Abstract
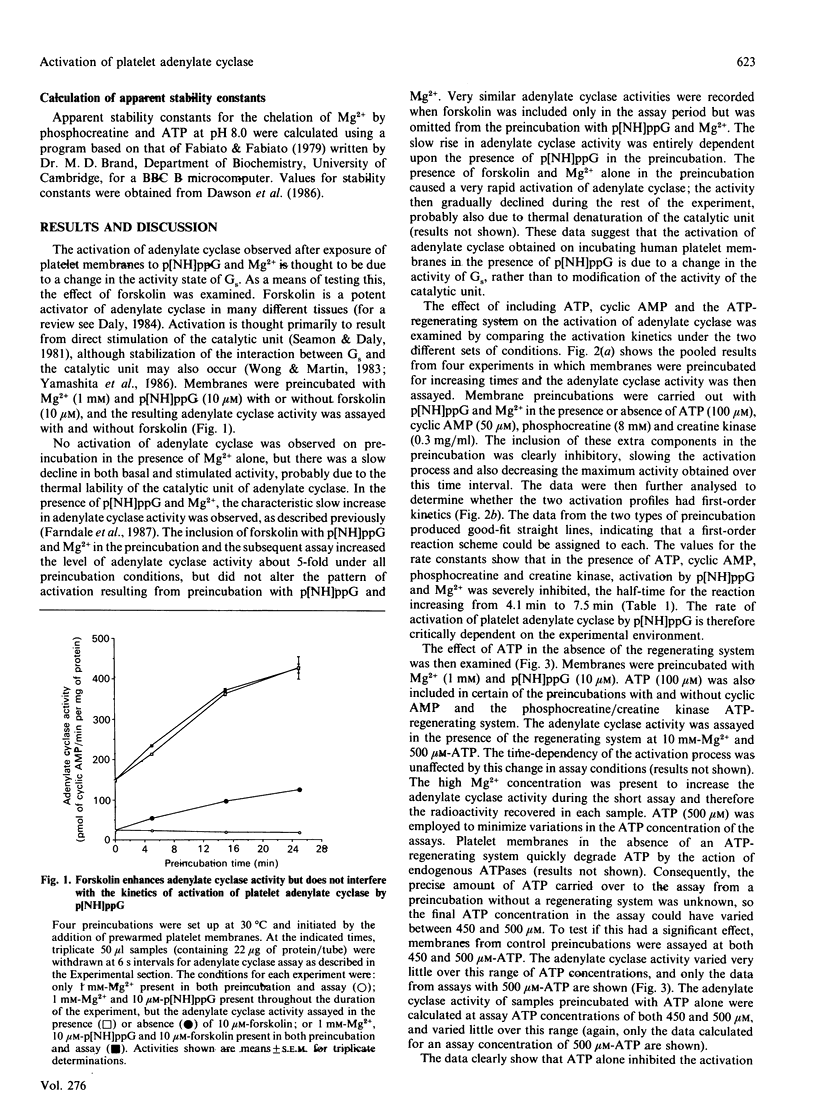
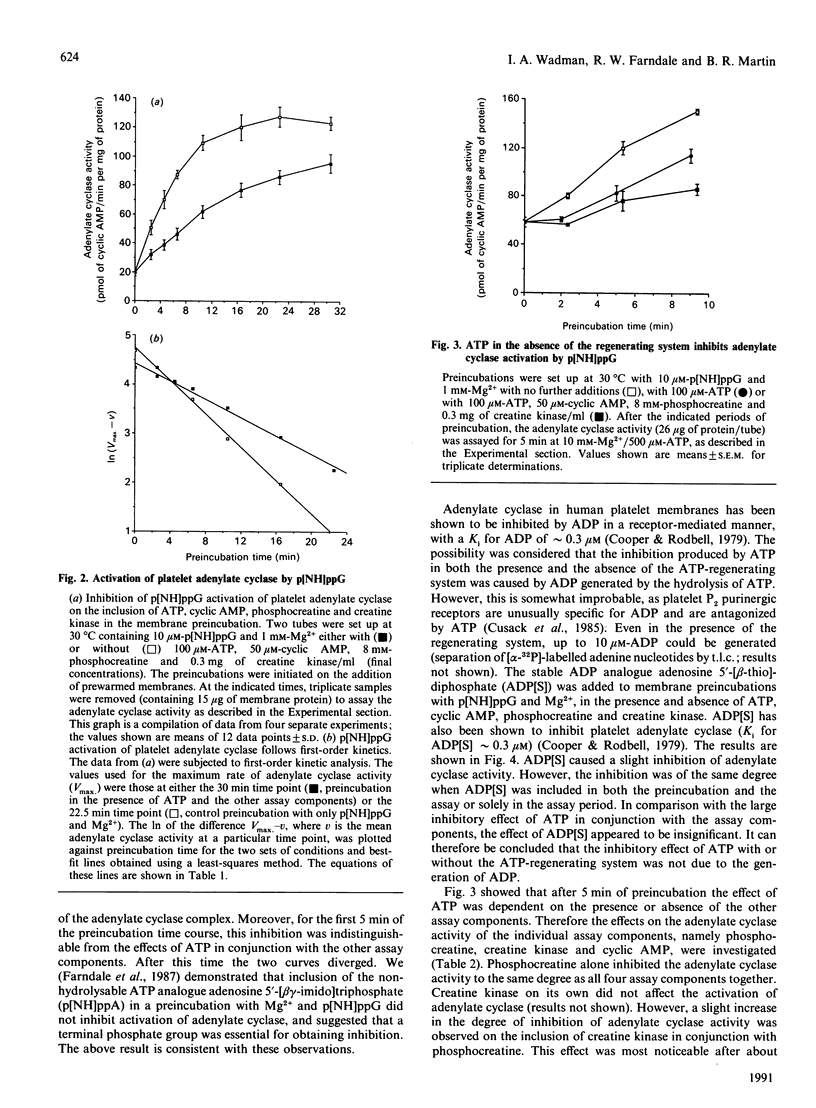
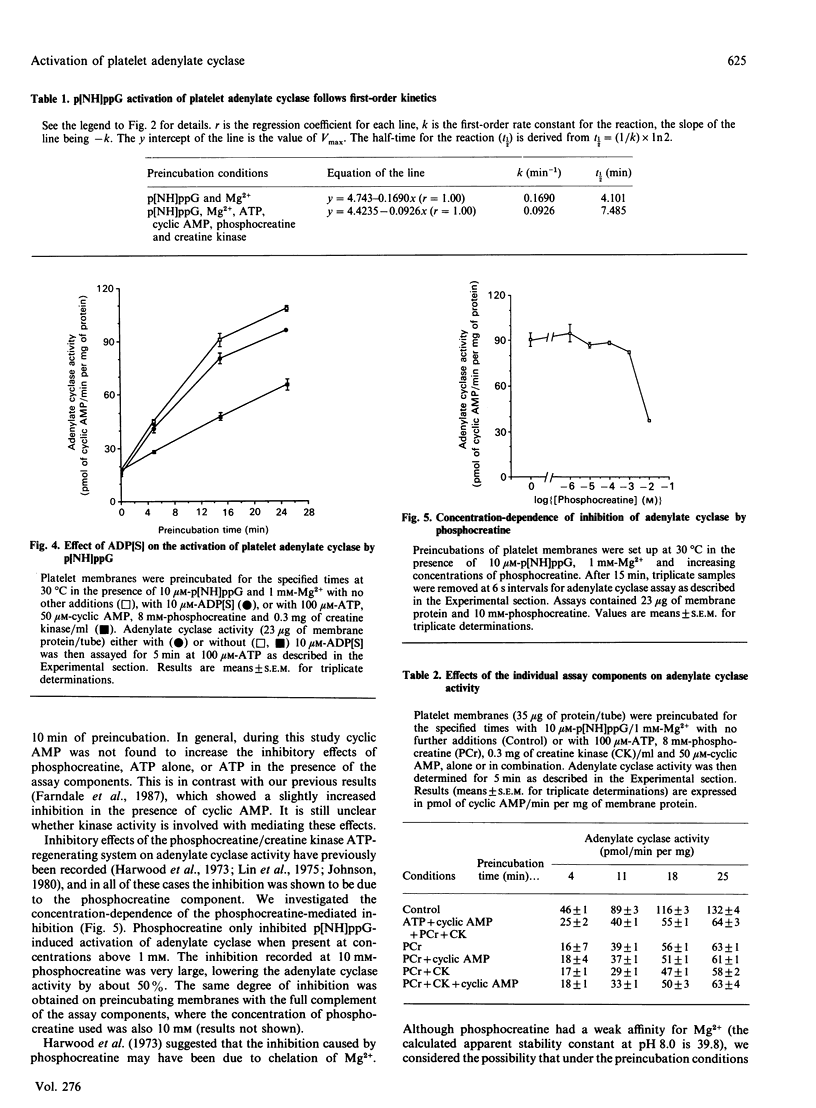
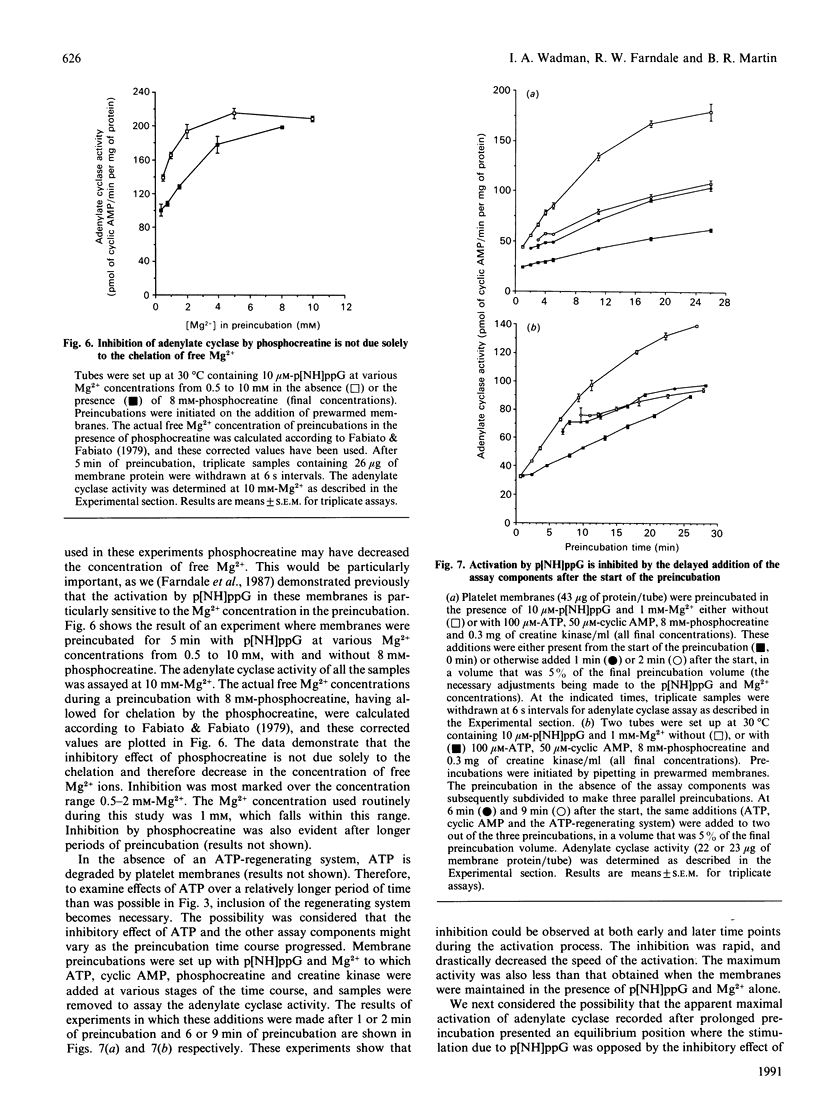
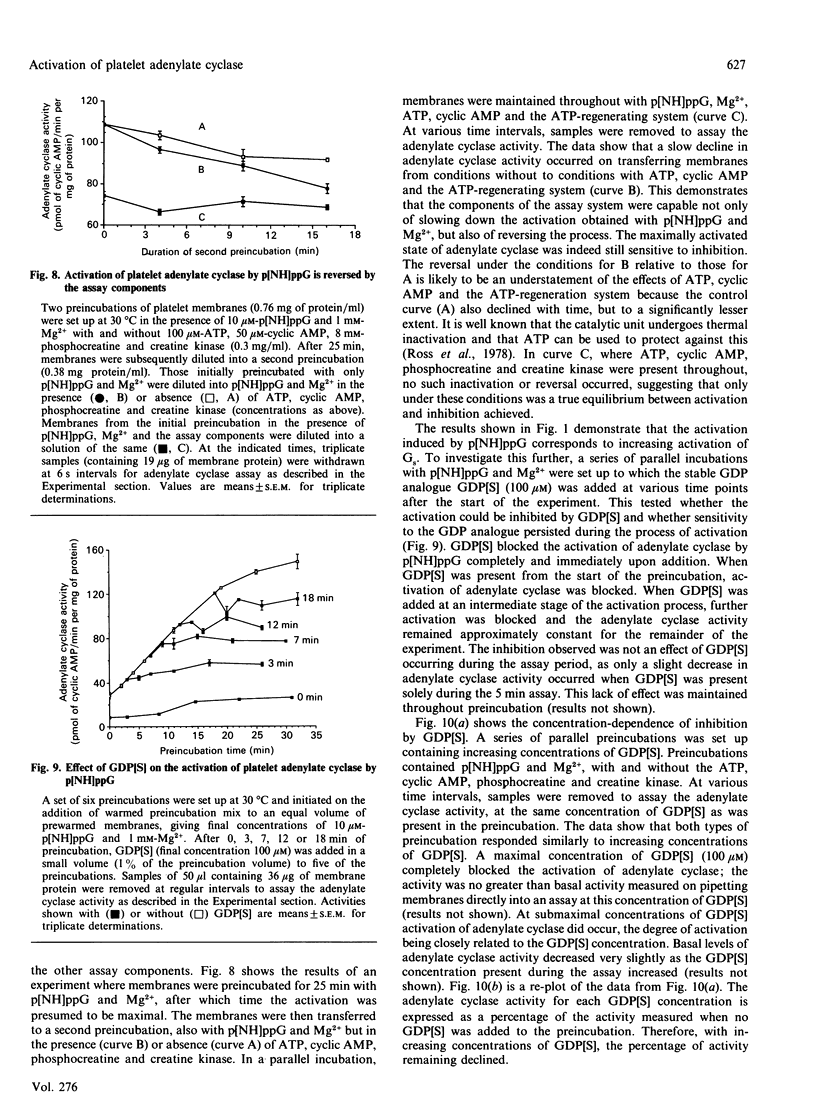
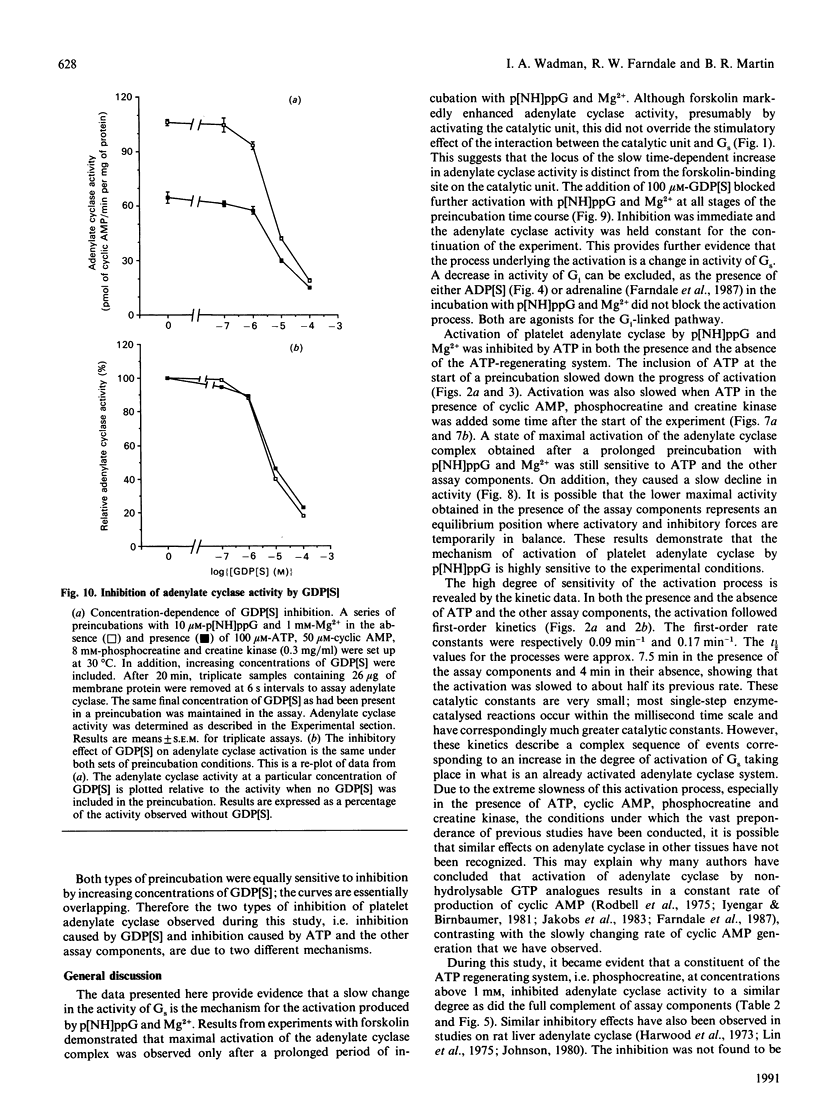
1. Incubation of human platelet membranes with guanosine 5'-[beta gamma-imido]triphosphate (p[NH]ppG) causes a time-dependent increase in the activation of adenylate cyclase due to Gs (the stimulatory GTP-binding protein). Forskolin enhances adenylate cyclase activity but does not interfere with the process of activation. The activation follows first-order kinetics in both the presence and the absence of the assay components. 2. ATP in the presence or the absence of an ATP-regenerating system of phosphocreatine and creatine kinase inhibits activation. 3. Hydrolysis of ATP to ADP does not lead to receptor-mediated inhibition of adenylate cyclase acting via Gi (the inhibitory GTP-binding protein). The ADP analogue adenosine 5'-[beta-thio]diphosphate (ADP[S]) does not inhibit the activation process. 4. Phosphocreatine alone inhibits adenylate cyclase activation at concentrations above 1 mM. 5. Inhibition by phosphocreatine is not due to the chelation of free Mg2+ ions. 6. Inhibition by ATP and the other assay components occurs throughout the activation process, decreasing both the rate of activation and the maximum activity obtained. 7. Maximal activation of adenylate cyclase after prolonged incubation with p[NH]ppG slowly reverses in the presence of the assay components. 8. A 10-fold excess of the GDP analogue guanosine 5'-[beta-thio]diphosphate (GDP[S]) over p[NH]ppG inhibits the activation process completely, at all stages of the time course. 9. Preincubations in the presence and absence of ATP, cyclic AMP, phosphocreatine and creatine kinase show equal sensitivity to increasing GDP[S] concentration. These data show that the inhibition observed in the presence of ATP is not due to endogenous or contaminating guanine nucleotides, and suggest that phosphoryl transfer may regulate adenylate cyclase activity.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L., Pohl S. L., Rodbell M. Adenyl cyclase in fat cells. 1. Properties and the effects of adrenocorticotropin and fluoride. J Biol Chem. 1969 Jul 10;244(13):3468–3476. [PubMed] [Google Scholar]
- Carlson K. E., Brass L. F., Manning D. R. Thrombin and phorbol esters cause the selective phosphorylation of a G protein other than Gi in human platelets. J Biol Chem. 1989 Aug 5;264(22):13298–13305. [PubMed] [Google Scholar]
- Cooper D. M., Rodbell M. ADP is a potent inhibitor of human platelet plasma membrane adenylate cyclase. Nature. 1979 Nov 29;282(5738):517–518. doi: 10.1038/282517a0. [DOI] [PubMed] [Google Scholar]
- Crouch M. F., Lapetina E. G. A role for Gi in control of thrombin receptor-phospholipase C coupling in human platelets. J Biol Chem. 1988 Mar 5;263(7):3363–3371. [PubMed] [Google Scholar]
- Cusack N. J., Hourani S. M., Welford L. A. Characterisation of ADP receptors. Adv Exp Med Biol. 1985;192:29–39. doi: 10.1007/978-1-4615-9442-0_3. [DOI] [PubMed] [Google Scholar]
- Daly J. W. Forskolin, adenylate cyclase, and cell physiology: an overview. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:81–89. [PubMed] [Google Scholar]
- Eckstein F., Cassel D., Levkovitz H., Lowe M., Selinger Z. Guanosine 5'-O-(2-thiodiphosphate). An inhibitor of adenylate cyclase stimulation by guanine nucleotides and fluoride ions. J Biol Chem. 1979 Oct 10;254(19):9829–9834. [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Farndale R. W., Wong S. K., Martin B. R. Activation of adenylate cyclase in human platelet membranes by guanosine 5'-[beta gamma-imido]triphosphate is inhibited by cyclic-AMP-dependent phosphorylation. Slow activation occurs in the absence of ATP. Biochem J. 1987 Mar 15;242(3):637–643. doi: 10.1042/bj2420637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong H. K., Yoshimoto K. K., Eversole-Cire P., Simon M. I. Identification of a GTP-binding protein alpha subunit that lacks an apparent ADP-ribosylation site for pertussis toxin. Proc Natl Acad Sci U S A. 1988 May;85(9):3066–3070. doi: 10.1073/pnas.85.9.3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Harwood J. P., Löw H., Rodbell M. Stimulatory and inhibitory effects of guanyl nucleotides on fat cell adenylate cyclase. J Biol Chem. 1973 Sep 10;248(17):6239–6245. [PubMed] [Google Scholar]
- Iyengar R., Birnbaumer L. Hysteretic activation of adenylyl cyclases. I. Effect of Mg ion on the rate of activation by guanine nucleotides and fluoride. J Biol Chem. 1981 Nov 10;256(21):11036–11041. [PubMed] [Google Scholar]
- Jakobs K. H., Schultz G., Gaugler B., Pfeuffer T. Inhibition of NS-protein-stimulated human-platelet adenylate cyclase by epinephrine and stable GTP analogs. Eur J Biochem. 1983 Aug 1;134(2):351–354. doi: 10.1111/j.1432-1033.1983.tb07574.x. [DOI] [PubMed] [Google Scholar]
- Johnson R. A. Stimulatory and inhibitory effects of ATP-regenerating systems on liver adenylate cyclase. J Biol Chem. 1980 Sep 10;255(17):8252–8258. [PubMed] [Google Scholar]
- KUBY S. A., NODA L., LARDY H. A. Adenosinetriphosphate-creatine transphosphorylase. III. Kinetic studies. J Biol Chem. 1954 Sep;210(1):65–82. [PubMed] [Google Scholar]
- Katada T., Gilman A. G., Watanabe Y., Bauer S., Jakobs K. H. Protein kinase C phosphorylates the inhibitory guanine-nucleotide-binding regulatory component and apparently suppresses its function in hormonal inhibition of adenylate cyclase. Eur J Biochem. 1985 Sep 2;151(2):431–437. doi: 10.1111/j.1432-1033.1985.tb09120.x. [DOI] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- Krupinski J., Coussen F., Bakalyar H. A., Tang W. J., Feinstein P. G., Orth K., Slaughter C., Reed R. R., Gilman A. G. Adenylyl cyclase amino acid sequence: possible channel- or transporter-like structure. Science. 1989 Jun 30;244(4912):1558–1564. doi: 10.1126/science.2472670. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin M. C., Salomon Y., Rendell M., Rodbell M. The hepatic adenylate cyclase system. II. Substrate binding and utilization and the effects of magnesium ion and pH. J Biol Chem. 1975 Jun 10;250(11):4246–4252. [PubMed] [Google Scholar]
- Londos C., Salomon Y., Lin M. C., Harwood J. P., Schramm M., Wolff J., Rodbell M. 5'-Guanylylimidodiphosphate, a potent activator of adenylate cyclase systems in eukaryotic cells. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3087–3090. doi: 10.1073/pnas.71.8.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. R., Voorheis H. P. A simple enzymic method for the synthesis of adenosine 5'-[alpha-32P]triphosphate on a preparative scale. Biochem J. 1977 Mar 1;161(3):555–559. doi: 10.1042/bj1610555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northup J. K., Smigel M. D., Gilman A. G. The guanine nucleotide activating site of the regulatory component of adenylate cyclase. Identification by ligand binding. J Biol Chem. 1982 Oct 10;257(19):11416–11423. [PubMed] [Google Scholar]
- Pyne N. J., Murphy G. J., Milligan G., Houslay M. D. Treatment of intact hepatocytes with either the phorbol ester TPA or glucagon elicits the phosphorylation and functional inactivation of the inhibitory guanine nucleotide regulatory protein Gi. FEBS Lett. 1989 Jan 16;243(1):77–82. doi: 10.1016/0014-5793(89)81221-9. [DOI] [PubMed] [Google Scholar]
- Rodbell M., Lin M. C., Salomon Y., Londos C., Harwood J. P., Martin B. R., Rendell M., Berman M. Role of adenine and guanine nucleotides in the activity and response of adenylate cyclase systems to hormones: evidence for multisite transition states. Adv Cyclic Nucleotide Res. 1975;5:3–29. [PubMed] [Google Scholar]
- Rodbell M. Metabolism of isolated fat cells. V. Preparation of "ghosts" and their properties; adenyl cyclase and other enzymes. J Biol Chem. 1967 Dec 25;242(24):5744–5750. [PubMed] [Google Scholar]
- Ross E. M., Howlett A. C., Ferguson K. M., Gilman A. G. Reconstitution of hormone-sensitive adenylate cyclase activity with resolved components of the enzyme. J Biol Chem. 1978 Sep 25;253(18):6401–6412. [PubMed] [Google Scholar]
- Sagi-Eisenberg R. GTP-binding proteins as possible targets for protein kinase C action. Trends Biochem Sci. 1989 Sep;14(9):355–357. doi: 10.1016/0968-0004(89)90001-7. [DOI] [PubMed] [Google Scholar]
- Salomon Y., Londos C., Rodbell M. A highly sensitive adenylate cyclase assay. Anal Biochem. 1974 Apr;58(2):541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- Seamon K., Daly J. W. Activation of adenylate cyclase by the diterpene forskolin does not require the guanine nucleotide regulatory protein. J Biol Chem. 1981 Oct 10;256(19):9799–9801. [PubMed] [Google Scholar]
- Sibley D. R., Benovic J. L., Caron M. G., Lefkowitz R. J. Regulation of transmembrane signaling by receptor phosphorylation. Cell. 1987 Mar 27;48(6):913–922. doi: 10.1016/0092-8674(87)90700-8. [DOI] [PubMed] [Google Scholar]
- Steer M. L., Wood A. Regulation of human platelet adenylate cyclase by epinephrine, prostaglandin E1, and guanine nucleotides. Evidence for separate guanine nucleotide sites mediating stimulation and inhibition. J Biol Chem. 1979 Nov 10;254(21):10791–10797. [PubMed] [Google Scholar]
- Stein J. M., Martin B. R. The role of GTP in prostaglandin E1 stimulation of adenylate cyclase in platelet membranes. Biochem J. 1983 Jul 15;214(1):231–234. doi: 10.1042/bj2140231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallant E. A., Wallace R. W. Characterization of a calmodulin-dependent protein phosphatase from human platelets. J Biol Chem. 1985 Jun 25;260(12):7744–7751. [PubMed] [Google Scholar]
- Wong S. K., Martin B. R. Activation of rat liver adenylate cyclase by guanosine 5'-[beta,gamma-imido]triphosphate and glucagon. Existence of reversibly and irreversibly activated states of the stimulatory GTP-binding protein. Biochem J. 1986 Feb 1;233(3):845–851. doi: 10.1042/bj2330845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. K., Martin B. R. The role of a guanine nucleotide-binding protein in the activation of rat liver plasma-membrane adenylate cyclase by forskolin. Biochem J. 1983 Dec 15;216(3):753–759. doi: 10.1042/bj2160753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita A., Kurokawa T., Higashi K., Dan'ura T., Ishibashi S. Forskolin stabilizes a functionally coupled state between activated guanine nucleotide-binding stimulatory regulatory protein, Ns, and catalytic protein of adenylate cyclase system in rat erythrocytes. Biochem Biophys Res Commun. 1986 May 29;137(1):190–194. doi: 10.1016/0006-291x(86)91194-0. [DOI] [PubMed] [Google Scholar]
- Yoshimasa T., Sibley D. R., Bouvier M., Lefkowitz R. J., Caron M. G. Cross-talk between cellular signalling pathways suggested by phorbol-ester-induced adenylate cyclase phosphorylation. Nature. 1987 May 7;327(6117):67–70. doi: 10.1038/327067a0. [DOI] [PubMed] [Google Scholar]
- Zucker M. B., Nachmias V. T. Platelet activation. Arteriosclerosis. 1985 Jan-Feb;5(1):2–18. doi: 10.1161/01.atv.5.1.2. [DOI] [PubMed] [Google Scholar]


