobacteria. Water Air Soil. Pollut. 2023;234:731. doi: 10.1007/s11270-023-06729-3. [DOI] [Google Scholar]
- 42.Carlton J.D., Langwig M.V., Gong X., Aguilar-Pine E.J., Vázquez-Rosas-Landa M., Seitz K.W., Baker B.J., De Anda V. Expansion of Armatimonadota through marine sediment sequencing describes two classes with unique ecological roles. ISME Commun. 2023;3:64. doi: 10.1038/s43705-023-00269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun X., Xu Z., Xie J., Hesselberg-Thomsen V., Tan T., Zheng D., Strube M.L., Dragoš A., Shen Q., Zhang R., et al. Bacillus velezensis stimulates resident rhizosphere Pseudomonas stutzeri for plant health through metabolic interactions. ISME J. 2022;16:774–787. doi: 10.1038/s41396-021-01125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Y., Tian C., Xiao J.L., Wei L., Tian Y., Liang Z.H. Soil inoculation of Trichoderma asperellum M45a regulates rhizosphere microbes and triggers watermelon resistance to Fusarium wilt. AMB Express. 2020;10:189. doi: 10.1186/s13568-020-01126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang G.S., Yin C.M., Pan F.B., Wang X.B., Xiang L., Wang Y.F., Wang J.Z., Tian C.P., Chen J., Mao Z.Q. Analysis of the Fungal Community in Apple Replanted Soil Around Bohai Gulf. Hortic. Plant J. 2018;4:175–181. doi: 10.1016/j.hpj.2018.05.003. [DOI] [Google Scholar]
- 46.Hao W.Y., Ren L.X., Ran W., Shen Q.R. Allelopathic effects of root exudates from watermelon and rice plants on Fusarium oxysporum f.sp. niveum. Plant Soil. 2010;336:485–497. doi: 10.1007/s11104-010-0505-0. [DOI] [Google Scholar]
- 47.Wang H.Y., Zhang R., Mao Y.F., Jiang W.T., Chen X.S., Shen X., Yin C.M., Mao Z.Q. Effects of Trichoderma asperellum 6S-2 on Apple Tree Growth and Replanted Soil Microbial Environment. J. Fungi. 2022;8:63. doi: 10.3390/jof8010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Song J., Xi X., Qian X., Li J., Tao Y., Li L., Zhou G. Revealing the microbial composition changes and relationship with Fusarium caused by rot disease in the Crocus sativus L. Ann. Microbiol. 2024;74:24. doi: 10.1186/s13213-024-01770-x. [DOI] [Google Scholar]
- 49.Xue T., Fang Y., Li H., Li M., Li C. The Effects of Exogenous Benzoic Acid on the Physicochemical Properties, Enzyme Activities and Microbial Community Structures of Perilla frutescens Inter-Root Soil. Microorganisms. 2024;12:1190. doi: 10.3390/microorganisms12061190. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.