Abstract
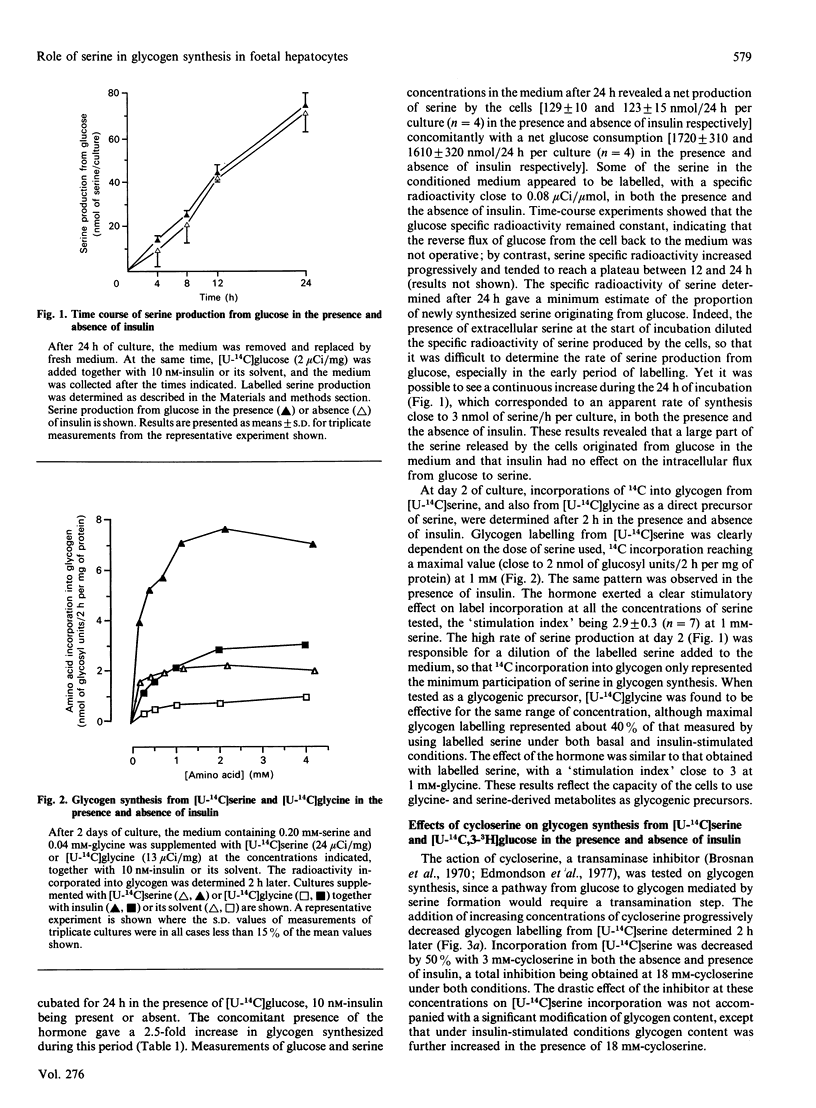
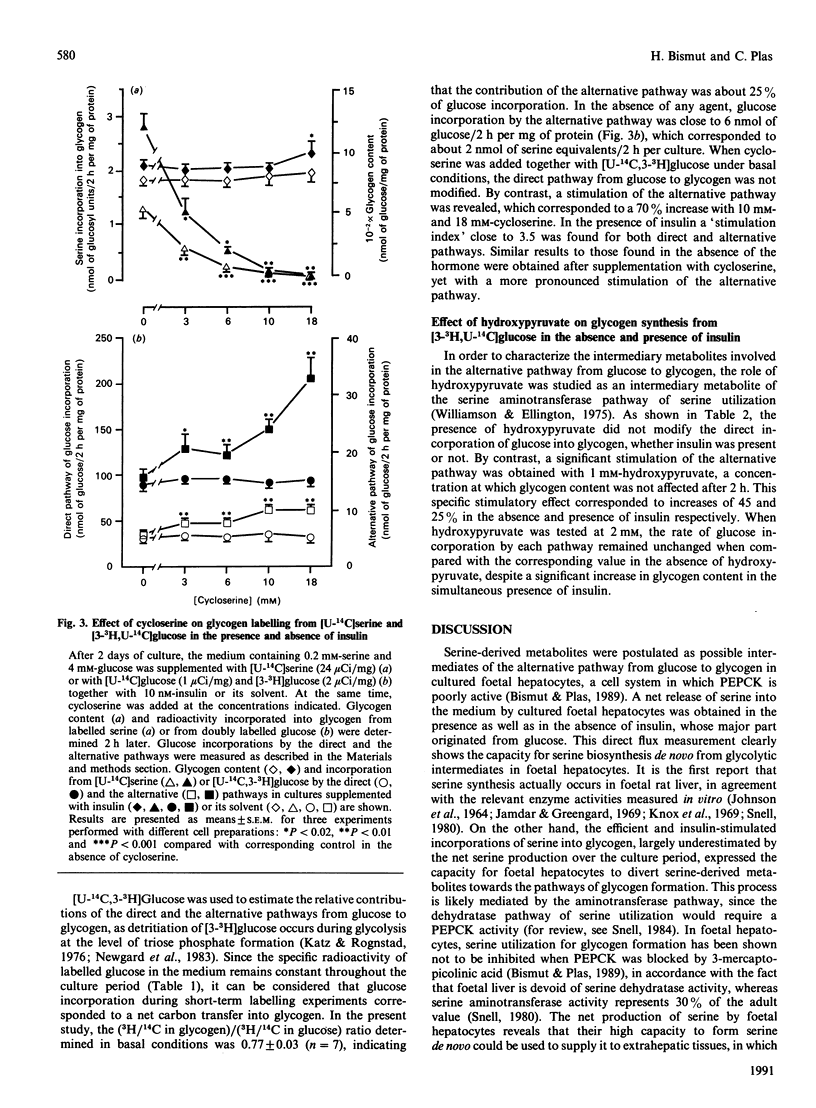
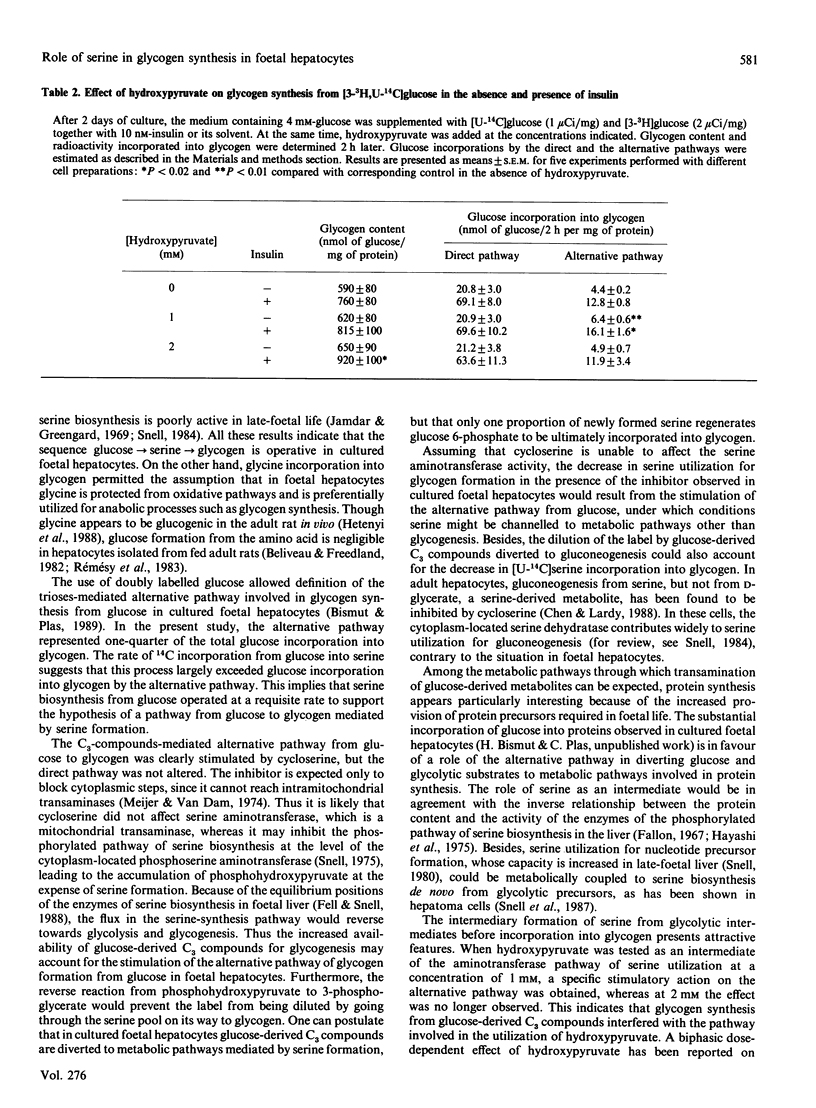
The role of serine as a possible intermediate of the alternative pathway from glucose to glycogen was investigated under basal and insulin-stimulated conditions in 18-day cultured foetal-rat hepatocytes because these cells cannot use pyruvate-derived metabolites [Bismut & Plas (1989) Biochem. J. 263, 889-895]. Incubation of cells with [U-14C]glucose for 24 h led to a release of labelled serine in the medium concomitantly with a net serine production (100 nmol/24 h per culture). The rate of [14C]serine formation (close to 3 nmol/h per culture) indicated that a large part of newly formed serine originated from glucose. When short-term experiments were performed at day 2, glycogen labelling from [U-14C]serine or [U-14C]glycine, which was increased 3-fold by insulin after 2 h, evidenced their participation as glycogenic precursors. When a double-isotope procedure with [U-14C,3-3H]glucose was used, the direct and the alternative pathways from glucose were found to contribute to glycogenesis by 75 and 25% respectively. Cycloserine (18 mM), a transaminase inhibitor, strongly inhibited glycogen labelling from [U-14C] serine while producing a 70% increase in glucose incorporation by the alternative pathway, in both the presence and the absence of insulin. The inhibitor had no effect on the direct pathway from glucose to glycogen. Supplementation with 1 mM-hydroxypyruvate, a serine-derived metabolite, did not affect direct glucose incorporation, whereas the alternative pathway was stimulated whether insulin was present or not. These results indicate that the sequence glucose----serine----glycogen is operative in cultured foetal hepatocytes. The alternative pathway interferes with hydroxypyruvate utilization, and is likely mediated by the serine aminotransferase pathway, independently of the acute glycogenic action of insulin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beliveau G. P., Freedland R. A. Metabolism of serine, glycine and threonine in isolated cat hepatocytes Felis domestica. Comp Biochem Physiol B. 1982;71(1):13–18. doi: 10.1016/0305-0491(82)90168-7. [DOI] [PubMed] [Google Scholar]
- Bismut H., Plas C. Pathways of glycogen synthesis from glucose during the glycogenic response to insulin in cultured foetal hepatocytes. Biochem J. 1989 Nov 1;263(3):889–895. doi: 10.1042/bj2630889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan J. T., Krebs H. A., Williamson D. H. Effects of ischaemia on metabolite concentrations in rat liver. Biochem J. 1970 Mar;117(1):91–96. doi: 10.1042/bj1170091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen K. S., Lardy H. A. Pathway of gluconeogenesis from D- and L-glycerate in rat hepatocytes. Arch Biochem Biophys. 1988 Sep;265(2):433–440. doi: 10.1016/0003-9861(88)90146-4. [DOI] [PubMed] [Google Scholar]
- EVANS V. J., BRYANT J. C., KERR H. A., SCHILLING E. L. CHEMICALLY DEFINED MEDIA FOR CULTIVATION OF LONG-TERM CELL STRAINS FROM FOUR MAMMALIAN SPECIES. Exp Cell Res. 1964 Dec;36:439–474. doi: 10.1016/0014-4827(64)90302-7. [DOI] [PubMed] [Google Scholar]
- Edmondson J. W., Lumeng L., Li T. K. Direct measurement of active transport systems for alanine in freshly isolated rat liver cells. Biochem Biophys Res Commun. 1977 Jun 6;76(3):751–757. doi: 10.1016/0006-291x(77)91564-9. [DOI] [PubMed] [Google Scholar]
- Fallon H. J. Regulatory phenomena in mammalian serine metabolism. Adv Enzyme Regul. 1967;5:107–120. doi: 10.1016/0065-2571(67)90012-x. [DOI] [PubMed] [Google Scholar]
- Fell D. A., Snell K. Control analysis of mammalian serine biosynthesis. Feedback inhibition on the final step. Biochem J. 1988 Nov 15;256(1):97–101. doi: 10.1042/bj2560097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard J. Gluconeogenesis in late fetal and early neonatal life. Biol Neonate. 1986;50(5):237–258. doi: 10.1159/000242605. [DOI] [PubMed] [Google Scholar]
- Hanson R. W., Ballard J. Hormonal regulation of hepatic P-enolpyruvate carboxykinase (GTP) during development. Fed Proc. 1975 Feb;34(2):166–171. [PubMed] [Google Scholar]
- Hayashi S., Tanaka T., Naito J., Suda M. Dietary and hormonal regulation of serine synthesis in the rat. J Biochem. 1975 Jan 1;77(1?):207–219. [PubMed] [Google Scholar]
- Hetenyi G., Jr, Anderson P. J., Raman M., Ferrarotto C. Gluconeogenesis from glycine and serine in fasted normal and diabetic rats. Biochem J. 1988 Jul 1;253(1):27–32. doi: 10.1042/bj2530027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON B. E., WALSH D. A., SALLACH H. J. CHANGES IN THE ACTIVITIES OF D-GLYCERATE AND D-3-PHOSPHOGLYCERATE DEHYDROGENASES IN THE DEVELOPING RAT LIVER. Biochim Biophys Acta. 1964 May 4;85:202–205. doi: 10.1016/0926-6569(64)90241-x. [DOI] [PubMed] [Google Scholar]
- Jamdar S. C., Greengard O. Phosphoserine phosphatase: development formation and hormonal regulation in rat tissues. Arch Biochem Biophys. 1969 Oct;134(1):228–232. doi: 10.1016/0003-9861(69)90270-7. [DOI] [PubMed] [Google Scholar]
- Katz J., McGarry J. D. The glucose paradox. Is glucose a substrate for liver metabolism? J Clin Invest. 1984 Dec;74(6):1901–1909. doi: 10.1172/JCI111610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Rognstad R. Futile cycles in the metabolism of glucose. Curr Top Cell Regul. 1976;10:237–289. doi: 10.1016/b978-0-12-152810-2.50013-9. [DOI] [PubMed] [Google Scholar]
- Knox W. E., Herzfeld A., Hudson J. Phosphoserine phosphatase distribution in normal and neoplastic rat tissues. Arch Biochem Biophys. 1969 Jul;132(2):397–403. doi: 10.1016/0003-9861(69)90381-6. [DOI] [PubMed] [Google Scholar]
- Lemonnier F., Gautier M., Moatti N., Lemonnier A. Comparative study of extracellular amino acids in culture of human liver and fibroblastic cells. In Vitro. 1976 Jun;12(6):460–466. doi: 10.1007/BF02806026. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Kuwajima M., Newgard C. B., Foster D. W., Katz J. From dietary glucose to liver glycogen: the full circle round. Annu Rev Nutr. 1987;7:51–73. doi: 10.1146/annurev.nu.07.070187.000411. [DOI] [PubMed] [Google Scholar]
- Meijer A. J., Van Dam K. The metabolic significance of anion transport in mitochondria. Biochim Biophys Acta. 1974 Dec 30;346(3-4):213–244. doi: 10.1016/0304-4173(74)90001-9. [DOI] [PubMed] [Google Scholar]
- Menuelle P., M'zali H., Forest N., Plas C. Compared roles of glucose, galactose and fructose as glycogen precursors during the acute response to insulin in cultured rat foetal hepatocytes. Int J Biochem. 1988;20(8):777–782. doi: 10.1016/0020-711x(88)90063-8. [DOI] [PubMed] [Google Scholar]
- Newgard C. B., Hirsch L. J., Foster D. W., McGarry J. D. Studies on the mechanism by which exogenous glucose is converted into liver glycogen in the rat. A direct or an indirect pathway? J Biol Chem. 1983 Jul 10;258(13):8046–8052. [PubMed] [Google Scholar]
- Parniak M., Kalant N. Incorporation of glucose into glycogen in primary cultures of rat hepatocytes. Can J Biochem Cell Biol. 1985 May;63(5):333–340. doi: 10.1139/o85-049. [DOI] [PubMed] [Google Scholar]
- Plas C., Chapeville F., Jacquot R. Development of glycogen storage ability under cortisol control in primary cultures of rat fetal hepatocytes. Dev Biol. 1973 May;32(1):82–91. doi: 10.1016/0012-1606(73)90221-2. [DOI] [PubMed] [Google Scholar]
- Plas C., Menuelle P., Moncany M. L., Fulchignoni-Lataud M. C. Time dependence of the glycogenic effect of insulin in cultured fetal hepatocytes. Diabetes. 1979 Aug;28(8):705–712. doi: 10.2337/diab.28.8.705. [DOI] [PubMed] [Google Scholar]
- Plas C., Nunez J. Role of cortisol on the glycogenolytic effect of glucagon and on the glycogenic response to insulin in fetal hepatocyte culture. J Biol Chem. 1976 Mar 10;251(5):1431–1437. [PubMed] [Google Scholar]
- Remesy C., Fafournoux P., Demigne C. Control of hepatic utilization of serine, glycine and threonine in fed and starved rats. J Nutr. 1983 Jan;113(1):28–39. doi: 10.1093/jn/113.1.28. [DOI] [PubMed] [Google Scholar]
- Salhanick A. I., Chang C. L., Amatruda J. M. Hormone and substrate regulation of glycogen accumulation in primary cultures of rat hepatocytes. Biochem J. 1989 Aug 1;261(3):985–992. doi: 10.1042/bj2610985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval I. V., Sols A. Gluconeogenesis from serine by the serine-dehydratase-dependent pathway in rat liver. Eur J Biochem. 1974 Apr 16;43(3):609–616. doi: 10.1111/j.1432-1033.1974.tb03448.x. [DOI] [PubMed] [Google Scholar]
- Snell K. Enzymes of serine metabolism in normal, developing and neoplastic rat tissues. Adv Enzyme Regul. 1984;22:325–400. doi: 10.1016/0065-2571(84)90021-9. [DOI] [PubMed] [Google Scholar]
- Snell K. Liver enzymes of serine metabolism during neonatal development of the rat. Biochem J. 1980 Aug 15;190(2):451–455. doi: 10.1042/bj1900451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell K. Mitochondrial-cytosolic interrelationships involved in gluconeogenesis from serine in rat liver. FEBS Lett. 1975 Jul 15;55(1):202–205. doi: 10.1016/0014-5793(75)80992-6. [DOI] [PubMed] [Google Scholar]
- Snell K., Natsumeda Y., Weber G. The modulation of serine metabolism in hepatoma 3924A during different phases of cellular proliferation in culture. Biochem J. 1987 Jul 15;245(2):609–612. doi: 10.1042/bj2450609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence J. T., Koudelka A. P. Pathway of glycogen synthesis from glucose in hepatocytes maintained in primary culture. J Biol Chem. 1985 Feb 10;260(3):1521–1526. [PubMed] [Google Scholar]
- Williamson D. H., Ellington E. V. Hydroxypyruvate as a gluconeogenic substrate in rat hepatocytes. Biochem J. 1975 Jan;146(1):277–279. doi: 10.1042/bj1460277. [DOI] [PMC free article] [PubMed] [Google Scholar]


