Abstract
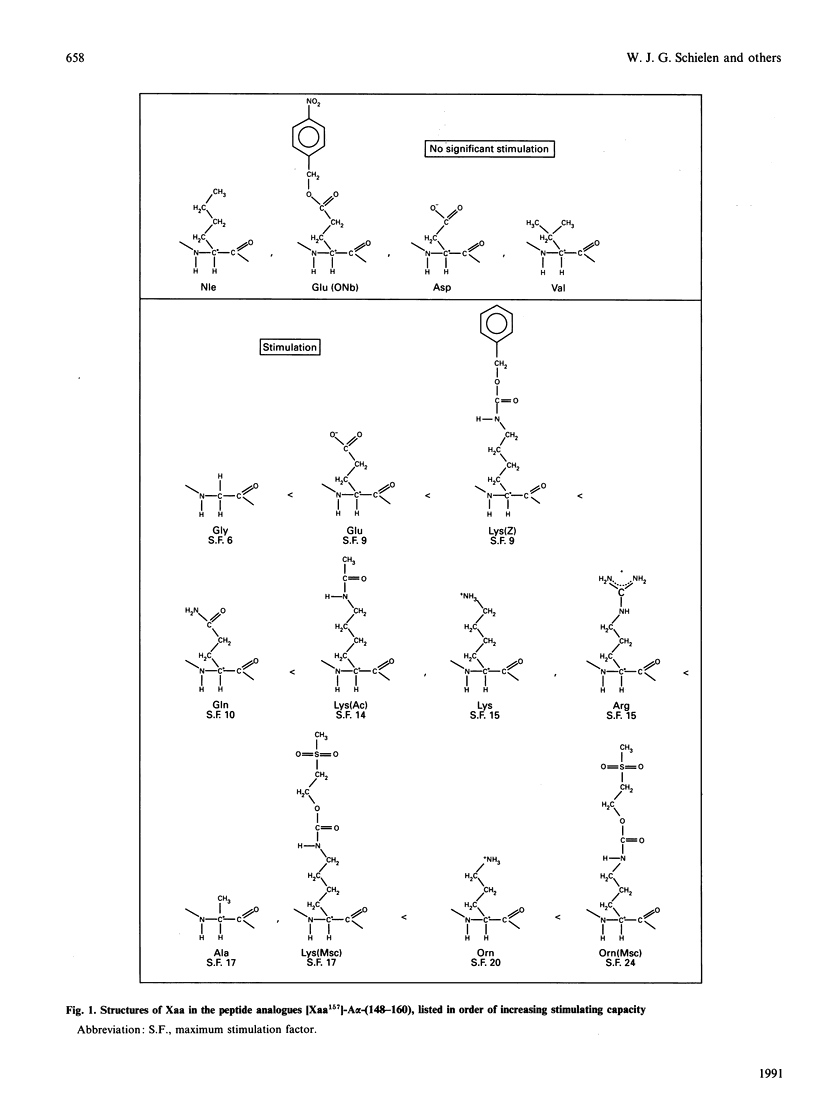
The sequence fibrinogen-A alpha-(148-160) can mimic part of the fibrin-induced rate enhancement of the activation of plasminogen by tissue-type plasminogen activator. Previously we have reported that the lysine residue at position A alpha-157 is crucial. During our further investigations on A alpha-157 we found that lysine at position A alpha-157 may be replaced by glutamic acid. This unexpected finding prompted us to re-investigate the requirements of this position. We prepared analogues of A alpha-(148-160) in which the lysine residue at position A alpha-157 was replaced by lysine derivatives (acetyl-lysine, benzyloxycarbonyl-lysine and methanesulphonylethyloxycarbonyl-lysine), acidic residues (aspartic acid and glutamic acid), basic residues (arginine and ornithine), polar residues (glutamine and methanesulphonylethyloxycarbonylornithine), apolar residues (alanine, valine, norleucine and glutamic acid 4-nitrobenzyl ester) and glycine. These analogues were tested for their stimulatory activity. When aspartic acid, glutamic acid 4-nitrobenzyl ester or norleucine is present at position A alpha-157 in A alpha-(148-160) virtually all stimulatory capacity is lost. With valine at position A alpha-157 the stimulatory activity is marginal. None of the other replacements at position A alpha-157 caused loss of rate-enhancing properties. From these results we conclude that for the rate-enhancing effect of A alpha-(148-160) the side chain of the amino acid residue at position A alpha-157 must fulfill certain requirements: there must be one (as in alanine) or no (as in glycine) carbon atom in the side chain, or at least two carbon atoms and a polar group (charged or uncharged) to which a rather bulky group (such as the benzyloxycarbonyl group) or a polar group (such as the methanesulphonylethyloxycarbonyl group) may be attached. The highest activity [even higher than native A alpha-(148-160)] was obtained with ornithine, methanesulphonylethyloxycarbonylornithine or methanesulphonylethyloxycarbonyl-lysine at position A alpha-157.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen R. A., Pepper D. S. Isolation and properties of human vascular plasminogen activator. Thromb Haemost. 1981 Feb 23;45(1):43–50. [PubMed] [Google Scholar]
- Christensen U. The AH-site of plasminogen and two C-terminal fragments. A weak lysine-binding site preferring ligands not carrying a free carboxylate function. Biochem J. 1984 Oct 15;223(2):413–421. doi: 10.1042/bj2230413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collen D. On the regulation and control of fibrinolysis. Edward Kowalski Memorial Lecture. Thromb Haemost. 1980 Jun 18;43(2):77–89. [PubMed] [Google Scholar]
- Deutsch D. G., Mertz E. T. Plasminogen: purification from human plasma by affinity chromatography. Science. 1970 Dec 4;170(3962):1095–1096. doi: 10.1126/science.170.3962.1095. [DOI] [PubMed] [Google Scholar]
- Doolittle R. F., Goldbaum D. M., Doolittle L. R. Designation of sequences involved in the "coiled-coil" interdomainal connections in fibrinogen: constructions of an atomic scale model. J Mol Biol. 1978 Apr 5;120(2):311–325. doi: 10.1016/0022-2836(78)90070-0. [DOI] [PubMed] [Google Scholar]
- Hoylaerts M., Rijken D. C., Lijnen H. R., Collen D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J Biol Chem. 1982 Mar 25;257(6):2912–2919. [PubMed] [Google Scholar]
- Kaiser E., Colescott R. L., Bossinger C. D., Cook P. I. Color test for detection of free terminal amino groups in the solid-phase synthesis of peptides. Anal Biochem. 1970 Apr;34(2):595–598. doi: 10.1016/0003-2697(70)90146-6. [DOI] [PubMed] [Google Scholar]
- Laudano A. P., Doolittle R. F. Influence of calcium ion on the binding of fibrin amino terminal peptides to fibrinogen. Science. 1981 Apr 24;212(4493):457–459. doi: 10.1126/science.7209542. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuizen W., Verheijen J. H., Vermond A., Chang G. T. Plasminogen activation by tissue activator is accelerated in the presence of fibrin(ogen) cyanogen bromide fragment FCB-2. Biochim Biophys Acta. 1983 Feb 22;755(3):531–533. doi: 10.1016/0304-4165(83)90261-1. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuizen W., Vermond A., Voskuilen M., Traas D. W., Verheijen J. H. Identification of a site in fibrin(ogen) which is involved in the acceleration of plasminogen activation by tissue-type plasminogen activator. Biochim Biophys Acta. 1983 Oct 17;748(1):86–92. doi: 10.1016/0167-4838(83)90030-4. [DOI] [PubMed] [Google Scholar]
- Norrman B., Wallén P., Rånby M. Fibrinolysis mediated by tissue plasminogen activator. Disclosure of a kinetic transition. Eur J Biochem. 1985 May 15;149(1):193–200. doi: 10.1111/j.1432-1033.1985.tb08911.x. [DOI] [PubMed] [Google Scholar]
- Radcliffe R. A critical role of lysine residues in the stimulation of tissue plasminogen activator by denatured proteins and fibrin clots. Biochim Biophys Acta. 1983 Mar 30;743(3):422–430. doi: 10.1016/0167-4838(83)90401-6. [DOI] [PubMed] [Google Scholar]
- Rijken D. C., Wijngaards G., Zaal-de Jong M., Welbergen J. Purification and partial characterization of plasminogen activator from human uterine tissue. Biochim Biophys Acta. 1979 Sep 29;580(1):140–153. doi: 10.1016/0005-2795(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Schielen W. J., Voskuilen M., Tesser G. I., Nieuwenhuizen W. The sequence A alpha-(148-160) in fibrin, but not in fibrinogen, is accessible to monoclonal antibodies. Proc Natl Acad Sci U S A. 1989 Nov;86(22):8951–8954. doi: 10.1073/pnas.86.22.8951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suenson E., Lützen O., Thorsen S. Initial plasmin-degradation of fibrin as the basis of a positive feed-back mechanism in fibrinolysis. Eur J Biochem. 1984 May 2;140(3):513–522. doi: 10.1111/j.1432-1033.1984.tb08132.x. [DOI] [PubMed] [Google Scholar]
- Verheijen J. H., Nieuwenhuizen W., Traas D. W., Chang G. T., Hoegee E. Differences in effects of fibrin(ogen) fragments on the activation of 1-glu-plasminogen and 442-val-plasminogen by tissue-type plasminogen activator. Thromb Res. 1983 Oct 1;32(1):87–92. doi: 10.1016/0049-3848(83)90157-3. [DOI] [PubMed] [Google Scholar]
- Verheijen J. H., Nieuwenhuizen W., Wijngaards G. Activation of plasminogen by tissue activator is increased specifically in the presence of certain soluble fibrin(ogen) fragments. Thromb Res. 1982 Aug 15;27(4):377–385. doi: 10.1016/0049-3848(82)90055-x. [DOI] [PubMed] [Google Scholar]
- Voskuilen M., Vermond A., Veeneman G. H., van Boom J. H., Klasen E. A., Zegers N. D., Nieuwenhuizen W. Fibrinogen lysine residue A alpha 157 plays a crucial role in the fibrin-induced acceleration of plasminogen activation, catalyzed by tissue-type plasminogen activator. J Biol Chem. 1987 May 5;262(13):5944–5946. [PubMed] [Google Scholar]
- Wang S. S. p-alkoxybenzyl alcohol resin and p-alkoxybenzyloxycarbonylhydrazide resin for solid phase synthesis of protected peptide fragments. J Am Chem Soc. 1973 Feb 21;95(4):1328–1333. doi: 10.1021/ja00785a602. [DOI] [PubMed] [Google Scholar]


