Abstract
Jaboticaba (Myrciaria jaboticaba) is a recognizable and unique crop from Brazil. The fruit’s byproducts are currently being studied, given their bioactive composition and promising anti-cancer potential. It is not evident, however, if different harvesting seasons can modify the chemical profile and antioxidant capacity of jaboticaba fruit fractions. Furthermore, as there is limited data for jaboticaba’s anti-proliferative effects, additional assessments are required to improve the robustness of these findings. Therefore, this study aimed to determine the composition of the peel of jaboticaba collected in two periods (May—off-season, sample 1—and August–October—peak season, sample 2) and test the peel’s richest anthocyanin sample against colorectal cancer (CRC) cell lines. To accomplish this, proximate, spectrophotometric, and chromatographic analyses were performed in two freeze-dried samples; and anti-proliferative and/or colony-forming assays were carried out in Caco-2, HT29, and HT29-MTX cells. As a result, sample 2 showed the highest levels of polyphenols overall, including flavonoids and anthocyanins. This sample displayed significative higher contents of cyanidin-3-O-glucoside (48%) and delphinidin-3-O-glucoside (105%), in addition to a superior antioxidant capacity (23% higher). Sample 1 showed higher amounts of total protein, gallic acid (20% higher), and specific carotenoids. An aqueous extract from sample 2 was tested against CRC, showing anti-proliferative effects for Caco-2 cells at 1 and 2 mg/mL concentrations, with IC50 values of 1.2–1.3 mg/mL. Additionally, the extract was able to inhibit cell colony formation when tested at both low and high concentrations. In conclusion, jaboticaba collected in the main season stands out regarding its polyphenol composition and holds potential against cancer cell growth.
Keywords: agro-industrial byproduct, anthocyanins, anti-colony-forming activity, anti-proliferative action, Caco-2 cells, colorectal cancer, cyanidin-3-O-glucoside, delphinidin-3-O-glucoside, gallic acid, phenolic compounds
1. Introduction
Brazil contains a diversity of native plants throughout its territory [1]. Biomes such as the Amazon, Atlantic Forest, and Cerrado are home to non-conventional tree exemplars, which provide tasteful and nutritious fruits of enormous industrial and commercial potential [2]. In recent years, a few indigenous samples have been studied regarding their bioactive composition and potential health effects. Jaboticaba (Myrciaria spp.), from the Atlantic Forest, stands out as one the most promising crops of Brazil [3,4,5]. The tree provides characteristic red-to-purple globose berries of sweet and appreciable pulp. Among jaboticaba fractions, however, the peel and seed have shown the highest bioactive potential [6]. These byproducts are regarded as rich sources of dietary fibers and polyphenols [7]. Most commonly, studies have suggested that jaboticaba peel possesses a high pro-health potential given its abundance in anthocyanins, phenolic acids, and tannins, in addition to a high antioxidant capacity [8]. The jaboticaba fruit is largely harvested for consumption or commercial purposes in the year’s second semester, mostly during August–November [9], with collection at other times being less common. Studies addressing possible composition differences between jaboticaba’s harvest seasons are non-existent and may be important to reveal the fruit’s other chemical qualities, in addition to its best industrial and pharmacological utilization.
In preclinical studies, jaboticaba’s powder and extracts have shown the capacity to inhibit the proliferation of breast, colon, and lung cancer cells in vitro, in addition to preventing colorectal cancer (CRC) in animals [10,11,12]. A recent study showed the positive effects of jaboticaba’s pectin on CRC cells in vitro. The treatment with purified pectin was able to reduce the proliferation of HCT116 and HT29 cells, as well as to inhibit galectin-3, a protein associated with aggressive types of cancer [13]. In another study, diet-enriched jaboticaba peel powder was capable of inhibiting intestinal inflammation, consequently preventing any formation of carcinoma [14]. Other investigations have suggested the benefits of jaboticaba’s anthocyanins on CRC by testing various extracts in both in vitro and animal models. While the in vitro studies demonstrated that the extracts of jaboticaba peel can reduce the proliferation of HT29 monolayers and spheroids [15,16], the ones using animals showed that anthocyanin-rich preparations from the whole fruit or seed can suppress the formation of aberrant crypts and modulate the intestinal microbiota of rats, respectively [17,18]. Despite a few investigations having indicated the anti-CRC potential of jaboticaba peel, the evidence is still not robust enough to list the fruit as a new therapeutic candidate. Since conventional medicine can sometimes be ineffective and offer resistance [19], the continuing search for complementary ways of handling CRC may eventually help improve health care. Disease initiation blockage by natural products could have an enormous impact on health expenditure, considering that up to 90% of CRC cases are said to be preventable [20].
All things considered, our study had as main objectives to (a) understand the differences in chemical composition and antioxidant capacity between the peel of jaboticaba collected in two distinct harvest periods (peak season and off-season), and (b) investigate the action of the richest anthocyanin sample against CRC cell lines (Caco-2, HT29, and HT29-MTX).
2. Material and Methods
2.1. Chemicals
The MTT assay kit (cell proliferation) was purchased from Abcam (Cambridge, UK). 2,2′-Azobis(2-amidinopropane) dihydrochloride was purchased from Cayman Chemical (Ann Arbor, MI, USA). Pelargonidin-3-O-glucoside (P3G) standard (CAS 18466-51-8), with ≥95% purity, was purchased from PhytoLab (Vestenbergsgreuth, Germany). 4-Hydroxybenzoic acid (CAS 99-96-7), catechin (CAS 154-23-4), cyanidin-3-O-glucoside (C3G) or kuromanin chloride (CAS 7084-24-4), delphinidin-3-O-glucoside (D3G) (CAS 6906-38-3), ellagic acid (CAS 476-66-4), epicatechin (CAS 490-46-0), ferulic acid (CAS 537-98-4), gallic acid (CAS 149-91-7), p-coumaric acid (CAS 501-98-4), protocatechuic acid (CAS 99-50-3), quercetin (CAS 117-39-5), rutin (CAS 153-18-4), and syringic acid (CAS 530-57-4) standards, all with ≥95% purity, were purchased from Sigma Aldrich (St. Louis, MO, USA). The analytical standards for carotenoids were previously isolated in the laboratory (purity grade ≥ 95%), according to Kimura and Rodriguez-Amaya [21]. 2,4,6-Tripyridyl-S-triazine, 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid or Trolox, bovine serum albumin, and Folin–Ciocalteu reagent were all purchased from Sigma Aldrich (St. Louis, MO, USA). Fluorescein was purchased from Synth (Diadema, SP, Brazil). Acetone, acetonitrile, methanol, methyl tert-butyl ether, and petroleum ether, all high-performance liquid chromatography grade, were all purchased from Tedia (Rio de Janeiro, RJ, Brazil). Hydrochloric acid (37%) and phosphoric acid (85%) were purchased from Tedia (Rio de Janeiro, RJ, Brazil). Dulbecco’s Modified Eagle Medium (DMEM), penicillin 5 units/mL and streptomycin 5 μg/mL, phosphate-buffered saline (PBS) pH 7.4, and trypsin-EDTA 0.25% were all purchased from Thermo Fisher (Paisley, UK).
2.2. Fruit Registry, Collection, and Processing
As jaboticaba is a native Brazilian crop, the local and overseas studies were registered at SisGen/Brazil, protocol numbers AD872CA and RA12F13, respectively. The fruit from the Myrciaria jaboticaba (Vell.) O. Berg tree, jaboticaba Sabará, was obtained during the year 2019 in two distinct harvest periods, these being May (autumn, off-season)—sample 1—and August–October (winter/spring, peak season)—sample 2. The samples were collected at Casa Branca, São Paulo state, Brazil, coordinates 21°53′42.1″ S 47°02′10.9″ W. The fruits were transported to the school facilities and stored at −20 °C. Afterward, they were thawed and properly sanitized using 1% sodium hydrochloride in tap water (v/v). Only the fruit peel was used for the experiments. The peel was manually separated and frozen at −20 °C for freeze-drying. The freeze-drying conditions followed the equipment and conditions described by Leite-Legatti et al. [22]. Briefly, once frozen, the peel samples were dried in equipment (LP1010, Liobras, São Carlos, SP, Brazil) programmed at 30 °C and 300 μmHg for 95 h [22]. After lyophilization, the materials were ground in a mill to a powder, being subsequently stored at −20 °C for further analyses.
2.3. Proximate and Spectrophotometric Analyses
Proximate analyses were performed in the freeze-dried samples, including moisture, ashes [23], lipids [24], and protein [25]. The total carbohydrate content was estimated based on the sum of moisture, ashes, lipids, and protein. For the spectrophotometric analyses, the samples were extracted according to Nascimento et al. [26]. Initially, the powders were mixed with a hydroalcoholic solution composed of 46% ethanol in distilled water, with pH 1 adjusted with hydrochloric acid. The sample-to-solvent ratio used was 1:20. The mixture was then subjected to an ultrasound bath for 10 min, with the water temperature at 30 °C. After 10 min, filtration was performed using a 15 cm diameter paper filter. The procedure was repeated three times with the residues of the filtration in order to extract more compounds from the samples [26]. The extracts were produced in triplicate and kept at −20 °C. Afterward, the following analyses were carried out: total phenolic content (TPC) by Folin–Ciocalteu (gallic acid curve: 16–100 µg/mL) [27], total flavonoids (catechin curve: 8–192 µg/mL) [28], monomeric anthocyanins by the pH differential method [29], and antioxidant capacity assays ferric-reducing antioxidant power (FRAP) [30] and oxygen radical absorbance capacity (ORAC) (Trolox curves) [31]. A microplate spectrophotometer coupled with Gen5™ 2.0 data analysis software was used for the analyses. Additionally, an analysis of total carotenoids was performed by ultraviolet–visible spectroscopy, according to the extraction and analytic procedures by Rodriguez-Amaya [32]. The analyses were carried out in duplicate or triplicate. Except for moisture, all proximate, spectrophotometric, and antioxidant capacity results were demonstrated as dry weight of jaboticaba peel powder.
2.4. Chromatographic Analyses
The samples were also analyzed by high-performance liquid chromatography coupled with a diode array detector (HPLC-DAD). The chromatographic analyses were carried out in a WatersTM Alliance model 2690/5 coupled with a WatersTM DAD model 2996, using EmpowerTM software version 1. Ultrapure water (0.054 µS/cm) from the Millipore® Milli-Q system (Milford, MA, USA) was used throughout the analyses. Anthocyanins, other flavonoids and phenolic acids, and carotenoids were extracted and quantified following the studies of Gouvêa et al. [33], Nascimento et al. [34], and Rodriguez-Amaya [32] and Pacheco et al. [35], respectively. All procedures were carried out in triplicate.
For anthocyanins, the freeze-dried samples were mixed with a 10% formic acid solution in methanol (sample-to-solvent ratio of 1:20) with stirring for 1 min, followed by a 10 min ultrasound extraction, and centrifugation at 6000 rpm for 10 min. The extraction process was repeated until solution discoloration. The volume obtained was completed to 10 mL by adding more 10% formic acid in methanol. About 1 mL of the solution was microcentrifuged at 12,000 rpm for 3 min. The solvent was evaporated under nitrogen flow until dry and then resuspended with the same amount (1 mL) of 10% formic acid in methanol. The extract was transferred to a vial and taken to the chromatograph injector. The following were the conditions of the chromatograph: Thermo Scientific C18 BDS (100 mm × 4.6 mm; 2.4 μm) column, column temperature at 40 °C, flow of 1.0 mL/minute, 20 µL injection. The elution mode gradient used 5% formic acid (phase A) and acetonitrile (phase B), starting with 95% of phase A and 5% of phase B; 15 min 87% A and 13% B; 16.5 min 86% A and 14% B; 18 min 95% A and 5% B, with a run time of 20 min [33]. The compounds were identified by comparing their retention time and ultraviolet/visible absorption spectra with those of the analytical standards. The quantification of anthocyanins (C3G, D3G, and P3G) was performed by external standardization.
For other flavonoids and phenolic acids, the samples were extracted with methanol–water (50:50, pH 2) (sample-to-solvent ratio of 1:20) followed by mechanical stirring for 1 h and centrifugation (5000× g) for 10 min. A solution of acetone–water (70:30, v/v) was added to the residue, and the mechanical stirring and centrifugation steps were repeated. About 3 mL of the two supernatants were mixed and transferred to a 1.5 mL vial for the chromatographic injection. Additionally, the solid sample residues were submitted to the extraction of hydrolyzed phenolic compounds. Alkaline hydrolysis was continued with a 5 mL solution of 2 M Sodium hydroxide containing 1% ascorbic acid and 10 mM EDTA. This solution was added to the samples, followed by heating at 61–63 °C for 60 min, and immediately after this 1.5 mL of 6 M hydrochloric acid was added for the acid hydrolysis. This solution was vortexed for 10 s, left to cool to room temperature, and then centrifuged (2700 rpm) for 10 min. The supernatant was collected and 6.5 mL of ethyl acetate was added. The organic phase was separated and the extraction with ethyl acetate was repeated. The organic fraction was dried under a nitrogen gas flow and then diluted in methanol for the chromatographic analysis. The following were the conditions of the chromatograph: Thermo Hypersil BDS C18 column (100 × 4.6 mm × 2.4 µm), mobile phase flow at 1 mL/minute, injection volume of 10 µL, run time of 28 min, and an elution mode gradient composed of an aqueous solution of 0.15% phosphoric acid (95%) and acetonitrile (5%) [34]. The compounds were identified by comparing their retention time and the ultraviolet–visible absorption spectra with those of the analytical standards. The quantifications of other flavonoids (catechin, epicatechin, quercetin, and rutin) and phenolic acids (4-hydroxybenzoic acid, ellagic acid, ferulic acid, p-coumaric acid, protocatechuic acid, and syringic acid) were performed by external standardization.
Finally, for carotenoids, the extraction was conducted with approximately 0.2 g of the samples, which were macerated with celite and acetone. The extraction procedure was repeated until the sample no longer exhibited the characteristic color of carotenoids. The acetone extract was transferred quantitatively to a separator funnel containing petroleum ether and washed, at least five times, with ultrapure water. The ether extract was filtered through anhydrous sodium sulfate, collected in 100 mL volumetric flasks, and completed with petroleum ether. A 5 mL aliquot was separated for a further saponification reaction with 5 mL of potassium hydroxide solution 10% in methanol (10:90, v/v) at room temperature and a reaction time of 16 h [32]. The carotenoid profile was determined by HPLC as described by Pacheco et al. [35], using a C30 column (S-3 Carotenoid, 4.6 mm × 250 mm, YMC™), a gradient elution of methanol, and methyl terc-butyl ether, with DAD. The flow rate was 0.8 mL/minute and the run time was 28 min. The compounds were identified by comparing their retention time and ultraviolet–visible absorption spectra with those of the analytical standards. The quantification of carotenoids (α-carotene, β-carotene, β-cryptoxanthin, and lutein) was performed by external standardization.
All chromatographic results were demonstrated as dry weight of jaboticaba peel powder.
2.5. Anti-Proliferative and Colony Formation Assays
An aqueous extract of the richest anthocyanin sample was prepared for use in the anti-cancer assays. We decided to use an aqueous extract for the health-related studies. This represents an easy and practical preparation, in addition to being a more green/less toxic option, but still highly functional, which could be well translated for human handling and consumption. Moreover, this aqueous extract has shown positive effects in studies conducted by our research group through pre-clinical models of chronic diseases [36,37].
The extraction method followed the study of Silva-Maia et al. [37]. The powder was mixed with distilled water (boiling, 100 °C) in a glass beaker. A sample-to-solvent ratio of 1:20 was applied. The mixture was allowed to rest for 30 min at room temperature with eventual mixing at 0, 15, and 30 min. The resulting infusion was filtered through a paper filter (0.2 mm) and stored at −20 °C. The residue was discarded. The extract was freeze-dried and quantified regarding the levels of monomeric anthocyanins [29]. The extract preparation and analysis were performed in triplicate.
Caco-2, HT29, and HT29-MTX cell lines were obtained from the European Collection of Authenticated Cell Cultures (ECACC, Salisbury, UK). They were grown in high-glucose DMEM, supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin solution, and kept at 37°C with 5% CO2.
For the cell viability assay, human CRC cells, Caco-2, HT29, and HT29-MTX, were seeded at 10,000 cells per well (150 μL) in 96-well flat-bottom plates. The treatment was carried out after 72 h of seeding (cells have reached at least 70% confluence). For the treatment, the following concentrations (mg/mL) of freeze-dried extract were applied: 0, 0.025, 0.05, 0.1, 0.5, 1, and 2. The extracts were diluted in fresh media. After 24 or 48 h of treatment, to measure cell death/survival, the cells were submitted to the MTT protocol. Briefly, the wells were added with 50 μL serum-free media and 50 μL MTT solution, and incubated at 37 °C for 3 h. After incubation, the content was retrieved and 150 μL of MTT solvent was added to each well, followed by orbital shaking until homogenization. The absorbance was read at 590 nm. The values were subtracted from blank values and the absorbance readings of the treatments were compared to those of the control (concentration of 0 mg/mL) in order to compose the final percentage results, which were indicative of cell survival or cell death/toxicity. The half maximal inhibitory concentration (IC50) was calculated using the percentage values of cell death; the ED50plus v1.0 program was used. Three replicates were performed for each cell line tested.
For the colony formation assay, the cell line with the most effective anti-proliferative effect in the MTT assay was tested. Two assays were carried out with distinct seeding and extract concentrations. The cells were seeded at 500 or 1000 cells per well using 6- or 24-well flat-bottom plates, respectively. After 24 or 48 h, the wells were added with the extracts at concentrations of 0, 0.5, 1, and 2 mg/mL (6-well plate) or 0, 0.025, 0.05, 0.1, 0.25, 0.5, 0.75, 1, 1.5, and 2 mg/mL (24-well plate), respectively. The extracts were diluted in fresh media. Every three days, new media was provided to cells. After a total of 8 days, the crystal violet protocol was applied. For the crystal violet protocol, cells were washed twice with PBS and incubated with absolute methanol for 20 min. The content was retrieved and a 0.5% crystal violet solution was added to the wells for 40 min. After that, the crystal violet solution was retrieved and the wells were washed with PBS up to four times. The plates were left at room temperature to dry. Photos of the wells were taken in the GelCountTM equipment (Oxford Optronix, Oxford, UK) for a visual comparison between the treatments. The assays were performed in triplicate.
2.6. Statistical Analysis
Data are represented by mean ± standard deviation (SD). Data were initially submitted to a normality test considering kurtosis and skewness values between −2 and 2 [38]. The composition differences between jaboticaba harvest periods (two samples) were analyzed by Student’s t-test (Welch’s correction, two-tailed). Data from the antiproliferative experiments were compared by One-way ANOVA followed by Tukey; the jaboticaba treatments (0.025–2 mg/mL) were compared to the control wells (concentration of 0 mg/mL). A p < 0.05 was considered to determine statistical difference, being represented in figures by the asterisk symbol (*).
3. Results and Discussion
3.1. Chemical Composition and Antioxidant Capacity
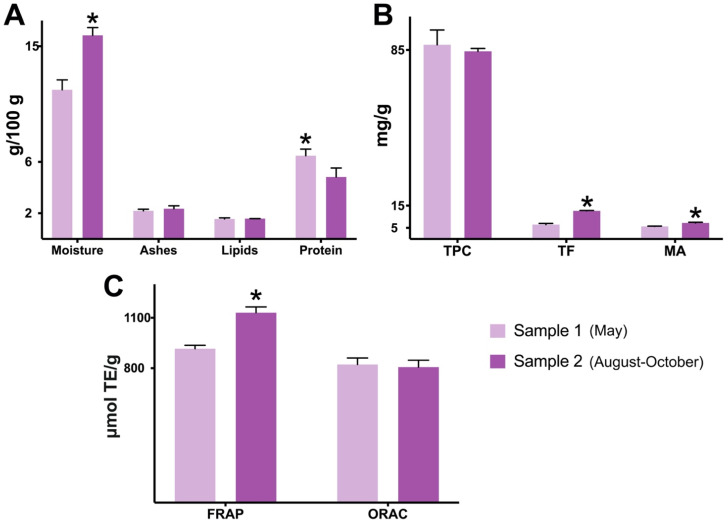
Sample 2 (August–October) showed a significantly higher (p < 0.05) moisture content after lyophilization when compared to sample 1 (May). Both samples went through the same freezing, thawing, and lyophilization procedures; therefore, differences in moisture after drying are probably an influence of jaboticaba’s collection periods. An aspect that may have exerted influence is that sample 2 comes from jaboticaba collected during three months, while sample 1 was collected over only one month. For example, the study of Bower and Papli [39] found variable moisture percentages (~60–80%) in the avocado fruit collected in different months (April–September). Also, Garçoa et al. [40] found moisture values varying greatly between jaboticaba’s developing moments, with differences between them reaching 14% within 34 days.
Despite having the highest moisture amount, sample 2 displayed a lower content of protein (4.82 ± 0.70) when compared with sample 1 (6.47 ± 0.51 g/100 g, dry weight) (p < 0.05) (Figure 1A). Other literature findings indicate that jaboticaba peel powders are usually low sources of protein, ranging between 3.77 and 7.31 g/100 g [22,36,41,42]. The powders’ contents in ashes and lipids were also relatively low and similar between the samples (Figure 1A). The total carbohydrate content for both jaboticaba powders was estimated at 89–92% (dry weight).
Figure 1.
Proximate and spectrophotometric composition differences between two samples of jaboticaba peel powder. Except for moisture, all results are in dry weight of jaboticaba peel powder. Sample 1: jaboticaba collected in May; sample 2: jaboticaba collected in August–October. Data are represented by mean ± standard deviation (SD). Student’s t-test (Welch’s correction, two-tailed); the asterisk symbol (*) indicates statistical difference (p < 0.05) between samples 1 and 2. (A) Proximate composition. (B) Spectrophotometric composition. (C) Antioxidant capacity. Abbreviations: FRAP—ferric-reducing antioxidant power; MA—monomeric anthocyanins; ORAC—oxygen radical absorbance capacity; TE—Trolox equivalent; TF—total flavonoids; TPC—total phenolic content.
In terms of bioactive compounds, sample 2 showed significantly higher (p < 0.05) amounts of total flavonoids (92% richer; 12.62 ± 0.19 mg/g, dry weight) and monomeric anthocyanins (28% richer; 7.22 ± 0.28 mg/g, dry weight) when compared to sample 1 (p < 0.05) (Figure 1B). Overall, both jaboticaba samples were revealed as “high sources” of TPC, since the samples surpassed 50 mg/g (dry weight), based on the classification of Rufino et al. [43]. Despite sample 2 having more content in flavonoids, the levels of TPC were not significantly changed by jaboticaba’s distinct harvest seasons (Figure 1B). The lower content of flavonoids found in sample 1 was compensated by the richness in gallic acid, which explains the lack of difference between the samples’ TPC. Another possibility is that sample 1 has more interfering agents, such as reducing sugars and/or ascorbic acid, factors known to overestimate the analysis values [44].
Sample 2 also showed a higher antioxidant capacity by FRAP (p < 0.05), which is probably associated with its superior levels of flavonoids and anthocyanins. However, the ORAC method did not capture any significant difference, since sample 2 showed a similar value to sample 1 (Figure 1C). The ORAC values found by our study (806.17 ± 41.09 and 821.80 ± 38.61) were similar to the one of Inada et al. [6] (827 µmol Trolox equivalent/g), also using M. jaboticaba peel powder. Among jaboticaba’s various fractions (whole fruit, pulp, peel, seed, and depulping residue), the authors confirmed that the peel possesses the most promising composition, given its significantly elevated contents in TPC and anthocyanins, in addition to a higher antioxidant capacity measure by ORAC [6]. When the ORAC values of well-known berries, including blackberry (423), raspberry (161), and strawberry (356 µmol Trolox equivalent/g) [45], are compared with the ones of jaboticaba peel (>806 µmol Trolox equivalent/g), the latter stands out as being a more promising antioxidant crop.
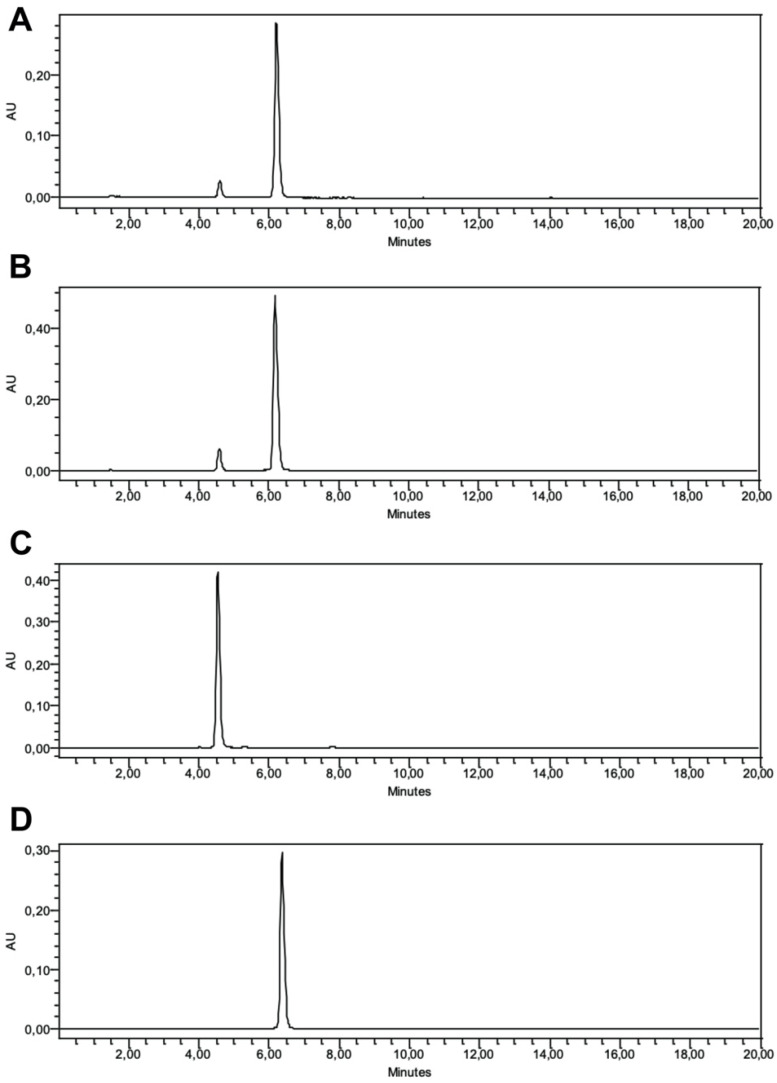
Following spectrophotometry, chromatography was performed for specific information on jaboticaba’s bioactive profile. The anthocyanin chromatograms revealed two major peaks for both samples 1 (Figure 2A) and 2 (Figure 2B). With the aid of purified standards, these were identified (from left to right) as D3G for the first peak (Figure 2C) and C3G for the second peak (Figure 2D). The peak for C3G was way more prominent than D3G, and sample 2 showed a higher AU reach for both C3G and D3G in comparison with sample 1. The P3G peak was not seen in any of the jaboticaba samples, indicating its absence in the fruit. The results found are in concordance with most HPLC investigations performed in jaboticaba peel powders, which indicate a clear dominance of C3G among total anthocyanins (about 80–90%) [46,47,48]. Unlike our study, a few other investigations have identified P3G in the peel, in addition to other less common anthocyanin derivatives from cyanidin, malvidin, pelargonidin, and petunidin compounds [49,50,51,52].
Figure 2.
Anthocyanin chromatograms of jaboticaba peel samples and standards. The analyses were carried out in a high-performance liquid chromatography coupled with a diode array detector. (A) Jaboticaba peel, sample 1 (May). (B) Jaboticaba peel, sample 2 (August–October). (C) Delphinidin-3-O-glucoside standard. (D) Cyanidin-3-O-glucoside standard.
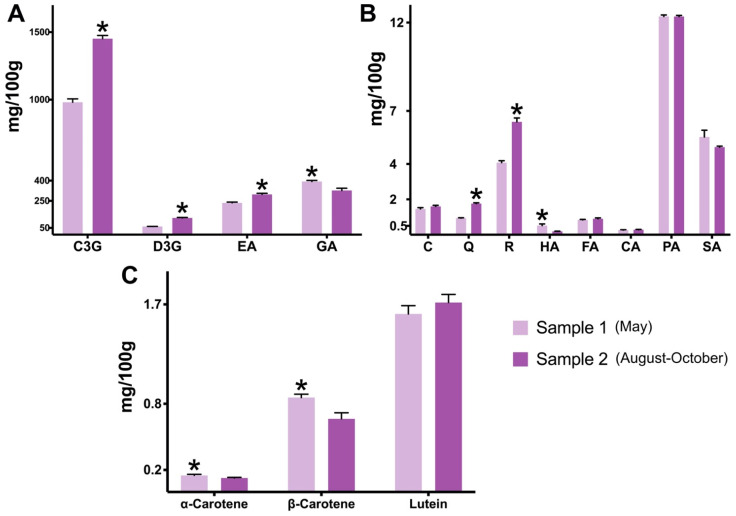
In general, the chromatographic analyses revealed that both powders are major sources (descending order) of C3G, gallic acid, ellagic acid, and D3G (Figure 3A), with fewer contents (<13 mg/100 g) of other flavonoids and phenolic acids (Figure 3B). The samples’ spectrophotometric differences in terms of flavonoids and anthocyanins were also seen in chromatography. When compared to sample 1, sample 2 showed a 48% higher content in C3G (1451 ± 23.29 mg/100 g) and 105% higher in D3G (123.88 ± 3.58 mg/100 g) (p < 0.05) (Figure 3A), in addition to significantly elevated contents of free and hydrolyzed fractions of quercetin and rutin (p < 0.05) (Figure 3B). The C3G amount in sample 2 was similar to or higher than the ones found by the studies of Resende et al. [47,53] (Plinia spp., 352–1008), Leite-Legatti et al. [22] (M. jaboticaba, 1514), and Quatrin et al. [51] (M. trunciflora, 1039, and M. jaboticaba, 1636 mg/100 g), but lower than those reported by Leite et al. [54] (M. jaboticaba, 1966) and Plaza et al. [48] (M. jaboticaba, 2866 mg/100 g). In general, most studies using jaboticaba peel, including ours, have employed acetic or formic acid to acidify the extraction solutions and obtain high yields of anthocyanins for HPLC analyses. These weakly acid solvents are known to provide a favorable extraction and avoid the hydrolysis of not only common anthocyanins but also acylated and 3,5-diglucosides, therefore increasing their specific and total content [55].
Figure 3.
Chromatographic composition differences between two samples of jaboticaba peel powder. The analyses were carried out in a high-performance liquid chromatography coupled with a diode array detector. All results are in dry weight of jaboticaba peel powder. Sample 1: jaboticaba collected in May; sample 2: jaboticaba collected in August–October. Data are represented by mean ± standard deviation (SD). Student’s t-test (Welch’s correction, two-tailed); the asterisk symbol (*) indicates statistical difference (p < 0.05) between samples 1 and 2. (A) The composition of jaboticaba peel’s main polyphenols. The values for ellagic acid represent the sum of free and hydrolyzed fractions. The values for gallic acid represent the hydrolyzed fraction. (B) Other flavonoids and phenolic acids composition. The values for catechin, quercetin, rutin, and protocatechuic acid represent the sum of free and hydrolyzed fractions. The values for epicatechin, 4-hydroxybenzoic acid, ferulic acid, and p-coumaric acid represent the hydrolyzed fraction. The values for syringic acid represent the free fraction. (C) Carotenoids’ composition. Abbreviations: C3G—cyanidin-3-O-glucoside; D3G—delphinidin-3-O-glucoside; C—catechin; CA—p-coumaric acid; EA—ellagic acid; FA—ferulic acid; GA—gallic acid; HA—4-hydroxybenzoic acid; PA—protocatechuic acid; Q—quercetin; R—rutin; SA—syringic acid.
Gallic and ellagic acids were by far the most abundant phenolic acids found in the jaboticaba powders, followed by (in descending order) protocatechuic acid and syringic acid (Figure 3B). While sample 2 showed the highest levels of total ellagic acid (free and hydrolyzed fractions) (p < 0.05), sample 1 displayed the most significant contents in hydrolyzed phenolic acids, these being gallic acid and 4-hydroxybenzoic acid (p < 0.05) (Figure 3B). Especially, sample 1 demonstrating a significantly higher (p < 0.05) content in gallic acid (393.66 ± 7.84 mg/100 g), by 20%, should be highlighted, as this phenolic acid has been considered a possible candidate for the treatment of gastrointestinal diseases, given its modulatory interaction with the gut microbiota and immune response [56]. Recently, jaboticaba peel’s richness in not only anthocyanins but also phenolic acids, including gallic acid, has been associated with the inhibition of colorectal carcinoma in mice [14]. The levels of catechin, ferulic acid, p-coumaric acid, protocatechuic acid, and syringic acid did not differ statistically between the samples (Figure 3B); and epicatechin was not identified in either jaboticaba powder.
The characterization of carotenoids was performed to facilitate an extended comparison between the harvest seasons. Jaboticaba’s carotenoid composition, especially, has been poorly documented in the literature. The powders’ chromatographic levels of α-carotene, β-carotene, and lutein, when combined (2.5–2.7), were slightly lower than the ones shown by the total carotenoids analysis (3.07 ± 0.12 and 3.21 ± 0.10 mg/100 g). The literature has reported a total carotenoid content varying between 1 and 5 mg/100 g for jaboticaba peel powders [57,58,59], which is in accordance with our study. No significant differences in total carotenoid content were found between the samples. Regarding specific carotenoids, lutein was the most common compound found in the jaboticaba powders (Figure 3C), representing between 60 and 70% of the total carotenoid content shown by chromatography. Similarly, Biazotto et al. [60] showed that lutein is the most common carotenoid found in the pulp, along with the peel of P. cauliflora. On the other side, Inada et al. [6] only found β-carotene among the carotenoids profile of M. jaboticaba’s whole fruit, with a content (0.87) similar to our study (0.85 ± 0.03 mg/100 g, sample 1). β-cryptoxanthin was not identified in the jaboticaba powders of the present study. When the harvesting periods were compared, sample 1 was revealed as a better source of α-carotene and β-carotene when compared to sample 2 (p < 0.05) (Figure 3C). However, overall, jaboticaba’s carotenoid content is less relevant than other compounds, such as flavonoids and phenolic acids.
The present study is a rare report on the composition differences between two jaboticaba harvesting seasons. Additionally, it highlights an uncommon collection period (May) for the fruit. Previous investigations have shown that the levels of bioactive compounds can change depending on the physiological and maturation developments of the fruit, but always analyzing within the year’s second semester or jaboticaba peak season. These studies have been conducted in the various fractions (peel, pulp, and seed) of M. cauliflora, variety ‘Pingo de mel’, and showed that, to obtain the highest levels of anthocyanins, essential oils, and vitamin C, the collection should be carried out 30 to 37 days after flowering [61,62,63]. More investigations with jaboticaba collected in unusual seasons may reveal the fruit’s other interesting characteristics and chemical attributes. In addition, it is also important that further studies collect and analyze samples from different years. In our study, the fruits from both peak season and off-season were obtained in the same year, limiting a more accurate comparison in the long-term and a better comparison with the literature.
All numeric values from the composition and antioxidant capacity analyses can be seen in the Supplementary Materials.
3.2. Anti-Cancer Activity
For the anti-cancer analyses, the treatment of choice was the freeze-dried aqueous peel extract from the jaboticaba collected in August–October (peak season, sample 2), as this sample showed the most content of anthocyanins from both spectrophotometric and chromatographic analyses. When analyzed for monomeric anthocyanins, the extract showed a value of 8.13 ± 1.32 mg/g. Additionally, a study of our research group [64], using the same extraction method, as well as the same fruit fraction, species, and supplier, identified and quantified the following compounds as the main polyphenols (in descending order) in the aqueous sample: C3G, gallic acid, rutin, kaempferol-3-O-glucoside, and D3G. C3G appears to be a remarkable and sometimes dominant compound in jaboticaba extracts, independent of the extraction method.
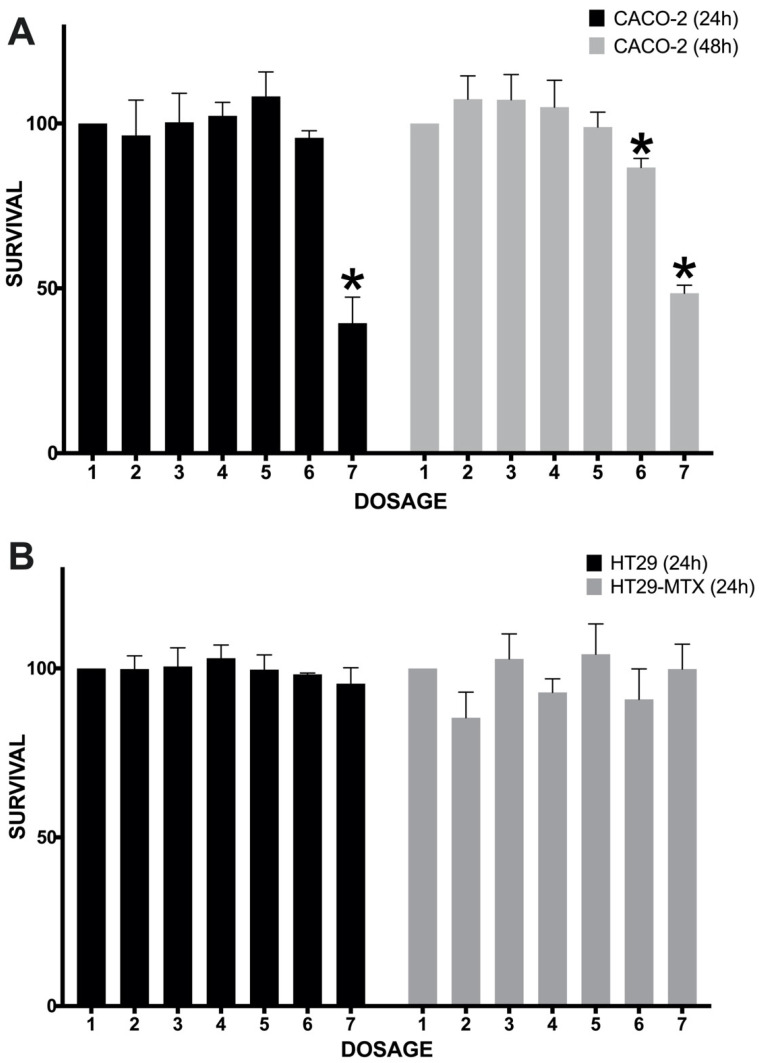
The extract’s highest concentrations, 1 and 2 mg/mL, were capable of significantly reducing the survival of Caco-2 cells after 48 h and 24/48 h of extract exposure, respectively (Figure 4A). Especially, the 2 mg/mL concentration was powerful enough to kill on average about 50–60% of all cells. The IC50 values were calculated as 1.32 ± 0.06 and 1.21 ± 0.07 mg/mL for the treatment times of 24 and 48 h, respectively. The 48 h treatment was slightly more effective, as two concentrations reduced cancer cell proliferation (Figure 4A). When compared to other investigations, the IC50 found by our study may be considered higher than expected, although still effective for an extract prepared without toxic and non-green solvents. Studies using other jaboticaba-based extracts have shown the following best IC50 values against CRC cell lines: 0.14 (HT29) [22], 0.34 (HCT116) [13], 0.45 (Caco-2) [18], 0.76 (HT29) [15], and 37.52 mg/mL (HT29 cells) [16].
Figure 4.
The anti-proliferative activity of jaboticaba peel extract (August–October sample) on colorectal cancer cell lines. Seeding: 10,000 cells per well. Extract concentrations: 1/control (0), 2 (0.025), 3 (0.05), 4 (0.1), 5 (0.5), 6 (1), and 7 (2 mg/mL). Cell death/survival was measured by the MTT assay. Data are represented by mean ± standard deviation (SD). One-way ANOVA followed by Tukey; the asterisk symbol (*) indicates statistical difference (p < 0.05) (the jaboticaba treatments were compared to the control concentration). (A) Survival of Caco-2 cells after exposure for 24 or 48 h of jaboticaba peel extract. (B) The survival of HT29 and HT29-MTX cells after exposure for 24 h of jaboticaba peel extract.
Previously, the cell viability of Caco-2 cells after treatment with a jaboticaba-based product has only been investigated once. Fidelis et al. [18] treated Caco-2 cells for 48 h with an aqueous/propanone-based jaboticaba (M. jaboticaba) seed extract, finding significative antiproliferative effects starting at a concentration of 0.5 mg/mL. In addition, investigations have confirmed the ability of anthocyanin-rich extracts to reduce the proliferation of Caco-2 cells. These include grape byproducts (seed, pomace) [65], black raspberry [66], and other Brazilian fruits, such as açaí (Euterpe oleracea) [67] and tucum-do-Cerrado (Bactris setosa) [68]. Anthocyanins’ mechanisms on CRC cells are still being understood, with experimental articles also indicating the participation of a few extrinsic mediators. C3G, for example, which is the main anthocyanin found in jaboticaba, has been linked with the blockage of programmed death-ligand 1 in HCT-116 cells [69], a scenario said to reduce the protein’s bond with programmed death-1 receptor and promote the reactivation and anti-cancer action of cytotoxic T lymphocytes [70]. Another report suggests that C3G may also act by downregulating the epidermal growth factor receptor (EGFR) [71], therefore promoting increased apoptosis and reduced angiogenesis mechanisms [72]. The anti-proliferative effects found by the present study are more likely to be associated with a blockage of EGFR, as we did not culture CRC cells with lymphocytes.
The present study also tested the effects of jaboticaba against other CRC cell lines. In general, HT-29 has been the most tested cell line on investigations with jaboticaba extracts. Previous studies have found anti-proliferative effects against HT29 by using aqueous or dichloromethane/ethanol extracts from jaboticaba peel [22,46], as well as colon-fermented samples [15]. However, our results with HT29-based cell lines did not come out as promising. The treatment with the peel’s aqueous extract had no inhibitory effects on HT29 or HT29-MTX cell lines (Figure 4B). Similarly, Nascimento et al. [13] did not manage to abrogate HT29 cells when testing a pectin-rich extract from jaboticaba (M. cauliflora) whole fruit. Such inefficacy is probably related to one or more of the following aspects of our study: (a) the concentrations applied may be too low to achieve proper inhibition results; (b) an aqueous extract usually has a lower content in total polyphenols and anthocyanins when compared with other commonly used solvent-based extracts; and (c) more treatment time may be necessary to achieve better responses. Caco-2 and HT29 cells’ characteristics and metabolism differences could also help explain such responses. When uncovering the gene expression profile between various CRC cell lines, Bourgine et al. [73] found the lowest correlation coefficients for Caco-2 and HT29 cells. While Caco-2 cells present a higher homology to enterocytes in the intestinal epithelium, in addition to most receptors, transporters, and drug-metabolizing enzymes, HT29 cells express more mucin and linked factors [74,75]. In our study, only Caco-2 cells showed sensitivity towards the aqueous extract of jaboticaba peel.
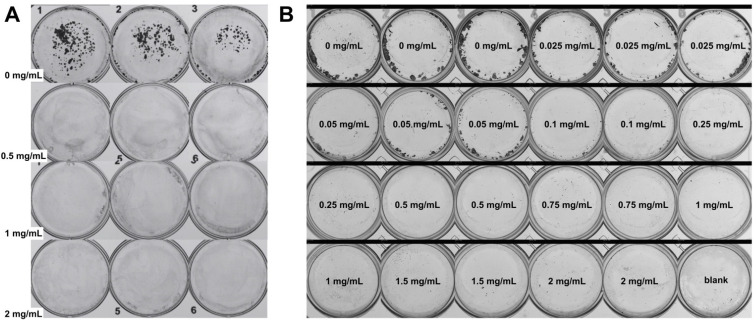
Considering the promising anti-proliferative effects of jaboticaba against Caco-2 cells, we used this cell line to identify if the extracts could inhibit cell colony formation. As a result, in the first assay, colonies were formed only in the first three control wells (horizontal direction), where no treatment was applied. The 0.5, 1, and 2 mg/mL concentrations were able to effectively inhibit colony formation (Figure 5A). In the second assay, on which 10 concentrations were tested, it was possible to notice that increasing the concentration lowered the number of colonies. Starting at concentration 0.1 mg/mL, almost no colonies were formed (Figure 5B). When compared to the MTT assay, the colony formation scheme showed more sensitivity towards the action of jaboticaba peel, as low concentrations were revealed to be efficient. Technically, the longer treatment time and fewer cells seeded could have contributed to more significant results in the colony formation assay. In terms of the biological mechanism, however, the results may be explained by the extract’s capacity to positively alter Caco-2’s genes and markers associated with intestinal stem cells, which are considered a hallmark of CRC initiation [76]. Recently, May et al. [76] showed that anthocyanin-rich black raspberry holds potential for CRC prevention, as it effectively reduces cancer cell stemness and linked markers of early tumorigenesis, consequently extending lifespan and decreasing tumor development. Natural plant extracts could be considered a strategy in progress to block CRC initiation, especially for those at high risk of obtaining the disease.
Figure 5.
The anti-colony-forming activity of jaboticaba peel extract (August–October sample) on Caco-2 cells. The crystal violet protocol was applied to observe cell colony formation. (A) First assay. Seeding: 500 cells per well. Extract concentrations: 0, 0.5, 1, and 2 mg/mL (6-well plate). (B) Second assay. Seeding: 1000 cells per well. Extract concentrations: 0, 0.025, 0.05, 0.1, 0.25, 0.5, 0.75, 1, 1.5, and 2 mg/mL.
A recent investigation by Silva-Maia et al. [77] showed that M. jaboticaba has anti-colony-forming properties against breast cancer cells. The authors tested the peel’s hydroethanolic- and ethyl acetate-based extracts and found reduced numbers of colonies for MDA-MB-231 cells when applied at the concentrations 0.01–0.025 and 0.1 mg/mL, respectively [77]. According to our search, the present study is the first report on jaboticaba’s anti-colony effects in a CRC cell line.
It is important to highlight the limitations of the in vitro study. First, we did not perform comparisons between distinct extraction methods or solvents; therefore, it is not possible to confirm that an easy-to-prepare aqueous extract would be more or less effective (or the best option) against CRC cell lines. Additionally, we did not perform comparisons with other fruit-like products or drugs, limiting our analogies to the results of the literature. Finally, our study did not investigate the metabolism and cellular mechanisms of jaboticaba compounds in vitro, a piece of information that could help explain with more certainty the antiproliferative and anti-colony-forming results found. It would be important for future studies to analyze the expression or concentration of markers related to inflammation, oxidative stress, and apoptosis, including cytokines, caspases, and binding proteins.
4. Conclusions
Jaboticaba peel’s chemical composition can be considerably changed depending on the fruit’s harvesting season. If the goal is obtaining the highest bioactive-enriched product, the peel from the fruit collected in the peak season (August–October) presents the most potential, given its superior levels of anthocyanins. Despite that, the May sample must not disregarded, as it still contains high amounts of polyphenols, especially gallic acid, and therefore, holds pro-health potential as well. In that respect, our study also showed that an eco-friendly aqueous extract from jaboticaba peel has anti-proliferative and anti-colony-forming effects on Caco-2 cells. The effects found strengthen the current knowledge regarding the fruit’s applicability against intestinal malignancies. Future pre-clinical studies should promote better insight into the molecular mechanisms by which jaboticaba peel acts to block the growth of CRC cells. In addition, clinical trials could be addressed in the same way as studies with other anthocyanin-rich fruits (bilberry, black raspberry) have been conducted in the last decade.
Acknowledgments
L.P. acknowledges the support of the Wales Cancer Research Centre. Special thanks to the Fagan family (Casa Branca, SP, Brazil), who provided the jaboticaba for the study.
Abbreviations
| C3G | cyanidin-3-O-glucoside |
| CRC | colorectal cancer |
| D3G | delphinidin-3-O-glucoside |
| DAD | diode array detector |
| DMEM | Dulbecco’s Modified Eagle Medium |
| EGFR | epidermal growth factor receptor |
| FRAP | ferric-reducing antioxidant power |
| HPLC | high-performance liquid chromatography |
| IC50 | half maximal inhibitory concentration |
| ORAC | oxygen radical absorbance capacity |
| P3G | pelargonidin-3-O-glucoside |
| PBS | phosphate-buffered saline |
| TPC | total phenolic content |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants13202907/s1.
Author Contributions
Conceptualization, R.d.P.d.N.; Formal analysis, R.d.P.d.N., J.S.R., G.P., R.G.B. and M.C.P.d.A.S.; Funding acquisition, M.R.M.J. and L.P.; Investigation, R.d.P.d.N., J.S.R., G.P., H.B., N.G.W., R.G.B. and M.C.P.d.A.S.; Methodology, R.d.P.d.N., H.B., N.G.W., R.G.B. and M.C.P.d.A.S.; Resources, M.R.M.J. and L.P.; Supervision, M.R.M.J. and L.P.; Validation, R.G.B. and M.C.P.d.A.S.; Writing—original draft, R.d.P.d.N.; Writing—review and editing, M.R.M.J. and L.P. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
For the purpose of Open Access, the author has applied a CC BY public copyright license to any Author Accepted Manuscript (AAM) version arising from this submission.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil—Finance Code 001; Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil—processes 140812/2019-9, 403328/2016-0, 301496/2019-6, 307314/2023-5, 117020/2019-2, and 120659/2019-0; and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil—processes 2019/03228-9, 2022/10485-0, 2022/09493-9, and 2020/00414-3. HB was supported by a predoctoral fellowship (“Plan Propio IBIMA 2020 A.1 Contratos predoctorales”, Ref.: predoc20_002, “Sara Borrell” postdoctoral contract CD22/00053) from the Instituto de Salud Carlos III-Madrid (Spain), “Financiado por la Unión Europea-NextGenerationEU” and the plan Recuperación, Transformación y Resiliencia, and with an EMBO Scientific Exchange Grants Fellowship (Number 9573). MRMJ acknowledges the Red Iberoamericana de Alimentos Autoctonos Subutilizados—Spain (ALSUB-CYTED, 118RT0543).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ministério do Meio Ambiente . Espécies Nativas Da Sociobiodiversidade Brasileira de Valor Alimentício. Ministério do Meio Ambiente; Brasília, Brazil: 2018. pp. 92–94. [Google Scholar]
- 2.Sviech F., Ubbink J., Prata A.S. Potential for the Processing of Brazilian Fruits—A Review of Approaches Based on the State Diagram. LWT. 2022;156:113013. doi: 10.1016/j.lwt.2021.113013. [DOI] [Google Scholar]
- 3.Schulz M., Seraglio S.K.T., Brugnerotto P., Gonzaga L.V., Costa A.C.O., Fett R. Composition and Potential Health Effects of Dark-Colored Underutilized Brazilian Fruits—A Review. Food Res. Int. 2020;137:109744. doi: 10.1016/j.foodres.2020.109744. [DOI] [PubMed] [Google Scholar]
- 4.Neri-Numa I.A., Soriano Sancho R.A., Pereira A.P.A., Pastore G.M. Small Brazilian Wild Fruits: Nutrients, Bioactive Compounds, Health-Promotion Properties and Commercial Interest. Food Res. Int. 2018;103:345–360. doi: 10.1016/j.foodres.2017.10.053. [DOI] [PubMed] [Google Scholar]
- 5.Fernandes I.d.A.A., Maciel G.M., Maroldi W.V., Bortolini D.G., Pedro A.C., Haminiuk C.W.I. Bioactive Compounds, Health-Promotion Properties and Technological Applications of Jabuticaba: A Literature Overview. Meas. Food. 2022;8:100057. doi: 10.1016/j.meafoo.2022.100057. [DOI] [Google Scholar]
- 6.Inada K.O.P., Oliveira A.A., Revorêdo T.B., Martins A.B.N., Lacerda E.C.Q., Freire A.S., Braz B.F., Santelli R.E., Torres A.G., Perrone D., et al. Screening of the Chemical Composition and Occurring Antioxidants in Jabuticaba (Myrciaria jaboticaba) and Jussara (Euterpe edulis) Fruits and Their Fractions. J. Funct. Foods. 2015;17:422–433. doi: 10.1016/j.jff.2015.06.002. [DOI] [Google Scholar]
- 7.Benvenutti L., Zielinski A.A.F., Ferreira S.R.S. Jaboticaba (Myrtaceae Cauliflora) Fruit and Its by-Products: Alternative Sources for New Foods and Functional Components. Trends Food Sci. Technol. 2021;112:118–136. doi: 10.1016/j.tifs.2021.03.044. [DOI] [Google Scholar]
- 8.Inada K.O.P., Leite I.B., Martins A.B.N., Fialho E., Tomás-Barberán F.A., Perrone D., Monteiro M. Jaboticaba Berry: A Comprehensive Review on Its Polyphenol Composition, Health Effects, Metabolism, and the Development of Food Products. Food Res. Int. 2021;147:110518. doi: 10.1016/j.foodres.2021.110518. [DOI] [PubMed] [Google Scholar]
- 9.Vieira V.L.L.P., Ferreira W.R. A Festa Da Jabuticaba e o Empreendedorismo Feminino No Município de Sabará/MG. Rev. Bras. Gestão Eng. 2013;4:1–28. [Google Scholar]
- 10.do Nascimento R.d.P., Reguengo L.M., da Fonseca Machado A.P., Marostica Junior M.R. The Preventive and Therapeutic Potential of Native Brazilian Fruits on Colorectal Cancer. Food Biosci. 2022;46:101539. doi: 10.1016/j.fbio.2021.101539. [DOI] [Google Scholar]
- 11.Reguengo L.M., do Nascimento R.d.P., da Fonseca Machado A.P., Marostica Junior M.R. Signaling Pathways and the Potential Anticarcinogenic Effect of Native Brazilian Fruits on Breast Cancer. Food Res. Int. 2022;155:111117. doi: 10.1016/j.foodres.2022.111117. [DOI] [PubMed] [Google Scholar]
- 12.da Fonseca Machado A.P., da Rocha Alves M., do Nascimento R.d.P., Reguengo L.M., Marostica Junior M.R. Antiproliferative Effects and Main Molecular Mechanisms of Brazilian Native Fruits and Their By-Products on Lung Cancer. Food Res. Int. 2022;162:111953. doi: 10.1016/j.foodres.2022.111953. [DOI] [PubMed] [Google Scholar]
- 13.do Nascimento R.S., de Freitas Pedrosa L., Diethelm L.T.H., Souza T., Shiga T.M., Fabi J.P. The Purification of Pectin from Commercial Fruit Flours Results in a Jaboticaba Fraction That Inhibits Galectin-3 and Colon Cancer Cell Growth. Food Res. Int. 2020;137:109747. doi: 10.1016/j.foodres.2020.109747. [DOI] [PubMed] [Google Scholar]
- 14.do Nascimento R.d.P., Rizzato J.S., Polezi G., Moya A.M.T.M., Silva M.F., da Fonseca Machado A.P., Franchi Junior G.C., Borguini R.G., de Araújo Santiago M.C.P., Paiotti A.P.R., et al. Freeze-Dried Jaboticaba (Myrciaria jaboticaba) Peel Powder, a Rich Source of Anthocyanins and Phenolic Acids, Mitigates Inflammation-Driven Colorectal Cancer in Mice. Food Biosci. 2023;53:102578. doi: 10.1016/j.fbio.2023.102578. [DOI] [Google Scholar]
- 15.Augusti P.R., Quatrin A., Mello R., Bochi V.C., Rodrigues E., Prazeres I.D., Macedo A.C., Oliveira-Alves S.C., Emanuelli T., Bronze M.R., et al. Antiproliferative Effect of Colonic Fermented Phenolic Compounds from Jaboticaba (Myrciaria trunciflora) Fruit Peel in a 3D Cell Model of Colorectal Cancer. Molecules. 2021;26:4469. doi: 10.3390/molecules26154469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holkem A.T., Robichaud V., Favaro-Trindade C.S., Lacroix M. Chemopreventive Properties of Extracts Obtained from Blueberry (Vaccinium myrtillus L.) and Jabuticaba (Myrciaria cauliflora Berg.) in Combination with Probiotics. Nutr. Cancer. 2021;73:671–685. doi: 10.1080/01635581.2020.1761986. [DOI] [PubMed] [Google Scholar]
- 17.Ardanareswari K., Lowisia W., Soedarini B., Liao J.W., Chung Y.C. Jaboticaba (Myrciaria cauliflora) Fruit Extract Suppressed Aberrant Crypt Formation in 1,2-Dimetylhydrazine-Induced Rats. Plant Foods Hum. Nutr. 2023;78:286–291. doi: 10.1007/s11130-023-01051-z. [DOI] [PubMed] [Google Scholar]
- 18.Fidelis M., Santos J.S., Escher G.B., Rocha R.S., Cruz A.G., Cruz T.M., Marques M.B., Nunes J.B., do Carmo M.A.V., de Almeida L.A., et al. Polyphenols of Jabuticaba [Myrciaria jaboticaba (Vell.) O. Berg] Seeds Incorporated in a Yogurt Model Exert Antioxidant Activity and Modulate Gut Microbiota of 1,2-Dimethylhydrazine-Induced Colon Cancer in Rats. Food Chem. 2021;334:127565. doi: 10.1016/j.foodchem.2020.127565. [DOI] [PubMed] [Google Scholar]
- 19.Van der Jeught K., Xu H.-C., Li Y.-J., Lu X.-B., Ji G. Drug Resistance and New Therapies in Colorectal Cancer. World J. Gastroenterol. 2018;24:3834–3848. doi: 10.3748/wjg.v24.i34.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boursi B., Arber N. Current and Future Clinical Strategies in Colon Cancer Prevention and the Emerging Role of Chemoprevention. Curr. Pharm. Des. 2007;13:2274–2282. doi: 10.2174/138161207781368783. [DOI] [PubMed] [Google Scholar]
- 21.Kimura M., Rodriguez-Amaya D.B. A Scheme for Obtaining Standards and HPLC Quantification of Leafy Vegetable Carotenoids. Food Chem. 2002;78:389–398. doi: 10.1016/S0308-8146(02)00203-0. [DOI] [Google Scholar]
- 22.Leite-Legatti A.V., Batista A.G., Dragano N.R.V., Marques A.C., Malta L.G., Riccio M.F., Eberlin M.N., Machado A.R.T., de Carvalho-Silva L.B., Ruiz A.L.T.G., et al. Jaboticaba Peel: Antioxidant Compounds, Antiproliferative and Antimutagenic Activities. Food Res. Int. 2012;49:596–603. doi: 10.1016/j.foodres.2012.07.044. [DOI] [Google Scholar]
- 23.Instituto Adolfo Lutz . Métodos Físico-Químicos Para Análise de Alimentos. ANVISA; São Paulo, Brazil: 2008. [Google Scholar]
- 24.Association of Official Analytical Chemists . Official Methods of Analysis of the Association of Official Analytical Chemists. Association of Official Analytical Chemists; Arlington, VA, USA: 1995. [Google Scholar]
- 25.Bligh E.G., Dyer W.J. A Rapid Method of Total Lipid Extraction and Purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/y59-099. [DOI] [PubMed] [Google Scholar]
- 26.do Nascimento R.d.P., Polezi G., Rizzato J.S., Batista P.B., dos Santos N.M., Loubet Filho P.S., Reguengo L.M., Morari J., Bogusz Junior S., Paiotti A.P.R., et al. Brazilian Berries Prevent Colitis Induced in Obese Mice by Reducing the Clinical Signs and Intestinal Damage. Food Biosci. 2021;44:101447. doi: 10.1016/j.fbio.2021.101447. [DOI] [Google Scholar]
- 27.Swain T., Hillis W.E. The Phenolic Constituents of Prunus Domestica. I.—The Quantitative Analysis of Phenolic Constituents. J. Sci. Food Agric. 1959;10:63–68. doi: 10.1002/jsfa.2740100110. [DOI] [Google Scholar]
- 28.Jia Z., Tang M., Wu J. The Determination of Flavonoid Contents in Mulberry and Their Scavenging Effects on Superoxide Radicals. Food Chem. 1999;64:555–559. doi: 10.1016/S0308-8146(98)00102-2. [DOI] [Google Scholar]
- 29.Abe L.T., Da Mota R.V., Lajolo F.M., Genovese M.I. Compostos Fenólicos e Capacidade Antioxidante de Cultivares de Uvas Vitis labrusca L. e Vitis vinifera L. Food Sci. Technol. 2007;27:394–400. doi: 10.1590/S0101-20612007000200032. [DOI] [Google Scholar]
- 30.Benzie I.F.F., Strain J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 31.Dávalos A., Gómez-Cordovés C., Bartolomé B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC-Fluorescein) Assay. J. Agric. Food Chem. 2004;52:48–54. doi: 10.1021/jf0305231. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Amaya D.B. A Guide to Carotenoid Analysis in Foods. ILSI Press; Washington, DC, USA: 2001. [Google Scholar]
- 33.Gouvêa A.C.M.S., Melo A., Santiago M.C.P.A., Peixoto F.M., Freitas V., Godoy R.L.O., Ferreira I.M.P.L.V.O. Identification and Quantification of Anthocyanins in Fruits from Neomitranthes Obscura (DC.) N. Silveira an Endemic Specie from Brazil by Comparison of Chromatographic Methodologies. Food Chem. 2015;185:277–283. doi: 10.1016/j.foodchem.2015.02.086. [DOI] [PubMed] [Google Scholar]
- 34.Nascimento L., Santiago M., Oliveira E., Borguini R., Braga E., Martins V., Pacheco S., Souza M., Gogoy R. Characterization of Bioactive Compounds in Eugenia Brasiliensis, Lam. (Grumixama) Nutr. Food Technol. 2017;3:1–7. doi: 10.16966/2470-6086.146. [DOI] [Google Scholar]
- 35.Pacheco S., Peixoto F.M., Borguini R.G., do Nascimento L.d.S.d.M., Bobeda C.R.R., Santiago M.C.P.d.A., Godoy R.L.d.O. Microscale Extraction Method for HPLC Carotenoid Analysis in Vegetable Matrices. Sci. Agric. 2014;71:416–419. doi: 10.1590/0103-9016-2013-0402. [DOI] [Google Scholar]
- 36.Lenquiste S.A., da Silva Marineli R., Moraes É.A., Dionísio A.P., de Brito E.S., Maróstica M.R. Jaboticaba Peel and Jaboticaba Peel Aqueous Extract Shows in Vitro and in Vivo Antioxidant Properties in Obesity Model. Food Res. Int. 2015;77:162–170. doi: 10.1016/j.foodres.2015.07.023. [DOI] [Google Scholar]
- 37.da Silva-Maia J.K., Batista Â.G., Cazarin C.B.B., Soares E.S., Junior S.B., Leal R.F., da Cruz-Höfling M.A., Maróstica Junior M.R. Aqueous Extract of Brazilian Berry (Myrciaria jaboticaba) Peel Improves Inflammatory Parameters and Modulates Lactobacillus and Bifidobacterium in Rats with Induced-Colitis. Nutrients. 2019;11:2776. doi: 10.3390/nu11112776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.George D., Mallery P. SPSS for Windows Step by Step: A Simple Guide and Reference. 11.0 Update. Allyn & Bacon, Inc.; Boston, MA, USA: 2003. [Google Scholar]
- 39.Bower J.P., Papli G. Effect of Fruit Coatings and Packaging on Chilling Injury of ‘Hass ’ Avocados. S. Afr. Avocado Grow. Assoc. Yearb. 2006;29:69–72. [Google Scholar]
- 40.Garçoa L.G.C., Da Solva F.A., Asquoero E.R., Volas Bdas E.V.D.B., Dgandd F.O.B., De Aguoar C.L., Damoano C. Proximate Composition, Minerals Profile, and Predominant Sugars by Ion Chromatograph along the Physiological Development of Jabuticaba Var. Pingo de Mel. Food Sci. Technol. 2018;38:16–21. doi: 10.1590/fst.08117. [DOI] [Google Scholar]
- 41.Marquetti C., Dos Santos T.B., Kaipers K.F.C., Böger B.R., Tonial I.B., Wagner Junior A., Lucchetta L., Do Prado N.V. Jaboticaba Skin Flour: Analysis and Sustainable Alternative Source to Incorporate Bioactive Compounds and Increase the Nutritional Value of Cookies. Food Sci. Technol. 2018;38:629–638. doi: 10.1590/fst.06717. [DOI] [Google Scholar]
- 42.Appelt P., da Cunha M.A.A., Guerra A.P., Kalinke C., da Lima V.A. Desenvolvimento e Caracterização de Barras de Cereais Produzidas Com Farinhas de Casca de Jabuticaba e Okara. Acta Sci.—Technol. 2015;37:117–122. doi: 10.4025/actascitechnol.v37i1.21070. [DOI] [Google Scholar]
- 43.Rufino S.M., Alves R.E., De Brito E.S., Pérez-jiménez J., Saura-calixto F., Mancini-filho J. Bioactive Compounds and Antioxidant Capacities of 18 Non-Traditional Tropical Fruits from Brazil. Food Chem. 2010;121:996–1002. doi: 10.1016/j.foodchem.2010.01.037. [DOI] [Google Scholar]
- 44.Singleton V.L., Orthofer R., Lamuela-Raventós R.M. Oxidants and Antioxidants Part A. Volume 299. Academic Press; Cambridge, MA, USA: 1999. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent; pp. 152–178. Methods in Enzymology. [Google Scholar]
- 45.Prior R.L., Sintara M., Chang T. Multi-Radical (ORAC MR5) Antioxidant Capacity of Selected Berries and Effects of Food Processing. J. Berry Res. 2016;6:159–173. doi: 10.3233/JBR-160127. [DOI] [Google Scholar]
- 46.Frauches N.S., Montenegro J., Amaral T., Abreu J.P., Laiber G., Junior J., Borguini R., Santiago M., Pacheco S., Nakajima V.M., et al. Antiproliferative Activity on Human Colon Adenocarcinoma Cells and In Vitro Antioxidant Effect of Anthocyanin-Rich Extracts from Peels of Species of the Myrtaceae Family. Molecules. 2021;26:564. doi: 10.3390/molecules26030564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Resende L.M., Oliveira L.S., Franca A.S. Polyphenols in Jabuticaba (Plinia Spp.) Peel Flours: Extraction and Comparative Evaluation of FTIR and HPLC for Quantification of Individual Compounds. Foods. 2023;12:1488. doi: 10.3390/foods12071488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Plaza M., Batista Â.G., Cazarin C.B.B., Sandahl M., Turner C., Östman E., Maróstica Júnior M.R. Characterization of Antioxidant Polyphenols from Myrciaria jaboticaba Peel and Their Effects on Glucose Metabolism and Antioxidant Status: A Pilot Clinical Study. Food Chem. 2016;211:185–197. doi: 10.1016/j.foodchem.2016.04.142. [DOI] [PubMed] [Google Scholar]
- 49.Loubet Filho P.S., Baseggio A.M., Vuolo M.M., Reguengo L.M., Telles Biasoto A.C., Correa L.C., Junior S.B., Alves Cagnon V.H., Betim Cazarin C.B., Maróstica Júnior M.R. Gut Microbiota Modulation by Jabuticaba Peel and Its Effect on Glucose Metabolism via Inflammatory Signaling. Curr. Res. Food Sci. 2022;5:382–391. doi: 10.1016/j.crfs.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Correia V.T.d.V., Silva V.D.M., Mendonça H.d.O.P., Ramos A.L.C.C., Silva M.R., Augusti R., de Paula A.C.C.F.F., Ferreira R.M.d.S.B., Melo J.O.F., Fante C.A. Efficiency of Different Solvents in the Extraction of Bioactive Compounds from Plinia cauliflora and Syzygium cumini Fruits as Evaluated by Paper Spray Mass Spectrometry. Molecules. 2023;28:2359. doi: 10.3390/molecules28052359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quatrin A., Pauletto R., Maurer L.H., Minuzzi N., Nichelle S.M., Carvalho J.F.C., Maróstica M.R., Rodrigues E., Bochi V.C., Emanuelli T. Characterization and Quantification of Tannins, Flavonols, Anthocyanins and Matrix-Bound Polyphenols from Jaboticaba Fruit Peel: A Comparison between Myrciaria Trunciflora and M. Jaboticaba. J. Food Compos. Anal. 2019;78:59–74. doi: 10.1016/j.jfca.2019.01.018. [DOI] [Google Scholar]
- 52.de Andrade Neves N., Stringheta P.C., da Silva I.F., García-Romero E., Gómez-Alonso S., Hermosín-Gutiérrez I. Identification and Quantification of Phenolic Composition from Different Species of Jabuticaba (Plinia spp.) by HPLC-DAD-ESI/MSn. Food Chem. 2021;355:129605. doi: 10.1016/j.foodchem.2021.129605. [DOI] [PubMed] [Google Scholar]
- 53.Resende L.M., Franca A.S. Jabuticaba (Plinia sp.) Peel as a Source of Pectin: Characterization and Effect of Different Extraction Methods. Foods. 2023;12:117. doi: 10.3390/foods12010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Leite A.V., Malta L.G., Riccio M.F., Eberlin M.N., Pastore G.M., Maróstica Júnior M.R. Antioxidant Potential of Rat Plasma by Administration of Freeze-Dried Jaboticaba Peel (Myrciaria jaboticaba Vell Berg) J. Agric. Food Chem. 2011;59:2277–2283. doi: 10.1021/jf103181x. [DOI] [PubMed] [Google Scholar]
- 55.Castañeda-Ovando A., de Lourdes Pacheco-Hernández M., Páez-Hernández M.E., Rodríguez J.A., Galán-Vidal C.A. Chemical Studies of Anthocyanins: A Review. Food Chem. 2009;113:859–871. doi: 10.1016/j.foodchem.2008.09.001. [DOI] [Google Scholar]
- 56.Yang K., Zhang L., Liao P., Xiao Z., Zhang F., Sindaye D., Xin Z., Tan C., Deng J., Yin Y., et al. Impact of Gallic Acid on Gut Health: Focus on the Gut Microbiome, Immune Response, and Mechanisms of Action. Front. Immunol. 2020;11:580208. doi: 10.3389/fimmu.2020.580208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Araujo Santiago M.C.P., Galhardo Borguini R., Da Silva de Mattos do Nascimento L., De Oliveira Braga E.C., De Carvalho Martins V., Senna Gouvêa A.C.M., Marques Peixoto F., Pacheco S., Nogueira R.I., De Oliveira Godoy R.L. Jabuticaba (Myrciaria jaboticaba (Vell.) O. Berg) Peel Powder Produced by Convective Drying Process: A Rich Anthocyanin Product. Fruits. 2018;73:201–208. doi: 10.17660/th2018/73.4.1. [DOI] [Google Scholar]
- 58.Resende L.M., Oliveira L.S., Franca A.S. Characterization of Jabuticaba (Plinia cauliflora) Peel Flours and Prediction of Compounds by FTIR Analysis. LWT. 2020;133:110135. doi: 10.1016/j.lwt.2020.110135. [DOI] [Google Scholar]
- 59.Almeida R.L.J., dos Santos Pereira T., Almeida R.D., Santiago Â.M., de Lima Marsiglia W.I.M., Nabeshima E.H., de Sousa Conrado L., de Gusmão R.P. Rheological and Technological Characterization of Red Rice Modified Starch and Jaboticaba Peel Powder Mixtures. Sci. Rep. 2021;11:9284. doi: 10.1038/s41598-021-88627-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Biazotto K.R., De Souza Mesquita L.M., Neves B.V., Braga A.R.C., Tangerina M.M.P., Vilegas W., Mercadante A.Z., De Rosso V.V. Brazilian Biodiversity Fruits: Discovering Bioactive Compounds from Underexplored Sources. J. Agric. Food Chem. 2019;67:1860–1876. doi: 10.1021/acs.jafc.8b05815. [DOI] [PubMed] [Google Scholar]
- 61.Garcia L.G.C., da Silva F.A., Asquieri E.R., Vilas Boas E.V.d.B., Silva M.M.M., Damiani C. Harvesting Period of Jabuticaba Fruits Var. “Pingo de Mel” in Relation to the Physicochemical Characterization Evaluated during Their Development. Emirates J. Food Agric. 2018;30:232–239. doi: 10.9755/ejfa.2018.v30.i3.1637. [DOI] [Google Scholar]
- 62.Garcia L.G.C., da Silva F.A., Asquieri E.R., Vilas Boas E.V.d.B., Damiani C. Bioactive Compounds and Antioxidant Activity of Jabuticaba Var. Pingo de Mel during Its Physiological Development. Food Sci. Technol. 2019;2061:556–562. doi: 10.1590/fst.25218. [DOI] [Google Scholar]
- 63.Fortes G.A.C., Naves S.S., Godoi F.F.F., Duarte A.R., Ferri P.H., Santos S.C. Assessment of a Maturity Index in Jabuticaba Fruit by the Evaluation of Phenolic Compounds, Essential Oil Components, Sugar Content and Total Acidity. Am. J. Food Technol. 2011;6:974–984. doi: 10.3923/ajft.2011.974.984. [DOI] [Google Scholar]
- 64.da Silva J.K., Batista Â.G., Cazarin C.B.B., Dionísio A.P., de Brito E.S., Marques A.T.B., Maróstica Junior M.R. Functional Tea from a Brazilian Berry: Overview of the Bioactives Compounds. LWT—Food Sci. Technol. 2017;76:292–298. doi: 10.1016/j.lwt.2016.06.016. [DOI] [Google Scholar]
- 65.Pérez-Ortiz J.M., Alguacil L.F., Salas E., Hermosín-Gutiérrez I., Gómez-Alonso S., González-Martín C. Antiproliferative and Cytotoxic Effects of Grape Pomace and Grape Seed Extracts on Colorectal Cancer Cell Lines. Food Sci. Nutr. 2019;7:2948–2957. doi: 10.1002/fsn3.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li X., Chen L., Gao Y., Zhang Q., Chang A.K., Yang Z., Bi X. Black Raspberry Anthocyanins Increased the Antiproliferative Effects of 5-Fluorouracil and Celecoxib in Colorectal Cancer Cells and Mouse Model. J. Funct. Foods. 2021;87:104801. doi: 10.1016/j.jff.2021.104801. [DOI] [Google Scholar]
- 67.Wang X., Zhang J., Cock I.E. Acai, Cacao and Maca Extracts: Anticancer Activity and Growth Inhibition of Microbial Triggers of Selected Autoimmune Inflammatory Diseases. Pharmacogn. Commun. 2016;6:204–214. doi: 10.5530/pc.2016.4.3. [DOI] [Google Scholar]
- 68.da Silva R.C., Fagundes R.R., Faber K.N., Campos É.G. Pro-Oxidant and Cytotoxic Effects of Tucum-Do-Cerrado (Bactris Setosa Mart.) Extracts in Colorectal Adenocarcinoma Caco-2 Cells. Nutr. Cancer. 2022;74:3723–3734. doi: 10.1080/01635581.2022.2086704. [DOI] [PubMed] [Google Scholar]
- 69.Mazewski C., Kim M.S., Gonzalez de Mejia E. Anthocyanins, Delphinidin-3-O-Glucoside and Cyanidin-3-O-Glucoside, Inhibit Immune Checkpoints in Human Colorectal Cancer Cells In Vitro and In Silico. Sci. Rep. 2019;9:11560. doi: 10.1038/s41598-019-47903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Passardi A., Canale M., Valgiusti M., Ulivi P. Immune Checkpoints as a Target for Colorectal Cancer Treatment. Int. J. Mol. Sci. 2017;18:1324. doi: 10.3390/ijms18061324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mazewski C., Liang K., Gonzalez de Mejia E. Comparison of the Effect of Chemical Composition of Anthocyanin-Rich Plant Extracts on Colon Cancer Cell Proliferation and Their Potential Mechanism of Action Using in Vitro, in Silico, and Biochemical Assays. Food Chem. 2018;242:378–388. doi: 10.1016/j.foodchem.2017.09.086. [DOI] [PubMed] [Google Scholar]
- 72.Pabla B., Bissonnette M., Konda V.J. Colon Cancer and the Epidermal Growth Factor Receptor: Current Treatment Paradigms, the Importance of Diet, and the Role of Chemoprevention. World J. Clin. Oncol. 2015;6:133–141. doi: 10.5306/wjco.v6.i5.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bourgine J., Billaut-Laden I., Happillon M., Lo-Guidice J.M., Maunoury V., Imbenotte M., Broly F. Gene Expression Profiling of Systems Involved in the Metabolism and the Disposition of Xenobiotics: Comparison between Human Intestinal Biopsy Samples and Colon Cell Lines. Drug Metab. Dispos. 2012;40:694–705. doi: 10.1124/dmd.111.042465. [DOI] [PubMed] [Google Scholar]
- 74.Lea T. Caco-2 Cell Line. In: Verhoeckx K., Cotter P., López-Expósito I., Kleiveland C., Lea T., Mackie A., Requena T., Swiatecka D., Wichers H., editors. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models. Springer International Publishing; Cham, Switzerland: 2015. pp. 103–111. [PubMed] [Google Scholar]
- 75.Martínez-Maqueda D., Miralles B., Recio I. HT29 Cell Line. In: Verhoeckx K., Cotter P., López-Expósito I., Kleiveland C., Lea T., Mackie A., Requena T., Swiatecka D., Wichers H., editors. The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models. Springer International Publishing; Cham, Switzerland: 2015. pp. 113–124. [Google Scholar]
- 76.May S., Greenow K.R., Higgins A.T., Derrick A.V., Taylor E., Pan P., Konstantinou M., Nixon C., Wooley T.E., Sansom O.J., et al. Modification of Diet to Reduce the Stemness and Tumorigenicity of Murine and Human Intestinal Cells. Mol. Nutr. Food Res. 2022;66:2200234. doi: 10.1002/mnfr.202200234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.da Silva-Maia J.K., Nagalingam A., Cazarin C.B.B., Marostica Junior M.R., Sharma D. Jaboticaba (Myrciaria jaboticaba) Peel Extracts Induce Reticulum Stress and Apoptosis in Breast Cancer Cells. Food Chem. Mol. Sci. 2023;6:100167. doi: 10.1016/j.fochms.2023.100167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.