Abstract
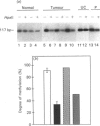
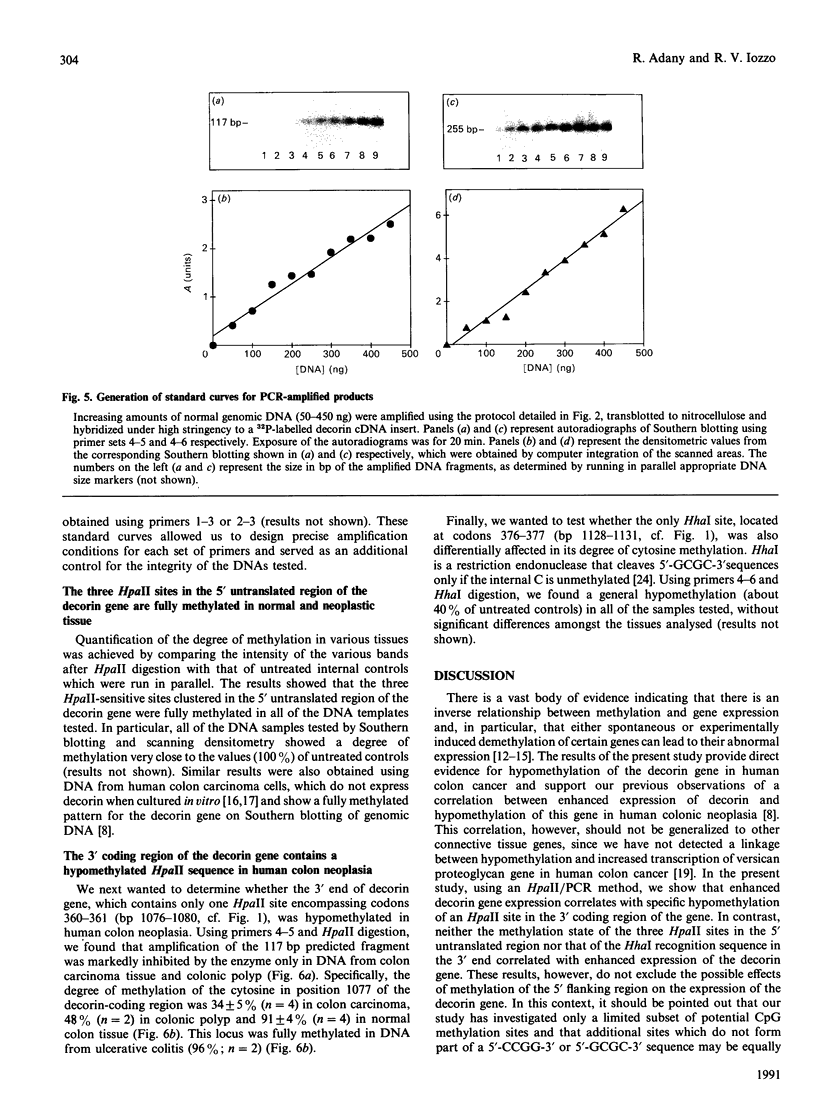
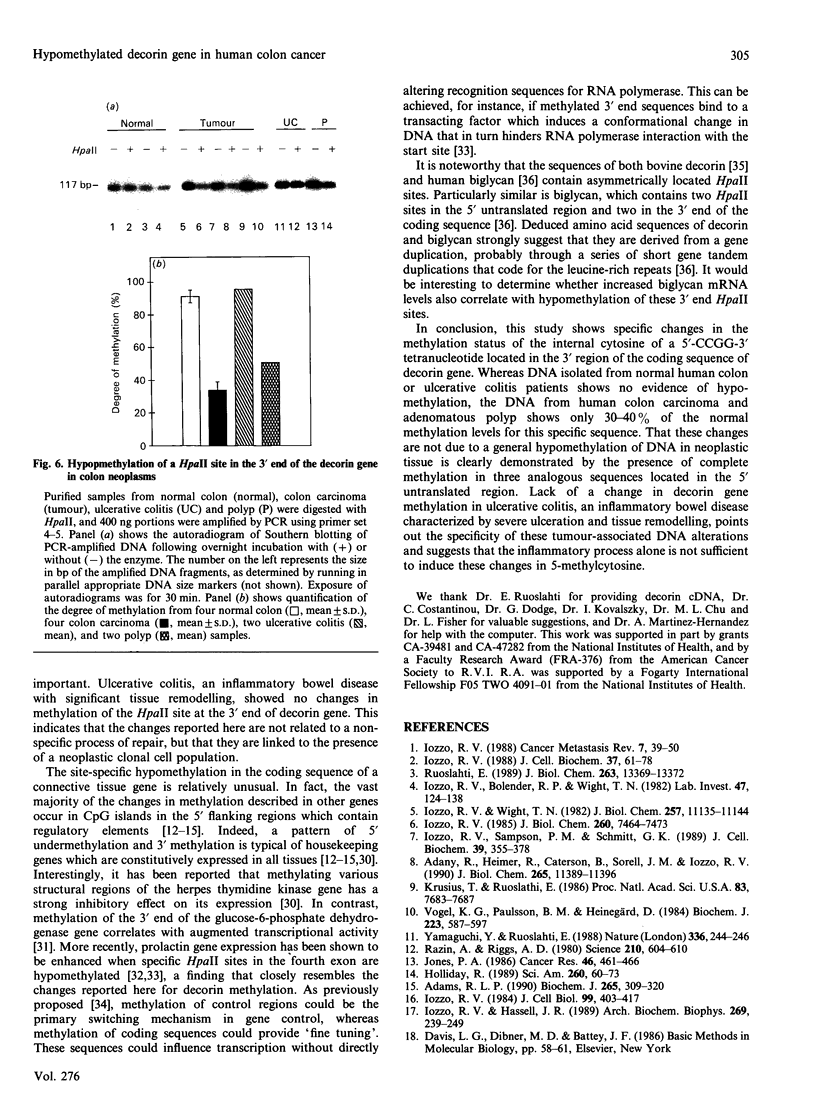
We have previously reported that the connective tissue stroma of human colon carcinoma contains elevated amounts of decorin, a small proteoglycan involved in the regulation of matrix formation and cell proliferation. These biochemical changes were correlated with increased mRNA levels and general hypomethylation of the decorin gene in human colon cancer DNA. In this report we use a quantitative polymerase chain reaction method coupled with digestion of the DNA template by methylation-sensitive restriction endonucleases to investigate in detail the location of hypomethylated sites in decorin gene. We demonstrate that a specific site in the 3' region of the gene, encompassing codons 360-361, is specifically hypomethylated in both colon carcinoma and benign polyp. In contrast, three HpaII sites, clustered in the 5' untranslated region, show full methylation in normal and neoplastic DNA. The lack of such changes in ulcerative colitis DNA suggests that chronic inflammation alone is not sufficient to alter cytosine methylation in the decorin gene. These results suggest the possibility that the 3' region of the decorin-coding sequence may be involved in the control of decorin gene expression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams R. L. DNA methylation. The effect of minor bases on DNA-protein interactions. Biochem J. 1990 Jan 15;265(2):309–320. doi: 10.1042/bj2650309. [DOI] [PMC free article] [PubMed] [Google Scholar]
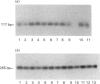
- Adany R., Heimer R., Caterson B., Sorrell J. M., Iozzo R. V. Altered expression of chondroitin sulfate proteoglycan in the stroma of human colon carcinoma. Hypomethylation of PG-40 gene correlates with increased PG-40 content and mRNA levels. J Biol Chem. 1990 Jul 5;265(19):11389–11396. [PubMed] [Google Scholar]
- Adany R., Iozzo R. V. Altered methylation of versican proteoglycan gene in human colon carcinoma. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1402–1413. doi: 10.1016/0006-291x(90)90841-a. [DOI] [PubMed] [Google Scholar]
- Battistuzzi G., D'Urso M., Toniolo D., Persico G. M., Luzzatto L. Tissue-specific levels of human glucose-6-phosphate dehydrogenase correlate with methylation of specific sites at the 3' end of the gene. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1465–1469. doi: 10.1073/pnas.82.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird A. P., Southern E. M. Use of restriction enzymes to study eukaryotic DNA methylation: I. The methylation pattern in ribosomal DNA from Xenopus laevis. J Mol Biol. 1978 Jan 5;118(1):27–47. doi: 10.1016/0022-2836(78)90242-5. [DOI] [PubMed] [Google Scholar]
- Day A. A., McQuillan C. I., Termine J. D., Young M. R. Molecular cloning and sequence analysis of the cDNA for small proteoglycan II of bovine bone. Biochem J. 1987 Dec 15;248(3):801–805. doi: 10.1042/bj2480801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Fisher L. W., Termine J. D., Young M. F. Deduced protein sequence of bone small proteoglycan I (biglycan) shows homology with proteoglycan II (decorin) and several nonconnective tissue proteins in a variety of species. J Biol Chem. 1989 Mar 15;264(8):4571–4576. [PubMed] [Google Scholar]
- Holliday R. A different kind of inheritance. Sci Am. 1989 Jun;260(6):60-5, 68-70, 73. doi: 10.1038/scientificamerican0689-60. [DOI] [PubMed] [Google Scholar]
- Iozzo R. V. Biosynthesis of heparan sulfate proteoglycan by human colon carcinoma cells and its localization at the cell surface. J Cell Biol. 1984 Aug;99(2):403–417. doi: 10.1083/jcb.99.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo R. V., Bolender R. P., Wight T. N. Proteoglycan changes in the intercellular matrix of human colon carcinoma: an integrated biochemical and stereologic analysis. Lab Invest. 1982 Aug;47(2):124–138. [PubMed] [Google Scholar]
- Iozzo R. V. Cell surface heparan sulfate proteoglycan and the neoplastic phenotype. J Cell Biochem. 1988 May;37(1):61–78. doi: 10.1002/jcb.240370107. [DOI] [PubMed] [Google Scholar]
- Iozzo R. V., Hassell J. R. Identification of the precursor protein for the heparan sulfate proteoglycan of human colon carcinoma cells and its post-translational modifications. Arch Biochem Biophys. 1989 Feb 15;269(1):239–249. doi: 10.1016/0003-9861(89)90105-7. [DOI] [PubMed] [Google Scholar]
- Iozzo R. V. Neoplastic modulation of extracellular matrix. Colon carcinoma cells release polypeptides that alter proteoglycan metabolism in colon fibroblasts. J Biol Chem. 1985 Jun 25;260(12):7464–7473. [PubMed] [Google Scholar]
- Iozzo R. V. Proteoglycans and neoplasia. Cancer Metastasis Rev. 1988 Apr;7(1):39–50. doi: 10.1007/BF00048277. [DOI] [PubMed] [Google Scholar]
- Iozzo R. V., Sampson P. M., Schmitt G. K. Neoplastic modulation of extracellular matrix: stimulation of chondroitin sulfate proteoglycan and hyaluronic acid synthesis in co-cultures of human colon carcinoma and smooth muscle cells. J Cell Biochem. 1989 Apr;39(4):355–378. doi: 10.1002/jcb.240390403. [DOI] [PubMed] [Google Scholar]
- Iozzo R. V., Wight T. N. Isolation and characterization of proteoglycans synthesized by human colon and colon carcinoma. J Biol Chem. 1982 Sep 25;257(18):11135–11144. [PubMed] [Google Scholar]
- Jones P. A. DNA methylation and cancer. Cancer Res. 1986 Feb;46(2):461–466. [PubMed] [Google Scholar]
- Keshet I., Yisraeli J., Cedar H. Effect of regional DNA methylation on gene expression. Proc Natl Acad Sci U S A. 1985 May;82(9):2560–2564. doi: 10.1073/pnas.82.9.2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krusius T., Ruoslahti E. Primary structure of an extracellular matrix proteoglycan core protein deduced from cloned cDNA. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7683–7687. doi: 10.1073/pnas.83.20.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V., Biswas D. K. Dynamic state of site-specific DNA methylation concurrent to altered prolactin and growth hormone gene expression in the pituitary gland of pregnant and lactating rats. J Biol Chem. 1988 Sep 5;263(25):12645–12652. [PubMed] [Google Scholar]
- Mann M. B., Smith H. O. Specificity of Hpa II and Hae III DNA methylases. Nucleic Acids Res. 1977 Dec;4(12):4211–4221. doi: 10.1093/nar/4.12.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland M., Hanish J., Nelson M., Patel Y. KGB: a single buffer for all restriction endonucleases. Nucleic Acids Res. 1988 Jan 11;16(1):364–364. doi: 10.1093/nar/16.1.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Razin A., Riggs A. D. DNA methylation and gene function. Science. 1980 Nov 7;210(4470):604–610. doi: 10.1126/science.6254144. [DOI] [PubMed] [Google Scholar]
- Riggs A. D., Jones P. A. 5-methylcytosine, gene regulation, and cancer. Adv Cancer Res. 1983;40:1–30. doi: 10.1016/s0065-230x(08)60678-8. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Proteoglycans in cell regulation. J Biol Chem. 1989 Aug 15;264(23):13369–13372. [PubMed] [Google Scholar]
- Singer-Sam J., LeBon J. M., Tanguay R. L., Riggs A. D. A quantitative HpaII-PCR assay to measure methylation of DNA from a small number of cells. Nucleic Acids Res. 1990 Feb 11;18(3):687–687. doi: 10.1093/nar/18.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vogel K. G., Paulsson M., Heinegård D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984 Nov 1;223(3):587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waalwijk C., Flavell R. A. MspI, an isoschizomer of hpaII which cleaves both unmethylated and methylated hpaII sites. Nucleic Acids Res. 1978 Sep;5(9):3231–3236. doi: 10.1093/nar/5.9.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi Y., Ruoslahti E. Expression of human proteoglycan in Chinese hamster ovary cells inhibits cell proliferation. Nature. 1988 Nov 17;336(6196):244–246. doi: 10.1038/336244a0. [DOI] [PubMed] [Google Scholar]
- Zhang Z. X., Kumar V., Rivera R. T., Pasion S. G., Chisholm J., Biswas D. K. Suppression of prolactin gene expression in GH cells correlates with site-specific DNA methylation. DNA. 1989 Oct;8(8):605–613. doi: 10.1089/dna.1989.8.605. [DOI] [PubMed] [Google Scholar]