Abstract
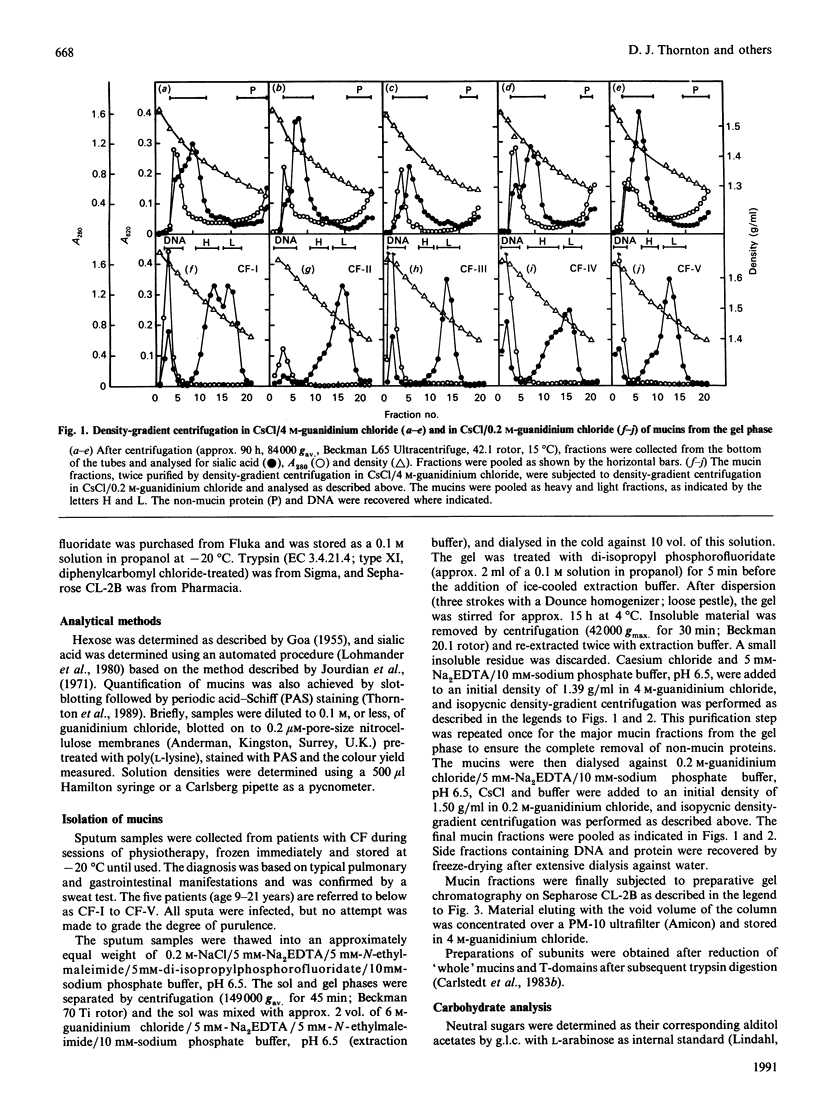
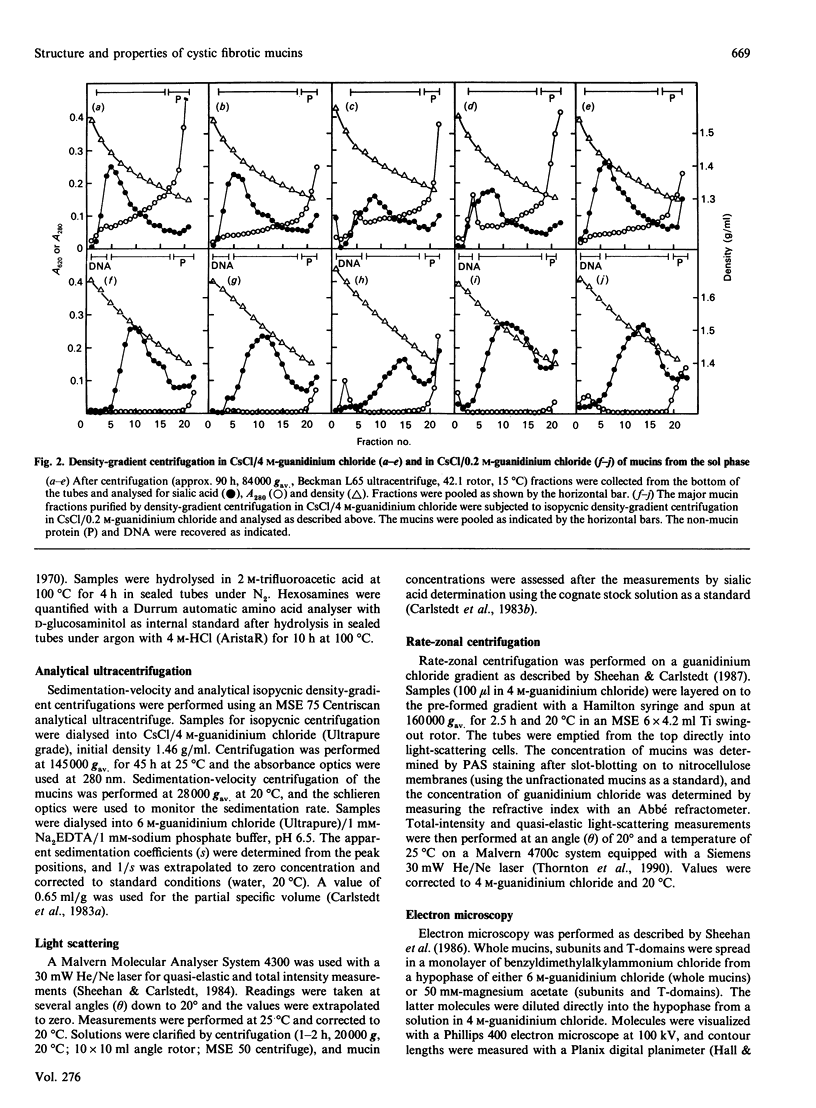
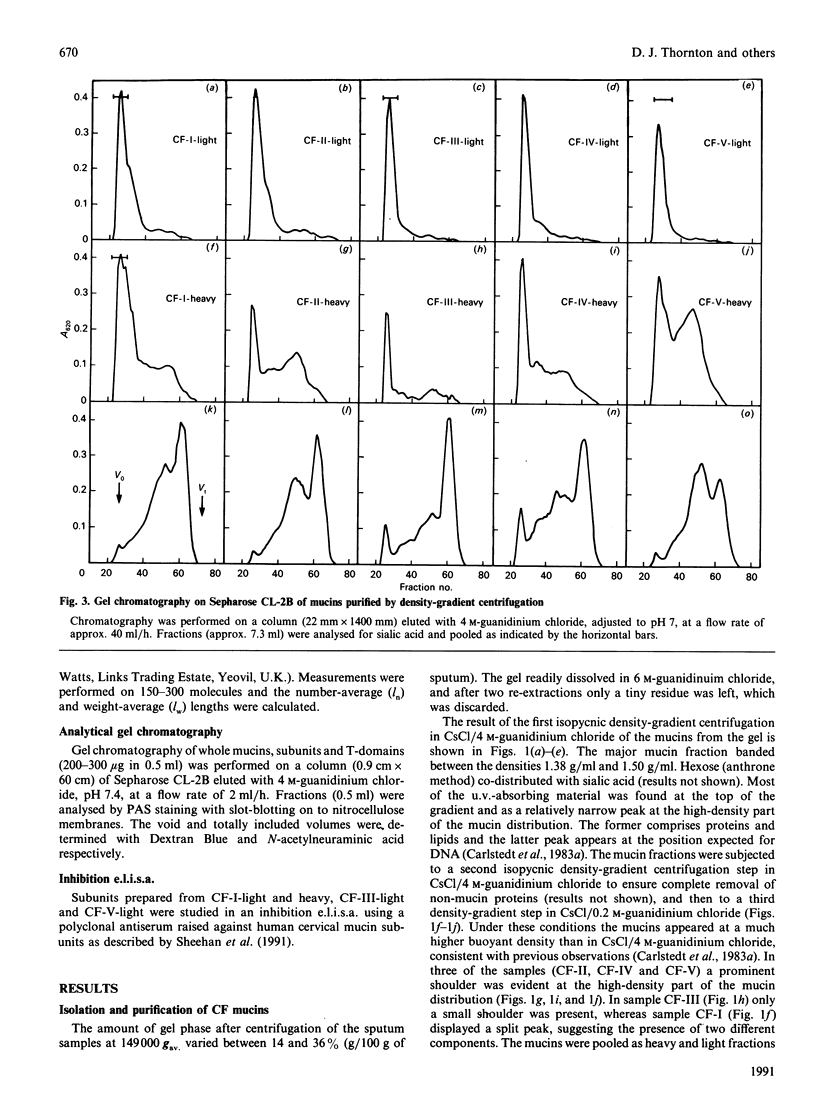
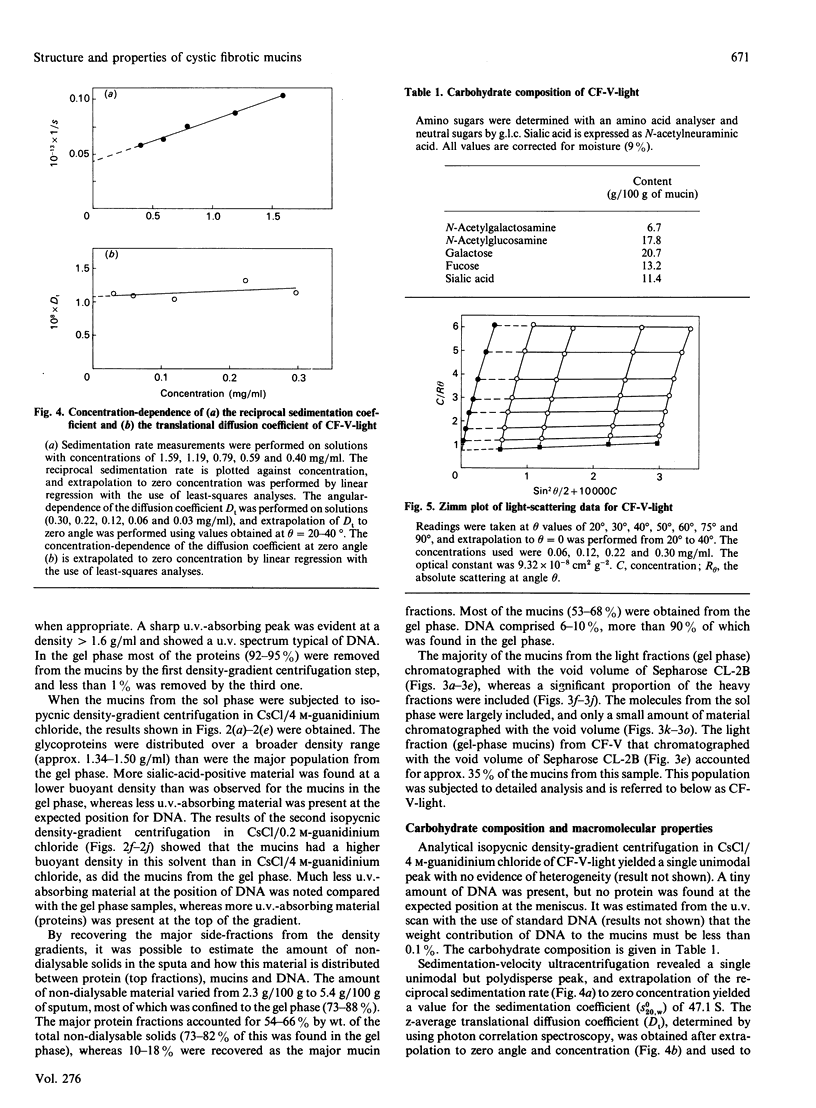
Mucus glycoproteins (mucins) were isolated from sputum of patients with cystic fibrosis (CF) after separation into sol and gel phases. The mucus gel was solubilized with gentle stirring in 6 M-guanidinium chloride supplemented with proteinase inhibitors, and purification of mucins was subsequently achieved by isopycnic density-gradient centrifugation in CsCl/guanidinium chloride. Density-gradient centrifugation also revealed a heterogeneity of the macromolecules, the pattern of which varied between individuals, and mucins from the gel phase was pooled as 'heavy' and 'light' fractions. Gel chromatography on Sepharose CL-2B showed that the heavy fraction contained a larger proportion of smaller species than the 'light' fraction and that the gel phase mucins were much larger than those from the sol. An apparently homogeneous high-Mr mucin population from one individual contained approx. 70% (w/w) carbohydrate, the major sugars being N-acetylglucosamine (17.8%), N-acetylgalactosamine (6.7%), galactose (20.7%), fucose (13.2%) and sialic acid (11.4%). These mucins had an S020.w of 47 S, and an Mr of 15 x 10(6) -20 x 10(6), and rate-zonal centrifugation revealed a polydisperse size distribution [range (5-30) x 10(6)] with a weight-average Mr of 17 x 10(6). The whole mucins were visualized with electron microscopy as linear and apparently flexible threads, disperse in size. Reduction produced subunits which were included on Sepharose CL-2B, and subsequent trypsin digestion yielded high-Mr glycopeptides which were further retarded. The size distributions and fragmentation patterns of mucin from two other CF patients were the same, as studied by gel chromatography, rate-zonal centrifugation and electron microscopy. We conclude that CF mucins are heterogeneous in both size and buoyant density and that the various populations, though differing in buoyant density, share the same architecture and macromolecular properties and are, in this respect, similar to mucins from normal respiratory secretions [Thornton, Davies, Kraayenbrink, Richardson, Sheehan & Carlstedt (1990) Biochem. J. 265, 179-186] and human cervical mucus [Carlstedt & Sheehan (1989) SEB Symp. XLIII 289-316].
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhaskar K. R., O'Sullivan D. D., Opaskar-Hincman H., Reid L. M., Coles S. J. Density gradient analysis of secretions produced in vitro by human and canine airway mucosa: identification of lipids and proteoglycans in such secretions. Exp Lung Res. 1986;10(4):401–422. doi: 10.3109/01902148609058290. [DOI] [PubMed] [Google Scholar]
- Bhaskar K. R., O'Sullivan D. D., Seltzer J., Rossing T. H., Drazen J. M., Reid L. M. Density gradient study of bronchial mucus aspirates from healthy volunteers (smokers and nonsmokers) and from patients with tracheostomy. Exp Lung Res. 1985;9(3-4):289–308. doi: 10.3109/01902148509057529. [DOI] [PubMed] [Google Scholar]
- Boat T. F., Cheng P. W., Iyer R. N., Carlson D. M., Polony I. Human respiratory tract secretion. Mucous glycoproteins of nonpurulent tracheobronchial secretions, and sputum of patients with bronchitis and cystic fibrosis. Arch Biochem Biophys. 1976 Nov;177(1):95–104. doi: 10.1016/0003-9861(76)90419-7. [DOI] [PubMed] [Google Scholar]
- Carlstedt I., Lindgren H., Sheehan J. K. The macromolecular structure of human cervical-mucus glycoproteins. Studies on fragments obtained after reduction of disulphide bridges and after subsequent trypsin digestion. Biochem J. 1983 Aug 1;213(2):427–435. doi: 10.1042/bj2130427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstedt I., Lindgren H., Sheehan J. K., Ulmsten U., Wingerup L. Isolation and characterization of human cervical-mucus glycoproteins. Biochem J. 1983 Apr 1;211(1):13–22. doi: 10.1042/bj2110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlstedt I., Sheehan J. K. Is the macromolecular architecture of cervical, respiratory and gastric mucins the same? Biochem Soc Trans. 1984 Aug;12(4):615–617. doi: 10.1042/bst0120615. [DOI] [PubMed] [Google Scholar]
- Carlstedt I., Sheehan J. K. Macromolecular properties and polymeric structure of mucus glycoproteins. Ciba Found Symp. 1984;109:157–172. doi: 10.1002/9780470720905.ch11. [DOI] [PubMed] [Google Scholar]
- Carlstedt I., Sheehan J. K. Structure and macromolecular properties of cervical mucus glycoproteins. Symp Soc Exp Biol. 1989;43:289–316. [PubMed] [Google Scholar]
- Chace K. V., Flux M., Sachdev G. P. Comparison of physicochemical properties of purified mucus glycoproteins isolated from respiratory secretions of cystic fibrosis and asthmatic patients. Biochemistry. 1985 Dec 3;24(25):7334–7341. doi: 10.1021/bi00346a047. [DOI] [PubMed] [Google Scholar]
- Creeth J. M., Bhaskar K. R., Horton J. R., Das I., Lopez-Vidriero M. T., Reid L. The separation and characterization of bronchial glycoproteins by density-gradient methods. Biochem J. 1977 Dec 1;167(3):557–569. doi: 10.1042/bj1670557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldhoff P. A., Bhavanandan V. P., Davidson E. A. Purification, properties, and analysis of human asthmatic bronchial mucin. Biochemistry. 1979 May 29;18(11):2430–2436. doi: 10.1021/bi00578a044. [DOI] [PubMed] [Google Scholar]
- Gupta R., Jentoft N., Jamieson A. M., Blackwell J. Structural analysis of purified human tracheobronchial mucins. Biopolymers. 1990 Feb 5;29(2):347–355. doi: 10.1002/bip.360290207. [DOI] [PubMed] [Google Scholar]
- Harding S. E., Rowe A. J., Creeth J. M. Further evidence for a flexible and highly expanded spheroidal model for mucus glycoproteins in solution. Biochem J. 1983 Mar 1;209(3):893–896. doi: 10.1042/bj2090893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastie A. T., Hingley S. T., Kueppers F., Higgins M. L., Tannenbaum C. S., Weinbaum G. Protease production by Pseudomonas aeruginosa isolates from patients with cystic fibrosis. Infect Immun. 1983 May;40(2):506–513. doi: 10.1128/iai.40.2.506-513.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdret N., Ramphal R., Scharfman A., Perini J. M., Filliat M., Lamblin G., Roussel P. Evidence for the in vivo degradation of human respiratory mucins during Pseudomonas aeruginosa infection. Biochim Biophys Acta. 1989 Jul 21;992(1):96–105. doi: 10.1016/0304-4165(89)90055-x. [DOI] [PubMed] [Google Scholar]
- Jackson A. H., Hill S. L., Afford S. C., Stockley R. A. Sputum sol-phase proteins and elastase activity in patients with cystic fibrosis. Eur J Respir Dis. 1984 Feb;65(2):114–124. [PubMed] [Google Scholar]
- Jourdian G. W., Dean L., Roseman S. The sialic acids. XI. A periodate-resorcinol method for the quantitative estimation of free sialic acids and their glycosides. J Biol Chem. 1971 Jan 25;246(2):430–435. [PubMed] [Google Scholar]
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989 Sep 8;245(4922):1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Lamb D., Reid L. The tracheobronchial submucosal glands in cystic fibrosis: a qualitative and quantitative histochemical study. Br J Dis Chest. 1972 Oct;66(4):239–247. doi: 10.1016/0007-0971(72)90042-3. [DOI] [PubMed] [Google Scholar]
- Lamblin G., Lafitte J. J., Lhermitte M., Degand P., Roussel P. Mucins from cystic fibrosis sputum. Mod Probl Paediatr. 1976 Oct 24;19:153–164. [PubMed] [Google Scholar]
- Lindahl U. Attempted isolation of a heparin proteoglycan from bovine liver capsule. Biochem J. 1970 Jan;116(1):27–34. doi: 10.1042/bj1160027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmander L. S., De Luca S., Nilsson B., Hascall V. C., Caputo C. B., Kimura J. H., Heinegard D. Oligosaccharides on proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1980 Jul 10;255(13):6084–6091. [PubMed] [Google Scholar]
- Lopez-Vidriero M. T., Reid L. Pathophysiology of mucus secretion in cystic fibrosis. Mod Probl Paediatr. 1976 Oct 24;19:120–128. [PubMed] [Google Scholar]
- Marianne T., Perini J. M., Lafitte J. J., Houdret N., Pruvot F. R., Lamblin G., Slayter H. S., Roussel P. Peptides of human bronchial mucus glycoproteins. Size determination by electron microscopy and by biosynthetic experiments. Biochem J. 1987 Nov 15;248(1):189–195. doi: 10.1042/bj2480189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzuca M., Lhermitte M., Lafitte J. J., Roussel P. Use of lectins for detection of glycoconjugates in the glandular cells of the human bronchial mucosa. J Histochem Cytochem. 1982 Sep;30(9):956–966. doi: 10.1177/30.9.7130674. [DOI] [PubMed] [Google Scholar]
- Mikkelsen A., Stokke B. T., Christensen B. E., Elgsaeter A. Flexibility and length of human bronchial mucin studied using low-shear viscometry, birefringence relaxation analysis, and electron microscopy. Biopolymers. 1985 Sep;24(9):1683–1704. doi: 10.1002/bip.360240904. [DOI] [PubMed] [Google Scholar]
- Riordan J. R., Rommens J. M., Kerem B., Alon N., Rozmahel R., Grzelczak Z., Zielenski J., Lok S., Plavsic N., Chou J. L. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989 Sep 8;245(4922):1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rommens J. M., Iannuzzi M. C., Kerem B., Drumm M. L., Melmer G., Dean M., Rozmahel R., Cole J. L., Kennedy D., Hidaka N. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science. 1989 Sep 8;245(4922):1059–1065. doi: 10.1126/science.2772657. [DOI] [PubMed] [Google Scholar]
- Rose M. C., Brown C. F., Jacoby J. Z., 3rd, Lynn W. S., Kaufman B. Biochemical properties of tracheobronchial mucins from cystic fibrosis and non-cystic fibrosis individuals. Pediatr Res. 1987 Nov;22(5):545–551. doi: 10.1203/00006450-198711000-00015. [DOI] [PubMed] [Google Scholar]
- Rose M. C., Voter W. A., Brown C. F., Kaufman B. Structural features of human tracheobronchial mucus glycoprotein. Biochem J. 1984 Sep 1;222(2):371–377. doi: 10.1042/bj2220371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel P., Lamblin G., Degand P. Heterogeneity of the carbohydrate chains of sulfated bronchial glycoproteins isolated from a patient suffering from cystic fibrosis. J Biol Chem. 1975 Mar 25;250(6):2114–2122. [PubMed] [Google Scholar]
- Sheehan J. K., Boot-Handford R. P., Chantler E., Carlstedt I., Thornton D. J. Evidence for shared epitopes within the 'naked' protein domains of human mucus glycoproteins. A study performed by using polyclonal antibodies and electron microscopy. Biochem J. 1991 Feb 15;274(Pt 1):293–296. doi: 10.1042/bj2740293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan J. K., Carlstedt I. Electron microscopy of cervical-mucus glycoproteins and fragments therefrom. The use of colloidal gold to make visible 'naked' protein regions. Biochem J. 1990 Jan 1;265(1):169–177. doi: 10.1042/bj2650169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan J. K., Carlstedt I. Hydrodynamic properties of human cervical-mucus glycoproteins in 6M-guanidinium chloride. Biochem J. 1984 Jan 1;217(1):93–101. doi: 10.1042/bj2170093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan J. K., Carlstedt I. Size heterogeneity of human cervical mucus glycoproteins. Studies performed with rate-zonal centrifugation and laser light-scattering. Biochem J. 1987 Aug 1;245(3):757–762. doi: 10.1042/bj2450757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan J. K., Oates K., Carlstedt I. Electron microscopy of cervical, gastric and bronchial mucus glycoproteins. Biochem J. 1986 Oct 1;239(1):147–153. doi: 10.1042/bj2390147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sleigh M. A., Blake J. R., Liron N. The propulsion of mucus by cilia. Am Rev Respir Dis. 1988 Mar;137(3):726–741. doi: 10.1164/ajrccm/137.3.726. [DOI] [PubMed] [Google Scholar]
- Spicer S. S., Schulte B. A., Chakrin L. W. Ultrastructural and histochemical observations of respiratory epithelium and gland. Exp Lung Res. 1983 Feb;4(2):137–156. doi: 10.3109/01902148309055010. [DOI] [PubMed] [Google Scholar]
- Suter S., Schaad U. B., Roux L., Nydegger U. E., Waldvogel F. A. Granulocyte neutral proteases and Pseudomonas elastase as possible causes of airway damage in patients with cystic fibrosis. J Infect Dis. 1984 Apr;149(4):523–531. doi: 10.1093/infdis/149.4.523. [DOI] [PubMed] [Google Scholar]
- Thornton D. J., Davies J. R., Kraayenbrink M., Richardson P. S., Sheehan J. K., Carlstedt I. Mucus glycoproteins from 'normal' human tracheobronchial secretion. Biochem J. 1990 Jan 1;265(1):179–186. doi: 10.1042/bj2650179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton D. J., Sheehan J. K., Carlstedt I. Heterogeneity of mucus glycoproteins from cystic fibrotic sputum. Are there different families of mucins? Biochem J. 1991 Jun 15;276(Pt 3):677–682. doi: 10.1042/bj2760677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward H., Horsey B., Bhavanandan V. P., Davidson E. A. Isolation, purification, and properties of respiratory mucus glycoproteins. Biochemistry. 1982 Feb 16;21(4):694–701. doi: 10.1021/bi00533a017. [DOI] [PubMed] [Google Scholar]



