Abstract
(1) Background/Objectives: Nail psoriasis (NP) is a chronic and difficult-to-treat disease, which causes significant social stigma and impairs the patients’ quality of life. Moreover, nail psoriasis is a true therapeutic challenge for clinicians. The presence of nail psoriasis can be part of a severe form of psoriasis and can have predictive value for the development of psoriatic arthritis. Our real-world-evidence multicenter study aims to evaluate the efficacy of bimekizumab in nail psoriasis. (2) Methods: A retrospective analysis of a multicenter observational study included 834 patients affected by moderate-to-severe psoriasis, in 33 Dermatologic Units in Italy, treated with bimekizumab from December 2022 to September 2023. Clinimetric assessments were based on Psoriasis Area and Severity Index (PASI), Dermatology Life Quality Index (DLQI), and Physician’s Global Assessment of Fingernail Psoriasis (PGA-F) for the severity of nail psoriasis at 0, 12, 24, and 36 weeks. (3) Results: Psoriatic nail involvement was present in 27.95% of patients. The percentage of patients who achieved a complete clearance of NP in terms of PGA-F 0 was 31.7%, 57%, and 88.5% at week 4, 16, and 36, respectively. PASI 100 was achieved by 32.03% of patients at week 4, by 61.8% at week 16, and by 78.92% of patients at week 36. The mean baseline PASI was 16.24. The mean DLQI values for the entire group of patients at baseline, at week 4, at week 16, and at week 36 were 14.62, 3.02, 0.83, and 0.5, respectively. (4) Conclusions: Therapies that promote the healing of both the skin and nails in a short time can also ensure a lower risk of subsequently developing arthritis which is disabling over time. Bimekizumab proved to be particularly effective to treat NP, with a fast response in terms of complete clearance, with over 88.5% of patients free from NP after 36 weeks. The findings of our real-world study showed that patients with moderate-to-severe PsO and concomitant NP had significantly faster and more substantial improvements in NP up to 36 weeks with respect to previous research findings. Considering the rapid healing of the nail, the dual inhibition of IL17 A and F might have a great value in re-establishing the dysregulation of keratin 17 at the nail level.
Keywords: bimekizumab, nail psoriasis, interleukin 17, psoriasis, PGA-F, PASI
1. Introduction
Psoriasis is a chronic inflammatory disease that affects up to 3% of the general population. It is more frequent in the white ethnic group and in Northern European countries; it has a lower incidence in Africans, Japanese, and Eskimos, and a very rare incidence in South Americans. Two incidence peaks have been reported, between the second and third, and between the fourth and sixth decades [1,2]. Psoriasis is a systemic inflammatory disease in which the dysregulation of the immune system results in an overexpression of consequently activated T-helper (Th) 17, leading to the uncontrolled proliferation of keratinocytes, acanthosis, neovascularization, and potent skin infiltration by immune cells [3]. The disease is characterized by epidermal hyperproliferation with the formation of erythematous squamous skin plaques that can cover large areas of the body; it is considered a multiorgan disease that requires a multidisciplinary approach and adequate management, taking into account a number of comorbidities [1,2,4]. In the early pathogenic stages of psoriasis, several cell types are present, including plasmacytoid dendritic cells, keratinocytes, natural killer T cells, and macrophages, that secrete cytokines, which activate myeloid dendritic cells [2,5]. Dendritic cells produce TNF-α and IL-23 to promote T cell differentiation toward TH17 cells that produce key psoriatic cytokines IL-17, IFN-γ, and IL-22 [2].
Interleukin 17, in particular, plays a key role in the resulting inflammation and joint injury [3]. It has been initially described as a Th17-produced cytokine, but it is now established that other cell types can also be a source, such as gamma delta T lymphocytes, Mucosal-Associated Invariant T (MAIT) cells, and Innate Lymphoid Cells 3; those cells act as an IL-23-independent source of IL17 in the skin in response to inflammatory stimuli [6]. Interleukin 17, first described by Yao et al. in 1995, is a family of pro-inflammatory cytokines composed of IL-17A, IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F, secreted by T cells, natural killer cells, mast cells, and neutrophils [7]. While IL-17A is more potent, IL-17F is more abundant in skin lesions of psoriasis (by approximately 30-fold), and can drive inflammation independently of IL-17A [7,8,9]. When psoriasis affects visible areas of the body including the face, hands, scalp, and nails, it is strongly associated with physical and quality-of-life impairment, including a negative notable impact on social relationships, mental health, and work activities [10]. The prevalence of nail involvement in psoriatic patients varies between 10% and 90% [9,11,12], with a prevalence of about 32% in children [12]. Approximately 90% of psoriatic patients develop nail psoriasis (NP) during their lifetime and this is not related to gender or age [13,14]. The pathogenesis of NP has not been fully clarified yet, although some peculiar inflammatory cytokines and chemokines seems to be the same as those described in psoriatic skin lesions [15,16,17,18,19]. Nail psoriasis (NP) is often related to long-lasting psoriasis and severity of skin and joint involvement [18,19,20]. Furthermore, psoriatic nail disease may be considered a risk predictor factor for the development of psoriatic arthritis (PSA) and could be considered a form of enthesitis in the early stage of rheumatic disease [19]. Nail lesions, including pitting and onycholysis, occur in approximately 80% to 90% of patients with PsA [21]. The nail bed, nail matrix, hyponychium, and nail folds can be affected by NP. The most observed forms are psoriasis of the nail matrix, the nail bed, and the nail fold [13]. Pitting, leukonychia, red spots of the lunula, transverse furrows (Beau’s lines), and crumbling of the nail plates are the typical signs of nail matrix psoriasis [20]. Oil drop discoloration, splinter hemorrhages involving the distal third of the nail plate, subungual hyperkeratosis, and/or detachment of the nail plate from the nail bed (onycholysis) are characteristic signs of nail bed involvement [13]. Psoriasis of the periungual region is characterized by paronychia [13]. The severity of NP is assessed using the Nail Psoriasis Severity Index (NAPSI) which is a numerical, reproducible, objective, and simple tool. Another tool for a better monitoring of the nail treatment outcome is the Physician’s Global Assessment of Fingernail Psoriasis (PGA-F) [21,22].
PGA-F, developed by Hudgens et al., according to best practices [22], was designed to combine ratings across multiple components of nail severity into one of five distinct classifications ranging from clear to severe. The usefulness of this classification system is that total scores, and changes in scores, are easy to quantify and clinically relevant [22].
Differential diagnosis between onychomycosis and psoriatic nail may be difficult; however, there may even be a coexistence of onychomycosis and NP, and both of them are common disorders in the general population [23,24,25,26,27,28].
Previous findings have shown an increased expression of tumor necrosis factor (TNF)-α, nuclear factor kappa B, IL-6, and IL-8 in psoriasis-affected nails [29,30,31,32]. Several studies have shown a downregulation of IL-10 in psoriatic skin lesions [32]. In contrast, Saulite et al. found an increased IL-10 expression in the affected nail bed, suggesting unique pathways of psoriatic nail disease and toenail as an immune-privileged site [33,34,35].
Nail therapeutic management is based on clinical presentation and patient-related factors. Most patients have mild NP without arthropathic disease or severe skin psoriasis [1]. Topical therapy may be suggested for these patients, while systemic therapy is indicated for patients with severe NP and those with a greater impact on the quality of life or with moderate-to-severe psoriasis. Age, disease burden, comorbidities, individual patient treatment preferences, and treatment risks should be considered to establish the treatment strategy [1]. First-line treatments for few-nail disease (≤3 nails involved) include topicals and intralesional injections. Topical treatments are steroids, vitamin D3 analog calcipotriol or tacalcitol or calcitriol used as monotherapy or combined with corticosteroids, tazarotene, topical calcineurin inhibitors, and 5-fluorouracil [35,36,37,38,39].
Regarding biologic agents used to treat NP, most data recorded to date are on adalimumab which is also the only biologic therapy with efficacy data cited in the US Food and Drug Administration’s package insert [35,36,37,38,39,40]. However, many other biologic and systemic therapies have been studied for the treatment of NP, including certolizumab, golimumab, etanercept, infliximab, ustekinumab, guselkumab, tildrakizumab, risankizumab, ixekizumab, secukinumab, brodalumab, and bimekizumab [41]. NP has also been treated with apremilast, a small molecule, that, as a selective inhibitor of phosphodiesterase 4, leads to an increase in cAMP levels, downregulating the expressions of TNFα, IL-17, and IL-23 with an upregulation of the anti-inflammatory IL-10 [41,42].
A systematic literature review gave a strong recommendation for the use of biologic agents including tumor necrosis factor inhibitors (TNFi) and interleukin (IL)-12/23, 17, and 23 inhibitors in patients with NP [38]. Although outcome data are difficult to compare, interleukin (IL)-17 inhibitors may have superior short-term efficacy for NP when compared to IL-23 inhibitors and tumor necrosis factor (TNF)-alpha inhibitors, although long-term efficacy is similar to TNF-alpha inhibitors [42].
Based on the available evidence regarding the role of IL17 in psoriasis and PSA, four therapeutic agents against IL-17A, IL17F, or its receptor have been developed: secukinumab, ixekizumab, brodalumab, and bimekizumab. Significant reductions in NAPSI scores were observed using ixekizumab as early as 2 weeks, up to 20 weeks, and in an open-label extension [43,44,45]. The complete remission of NP was achieved in a high percentage of patients: 43% at week 44 and 51% at week 68 [44]. Brodalumab showed great efficacy in reducing NAPSI in several studies, including three phase 3 trials, ensuring the complete clearance of psoriatic onychopathy at week 52 in almost 64% of affected patients [45]. In a network meta-analysis, brodalumab had the absolute probability of achieving a complete resolution of NP at weeks 24–28 in 37.1% of patients. For bimekizumab, the authors reported an NP complete resolution for 26.7%, 62.1%, and 70.7% of patients at weeks 16, 32, and 48, respectively [46].
The high response rate of NP during anti-IL-17 therapy is also documented by the immunohistochemical evidence of pathogenetically relevant molecules in psoriasis, in particular in NP and PSA [35].
Within this context, cathelicidin (LL-37) is an antimicrobial peptide whose cellular expression levels have been found to be higher in the psoriatic nail bed compared to the control nail bed [35]. IL-17A seems to enhance keratin 17 expression by keratinocytes [47].
A recent study found significantly higher serum IL-17 levels in psoriatic patients with nail involvement compared to those without nail involvement (p = 0.002) [48].
Bimekizumab is a humanized IgG1/κ monoclonal antibody that binds selectively and with high affinity to the cytokines IL-17A, IL-17F, and IL-17A/F, blocking their interaction with the IL-17RA receptor complex IL-17RC [49].
Bimekizumab was approved in 2021 by the European Medicine Agency for chronic plaque psoriasis in adult patients eligible for systemic therapy [50].
Real-life data of bimekizumab are quite aligned, showing a fast response in terms of reduction in PASI and DLQI, with approximately 43% of patients able to reach PASI100 after just 4 weeks of therapy and over 70% after 16 weeks, with infrequent adverse reactions of limited severity [51].
In this regard, a multicenter retrospective real-life clinical study is presented to document the efficacy of bimekizumab in NP in a cohort of 834 patients affected by moderate-to-severe psoriasis followed during 36 weeks.
2. Results
The clinical and demographic characteristics of our 834 patients recorded at baseline (W0) are shown in Table 1. In particular, 543 were male and 291 were female (Table 2).
Table 1.
Demographic and clinical characteristic of enrolled patients.
| Variable | N | Mean | SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Age at BKZ first dose (years) | 834 | 50.13 | 14.77 | 51.00 | 18.00 | 87.00 |
| Weight (kg) | 792 | 81.01 | 17.63 | 80.00 | 46.00 | 178.00 |
| Height (cm) | 791 | 171.93 | 10.04 | 173.00 | 72.00 | 201.00 |
| BMI | 791 | 27.49 | 6.99 | 26.31 | 16.90 | 125.00 |
| Time from PSO diagnosis (years) | 697 | 14.83 | 12.57 | 12.00 | 0.00 | 57.00 |
Table 2.
Gender distribution and other clinical characteristics of enrolled patients.
| Gender | Frequency | Percent |
|---|---|---|
| Female | 291 | 34.89 |
| Male | 543 | 65.11 |
| Covid-19 | Frequency | Percent |
| NO | 557 | 66.79 |
| YES | 277 | 33.21 |
| Naive to systemic treatment | Frequency | Percent |
| NO | 703 | 84.29 |
| YES | 131 | 15.71 |
| Naive to biologic therapies | Frequency | Percent |
| NO | 385 | 46.1% |
| YES | 359 | 43.05% |
| Undefined | 32 | 3.84% |
| Arthritis | Frequency | Percent |
| NO | 693 | 83.49 |
| YES | 109 | 13.13 |
| Undefined | 28 | 3.37 |
| Other comorbidities | Frequency | Percent |
| None | 370 | 44.36 |
| At least one comorbidity | 464 | 55.64 |
| Cardiovascular disease | 70 | 8.4 |
| Diabetes | 93 | 11.2 |
| Hypertension | 254 | 30.5 |
| Hyperlipidemia | 142 | 17.0 |
| Neoplasias | 31 | 3.7 |
| Other | 198 | 23.7 |
Patients with at least one comorbidity were 464 out of 834 (55.6%); in particular, they were affected by arterial hypertension as the most frequent comorbidity (30.5%), followed by hypercholesterolemia (17%), PsA (13.3%), diabetes (11.2%), heart disease (8.4%), and cancer (3.7%), whereas 23.7% patients were affected by other pathologies (Table 2).
All patients were affected by moderate-to-severe plaque-type psoriasis. Furthermore, 109 patients (13.13%) had concomitant PsA (Table 2).
The involvement of difficult-to-treat sites was also evaluated: 342 (41.0%) patients were affected by scalp psoriasis, 232 (27.95%) had NP, and a further 153 (18.3%) had genital involvement. Furthermore, 11 (1.3%) patients showed palmoplantar psoriasis.
In addition, stratification was carried out for previous treatments (Table 2): 131 (15.7%) of subjects were naïve to systemic treatment, and 359 (43.05%) were naïve to biologic therapies. A previous biologic therapy failed in 443 patients (53.12%).
In particular, 173 patients (38%) used Adalimumab, 57 (12%) Secukinumab, 53 (11%) Etanercept, 38 (8%) Ustekinumab, 35 (8%) Ixekizumab, 26 (6%) Adalimumab biosimilar, 18 (4%) Brodalumab, 16 (3%) Risankizumab, 13 (3%) Apremilast, 10 (2%) Infliximab, 8 (2%) Guselkumab, 6 (1%) Tildrakizumab, 4 (1%) Etanercept biosimilar, and 4 (1%) Infliximab biosimilar. Some patients had previously undergone more than one treatment with a biologic drug.
The mean baseline PASI was 16.24 (9.03) in the overall patient population (Table 3).
Table 3.
PASI and DLQI at baseline.
| Variable | n | Mean | SD | Median |
|---|---|---|---|---|
| PASI | 834 | 16.24 | 9.03 | 15.00 |
| DLQI | 830 | 14.62 | 8.81 | 15.00 |
A score of PASI 100 was reached by 32.03% of patients at week 4, by 61.8% at week 16, and by 78.92% of patients at week 36 (Table 4).
Table 4.
PASI100 at each time point.
| PASI100 | Frequency | Percent |
|---|---|---|
| Week 4 (n = 512) | 164 | 32.03 |
| Week 16 (n = 411) | 254 | 61.80 |
| Week 36 (n = 223) | 176 | 78.92 |
Psoriatic nail involvement was present in 27.95% of patients (Table 5), and the clinical and demographic characteristics of this subpopulation of patients are described in Table 6. Patients affected by NP were predominantly male (71.55%), and 84.05% of the entire group had already received previous systemic conventional therapy, whilst 49.14% had one or more previous treatments with a biological drug (Table 7).
Table 5.
Presence of nail involvement at baseline.
| Nail Involvement | Frequency | Percent |
|---|---|---|
| NO | 579 | 69.76 |
| YES | 232 | 27.95 |
| Undefined | 19 | 2.29 |
| Frequency Missing = 4 | ||
Table 6.
Demographic data and baseline characteristics in patients with nail involvement at baseline.
| Variable | n | Mean | SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Age at BKZ first dose (years) | 232 | 51.52 | 14.21 | 53.00 | 19.00 | 87.00 |
| Weight (kg) | 226 | 82.35 | 16.77 | 80.00 | 46.00 | 178.00 |
| Height (cm) | 225 | 173.13 | 8.48 | 175.00 | 148.00 | 193.00 |
| BMI | 225 | 27.44 | 5.02 | 26.56 | 17.63 | 54.94 |
| Time from diagnosis (years) | 183 | 14.28 | 11.86 | 12.00 | 0.00 | 48.00 |
Table 7.
Gender distribution and other clinical characteristics of patients with nail involvement at baseline.
| Frequency | Percent | |
|---|---|---|
| Female | 66 | 28.45 |
| Male | 166 | 71.55 |
| Covid-19 | Frequency | Percent |
| NO | 135 | 58.19 |
| YES | 97 | 41.81 |
| Systemic therapy | Frequency | Percent |
| NO | 195 | 84.05 |
| YES | 37 | 15.95 |
| Bio-naive | Frequency | Percent |
| NO | 105 | 49.53 |
| YES | 107 | 50.47 |
| Comorbidities | Frequency | Percent |
| None | 93 | 40.1% |
| Comorbidities ≥ 1 | 139 | 59.9% |
| Cardiovascular diseases | 19 | 8.2% |
| Diabetes | 28 | 12.1% |
| Hypertension | 81 | 34.9% |
| Hyperlipidemia | 45 | 19.4% |
| Neoplasias | 10 | 4.3% |
| Other | 71 | 30.6% |
The comorbidities found in the NP patient population were mostly similar to those found for the entire patient group (Table 7).
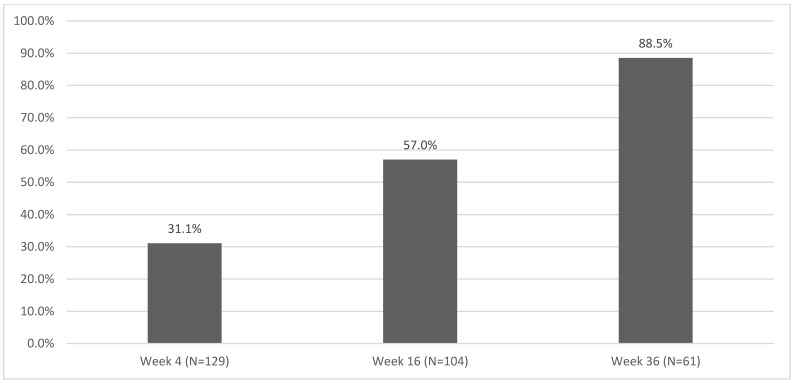
Figure 1 shows the percentages of patients who achieved a complete clearance of NP. In total, 31.7%, 57%, and 88.5% of patients achieved PGA-F 0 at week 4, 16, and 36, respectively.
Figure 1.
Percentage of patients with PGA-F = 0 at different time points.
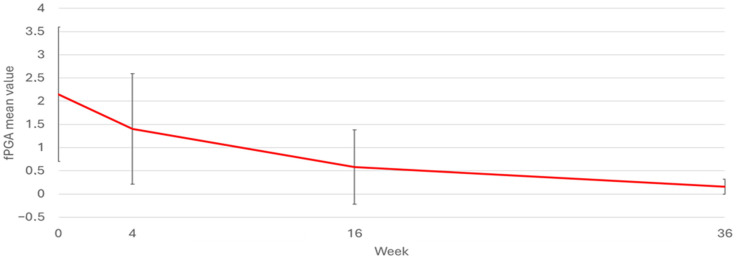
In Figure 2, it is possible to appreciate the trend in PGA-F at the various time points for the entire group of patients affected by NP. The values of PGA-F at baseline, week 4, week 16, and week 36 were 2.15, 1.4, 0.58, and 0.16, respectively. The mean percentage reduction in PGA-F from baseline to week 36 was 92.5% (p-value < 0.0001).
Figure 2.
PGA−F mean value over time. Values of PGA−F at baseline, week 4, week 16, and week 36 were 2.15, 1.4, 0.58, and 0.16, respectively.
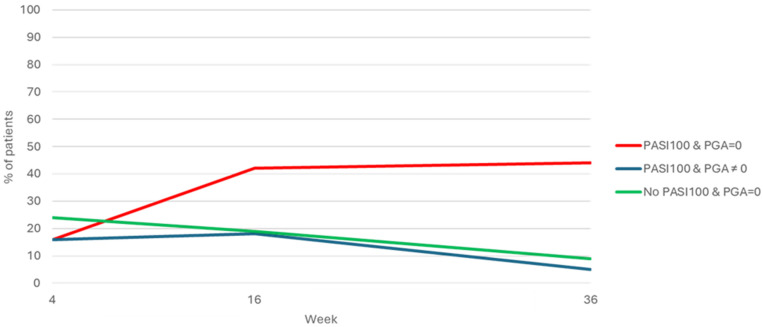
In the subgroup of patients with nail involvement, the percentage of patients who achieved complete skin and nail clearance was reported at different time points (Figure 3). At W4, the population reaching PASI 100 and PGA-F = 0, PASI 100 and PGA-F ≠ 0, and no-PASI 100 and PGA-F = 0 was 12.21%, 12.21%, and 18.32%, respectively. At W16, the population reaching PASI 100 and PGA-F = 0, PASI 100 and PGA-F ≠ 0, and no-PASI 100 and PGA-F = 0 was 39.25%, 16.82%, and 17.76%, respectively. At W36, the population reaching PASI 100 and PGA-F = 0, PASI 100 and PGA-F ≠ 0, and no-PASI 100 and PGA-F = 0 was 73.33%, 8.33%, and 15%, respectively (Figure 3). Figure 1 shows the group that did not achieve complete clearance at the various control time points, corresponding to the smallest one.
Figure 3.
Percentage of patients with complete clearance of NP and PSO assessed by PGA-F = 0 and PASI100 at different time points.
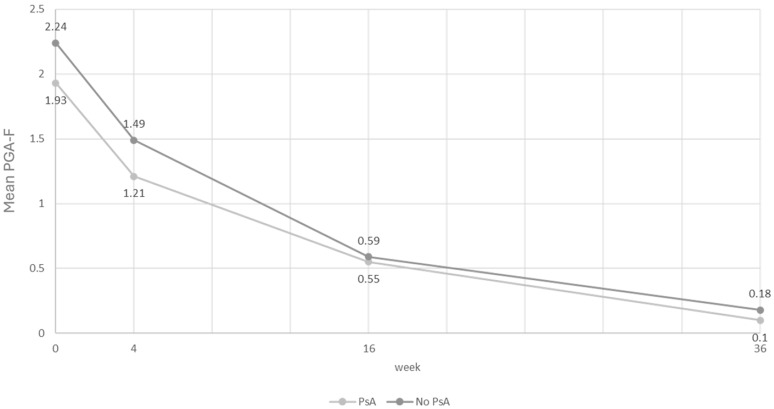
Among patients suffering from NP, those also suffering from PSA showed slightly lower mean baseline PGA-F values than patients without PSA (Table 8). The difference in the reduction in PGA-F in the two subgroups of patients did not show any statistical significance, although there was an appreciable reduction at W36 of 2.27 times and 2.4 times the average value of PGA-F in the population without PsA and in that with PsA, respectively (Table 8). The trend at the various time points is comparable between the two groups, showing a rapid and parallel reduction in PGA-F values (Figure 4).
Table 8.
PGA-F stratified by PsA presence/absence at baseline.
| PsA | Variable: PGA-F | N | Mean | SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|---|
| NO | Baseline | 158 | 2.24 | 1.43 | 3.00 | 0.00 | 4.00 |
| Week 4 | 101 | 1.49 | 1.22 | 2.00 | 0.00 | 4.00 | |
| Week 16 | 83 | 0.59 | 0.84 | 0.00 | 0.00 | 4.00 | |
| Week 36 | 49 | 0.18 | 0.63 | 0.00 | 0.00 | 4.00 | |
| Change w4 vs. baseline | 99 | −0.91 | 1.09 | −1.00 | −4.00 | 1.00 | |
| Change w16 vs. baseline | 81 | −1.72 | 1.25 | −2.00 | −4.00 | 2.00 | |
| Change w36 vs. baseline | 49 | −2.27 | 1.25 | −2.00 | −4 | 0.00 | |
| YES | Baseline | 43 | 1.93 | 1.5 | 2.00 | 0.00 | 4.00 |
| Week 4 | 28 | 1.21 | 1.07 | 1.00 | 0.00 | 3.00 | |
| Week 16 | 22 | 0.55 | 0.67 | 0.00 | 0.00 | 2.00 | |
| Week 36 | 10 | 0.10 | 0.32 | 0.00 | 0.00 | 1.00 | |
| Change w4 vs. baseline | 27 | −1.33 | 1.24 | −1.00 | −4.00 | 0.00 | |
| Change w16 vs. baseline | 21 | −1.81 | 1.17 | −2.00 | −3.00 | 0.00 | |
| Change w36 vs. baseline | 10 | −2.4 | 1.07 | −3.00 | −4.00 | −1.00 |
Figure 4.
Graphical trend in PGA-F stratified by presence or absence of PSA in patients with NP.
Wilcoxon p-value between PsA vs. no PsA. Change at week 4 vs. baseline: 0.1375. Change at week 16 vs. baseline: 0.7175. Change at week 36 vs. baseline: 0.7939.
The mean DLQI values for the entire group of patients at baseline, at week 4, at week 16, and at week 36 were 14.62, 3.02, 0.83, and 0.5, respectively. The mean DLQI reduction from baseline to week 36 was 96.6% (Table 9). Two clinical cases of nail psoriasis can be seen in Figure 5 and Figure 6.
Table 9.
Evaluation of DLQI at baseline, and at weeks 4, 16, 36.
| Variable | N° | Mean | Std Dev | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Baseline | 830 | 14.62 | 8.81 | 15.00 | 0.00 | 30.00 |
| Week 4 | 512 | 3.02 | 4.06 | 2.00 | 0.00 | 23.00 |
| Week 16 | 411 | 0.83 | 1.87 | 0.00 | 0.00 | 15.00 |
| Week 36 | 225 | 0.50 | 1.66 | 0.00 | 0.00 | 15.00 |
Figure 5.
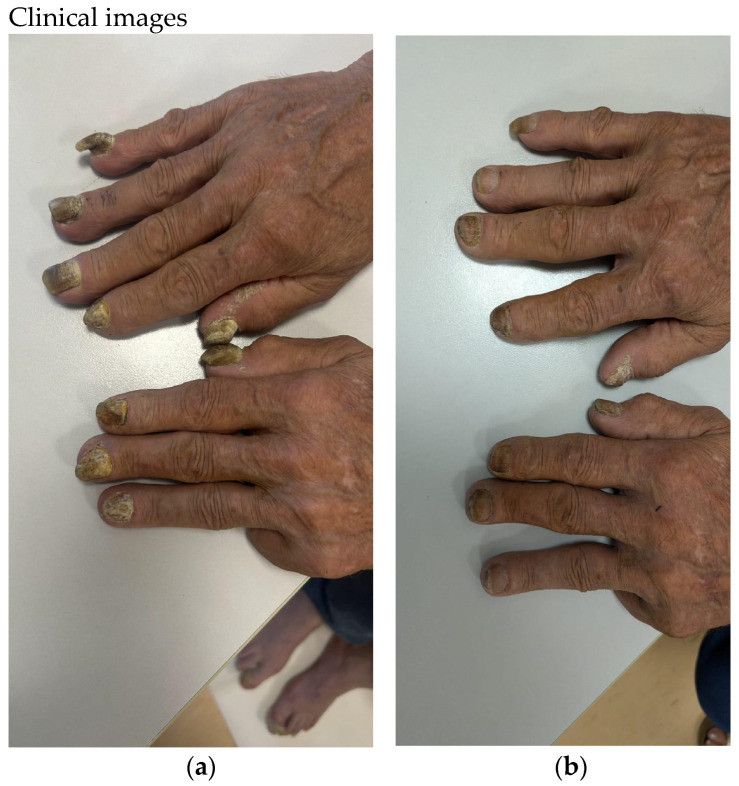
(a) Case 1—Nail psoriasis in male patient at baseline, PGA-F: 4. (b) After 36 weeks of treatment with bimekizumab, PGA-F: 2.
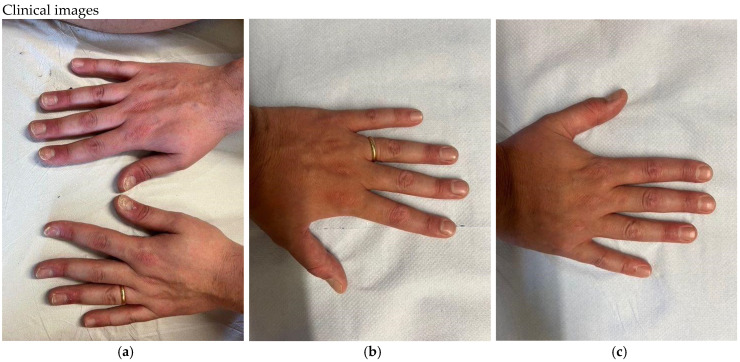
Figure 6.
(a) Nail psoriasis in female at baseline. PGA-F: 3. (b,c) After 36 weeks of bimekizumab treatment- PGA-F: 0.
The mean DLQI values for the group of NP-affected patients were 15.33, 3.70, 1.08, and 0.57 at baseline, week 4, week 16, and week 36, respectively (Table 10).
Table 10.
DLQI—Nail psoriasis population.
| Variable | N° | Mean | Std Dev | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|
| Baseline | 210 | 15.33 | 9.19 | 17.00 | 0.00 | 30.00 |
| Week 4 | 136 | 3.70 | 4.74 | 2.00 | 0.00 | 23.00 |
| Week 16 | 108 | 1.08 | 2.23 | 0.00 | 0.00 | 11.00 |
| Week 36 | 61 | 0.57 | 1.62 | 0.00 | 0.00 | 8.00 |
3. Materials and Methods
A retrospective multicenter clinical study was conducted in 33 Italian Dermatologic Units. We collected data on 834 patients aged ≥ 18 years with moderate-to-severe plaque psoriasis, treated with bimekizumab, a new inhibitor of IL17A and IL17F. The recommended bimekizumab regimen consisted in two subcutaneous injections of 160 mg administered at week 0, 4, 8, and 16 and then every 8 weeks. Patients were monitored for 36 weeks from baseline (visit 1, day 0 prior to the first dose). The study was conducted in accordance with Good Clinical Practice, the applicable regulatory requirements, and the Declaration of Helsinki. All patients provided written informed consent prior to participating in the study.
3.1. Assessments and Outcomes
Patient demographics and psoriasis disease characteristics were collected at the baseline visit. The assessment criteria used to evaluate the severity of skin disease were the Psoriasis Area and Severity Index (PASI) and the Dermatology Life Quality Index (DLQI). Nail psoriasis was evaluated by measuring PGA-F. The score includes the assessment of symptoms and scoring table to individually rate the level of involvement of the nail bed and nail matrix using predefined categories of “clear” (0), “minimal” (1), “mild” (2), “moderate” (3), and “severe” (4). Each grade was supplemented by a general description of the criteria needed to qualify for assignment. Patients whose signs and symptoms were intermediate between two grades were assigned the higher of the two. The score from the nail area (matrix or nail bed) with the highest level of involvement was then selected to generate a total global assessment score [21].
3.2. Statistical Analysis
Quantitative variables were described using mean, standard deviation (SD), median, and minimum and maximum values, while qualitative variables were reported using absolute and relative frequencies.
Missing values were neither replaced nor considered for the analysis of that variable. The assessment of PASI values was calculated at baseline. The proportion of patients reaching PASI100 and PGA-F equal to zero were calculated at week 4, 16, and 36.
A subgroup of patients with nail psoriasis were also described using the same methods described above. Among these patients were a class of patients with PsA and diabetes. Comparison between cohorts was carried out using the Wilcoxon test. All p-values < 0.05 were considered significant.
4. Discussion
Nail psoriasis is a chronic and difficult-to-treat disease, which causes significant social stigma, impairs the patients’ quality of life, and is a true therapeutic challenge for clinicians. The presence of NP could be associated with a more severe form of psoriasis but also has a predictive value for the development of PsA. The nail bed is close to the periosteum of the distal phalanx, and the nail matrix lies near the distal interphalangeal joint and the insertion of the digital extensor tendon. This anatomic structure favors the common association of NP with distal interphalangeal joint arthritis and enthesitis [17,19]. An early diagnosis of enthesitis and distal interphalangeal joint arthritis by radiological imaging (ultrasonography or magnetic resonance imaging) allows clinicians to establish the appropriate treatment, and thus it enables optimal outcomes, improves prognosis, and prevents deformities [17,51]. Therefore, an early treatment is mandatory since it is decisive in blocking the onset of PsA; thus, biologics are a promising option for NP. The data about network meta-analyses (NMAs) of randomized clinical trials have documented the relative efficacy of different biologics [11,46].
The upregulation of several cytokines and kinases has been implicated in the pathogenesis of psoriasis, including tumor necrosis factor-α (TNF-α), IL-17A, IL-17F, and IL-23. These inflammatory mediators are crucial in the development and maintenance of the changes in skin observed in psoriasis.
The IL-17 family is one of the main effectors for the development of cutaneous and nail psoriatic lesions. Interleukin-17F shares 50% homology with IL-17A. It also causes the release of pro-inflammatory cytokines and mobilizes neutrophils [52,53]. Therefore, in psoriasis lesions, the pro-inflammatory effects on keratinocytes and neutrophils are due to both members of the IL-17 family.
Based on the available evidence regarding the role of IL17 in psoriasis and PSA, four therapeutic agents against IL-17A, IL17F or its receptor have been developed: secukinumab, ixekizumab, brodalumab, and bimekizumab. Significant reductions in NAPSI scores were observed using ixekizumab as early as 2 weeks, up to 20 weeks, and in an open-label extension [43,44,45]. The complete remission of NP was achieved in a high percentage of patients: 43 % at week 44 and 51% at week 68 [44]. Brodalumab showed great efficacy in reducing NAPSI in several studies, including three phase 3 trials, ensuring the complete clearance of psoriatic onychopathy at week 52 in almost 64% of affected patients [45]. In a network meta-analysis, brodalumab had the absolute probability of achieving a complete resolution of NP at weeks 24–28 in 37.1% of patients. For bimekizumab, the authors reported a NP complete resolution for 26.7%, 62.1%, and 70.7% of patients at week 16, 32, and 48, respectively [46]. Bimekizumab showed superior efficacy respect to comparators in pivotal phase 3 studies with the same safety profile. The PsO clinical trial program included four phase 3 studies enrolling a total of 2223 patients with moderate-to-severe plaque psoriasis: BE VIVID (compared to placebo and ustekinumab), BE READY (compared to placebo), BE SURE (compared to adalimumab), and BE RADIANT (compared to secukinumab). The primary endpoints were superior to placebo (in the BE VIVID and BE READY studies), to adalimumab (BE SURE study) and to secukinumab (BE RADIANT study); bimekizumab was also superior to ustekinumab in secondary endpoints classified in BE VIVID [46]. A BE VIVID post hoc subanalysis showed the higher efficacy of bimekizumab on NP, with a modified nail psoriasis index score (mNAPSI) of 0 achieved by 54% of patients after 52 weeks of treatment [54].
In this multicenter clinical study, 28% of patients were affected by NP, in line with the evidence in the literature, since it affects a percentage of 10–82% of psoriasis patients [55,56]. Furthermore, NP was found more frequently in men (71.55%) than in women (28.45%).
Our results highlighted a higher PASI decrease during treatment with bimekizumab than previously reported [57], with PASI 100 response in 62% of patients after 16 weeks and 79% after 36 weeks.
Bimekizumab also proved to be particularly effective to treat NP, with a fast response in terms of complete clearance, with almost 32% of patients achieving PGA-F 0 after just 4 weeks. The PGA-F score, in fact, considers not only nail bed alterations with a growth slower than 4 weeks, but also the presence of splinter hemorrhages, nail bed erythema, and subungual hyperkeratosis. It should be noted that in our cohort, bimekizumab had superior efficacy when compared to other anti-IL-17 drugs such as brodalumab and ixekizumab [44], demonstrating a complete response of NP (PGA-F 0) in 88.5% of patients after 36 weeks.
In their NMA, Egberg et al. considered a score of zero on the NAPSI, modified NAPSI, or Physician’s Global Assessment of Fingernails (PGA-F) as an outcome of complete clinical resolution for NP [46]. PGA-F is a rapid, valid, and reliable measure of psoriatic nail disease compared to other NP scoring systems. PGA-F was developed according to best practices for instrument development described by the FDA, and is the only system with established content validity [21]. The PGA-F total score accurately reflects the overall severity of NP [21]. Our data underlined a great outcome, with a mean reduction in PGA-F during follow-up of 92.5%, despite 56.9% of patients being bio-experienced, so at risk of higher rates of primary and secondary therapeutic ineffectiveness [58]. Both skin and nails achieved a quick clearance. Indeed, in patients with both PsO and NP, the simultaneous achievement of skin and nail clearance was the most observed event, since these patients represented the largest group at week 16 (39.25%) and week 36 (73.33%). On the other hand, the two populations that separately achieved PASI100 or PGA-F 0 tended to progressively decrease, reaching a minimum percentage of enrolled patients at week 36 (8.33% and 15%; Figure 3). Elisabeth Riedl et al. also confirmed that patients with PsO and concomitant NP have significantly better and faster improvements in their NP when treated with anti-IL-17 drugs, compared to other classes of biological drugs [59]. Therefore, our data confirm the strong rationale of the use of bimekizumab in patients with PsO and concomitant NP.
No significant difference was recorded in the trend at different time points of NP when patients were divided by presence or absence of PSA, which leads us to also hypothesize a simultaneous action on the latter, since the two entities are intimately related both at the pathogenetic level and for clinical progression [60]. However, data related to the response of PSA to bimekizumab were not the aim of this paper and will be the subject of future publications.
Furthermore, the rapid response to the drug in terms of the complete healing of the skin and nails was reflected in the quality of life of the patients, with 96.6% reduction in DLQI from baseline.
5. Conclusions
The results of our real-world study showed that patients with moderate-to-severe PsO and concomitant NP had significantly faster and more substantial improvements in NP up to 36 weeks with respect to previous findings. Considering the complete healing of the nail, the dual inhibition of IL17 A and F probably has a greater value in re-establishing the dysregulation of keratin 17 at the nail level. The use of bimekizumab in a greater number of subjects also suffering from PsA will give us even more indications in clinical practice to choose the most suitable drugs for our patients.
Author Contributions
Conceptualization, E.C. and F.A.; methodology, L.B.; software, E.C.; validation, L.B.; formal analysis, E.C. and F.A.; investigation, E.C., F.A., R.G.S., A.G., G.A., C.A., A.B., N.B., A.M.G.B., M.B., G.C., A.C. (Anna Campanati), A.C. (Andrea Carugno), F.C., A.C. (Andrea Conti), A.C. (Antonio Costanzo), A.C. (Aldo Cuccia), P.D., A.D., C.D.S., V.D.L., V.D., E.E., M.E., M.C.F., A.F., C.F., L.G., P.G., C.G., A.L., S.L., F.L., P.M., A.V.M., S.R.M., M.M., G.M., E.M., M.L.M., A.N., A.M.O., D.O., G.P. (Giovanni Paolino), G.P. (Giovanni Pellacani), K.P., C.P. (Concetta Potenza), F.P., P.Q., A.R., M.R., A.G.R., D.S., E.T., M.V., S.R. and M.D.; resources, E.C.; data curation, F.A.; writing—original draft preparation, F.A. and E.C.; writing—review and editing, F.A., E.C. and L.B.; visualization, L.B.; supervision, E.C.; project administration, L.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical approval is not required for this study in accordance with local or national guidelines since this is a retrospective study conducted on cases of common clinical practice.
Informed Consent Statement
Written informed consent was obtained from the patients for the publication of the details of their medical cases and any accompanying images.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
E. Campione has served as advisory board member and received fees for lectures and/or research grants by Almirall, Amgen, Abbvie, Bristol Myers Squibb, Incyte, Leo Pharma, and UCB. F. Artosi declares no conflicts of interest. R.Gaeta Shumack declares no conflicts of interest. A. Giunta has received grants and has been a consultant and speaker for AbbVie, Almirall, Celgene, Janssen, Leo Pharma, Eli Lilly, Merck Sharpe Dohme, Novartis, Pfizer, Sandoz, and UCB. L. Bianchi has served as speaker and consultant for AbbVie, Novartis, Janssen-Cilag, Pfizer, UCB, and LeoPharma. D. Orsini has been a speaker and/or consultant for Abbvie, LeoPharma, UCB, Bristol-Meyer-Squibb, and Boehringer-Ingelheim. P. Malagoli has been a speaker for AbbVie, Lilly, Novartis, Janssen-Cilag, Celgene, Leopharma, and Almirall. A. Balato has received honoraria for participation in advisory boards and meetings, or as speaker for AbbVie, Celgene, Janssen-Cilag, Eli Lilly, Novartis Pharma, Pfizer, Sanofi-Genzyme, and UCB Pharma. G. Caldarola reports consulting fees or honorarium and payment for lectures from Lilly and Novartis. A. Narcisi has served on advisory boards and received honoraria for lectures and research grants from Almirall, Abbvie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi-Genzyme, Amgen, and Boehringer Ingelheim. A. Offidani has acted as a speaker and consultant for Abbvie, Eli Lilly, Novartis, Celgene, Sanofi, Galderma, Leo Pharma, and Pierre Fabre. A. Costanzo has served as advisory board member and consultant and received fees and speaker’s honoraria or participated in clinical trials for Abbvie, Almirall, Biogen, LEO Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. F. Loconsole declares no conflicts of interest. E. Mortato declares no conflicts of interest. M. Megna has acted as a speaker or consultant for Abbvie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Leo Pharma, Janssen, Novartis, Sandoz, and UCB. L. Gargiulo has been a consultant and/or speaker and participated in advisory boards for Abbvie, Almirall, Eli Lilly, Pfizer, Sanofi, and UCB Pharma. A. Carugno has been a consultant and/or speaker for Abbvie, Almirall, Amgen, Boehringer Ingelheim, Eli Lilly, Leo Pharma, Janssen-Cilag, Novartis, and UCB Pharma. V. Di Lernia has served as consultant and/or member of Data Safety Monitoring Boards or advisory boards and/or received speaker honoraria from Abbvie, Amgen, Eli Lilly, Janssen, and Novartis. A.V. Marzano has received consulting fees and disease-relevant honoraria from ABBVIE, AMGEN, BOEHRINGER INGELHEIM, BMS, INCYTE, LEOPHARMA, Novartis, Pfizer, SANOFI, and UCB. D. Strippoli has been a consultant and/or speaker for Almirall. Amgen, Janssen-Cilag, Sun-Pharma, Leo Pharma, Novartis, and UCB Pharma. G. Paolino has been a consultant for Abbvie, Pierre-Fabre, Janssen-Cilag, and Almirall. S. Lembo has acted as a speaker or consultant for Abbvie, Almirall, Eli Lilly, Leo Pharma, Janssen, Novartis, UCB, Sanofi, and Pfizer. M. Esposito has served as speaker and/or consultant for AbbVie, Almirall, Biogen, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, Sanofi Genzyme, and UCB. M. C. Fargnoli has served on advisory boards and received honoraria for lectures and/or research grants from Amgen, Almirall, Abbvie, Boehringer-Ingelheim, BMS, Galderma, Kyowa Kyrin, Incyte, LEO Pharma, Pierre Fabre, UCB, Lilly, Pfizer, Janssen, MSD, Novartis, Sanofi, Regeneron, and Sun Pharma. A. Dattola has served as a speaker, consultant, or advisory board member for Abbvie, Almirall, Amgen, Eli Lilly, Leo Pharma, Janssen, Novartis, Boehringer Ingelheim, and UCB Pharma outside the submitted work. G.Pellacani has served as advisory board member for Abbvie, Almirall, Amgen, Leo Pharma, Janssen, Novartis, Canfield, Galderma, Lorèal, Sanofi, and UCB Pharma outside the submitted work. C.S. Fiorella has acted as a speaker or consultant for Abbvie, Almirall, Amgen, Eli Lilly, Leo Pharma, Janssen, Novartis, and UCB Pharma. E.Errichetti has been a consultant/speaker for Eli Lilly, Abbvie, Almirall, Amgen, LeoPharma, Janssen, Novartis, and UCB Pharma. M. Donini has been a consultant/speaker for Almirall, Leopharma, Novartis, Eli-Lilly, Abbvie, and Sanofi. P. Gisondi has served as a speaker, consultant, or advisory board member for Abbvie, Almirall, Amgen, Eli Lilly, Janssen, Leo Pharma, Novartis, Pierre Fabre, Sanofi, Sandoz, and UCB Pharma. E. Trovato declares no conflicts of interest. M.L. Musumeci has served as consultant and advisory board member and has participated in clinical trials for Janssen-Cilag, AbbVie, Almirall, Leo Pharma, Eli-Lilly, Novartis, Sanofi, UCB, and Boehringer-Ingelheim. C. De Simone has received honoraria from Amgen, Almirall, Abbvie, Boehringer-Ingelheim, Eli Lilly Incyte, LEO Pharma, UCB, Pfizer, Janssen, Novartis, and Sanofi, for serving as advisory board member, consultant, and/or speaker. F. Prignano has served as advisory board member and consultant and has received fees and speaker’s honoraria or has participated in clinical trials for AbbVie, Almirall, Leo Pharma, Eli-Lilly, Janssen, Novartis, Biogen, Sanofi Genzyme, UCB, and Boehringer-Ingelheim. M. Venturini has served as advisory board member and/or consultant and has received fees/speaker’s honoraria and/or has participated in clinical trials for AbbVie, Almirall, Amgen, Bristol Myers Squibb, Boehringer-Ingelheim, Eli Lilly, Galderma, Janssen, Leo Pharma, Novartis, Pierre Fabre, and UCB Pharma. No other disclosures have been made.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ventura A., Mazzeo M., Gaziano R., Galluzzo M., Bianchi L., Campione E. New insight into the pathogenesis of nail psoriasis and overview of treatment strategies. Drug Des. Dev. Ther. 2017;11:2527–2535. doi: 10.2147/DDDT.S136986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanda N. Psoriasis: Pathogenesis, Comorbidities, and Therapy Updated. Int. J. Mol. Sci. 2021;22:2979. doi: 10.3390/ijms22062979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blauvelt A., Chiricozzi A. The Immunologic Role of IL-17 in Psoriasis and Psoriatic Arthritis Pathogenesis. Clin. Rev. Allergy Immunol. 2018;55:379–390. doi: 10.1007/s12016-018-8702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang Y., Chen Y., Yu Q., Shi Y. Biologic and Small-Molecule Therapies for Moderate-to-Severe Psoriasis: Focus on Psoriasis Comorbidities. BioDrugs. 2023;37:35–55. doi: 10.1007/s40259-022-00569-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanna C., Mancini M., Gaziano R., Cannizzaro M.V., Galluzzo M., Talamonti M., Rovella V., Annicchiarico-Petruzzelli M., Melino G., Wang Y., et al. Skin immunity and its dysregulation in psoriasis. Cell Cycle. 2019;18:2581–2589. doi: 10.1080/15384101.2019.1653099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang P., Su Y., Li S., Chen H., Wu R., Wu H. The roles of T cells in psoriasis. Front. Immunol. 2023;14:1081256. doi: 10.3389/fimmu.2023.1081256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yao Z., Fanslow W.C., Seldin M.F., Rousseau A.M., Painter S.L., Comeau M.R., Cohen J.I., Spriggs M.K. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 8.Kolbinger F., Loesche C., Valentin M.A., Jiang X., Cheng Y., Jarvis P., Peters T., Calonder C., Bruin G., Polus F., et al. β-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J. Allergy Clin. Immunol. 2017;139:923–932.e8. doi: 10.1016/j.jaci.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 9.Reich K. Approach to managing patients with nail psoriasis. J. Eur. Acad. Dermatol. Venereol. 2009;23((Suppl. S1)):15–21. doi: 10.1111/j.1468-3083.2009.03364.x. [DOI] [PubMed] [Google Scholar]
- 10.Prignano F., Campione E., Parodi A., Vegni E., Bardazzi F., Borroni R.G., Burlando M., Cinotti E., Dini V., Giacchetti A., et al. EMPATHY Life in Psoriasis: Embracing Patients’ Well-Being in Their Journey of Moderate-to-Severe Psoriasis. J. Clin. Med. 2024;13:4469. doi: 10.3390/jcm13154469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasch M.C. Nail psoriasis: A review of treatment options. Drugs. 2016;76:675–705. doi: 10.1007/s40265-016-0564-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pourchot D., Bodemer C., Phan A., Bursztejn A., Hadj-Rabia S., Boralevi F., Miquel J., Hubiche T., Puzenat E., Souillet A., et al. Nail Psoriasis: A Systematic Evaluation in 313 Children with Psoriasis. Pediatr. Dermatol. 2017;34:58–63. doi: 10.1111/pde.13028. [DOI] [PubMed] [Google Scholar]
- 13.Lawry M. Biological therapy and nail psoriasis. Dermatol. Ther. 2007;20:60–67. doi: 10.1111/j.1529-8019.2007.00112.x. [DOI] [PubMed] [Google Scholar]
- 14.Armesto S., Esteve A., Coto-Segura P., Drake M., Galache C., Martínez-Borra J., Santos-Juanesc J. Nailpsoriasisinindividuals with psoriasis vulgaris: A study of 661 patients. Actas Dermosifiliogr. 2011;102:365–372. doi: 10.1016/j.ad.2011.02.007. (In Spanish) [DOI] [PubMed] [Google Scholar]
- 15.Klaassen K.M., van de Kerkhof P.C., Pasch M.C. Nail psoriasis: A questionnaire-based survey. Br. J. Dermatol. 2013;169:314–319. doi: 10.1111/bjd.12354. [DOI] [PubMed] [Google Scholar]
- 16.Jiaravuthisan M.M., Sasseville D., Vender R.B., Murphy F., Muhn C.Y. Psoriasis of the nail: Anatomy, pathology, clinical presentation, and a review of the literature on therapy. J. Am. Acad. Dermatol. 2007;57:1–27. doi: 10.1016/j.jaad.2005.07.073. [DOI] [PubMed] [Google Scholar]
- 17.Langenbruch A., Radtke M.A., Krensel M., Jacobi A., Reich K., Augustin M. Nail involvement as a predictor of concomitant psoriatic arthritis in patients with psoriasis. Br. J. Dermatol. 2014;171:1123–1128. doi: 10.1111/bjd.13272. [DOI] [PubMed] [Google Scholar]
- 18.Zaias N. Psoriasis of the nail. A clinical-pathologic study. Arch. Dermatol. 1969;99:567–579. doi: 10.1001/archderm.1969.01610230059011. [DOI] [PubMed] [Google Scholar]
- 19.Zabotti A., De Marco G., Gossec L., Baraliakos X., Aletaha D., Iagnocco A., Gisondi P., Balint P.V., Bertheussen H., Boehncke W.-H., et al. EULAR points to consider for the definition of clinical and imaging features suspicious for progression from psoriasis to psoriatic arthritis. Ann. Rheum. Dis. 2023;82:1162–1170. doi: 10.1136/ard-2023-224148. [DOI] [PubMed] [Google Scholar]
- 20.Alinaghi F., Calov M., Kristensen L.E., Gladman D.D., Coates L.C., Jullien D., Gottlieb A.B., Gisondi P., Wu J.J., Thyssen J.P., et al. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. J. Am. Acad. Dermatol. 2019;80:251–265.e19. doi: 10.1016/j.jaad.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 21.Hudgens S., Rich P., Geng Z., Williams D., Fleischer A., Ganguli A. Development and validation of the Physician’s Global Assessment of Fingernail Psoriasis. J. Eur. Acad. Dermatol. Venereol. 2021;35:2324–2330. doi: 10.1111/jdv.17387. [DOI] [PubMed] [Google Scholar]
- 22.Rich P., Scher R.K. Nail psoriasis severity index: A useful tool for evaluation of nail psoriasis. J. Am. Acad. Dermatol. 2003;49:206–212. doi: 10.1067/S0190-9622(03)00910-1. [DOI] [PubMed] [Google Scholar]
- 23.Szepietowski J.C., Salomon J. Do fungi play a role in psoriatic nails? Mycoses. 2007;50:437–442. doi: 10.1111/j.1439-0507.2007.01405.x. [DOI] [PubMed] [Google Scholar]
- 24.Rigopoulos D., Papanagiotou V., Daniel R., 3rd, Piraccini B.M. Onychomycosis in patients with nail psoriasis: A point to point discussion. Mycoses. 2017;60:6–10. doi: 10.1111/myc.12542. [DOI] [PubMed] [Google Scholar]
- 25.Elewski B.E. Onychomycosis: Pathogenesis, diagnosis, and manage-ment. Clin. Microbiol. Rev. 1998;11:415–429. doi: 10.1128/CMR.11.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaias N., Escovar S.X., Zaiac M.N. Finger and toenail onycholysis. J. Eur. Acad. Dermatol. Venereol. 2015;29:848–853. doi: 10.1111/jdv.12862. [DOI] [PubMed] [Google Scholar]
- 27.Zisova L., Valtchev V., Sotiriou E., Gospodinov D., Mateev G. Onycho-mycosis in patients with psoriasis: A multicentre study. Mycoses. 2012;55:143–147. doi: 10.1111/j.1439-0507.2011.02053.x. [DOI] [PubMed] [Google Scholar]
- 28.De Luca A., Zelante T., D’Angelo C., Zagarella S., Fallarino F., Spreca A., Iannitti R.G., Bonifazi P., Renauld J.-C., Bistoni F., et al. IL-22 defines a novel immune pathway of antifungal resistance. Mucosal Immunol. 2010;3:361–373. doi: 10.1038/mi.2010.22. [DOI] [PubMed] [Google Scholar]
- 29.Fry L., Baker B.S. Triggering psoriasis: The role of infections and medications. Clin. Dermatol. 2007;25:606–615. doi: 10.1016/j.clindermatol.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Goldminz A.M., Au S.C., Kim N., Gottlieb A.B., Lizzul P.F. NF-kB: An essential transcription factor in psoriasis. J. Dermatol. Sci. 2013;69:89–94. doi: 10.1016/j.jdermsci.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Di Meglio P., Perera G.K., Nestle F.O. The multitasking organ: Recent insights into skin immune function. Immunity. 2011;35:857–869. doi: 10.1016/j.immuni.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 32.Iyer S.S., Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit. Rev. Immunol. 2012;32:23–63. doi: 10.1615/CritRevImmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saulite I., Pilmane M., Kisis J. Expression of antimicrobial peptides in nail psoriasis and normal nails. Acta Derm. Venereol. 2017;97:644–645. doi: 10.2340/00015555-2605. [DOI] [PubMed] [Google Scholar]
- 34.Gniadecki R. Next-generation antipsoriatic drugs: Small molecules join. Br. J. Dermatol. 2015;173:1355–1356. doi: 10.1111/bjd.14249. [DOI] [PubMed] [Google Scholar]
- 35.Crowley J.J., Weinberg J.M., Wu J.J., Robertson A.D., Van Voorhees A.S., National Psoriasis Foundation Treatment of nail psoriasis: Best practice recommendations from the Medical Board of the National Psoriasis Foundation. JAMA Dermatol. 2015;151:87–94. doi: 10.1001/jamadermatol.2014.2983. [DOI] [PubMed] [Google Scholar]
- 36.Laheru D., Antony A., Carneiro S., Di Lernia V., Garg A., Love T.J., Garcia K.d.R.M., Mendonça J.A., Mukherjee S., Olteanu R., et al. Management of Nail Disease in Patients With Psoriatic Arthritis: An Updated Literature Review Informing the 2021 GRAPPA Treatment Recommendations. J. Rheumatol. 2023;50:433–437. doi: 10.3899/jrheum.220313. [DOI] [PubMed] [Google Scholar]
- 37.Marquez Balbas G., Sanchez Regana M., Umbert Millet P. Tacalcitol ointment for the treatment of nail psoriasis. J. Dermatol. Treat. 2009;20:308–310. doi: 10.1080/09546630902787585. [DOI] [PubMed] [Google Scholar]
- 38.Reichrath J. Vitamin D and the skin: An ancient friend, revisited. Exp. Dermatol. 2007;16:618–625. doi: 10.1111/j.1600-0625.2007.00570.x. [DOI] [PubMed] [Google Scholar]
- 39.Fischer-Levancini C., Sanchez-Regana M., Llambi F., Collgros H., Exposito-Serrano V., Umbert-Millet P. Nail psoriasis: Treatment with tazarotene 0.1% hydrophilic ointment. Actas Dermosifiliogr. 2012;103:725–728. doi: 10.1016/j.ad.2012.04.008. (In Spanish) [DOI] [PubMed] [Google Scholar]
- 40.Hadeler E., Mosca M., Hong J., Brownstone N., Bhutani T., Liao W. Nail Psoriasis: A Review of Effective Therapies and Recommendations for Management. Dermatol. Ther. 2021;11:799–831. doi: 10.1007/s13555-021-00523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Papp K., Reich K., Leonardi C.L., Kircik L., Chimenti S., Langley R.G., Hu C., Stevens R.M., Day R.M., Gordon K.B., et al. Apremilast, an oral phosphodi- esterase 4 (PDE4) inhibitor, in patients with moderate to severe plaque psoriasis: Results of a phase III, randomized, controlled trial (Efficacy and Safety Trial Evaluating the Effects of Apremilast in Psoriasis [ESTEEM] 1) J. Am. Acad. Dermatol. 2015;73:37–49. doi: 10.1016/j.jaad.2015.03.049. [DOI] [PubMed] [Google Scholar]
- 42.Lanna C., Cesaroni G.M., Mazzilli S., Vollono L., Gaziano R., Marino D., Bianchi L., Campione E. Apremilast as a target therapy for nail psoriasis: A real-life observational study proving its efficacy in restoring the nail unit. J. Dermatol. Treat. 2022;33:1097–1101. doi: 10.1080/09546634.2020.1801976. [DOI] [PubMed] [Google Scholar]
- 43.Langley R.G., Rich P., Menter A., Krueger G., Goldblum O., Dutronc Y., Zhu B., Wei H., Cameron G., Heffernan M. Improvement of scalp and nail lesions with ixekizumab in a phase 2 trial in patients with chronic plaque psoriasis. J. Eur. Acad. Dermatol. Venereol. 2015;29:1763–1770. doi: 10.1111/jdv.12996. [DOI] [PubMed] [Google Scholar]
- 44.Thaçi D., Kimball A., Foley P., Poulin Y., Levi E., Chen R., Feldman S.R. Apremilast, an oral phospho-diesterase 4 inhibitor, improves patient-reported outcomes in the treatment of moderate to severe psoriasis: Results of two phase III ran-domized, controlled trials. J. Eur. Acad. Dermatol. Venereol. 2016;31:498–506. doi: 10.1111/jdv.13918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elewski B., Rich P., Lain E., Soung J., Lewitt G.M., Jacobson A. Efficacy of brodalumab in the treatment of scalp and nail psoriasis: Results from three phase 3 trials. J. Dermatol. Treat. 2022;33:261–265. doi: 10.1080/09546634.2020.1749546. [DOI] [PubMed] [Google Scholar]
- 46.Egeberg A., Kristensen L.E., Puig L., Rich P., Smith S.D., Garrelts A., See K., Holzkaemper T., Fotiou K., Schuster C. Network meta-analyses comparing the efficacy of biologic treatments for achieving complete resolution of nail psoriasis at 24–28 and 48–52 weeks. J. Dermatol. Treat. 2023;34:2263108. doi: 10.1080/09546634.2023.2263108. [DOI] [PubMed] [Google Scholar]
- 47.Furue M., Furue K., Tsuji G., Nakahara T. Interleukin-17A and Keratinocytes in Psoriasis. Int. J. Mol. Sci. 2020;21:1275. doi: 10.3390/ijms21041275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bhoi A.K., Grover C., Singal A., Kashyap B., Dibyashree D. Serum levels of tumour necrosis factor (TNF-α) and interleukin-17 (IL-17) in patients with nail psoriasis: A cross-sectional study. Indian J. Dermatol. Venereol. Leprol. 2024;90:453–457. doi: 10.25259/IJDVL_367_2023. [DOI] [PubMed] [Google Scholar]
- 49.Adams R., Maroof A., Baker T., Lawson A.D.G., Oliver R., Paveley R., Rapecki S., Shaw S., Vajjah P., West S., et al. Bimekizumab, a Novel Humanized IgG1 Antibody That Neutralizes Both IL-17A and IL-17F. Front. Immunol. 2020;11:1894. doi: 10.3389/fimmu.2020.01894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.European Medicines Agency Summary of Product Characteristics: Bimzelx 160 mg Solution for Injection in Pre-Filled Syringe, Bimzelx 160 mg Solution for Injection in Pre-Filled Pen 2021. [(accessed on 1 January 2020)]. Available online: https://www.ema.europa.eu/en/documents/product-information/bimzelx-epar-product-information_en.pdf.
- 51.Gargiulo L., Narcisi A., Ibba L., Balato A., Bianchi L., Brianti P., Buononato D., Burlando M., Caldarola G., Campanati A., et al. Effectiveness and safety of bimekizumab for the treatment of plaque psoriasis: A real-life multicenter study-IL PSO (Italian landscape psoriasis) Front. Med. 2023;10:1243843. doi: 10.3389/fmed.2023.1243843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Malakouti M., Brown G.E., Wang E., Koo J., Levin E.C. The role of IL-17 in psoriasis. J. Dermatol. Treat. 2015;26:41–44. doi: 10.3109/09546634.2013.879093. [DOI] [PubMed] [Google Scholar]
- 53.Chiricozzi A. Pathogenic role of IL-17 in psoriasis and psoriatic arthritis. Actas Dermosifiliogr. 2014;105((Suppl. S1)):9–20. doi: 10.1016/S0001-7310(14)70014-6. [DOI] [PubMed] [Google Scholar]
- 54.Reich K., Papp K.A., Blauvelt A., Langley R.G., Armstrong A., Warren R.B., Gordon K.B., Merola J.F., Okubo Y., Madden C., et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): Efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397:487–498. doi: 10.1016/S0140-6736(21)00387-1. Erratum in Lancet 2021, 397, 670. [DOI] [PubMed] [Google Scholar]
- 55.Battista T., Scalvenzi M., Martora F., Potestio L., Megna M. Nail Psoriasis: An Updated Review of Currently Available Systemic Treatments. Clin. Cosmet. Investig. Dermatol. 2023;16:1899–1932. doi: 10.2147/CCID.S417679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ji C., Wang H., Bao C., Zhang L., Ruan S., Zhang J., Gong T., Cheng B. Challenge of Nail Psoriasis: An Update Review. Clin. Rev. Allergy Immunol. 2021;61:377–402. doi: 10.1007/s12016-021-08896-9. [DOI] [PubMed] [Google Scholar]
- 57.Armstrong A.W., Soliman A.M., Betts K.A., Wang Y., Gao Y., Stakias V., Puig L. Long-Term Benefit-Risk Profiles of Treatments for Moderate-to-Severe Plaque Psoriasis: A Network Meta-Analysis. Dermatol. Ther. 2022;12:167–184. doi: 10.1007/s13555-021-00647-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rompoti N., Politou M., Stefanaki I., Vavouli C., Papoutsaki M., Neofotistou A., Rigopoulos D., Stratigos A., Nicolaidou E. Brodalumab in plaque psoriasis: Real-world data on effectiveness, safety and clinical predictive factors of initial response and drug survival over a period of 104 weeks. J. Eur. Acad. Dermatol. Venereol. 2023;37:689–697. doi: 10.1111/jdv.18825. [DOI] [PubMed] [Google Scholar]
- 59.Riedl E., Pinter A., Zaheri S., Costanzo A., Brnabic A., Konicek B., McKenzie R., Lampropoulou A., Rayes M.E., Haustrup N., et al. Baseline Characteristics and mNAPSI Change from Baseline Scores Through Month 12 for Patients with Moderate-to-Severe Plaque Psoriasis and Concomitant Nail Psoriasis Treated with Biologics from PSoHO. Dermatol. Ther. 2024;14:1327–1335. doi: 10.1007/s13555-024-01150-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mease P., Elaine Husni M., Chakravarty S.D., Kafka S., Parenti D., Kim L., Hung Lo K., Hsia E.C., Kavanaugh A. Evaluation of Improvement in Skin and Nail Psoriasis in Bio-naïve Patients With Active Psoriatic Arthritis Treated With Golimumab: Results Through Week 52 of the GO-VIBRANT Study. ACR Open Rheumatol. 2020;2:640–647. doi: 10.1002/acr2.11180. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data is contained within the article.