Table 1.
Various nanoparticle systems for poorly soluble covalent drugs.
| Entry | Drug | Nanoparticle Type | Significant Findings | Ref. |
|---|---|---|---|---|
| 1 | Orlistat | PBA-hyaluronic acid nanoparticles | 97% encapsulation efficiency (EE); 19% drug loading capacity (LC) | [46] |
| 2 | Orlistat | Hyaluronic acid–lipid–polymer hybrid nanoparticles | 90% EE; 6% drug LC | [47] |
| 3 | Orlistat | PLGA-PEG nanoparticles | 72% EE; 7% drug LC | [48] |
| 4 | Orlistat | Polydopamine-coated hollow capsules | 91% EE (using Nile Red as proxy drug) | [49] |
| 5 | Ibrutinib | Pluronic-stabilised nanosuspension | 21-fold increase in solubility | [50] |
| 6 | Ibrutinib | Pluronic-stabilised PLGA nanoparticles | 4-fold enhancement of oral bioavailability | [51] |
| 7 | Ibrutinib | Cyclodextrin chitosan nanoparticles |
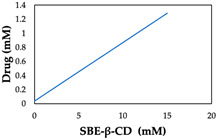 77% EE; 13% drug LC |
[52] |
