Abstract
Transmission of human immunodeficiency virus type 1 (HIV-1) is largely a result of heterosexual exposure, leading many investigators to evaluate mucosal vaccines for protection against intravaginal (i.vag.) transmission in macaque models of AIDS. Relatively little is known, however, about the dynamics of viral replication and the ensuing immune response following mucosal infection. We have utilized a simian-human immunodeficiency virus (SHIV) to study the differences in viremia, CD4 T-cell percentages, and mucosal and systemic anti-SHIV humoral and cellular immune responses during primary infection of animals infected either intravenously (i.v.) or i.vag. Positive viral cocultures, peripheral blood mononuclear cell viral load peaks, and CD4 cell declines were delayed by 1 week in the i.vag. inoculated animals compared to the animals infected i.v., demonstrating delayed viral spreading to the periphery. In contrast, mucosal anti-SHIV antibody levels were greater in magnitude and arose more rapidly and mucosal CD8+ T-cell responses were enhanced in the i.vag. group animals, whereas both the magnitudes and times of onset of systemic immune responses for the animals in the two groups did not differ. These observations demonstrate that compartmentalization of viral replication and induction of local antiviral immunity occur in the genital tract early after i.vag. but not i.v. inoculation. Induction of mucosal immunity to target this local, contained replication should be a goal in HIV vaccine development.
Worldwide, the most prevalent route of transmission of human immunodeficiency virus type 1 (HIV-1) occurs through heterosexual contact, especially in developing countries. Heterosexual transmission is highly prevalent in sub-Saharan Africa, where 55% of HIV-infected adults are women (29). Women are most likely infected as a result of coming into contact with HIV-infected cells or cell-free virus from infected semen during vaginal intercourse. The course of infection and progression to disease, once infection is established, appear to be similar regardless of the route of infection.
The mucosal surfaces of the vagina and ectocervix comprise multiple layers of stratified squamous epithelial cells (22), which presumably form an effective barrier against viral infection. As a consequence, the use of hormonal contraceptives can pose a significant risk for transmission in women due to thinning of the vaginal epithelium during high progesterone levels (16). How the virus crosses the epithelium and infects target cells is not completely understood. Recently Hu et al. showed that dendritic cells (DCs), macrophages, and CD4+ T lymphocytes in the vaginal mucosas of macaques were infected with simian immunodeficiency virus (SIV) 18 h after inoculation by this route (7). One to 5 days after infection via this route, lymph node cells were shown to contain SIV (7, 26). Although these papers describe the fate of individual target cells infected with HIV-1, they do not address the extent of viral replication that occurs locally after mucosal infection.
Recently, mucosal immune responses against HIV in women who have been highly exposed to HIV but who are persistently seronegative have been described in an effort to elucidate immune system correlates of protection against infection. In such cohorts, HIV-1-specific immunoglobulin A (IgA) antibodies were discovered in the vaginal secretions, suggesting that locally produced antibodies were important in protection of these women from overt infection (10, 18). In addition, numerous studies have shown induction of antigen-specific IgG and IgA in the genital tract by a variety of immunization methods, with various degrees of protection from viral infection (reviewed in references 3 and 6). In terms of mucosal cellular immune responses, HIV-specific cervical CD8+ T lymphocytes were found to be enriched in the cervices of the multiply exposed, seronegative women compared to levels in women who were HIV seropositive, suggesting that local CD8+ T cells are also important in protection against intravaginal (i.vag.) infection (9).
Studies have shown that atraumatic i.vag. inoculation of cell-free SIV can infect macaques, which have an anatomy similar to that of humans (19). However, nonhuman primate models for AIDS often use intravenous (i.v.) inoculation as the mode of infection. This emphasis on i.v. inoculation stems in part from the reproducibility of the system. The i.vag. inoculation of macaques generally requires high doses of virus and often does not result in productive infection (19), whereas i.v. infection requires much less virus to consistently produce infection. It is not clear whether the route of HIV infection results in differences in the systemic and mucosal antiviral immune responses. A previous study of the SIV macaque model has shown similar levels of vaginal antibody responses following either systemic or mucosal infection (21). However, this study did not investigate early time points after infection. No studies have evaluated quantitative or qualitative differences in mucosal cellular immunity against SIV or simian-human immunodeficiency virus (SHIV) in nonhuman primates during primary infection following systemic versus i.vag. challenge. If differences in these immune compartments exist at these early, critical time points, the nature of HIV immunization will need to be evaluated and optimized for better immune responses, especially in the female reproductive tract.
In this paper we have evaluated humoral and cellular immune responses in macaques infected with an SHIV chimera virus (SHIV89.6) either i.v. or i.vag. We have identified differences in the kinetics of primary viral replication and the early immune responses during acute SHIV infection between these two groups.
MATERIALS AND METHODS
Animal care.
Twelve adult female pigtailed macaques (Macaca nemestrina) which were negative for SIV, simian retrovirus type 2, and simian T-cell lymphotropic virus type 1 were used. These animals were between the ages of 7 and 15 and were experiencing menses. The animals were housed at the Washington Regional Primate Research Center. All macaque procedures were carried out with the approval of the Animal Care and Use Committee at the University of Washington. During all procedures the animals were anesthetized with an intramuscular injection of 10 to 15 mg of ketamine-HCl/kg of body weight.
Cell culture medium.
In vitro cultures were performed in RPMI 1640 (Gibco BRL, Gaithersburg, Md.) containing 25 mM HEPES, 10% fetal calf serum (HyClone, Logan, Utah), 100 U each of penicillin and streptomycin (Sigma, St. Louis, Mo.), and 50 μM 2-mercaptoethanol (J. T. Baker, Phillipsburg, N.J.).
Virus stock.
The virus used in this study was SHIV89.6, which has been described previously (24). The particular stock used here, designated SHIV89.6V, was passaged once in vivo in a rhesus macaque through i.vag. inoculation and briefly cultured on rhesus macaque peripheral blood mononuclear cells (PBMC; a kind gift from Y. Lu, AVANT Immunotherapeutics, Needham, Mass.). The viral concentration of the stock was determined to be 103 50% tissue culture infectious doses (TCID50)/ml by culture on CEMx174 cells and p27 production. The C2 through C4 envelope sequence was determined by PCR using the M7 and M10 primers described previously (27).
SHIV challenge.
Four weeks prior to and 1 week following challenge, all animals were given 30 mg of medroxyprogestrone acetate (Depo-Provera; Pharmacia & Upjohn, Kalamazoo, Mich.) intramuscularly (25). The i.v. inoculations were given by syringe into the femoral vein with 200, 20, and 2 TCID50 of SHIV89.6V. The i.vag. inoculations were given atraumatically through a small tube inserted into the vagina with 1,800, 600, or 200 TCID50 of SHIV89.6V.
Blood and mucosal samples for immunological assays.
Blood was taken at multiple time points throughout the study in tubes containing preservative-free heparin (immunological assays) or EDTA (virological assays). PBMC were separated over 95% Lymphocyte Separation Medium (immunological assays; Organon Teknika, Durham, N.C.) or 95% Lymphoprep medium (virological assays; Life Technologies, Rockville, Md.) gradient and used fresh or after thawing following cryopreservation in 10% dimethyl sulfoxide at −80°C or in liquid nitrogen. Plasma was also separated and frozen at −20°C. Vaginal washes were obtained by instilling and removing 1 ml of sterile phosphate-buffered saline (PBS) into the vagina. The washes were frozen at −20°C until ready for use. Cervical lymphocytes were obtained by inserting a plastic tube into the animal's vagina and placing a Cytobrush Plus (Medscand, Hollywood, Fla.) into the cervical os and rotating it. The cytobrush was removed from the tube without contacting the vaginal walls, and the cells were washed in medium and placed on ice until ready for use in immunological assays. Vaginal washes and cervical cytobrushes were discarded if they contained visible blood contamination.
Virus isolation.
PBMC (5 × 106) were cocultured 1:1 with CEMx174 cells in medium at 37°C for 1 week. Coculture supernatant was analyzed for viral antigens with the SIV-1 p27 antigen enzyme-linked immunosorbent assay (ELISA) kit (ZeptoMetrix, Buffalo, N.Y.). After sampling, half of the culture medium was replaced and additional CEMx174 cells were added if necessary. Sampling and medium replacement were repeated for 6 weeks. After two consecutive positive assays, the coculture was considered positive and discarded.
Viral DNA and RNA loads.
Quantification of SIV DNA in PBMC was determined by quantitative-competitive PCR as previously described (30). Briefly, DNA samples were mixed with competitor DNA of pCon-1 plasmid containing an SIV gag insert with an internal deletion. Primers used for amplification were AAAGCCTGTTGGAGAACAAAGAAG (5′) and AATTTTACCCAGGCATTTA (3′). The thermocycling conditions used for PCR were 96°C for 11 min, followed by 41 cycles of 96°C for 15 s, 55°C for 1 min, and 72°C for 30 s, followed by an extension at 72°C for 9 min. Amplified products were separated on 3% agarose gels and visualized on a Gel-Doc 2000 documentation system (Bio-Rad, Hercules, Calif.) to determine band intensities. The limit of detection was 101 copies/μg of DNA.
Plasma SIV branched-DNA (bDNA) assays were performed at Bayer Diagnostics as previously described (5). The limit of detection was 500 copies/ml of plasma.
PBMC subsets.
PBMC were stained with fluorescently labeled antibodies against surface CD4 (Becton Dickinson, Mountain View, Calif.) and CD8 (clone G10-1; a kind gift from E. Clark, University of Washington, Seattle). PBMC were analyzed by FACScan and CellQuest (Becton Dickinson). CD4+ and CD8+ T-cell percentages were expressed as the absolute count divided by the total number of lymphocytes per cubic millimeter.
SHIV antibody ELISA.
Maxisorp plates (Nunc, Naperville, Ill.) were coated overnight with 1 (plasma ELISA) or 3 μg (vaginal wash ELISA) of AT-2 inactivated SHIV89.6V lysate/ml in carbonate buffer. Plates were washed four times in wash buffer (0.1% Triton X-100 in PBS) and then blocked with 5% nonfat milk in PBS for 1 h at 37°C. Plasma was diluted to 1:50 in 1% Triton X-100 buffer and titrated in duplicate. Vaginal washes were diluted 2:1 in 1% Triton X-100 and tested in duplicate. The samples were incubated for 1 h at 37°C. After the plates were washed, rabbit anti-human IgG-horseradish peroxidase (HRP) or rabbit anti-human IgA-HRP (1:5,000; Dako, Carpinteria, Calif.) in 1% Triton X-100 was added and the plates were incubated for 1 h at 37°C. Turbo TMB (Pierce, Rockford, Ill.) was added to the plates, allowed to develop, and stopped with 1 M sulfuric acid. Plates were read at 450 nm on a THERMOmax ELISA plate reader with SOFTmax software (Molecular Devices, Sunnyvale, Calif.). Plasma titers are expressed as endpoint dilutions, which were defined as dilutions twofold above the background of prebleeds, and vaginal washes are expressed as optical density (OD)/100 μl of diluted wash.
Intracellular cytokine staining.
Intracellular gamma interferon (IFN-γ) cytokine staining was performed on PBMC and cervical lymphocytes after SHIV antigen stimulation. Monocyte target cells from PBMC in 48-well plates (Costar, Cambridge, Mass.) were infected with wild-type (WT) vaccinia virus (VV) (NYCBH) or recombinant VV (rVV) (vT107 or vAbT394, which expressed HIV-189.6 Env and SIVmac251 Gag-Pol, respectively; kind gifts from D. Panacali, Therion Biologics, Cambridge, Mass.) at a multiplicity of infection of 10 for 4 h. The targets then were washed with PBS, and autologous effector cells from monocyte-depleted PBMC or cervical lymphocytes were added to the targets at a ratio of 1:1. The cells were incubated for 12 h, of which the last 4 h were in the presence of brefeldin A (GolgiPlug; PharMingen, San Diego, Calif.). Effector cells were collected, washed in medium, and stained for surface antigens CD8 (biotinylated or conjugated to phycoerythrin [PE; Becton Dickinson]; detected with streptavidin-Tri-Color [Caltag, South San Francisco, Calif.]), CD3 (conjugated to fluorescein isothiocyanate [FITC; PharMingen]), and CD69 (conjugated to PE; Becton Dickinson). The cells were permeabilized with the Cytofix/Cytoperm kit (PharMingen) and stained intracellularly for IFN-γ (FITC or PE; PharMingen). Cells were evaluated by FACScan and CellQuest software.
Responses are expressed as the percentages of CD8+ CD69+ or CD3+ CD8+ cells that produce IFN-γ after stimulation with rVV above the percentage of cells that produce IFN-γ after stimulation with WT VV. Responses were not included if the percentage of IFN-γ-producing cells when stimulated with WT VV was above 1%.
Statistical analysis.
All statistical analyses were performed using GraphPad Prism software (GraphPad, San Diego, Calif.). Comparisons of dose inoculum to viremic peak or decline in CD4 percentages were done using a two-sided Pearson correlation test with 95% confidence interval (CI). Comparisons between i.v. and i.vag. viremic peaks, CD4 percentage declines, and antibody responses were done using two-sided unpaired t tests, with a 95% CI.
RESULTS
i.v. and i.vag. inoculations of macaques with SHIV89.6V.
In vivo titrations of SHIV89.6V were performed using 12 pigtailed macaques. PCR of the C2, C3, C4, V3, and V4 regions of the envelope of this previously in vivo-passaged virus showed the virus to be essentially identical to the original, unpassaged SHIV89.6 (data not shown). Six animals were inoculated i.v. and six animals were inoculated i.vag. with different doses of the viral stock (Table 1). Previously, 1,800 TCID50 of a stock of the original SHIV89.6 was used to inoculate two pigtailed macaques i.vag. In that study, neither animal became infected as determined by coculture and by measuring plasma viral RNA and PBMC proviral DNA (unpublished results). To increase the rate of infection in the i.vag. titrations in this study, all animals were treated with Depo-Provera 4 weeks before and 1 week after viral challenge, as such progesterone treatment has been shown to decrease the thickness of the vaginal epithelium and to lead to higher SIV transmission rates in rhesus macaques (17). This treatment was applied to all animals, irrespective of the subsequent route of viral inoculation, to cancel any bias that could potentially be introduced by the administered hormones.
TABLE 1.
i.v. and i.vag. titration groups and viral coculture results
| Animal no. | Inoculation route | Dose (TCID50)a | Coculture result at w.p.i.b:
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 10 | 12 | |||
| 99150 | i.v. | 200 | − | + | + | + | + | + | + | + | + | + | − |
| 99161 | i.v. | 200 | − | + | + | + | + | + | + | + | + | + | + |
| A99224 | i.v. | 20 | − | + | + | + | + | + | + | + | + | + | − |
| A99249 | i.v. | 20 | − | + | + | + | + | + | + | + | + | + | + |
| 99158 | i.v. | 2 | − | − | + | + | + | + | + | + | + | + | − |
| 99176 | i.v. | 2 | − | + | + | + | + | + | + | + | + | + | + |
| 97063 | i.vag. | 1,800 | − | − | + | + | + | + | + | + | + | − | + |
| 92218 | i.vag. | 1,800 | − | − | + | + | + | + | + | + | + | + | + |
| 94047 | i.vag. | 600 | − | + | + | + | + | + | + | + | + | − | − |
| F92261 | i.vag. | 600 | − | − | + | + | + | + | + | + | + | + | + |
| 97070 | i.vag. | 200 | − | + | + | + | + | + | + | + | + | + | + |
| 94075 | i.vag. | 200 | − | − | + | + | + | + | + | + | + | + | − |
Viral stock was 1,000 TCID50/ml.
Cocultures were done weekly or biweekly and sampled every 7 days for 35 days and were determined positive by ELISA.
Viral load (DNA and RNA) quantitation.
The infection status of the 12 animals was determined by three assays. A very sensitive nonquantitative coculture assay was performed on PBMC at multiple time points after inoculation (Table 1). Proviral loads were determined by SIV quantitative-competitive PCR on PBMC DNA (Fig. 1A and B), and the bDNA assay was used to test viral RNA concentrations in the plasma (Fig. 1C and D) at multiple time points postinfection. All 12 animals were positive for infection by all three assays.
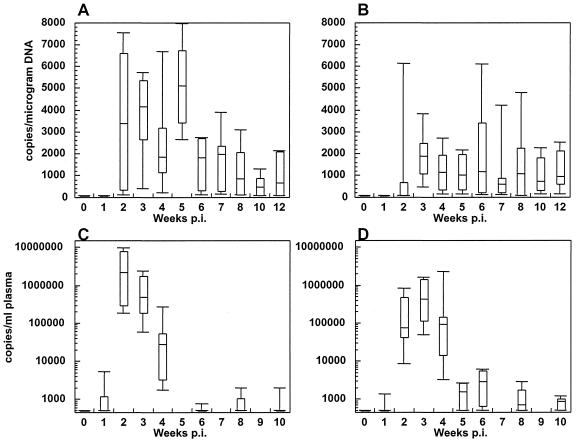
FIG. 1.
Viral loads from the i.v. and i.vag. groups of animals. Viral DNA in the PBMC as determined by PCR was assayed for the i.v. group (A) and the i.vag. group (B) from 0 to 12 w.p.i. Plasma viral RNA levels were determined by bDNA assay for the i.v. (C) and i.vag. (D) inoculated animals. Viral loads from animals of each group at the time points tested are represented by box plots. Boxes, 75th and 25th percentiles (top and bottom, respectively); lines in boxes, medians; outlier caps, spreads of values for each group.
Virus levels in plasma peaked between 2 and 4 weeks postinfection (w.p.i.) in both groups, with higher peak values of plasma viremia in the animals that were inoculated i.v. (3.8 × 106 versus 1.0 × 106 copies/ml; Fig. 1C and D). The peak plasma viral load within both groups showed a nonsignificant trend toward correlation with the dose of virus (i.v.: P = 0.07; i.vag.: P = 0.06). Proviral DNA could be detected in five of six animals inoculated i.v. (Fig. 1A) and in two of six animals inoculated i.vag. (Fig. 1B). All other animals had detectable provirus by 3 w.p.i. The differences in the slopes of proviral loads versus time postinfection between the two groups of animals showed a trend toward significance (P = 0.08). Likewise, the difference in mean peak proviral loads between the two groups (5,860 ± 864.7 versus 3,312 ± 966.6 copies/μg of DNA) also approached significance (P = 0.07). This lack of significance perhaps can be attributed to the large variation in proviral load peak values seen specifically for the individual i.vag. inoculated animals (Fig. 1B); these values appeared to be unrelated to the size of the viral inoculum.
Virus isolation from PBMC was performed using CEMx174 cells. The results obtained for the first few time points after inoculation suggest a delay in the appearance of infectious virus in the PBMC of the animals inoculated i.vag., similar to what was observed for the detection of proviral DNA. At 1 w.p.i., virus could be reisolated from the PBMC of five of six animals in the i.v. group but from the PBMC of only of two of six i.vag. inoculated animals (Table 1). This difference also showed a trend toward significance (P = 0.08).
Lymphocyte subset changes.
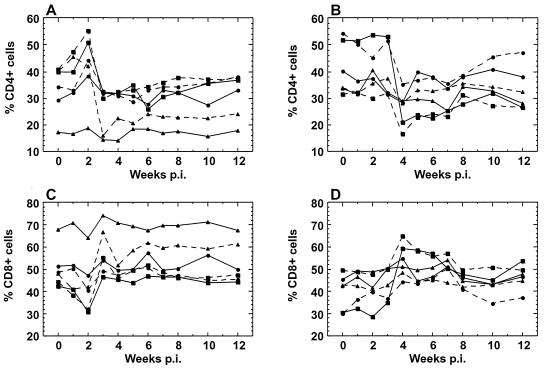
Infection with SHIV89.6V was not pathogenic in M. nemestrina during follow-up. However, we did observe a temporary decrease in CD4+ cell percentages in the circulation during infection (Fig. 2A and B). In the animals infected i.v., an initial increase in CD4+ cell percentages was observed during the first 2 w.p.i., followed by a decline in CD4+ T cells at 3 w.p.i. (Fig. 2A), concurrent with an increase in CD8+ cell percentages (Fig. 2C). The i.vag. infected animals did not have an initial increase in CD4+ cells, as observed in the i.v.-inoculated animals, but they had a similar decline in PBMC CD4 percentages (Fig. 2B), which occurred simultaneously with increases in CD8 percentages (Fig. 2D). This decline was delayed by 1 week compared to the CD4 percentage decline in the i.v. group (4 w.p.i. versus 3 w.p.i.), and the magnitude of the decline in individual animals correlated with inoculum dose (P = 0.03). The changes in lymphocyte subsets also were apparent in the absolute CD4 and CD8 counts (data not shown).
FIG. 2.
T-cell subsets of PBMC for all animals. Percentages of CD4+ lymphocytes were plotted for the i.v. (A) and i.vag. (B) groups. The difference in CD4+ percentages between baseline and the lowest value was not correlated with inoculum dose in the i.v. group (two-tailed Spearman correlation, P = 0.8) but was correlated with inoculum dose in the i.vag. group (two-tailed Spearman correlation, P = 0.03). Percentages of CD8+ lymphocytes were plotted for the i.v. (C) and i.vag. (D) groups. The doses for the i.v. and i.vag. animals, respectively, were as follows: high (squares), 200 and 1,800 TCID50; medium (circles), 20 and 600 TCID50; low (triangles), 2 and 200 TCID50.
SHIV-specific humoral immune responses.
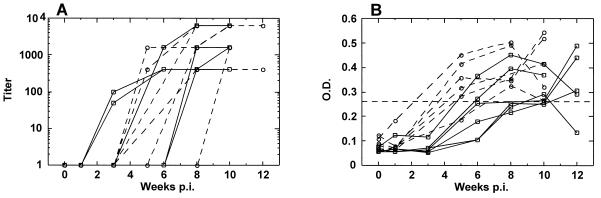
Anti-SHIV plasma IgG antibodies from the 12 animals were evaluated in the first 10 w.p.i. by ELISA (Fig. 3A). Animals inoculated i.v. had peak plasma endpoint titers ranging from 1:400 to 1:6,400 (mean of 1:2,200 ± 862.6) with the responses first detected at 3 to 8 w.p.i. Macaques infected i.vag. had endpoint IgG titers of 1:400 to 1:6,400 (mean of 1:3,800 ± 1,149), and responses were initially seen at 5 to 10 w.p.i., which appeared to be somewhat delayed relative to those of the i.v. group animals.
FIG. 3.
Anti-SHIV IgG responses in the plasma (A) and vaginal washes (B) of the i.v. (□) and the i.vag. (○) animals. Dashed line, cutoff level of detection, which was defined as a level twofold higher than the average of the washes obtained at week 0. There was no statistically significant difference in plasma IgG titer between the two groups (unpaired t test, P = 0.13). The magnitude of the vaginal IgG OD values was significantly lower at the first positive time point in the i.v. group than in the i.vag. group (unpaired t test, P = 0.02).
There were clear differences in both the onset and magnitude of the genital humoral immune responses against SHIV between the animals infected i.v. and those infected i.vag. (Fig. 3B). Five of the six macaques infected i.vag. had detectable vaginal IgG responses at 5 w.p.i., whereas none of the animals that were inoculated i.v. had detectable vaginal IgG responses prior to 6 w.p.i. The magnitude of the antibody responses was lower at the first positive time point in the animals infected i.v. (mean OD of 0.266 ± 0.021) than in those infected i.vag. (mean OD of 0.359 ± 0.026). This difference was statistically significant (P = 0.02). There was no apparent association between inoculum dose and the magnitude or time of onset of the vaginal IgG response in either group of animals. Consistent with other studies of HIV-infected humans (1, 15) and chimpanzees (8) and SIV-infected macaques (13, 21), we detected IgG and not IgA antibodies in vaginal secretions (Fig. 3B). It is possible that IgA antibodies were outcompeted in binding by higher-affinity IgG isotypes or that IgG was the dominant isotype found in the vaginal tract.
SHIV-specific cellular immune responses.
CD8+ T-cell responses against SHIV antigens were measured regularly in the PBMC from both groups of animals from 0 to 12 w.p.i. (Table 2). Strong, nonspecific cellular responses, characterized by a high percentage of CD8+ CD69+ cells responding to WT VV, were observed in all animals at early time points (1 to 4 w.p.i.; data not shown). Such nonspecific responses may have obscured SHIV-specific responses in those early samples. Responses to Env and Gag-Pol were detected in the PBMC of all the i.v. infected animals (17 of 51 assays, or 33%, showed detectable responses). The i.vag. infected animals had similar levels of responses to both antigens in PBMC (responses detected in 17 of 57 assays, or 30%).
TABLE 2.
CD8+ CD69+ PBMC IFN-γ responses to Env and Gag-Pol
| Animal | Routea | % of cells producing IFN-γ in response to Env/Gag-Polc at w.p.i.
|
|||||
|---|---|---|---|---|---|---|---|
| 1 | 3 | 5/6b | 8 | 10 | 12 | ||
| 99150 | i.v. | −/0.75 | −/ND | 0.18/0.10 | 0.54/0.61 | 0.71/− | ND |
| 99161 | i.v. | −/− | −/− | ND/− | −/0.36 | −/0.13 | −/ND |
| A99224 | i.v. | −/− | ND | −/− | −/0.93 | 0.33/0.74 | ND/− |
| A99249 | i.v. | −/− | ND | ND/− | ND/− | −/− | 1.93/ND |
| 99158 | i.v. | −/− | ND | 0.50/− | ND/− | −/− | ND |
| 99176 | i.v. | −/− | ND | 1.92/− | −/1.91 | −/ND | 1.05/0.98 |
| 97063 | i.vag. | −/− | −/ND | ND/− | 2.55/− | −/ND | −/− |
| 92218 | i.vag. | ND | −/− | 0.07/0.32 | ND | −/− | −/− |
| 94047 | i.vag. | 0.17/− | −/− | 0.04/0.81 | 0.05/− | −/0.56 | 1.11/0.78 |
| F92261 | i.vag | −/− | −/− | −/− | ND/− | ND | 0.02/ND |
| 97070 | i.vag. | −/− | −/− | 0.19/− | 0.17/− | ND/− | 0.27/ND |
| 94075 | i.vag. | −/− | 0.06/− | ND | −/− | 5.15/− | −/0.39 |
Route of SHIV inoculation.
i.v. inoculated animals were tested at 6 w.p.i., and i.vag. inoculated animals were tested 5 w.p.i.
Background responses to WT VV have been subtracted. ND, not done; −, no response detected.
Cervical lymphocytes also were evaluated for responses in CD8+ CD69+ cells (at 3, 5, 6, 8, 10, and 12 w.p.i.) and CD3+ CD8+ cells (at 1 w.p.i. for the i.vag. group animals only). The results are shown in Table 3. Cytobrushings were performed at every blood draw, but the number of samples available for evaluation was considerably less than could be obtained from PBMC due to the difficulty in obtaining sufficient numbers of cells for the assay and to the substantial activation of these lymphocytes, as evidenced by the production of high levels of IFN-γ in response to no stimulation or to WT VV alone (data not shown). The i.v. infected animals had few responses in their cervical T cells to Env or Gag-Pol (Table 3), although strong anti-Env and anti-Gag-Pol responses were seen in the PBMC of the same animals (Table 2). Positive IFN-γ responses were detected more frequently in cervical cells from the animals that had been inoculated i.vag. Sixty-two percent (16 of 26) of the assays in the i.vag. group were positive, whereas only 30% (7 of 23) of the cervical T-cell responses in the i.v. inoculated group were positive.
TABLE 3.
CD8+ CD69+ cervical lymphocyte IFN-γ responses to Env and Gag-Pola
| Animal | Routeb | % of cells producing IFN-γ in response to Env/Gag-Pold at w.p.i.:
|
|||||
|---|---|---|---|---|---|---|---|
| 1 | 3 | 5/6c | 8 | 10 | 12 | ||
| 99150 | i.v. | ND | ND | ND | −/0.68+ | ND | ND |
| 99161 | i.v. | ND/0.63 | ND | ND/0.43 | ND | ND | ND |
| A99224 | i.v. | ND | −/− | −/− | ND | ND | ND |
| A99249 | i.v. | −/− | −/− | −/ND | ND | /ND | ND |
| 99158 | i.v. | ND | ND/0.29 | 3.44/4.48 | ND | ND | ND |
| 99176 | i.v. | −/ND | −/− | ND/1.27 | −/− | ND | ND |
| 97063 | i.vag. | ND/− | ND | ND | −/− | ND | 0.61/0.34 |
| 92218 | i.vag. | ND | ND | ND | ND | ND | ND |
| 94047 | i.vag. | 0.44/0.50 | ND | ND | ND | ND | ND |
| F92261 | i.vag. | 0.24/0.44 | ND | 0.40/0.74 | 0.09/0.71 | ND | ND |
| 97070 | i.vag. | −/− | ND | 0.28/0.17 | −/− | 0.64/0.37 | ND |
| 94075 | i.vag. | ND/− | ND | −/1.71 | −/0.53 | ND | ND |
Week 1 samples for i.vag. inoculated animals were evaluated for responses in CD3+ CD8+ lymphocytes; most CD8+ IFN-γ+ cells are also CD69+ by this assay (data not shown).
Route of SHIV inoculation.
i.v.inoculated animals were tested at 6 w.p.i., and i.vag. inoculated animals were tested at 5 w.p.i.
Background responses to WT VV have been subtracted. ND, not done; −, no response detected.
DISCUSSION
There are several theories as to how HIV or SIV crosses the stratified epithelial layer of the vaginal tract (2, 12, 23, 28, 31). Recent studies with rhesus macaques have shown that local DCs, macrophages, and lymphocytes are infected with SIV early after atraumatic vaginal inoculation (7, 26). Presumably infected DCs and macrophages migrate to nearby lymph nodes to establish a systemic infection (7). Detectable virus in the draining lymph nodes of these animals can be detected between 1 to 5 days postinoculation (7, 26). These studies did not address whether local virus replication at or near the site of infection occurs or whether such replication can elicit a local immune response.
In this study, we found significant differences in viral dissemination and magnitude of the immune responses between the animals that were inoculated i.vag. and those inoculated i.v. with the same virus, SHIV89.6V. Our findings suggest a compartmentalization of the virus in i.vag. inoculated animals, in which SHIV replicates initially at the mucosal site and/or in the draining lymph nodes before spreading to the periphery. The evidence for these conclusions is found in three observed phenomena.
First, there is a delay in detectable provirus in the periphery upon i.vag. inoculation (Table 1 and Fig. 1), suggesting that virus-infected cells do not spread immediately to the periphery in these animals. This observation is consistent with earlier studies (7, 26). We did not observe a similar delay for plasma viral load measurements, which is indicative of similar levels of early viral replication in animals of both groups. In both groups of animals a correlation between inoculum dose and plasma viral load measurements was observed. Plasma viral load is considered to be a direct reflection of ongoing viral replication (4). Because such replication early in infection is not constrained by an antiviral immune response, the viral load in plasma can be interpreted as a direct reflection of the number of cells that are productively infected. The fact that these viral load measurements are potentially correlated with the size of the inoculum in animals from both groups directly supports that hypothesis. Two animals in both groups received 200 ΤCID50 of virus via i.vag. or i.v. inoculation. Of these four animals, the two that were inoculated i.vag. had considerably lower plasma viral load measurements than the other two animals (4.9 × 105 versus 9.0 × 106 copies/ml, respectively). Together, these data suggest that the physical barrier imposed by the mucosal tissue effectively limits the number of viral particles available for infection, consistent with previous reports (20), thereby limiting the number of cells that become infected immediately after exposure.
Second, a relative delay in the decline of circulating T cells of the animals infected i.vag. also was observed (Fig. 2). The fluctuation in CD4+ T-cell percentages of the i.v. infected animals was marked by an initial rise, followed by a sharp decrease. The initial rise of CD4+ cells of these animals coincides with the peak in viremia at 2 w.p.i. This is most likely due to an increase in activated, proliferating CD4+ cells in the circulating lymphocyte population, following the kinetics of virus replication (11). Consequently, the percentages of CD4+ T cells decreased at 3 w.p.i., likely due to redistribution of these cells from the periphery into the lymph nodes in response to viral antigen production there (11). The animals in the i.vag. group had no significant increases in CD4+ T cells, but all had equally sharp declines in circulating CD4 cell numbers, as was observed in the i.v. group animals. However, this decline occurred 1 week later in this group and was dose dependent. This delay in CD4+ T-cell decline could be related to the delay in the spread of the virus to the peripheral lymph nodes, which was likewise suggested by our observations on proviral distribution. Both of these observations suggest that mucosal infection results in a delay in viral spreading to the periphery, but they do not conclusively demonstrate whether local replication at or near the site of infection occurs.
The third observation, however, concerns the more rapid development of a local humoral immune response in the i.vag. infected animals, strongly supporting the idea that the virus does indeed replicate locally during that time (Fig. 3). We found that, early after infection, vaginal antibodies against the virus arose more rapidly and reached a higher magnitude in the animals infected i.vag. than in those infected i.v. The delay in the detection of vaginal IgG in the i.v. inoculated animals occurred despite relatively rapid systemic antibody production. This observation suggests that the early antiviral antibodies are produced locally and are not the result of transudation, disputing the theory that systemic antibodies transude the vaginal and cervical epithelia, as has been suggested by others (21). It has been demonstrated that antibody-secreting cells exist in the vaginal and cervical tissues (14), and these cells are a likely source for the early antiviral antibodies found in the vaginal secretions of these animals. In addition, it appears that Env-specific CD8+ T-cell responses in the cervix may have been enhanced in the i.vag. infected animals relative to those in the cervices of the i.v. infected animals (Table 3), although studies with more animals will be needed to confirm this observation. Such local production of cellular immunity against SHIV is consistent with studies of women exposed to HIV who remain seronegative (9).
It is interesting to speculate about the role of the mucosal immune system in i.vag. infection and subsequent dissemination of the virus to the periphery, although this was not addressed here. The combination of the architecture of the mucosa with the multiple steps needed for migration of SHIV+ or HIV+ cells to the lymph nodes, where significant viral replication can occur, may allow the local immune system to eliminate infected cells more efficiently than when the infection occurs at the periphery. Together these observations may explain the contrasts in viremia observed in the i.v. and i.vag. infected animals and reemphasize the value of testing vaccines designed to induce mucosal as well as systemic immune responses.
ACKNOWLEDGMENTS
We thank Yichen Lu for the SHIV89.6V virus, Dennis Panicali for the recombinant VVs, and Edward Clark for the G10-1 antibody. We also extend our appreciation to Jiangli Chen, Kurt Lustig, Heather Mack, Jeremy Capulangen, Jenny Booth, and Casey Wingfield for their technical assistance; Nancy Haigwood, M. Juliana McElrath, and Wesley Van Voorhis for helpful discussions; and Barbra Richardson for statistical analysis advice.
The work was supported by AIDS Vaccine Development grant AI26503.
REFERENCES
- 1.Artenstein A W, VanCott T C, Sitz K V, Robb M L, Wagner K F, Veit S C, Rogers A F, Garner R P, Byron J W, Burnett P R, Birx D L. Mucosal immune responses in four distinct compartments of women infected with human immunodeficiency virus type 1: a comparison by site and correlation with clinical information. J Infect Dis. 1997;175:265–271. doi: 10.1093/infdis/175.2.265. [DOI] [PubMed] [Google Scholar]
- 2.Bomsel M. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat Med. 1997;3:42–47. doi: 10.1038/nm0197-42. [DOI] [PubMed] [Google Scholar]
- 3.Brandtzaeg P. Mucosal immunity in the female genital tract. J Reprod Immunol. 1997;36:23–50. doi: 10.1016/s0165-0378(97)00061-2. [DOI] [PubMed] [Google Scholar]
- 4.Cohen O J, Pantaleo G, Schwartzentruber D J, Graziosi C, Vaccarezza M, Fauci A S. Pathogenic insights from studies of lymphoid tissue from HIV-infected individuals. J Acquir Immune Defic Syndr. 1995;10:S6–S14. [PubMed] [Google Scholar]
- 5.Dailey P J, Zamroud M, Kelso R, Kolberg J, Urdea M. Quantitation of simian immunodeficiency virus (SIV) RNA in plasma of acute and chronically infected macaques using a branched DNA (bDNA) signal amplification assay. J Med Primatol. 1995;24:209. [Google Scholar]
- 6.Haigwood N L, Zolla-Pazner S. Humoral immunity to HIV, SIV, and SHIV. AIDS. 1998;12:S121–S132. [PubMed] [Google Scholar]
- 7.Hu J, Gardner M B, Miller C J. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J Virol. 2000;74:6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Israel Z R, Marx P A. Nonclassical mucosal antibodies predominate in genital secretions of HIV-1 infected chimpanzees. J Med Primatol. 1995;24:53–60. doi: 10.1111/j.1600-0684.1995.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 9.Kaul R, Plummer F A, Kimani J, Dong T, Kiama P, Rostron T, Njagi E, MacDonald K S, Bwayo J J, McMichael A J, Rowland-Jones S L. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J Immunol. 2000;164:1602–1611. doi: 10.4049/jimmunol.164.3.1602. [DOI] [PubMed] [Google Scholar]
- 10.Kaul R, Trabattoni D, Bwayo J J, Arienti D, Zagliani A, Mwangi F M, Kariuki C, Ngugi E N, MacDonald K S, Ball T B. HIV-1-specific mucosal IgA in a cohort of HIV-1-resistant Kenyan sex workers. AIDS. 1999;13:23–29. doi: 10.1097/00002030-199901140-00004. [DOI] [PubMed] [Google Scholar]
- 11.Kaur A, Hale C L, Ramanujan S, Jain R K, Johnson R P. Differential dynamics of CD4+ and CD8+ T-lymphocyte proliferation and activation in acute simian immunodeficiency virus infection. J Virol. 2000;74:8413–8424. doi: 10.1128/jvi.74.18.8413-8424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreiss J, Carael M, Meheus A. Role of sexually transmitted diseases in transmitting human immunodeficiency virus. Genitourin Med. 1988;64:1–2. doi: 10.1136/sti.64.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuller L, Thompson J, Watanabe R, Iskandriati D, Alpers C E, Morton W R, Agy M B. Mucosal antibody expression following rapid SIV(Mne) dissemination in intrarectally infected Macaca nemestrina. AIDS Res Hum Retroviruses. 1998;14:1345–1356. doi: 10.1089/aid.1998.14.1345. [DOI] [PubMed] [Google Scholar]
- 14.Kutteh W H. Mucosal immunity in the human female reproductive tract. In: Ogra P L, Mestecky J, Lamm M E, Strober W, Bienenstock J, McGhee J R, editors. Mucosal immunology. 2nd ed. San Diego, Calif: Academic Press; 1999. pp. 1423–1434. [Google Scholar]
- 15.Lu X S, Belec L, Pillot J. Anti-gp160 IgG and IgA antibodies associated with a large increase in total IgG in cervicovaginal secretions from human immunodeficiency virus type 1-infected women. J Infect Dis. 1993;167:1189–1192. doi: 10.1093/infdis/167.5.1189. [DOI] [PubMed] [Google Scholar]
- 16.Martin H L, Jr, Nyange P M, Richardson B A, Lavreys L, Mandaliya K, Jackson D J, Ndinya-Achola J O, Kreiss J. Hormonal contraception, sexually transmitted diseases, and risk of heterosexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1998;178:1053–1059. doi: 10.1086/515654. [DOI] [PubMed] [Google Scholar]
- 17.Marx P A, Spira A I, Gettie A, Dailey P J, Veazey R S, Lackner A A, Mahoney C J, Miller C J, Claypool L E, Ho D D, Alexander N J. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat Med. 1996;2:1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 18.Mazzoli S, Trabattoni D, Caputo S L, Piconi S, Ble C, Meacci F, Ruzzante S, Salvi A, Semplici F, Longhi R, Fusi M L, Tofani N, Biasin M, Villa M L, Mazzotta F, Clerici M. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat Med. 1997;3:1250–1257. doi: 10.1038/nm1197-1250. [DOI] [PubMed] [Google Scholar]
- 19.Miller C J. Mucosal transmission of SIV. Curr Top Microbiol Immunol. 1994;188:107–122. doi: 10.1007/978-3-642-78536-8_6. [DOI] [PubMed] [Google Scholar]
- 20.Miller C J, Alexander N J, Sutjipto S, Lackner A A, Gettie A, Hendrickx A G, Lowenstine L J, Jennings M, Marx P A. Genital mucosal transmission of simian immunodeficiency virus: animal model for heterosexual transmission of human immunodeficiency virus. J Virol. 1989;63:4277–4284. doi: 10.1128/jvi.63.10.4277-4284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller C J, Kang D W, Marthas M, Moldoveanu Z, Kiyono H, Marx P, Eldridge J H, Mestecky J, McGhee J R. Genital secretory immune response to chronic simian immunodeficiency virus (SIV) infection: a comparison between intravenously and genitally inoculated rhesus macaques. Clin Exp Immunol. 1992;88:520–526. doi: 10.1111/j.1365-2249.1992.tb06481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishell D R, Stenchever M A, Droegemuller W, Herbst A L. Comprehensive gynecology. 3rd ed. Chicago, Ill: Mosby-Year Book; 1997. [Google Scholar]
- 23.Nuovo G J, Forde A, MacConnell P, Fahrenwald R. In situ detection of PCR-amplified HIV-1 nucleic acids and tumor necrosis factor cDNA in cervical tissues. Am J Pathol. 1993;143:40–48. [PMC free article] [PubMed] [Google Scholar]
- 24.Reimann K A, Li J T, Voss G, Lekutis C, Tenner-Racz K, Racz P, Lin W, Montefiori D C, Lee-Parritz D E, Lu Y, Collman R G, Sodroski J, Letvin N L. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J Virol. 1996;70:3198–3206. doi: 10.1128/jvi.70.5.3198-3206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sodora D L, Gettie A, Miller C J, Marx P A. Vaginal transmission of SIV: assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res Hum Retroviruses. 1998;14:S119–S123. [PubMed] [Google Scholar]
- 26.Spira A I, Marx P A, Patterson B K, Mahoney J, Koup R A, Wolinsky S M, Ho D D. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J Exp Med. 1996;183:215–225. doi: 10.1084/jem.183.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takehisa J, Zekeng L, Ido E, Mboudjeka I, Moriyama H, Miura T, Yamashita M, Gurtler L G, Hayami M, Kaptue L. Various types of HIV mixed infections in Cameroon. Virology. 1998;245:1–10. doi: 10.1006/viro.1998.9141. [DOI] [PubMed] [Google Scholar]
- 28.Tan X, Pearce-Pratt R, Phillips D M. Productive infection of a cervical epithelial cell line with human immunodeficiency virus: implications for sexual transmission. J Virol. 1993;67:6447–6452. doi: 10.1128/jvi.67.11.6447-6452.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.United Nations Programme on HIV/AIDS/World Health Organization. AIDS epidemic update: December 2000. United Nations Programme on HIV/AIDS/World Health Organization. 2000. [Google Scholar]
- 30.Watson A, Ranchalis J, Travis B, McClure J, Sutton W, Johnson P R, Hu S-L, Haigwood N L. Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load early in infection predicts survival. J Virol. 1997;71:284–290. doi: 10.1128/jvi.71.1.284-290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zacharopoulos V R, Perotti M E, Phillips D M. A role for cell migration in the sexual transmission of HIV-1? Curr Biol. 1997;7:534–537. doi: 10.1016/s0960-9822(06)00225-9. [DOI] [PubMed] [Google Scholar]