Abstract
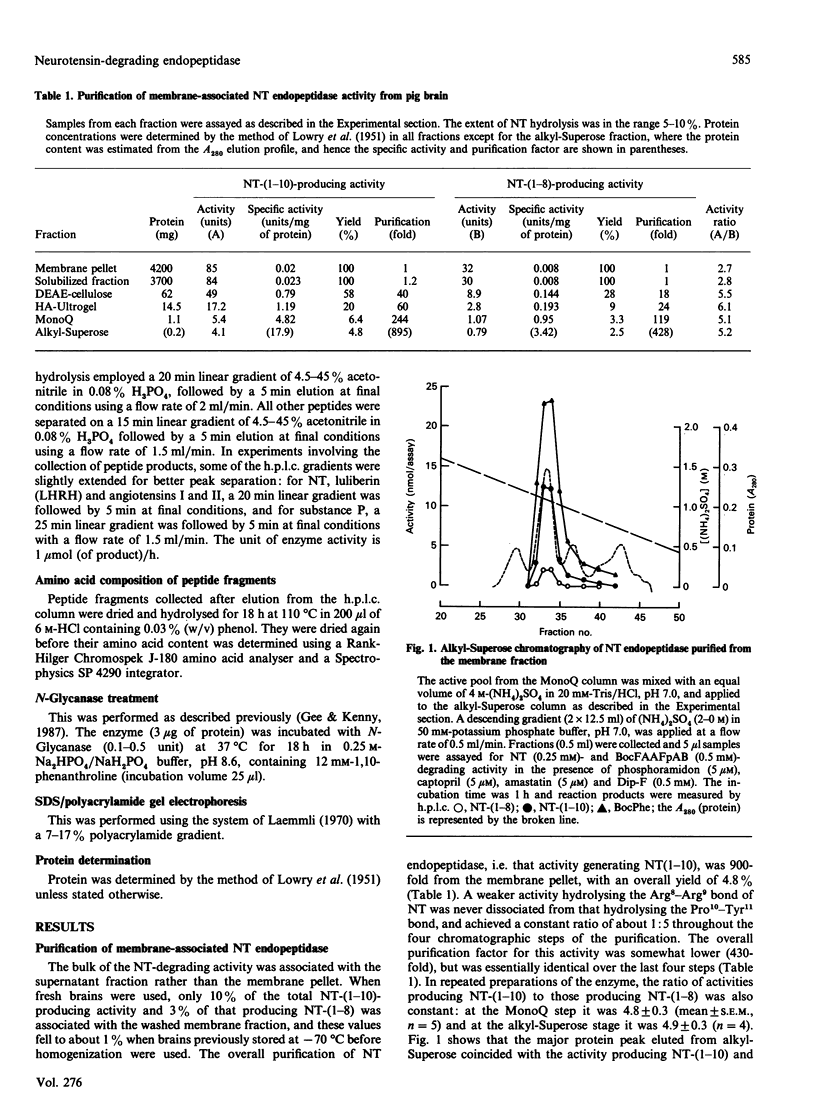
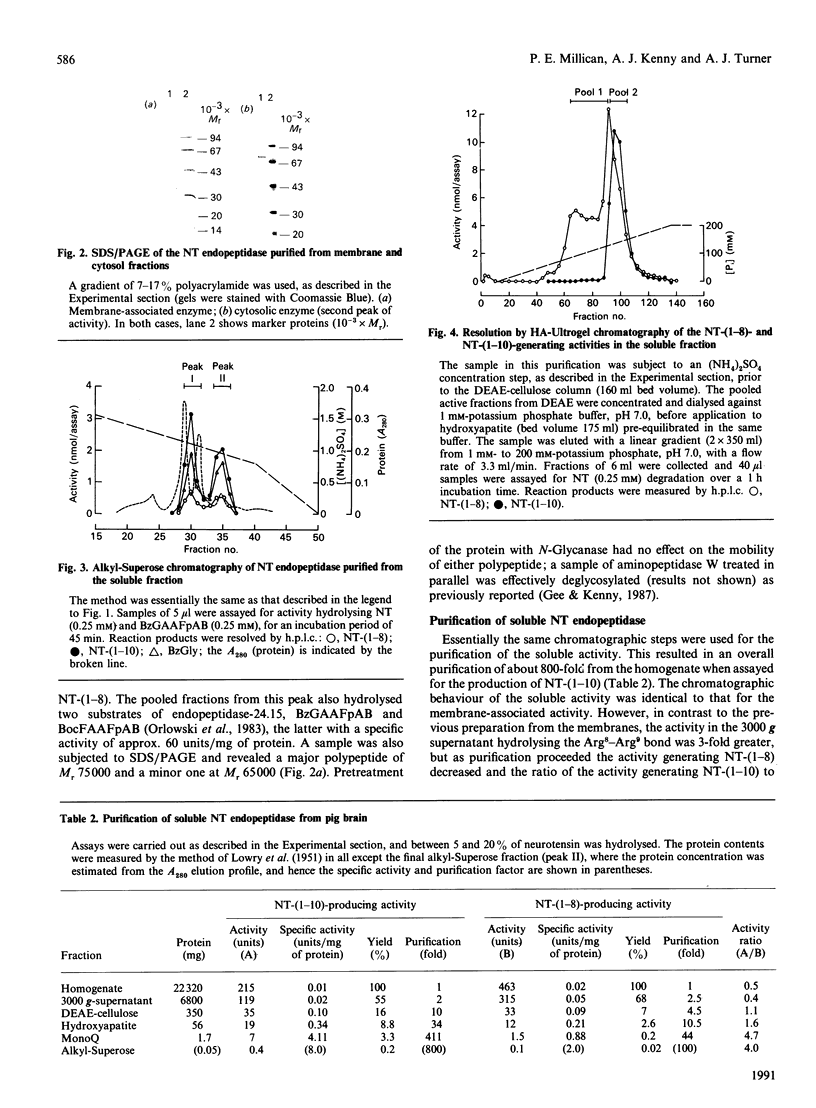
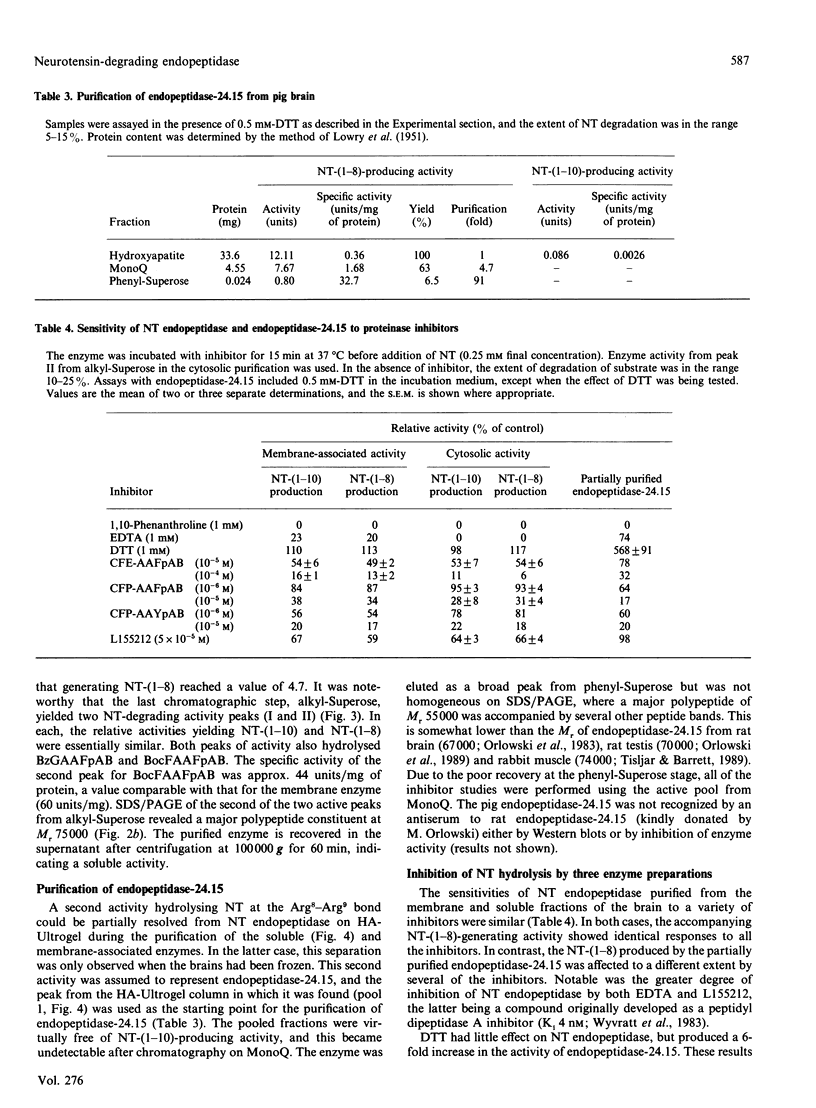
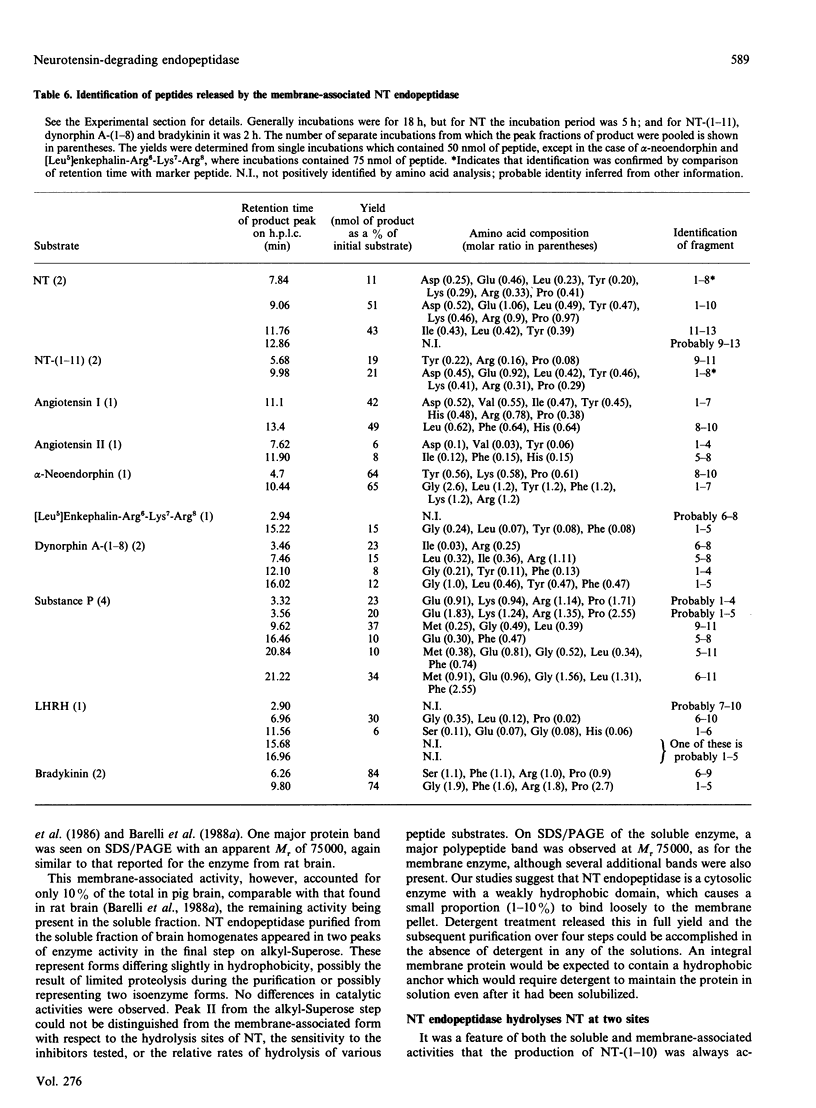
Neurotensin (NT) endopeptidase (EC 3.4.24.16) has been purified about 800-fold from pig brain by four sequential chromatographic steps depending on ion-exchange and hydrophobic interactions. Two types of preparation were studied: one from a Triton X-100-solubilized membrane fraction, and the other from the soluble fraction containing 90% or more of the total activity in the homogenate. NT endopeptidase activity was monitored by high-precision liquid chromatography of the two peptide products, characterized as NT-(1-10) and NT-(1-8), resulting from cleavage of the Pro10-Tyr11 and Arg8-Arg9 bonds respectively. As purification proceeded, from both membranes and cytosol, the yield of the two products achieved a constant ratio of 5:1 and this ratio was reproduced in repeated purifications. However, a distinct peptidase which hydrolysed exclusively at the Arg8-Arg9 bond was partially resolved from NT endopeptidase by chromatography on hydroxyapatite, and this activity was further purified and assigned to endopeptidase-24.15 (EC 3.4.24.15). SDS/PAGE of both preparations of neurotensin endopeptidase revealed a major band of apparent Mr 75000, and treatment of the membrane-associated form with N-Glycanase gave no evidence that the enzyme was a glycoprotein. The membrane-associated and cytosol forms of NT endopeptidase activities, monitored for both NT-(1-10) and NT-(1-8) products, were compared in their responses to 1,10-phenanthroline, EDTA, dithiothreitol (DTT) and some synthetic site-directed inhibitors of endopeptidase-24.15 or peptidyl dipeptidase A. The effects revealed no significant differences between the two preparations, nor did the reagents discriminate between the activities generating the two NT fragments. The partially purified form of endopeptidase-24.15 was also included in this comparison: while some responses were similar, this peptidase was distinguishable in its activation by DTT and its relative resistance to inhibition by EDTA. Both forms of NT endopeptidase were found to hydrolyse other substrates, including Boc-Phe-Ala-Ala-Phe-4-aminobenzoate, bradykinin and substance P (these at faster rates than neurotensin), as well as dynorphin A-(1-8) and luliberin. The bonds hydrolysed in these neuropeptides, as well as in angiotensins I and II and alpha-neoendorphin, were defined. These studies confirm that NT endopeptidase is distinct from endopeptidase-24.15. They further show that the former is a soluble enzyme, not an integral membrane protein, that it is not peptide-specific and that it might be more appropriately named. enzyme, not an integral membrane protein, that it is not peptide-specific and
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acker G. R., Molineaux C., Orlowski M. Synaptosomal membrane-bound form of endopeptidase-24.15 generates Leu-enkephalin from dynorphin1-8, alpha- and beta-neoendorphin, and Met-enkephalin from Met-enkephalin-Arg6-Gly7-Leu8. J Neurochem. 1987 Jan;48(1):284–292. doi: 10.1111/j.1471-4159.1987.tb13160.x. [DOI] [PubMed] [Google Scholar]
- Barelli H., Vincent J. P., Checler F. Peripheral inactivation of neurotensin. Isolation and characterization of a metallopeptidase from rat ileum. Eur J Biochem. 1988 Aug 15;175(3):481–489. doi: 10.1111/j.1432-1033.1988.tb14220.x. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Tisljar U. The activities of 'Pz-peptidase' and 'endopeptidase 24.15' are due to a single enzyme. Biochem J. 1989 Aug 1;261(3):1047–1050. doi: 10.1042/bj2611047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo A. C., Oliveira E. B., Toffoletto O., Metters K. M., Rossier J. Brain endo-oligopeptidase A, a putative enkephalin converting enzyme. J Neurochem. 1987 Apr;48(4):1258–1263. doi: 10.1111/j.1471-4159.1987.tb05655.x. [DOI] [PubMed] [Google Scholar]
- Checler F., Emson P. C., Vincent J. P., Kitabgi P. Inactivation of neurotensin by rat brain synaptic membranes. Cleavage at the Pro10-Tyr11 bond by endopeptidase 24.11 (enkephalinase) and a peptidase different from proline-endopeptidase. J Neurochem. 1984 Nov;43(5):1295–1301. doi: 10.1111/j.1471-4159.1984.tb05386.x. [DOI] [PubMed] [Google Scholar]
- Checler F., Vincent J. P., Kitabgi P. Degradation of neurotensin by rat brain synaptic membranes: involvement of a thermolysin-like metalloendopeptidase (enkephalinase), angiotensin-converting enzyme, and other unidentified peptidases. J Neurochem. 1983 Aug;41(2):375–384. doi: 10.1111/j.1471-4159.1983.tb04753.x. [DOI] [PubMed] [Google Scholar]
- Checler F., Vincent J. P., Kitabgi P. Inactivation of neurotensin by rat brain synaptic membranes partly occurs through cleavage at the Arg8-Arg9 peptide bond by a metalloendopeptidase. J Neurochem. 1985 Nov;45(5):1509–1513. doi: 10.1111/j.1471-4159.1985.tb07220.x. [DOI] [PubMed] [Google Scholar]
- Checler F., Vincent J. P., Kitabgi P. Purification and characterization of a novel neurotensin-degrading peptidase from rat brain synaptic membranes. J Biol Chem. 1986 Aug 25;261(24):11274–11281. [PubMed] [Google Scholar]
- Chu T. G., Orlowski M. Active site directed N-carboxymethyl peptide inhibitors of a soluble metalloendopeptidase from rat brain. Biochemistry. 1984 Jul 31;23(16):3598–3603. doi: 10.1021/bi00311a005. [DOI] [PubMed] [Google Scholar]
- Chu T. G., Orlowski M. Soluble metalloendopeptidase from rat brain: action on enkephalin-containing peptides and other bioactive peptides. Endocrinology. 1985 Apr;116(4):1418–1425. doi: 10.1210/endo-116-4-1418. [DOI] [PubMed] [Google Scholar]
- Coquerel A., Dubuc I., Menard J. F., Kitabgi P., Costentin J. Naloxone-insensitive potentiation of neurotensin hypothermic effect by the enkephalinase inhibitor thiorphan. Brain Res. 1986 Nov 29;398(2):386–389. doi: 10.1016/0006-8993(86)91501-5. [DOI] [PubMed] [Google Scholar]
- Emson P. C., Goedert M., Horsfield P., Rioux F., St Pierre S. The regional distribution and chromatographic characterisation of neurotensin-like immunoreactivity in the rat central nervous system. J Neurochem. 1982 Apr;38(4):992–999. doi: 10.1111/j.1471-4159.1982.tb05340.x. [DOI] [PubMed] [Google Scholar]
- Gee N. S., Kenny A. J. Proteins of the kidney microvillar membrane. Enzymic and molecular properties of aminopeptidase W. Biochem J. 1987 Aug 15;246(1):97–102. doi: 10.1042/bj2460097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen L. L., Iversen S. D., Bloom F., Douglas C., Brown M., Vale W. Calcium-dependent release of somatostatin and neurotensin from rat brain in vitro. Nature. 1978 May 11;273(5658):161–163. doi: 10.1038/273161a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McDermott J. R., Smith A. I., Edwardson J. A., Griffiths E. C. Mechanism of neurotensin degradation by rat brain peptidases. Regul Pept. 1982 May;3(5-6):397–404. doi: 10.1016/0167-0115(82)90062-3. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Michaud C., Chu T. G. A soluble metalloendopeptidase from rat brain. Purification of the enzyme and determination of specificity with synthetic and natural peptides. Eur J Biochem. 1983 Sep 1;135(1):81–88. doi: 10.1111/j.1432-1033.1983.tb07620.x. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Michaud C., Molineaux C. J. Substrate-related potent inhibitors of brain metalloendopeptidase. Biochemistry. 1988 Jan 26;27(2):597–602. doi: 10.1021/bi00402a015. [DOI] [PubMed] [Google Scholar]
- Orlowski M., Reznik S., Ayala J., Pierotti A. R. Endopeptidase 24.15 from rat testes. Isolation of the enzyme and its specificity toward synthetic and natural peptides, including enkephalin-containing peptides. Biochem J. 1989 Aug 1;261(3):951–958. doi: 10.1042/bj2610951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozsgay M., Michaud C., Liebman M., Orlowski M. Substrate and inhibitor studies of thermolysin-like neutral metalloendopeptidase from kidney membrane fractions. Comparison with bacterial thermolysin. Biochemistry. 1986 Mar 25;25(6):1292–1299. doi: 10.1021/bi00354a015. [DOI] [PubMed] [Google Scholar]
- Tisljar U., Barrett A. J. Purification and characterization of Pz-peptidase from rabbit muscle. Arch Biochem Biophys. 1989 Oct;274(1):138–144. doi: 10.1016/0003-9861(89)90424-4. [DOI] [PubMed] [Google Scholar]
- Tisljar U., Barrett A. J. Thiol-dependent metallo-endopeptidase characteristics of Pz-peptidase in rat and rabbit. Biochem J. 1990 Apr 15;267(2):531–533. doi: 10.1042/bj2670531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisljar U., de Camargo A. C., da Costa C. A., Barrett A. J. Activity of Pz-peptidase and endo-oligopeptidase are due to the same enzyme. Biochem Biophys Res Commun. 1989 Aug 15;162(3):1460–1464. doi: 10.1016/0006-291x(89)90838-3. [DOI] [PubMed] [Google Scholar]
- Uhl G. R., Snyder S. H. Regional and subcellular distributions of brain neurotensin. Life Sci. 1976 Dec 15;19(12):1827–1832. doi: 10.1016/0024-3205(76)90114-4. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Kuhar M. J. Neurotensin receptor localization by light microscopic autoradiography in rat brain. Brain Res. 1981 Feb 16;206(2):273–285. doi: 10.1016/0006-8993(81)90532-1. [DOI] [PubMed] [Google Scholar]