Abstract
Background: Following injury, older adults exhibit slow recovery of muscle function. Age-related impairment of sarcolemmal membrane repair may contribute to myocyte death, increasing the need for myogenesis and prolonging recovery. Dietary fish oil (FO) is a common nutritional supplement that may alter plasma membrane composition to enhance the response to membrane injury. Methods: We assessed effects of an 8-week dietary intervention on muscle contractile recovery in aged (22 mo.) rats on control (n = 5) or FO (control + 33 g/kg FO (45% eicosapentaenoic acid; 10% docosahexaenoic acid); n = 5) diets 1-week after contusion injury, as well as adult (8 mo., n = 8) rats on the control diet. Results: Recovery was reduced in aged rats on the control diet vs. adults (63 vs. 80%; p = 0.042), while those on the FO diet recovered similarly to (78%) adults. To directly assess sarcolemma injury, C2C12 cells were cultured in media with and without FO (1, 10, and 100 μg/mL; 24 or 48 h) and injured with an infrared laser in medium containing FM4-64 dye as a marker of sarcolemmal injury. FO reduced the area under the FM4-64 fluorescence-time curve at all concentrations after both 24 and 48 h supplementation. Conclusions: These preliminary data suggest FO might aid recovery of muscle function following injury in older adults by enhancing membrane resealing and repair.
Keywords: aging, contractility, membrane injury, eicosapentaenoic acid, docosahexaenoic acid, sarcopenia
1. Introduction
Although older adults (OAs) tend to participate less often in occupational and athletic activities typically associated with mechanical muscle injury, they are still at risk. Many OAs initiate exercise and mobility programs [1] that carry a risk of injury and falling; the most common mechanism of injury in OAs [2,3] carries a risk of muscle contusion [3,4,5,6]. Geriatric clinical care following falls understandably focuses on fractures [4], but muscle contusion can reduce function and mobility, exacerbating physical inactivity in the absence of fracture [5]. Even young adults can take weeks to fully recover from a muscle contusion [7,8]. However, contusion injury has not received much study in aging models. We have previously reported that the magnitude of acute functional muscle impairment following contusion was similar in adult and aged rats [9] but have not examined short- or long-term recovery from contusion.
Standard treatment of contusion involves non-steroidal anti-inflammatory drugs (NSAIDS), mostly for pain control. However, the direct benefits of NSAIDs to injured muscles remain unclear, especially after the acute phase [10]. Some data suggest they might even impair recovery [11], though the extent of this negative impact is equivocal [12,13]. Dietary supplements, such as fish oil (FO), might offer effective alternatives to, or additional benefits in combination with, standard drug treatments. Multiple beneficial effects on OAs have been ascribed to dietary FO, many linked to anti-oxidant and anti-inflammatory actions [14,15,16]. Indeed, a scoping review identified FO as one of only 16 micronutrient supplements with potential to improve musculoskeletal health in aging [17]. Moreover, unlike several other dietary supplements, FO does not appear to blunt muscle responses to exercise [18] and would thus be potentially equally useful in active and sedentary older adults. Although the anti-coagulant effects attributed to FO could conceivably worsen contusion injury, we have found that FO neither reduced nor increased the acute functional impairment following muscle contusion in adult and aged rodents [9].
Incomplete recovery from muscle injury is a frequently-reported consequence of old age [19,20,21,22,23] and has been observed even if the degree of acute injury is equal [23]. Muscle cells are exposed to regular mechanical injury during contraction and thus need a robust, rapid process to maintain sarcolemmal membrane integrity and maintain cell homeostasis. Inhibition of sarcolemmal repair processes can increase the muscle degeneration and resulting fibrosis [24,25,26], thus impairing and/or slowing recovery. We have previously reported that aged muscle exhibits increased content of dysferlin [27], a protein responsive to membrane injury [28,29], suggesting that this increased expression in aged muscle could be compensation for more frequent or long-lasting membrane stress in old age. Dysferlin is known to act in concert with Tripartite motif-containing protein 72 (TRIM72/MG53) to repair membrane disruption in response to injury [30], but we are unaware of any reported age-related differences in TRIM72/MG53. It has been shown that aging affects the lipid composition of cell membranes, which could impair the muscle membrane resealing process [25]. Dietary FO can also affect membrane lipid metabolism and composition [31,32] and thus might counteract age-related changes and, at least partially, restore recovery following injury. Thus, while FO did not provide prophylactic protection against injury in our earlier study [33], it might still enhance or accelerate recovery from injury, possibly by enhancing membrane repair/resealing.
In this preliminary report, we evaluated the effects of FO subacute (7-day) functional (i.e., contractile) recovery of in situ muscle contractility in aged rats. Because impaired recovery from injury has been reported with increased age, we also tested a group of adult rats on a standard diet compared to aged rats on the standard diet or one supplemented with FO. In addition, we examined the effects of age, injury, and FO on the abundance of the membrane repair proteins dysferlin and TRIM72/MG53. We also conducted an in vitro assay of direct membrane injury and resealing of myoblasts incubated with the same FO used in the in situ studies. Because poor membrane repair would be expected to result in greater injury, we also evaluated a marker of the endoplasmic/sarcoplasmic reticulum stress response. ER/SR stress is associated with atrophy and weakness during muscle disuse (i.e., hindlimb suspension) [34]. Among the factors in this stress response is the unfolded protein response, which involves molecular chaperones working to appropriately refold proteins damaged by injury [35]. Accordingly, we also probed the muscles tested in situ for 78 kD glucose-related protein (Grp78, A.K.A. BiP), a common marker of ER stress [35,36]. We hypothesized that FO supplementation would improve contractile recovery in aged rats to a level similar to that seen in adult animals and that FO would enhance the membrane resealing process.
2. Materials and Methods
2.1. In Vivo Testing
Experimental animals: Adult (6–8 months; n = 8) and aged (20 months; n = 10), male and female Sprague Dawley rats were purchased from Envigo (Indianapolis, IN, USA). All aged rats were acclimated for 1 week prior to assignment to the experimental diet group, with ad libitum access to standard natural chow (Envigo #T8640 Teklab 22/5, Madison, WI, USA) and water, while adult rats were maintained on the control diet throughout the study. All animal use and procedures were approved by the University of South Florida IACUC (approval code: 8039; approval date: 30 November 2020), and “Principles of laboratory animal care” (NIH publication No. 86-23, revised 1985) were followed throughout the study.
Dietary intervention in aged animals: Following acclimation, aged rats were balanced by body mass and food intake, and equal numbers in each age group were assigned to control (Ctl) (AIN-93M purified diet, Research Diets, New Brunswick, NJ, USA) or experimental (FO) diet (AIN-93M formulated with 33 g/kg commercial fish oil product (EPAX 4020TGN, EPAX, Ålesund, Norway AS; 10% docosahexaenoic acid (DHA) and 45% eicosapentaenoic acid (EPA)). Diets were maintained for 8 wks.
Injury protocol: After 8 wks, all animals were anesthetized with isoflurane, and the left hindlimb was placed in a custom injury apparatus we have described and utilized previously [9]. Muscle injury was produced by dropping a known weight and a known height onto a custom impactor placed on the medial gastrocnemius muscle (MG). Following injury, 1.0 mg/kg sustained-release buprenorphine was administered via subcutaneous injection. A second injection was administered 24 h later.
Grip strength: Bilateral forelimb grip strength was determined as previously described [37], using a Columbus Instruments 1027DR grip strength meter (Columbus, OH, USA) on Days 1 and 32 of the dietary intervention in aged rats only, as our previous study suggested that FO might slow the decline of grip strength in aging [33]. Peak forces of 5 trials were averaged for our measure of grip strength and were expressed in absolute terms and relative to body mass.
2.2. In Situ Testing
Muscle contractility: Prior to contractile testing, animals were anesthetized by injection of ketamine:xylazine (40:10 mg kg−1 body mass; IP). In situ contractility of the MG was assessed 7 d post-injury in the injured and uninjured hindlimbs of all rats using our published experimental setup [9] and the Aurora Muscle Testing System (Ontario, Canada). Peak force responses to single-pulse and 6-pulse (100 Hz), 200 µs pulse duration, and stimulation trains delivered to the sciatic nerve were evaluated because studies of awake moving rats show that most motor units fire in short (≤6 spikes) bursts [38]. Following testing, animals were euthanized by intracardiac injection (Euthasol, 100 mg kg−1). The ratio of force production by the injured MG to that of the uninjured MG was used as the index of recovery of muscle function.
Immunoblotting: The abundance of proteins associated with membrane injury was determined via Western blotting of injured and uninjured muscle tissue. Portions of the MG muscle were homogenized as described previously [27]. Proteins were separated by SDS-PAGE using 8–15% gels, depending on the weight of the protein of interest, then transferred overnight to PVDF membranes. After drying for 24 h, membranes were blocked and incubated overnight with at 4 °C primary antibodies to Dysferlin (Sigma-Aldrich, St. Louis, MO, USA, SAB4200453) and Trim72/MG53 (custom antibody [39]). The next day, blots were rinsed five times in TBS-T, incubated with secondary antibodies (LiCor) at room temperature for 2 h, rinsed 5 times with TBS-T, and scanned on an Odyssey fluorescence scanning system (LiCor, Lincoln, NE, USA). An additional protein, Grp78, which is associated with ER stress, and the unfolded protein response were also assessed using the same procedures with antibodies (Sigma-Aldrich SAB4501452). We have previously found that this protein is elevated in acutely injured muscle regardless of age [9]. Proteins were visualized and analyzed by densitometry using the scanner manufacturer’s software (Image Studio 5.2, LiCor, Lincoln, NE, USA). Membranes were later stripped and stained for total with Coomassie Brilliant Blue R25. Band intensities were normalized to total protein per lane.
2.3. In Vitro Testing
Cell culture conditions and fish oil treatment: C2C12 mouse myoblast cells (American Type Culture Collection, Manassas, VA, USA) were maintained in a humidified environment at 37 °C and 5% CO2 in Dulbecco’s modified Eagle’s medium (ThermoFisher, Waltham, MA, USA), supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 100 μg/mL streptomycin. C2C12 myoblasts were grown to 70% confluence on 35 mm glass-bottomed petri dishes (MatTek, Ashland, MA, USA) before the culture media was supplemented with various doses of fish oil (1 μg/mL, 10 μg/mL, and 100 μg/mL) for either 24 or 48 h.
Plasma membrane repair assay: The plasma membrane repair capacity of C2C12 cells was measured using laser scanning confocal microscopy, essentially as previously described [40]. In brief, C2C12 cells were cultured on 35 mm glass-bottomed petri dishes (MatTek, Ashland, MA, USA) and then washed and transitioned into modified Tyrode’s solution (140 mM NaCl, 5 mM KCl, 2 mM MgCl2, and 10 mM HEPES (pH 7.2). Membrane injury was induced using a FluoView FV1000 multi-photon confocal laser-scanning microscope (Olympus, Center Valley, PA, USA) on cells in Tyrode’s solution supplemented with 2.5 µM FM4-64 dye (Invitrogen, Eugene, OR, USA). A circular region of interest was selected along the edge of the plasma membrane and irradiated at 50% of maximum infrared laser power for 5 s. Pre- and post-damage images were captured every 3 s for a total of 60 s. The extent of membrane damage was analyzed using Fiji ImageJ software, Version 20170530 (National Institute of Health, Bethesda, MD, USA), measuring the fluorescence intensity encompassing the site of damage with results represented as ∆F/F0, as previously described [41]. Briefly, the fluorescent intensity at the injury site is measured, and then the fluorescence intensity level in the injury area before the injury is subtracted from that value. The remaining signal is divided by the fluorescence intensity level in the injury area before the injury. This calculation is repeated in each image captured over the 60 s time course. These ∆F/F0 results are plotted over time, and the area under the curve (AUC) was calculated using GraphPad Prism Version 10 (GraphPad Software, La Jolla, CA, USA) to provide a measurement of the total dye entry when the cell is injured.
2.4. Statistical Analysis
Group differences in initial body mass, food consumption, and food consumption normalized to body mass were initially analyzed by ANOVA, followed by ANCOVA with sex as a covariate. The percent changes over 8 weeks were assessed similarly. Group differences in the ratio of injured/uninjured forces were evaluated using the Kruskall–Wallis test. In the event of a significant omnibus test, post hoc testing was conducted via the Dunn–Bonferroni test. Grip strength was assessed using Wilcoxon signed-rank tests to compare differences within dietary conditions, and Mann–Whitney U-tests were used to compare differences across groups. Immunoblot data were analyzed by 3 × 2 (Group X Injury), with injury as a repeated factor. Post hoc testing in the case of significant main effects or interactions was conducted with Fisher’s LSD test. For the in vitro experiments, one-way ANOVAs were used to assess peak FM4-64 intensity and AUC for the 24 and 48 h incubation conditions, separately. Analyses were performed in SPSS (version 29, IBM Corp., Armonk, NY, USA)
3. Results
3.1. In Vivo Testing
Animal characteristics: Animal weights and food consumption (disappearance) are presented in Table 1. Based on these data, the average EPA dosage was 0.540 g/kg bw/day, while the DHA dosage in the aged FO group was 0.112 g/kg bw/day, calculated as we have previously performed [9]. Although there was no significant effect of group for initial body masses and rates of food disappearance (p = 0.547 and 0.219, respectively), differences emerged upon inclusion of sex as a covariate in the analysis due to the unbalanced distribution of males and females across groups (p = 0.001 and 0.002, respectively). The initial food consumption normalized to body mass exhibited no significant effects for either analysis. Similarly, none of the percent changes in these variables showed a significant effect of group.
Table 1.
Animal weights and food consumption (disappearance).
| Adult (4M/4F) | Aged (2M/3F) | Aged (2M/3F) | |
|---|---|---|---|
| Body Mass and Food Consumption | |||
| Diet: | Ctl | Ctl | FO |
| Week 1 body mass (g) | 286.9 ± 95.2 | 325.1 ± 62.3 | 332.2 ± 70.3 |
| Body mass change at week 8 (%) | 3.3 ± 3.5 | 1.2 ± 5.0 | 2.5 ± 4.5 |
| Week 1 food disappearance (g/week) | 65.7 ± 20.3 | 79.3 ± 15.4 | 83.5 ± 17.0 |
| Week 8 food disappearance (g/week) | −6.7 ± 3.8 | −3.9 ± 13.6 | −1.6 ± 8.7 |
| Week 1 food disappearance/body mass | 0.241 ± 0.009 | 0.246 ± 0.014 | 0.254 ± 0.017 |
| Week 8 food disappearance/body mass | −9.6 ± 5.2 | −4.7 ± 15.2 | −3.6 ± 11.7 |
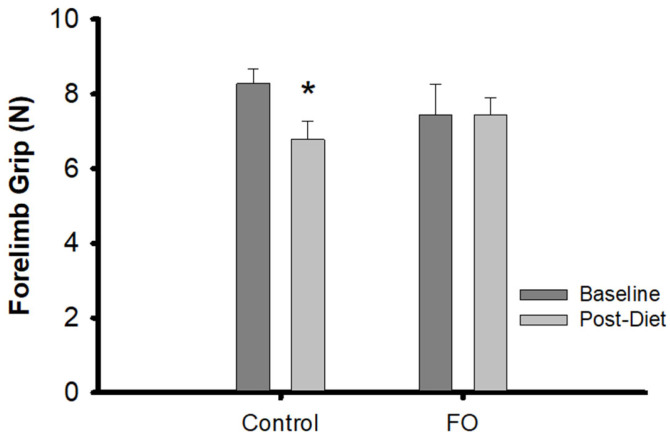
Grip strength: There were no differences in grip strength between the aged animals on the control and on the experimental diets. However, within-groups testing showed a significant (p = 0.028) decline over the 8-week dietary intervention in the aged rats on the control diet, but not the experimental diet (Figure 1).
Figure 1.
Changes in forelimb grip strength: Baseline and post-dietary intervention bilateral forelimb grip breaking force in aged rats on the control and FO diets. * = significant within group difference by Wilcoxon signed-rank tests (p = 0.028).
3.2. In Situ Testing
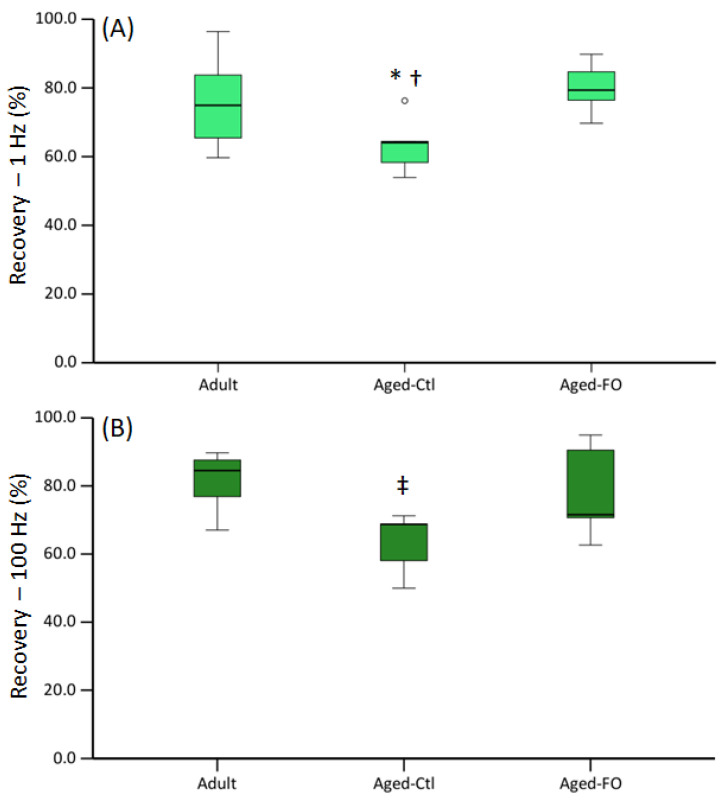
Muscle contractility: At 7 d post-injury, there was a significant effect of group on the ratio of injured/uninjured force in response to 1 and 100 Hz stimulation, with the aged/control diet group showing the least recovery of force (Figure 2).
Figure 2.
Muscle force at 7 d post-injury: Injured/uninjured peak force in response to (A) 1 Hz and (B) 100 Hz stimulation. Data are presented as boxplots showing median, IQR and CI. Kruskall–Wallis test showed significant group effect for 1 and 100 Hz (p = 0.050 and 0.040, respectively). * = significantly different from aged-FO; † = trend for difference from adult (p = 0.080), ‡ = significantly different from adult (p = 0.042).
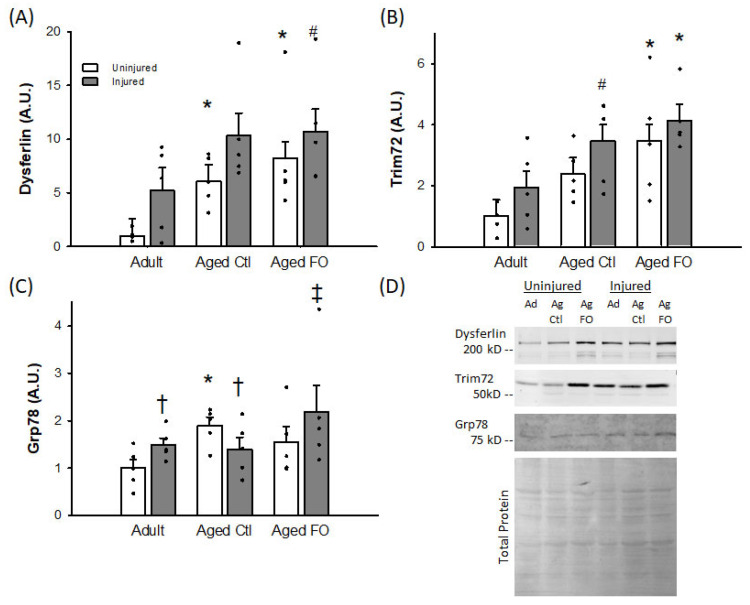
Immunoblotting: Dysferlin and Trim72/MG53 exhibited similar patterns of expression with a significant main effect of group (p = 0.006 and 0.010, respectively), due to increased levels in the aged groups relative to the young control animals (Figure 3A,B). Both proteins also exhibited an increase in response to injury, though this effect was significant for Dysferlin (p = 0.040) but not Trim72/MG53 (p = 0.053). No group X injury interaction was observed for either protein. In contrast to these membrane repair proteins, Grp78 did exhibit a significant group X injury interaction (p = 0.021), with no individual main effect for either factor. The interaction was largely driven by the aged-control diet group, which was increased in uninjured muscles but reduced in the injured muscles relative to the other two groups (Figure 3C).
Figure 3.
Mean (+SE) protein abundance in injured and uninjured muscles. Values are normalized to uninjured Ad means (arbitrary units, A.U.). (A) Dysferlin, (B) Trim72/MG53, (C) Grp78 and (D) representative blots for Dysferlin, Trim 72/MG53, and Grp78, along with total protein staining. Ad = adult; Ag Ctl = aged control diet; Ag FO = aged FO supplemented diet; n = 5/group. * = significantly different from corresponding Ad group (p < 0.050); # = trend for difference from corresponding Ad group (0.050 < p < 0.100); ‡ = significantly different from corresponding uninjured group (p < 0.050); † = trend for difference from corresponding uninjured group (0.050 < p < 0.100).
3.3. In Vitro Testing
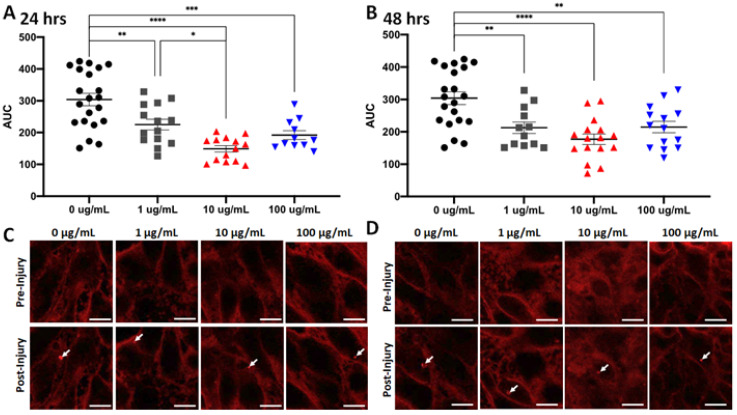
Plasma membrane repair assay: Generally, C2C12 cells incubated in the FO exhibited reduced AUC and peak FM4-64 fluorescence intensity at areas of membrane disruption following laser injury compared to those incubated in the control medium. There were some effects from dosage, as incubation in 10 µg/mL showed a greater effect than 1 µg/mL, but no additional effect was seen for 20 µg/mL. Of note, increasing the incubation time from 24 to 48 h did not appreciably change the results (Figure 4). These results suggest that FO enhances membrane resealing in the muscle-derived C2C12 cells.
Figure 4.
Fish oil exposure increases membrane repair in cultured skeletal myoblasts. (A) Fish oil was supplemented into the culture media for C2C12 cells at various concentrations (1 μg/mL, 10 μg/mL, and 100 μg/mL) for 24 h before the cells were subjected to laser injury in the presence of FM6-64 dye. FM-464 fluorescence signal at the laser injury were recorded by confocal microscopy for 60 s and then the area under curve (AUC) was determined for curves of the changes in fluorescent signal over time. Means of each treatment group were compared by one-way ANOVA with significance presented by * = p < 0.05, ** = p < 0.005, *** = p < 0.001, and **** = p < 0.0001. Data are represented as means ± SEM. (B) Similar results were seen in identical assays with cells exposed to fish oil for 48 h. (C) Representative images of C2C12 cells exposed to fish oil for 24 h before injury (top) or 60 s post-injury (bottom). Arrows indicate sites of laser injury. Scale bar represents 10 μm. (D) Representative images of C2C12 cells exposed to fish oil for 48 h before injury (top) or 60 s post-injury (bottom). Arrows indicate sites of laser injury. Scale bar represents 10 μm.
4. Discussion
The principal findings of this preliminary study were that an 8-week FO-supplemented diet improved muscle contractile function 7 days post-contusion injury and that the same FO product added to the incubation medium of C2C12 cells enhanced membrane resealing following laser injury of the plasmalemma.
Our previous study found that both adult and aged MG muscle exhibited similar (~45%) loss of contractile function acutely following contusion injury, with or without a FO-supplemented diet [9]. In the present study, adult MG muscles at 7 days post-injury exhibited 20–25% reduction of force vs. uninjured muscles, similar reports of contractility 7 days following a single, mechanical injury [7,42] that induced a comparable level of acute contractile impairment to that seen with our contusion protocol [9]. In contrast, the aged MG muscles from rats on the control diet exhibited a 30–40% reduction of force, while the aged MG muscles from rats on the experimental diet exhibited a reduction of force similar to that of the adult MG. These data suggest that increased age slows recovery of contractile function following contusion injury and that an 8-week FO-supplemented diet largely offsets this impairment.
The age-associated increase in dysferlin in uninjured muscles is similar to that which we have reported previously [27], and the observation of increased dysferlin abundance 1 week post-contusion is consistent with data from gastrocnemius muscles of young mice following electroporation injury [43]. It has been suggested that this sustained increase following acute injury may potentiate the membrane-sealing response to future injuries, which one might speculate are more likely in a previously-injured muscle. However, the similar pattern of expression seen in aged rats on the control and FO diets suggests that an effect of FO on dysferlin does not account for the enhanced recovery following injury. While we are unaware of studies reporting TRIM72/MG53 expression in the subacute and chronic injury phases, it seems reasonable that this protein would increase similarly to dysferlin, given the coordinated function of the two proteins in response to injury. It has also been reported that activation of AMPK is important for the membrane repair function of dysferlin, as rescued muscle phenotype in models of dysferlinopathy and enhanced myotube membrane recovery from injury, even when dysferlin levels were unchanged [44]. It could be that changes in p-AMPK/AMPK with FO could account for the improved contractile recovery despite the lack of effect on dysferlin. However, it is worth noting that aging is not a dysferlinopathy per se. Moreover, AMPK hyperphosphorylation has been reported in aging rat muscles [45], so a role for AMPK in aging membrane repair is not clear. Nevertheless, some [46,47,48], though not all [49,50,51], studies suggest that DHA and EPA can increase AMPK phosphorylation and activity, making this an appropriate target for future research.
The Grp78 response deviated somewhat from those of dysferlin and TRIM72/MG53. While aged muscle showed increased Grp78, consistent with previous observations [36,52], the injured muscles showed a different pattern than the membrane resealing proteins. We have previously shown that Grp78 is increased following acute contusion injury in adult and aged muscles [9], and elevated Grp78 has been reported 1 week following cardiotoxin-induced injury in young mice [53]. However, elevated Grp78 was seen in injured muscles of the adult and aged FO-supplemented rats, but not those in the aged control-diet group (Figure 3). Though associated with the ER stress and unfolded protein response resulting from proteins damaged by injury, Grp78 is also related to the chaperoning of newly synthesized proteins during tissue growth and regeneration [54,55]. It may be that the reduced Grp78 observed in the aged/control diet-fed rats compared to the adult and aged/FO diet-fed rats indicates an attenuated muscle regeneration with reduced new protein synthesis, contributing to the impaired functional recovery. It has also been reported that aging muscles contain tubular aggregates, abnormal cytoplasmic accumulations of membranes, typically enriched in SR markers [56]. Other investigators have reported that tubular aggregates also contain dysferlin and Grp78 [57]. A similar intramuscular aggregation pattern of TRIM72/MG53 has been reported in a mouse model of ALS with fragile membranes, though it was not definitively tied to tubular aggregates [58]. It is thus possible that the age-related increases seen in all three proteins evaluated in this study reflect increased tubular aggregates in addition to accumulated and/or ongoing focal membrane injuries. It has been suggested that tubular aggregates may be broken by degeneration/regeneration following significant muscle injury [59]. The differential changes in Grp78 and dysferlin (Figure 3) observed here suggest that a blanket change in tubular aggregates with injury is unlikely and an interaction between changes in tubular aggregates and other sites of protein localization is probably occurring.
Cultured C2C12 cells that received the same FO in their bathing medium exhibited a dose-dependent increase in membrane resealing following laser-induced membrane injury compared to those in medium without FO supplementation. These findings suggest a possible mechanism of action for the in vivo responses described above, since it has been observed that reduced sarcolemmal membrane repair increases susceptibility to mechanical muscle injury [29,39,40]. Though speculative, if membrane resealing is impaired in aged muscles and if dietary FO can correct that impairment, it could explain our in vivo findings. It is known that membrane composition and environment can affect its function and that age-related changes in membrane lipid makeup are associated with increased membrane fragility [60]. Moreover, both injury and dietary interventions, including FO, can alter membrane composition [31,61]. Thus, we speculate that changes in muscle membrane composition could explain the observed effects on membrane resealing observed in the current study. Further work is needed to explore these potential mechanisms in in vivo models of aging muscle injury.
The known antioxidant and anti-inflammatory effects attributed to FO might also speed recovery by, alone or in combination with effects on sarcolemmal repair, limiting the accumulation of chemical byproducts that could impair recovery. For example, it has been reported that reactive oxygen species induce greater inhibition of acetylcholine release at the neuromuscular junction of aged vs. adult rodents (NMJ) [62]. Thus, the anti-oxidant action of FO could limit this NMJ impairment post-injury and enhance recovery of contractile force. Interestingly, the decline in grip strength seen over 8 wks in the aged, control-fed rats was not observed in the aged rats on the experimental diet, similar to what we have observed previously, though in that study, the effect of FO showed only a trend for an effect on maintaining grip strength [9]. Thus, FO might enhance other aspects of muscle health in aging beyond recovery from mechanical injury.
These preliminary data suggest a potential role for dietary FO in promoting recovery of aging skeletal muscle function following mechanical injury. In addition, they provide proof-of-concept for at least one mechanism (improved sarcolemmal membrane resealing) for post-injury benefits of FO. However, as a preliminary study, there are limitations that must be acknowledged. First, we evaluated contractility and direct membrane resealing in different preparations: whole muscle and C2C12 myoblasts, respectively. In addition, we evaluated muscle function at a single time point—1 week post-injury. While our earlier study using the same injury model showed no acute benefit of FO, it would still have strengthened the study had we been able to test muscle function acutely and sub-acutely as well as during more prolonged recovery. We also tested only a few candidate mechanisms related to sarcolemmal injury (i.e., dysferlin, TRIM72/MG53), as these are known to be directly related to the membrane injury response. However, having identified a beneficial effect on subacute muscle function (contractility), future studies can examine other potential mechanisms, including, but not limited to, AMPK, membrane lipid composition, sarcoplasmic reticulum function, and myogenesis. Finally, because our focus was on improving aged muscle function, we did not evaluate the effects of FO in young animals.
Muscle function declines even in healthy, uncomplicated aging, and below a certain threshold, the risk of disability increases [63], whereby impaired muscle recovery is thought to contribute to the “negative staircase” effect in aging physical function [64]. It has been frequently observed that OAs exhibit reduced resiliency to muscle injury and disuse compared to younger adults [19,21,65,66,67,68], even when the magnitude of injury is no greater [69,70,71,72,73]. Thus, it is likely that impaired recuperative capacity plays a substantial role in the development of age-related weakness and physical disability [64]. Treatments that could enhance recovery might thus have great potential to improve health and wellness in old age. Further work is needed to evaluate this potential in dietary FO, in particular the extent to which FO supplementation could be used as a therapeutic intervention acutely following injury or if it needs to be consumed regularly as part of the normal diet.
5. Conclusions
The addition of an FO supplement to a standard diet appears to benefit aging muscle in rats by increasing recovery of muscle contractility in the sub-acute phase following a contusion injury. The same FO supplement, when included in a cell culture model, enhances sarcolemmal membrane resealing following acute laser injury. These data suggest that regular inclusion of FO in the diet may promote recovery from mechanical injury by enhancing membrane repair processes, though other effects of FO may be at work. Further study of these phenomena is needed to better understand the potential benefits of FO for aging muscle.
Author Contributions
Conceptualization, D.W.R. and N.L.W.; methodology, D.W.R. and N.L.W.; formal analysis, K.B.; investigation, C.S., K.B., and D.W.R.; resources, D.W.R. and N.L.W.; data curation, D.W.R.; writing—original draft preparation, D.W.R.; writing—review and editing, K.B., C.S., D.W.R., and N.L.W.; visualization, K.B. and D.W.R.; supervision, D.W.R. and N.L.W.; project administration, D.W.R. and N.L.W.; funding acquisition, D.W.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal study protocol was approved by the University of South Florida IACUC (approval code: 8039; approval date: 30 November 2020), and the “Principles of laboratory animal care” (NIH publication No. 86-23, revised 1985) were followed throughout the study.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
D.W.R., C.S., and K.B. have no conflicts to report. N.L.W. is a founder of TRIM-edicine and an inventor on multiple University-owned patents and patent applications relating to increasing membrane repair as a therapeutic intervention. These do not apply directly to the work presented here.
Funding Statement
University of South Florida Center for Neuromuscular Research (to D.W.R.).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.NCHS . National Center for Health Statistics. Health, U.S., 2016: With Chartbook on Long-Term Trends in Health. National Center for Health Statistics; Hyattsville, MD, USA: 2017. [PubMed] [Google Scholar]
- 2.Marrero J., Fortinsky R.H., Kuchel G.A., Robison J. Risk Factors for Falls Among Older Adults Following Transition From Nursing Home to the Community. Med. Care Res. Rev. MCRR. 2019;76:73–88. doi: 10.1177/1077558717697012. [DOI] [PubMed] [Google Scholar]
- 3.Rubenstein L.Z. Falls in older people: Epidemiology, risk factors and strategies for prevention. Age Ageing. 2006;35((Suppl. S2)):ii37–ii41. doi: 10.1093/ageing/afl084. [DOI] [PubMed] [Google Scholar]
- 4.Stevens J.A., Sogolow E.D. Gender differences for non-fatal unintentional fall related injuries among older adults. Inj. Prev. 2005;11:115–119. doi: 10.1136/ip.2004.005835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herala M., Luukinen H., Honkanen R., Koski K., Laippala P., Kivela S.L. Soft tissue injury resulting from falling predicts a future major falling injury in the home dwelling elderly. J. Epidemiol. Community Health. 2000;54:557–558. doi: 10.1136/jech.54.7.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Souza J., Gottfried C. Muscle injury: Review of experimental models. J. Electromyogr. Kinesiol. 2013;23:1253–1260. doi: 10.1016/j.jelekin.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Delos D., Leineweber M.J., Chaudhury S., Alzoobaee S., Gao Y., Rodeo S.A. The effect of platelet-rich plasma on muscle contusion healing in a rat model. Am. J. Sports Med. 2014;42:2067–2074. doi: 10.1177/0363546514540272. [DOI] [PubMed] [Google Scholar]
- 8.Naughton M., Miller J., Slater G.J. Impact-Induced Muscle Damage: Performance Implications in Response to a Novel Collision Simulator and Associated Timeline of Recovery. J. Sports Sci. Med. 2018;17:417–425. [PMC free article] [PubMed] [Google Scholar]
- 9.Russ D.W., Garvey S.M., Densmore C., Hawks T., Herman S., Pardi K. Effect of acute muscle contusion injury, with and without dietary fish oil, on adult and aged male rats: Contractile and biochemical responses. Exp. Gerontol. 2018;111:241–252. doi: 10.1016/j.exger.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Mackey A.L., Mikkelsen U.R., Magnusson S.P., Kjaer M. Rehabilitation of muscle after injury—The role of anti-inflammatory drugs. Scand. J. Med. Sci. Sports. 2012;22:e8–e14. doi: 10.1111/j.1600-0838.2012.01463.x. [DOI] [PubMed] [Google Scholar]
- 11.Bondesen B.A., Mills S.T., Kegley K.M., Pavlath G.K. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am. J. Physiol. Cell Physiol. 2004;287:C475–C483. doi: 10.1152/ajpcell.00088.2004. [DOI] [PubMed] [Google Scholar]
- 12.Morelli K.M., Brown L.B., Warren G.L. Effect of NSAIDs on Recovery from Acute Skeletal Muscle Injury: A Systematic Review and Meta-analysis. Am. J. Sports Med. 2018;46:224–233. doi: 10.1177/0363546517697957. [DOI] [PubMed] [Google Scholar]
- 13.Urso M.L. Anti-inflammatory interventions and skeletal muscle injury: Benefit or detriment? J. Appl. Physiol. 2013;115:920–928. doi: 10.1152/japplphysiol.00036.2013. [DOI] [PubMed] [Google Scholar]
- 14.de Magalhaes J.P., Muller M., Rainger G.E., Steegenga W. Fish oil supplements, longevity and aging. Aging. 2016;8:1578–1582. doi: 10.18632/aging.101021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Molfino A., Gioia G., Rossi Fanelli F., Muscaritoli M. The role for dietary omega-3 fatty acids supplementation in older adults. Nutrients. 2014;6:4058–4073. doi: 10.3390/nu6104058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casas-Agustench P., Cherubini A., Andres-Lacueva C. Lipids and physical function in older adults. Curr. Opin. Clin. Nutr. Metab. Care. 2017;20:16–25. doi: 10.1097/MCO.0000000000000333. [DOI] [PubMed] [Google Scholar]
- 17.Iolascon G., Gimigliano R., Bianco M., De Sire A., Moretti A., Giusti A., Malavolta N., Migliaccio S., Migliore A., Napoli N., et al. Are Dietary Supplements and Nutraceuticals Effective for Musculoskeletal Health and Cognitive Function? A Scoping Review. J. Nutr. Health Aging. 2017;21:527–538. doi: 10.1007/s12603-016-0823-x. [DOI] [PubMed] [Google Scholar]
- 18.Mendelsohn A.R., Larrick J.W. Trade-offs between anti-aging dietary supplementation and exercise. Rejuvenation Res. 2013;16:419–426. doi: 10.1089/rej.2013.1484. [DOI] [PubMed] [Google Scholar]
- 19.Alway S.E., Pereira S.L., Edens N.K., Hao Y., Bennett B.T. beta-Hydroxy-beta-methylbutyrate (HMB) enhances the proliferation of satellite cells in fast muscles of aged rats during recovery from disuse atrophy. Exp. Gerontol. 2013;48:973–984. doi: 10.1016/j.exger.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 20.McBride T.A., Gorin F.A., Carlsen R.C. Prolonged recovery and reduced adaptation in aged rat muscle following eccentric exercise. Mech. Ageing Dev. 1995;83:185–200. doi: 10.1016/0047-6374(95)01629-E. [DOI] [PubMed] [Google Scholar]
- 21.Morris R.T., Spangenburg E.E., Booth F.W. Responsiveness of cell signaling pathways during the failed 15-day regrowth of aged skeletal muscle. J. Appl. Physiol. 2004;96:398–404. doi: 10.1152/japplphysiol.00454.2003. [DOI] [PubMed] [Google Scholar]
- 22.English K.L., Paddon-Jones D. Protecting muscle mass and function in older adults during bed rest. Curr. Opin. Clin. Nutr. Metab. Care. 2010;13:34–39. doi: 10.1097/MCO.0b013e328333aa66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hui E.K., Rubenstein L.Z. Promoting physical activity and exercise in older adults. J. Am. Med. Dir. Assoc. 2006;7:310–314. doi: 10.1016/j.jamda.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Chakrabarti S., Kobayashi K.S., Flavell R.A., Marks C.B., Miyake K., Liston D.R., Fowler K.T., Gorelick F.S., Andrews N.W. Impaired membrane resealing and autoimmune myositis in synaptotagmin VII-deficient mice. J. Cell Biol. 2003;162:543–549. doi: 10.1083/jcb.200305131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X., Xie W., Zhang Y., Lin P., Han L., Han P., Wang Y., Chen Z., Ji G., Zheng M., et al. Cardioprotection of ischemia/reperfusion injury by cholesterol-dependent MG53-mediated membrane repair. Circ. Res. 2010;107:76–83. doi: 10.1161/CIRCRESAHA.109.215822. [DOI] [PubMed] [Google Scholar]
- 26.Rader E.P., Turk R., Willer T., Beltran D., Inamori K., Peterson T.A., Engle J., Prouty S., Matsumura K., Saito F., et al. Role of dystroglycan in limiting contraction-induced injury to the sarcomeric cytoskeleton of mature skeletal muscle. Proc. Natl. Acad. Sci. USA. 2016;113:10992–10997. doi: 10.1073/pnas.1605265113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russ D.W., Krause J., Wills A., Arreguin R. “SR stress” in mixed hindlimb muscles of aging male rats. Biogerontology. 2012;13:547–555. doi: 10.1007/s10522-012-9399-y. [DOI] [PubMed] [Google Scholar]
- 28.Bansal D., Miyake K., Vogel S.S., Groh S., Chen C.C., Williamson R., McNeil P.L., Campbell K.P. Defective membrane repair in dysferlin-deficient muscular dystrophy. Nature. 2003;423:168–172. doi: 10.1038/nature01573. [DOI] [PubMed] [Google Scholar]
- 29.Roche J.A., Lovering R.M., Bloch R.J. Impaired recovery of dysferlin-null skeletal muscle after contraction-induced injury in vivo. Neuroreport. 2008;19:1579–1584. doi: 10.1097/WNR.0b013e328311ca35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quinn C.J., Cartwright E.J., Trafford A.W., Dibb K.M. On the role of dysferlin in striated muscle: Membrane repair, t-tubules and Ca(2+) handling. J. Physiol. 2024;602:1893–1910. doi: 10.1113/JP285103. [DOI] [PubMed] [Google Scholar]
- 31.Henry R., Peoples G.E., McLennan P.L. Muscle fatigue resistance in the rat hindlimb in vivo from low dietary intakes of tuna fish oil that selectively increase phospholipid n-3 docosahexaenoic acid according to muscle fibre type. Br. J. Nutr. 2015;114:873–884. doi: 10.1017/S0007114515002512. [DOI] [PubMed] [Google Scholar]
- 32.Shaikh S.R., Dumaual A.C., Castillo A., LoCascio D., Siddiqui R.A., Stillwell W., Wassall S.R. Oleic and docosahexaenoic acid differentially phase separate from lipid raft molecules: A comparative NMR, DSC, AFM, and detergent extraction study. Biophys. J. 2004;87:1752–1766. doi: 10.1529/biophysj.104.044552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Russ D.W., Dimova K., Morris E., Pacheco M., Garvey S.M., Scordilis S.P. Dietary fish oil supplement induces age-specific contractile and proteomic responses in muscles of male rats. Lipids Health Dis. 2020;19:165. doi: 10.1186/s12944-020-01333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan A.A., Gul M.T., Karim A., Ranade A., Azeem M., Ibrahim Z., Ramachandran G., Nair V.A., Ahmad F., Elmoselhi A., et al. Mitigating sarcoplasmic reticulum stress limits disuse-induced muscle loss in hindlimb unloaded mice. NPJ Microgravity. 2022;8:24. doi: 10.1038/s41526-022-00211-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogborn D.I., McKay B.R., Crane J.D., Parise G., Tarnopolsky M.A. The unfolded protein response is triggered following a single, unaccustomed resistance-exercise bout. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014;307:R664–R669. doi: 10.1152/ajpregu.00511.2013. [DOI] [PubMed] [Google Scholar]
- 36.Russ D.W., Boyd I.M., McCoy K.M., McCorkle K.W. Muscle-specificity of age-related changes in markers of autophagy and sphingolipid metabolism. Biogerontology. 2015;16:747–759. doi: 10.1007/s10522-015-9598-4. [DOI] [PubMed] [Google Scholar]
- 37.Garvey S.M., Russ D.W., Skelding M.B., Dugle J.E., Edens N.K. Molecular and metabolomic effects of voluntary running wheel activity on skeletal muscle in late middle-aged rats. Physiol. Rep. 2015;3:e12319. doi: 10.14814/phy2.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hennig R., Lomo T. Firing patterns of motor units in normal rats. Nature. 1985;314:164–166. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- 39.Gushchina L.V., Bhattacharya S., McElhanon K.E., Choi J.H., Manring H., Beck E.X., Alloush J., Weisleder N. Treatment with Recombinant Human MG53 Protein Increases Membrane Integrity in a Mouse Model of Limb Girdle Muscular Dystrophy 2B. Mol. Ther. 2017;25:2360–2371. doi: 10.1016/j.ymthe.2017.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cai C., Weisleder N., Ko J.K., Komazaki S., Sunada Y., Nishi M., Takeshima H., Ma J. Membrane repair defects in muscular dystrophy are linked to altered interaction between MG53, caveolin-3, and dysferlin. J. Biol. Chem. 2009;284:15894–15902. doi: 10.1074/jbc.M109.009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weisleder N., Takizawa N., Lin P., Wang X., Cao C., Zhang Y., Tan T., Ferrante C., Zhu H., Chen P.J., et al. Recombinant MG53 protein modulates therapeutic cell membrane repair in treatment of muscular dystrophy. Sci. Transl. Med. 2012;4:139ra185. doi: 10.1126/scitranslmed.3003921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lovering R.M., Roche J.A., Bloch R.J., De Deyne P.G. Recovery of function in skeletal muscle following 2 different contraction-induced injuries. Arch. Phys. Med. Rehabil. 2007;88:617–625. doi: 10.1016/j.apmr.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Ishiba R., Santos A.L.F., Almeida C.F., Caires L.C., Jr., Ribeiro A.F., Jr., Ayub-Guerrieri D., Fernandes S.A., Souza L.S., Vainzof M. Faster regeneration associated to high expression of Fam65b and Hdac6 in dysferlin-deficient mouse. J. Mol. Histol. 2019;50:375–387. doi: 10.1007/s10735-019-09834-y. [DOI] [PubMed] [Google Scholar]
- 44.Ono H., Suzuki N., Kanno S.I., Kawahara G., Izumi R., Takahashi T., Kitajima Y., Osana S., Nakamura N., Akiyama T., et al. AMPK Complex Activation Promotes Sarcolemmal Repair in Dysferlinopathy. Mol. Ther. 2020;28:1133–1153. doi: 10.1016/j.ymthe.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomson D.M., Gordon S.E. Impaired overload-induced muscle growth is associated with diminished translational signalling in aged rat fast-twitch skeletal muscle. J. Physiol. 2006;574:291–305. doi: 10.1113/jphysiol.2006.107490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katsnelson G., Ceddia R.B. Docosahexaenoic and eicosapentaenoic fatty acids differentially regulate glucose and fatty acid metabolism in L6 rat skeletal muscle cells. Am. J. Physiol. Cell Physiol. 2020;319:C1120–C1129. doi: 10.1152/ajpcell.00304.2020. [DOI] [PubMed] [Google Scholar]
- 47.Martins A.R., Crisma A.R., Masi L.N., Amaral C.L., Marzuca-Nassr G.N., Bomfim L.H.M., Teodoro B.G., Queiroz A.L., Serdan T.D.A., Torres R.P., et al. Attenuation of obesity and insulin resistance by fish oil supplementation is associated with improved skeletal muscle mitochondrial function in mice fed a high-fat diet. J. Nutr. Biochem. 2018;55:76–88. doi: 10.1016/j.jnutbio.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 48.Wardle S.L., Macnaughton L.S., McGlory C., Witard O.C., Dick J.R., Whitfield P.D., Ferrando A.A., Wolfe R.R., Kim I.Y., Hamilton D.L., et al. Human skeletal muscle metabolic responses to 6 days of high-fat overfeeding are associated with dietary n-3PUFA content and muscle oxidative capacity. Physiol. Rep. 2020;8:e14529. doi: 10.14814/phy2.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deval C., Capel F., Laillet B., Polge C., Bechet D., Taillandier D., Attaix D., Combaret L. Docosahexaenoic acid-supplementation prior to fasting prevents muscle atrophy in mice. J. Cachexia Sarcopenia Muscle. 2016;7:587–603. doi: 10.1002/jcsm.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dobrzyn A., Dobrzyn P., Miyazaki M., Ntambi J.M. Polyunsaturated fatty acids do not activate AMP-activated protein kinase in mouse tissues. Biochem. Biophys. Res. Commun. 2005;332:892–896. doi: 10.1016/j.bbrc.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 51.McGlory C., Wardle S.L., Macnaughton L.S., Witard O.C., Scott F., Dick J., Bell J.G., Phillips S.M., Galloway S.D., Hamilton D.L., et al. Fish oil supplementation suppresses resistance exercise and feeding-induced increases in anabolic signaling without affecting myofibrillar protein synthesis in young men. Physiol. Rep. 2016;4:e12715. doi: 10.14814/phy2.12715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogata T., Machida S., Oishi Y., Higuchi M., Muraoka I. Differential cell death regulation between adult-unloaded and aged rat soleus muscle. Mech. Ageing Dev. 2009;130:328–336. doi: 10.1016/j.mad.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 53.He S., Fu T., Yu Y., Liang Q., Li L., Liu J., Zhang X., Zhou Q., Guo Q., Xu D., et al. IRE1alpha regulates skeletal muscle regeneration through Myostatin mRNA decay. J. Clin. Investig. 2021;131:e143737. doi: 10.1172/JCI143737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bohnert K.R., McMillan J.D., Kumar A. Emerging roles of ER stress and unfolded protein response pathways in skeletal muscle health and disease. J. Cell Physiol. 2018;233:67–78. doi: 10.1002/jcp.25852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhu G., Ye R., Jung D.Y., Barron E., Friedline R.H., Benoit V.M., Hinton D.R., Kim J.K., Lee A.S. GRP78 plays an essential role in adipogenesis and postnatal growth in mice. FASEB J. 2013;27:955–964. doi: 10.1096/fj.12-213330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boncompagni S., Pecorai C., Michelucci A., Pietrangelo L., Protasi F. Long-Term Exercise Reduces Formation of Tubular Aggregates and Promotes Maintenance of Ca(2+) Entry Units in Aged Muscle. Front. Physiol. 2020;11:601057. doi: 10.3389/fphys.2020.601057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ikezoe K., Furuya H., Ohyagi Y., Osoegawa M., Nishino I., Nonaka I., Kira J. Dysferlin expression in tubular aggregates: Their possible relationship to endoplasmic reticulum stress. Acta Neuropathol. 2003;105:603–609. doi: 10.1007/s00401-003-0686-1. [DOI] [PubMed] [Google Scholar]
- 58.Yi J., Li A., Li X., Park K., Zhou X., Yi F., Xiao Y., Yoon D., Tan T., Ostrow L.W., et al. MG53 Preserves Neuromuscular Junction Integrity and Alleviates ALS Disease Progression. Antioxidants. 2021;10:1522. doi: 10.3390/antiox10101522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.de Vasconcelos F., Ribeiro Junior A.F., Souza B.W., Zogbi I.A., Carvalho L.M.L., Feitosa L.N., Souza L.S., Saldys N.G., Ferrari M.F.R., Vainzof M. Induced degeneration and regeneration in aged muscle reduce tubular aggregates but not muscle function. Front. Neurol. 2024;15:1325222. doi: 10.3389/fneur.2024.1325222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hord J.M., Botchlett R., Lawler J.M. Age-related alterations in the sarcolemmal environment are attenuated by lifelong caloric restriction and voluntary exercise. Exp. Gerontol. 2016;83:148–157. doi: 10.1016/j.exger.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Helge J.W., Therkildsen K.J., Jorgensen T.B., Wu B.J., Storlien L.H., Asp S. Eccentric contractions affect muscle membrane phospholipid fatty acid composition in rats. Exp. Physiol. 2001;86:599–604. doi: 10.1113/eph8602196. [DOI] [PubMed] [Google Scholar]
- 62.Shakirzyanova A., Valeeva G., Giniatullin A., Naumenko N., Fulle S., Akulov A., Atalay M., Nikolsky E., Giniatullin R. Age-dependent action of reactive oxygen species on transmitter release in mammalian neuromuscular junctions. Neurobiol. Aging. 2016;38:73–81. doi: 10.1016/j.neurobiolaging.2015.10.023. [DOI] [PubMed] [Google Scholar]
- 63.Manini T.M., Visser M., Won-Park S., Patel K.V., Strotmeyer E.S., Chen H., Goodpaster B., De Rekeneire N., Newman A.B., Simonsick E.M., et al. Knee extension strength cutpoints for maintaining mobility. J. Am. Geriatr. Soc. 2007;55:451–457. doi: 10.1111/j.1532-5415.2007.01087.x. [DOI] [PubMed] [Google Scholar]
- 64.Faulkner J.A., Brooks S.V., Zerba E. Muscle atrophy and weakness with aging: Contraction-induced injury as an underlying mechanism. J. Gerontol. A Biol. Sci. Med. Sci. 1995;50A:124–129. doi: 10.1093/gerona/50a.special_issue.124. [DOI] [PubMed] [Google Scholar]
- 65.Baehr L.M., West D.W., Marcotte G., Marshall A.G., De Sousa L.G., Baar K., Bodine S.C. Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging. 2016;8:127–146. doi: 10.18632/aging.100879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rader E.P., Faulkner J.A. Effect of aging on the recovery following contraction-induced injury in muscles of female mice. J. Appl. Physiol. 2006;101:887–892. doi: 10.1152/japplphysiol.00380.2006. [DOI] [PubMed] [Google Scholar]
- 67.Brooks S.V., Faulkner J.A. Contraction-induced injury: Recovery of skeletal muscles in young and old mice. Am. J. Physiol. 1990;258:C436–C442. doi: 10.1152/ajpcell.1990.258.3.C436. [DOI] [PubMed] [Google Scholar]
- 68.Buford T.W., MacNeil R.G., Clough L.G., Dirain M., Sandesara B., Pahor M., Manini T.M., Leeuwenburgh C. Active muscle regeneration following eccentric contraction-induced injury is similar between healthy young and older adults. J. Appl. Physiol. 2014;116:1481–1490. doi: 10.1152/japplphysiol.01350.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hunter G.R., McCarthy J.P., Bamman M.M. Effects of resistance training on older adults. Sports Med. 2004;34:329–348. doi: 10.2165/00007256-200434050-00005. [DOI] [PubMed] [Google Scholar]
- 70.Cutlip R.G., Baker B.A., Geronilla K.B., Kashon M.L., Wu J.Z. The influence of velocity of stretch-shortening contractions on muscle performance during chronic exposure: Age effects. Appl. Physiol. Nutr. Metab. 2007;32:443–453. doi: 10.1139/H07-014. [DOI] [PubMed] [Google Scholar]
- 71.Jarvinen M., Aho A.J., Lehto M., Toivonen H. Age dependent repair of muscle rupture. A histological and microangiographical study in rats. Acta Orthop. Scand. 1983;54:64–74. doi: 10.3109/17453678308992871. [DOI] [PubMed] [Google Scholar]
- 72.Roth S.M., Martel G.F., Ivey F.M., Lemmer J.T., Tracy B.L., Hurlbut D.E., Metter E.J., Hurley B.F., Rogers M.A. Ultrastructural muscle damage in young vs. older men after high-volume, heavy-resistance strength training. J. Appl. Physiol. 1999;86:1833–1840. doi: 10.1152/jappl.1999.86.6.1833. [DOI] [PubMed] [Google Scholar]
- 73.Brooks S.V., Faulkner J.A. The magnitude of the initial injury induced by stretches of maximally activated muscle fibres of mice and rats increases in old age. Pt 2J. Physiol. 1996;497:573–580. doi: 10.1113/jphysiol.1996.sp021790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.