Abstract
T-20 is a synthetic peptide that corresponds to 36 amino acids within the C-terminal heptad repeat region (HR2) of human immunodeficiency virus type 1 (HIV-1) gp41. T-20 has been shown to potently inhibit viral replication of HIV-1 both in vitro and in vivo and is currently being evaluated in a Phase III clinical trial. T-649 is an inhibitory peptide that also corresponds to 36 amino acids within HR2. This sequence overlaps the T-20 sequence but is shifted 10 residues toward the N terminus of gp41. Both inhibitors are thought to exert their antiviral activity by interfering with the conformational changes that occur within gp41 to promote membrane fusion following gp120 interactions with CD4 and coreceptor molecules. We have shown previously that coreceptor specificity defined by the V3 loop of gp120 modulates sensitivity to T-20 and that a critical region within the N-terminal heptad repeat (HR1) of gp41 is the major determinant of sensitivity (C. A. Derdeyn et al., J. Virol. 74:8358–8367, 2000). This report shows that (i) regions within gp41 distinct from those associated with T-20 sensitivity govern the baseline sensitivity to T-649 and (ii) T-649 sensitivity of chimeric viruses that contain sequences derived from CXCR4- and CCR5-specific envelopes is also modulated by coreceptor specificity. Moreover, the pattern of sensitivity of CCR5-specific chimeras with only minor differences in their V3 loop was consistent for both inhibitors, suggesting that the individual affinity for coreceptor may influence accessibility of these inhibitors to their target sequence. Finally, an analysis of the sensitivity of 55 primary, inhibitor-naive HIV-1 isolates found that higher concentrations of T-20 (P < 0.001) and T-649 (P = 0.016) were required to inhibit CCR5-specific viruses compared to viruses that utilize CXCR4. The results presented here implicate gp120-coreceptor interactions in driving the complex conformational changes that occur in gp41 to promote fusion and entry and suggest that sensitivity to different HR1-directed fusion inhibitors is governed by distinct regions of gp41 but is consistently modulated by coreceptor specificity.
The surface glycoprotein of human immunodeficiency virus type 1 (HIV-1), gp120, mediates interactions with receptor molecules expressed on the target cell surface as one of the earliest events leading to entry into the host cell (20). All naturally occurring isolates of HIV-1 require, in addition to the CD4 molecule, interaction with a chemokine receptor, usually CCR5 or CXCR4, and the specificity of this interaction is determined by the third variable (V3) loop of gp120 (2, 6, 14–16, 25, 28, 30, 32, 33, 35, 39). Viruses that interact with CXCR4 or CXCR4 and CCR5 display phenotypes distinct from those viruses that interact only with CCR5 in vitro and in vivo (reviewed in reference 1). Interactions between gp120 and CD4 together with coreceptor molecules trigger conformational changes within the HIV-1 transmembrane protein, gp41, that promote fusion between the viral and cellular membranes (4). It has been postulated that two heptad repeat regions (HR1 and HR2; Fig. 1) located within gp41 associate to form a coiled-coil bundle that brings the bridged viral and cellular membranes together (4, 11, 21, 31, 34, 36). Short synthetic peptides that target sequences within these heptad repeats have been used successfully to inhibit viral entry and cell-to-cell fusion in vitro (8, 23, 27, 37, 38) and viral replication in vivo (18).
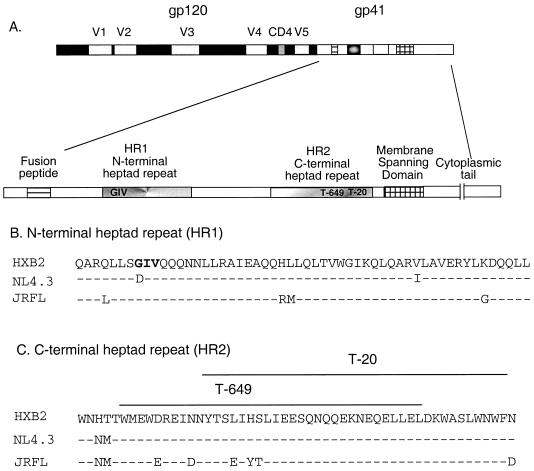
FIG. 1.
The HIV-1 env gene. (A) The HIV-1 env gene produces surface (gp120) and transmembrane (gp41) glycoprotein subunits from a precursor molecule through posttranslational proteolytic cleavage. The five variable regions of gp120 and a conserved CD4 binding site are indicated. The enlarged inset depicts the major structural domains of gp41 (not to scale). Amino acid alignments of the N-terminal (B) and C-terminal (C) heptad repeat regions are shown for HXB2 (used as the reference sequence), NL4.3, and JRFL. Residues that are identical to the HXB2 sequence are indicated by dashes, and substitutions are indicated by the amino acid letter. (B) The T-20 interaction site in HR1, residues 36 to 38, is shown in bold. (C) The corresponding positions of T-20 and T-649 in HR2 are indicated above the alignments. Both peptides are identical to the HXB2 sequence.
T-20 is a synthetic peptide that corresponds to a linear 36-amino-acid sequence within HR2 of gp41 (27). T-20 potently inhibits entry of HIV-1 into host cells, most likely by interfering with the intramolecular interactions of gp41 that define its fusion-active state (12, 19, 38) and is currently being evaluated in a Phase III clinical trial. T-649 is also a synthetic 36-mer that corresponds to a linear sequence within HR2 and shares its C terminus with the N-terminal region of T-20 (Fig. 1C) (27). The mechanism of action of both inhibitors is proposed to be an interaction with a target sequence within HR1 that inhibits association with the native HR2 and prevents apposition of the viral and cellular membranes (5, 18). However, these overlapping peptides are believed to target distinct regions within HR1, evidenced by the fact that T-20 resistant viruses remain fully sensitive to T-649 (27). A contiguous three-amino-acid sequence within HR1 (GIV at positions 36 to 38) is associated with sensitivity to T-20 and most likely defines an important interaction site (8, 27). Rimsky et al. suggested that T-649-resistant viruses generated in vitro contained mutations in regions C terminal to the GIV motif, indicating that although these peptides overlap, they target distinct sites within HR1.
We have previously reported that although the baseline sensitivity of a virus to T-20 is defined by the GIV motif, coreceptor specificity defined by the V3 loop of gp120 modulates this sensitivity (8). In this study, we sought to confirm our hypothesis that differences in gp120-coreceptor interactions drive the accessibility of HR1 to peptide inhibitors by analyzing the effect of coreceptor specificity on sensitivity to T-649. In addition, we investigated whether the GIV motif influences sensitivity to T-649 in a fashion similar to that observed for T-20. The results support evidence that gp120 interactions with CCR5 are more vigorous than interactions with CXCR4 and suggest that coreceptor influence on HR1-targeted peptide inhibitors is a general phenomenon. Furthermore, fusion inhibitors, even when they contain overlapping sequences, target distinct regions of HR1, suggesting that combination peptide therapy could reduce the likelihood of viral resistance developing.
MATERIALS AND METHODS
Primary HIV-1 isolates.
Primary isolates of HIV-1 were obtained from infected patients with Institutional Review Board-approved informed consent at the University of Alabama at Birmingham 1917 Clinic. Peripheral blood mononuclear cells (PBMC) were isolated from whole blood by Ficoll-Hypaque (Pharmacia, Piscataway, N.J.) gradient centrifugation and then cocultured with normal donor PBMC that had been cultured for 1 to 3 days in the presence of 3 μg of phytohemagglutinin (PHA)/ml to activate T cells. Cultures were maintained in RPMI supplemented with 15% fetal bovine serum (Hyclone, Logan, Utah), 2 mM glutamine, 1× nonessential amino acids, 1× penicillin-streptomycin, and 20 U of recombinant human interleukin-2 (IL-2) (Roche, Indianapolis, Ind.)/ml for 7 days. At day 7, one-half of the media was removed and replaced with IL-2-supplemented media containing fresh PHA-activated blasts. Production of virus was monitored by enzyme-linked immunosorbent assay (Coulter, Miami, Fla.) to detect HIV-1 p24 in the culture supernatant. At day 14, the virus-containing supernatant was collected, clarified by low-speed centrifugation, and passed through a 0.45-μm-pore-size filter to produce cell-free virus stocks. Stocks were stored in 1-ml aliquots at −70°C.
Determination of SI and NSI phenotypes and coreceptor specificity of primary isolates.
Primary isolates were used to infect MT-2 cells to determine their syncytium-inducing (SI) phenotype by using the AIDS Clinical Trials Group (ACTG) Virology Manual MT-2 assay (17). Briefly, 5 × 104 MT-2 cells were plated into 96-well plates and 50 μl of cell-free virus stock was added. Cultures were monitored for the presence of syncytia at days 3, 6, 9, 12, and 14 and scored positive (SI) if syncytia were present or negative (non-syncytium inducing [NSI]) if no syncytia were observed during the 14-day period. All virus stocks were also tested for infectivity in PBMC. Coreceptor specificity was determined using GHOST4 HIV indicator cells expressing CCR5, CXCR4, or no coreceptor (7). Briefly, 2 × 104 cells were plated into 12-well plates in Dulbecco modified Eagle medium (DMEM) containing the selective antibiotics puromycin (except the parental line), hygromycin, and G418. The next day, the cells were infected with 0.3 ml of cell-free virus stock for 2 h at 37°C and the culture volume was adjusted to 1 ml with complete DMEM with antibiotics. Coreceptor utilization was indicated by detection of long terminal repeat-driven green fluorescence protein by flow cytometry at 48 or 72 h postinfection over background fluorescence in the coreceptor-negative parental cells.
Phenotypic sensitivity assays.
JC53-BL HIV-1 indicator cells (kindly provided by Tranzyme Inc., Birmingham, Ala.) are a derivative of HeLa cells that express high levels of CD4 and the HIV-1 coreceptors CCR5 and CXCR4 and have been described previously (8). The infectious titer of all virus stocks was determined on JC53-BL cells by direct counting of blue foci prior to analysis (data not shown). For each set of analyses, JC53-BL cells were infected with equivalent amounts of infectious units for each virus as described previously except that the infections were scaled down to a 96-well format and were set up in quadruplicate (8). Infections were performed and maintained in the absence of (control) or in the presence of 0.008, 0.04, 0.2, 1, and 5 μg of T-20 and T-649/ml in parallel. Luciferase activity was measured 48 h after infection as described previously (8). The 50% inhibitory concentration (IC50) was calculated for each virus from the infectivity data by using the predicted exponential growth function in Microsoft Excel. Each quadruplicate infection was repeated independently between two and eight times. Mean IC50s (± standard deviation [SD]) were calculated for each virus by using all replicates and were used for comparison. The Wilcoxon rank sum test was applied to the replicate IC50s to determine whether the differences between pairs of chimeras were significantly different for both inhibitors. The Wilcoxon rank sum test and the t test for independent samples were applied to comparisons of CCR5 and CXCR4 primary viruses to determine whether their IC50s (and logarithmic IC50s) were significantly different for both T-20 and T-649.
For infections performed with PBMC, the titer of each virus stock was individually determined on a wild-type CCR5 donor by measuring p24 production at day 7 in wells containing serial dilutions of virus and calculating the 50% tissue culture infective dose (TCID50) by using the Spearman-Karber formula. PBMC from the same donor were used for the inhibition assay, in which 1,000 TCID50s of each virus stock was used to infect 106 PHA-activated PBMC in the presence or absence of 0.0016, 0.008, 0.04, 0.2, and 1 μg of inhibitor/ml by using a modification of the ACTG Virology Manual HIV Drug Susceptibility Assay. Supernatant p24 was measured at day 7 postinfection for each virus and used to calculate virus infectivity and IC50s.
HIV-1 envelope chimeras.
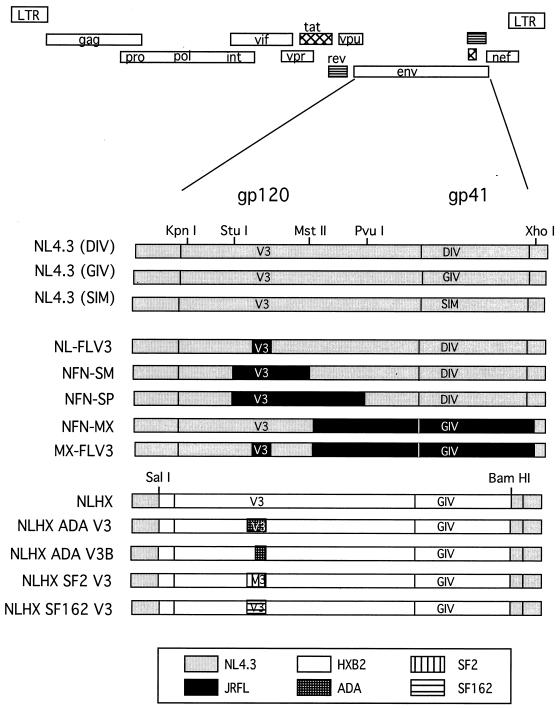
Plasmids containing envelope sequences derived from HIV-1 strains NL4.3 or JRFL in an NL4.3 proviral background have been previously described (24–26). Plasmids containing gp120 V3 loops from HIV-1 strains ADA, SF162, and SF2 in an NLHX (NL4.3 containing the HXB2 envelope) proviral background have been previously described (15). Restriction sites used for subcloning are shown in Fig. 2, and their nucleotide positions are numbered according to NL4.3 numbering and are as follows: SalI, 5785; KpnI, 6343; StuI, 6822; MstII, 7305; PvuI, 7655; BamHI, 8474; and XhoI, 8887. The envelope coding region begins at position 6224, the V3 loop is at positions 7103 to 7267, the gp120-gp41 cleavage site is at 7747, and the coding region ends at 8785. Infectious viral stocks representing each proviral construct were prepared as previously described (8). The proviral clones NL4.3 (GIV) and NL4.3 (SIM) were kind gifts from Trimeris, Inc., Durham, N.C.
FIG. 2.
Chimeric proviruses containing substitutions in env. All proviruses were created in an NL4.3 proviral background. Constructs designated NLHX contain HXB2-derived env sequences. V3, the gp120 V3 loop; GIV, DIV, or SIM, the residues comprising the critical T-20 interaction site. Restriction sites used for construction are shown. Shown are NL4.3 sequences, HXB2 sequences, JRFL sequences, and sequences from CCR5-specific V3 loop donors ADA, SF2, and SF162.
RESULTS
Substitution of multiple CCR5 V3 loops into a CXCR4 envelope background decreases sensitivity to fusion inhibitors.
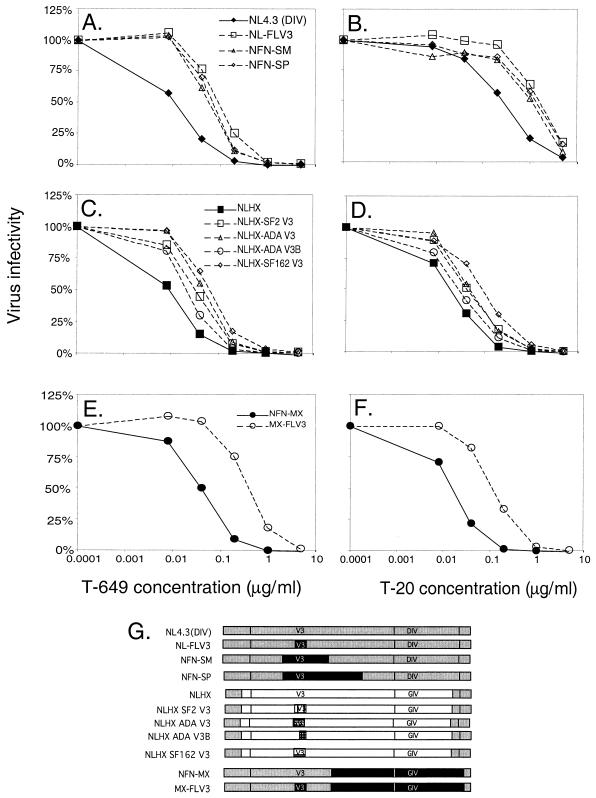
To carefully analyze the contribution of coreceptor specificity to inhibition by peptide inhibitors, we employed different panels of well-characterized proviruses containing chimeric envelope genes with different CCR5 V3 loops substituted into an CXCR4 envelope gene background (Fig. 3G) (15, 24–26). The first set of chimeric proviruses analyzed was constructed in an NL4.3 proviral background and contains envelope sequences derived from either NL4.3, which is CXCR4 specific, or JRFL, which is CCR5 specific. To determine whether coreceptor specificity modulates sensitivity in a similar manner to both T-649 and T-20, parallel infections were performed in which viruses were exposed to increasing amounts of each inhibitor. Figures 3A and B show that the parent virus, NL4.3 (DIV), is more sensitive to inhibition by T-649 and T-20 than the three viruses containing the JRFL-derived V3 loop. The IC50 of T-649 for NL4.3 (DIV) was 0.01 μg/ml (Fig. 3A). Substitution of the JRFL V3 loop (NL-FLV3) or the V3 loop plus flanking sequences (NFN-SM, NFN-SP) into NL4.3 (DIV) resulted in decreased sensitivity to T-649, ranging from 0.03 to 0.06 μg/ml (mean [± SD] of 0.04 ± 0.01 μg/ml, a difference of 0.6 log10) (Fig. 3A). The difference between NL4.3 and NL-FLV3 was statistically significant (P = 0.007). Comparisons of NL4.3 with NFN-SM and NFN-SP did not reach statistical significance, despite the fact that their IC50s were 0.4 log10 and 0.6 log10 higher, respectively, than that of NL4.3. It is likely that the small number of analyses that were performed with NFN-SM and NFN-SP (two independent experiments with quadruplicate wells) and the relatively small differences between IC50s (less than 1.0 log10) make reaching significance difficult, even though a clear and consistent difference was observed. Similar decreases in sensitivity associated with CCR5 specificity were observed for T-20 (Fig. 3B). The IC50 increased from 0.15 μg/ml to 0.38 to 0.84 μg/ml (mean [± SD] of 0.66 ± 0.24 μg/ml, a difference of 0.6 log10) when the JRFL V3 loop or the V3 loop plus flanking sequences was present (Fig. 3B), consistent with our previous observations. Interestingly, the pattern of sensitivity of the different CCR5 V3 loop constructs was consistent between the two inhibitors: NL-FLV3 was the least sensitive to inhibition, followed by NFN-SP and then NFN-SM (compare Fig. 3A and B). This finding suggests that a deliberate relationship exists between the characteristics of each individual Env structure and sensitivity to these inhibitors.
FIG. 3.
Effect of coreceptor utilization on sensitivity to fusion inhibitors. JC53-BL indicator cells were infected with each chimeric virus in the presence of increasing concentrations of T-649 (left) or T-20 (right). Inhibitor concentrations are plotted along the horizontal axes. Luciferase activities in the infected cell lysates were measured at 48 h postinfection and were used to calculate virus infectivity relative to that of the control (vertical axes) and IC50s for each inhibitor. (A and B) Comparison of the infectivity of NL4.3 (DIV) and the JRFL-derived CCR5 V3 loop chimeras in the presence of T-649 and T-20, respectively. (C and D) Comparison of the infectivity of NLHX and the four CCR5-specific V3 loop chimeras in the presence of T-649 and T-20, respectively. (E and F) Comparison of the infectivity of two additional NL4.3-derived viruses that differed only in substitution of JRFL V3 loop in the presence of T-649 and T-20, respectively. (G) Chimeric constructs that correspond to the infectivity graphs.
To further validate the modulatory effect of coreceptor specificity on sensitivity to both peptide inhibitors, we analyzed a different set of chimeras (15). These viruses were constructed in an NL4.3 proviral background but contained the HXB2 envelope gene with or without CCR5 V3 loop substitutions (Fig. 3G). The IC50 of T-649 for the parent virus NLHX, whose coreceptor specificity is CXCR4, was determined to be 0.01 μg/ml (Fig. 3C). Substitution of all four CCR5-specific V3 loops resulted in a decrease in sensitivity to T-649, ranging from 0.02 to 0.05 μg/ml (mean [± SD] of 0.03 ± 0.01 μg/ml, an increase of 0.6 log10) (Fig. 3C). The difference between NLHX and three of the four V3 loop chimeras were statistically significant, with a P value of 0.05 for NLHX SF162 V3, a P value of 0.03 for NLHX SF2 V3, and a P value of 0.05 for NLHX ADA V3. NLHX ADA V3B was not significantly different from NLHX, which is consistent with the fact that this virus has acquired the ability to use CCR5 but derives the N-terminal half of its V3 loop from the CXCR4-specific virus HXB2. The IC50 of T-20 for NLHX was also 0.01 μg/ml (Fig. 3D). Once again, substitution of all four CCR5 V3 loops resulted in a decreased sensitivity to the inhibitor, with IC50s ranging from 0.02 to 0.06 μg/ml (mean [± SD] of 0.04 ± 0.01 μg/ml, an increase of 0.5 log10) (Fig. 3D). Again, NLHX SF162 V3, NLHX SF2 V3, and NLHX ADA V3 were significantly different from NLHX (P values of 0.05, 0.03, and 0.05, respectively) but the difference of NLHX versus NLHX ADA V3B was not. Consistent with the first set of chimeras, the pattern of sensitivity for the four CCR5 V3 loop chimeras was the same for both inhibitors (compare Fig. 3C and D). NLHX SF162 was the least sensitive to inhibition, followed by NLHX ADA and NLHX SF2, which displayed moderate and similar levels of sensitivity. ADA V3B (which contains only the C-terminal half of the ADA V3 loop) was the most sensitive to inhibition by both T-649 and T-20, suggesting a correlation between the structure of each CCR5 V3 loop and level of sensitivity.
Finally, we analyzed a third pair of chimeric viruses that differ only in their V3 loop. NFN-MX contains the C-terminal portion of gp120 and most of gp41 derived from JRFL and its V3 loop is derived from NL4.3, conferring CXCR4 specificity (Fig. 3G). MX-FLV3 is an identical virus except that the NL4.3-derived V3 loop has been replaced by the JRFL-derived CCR5-specific V3 loop (Fig. 3G). The IC50 of T-649 for NFN-MX was 0.03 μg/ml, but substitution with the CCR5 V3 loop again resulted in a decreased sensitivity of 0.7 log10 to 0.15 μg/ml (Fig. 3E) (P = 0.01). When exposed to T-20, these viruses displayed similar phenotypes (Fig. 3F). The IC50 of T-20 for NFN-MX was 0.01 μg/ml, which increased to 0.07 μg/ml for MX-FLV3, a difference of 0.7 log10 (P = 0.01). Using three independent sets of CCR5 V3 loop-substituted envelope chimeras, remarkable consistency in the influence of coreceptor specificity was observed for T-649 and T-20, suggesting similar modes of action for these two inhibitors and a similar mechanism for modulation by coreceptor utilization.
Modulation by coreceptor specificity in primary cells.
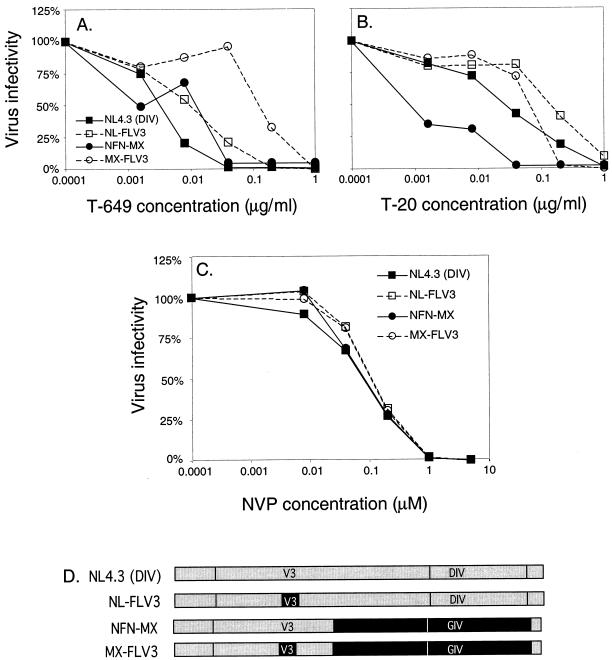
To confirm that our results were not confined to use of the JC53-BL cells, we chose two pairs of isogenic CXCR4-CCR5 viruses [NL4.3 (DIV) and NL-FLV3, NFN-MX and MX-FLV3] to analyze in PBMC. PBMC were infected in the presence of increasing doses of each inhibitor, and the production of p24 in each well was measured 7 days after infection (Fig. 4A and B). The IC50 of T-649 for NL4.3 (DIV) in PBMC was 0.004 μg/ml, compared to 0.008 μg/ml for NL-FLV3 (a difference of 0.3 log10) (Fig. 4A). Similarly, the IC50s of T-20 for these two viruses were 0.02 and 0.06 μg/ml, respectively, representing an increase of 0.5 log10 (Fig. 4B). For NFN-MX, the IC50 of T-649 was 0.006 μg/ml, compared to 0.04 μg/ml for MX-FLV3, representing a change of 0.8 log10 (Fig. 4A). Finally, the IC50 of T-20 for NFN-MX increased about 1.0 log10 from 0.003 to 0.03 μg/ml with substitution of the JRFL V3 loop (Fig. 4B). Although the effects of coreceptor specificity were more variable in this system, which requires multiple rounds of infection and is more difficult to quantitate than luciferase activity in infected JC53-BL cells, a consistent decrease in sensitivity to both T-649 and T-20 was observed in primary cells with CCR5 viruses compared to the parent CXCR4 viruses, suggesting that this phenomenon is not specific to the JC53-BL indicator cells.
FIG. 4.
Effect of coreceptor utilization in primary cells and in the presence of nevirapine. PBMC were infected with two isogenic CXCR4-CCR5 pairs of chimeric viruses in the presence of increasing concentrations of T-649 (A) or T-20 (B), which are plotted along the horizontal axes. Production of HIV-1 p24 in the supernatant was measured 7 days later and was used to calculate relative virus infectivity (vertical axes) and IC50s for T-649 and T-20. (C) JC53-BL cells were infected with the same virus pairs in the presence of increasing concentrations of the nonnucleoside reverse transcriptase inhibitor nevirapine (horizontal axis), which targets the pol gene. Luciferase activity was measured 48 h later and was used to calculate virus infectivity (plotted along the vertical axes). (D) Pairs of chimeric constructs shown in the infectivity graphs.
Effect of coreceptor specificity on sensitivity to the reverse transcriptase inhibitor nevirapine.
To further validate that our results were specific for entry inhibitors that target the intramolecular interactions of gp41 and were not observed with other types of antiviral agents, we analyzed the sensitivity of the two pairs of viruses described above to the nonnucleoside reverse transcriptase inhibitor nevirapine (NVP) by using JC53-BL cells. Because all of the proviruses used in our analysis contained identical pol genes derived from NL4.3, different phenotypes based on coreceptor specificity would not be expected. Figure 4C shows that all four viruses displayed comparable sensitivities to NVP regardless of their coreceptor specificity or sequence variation within HR1. The IC50s ranged from 0.05 to 0.07 μM, and the mean IC50 (± SD) was 0.06 ± 0.01 μM (the NL4.3 mean IC50 was 0.05 μM, the NL-FLV3 mean IC50 was 0.07 μM, the NFN-MX mean IC50 was 0.06 μM, and the MX-FLV3 mean IC50 was 0.07 μM). These results support that the observed modulatory effects of coreceptor specificity are specific for inhibitors that target the heptad repeat regions of gp41, such as T-649 and T-20.
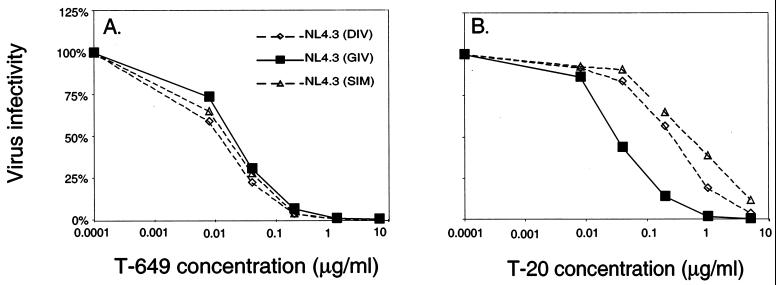
Role of the GIV motif in sensitivity to T-649.
Since our group and others have shown that the GIV sequence in HR1 is a critical interaction site for T-20, we investigated whether differences in this site affect the sensitivity of viruses to T-649. To do this, we analyzed the sensitivities of three NL4.3 proviruses that differ only in residues 36 to 38 of gp41 HR1. NL4.3 (DIV) contains the wild-type sequence, NL4.3 (SIM) was derived from a T-20-resistant virus, and NL4.3 (GIV) contains the more prevalent sequence that confers increased sensitivity to T-20. Substitutions in the GIV motif did not affect the level of sensitivity to T-649 (Fig. 5A). The IC50s of T-649 were similar for all three NL4.3-based viruses: 0.01 μg/ml for NL4.3 (SIM), 0.01 μg/ml for NL4.3 (DIV), and 0.02 μg/ml for NL4.3 (GIV) (Fig. 5A). In contrast, as shown in Fig. 5B, the baseline sensitivity to T-20 was drastically altered by substitutions in this motif. The IC50 of T-20 for NL4.3 (GIV) was 0.03 μg/ml, compared to 0.15 μg/ml when G was replaced by D (an increase of about 0.7 log10). When both G→S and V→M substitutions were made, the T-20 IC50 increased further to 0.29 μg/ml, a difference of about 1.0 log10 (Fig. 5B). It is important to note that the T-649 IC50 of NL4.3 (DIV) and NL4.3 (SIM) were 1.1 log10 and 1.5 log10 lower, respectively, than the T-20 IC50 for each virus, while the IC50s for NL4.3 (GIV) were almost identical (0.02 μg/ml for T-649 and 0.03 μg/ml for T-20) (compare Fig. 5A and B). Taken together, these results confirm structural predictions that T-649 targets a distinct site within HR1 and that alterations in the GIV motif can lead to resistance to T-20 but not T-649.
FIG. 5.
Effect of changes within the GIV motif on inhibition by T-649 and T-20. JC53-BL cells were infected with each chimeric virus in the presence of increasing concentrations of T-649 (A) or T-20 (B), which are plotted along the horizontal axes. Luciferase activity was measured at 48 h postinfection and was used to calculate the virus infectivity relative to the control, which is plotted along the vertical axes. Shown are the infectivities of NL4.3-based isogenic viruses containing DIV, GIV, or SIM in the T-20 interaction site (residues 36 to 38) in the presence of each inhibitor.
Primary CXCR4 SI isolates are more sensitive to inhibition by T-649 and T-20 than CCR5 NSI isolates.
Previously, we showed that primary CXCR4 SI isolates were more sensitive to inhibition by T-20 than were CCR5 NSI isolates by using a panel of 14 inhibitor-naive isolates for which coreceptor specificity and MT-2 phenotype had been determined (8). In the present study, we performed parallel infections in the presence of increasing doses of T-649 and T-20 to determine whether the influence of coreceptor specificity was (i) reproducible in a larger, more diverse panel of virus isolates, (ii) generalizable to fusion inhibitors other than T-20, and (iii) consistent with the results obtained using the chimeric viruses. Each of the 55 primary isolates analyzed here was first characterized as CXCR4 SI or CCR5 NSI by its ability to use the coreceptors CCR5 or CXCR4 on GHOST indicator cells and induce syncytia in cultures of MT-2 cells (data not shown). For all isolates, the ability to use CXCR4 was correlated with an SI phenotype in MT-2 cultures, whereas CCR5 specificity correlated with an NSI phenotype. Thus, for this analysis, dualtropic viruses (those that use CXCR4 and CCR5) were characterized as CXCR4 SI viruses and compared against the CCR5 NSI viruses.
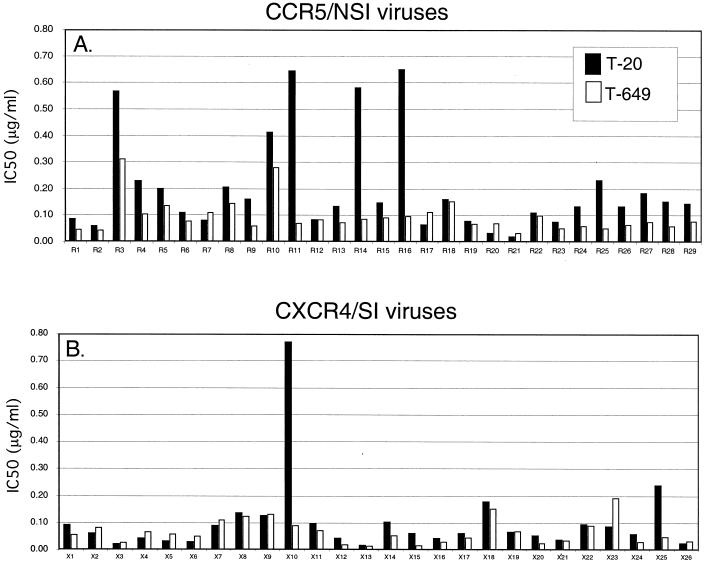
For each isolate, IC50s were calculated from infectivity curves and used for comparisons (Fig. 6). In general, the 55 isolates showed greater sensitivity to inhibition by T-649 than T-20. The overall mean (± SD) IC50 for T-20 was 0.16 ± 0.17 μg/ml and that for T-649 was 0.08 ± 0.06 μg/ml, a difference of about 0.3 log10. The range of IC50s for inhibition by T-20 was 0.02 to 0.77 μg/ml (1.7 log10) while the range for T-649 was 0.02 to 0.31 μg/ml (1.2 log10).
FIG. 6.
Sensitivity of CCR5 NSI and CXCR4 SI primary isolates to fusion inhibitors. JC53-BL cells were infected with 29 CCR5 NSI (A) and 26 CXCR4 SI (B) patient isolates in the presence of increasing doses of T-20 or T-649. Luciferase activity was measured at 48 h postinfection, and the relative infectivities for each virus were used to calculate the IC50s for T-20 and T-649 (vertical axis). The mean (±SD) IC50 for T-20 was 0.20 ± 0.18 μg/ml for CCR5 viruses and 0.10 ± 0.15 μg/ml for CXCR4 viruses. The mean (±SD) IC50 for T-649 was 0.10 ± 0.06 μg/ml for CCR5 viruses and 0.07 ± 0.05 μg/ml for CXCR4 viruses.
When isolates were grouped by coreceptor specificity and compared, higher concentrations of both peptides were required to inhibit CCR5 viruses than to inhibit CXCR4 viruses. Using the t test for independent samples, CCR5 viruses were significantly different from CXCR4 viruses for both T-20 (P = 0.03) and T-649 (P = 0.05) IC50s. When the Wilcoxon rank sum test was performed, there was also a significant difference between CCR5 viruses and CXCR4 viruses with respect to T-20 (P < 0.001) and T-649 (P = 0.016). When the t test was performed on the logarithmic values of the IC50s, the CCR5 viruses were again significantly different from the CXCR4 viruses for T-20 (P = 0.001) and T-649 (P = 0.009). The Wilcoxon rank sum test performed on the logarithmic IC50s yielded the same results as described above for the actual values. Although the differences between the sensitivity of CCR5 viruses and CXCR4 viruses reported here are less than those we described previously for T-20, they were found to be statistically significant for both T-20 and T-649 by a variety of methods. These results, combined with those obtained using the chimeric virus panels, suggest that coreceptor specificity consistently modulates the baseline sensitivity to fusion inhibitors and that this effect is inevitably less pronounced when comparing a large panel of patient-derived isolates than when comparing isogenic V3 loop chimeras.
DISCUSSION
In this study, we investigated the effects of coreceptor specificity on sensitivity to two fusion inhibitors that target the intramolecular interactions within gp41 that promote membrane fusion. We showed that the sensitivity of patient-derived HIV-1 isolates to both T-649 and T-20 is governed by coreceptor specificity in a similar manner. A statistically significant difference was observed between the IC50s of CCR5 and CXCR4 viruses for both peptides by using two independent statistical tests, suggesting that this type of modulation is a general phenomenon of fusion inhibitors. In our original study, we compared the sensitivity of a small panel of patient-derived viruses to T-20 (eight CCR5 and six CXCR4 viruses) and found that ∼0.8-log10-higher concentrations of T-20 were required to inhibit the CCR5 viruses (8). In the present study, we analyzed a larger sampling of primary isolates and found that CCR5 viruses are about 0.3 log10 less sensitive to inhibition by T-20 than CXCR4 isolates (based on the mean IC50 for each group). That the magnitude of this difference is 0.5 log10 less is not surprising, since this larger panel of isolates represents more diverse sequences in env that may contribute to sensitivity to inhibition independently of coreceptor specificity. It is important to note that in this study, all dualtropic viruses were classified as CXCR4 SI, although it is possible that some of these viruses prefer to utilize CCR5 on JC53-BL cells. To address this possibility, studies to measure the IC50 of a subset of dualtropic viruses in the presence of CXCR4- or CCR5 specific inhibitors are currently underway. CCR5 viruses were also less sensitive to inhibition by T-649, although the mean IC50 was only 0.2-log10 higher than the CXCR4 viruses. A specific region in HR1 that determines baseline sensitivity to T-649 has not yet been identified, so it is difficult to assess the influence of other regions of gp41 on sensitivity to this inhibitor. Interestingly, T-649 was generally more potent than T-20 against both CCR5 and CXCR4 viruses, although the basis for this observation is not immediately clear.
Since two of the CCR5 viruses, R3 and R10, required relatively high levels of both T-20 and T-649 while three CCR5 viruses (R11, R14, and R16) required high levels of T-20 but low levels of T-649 to achieve inhibition (Fig. 6A), we postulated that different mechanisms of resistance could be operative in viruses that share specificity for CCR5. Viruses that require higher levels of both inhibitors could possess an increased affinity for CCR5 (compared to the other CCR5 viruses) that could contribute to their decreased sensitivity to both T-20 and T-649. On the other hand, viruses that require much higher concentrations of T-20 than T-649 resemble the viruses containing substitutions in the GIV sequence of HR1 characterized here, suggesting that similar mutations might exist in these natural isolates. The phenotypes of these isolates further support the concept that factors other than coreceptor specificity determine baseline sensitivity. Since a different region of gp41 is evidently targeted by T-20 and T-649, combination fusion inhibitor therapy could delay the emergence of resistant viruses in vivo, since sensitivity to one peptide does not necessarily correlate with sensitivity to the other. One CXCR4 virus, X10, required very high levels of T-20 but not T-649 for inhibition (Fig. 6B). In fact, this virus displayed the highest IC50 for T-20 (0.78 μg/ml) among all of the primary viruses analyzed. To gain a better understanding of how T-20 and T-649 inhibit natural isolates, studies to compare affinities for CCR5 and determine the sequences of HR1 and HR2 using these primary isolates are currently underway.
By extending our analyses to isogenic viruses that contain chimeric CXCR4 or CCR5 envelope genes, we confirmed that CXCR4 viruses containing CCR5 V3 loop substitutions are generally ∼0.6-log10 less sensitive to the inhibitory effects of both T-649 and T-20 than the corresponding CXCR4-specific viruses. Moreover, this effect can be directly attributed to the presence of a CCR5 V3 loop. For both T-20 and T-649, all groups of CCR5 viruses were less sensitive to inhibition compared to related CXCR4 viruses. We also confirmed the effects of the GIV motif on sensitivity to T-20, showing that the G→D substitution accounts for a ∼0.7-log10 decrease and the combined G→S and V→M substitutions account for about ∼1.0-log10 less sensitivity to T-20 independently of coreceptor specificity. In contrast, neither substitution within GIV had any appreciable effect on sensitivity to T-649, firmly supporting that T-649 targets a different site.
Studies of transdominant peptide interactions with HR1 have proven to be very informative, especially considering their clinical applicability. However, it is important to consider that the native interactions between HR1 and HR2 occurring within the context of the multiple surfaces of gp41 may differ. For example, if one inspects the N- and C-terminal heptad repeat regions derived from NL4.3 and HXB2, no compensatory substitutions are apparent in the NL4.3 HR2 that would complement the G→D substitution in the NL4.3 HR1. Likewise, T-20-resistant viruses containing substitutions within the GIV sequence lack compensatory changes within the corresponding region in HR2 (27). Moreover, the development of resistance through changes within the GIV sequence does not appear to be detrimental to viral fitness (27) and no differences in infectivity between NL4.3 (DIV), NL4.3 (GIV), and NL4.3 (SIM) were observed in the current study. Although much information has been gained on the conformational changes that occur within gp41 following gp120-receptor interactions, the mechanisms by which peptides inhibit these changes may be quite complex, involving sequences within both heptad repeats.
Previous studies support the conformational exposure of the T-20 interaction site in HR1 upon gp120 binding to CD4 (12, 19, 22). Based on these observations and our previous studies (8), we propose that differences in the affinity of the gp120-coreceptor interactions may result in the relative resistance of CCR5 viruses to inhibition compared to that of CXCR4 viruses. In support of this idea, the pattern of sensitivity of two independent sets of CCR5 V3 loop chimeras was consistent for T-20 and T-649. For example, in the NLHX-based V3 chimeras. NLHX SF162 was consistently the most resistant to inhibition, while NLHX ADA V3B was the most sensitive to inhibition. Moreover, the N-terminal half of the V3 loop in NLHX ADA V3B was derived from HXB2, while only the C-terminal half provides specificity for CCR5. The subtle difference in V3 loop structure between NLHX ADA and NLHX ADA V3B accounts for approximately a ∼0.3-log10 difference in sensitivity to both inhibitors. A possible explanation for this observation is that although all of these viruses utilize CCR5, their affinities for coreceptor differ, leading to variable sensitivity to inhibition. If NLHX SF162 binds to CCR5 with the greatest affinity, the conformational changes that are triggered within gp41 may occur more efficiently, leaving a smaller “window of opportunity” for interaction of the peptides with HR1 compared to the other V3 loop chimeras. Studies to measure the affinities of the surface glycoproteins of NLHX ADA, NLHX ADA V3B, NLHX SF2, and NLHX SF162 for CCR5 are currently underway to determine whether coreceptor affinity correlates with sensitivity to inhibition. Our observations lend support to the model in which fusion inhibitors bind to gp41 after CD4 binding but prior to coreceptor binding (12, 19, 22). This window of opportunity for peptide-gp41 interaction would then be dictated not only by the affinity of T-20 or T-649 for HR1 but also by the affinity of gp120 for its coreceptor. The higher affinity of CCR5-gp120 interactions versus CXCR4-gp120 interactions (9, 10, 13) would shorten the time that the peptide binding sites are exposed. The data would also suggest that drugs that block Env-coreceptor interactions could be more efficacious if the coreceptor binding drug induces endocytosis of the coreceptor (3, 29). Lowering surface coreceptor concentrations would effectively decrease the rate at which the Env protein could complete the conformational changes required to induce fusion. This could also be demonstrated by repeating our assay in cell lines that express lower levels of surface coreceptor. The time it takes for Env to bind coreceptor and complete the conformational change would be expected to increase if coreceptor concentrations were limiting. Thus, one would predict that cell lines that express lower concentrations of surface CCR5 would effectively increase the sensitivity of CCR5 viruses to fusion inhibitors, possibly neutralizing the differences seen between the sensitivity of CXCR4 versus CCR5 viruses. Of note, Greenberg et al. were unable to detect differences between the sensitivities of CXCR4 and CCR5 primary viruses to fusion inhibitors T-20 and T-1249 by using a cMAGI-MAGI cell-based assay (M. L. Greenberg, C. B. McDanal, S. A. Stanfield-Oakley, L. Jin, C. Tremblay, P. Sista, M. Hirsch, and T. J. Matthews, 8th Conf. Retrovir. Opportunistic Infect., abstr. 473, 2001). This discrepancy may be due to a lower level of CCR5 expression on cMAGI cells compared to the JC53-BL cells used here. The modulation of sensitivity to fusion inhibitors by coreceptor specificity appears to be a consistent phenomenon, although other regions of env, especially within gp41, clearly influence the overall sensitivity. The results presented here imply that different peptide inhibitors directed against HR1 will most likely target distinct sites, even if the peptides contain overlapping sequences, but their efficacy will be subject to influence by specific gp120-coreceptor interactions governed by the V3 loop and levels of coreceptor expressed on the target cell surface.
ACKNOWLEDGMENTS
We thank Trimeris, Inc., Durham, N.C., for providing T-20 and T-649; Tranzyme, John Kappes, and Xiaoyun Wu for providing JC53-BL cells; and Jeannette Lee and the UAB CFAR Biostatistics Core for performing statistical analysis of the data, supported by UAB Center for AIDS Research grant P30-A1-27767.
This work was supported by NIH grants R37AI33319 (E.H.), R01AI24745 (L.R.), and the Howard Hughes Medical Institute (G.M.S.). The experiments were performed in the Central Virus Core of the UAB Center for AIDS Research supported by grant P30-A1-27767.
REFERENCES
- 1.Berger E A, Murphy P M, Farber J M. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu Rev Immunol. 1999;17:657–700. doi: 10.1146/annurev.immunol.17.1.657. [DOI] [PubMed] [Google Scholar]
- 2.Bieniasz P D, Fridell R A, Aramori I, Ferguson S S, Caron M G, Cullen B R. HIV-1-induced cell fusion is mediated by multiple regions within both the viral envelope and the CCR-5 co-receptor. EMBO J. 1997;16:2599–2609. doi: 10.1093/emboj/16.10.2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanpain C, Migeotte I, Lee B, Vakili J, Doranz B J, Govaerts C, Vassart G, Doms R W, Parmentier M. CCR5 binds multiple CC-chemokines: MCP-3 acts as a natural antagonist. Blood. 1999;94:1899–1905. [PubMed] [Google Scholar]
- 4.Chan D C, Fass D, Berger J M, Kim P S. Core structure of gp41 from the HIV envelope glycoprotein. Cell. 1997;89:263–273. doi: 10.1016/s0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 5.Chan D C, Kim P S. HIV entry and its inhibition. Cell. 1998;93:681–684. doi: 10.1016/s0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 6.Cocchi F, DeVico A L, Garzino-Demo A, Cara A, Gallo R C, Lusso P. The V3 domain of the HIV-1 gp120 envelope glycoprotein is critical for chemokine-mediated blockade of infection. Nat Med. 1996;2:1244–1247. doi: 10.1038/nm1196-1244. [DOI] [PubMed] [Google Scholar]
- 7.Deng H K, Unutmaz D, KewalRamani V N, Littman D R. Expression cloning of new receptors used by simian and human immunodeficiency viruses. Nature. 1997;388:296–300. doi: 10.1038/40894. [DOI] [PubMed] [Google Scholar]
- 8.Derdeyn C A, Decker J M, Sfakianos J N, Wu X, O'Brien W A, Ratner L, Kappes J C, Shaw G M, Hunter E. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J Virol. 2000;74:8358–8367. doi: 10.1128/jvi.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doranz B J, Baik S S, Doms R W. Use of a gp120 binding assay To dissect the requirements and kinetics of human immunodeficiency virus fusion events. J Virol. 1999;73:10346–10358. doi: 10.1128/jvi.73.12.10346-10358.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doranz B J, Orsini M J, Turner J D, Hoffman T L, Berson J F, Hoxie J A, Peiper S C, Brass L F, Doms R W. Identification of CXCR4 domains that support coreceptor and chemokine receptor functions. J Virol. 1999;73:2752–2761. doi: 10.1128/jvi.73.4.2752-2761.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubay J W, Roberts S J, Brody B, Hunter E. Mutations in the leucine zipper of the human immunodeficiency virus type 1 transmembrane glycoprotein affect fusion and infectivity. J Virol. 1992;66:4748–4756. doi: 10.1128/jvi.66.8.4748-4756.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furuta R A, Wild C T, Weng Y, Weiss C D. Capture of an early fusion-active conformation of HIV-1 gp41. Nat Struct Biol. 1998;5:276–279. doi: 10.1038/nsb0498-276. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman T L, Canziani G, Jia L, Rucker J, Doms R W. A biosensor assay for studying ligand-membrane receptor interactions: binding of antibodies and HIV-1 env to chemokine receptors. Proc Natl Acad Sci USA. 2000;97:11215–11220. doi: 10.1073/pnas.190274097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffman T L, Doms R W. HIV-1 envelope determinants for cell tropism and chemokine receptor use. Mol Membr Biol. 1999;16:57–65. doi: 10.1080/096876899294760. [DOI] [PubMed] [Google Scholar]
- 15.Hung C S, Vander Heyden N, Ratner L. Analysis of the critical domain in the V3 loop of human immunodeficiency virus type 1 gp120 involved in CCR5 utilization. J Virol. 1999;73:8216–8226. doi: 10.1128/jvi.73.10.8216-8226.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hwang S S, Boyle T J, Lyerly H K, Cullen B R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991;253:71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- 17.Japour A J, Fiscus S A, Arduino J M, Mayers D L, Reichelderfer P S, Kuritzkes D R. Standardized microtiter assay for determination of syncytium-inducing phenotypes of clinical human immunodeficiency virus type 1 isolates. J Clin Microbiol. 1994;32:2291–2294. doi: 10.1128/jcm.32.9.2291-2294.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kilby J M, Hopkins S, Venetta T M, DiMassimo B, Cloud G A, Lee J Y, Alldredge L, Hunter E, Lambert D, Bolognesi D, Matthews T, Johnson M R, Nowak M A, Shaw G M, Saag M S. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat Med. 1998;4:1302–1307. doi: 10.1038/3293. [DOI] [PubMed] [Google Scholar]
- 19.Kliger Y, Shai Y. Inhibition of HIV-1 entry before gp41 folds into its fusion-active conformation. J Mol Biol. 2000;295:163–168. doi: 10.1006/jmbi.1999.3368. [DOI] [PubMed] [Google Scholar]
- 20.Kwong P D, Wyatt R, Robinson J, Sweet R W, Sodroski J, Hendrickson W A. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu M, Blacklow S C, Kim P S. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat Struct Biol. 1995;2:1075–1082. doi: 10.1038/nsb1295-1075. [DOI] [PubMed] [Google Scholar]
- 22.Melikyan G B, Markosyan R M, Hemmati H, Delmedico M K, Lambert D M, Cohen F S. Evidence that the transition of HIV-1 gp41 into a six-helix bundle, not the bundle configuration, induces membrane fusion. J Cell Biol. 2000;151:413–423. doi: 10.1083/jcb.151.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munoz-Barroso I, Durell S, Sakaguchi K, Appella E, Blumenthal R. Dilation of the human immunodeficiency virus-1 envelope glycoprotein fusion pore revealed by the inhibitory action of a synthetic peptide from gp41. J Cell Biol. 1998;140:315–323. doi: 10.1083/jcb.140.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Brien W A, Chen I S, Ho D D, Daar E S. Mapping genetic determinants for human immunodeficiency virus type 1 resistance to soluble CD4. J Virol. 1992;66:3125–3130. doi: 10.1128/jvi.66.5.3125-3130.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Brien W A, Koyanagi Y, Namazie A, Zhao J Q, Diagne A, Idler K, Zack J A, Chen I S. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990;348:69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- 26.O'Brien W A, Summer-Smith M, Mao S H, Sadeghi S, Zhao J Q, Chen I S. Anti-human immunodeficiency virus type 1 activity of an oligocationic compound mediated via gp120 V3 interactions. J Virol. 1996;70:2825–2831. doi: 10.1128/jvi.70.5.2825-2831.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rimsky L T, Shugars D C, Matthews T J. Determinants of human immunodeficiency virus type 1 resistance to gp41-derived inhibitory peptides. J Virol. 1998;72:986–993. doi: 10.1128/jvi.72.2.986-993.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shioda T, Levy J A, Cheng-Mayer C. Macrophage and T cell-line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature. 1991;349:167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- 29.Signoret N, Pelchen-Matthews A, Mack M, Proudfoot A E, Marsh M. Endocytosis and recycling of the HIV coreceptor CCR5. J Cell Biol. 2000;151:1281–1294. doi: 10.1083/jcb.151.6.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Speck R F, Wehrly K, Platt E J, Atchison R E, Charo I F, Kabat D, Chesebro B, Goldsmith M A. Selective employment of chemokine receptors as human immunodeficiency virus type 1 coreceptors determined by individual amino acids within the envelope V3 loop. J Virol. 1997;71:7136–7139. doi: 10.1128/jvi.71.9.7136-7139.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tan K, Liu J, Wang J, Shen S, Lu M. Atomic structure of a thermostable subdomain of HIV-1 gp41. Proc Natl Acad Sci USA. 1997;94:12303–12308. doi: 10.1073/pnas.94.23.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trkola A, Dragic T, Arthos J, Binley J M, Olson W C, Allaway G P, Cheng-Mayer C, Robinson J, Maddon P J, Moore J P. CD4-dependent, antibody-sensitive interactions between HIV-1 and its co-receptor CCR-5. Nature. 1996;384:184–187. doi: 10.1038/384184a0. [DOI] [PubMed] [Google Scholar]
- 33.Trujillo J R, Wang W K, Lee T H, Essex M. Identification of the envelope V3 loop as a determinant of a CD4-negative neuronal cell tropism for HIV-1. Virology. 1996;217:613–617. doi: 10.1006/viro.1996.0158. [DOI] [PubMed] [Google Scholar]
- 34.Weissenhorn W, Dessen A, Harrison S C, Skehel J J, Wiley D C. Atomic structure of the ectodomain from HIV-1 gp41. Nature. 1997;387:426–430. doi: 10.1038/387426a0. [DOI] [PubMed] [Google Scholar]
- 35.Westervelt P, Trowbridge D B, Epstein L G, Blumberg B M, Li Y, Hahn B H, Shaw G M, Price R W, Ratner L. Macrophage tropism determinants of human immunodeficiency virus type 1 in vivo. J Virol. 1992;66:2577–2582. doi: 10.1128/jvi.66.4.2577-2582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wild C, Dubay J W, Greenwell T, Baird T, Jr, Oas T G, McDanal C, Hunter E, Matthews T. Propensity for a leucine zipper-like domain of human immunodeficiency virus type 1 gp41 to form oligomers correlates with a role in virus-induced fusion rather than assembly of the glycoprotein complex. Proc Natl Acad Sci USA. 1994;91:12676–12680. doi: 10.1073/pnas.91.26.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wild C, Oas T, McDanal C, Bolognesi D, Matthews T. A synthetic peptide inhibitor of human immunodeficiency virus replication: correlation between solution structure and viral inhibition. Proc Natl Acad Sci USA. 1992;89:10537–10541. doi: 10.1073/pnas.89.21.10537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wild C T, Shugars D C, Greenwell T K, McDanal C B, Matthews T J. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc Natl Acad Sci USA. 1994;91:9770–9774. doi: 10.1073/pnas.91.21.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu L, Gerard N P, Wyatt R, Choe H, Parolin C, Ruffing N, Borsetti A, Cardoso A A, Desjardin E, Newman W, Gerard C, Sodroski J. CD4-induced interaction of primary HIV-1 gp120 glycoproteins with the chemokine receptor CCR-5. Nature. 1996;384:179–183. doi: 10.1038/384179a0. [DOI] [PubMed] [Google Scholar]