Abstract
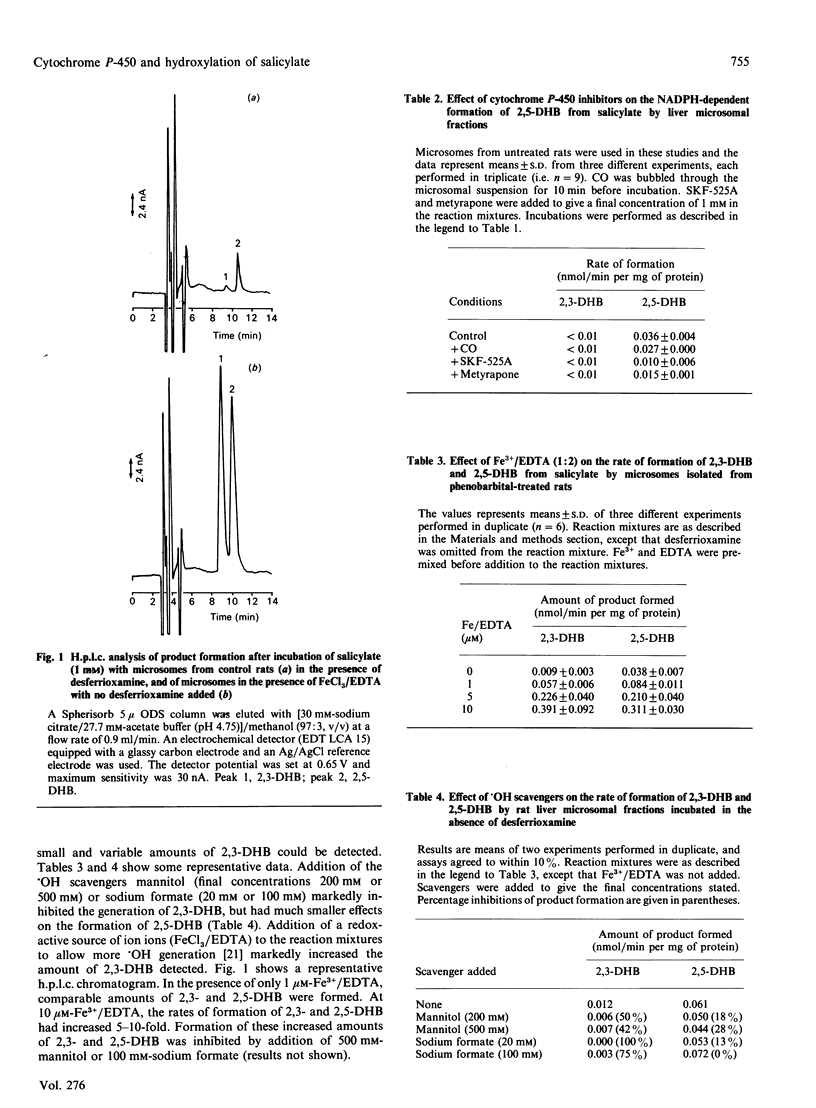
Attack by hydroxyl radicals (.OH) upon salicylate (2-hydroxybenzoate) leads to formation of both 2,3-dihydroxybenzoate (2,3-DHB) and 2,5-dihydroxybenzoate (gentisate, 2,5-DHB). It has been suggested that formation of 2,3-DHB from salicylate is a means of monitoring .OH formation. Production of 2,3-DHB and 2,5-DHB by liver microsomal fractions and isoforms of cytochrome P-450 was investigated. Liver microsomes prepared from variously treated rats and rabbits catalysed the formation of 2,5-DHB but not 2,3-DHB. Formation of 2,5-DHB was inhibited by CO, metyrapone and SKF-525A, but not by the .OH scavengers mannitol and formate or by the iron chelator desferrioxamine. Purified P-450s IIE1, IIB4 or IA2 from rabbit liver microsomes, reconstituted together with NADPH-cytochrome P-450 reductase, led to formation of equal amounts of 2,3-DHB and 2,5-DHB in reactions that were almost completely inhibited by mannitol or formate. Addition of Fe3+/EDTA either to microsomes or to membranes containing reconstituted P-450 caused formation of approximately equal amounts of 2,3-DHB and 2,5-DHB, consistent with an .OH-dependent attack on salicylate. The data indicate that the microsomal P-450 system catalyses hydroxylation of salicylate to 2,5-DHB, but not formation of 2,3-DHB. Hence measurement of 2,3-DHB might provide a means of monitoring .OH formation. Care must be taken in studies of substrate hydroxylation by microsomes or reconstituted P-450 systems to avoid artefacts resulting from .OH generation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S., Korchak H., Ludewig R., Edelson H., Haines K., Levin R. I., Herman R., Rider L., Kimmel S., Weissmann G. Modes of action of aspirin-like drugs. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7227–7231. doi: 10.1073/pnas.82.21.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aruoma O. I., Bomford A., Polson R. J., Halliwell B. Nontransferrin-bound iron in plasma from hemochromatosis patients: effect of phlebotomy therapy. Blood. 1988 Oct;72(4):1416–1419. [PubMed] [Google Scholar]
- Aruoma O. I., Halliwell B. The iron-binding and hydroxyl radical scavenging action of anti-inflammatory drugs. Xenobiotica. 1988 Apr;18(4):459–470. doi: 10.3109/00498258809041682. [DOI] [PubMed] [Google Scholar]
- Chand P., Clausen J. Effects of phenobarbital and sodium salicylate on cytochrome P-450 mixed function oxygenase and glutathione S-transferase activities in rat brain. Chem Biol Interact. 1982 Jul 1;40(3):357–363. doi: 10.1016/0009-2797(82)90158-2. [DOI] [PubMed] [Google Scholar]
- Davison C. Salicylate metabolism in man. Ann N Y Acad Sci. 1971 Jul 6;179:249–268. doi: 10.1111/j.1749-6632.1971.tb46905.x. [DOI] [PubMed] [Google Scholar]
- Eliasson E., Johansson I., Ingelman-Sundberg M. Ligand-dependent maintenance of ethanol-inducible cytochrome P-450 in primary rat hepatocyte cell cultures. Biochem Biophys Res Commun. 1988 Jan 15;150(1):436–443. doi: 10.1016/0006-291x(88)90539-6. [DOI] [PubMed] [Google Scholar]
- Eynard A. R., Galli G., Tremoli E., Maderna P., Magni F., Paoletti R. Aspirin inhibits platelet 12-hydroxy-eicosatetraenoic acid formation. J Lab Clin Med. 1986 Jan;107(1):73–78. [PubMed] [Google Scholar]
- Floyd R. A., Watson J. J., Wong P. K. Sensitive assay of hydroxyl free radical formation utilizing high pressure liquid chromatography with electrochemical detection of phenol and salicylate hydroxylation products. J Biochem Biophys Methods. 1984 Dec;10(3-4):221–235. doi: 10.1016/0165-022x(84)90042-3. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J. The molecular biology of cytochrome P450s. Pharmacol Rev. 1988 Dec;40(4):243–288. [PubMed] [Google Scholar]
- Grootveld M., Bell J. D., Halliwell B., Aruoma O. I., Bomford A., Sadler P. J. Non-transferrin-bound iron in plasma or serum from patients with idiopathic hemochromatosis. Characterization by high performance liquid chromatography and nuclear magnetic resonance spectroscopy. J Biol Chem. 1989 Mar 15;264(8):4417–4422. [PubMed] [Google Scholar]
- Grootveld M., Halliwell B. 2,3-Dihydroxybenzoic acid is a product of human aspirin metabolism. Biochem Pharmacol. 1988 Jan 15;37(2):271–280. doi: 10.1016/0006-2952(88)90729-0. [DOI] [PubMed] [Google Scholar]
- Grootveld M., Halliwell B. An aromatic hydroxylation assay for hydroxyl radicals utilizing high-performance liquid chromatography (HPLC). Use to investigate the effect of EDTA on the Fenton reaction. Free Radic Res Commun. 1986;1(4):243–250. doi: 10.3109/10715768609051634. [DOI] [PubMed] [Google Scholar]
- Grootveld M., Halliwell B. Aromatic hydroxylation as a potential measure of hydroxyl-radical formation in vivo. Identification of hydroxylated derivatives of salicylate in human body fluids. Biochem J. 1986 Jul 15;237(2):499–504. doi: 10.1042/bj2370499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Grootveld M. The measurement of free radical reactions in humans. Some thoughts for future experimentation. FEBS Lett. 1987 Mar 9;213(1):9–14. doi: 10.1016/0014-5793(87)81455-2. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Hoult J. R., Blake D. R. Oxidants, inflammation, and anti-inflammatory drugs. FASEB J. 1988 Oct;2(13):2867–2873. doi: 10.1096/fasebj.2.13.2844616. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Protection against tissue damage in vivo by desferrioxamine: what is its mechanism of action? Free Radic Biol Med. 1989;7(6):645–651. doi: 10.1016/0891-5849(89)90145-7. [DOI] [PubMed] [Google Scholar]
- Hiller K. O., Hodd P. L., Willson R. L. Antiinflammatory drugs: protection of a bacterial virus as an in vitro biological measure of free radical activity. Chem Biol Interact. 1983 Dec;47(3):293–305. doi: 10.1016/0009-2797(83)90165-5. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M., Glaumann H. Incorporation of purified components of the rabbit liver microsomal hydroxylase system into phospholipid vesicles. Biochim Biophys Acta. 1980 Jul;599(2):417–435. doi: 10.1016/0005-2736(80)90188-1. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M., Johansson I. Mechanisms of hydroxyl radical formation and ethanol oxidation by ethanol-inducible and other forms of rabbit liver microsomal cytochromes P-450. J Biol Chem. 1984 May 25;259(10):6447–6458. [PubMed] [Google Scholar]
- Johansson I., Ekström G., Scholte B., Puzycki D., Jörnvall H., Ingelman-Sundberg M. Ethanol-, fasting-, and acetone-inducible cytochromes P-450 in rat liver: regulation and characteristics of enzymes belonging to the IIB and IIE gene subfamilies. Biochemistry. 1988 Mar 22;27(6):1925–1934. doi: 10.1021/bi00406a019. [DOI] [PubMed] [Google Scholar]
- Kato S., Kawase T., Alderman J., Inatomi N., Lieber C. S. Role of xanthine oxidase in ethanol-induced lipid peroxidation in rats. Gastroenterology. 1990 Jan;98(1):203–210. doi: 10.1016/0016-5085(90)91311-s. [DOI] [PubMed] [Google Scholar]
- Reidl U. Determination of acetylsalicylic acid and metabolites in biological fluids by high-performance liquid chromatography. J Chromatogr. 1983 Feb 11;272(2):325–331. doi: 10.1016/s0378-4347(00)86135-6. [DOI] [PubMed] [Google Scholar]
- Rumble R. H., Roberts M. S., Wanwimolruk S. Determination of aspirin and its major metabolites in plasma by high-performance liquid chromatography without solvent extraction. J Chromatogr. 1981 Sep 11;225(1):252–260. doi: 10.1016/s0378-4347(00)80270-4. [DOI] [PubMed] [Google Scholar]
- Vane J., Botting R. Inflammation and the mechanism of action of anti-inflammatory drugs. FASEB J. 1987 Aug;1(2):89–96. [PubMed] [Google Scholar]


